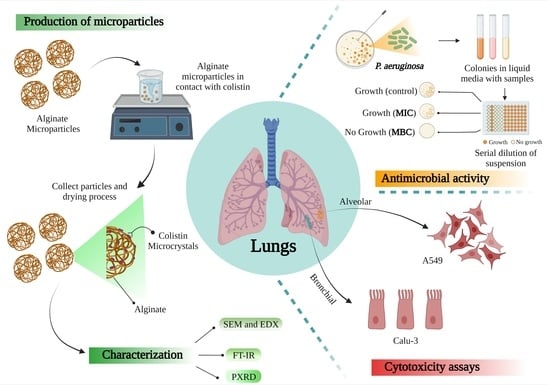
Engineering Alginate-Based Dry Powder Microparticles to a Size Suitable for the Direct Pulmonary Delivery of Antibiotics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
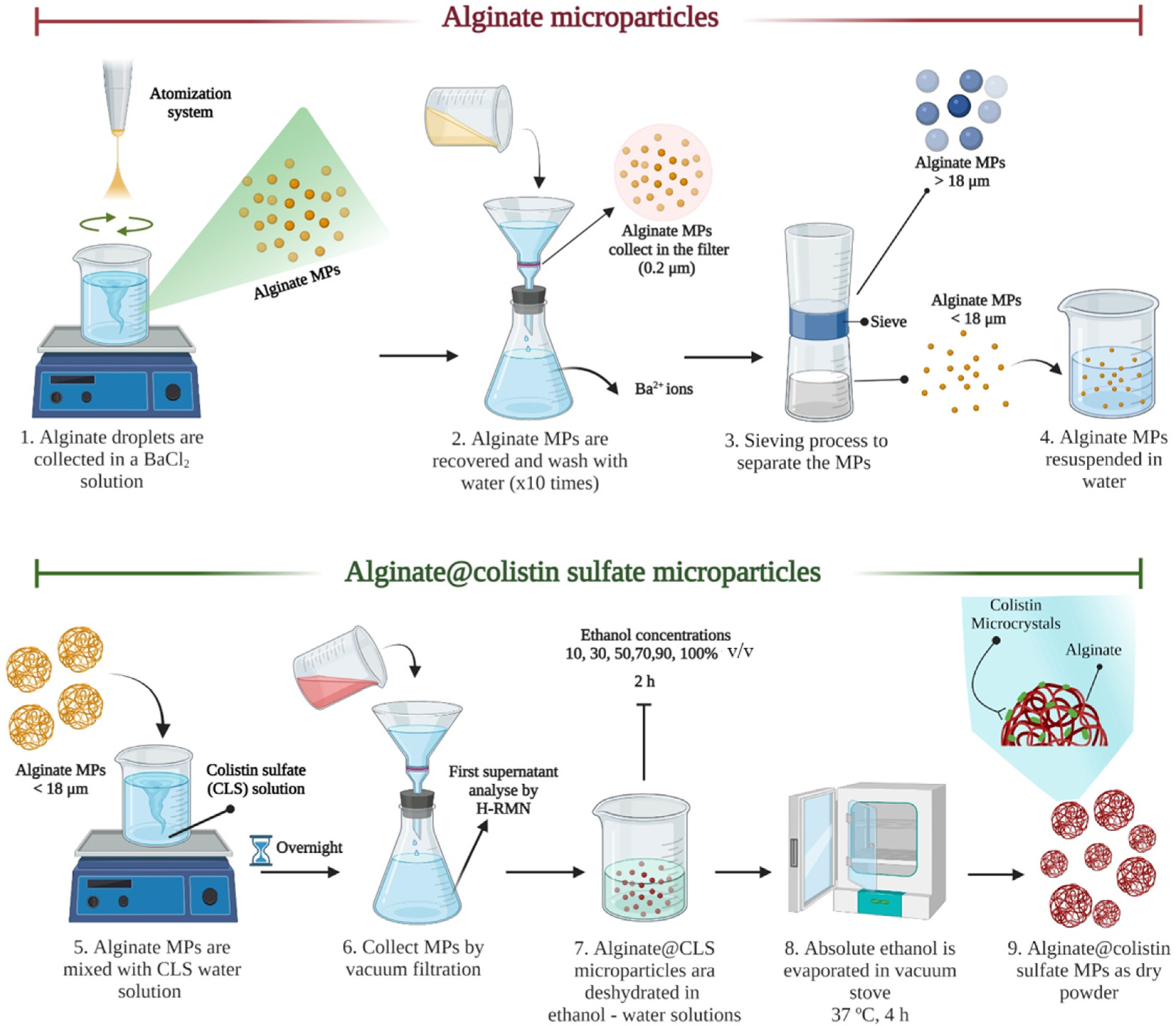
2.2. Atomization Device and Production of Inhalable Powder Formulation
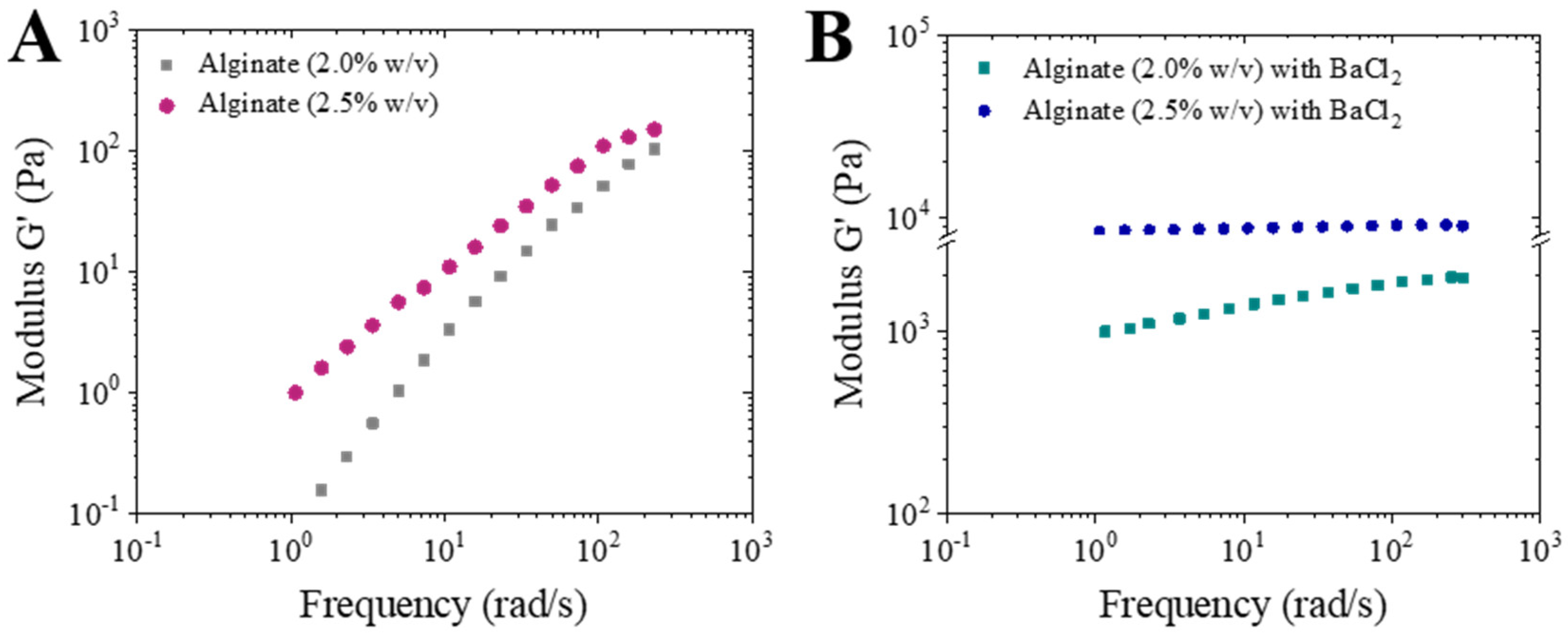
2.3. Rheological Experiments
2.4. Size and Zeta Potential Measurement
2.5. Powder X-ray Diffraction (XRD)
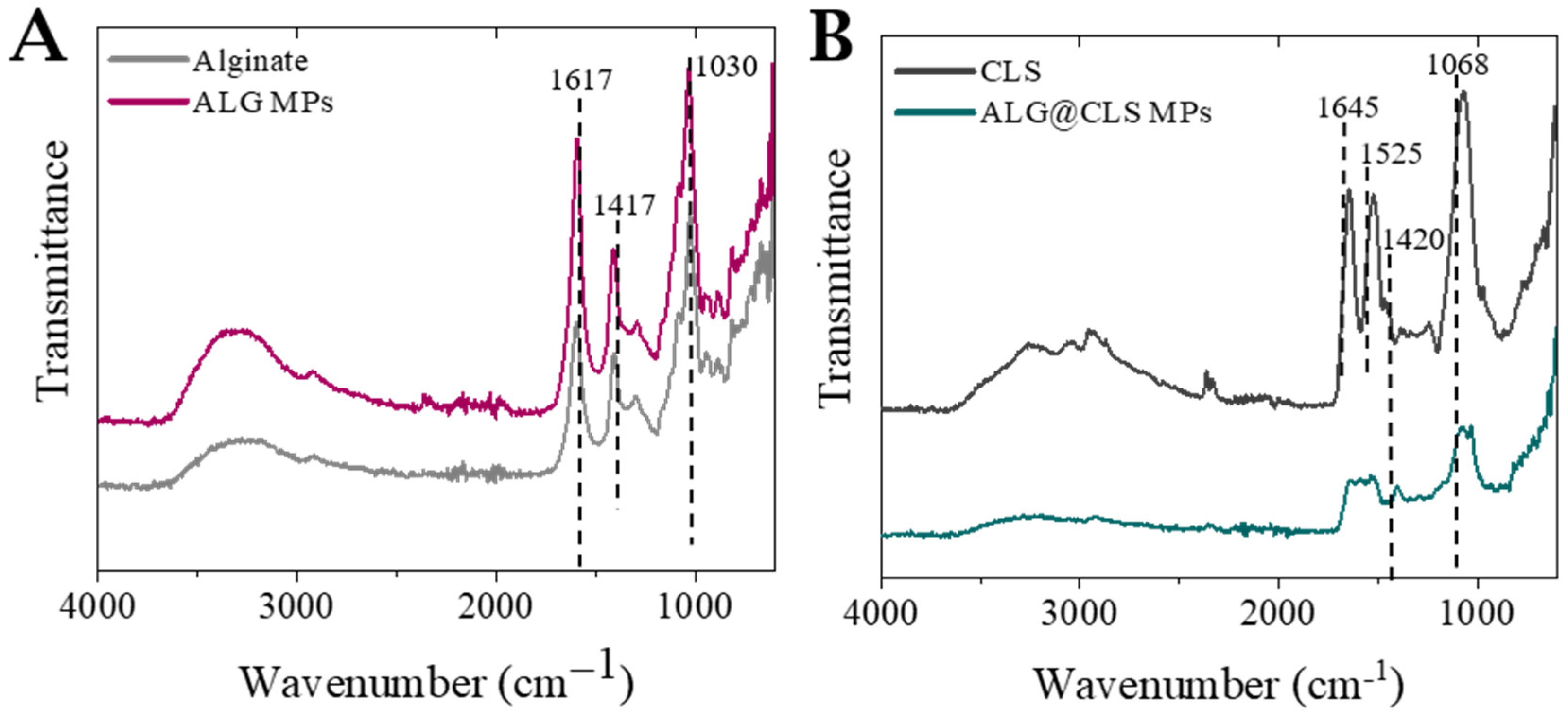
2.6. Solid State Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Drug Quantification
2.7.1. Encapsulation Efficiency of Microparticles (% EE) and Drug Loading (% DL)
2.7.2. In Vitro Drug Release
2.7.3. Ultra-Performance Liquid Chromatography (UPLC)
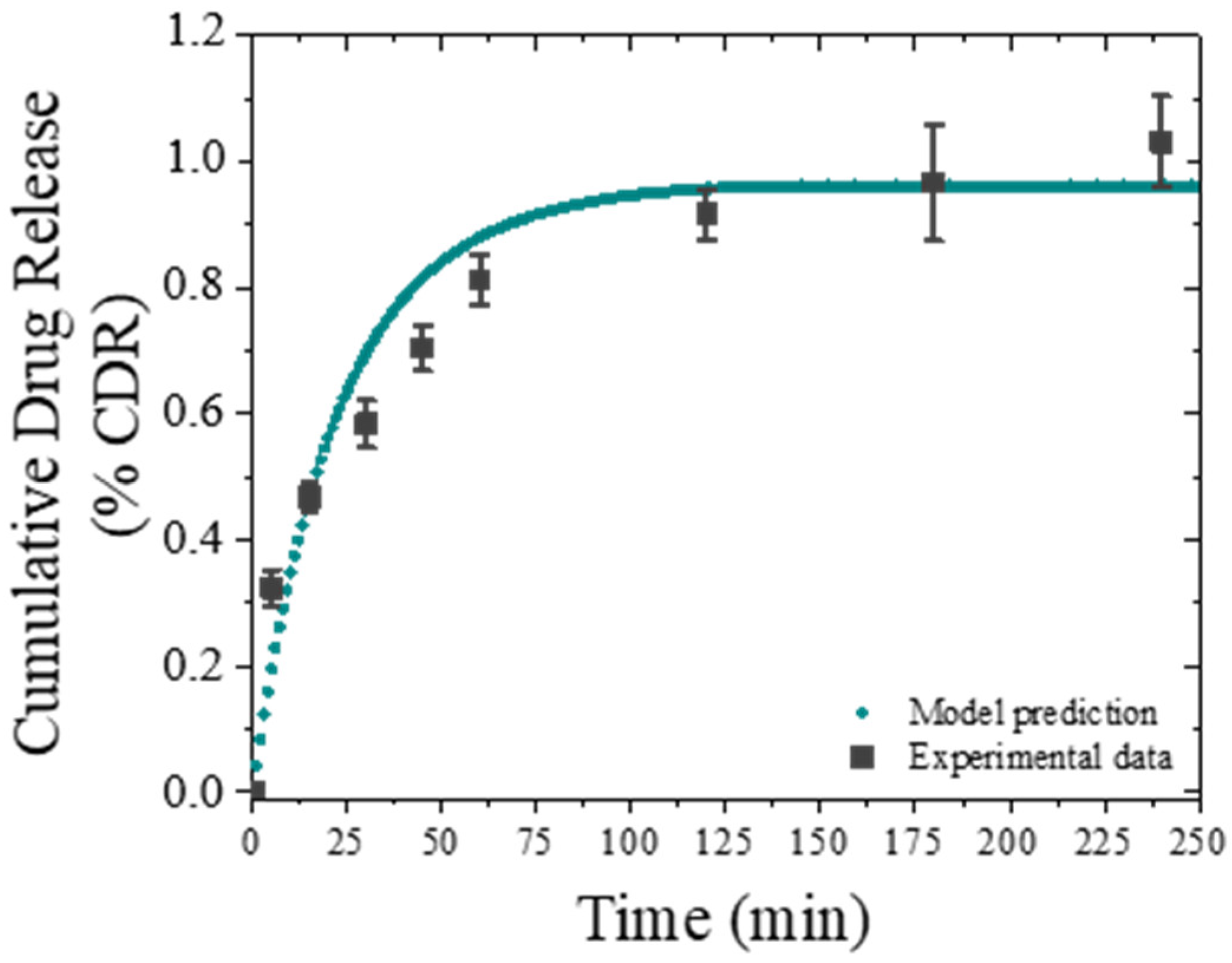
2.8. Drug Release Modelling
2.9. Antimicrobial Activity
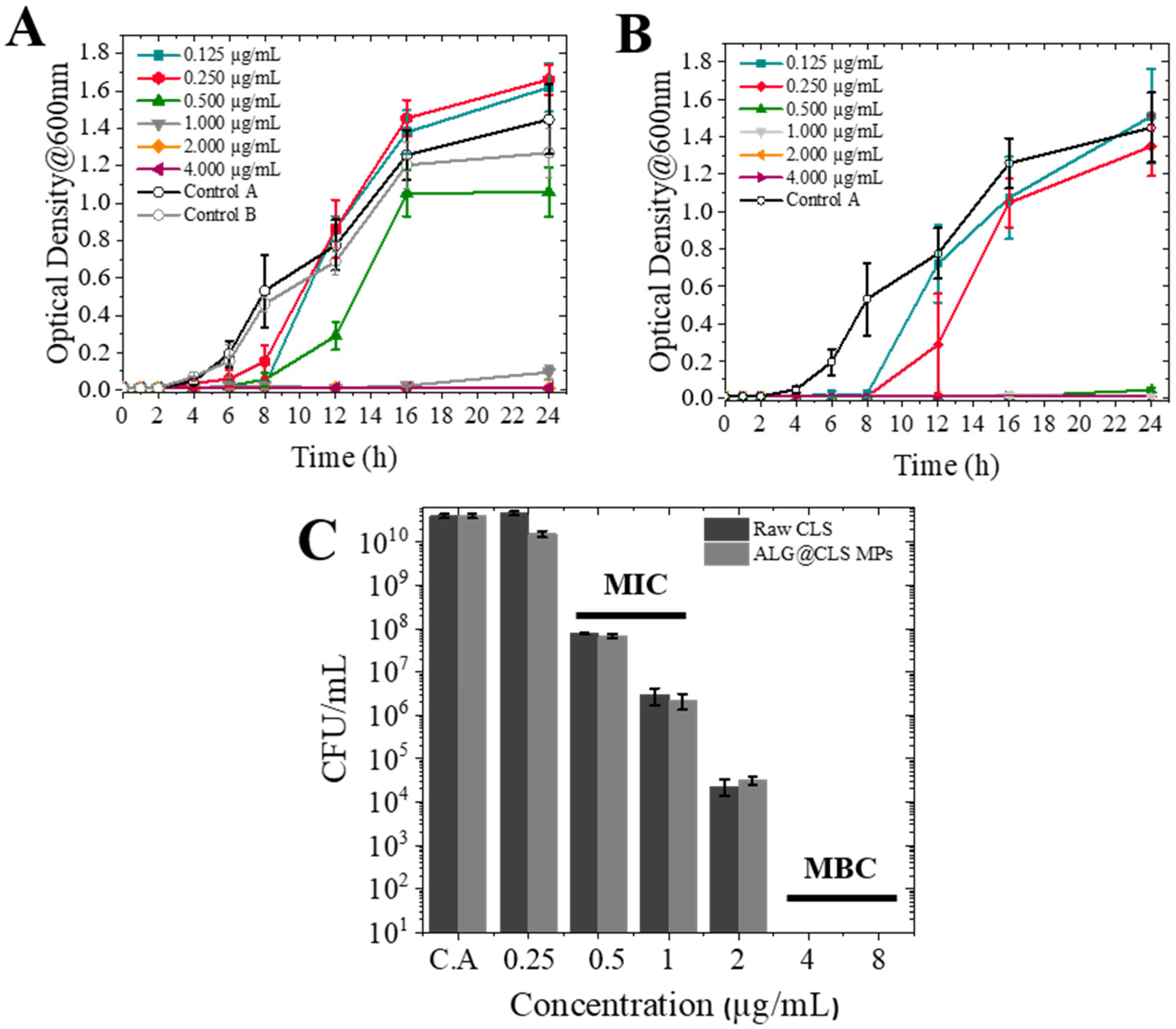
2.9.1. Optical Density (OD600) Measurements
2.9.2. Agar Dilution Method
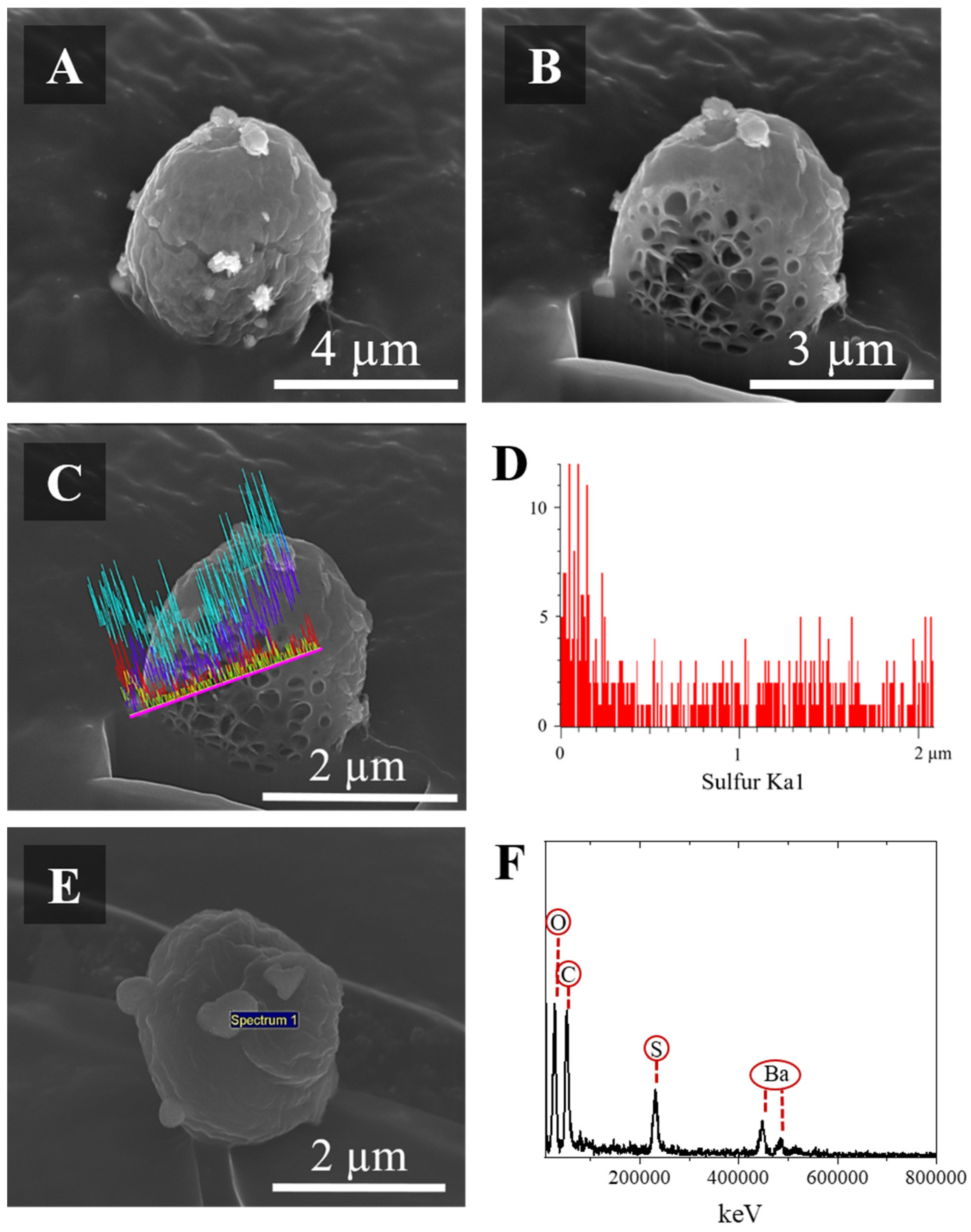
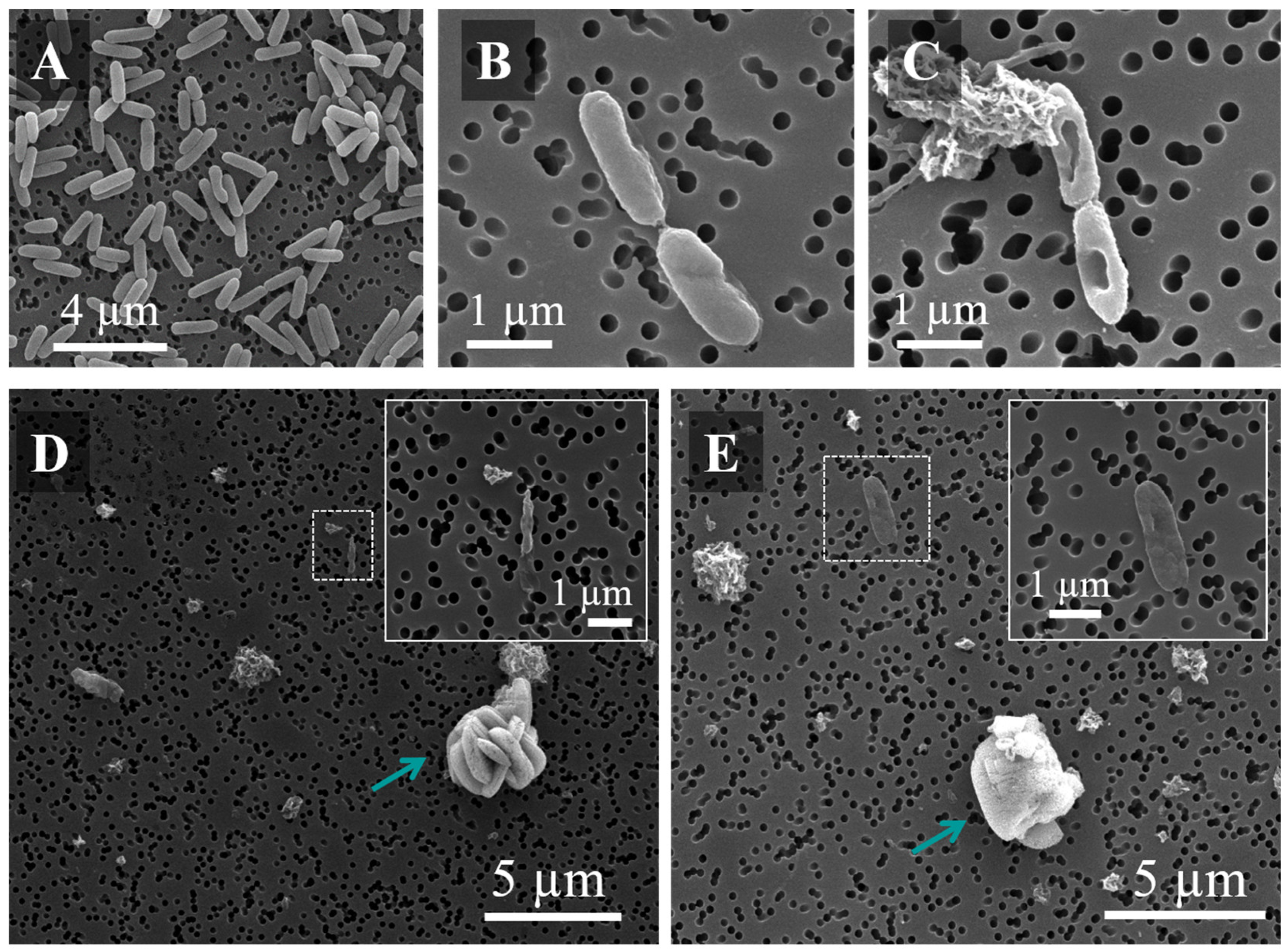
2.9.3. SEM Analysis
2.10. Cytotoxicity Studies
2.10.1. Cell Culture Conditions
2.10.2. Alamar Blue (AB) Assay
3. Results and Discussion
3.1. Rheological Analysis, Atomization and Concentration Effect in Microparticles Generation
3.2. Characterization of Microparticles
3.3. In Vitro Release
3.4. Antimicrobial Activity against Pseudomonas aeruginosa
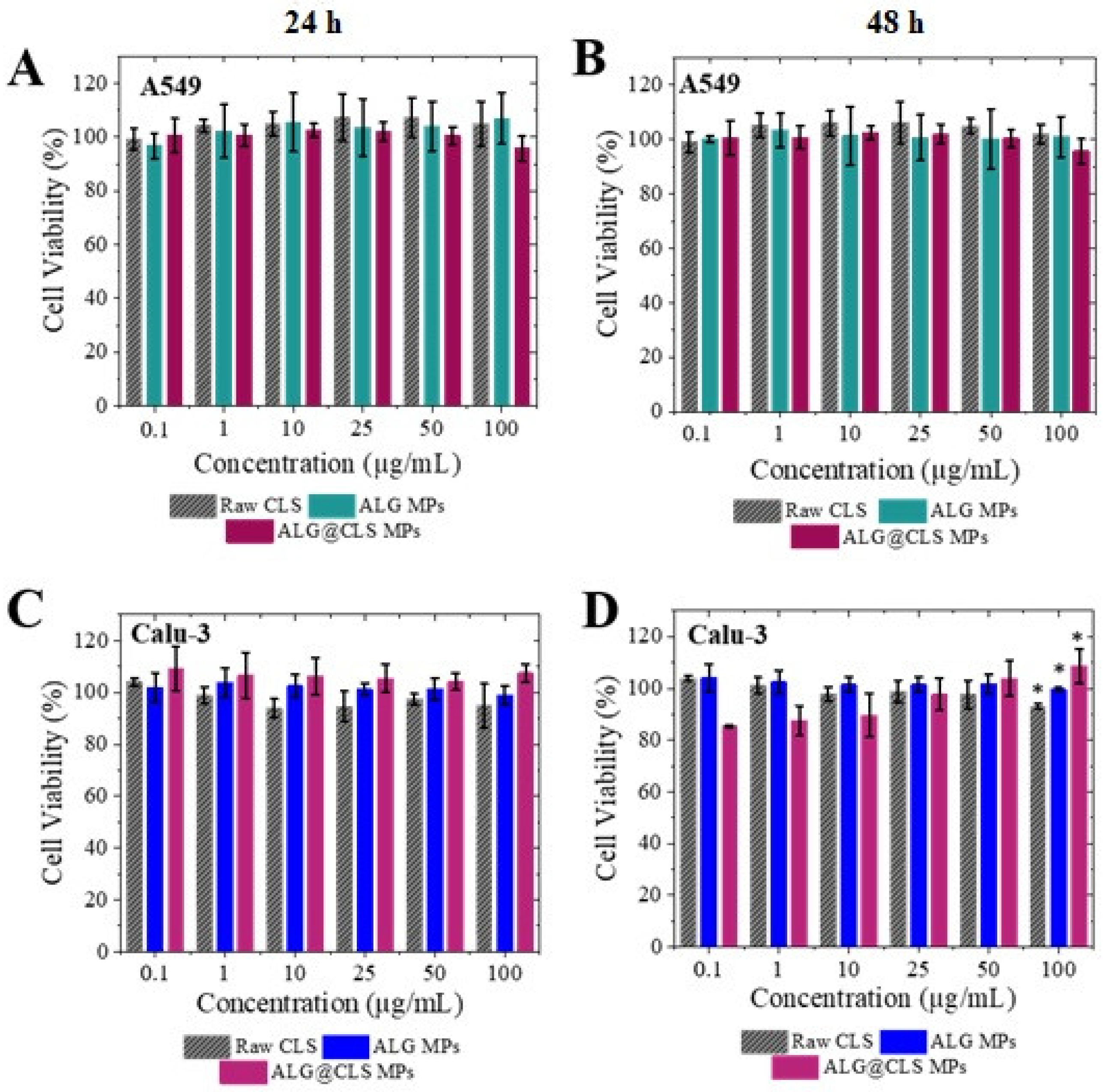
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shetty, N.; Ahn, P.; Park, H.; Bhujbal, S.; Zemlyanov, D.; Cavallaro, A.; Mangal, S.; Li, J.; Zhou, Q.T. Improved physical stability and aerosolization of inhalable amorphous ciprofloxacin powder formulations by incorporating synergistic colistin. Mol. Pharm. 2018, 15, 4004–4020. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, D.; Chan, H.K. Inhaled antibiotics to treat lung infection. Pharm. Pat. Anal. 2013, 2, 647–663. [Google Scholar] [CrossRef] [PubMed]
- Michalopoulos, A.S. Aerosolized antibiotics: The past, present and future, with a special emphasis on inhaled colistin. Expert Opin. Drug Deliv. 2012, 9, 493–495. [Google Scholar] [CrossRef] [PubMed]
- Mangal, S.; Park, H.; Zeng, L.; Yu, H.H.; Lin, Y.W.; Velkov, T.; Denman, J.A.; Zemlyanov, D.; Li, J.; Zhou, Q.T. Composite particle formulations of colistin and meropenem with improved in-vitro bacterial killing and aerosolization for inhalation. Int. J. Pharm. 2018, 548, 443–453. [Google Scholar] [CrossRef]
- Mangal, S.; Park, H.; Nour, R.; Shetty, N.; Cavallaro, A.; Zemlyanov, D.; Thalberg, K.; Puri, V.; Nicholas, M.; Narang, A.S.; et al. Correlations between surface composition and aerosolization of jet-milled dry powder inhaler formulations with pharmaceutical lubricants. Int. J. Pharm. 2019, 568, 118504. [Google Scholar] [CrossRef] [PubMed]
- Jong, T.; Li, J.; Mortonx, D.A.V.; Zhou, Q.T.; Larson, I. Investigation of the changes in aerosolization behavior between the jet-milled and spray-dried colistin powders through surface energy characterization. J. Pharm. Sci. 2016, 105, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Wang, S.; Zou, P.; Chai, G.; Lin, Y.W.; Velkov, T.; Li, J.; Pan, W.; Zhou, Q.T. Inhalable liposomal powder formulations for co-delivery of synergistic ciprofloxacin and colistin against multi-drug resistant gram-negative lung infections. Int. J. Pharm. 2020, 575, 118915. [Google Scholar] [CrossRef]
- Sans-Serramitjana, E.; Fusté, E.; Martínez-Garriga, B.; Merlos, A.; Pastor, M.; Pedraz, J.L.; Esquisabel, A.; Bachiller, D.; Vinuesa, T.; Viñas, M. Killing effect of nanoencapsulated colistin sulfate on Pseudomonas aeruginosa from cystic fibrosis patients. J. Cyst. Fibros. 2016, 15, 611–618. [Google Scholar] [CrossRef]
- O’Driscoll, N.H.; Cushnie, T.P.T.; Matthews, K.H.; Lamb, A.J. Colistin causes profound morphological alteration but minimal cytoplasmic membrane perforation in populations of Escherichia coli and Pseudomonas aeruginosa. Arch. Microbiol. 2018, 200, 793–802. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, S.P.; Chan, J.G.Y.; Wang, P.; Barraud, N.; Rice, S.A.; Wang, J.; Li, J.; Chan, H.K. Novel Inhaled Combination Powder Containing Amorphous Colistin and Crystalline Rifapentine with Enhanced Antimicrobial Activities against Planktonic Cells and Biofilm of Pseudomonas aeruginosa for Respiratory Infections. Mol. Pharm. 2015, 12, 2594–2603. [Google Scholar] [CrossRef]
- Maselli, D.J.; Keyt, H.; Restrepo, M.I. Inhaled antibiotic therapy in chronic respiratory diseases. Int. J. Mol. Sci. 2017, 18, 1062. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Okuda, T.; Lu, X.Y.; Chan, H.K. Amorphous powders for inhalation drug delivery. Adv. Drug Deliv. Rev. 2016, 100, 102–115. [Google Scholar] [CrossRef]
- Zhou, Q.; Gengenbach, T.; Denman, J.A.; Yu, H.H.; Li, J.; Chan, H.K. Synergistic antibiotic combination powders of colistin and rifampicin provide high aerosolization efficiency and moisture protection. AAPS J. 2014, 16, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Wang, T.; Rintoul, L.; Ayoko, G.A.; Islam, N.; Izake, E.I. Development and characterization of meropenem dry powder inhaler formulation for pulmonary drug delivery. Int. J. Pharm. 2020, 587, 119684. [Google Scholar] [CrossRef] [PubMed]
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Leopold, C.S.; Gurikov, P.; Smirnova, I. Alginate and hybrid alginate-hyaluronic acid aerogel microspheres as potential carrier for pulmonary drug delivery. J. Supercrit. Fluids 2019, 150, 49–55. [Google Scholar] [CrossRef]
- Mahmoud, A.A.; Elkasabgy, N.A.; Abdelkhalek, A.A. Design and characterization of emulsified spray dried alginate microparticles as a carrier for the dually acting drug roflumilast. Eur. J. Pharm. Sci. 2018, 122, 64–76. [Google Scholar] [CrossRef]
- Abad, I.; Conesa, C.; Sánchez, L. Development of encapsulation strategies and composite edible films to maintain lactoferrin bioactivity: A review. Materials 2021, 14, 7358. [Google Scholar] [CrossRef] [PubMed]
- Dhanka, M.; Shetty, C.; Srivastava, R. Methotrexate loaded alginate microparticles and effect of Ca2+ post-crosslinking: An in vitro physicochemical and biological evaluation. Int. J. Biol. Macromol. 2018, 110, 294–307. [Google Scholar] [CrossRef]
- Trinh, K.T.L.; Le, N.X.T.; Lee, N.Y. Microfluidic-based fabrication of alginate microparticles for protein delivery and its application in the in vitro chondrogenesis of mesenchymal stem cells. J. Drug Deliv. Sci. Technol. 2021, 66, 102735. [Google Scholar] [CrossRef]
- del Valle, E.M.M.; Herrero, E.P.; Martins, D.A.O.; Galan, M.A. Immobilisation of cells in biocompatible films to cell therapy. Open Tissue Eng. Regen. Med. J. 2009, 2, 14–19. [Google Scholar] [CrossRef]
- Baimark, Y.; Srisuwan, Y. Preparation of alginate microspheres by water-in-oil emulsion method for drug delivery: Effect of Ca2+ post-cross-linking. Adv. Powder Technol. 2014, 25, 1541–1546. [Google Scholar] [CrossRef]
- Santa-Maria, M.; Scher, H.; Jeoh, T. Microencapsulation of bioactives in cross-linked alginate matrices by spray drying. J. Microencapsul. 2012, 29, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Cerveró, J.M.; Nogareda, J.; del Valle, E.M.M.; Galán, M.A. Development of a technology to produce monodispersed microparticles based on the formation of drops from viscous non-Newtonian liquids sprayed through a fan jet nozzle. Chem. Eng. J. 2011, 174, 699–708. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Q.T.; Sun, S.P.; Denman, J.A.; Gengenbach, T.R.; Barraud, N.; Rice, S.A.; Li, J.; Yang, M.; Chan, H.K. Effects of Surface Composition on the Aerosolisation and Dissolution of Inhaled Antibiotic Combination Powders Consisting of Colistin and Rifampicin. AAPS J. 2016, 18, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Herrero, E.P.; del Valle, E.M.M.; Galan, M.A. Modelling prediction of the microcapsule size of polyelectrolyte complexes produced by atomization. Chem. Eng. J. 2006, 121, 1–8. [Google Scholar] [CrossRef]
- Arauzo, B.; Gonzalez-Garcinuño, A.; Tabernero, A.; Lobera, M.; Santamaria, J.; del Valle, E.M. Tuning Alginate Microparticle Size via Atomization of Non-Newtonian Fluids. Materials 2021, 14, 7601. [Google Scholar] [CrossRef]
- National Formulary, United States Pharmacopeia. Validation of Compendial Methods: Twenty-Sixth Revision, 21st ed.; The United States Pharmacopeial Convention Inc.: Rockville, MD, USA, 2003. [Google Scholar]
- Magnuusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Eurachem: London, UK, 2014; Available online: www.eurachem.org (accessed on 19 November 2021).
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Moroney, K.M.; Vynnycky, M. Mathematical modelling of drug release from a porous granule. Appl. Math. Model. 2021, 100, 432–452. [Google Scholar] [CrossRef]
- Aaron, H.B.; Kotler, G.R. The effects of curvature on the dissolution kinetics of spherical precipitates. Met. Sci. J. 1970, 4, 222–225. [Google Scholar] [CrossRef]
- Lopez-Mendez, T.B.; Santos-Vizcaino, E.; Pedraz, J.L.; Orive, G.; Hernandez, R.M. Cell microencapsulation technologies for sustained drug delivery: Latest advances in efficacy and biosafety. J. Control. Release 2021, 335, 619–636. [Google Scholar] [CrossRef]
- Li, P.; Dail, Y.N.; Zhang, J.P.; Wang, A.Q.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedipine. Int. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar] [PubMed]
- Simsek-Ege, F.A.; Bond, G.M.; Stringer, J. Polyelectrolye complex formation between alginate and chitosan as a function of pH. J. Appl. Polym. Sci. 2003, 88, 346–351. [Google Scholar] [CrossRef]
- Strydom, S.; Liebenberg, W.; Yu, L.; de Villiers, M. The effect of temperature and moisture on the amorphous-to-crystalline transformation of stavudine. Int. J. Pharm. 2009, 379, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Douroumis, D.; Fahr, A. Stable carbamazepine colloidal systems using the cosolvent technique. Eur. J. Pharm. Sci. 2007, 30, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Rasenack, N.; Müller, B.W. Properties of ibuprofen crystallized under various conditions: A comparative study. Drug Dev. Ind. Pharm. 2002, 28, 1077–1089. [Google Scholar] [CrossRef]
- Raghavan, S.L.; Trividic, A.; Davis, A.F.; Hadgraft, J. Crystallization of hydrocortisone acetate: Influence of polymers. Int. J. Pharm. 2001, 212, 213–221. [Google Scholar] [CrossRef]
- Tan, J.; Namuangruk, S.; Kong, W.; Kungwan, N.; Guo, J.; Wang, C. Manipulation of Amorphous-to-Crystalline Transformation: Towards the Construction of Covalent Organic Framework Hybrid Microspheres with NIR Photothermal Conversion Ability. Angew. Chem. Int. Ed. 2016, 55, 13979–13984. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; O’Reilly, N.J. Polymeric Amorphous Solid Dispersions: A Review of Amorphization, Crystallization, Stabilization, Solid-State Characterization, and Aqueous Solubilization of Biopharmaceutical Classification System Class II Drugs. J. Pharm. Sci. 2016, 105, 2527–2544. [Google Scholar] [CrossRef]
- Shetty, N.; Zeng, L.; Mangal, S.; Nie, H.; Rowles, M.R.; Guo, R.; Han, Y.; Park, J.H.; Zhou, Q.T. Effects of moisture-induced crystallization on the aerosol performance of spray dried amorphous ciprofloxacin powder formulations. Pharm. Res. 2019, 35, 139–148. [Google Scholar] [CrossRef]


| SEM | Zeta Potential (mV) | % DL | % EE | |||||
|---|---|---|---|---|---|---|---|---|
| Samples | Mean ± SD (µm) | D 0.1 (µm) | D 0.1 (µm) | D 0.1 (µm) | Span | |||
| Colistin sulfate | 173.30 ± 47.5 | 108.97 | 177.05 | 229.97 | 0.68 | 7.37 ± 0.9 | - | - |
| Alginate MPs | 4.80 ± 1.6 | 2.87 | 4.62 | 6.76 | 0.84 | −35.14 ± 0.4 | - | - |
| Alg@CLS MPs | 4.45 ± 1.4 | 2.80 | 4.35 | 6.48 | 0.85 | −14.14 ± 0.1 | 8.50 ± 1.5 | 28.80 ± 1.1 |
| Parameters Estimated | Goodness of Fit Test | Lack of Fit Test | ||||
|---|---|---|---|---|---|---|
| D (m2/s) | K (1/m2s) | m | Weighted Residuals | X2 | F-Value | F-Critical |
| 7.84 × 10−9 | 2.35 × 108 | 1.81 × 10−9 | 10.98 | 11.07 | 0.069 | 3.326 |
| Confidence interval 95% | ||||||
| D (7.73 × 10−9–7.89 × 10−9) | k (2.30 × 108–2.40 × 108) | m (1.77 × 10−9–1.85 × 10−9) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arauzo, B.; González-Garcinuño, Á.; Tabernero, A.; Calzada-Funes, J.; Lobera, M.P.; del Valle, E.M.M.; Santamaria, J. Engineering Alginate-Based Dry Powder Microparticles to a Size Suitable for the Direct Pulmonary Delivery of Antibiotics. Pharmaceutics 2022, 14, 2763. https://doi.org/10.3390/pharmaceutics14122763
Arauzo B, González-Garcinuño Á, Tabernero A, Calzada-Funes J, Lobera MP, del Valle EMM, Santamaria J. Engineering Alginate-Based Dry Powder Microparticles to a Size Suitable for the Direct Pulmonary Delivery of Antibiotics. Pharmaceutics. 2022; 14(12):2763. https://doi.org/10.3390/pharmaceutics14122763
Chicago/Turabian StyleArauzo, Beatriz, Álvaro González-Garcinuño, Antonio Tabernero, Javier Calzada-Funes, María Pilar Lobera, Eva M. Martín del Valle, and Jesus Santamaria. 2022. "Engineering Alginate-Based Dry Powder Microparticles to a Size Suitable for the Direct Pulmonary Delivery of Antibiotics" Pharmaceutics 14, no. 12: 2763. https://doi.org/10.3390/pharmaceutics14122763
APA StyleArauzo, B., González-Garcinuño, Á., Tabernero, A., Calzada-Funes, J., Lobera, M. P., del Valle, E. M. M., & Santamaria, J. (2022). Engineering Alginate-Based Dry Powder Microparticles to a Size Suitable for the Direct Pulmonary Delivery of Antibiotics. Pharmaceutics, 14(12), 2763. https://doi.org/10.3390/pharmaceutics14122763