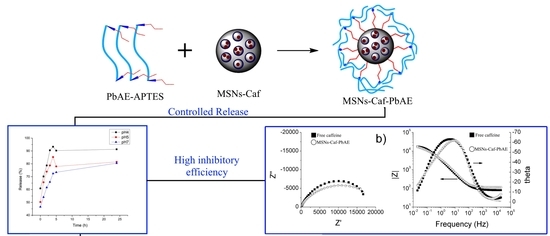
Synthesis and Electrochemical Evaluation of MSNs-PbAE Nanocontainers for the Controlled Release of Caffeine as a Corrosion Inhibitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
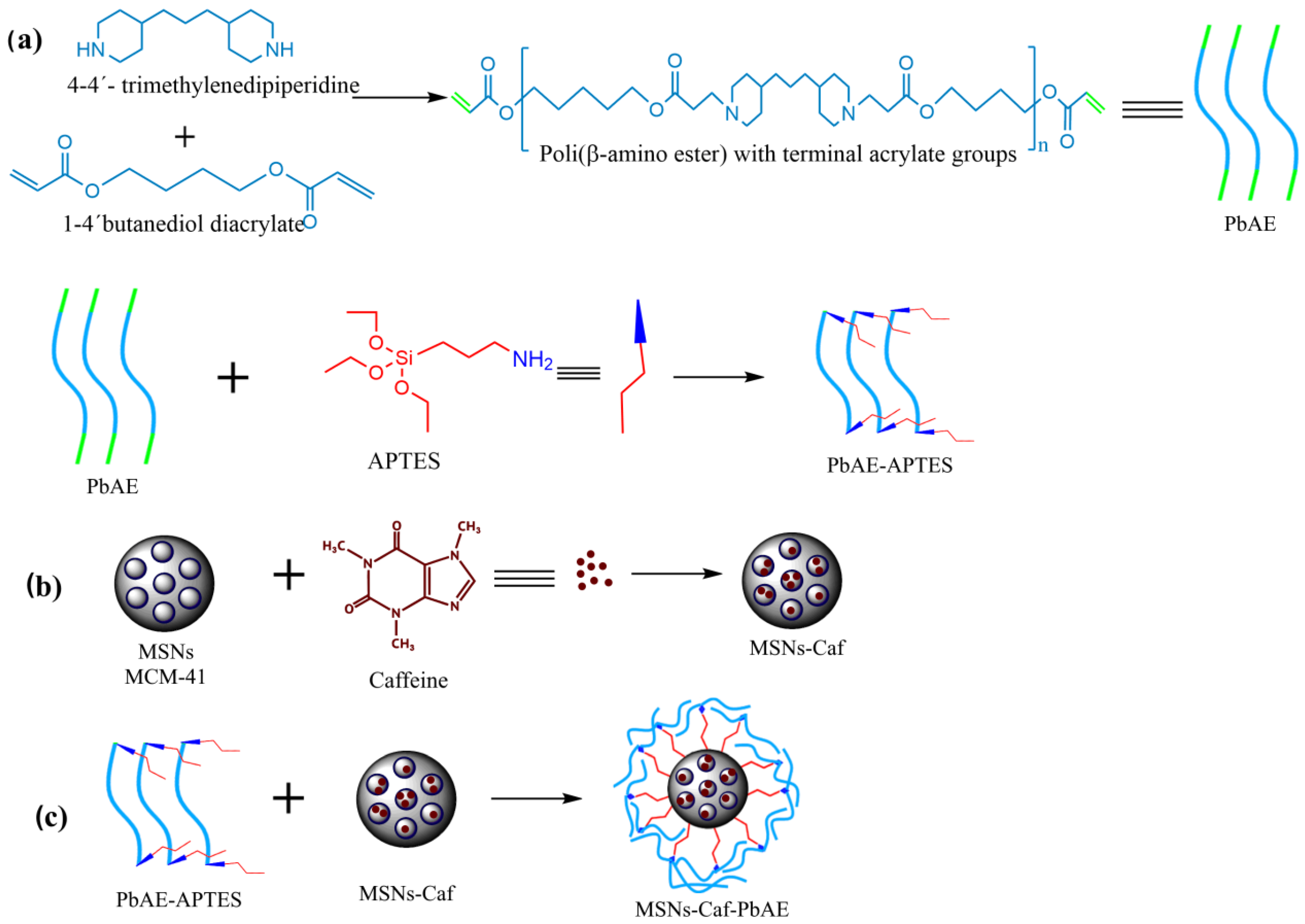
2.2. MSNs Synthesis
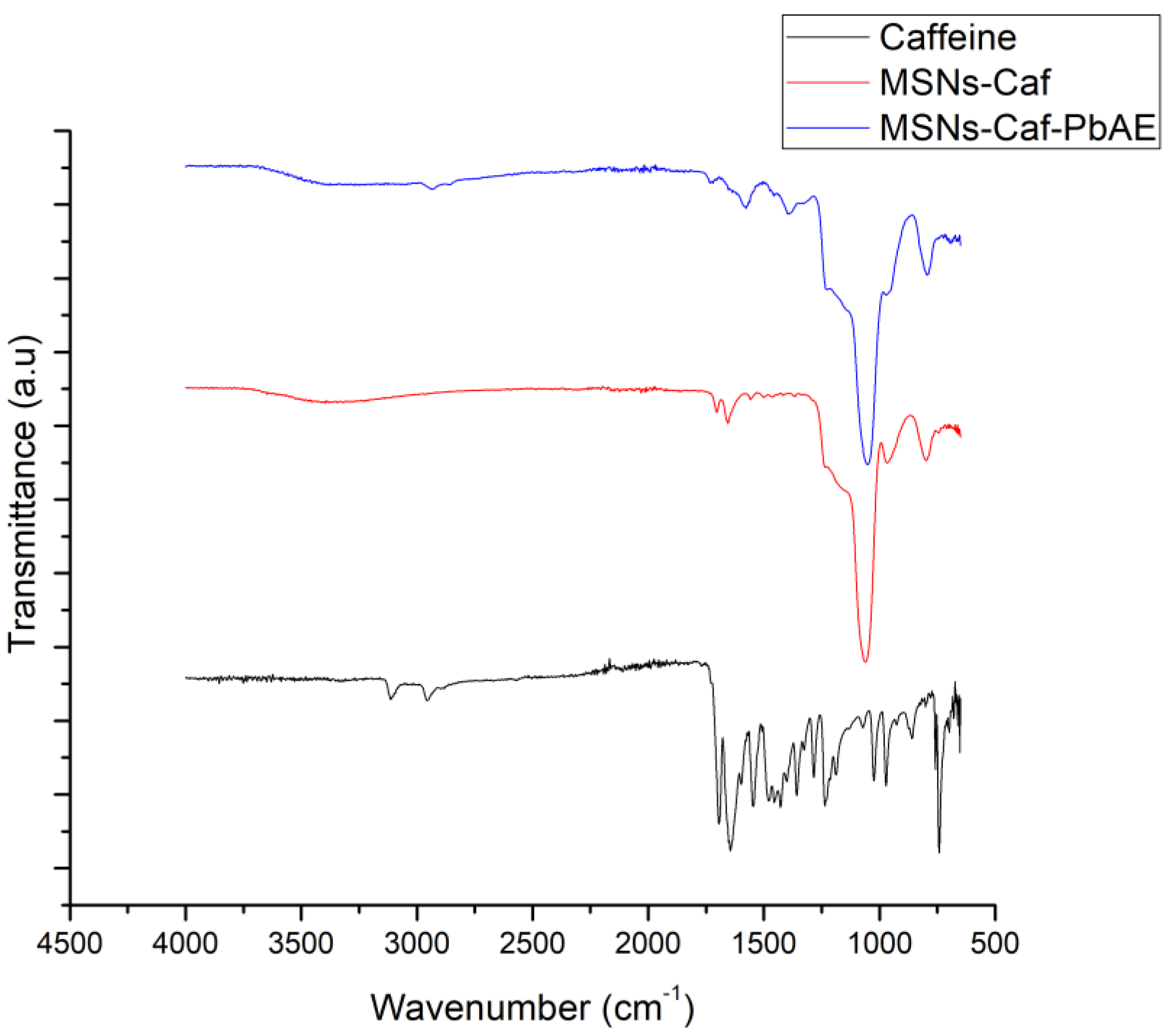
2.3. Caffeine Encapsulation
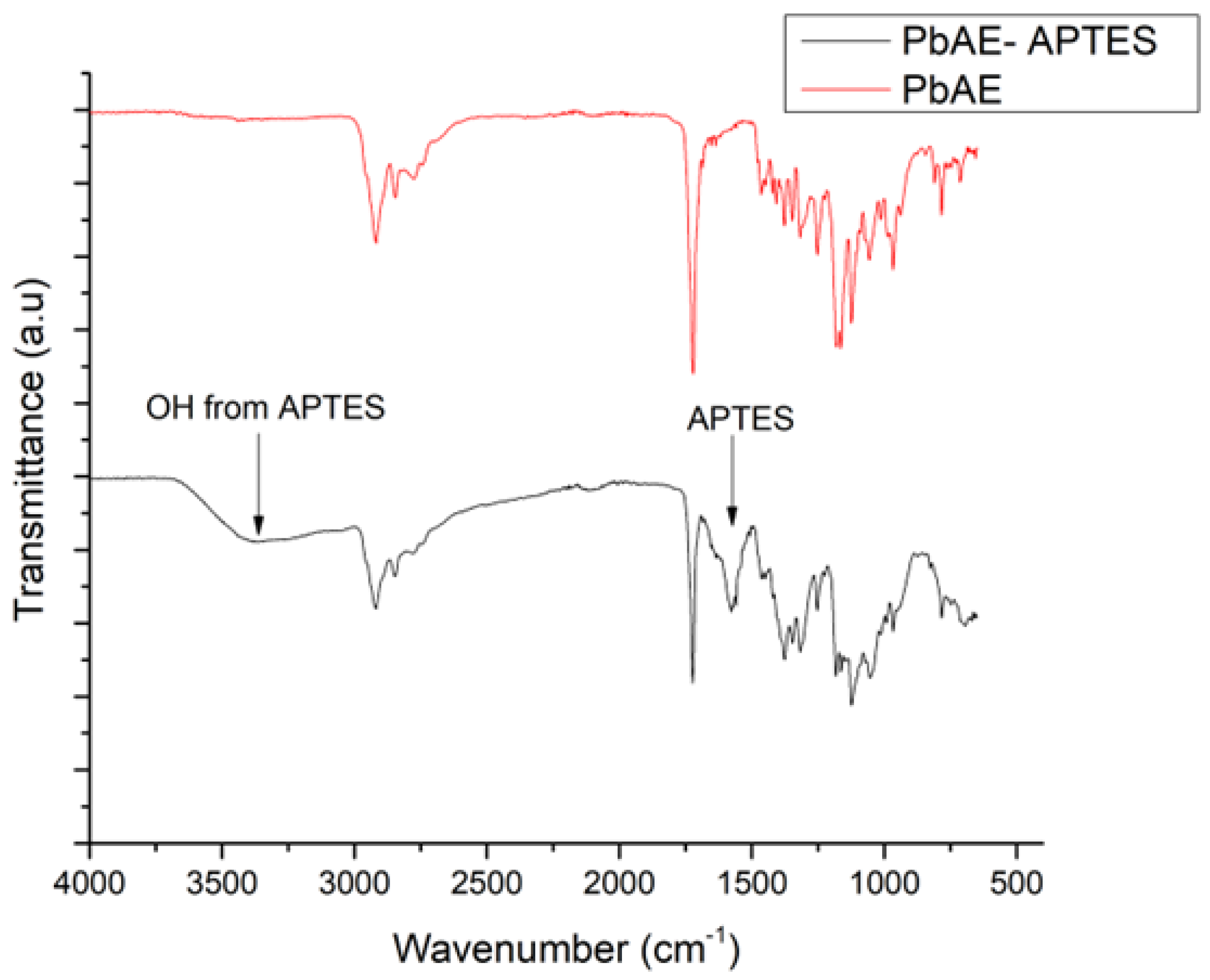
2.4. PbAE Synthesis
2.5. Modification of MSNs with PbAE
2.6. Characterization of Nanocontainers
2.7. Evaluation of Caffeine Release from MSNs Using UV–Vis Spectroscopy
2.8. Electrochemical Characterization
3. Results and Discussion
3.1. Characterization of MSNs
3.2. Characterization of Nanocontainers
3.2.1. FTIR
3.2.2. TGA
3.3. Controlled Release of Caffeine in Solutions with Different pH Values
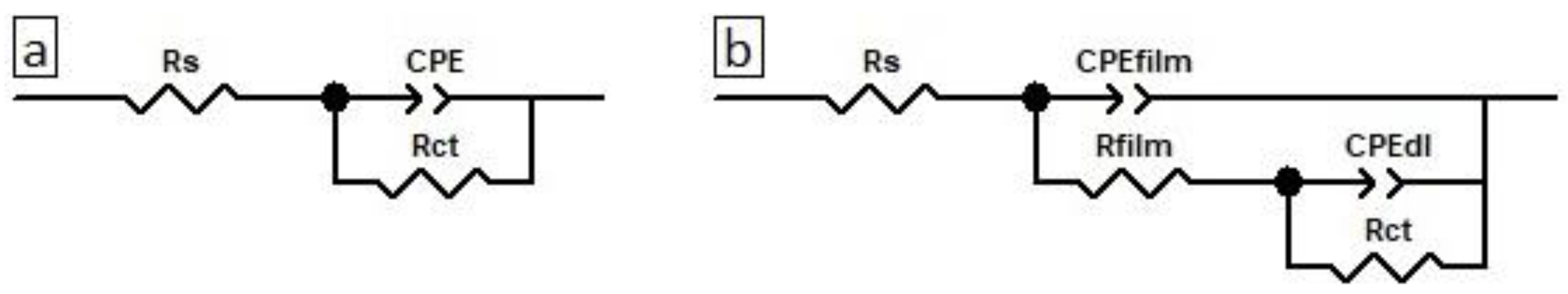
3.4. Electrochemical Evaluation of Caffeine and MSNs-Caf-PbAE Nanocontainers as Corrosion Inhibitors Using Potentiodynamic Polarization and Electrochemical Impedance Spectroscopy
- Rs = solution resistance.
- Rct = charge transfer resistance.
- n = exponent.
- D = fractal dimension.
- n = exponent.
| Caffeine Concentration ppm | Rs Ω | Ω−1 cm−2 sn | n | Rct Ω cm2 | Cdl μF cm−2 | D |
|---|---|---|---|---|---|---|
| 0 | 25.35 | 1.34 × 10−4 | 0.74 | 3135 | 18.13 | 2.35 |
| 150 | 24.63 | 2.40 × 10−4 | 0.77 | 3495 | 51.72 | 2.29 |
| 324 | 78.54 | 6.97 × 10−5 | 0.77 | 19,435 | 14.69 | 2.29 |
- Cfilm = film effective capacitance.
- Qfilm = film capacitance.
- n = exponent.
- Rfilm = film resistance.
| Rs Ω | Ω−1 cm−2 sn | n | Rfilm Ω cm2 | Cfilm μF cm−2 | Ω−1 cm−2 sn | n | Rct Ω cm2 | Cdl μF cm−2 | D |
|---|---|---|---|---|---|---|---|---|---|
| 112 | 3.90 × 10−5 | 0.86 | 4248 | 29.10 | 8.82 × 10−5 | 0.58 | 16,927 | 3.09 | 2.35 |
- θ = degree of inhibitory coating on the surface of the metal.
- icorr = corrosion rate without inhibitor (mA/cm2).
- icorr(inh) = rate of corrosion with inhibitor (mA/cm2).
- B = a constant of proportionality, which is equal to 0.026 V.
- Rp = resistance to polarization (Ω cm2).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedeferri, P. General Principles of Corrosion. In Corrosion Science and Engineering, 1st ed.; Lazzari, L., Pedeferri, M., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Schweitzer, P.A. Paint and Coatings: Applications and Corrosion Resistance, 1st ed.; Emerald Group Publishing Limited: Bingley, UK, 2006. [Google Scholar] [CrossRef]
- Ding, C.; Fu, J. Smart anticorrosion coatings based on nanocontainers. In Smart Nanocontainers; Elsevier: Amsterdam, The Netherlands, 2019; pp. 413–429. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.; Luo, J.L. Probing the corrosion resistance of a smart electroless Ni-P composite coating embedded with pH-responsive corrosion inhibitor-loaded nanocapsules. Chem. Eng. J. 2021, 421, 127752. [Google Scholar] [CrossRef]
- Chen, T.; Fu, J. An intelligent anticorrosion coating based on pH-responsive supramolecular nanocontainers. Nanotechnology 2012, 23, 505705. [Google Scholar] [CrossRef] [PubMed]
- Tejero-Martin, D.; Rad, M.R.; McDonald, A.; Hussain, T. Beyond Traditional Coatings: A Review on Thermal-Sprayed Functional and Smart Coatings; Springer: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Raja, P.B.; Assad, M.A.; Ismail, M. Inhibitor-Encapsulated Smart Nanocontainers for the Controlled Release of Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Makhlouf, A. Handbook of Smart Coatings for Materials Protection; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Li, G.L.; Zheng, Z.; Möhwald, H.; Shchukin, D.G. Silica/polymer double-walled hybrid nanotubes: Synthesis and application as stimuli-responsive nanocontainers in self-healing coatings. ACS Nano 2013, 7, 2470–2478. [Google Scholar] [CrossRef]
- Niculescu, V.C. Mesoporous Silica Nanoparticles for Bio-Applications. Front. Mater. 2011, 7, 36. [Google Scholar] [CrossRef]
- Liu, X.; Gu, C.; Wen, Z.; Hou, B. Improvement of active corrosion protection of carbon steel by water-based epoxy coating with smart CeO2 nanocontainers. Prog. Org. Coat. 2018, 115, 195–204. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Möhwald, H. Smart nanocontainers as depot media for feedback active coatings. Chem. Commun. 2011, 47, 8730–8739. [Google Scholar] [CrossRef]
- Elmsellem, H.; Aouniti, A.; Youssoufi, M.H.; Bendaha, H.; Hadda, T.b.; Chetouani, A.; Warad, I.; Hammouti, B. Caffeine as a corrosion inhibitor of mild steel in hydrochloric acid. Phys. Chem. News. 2013, 70, 84–90. Available online: https://www.researchgate.net/publication/263926168 (accessed on 8 November 2022).
- Fu, J.; Chen, T.; Wang, M.; Yang, N.; Li, S.; Wang, Y.; Liu, X. Acid and Alkaline Dual Stimuli-Responsive Mechanized Hollow Mesoporous Silica Nanoparticles as Smart Nanocontainers for Intelligent Anticorrosion Coatings. ACS Nano 2013, 7, 11397–11408. [Google Scholar] [CrossRef]
- da Silva, A.C.P.; Cordeiro, P.H.Y.; Estevão, B.M.; Caetano, W.; Eckert, H.; Santin, S.M.O.; Moisés, M.P.; Hioka, N.; Tessaro, A.L. Synthesis of highly ordered mesoporous MCM-41: Selective external functionalization by time control. J. Braz. Chem. Soc. 2019, 30, 1599–1607. [Google Scholar] [CrossRef]
- Sharifi, S.; Abdolahinia, E.D.; Ghavimi, M.A.; Dizaj, S.M.; Aschner, M.; Saso, L.; Khan, H. Effect of Curcumin-Loaded Mesoporous Silica Nanoparticles on the Head and Neck Cancer Cell Line, HN5. Curr. Issues Mol. Biol. 2022, 44, 5247–5259. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Khashab, N.M. Degradability and Clearance of Silicon, Organosilica, Silsesquioxane, Silica Mixed Oxide, and Mesoporous Silica Nanoparticles. Adv. Mater. 2017, 29, 1604634. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Schüth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering mesoporous silica nanoparticles for drug delivery: Where are we after two decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef]
- Yang, K.-N.; Zhang, C.-Q.; Wang, W.; Wang, P.C.; Zhou, J.-P.; Liang, X.-J. pH-responsive mesoporous silica nanoparticles employed in controlled drug delivery systems for cancer treatment. Cancer Biol. Med. 2014, 11, 34–43. [Google Scholar] [CrossRef]
- Racles, C.; Zaltariov, M.F.; Peptanariu, D.; Vasiliu, T.; Cazacu, M. Functionalized Mesoporous Silica as Doxorubicin Carriers and Cytotoxicity Boosters. Nanomaterials 2022, 12, 1823. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Yin, Q.; Zhang, Z.; Gu, W.; Chen, L.; Yu, H.; Huang, Y.; Chen, X.; Xu, M.; Li, Y. Co-delivery of doxorubicin and RNA using pH-sensitive poly (β-amino ester) nanoparticles for reversal of multidrug resistance of breast cancer. Biomaterials 2014, 35, 6047–6059. [Google Scholar] [CrossRef]
- Green, J.J.; Langer, R.; Anderson, D.G. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 2008, 41, 749–759. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Keskin, D.; Shi, L. Poly(β-Amino Esters): Synthesis, Formulations, and Their Biomedical Applications. Adv. Healthc. Mater. 2019, 8, 1801359. [Google Scholar] [CrossRef]
- Fernando, I.R.; Ferris, D.P.; Frasconi, M.; Malin, D.; Strekalova, E.; Yilmaz, M.D.; Ambrogio, M.W.; Algaradah, M.M.; Hong, M.P.; Chen, X.; et al. Esterase- and pH-responsive poly(β-amino ester)-capped mesoporous silica nanoparticles for drug delivery. Nanoscale 2015, 7, 7178–7183. [Google Scholar] [CrossRef]
- Talavera-Pech, W.A.; Esparza-Ruiz, A.; Quintana-Owen, P.; Vilchis-Nestor, A.R.; Barrón-Zambrano, J.A.; Ávila-Ortega, A. Synthesis of pH-sensitive poly(β-amino ester)-coated mesoporous silica nanoparticles for the controlled release of drugs. Appl. Nanosci. 2018, 8, 853–866. [Google Scholar] [CrossRef]
- Liédana, N.; Lozano, P.; Galve, A.; Téllez, C.; Coronas, J. The template role of caffeine in its one-step encapsulation in MOF NH 2-MIL-88B(Fe). J. Mater. Chem. B 2014, 2, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.K.; Boateng, J.S. Surface Modification of Mobile Composition of Matter (MCM)-41 Type Silica Nanoparticles for Potential Oral Mucosa Vaccine Delivery. J. Pharm. Sci. 2020, 109, 2271–2283. [Google Scholar] [CrossRef] [PubMed]
- Meka, A.K.; Niu, Y.; Karmakar, S.; Hartono, B.; Zhang, J. Facile Synthesis of Large-Pore Bicontinuous Cubic Mesoporous Silica Nanoparticles for Intracellular Gene Delivery. ChemNanoMat 2016, 2, 2–7. [Google Scholar] [CrossRef]
- Varache, M.; Bezverkhyy, I.; Saviot, L.; Bouyer, F.; Baras, F.; Bouyer, F. Optimization of MCM-41 type silica nanoparticles for biological applications: Control of size and absence of aggregation and cell cytotoxicity. J. Non Cryst. Solids 2015, 408, 87–97. [Google Scholar] [CrossRef]
- Castillo, R.R.; de la Torre, L.; García-Ochoa, F.; Ladero, M.; Vallet-Regí, M. Production of MCM-41 nanoparticles with control of particle size and structural properties: Optimizing operational conditions during scale-up. Int. J. Mol. Sci. 2020, 21, 7899. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Talavera-Pech, W.A.; Esparza-Ruiz, A.; Quintana-Owen, P.; Vilchis-Nestor, A.R.; Carrera-Figueiras, C.; Ávila-Ortega, A. Effects of different amounts of APTES on physicochemical and structural properties of amino-functionalized MCM-41-MSNs. J. Solgel. Sci. Technol. 2016, 80, 697–708. [Google Scholar] [CrossRef]
- Wang, R.; Xue, J.; Meng, L.; Lee, J.W.; Zhao, Z.; Sun, P.; Cai, L.; Huang, T.; Wang, Z.; Wang, Z.K.; et al. Caffeine Improves the Performance and Thermal Stability of Perovskite Solar Cells. Joule 2019, 3, 1464–1477. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, L.; Huang, P.; Wang, Z.Y.; Tan, Y.; Liu, H.; Pan, J.J.; He, C.Y.; Chen, Z.Y. Synthesis and characterization of low molecular weight polyethyleneimine-terminated Poly(β-amino ester) for highly efficient gene delivery of minicircle DNA. J. Colloid Interface Sci. 2016, 463, 93–98. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, S.S.; Rathore, A.S.; Singh, S.P.; Mishra, G.; Awasthi, R.; Mishra, S.K.; Gautam, V.; Singh, S.K. Lipid-Coated MCM-41 Mesoporous Silica Nanoparticles Loaded with Berberine Improved Inhibition of Acetylcholine Esterase and Amyloid Formation. ACS Biomater. Sci. Eng. 2021, 7, 3737–3753. [Google Scholar] [CrossRef]
- Amolegbe, S.A.; Hirano, Y.; Adebayo, J.O.; Ademowo, O.G.; Balogun, E.A.; Obaleye, J.A.; Krettli, A.U.; Yu, C.; Hayami, S. Mesoporous silica nanocarriers encapsulated antimalarials with high therapeutic performance. Sci. Rep. 2018, 8, 3078. [Google Scholar] [CrossRef]
- Feng, L.; Li, H.; Qu, Y.; Lü, C. Detection of TNT based on conjugated polymer encapsulated in mesoporous silica nanoparticles through FRET. Chem. Commun. 2012, 48, 4633–4635. [Google Scholar] [CrossRef] [PubMed]
- Maia, F.; Tedim, J.; Lisenkov, A.D.; Salak, A.N.; Zheludkevich, M.L.; Ferreira, M.G.S. Silica nanocontainers for active corrosion protection. Nanoscale 2012, 4, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Nairi, V.; Medda, S.; Piludu, M.; Casula, M.F.; Vallet-Regì, M.; Monduzzi, M.; Salis, A. Interactions between bovine serum albumin and mesoporous silica nanoparticles functionalized with biopolymers. Chem. Eng. J. 2018, 340, 42–50. [Google Scholar] [CrossRef]
- de Souza, F.S.; Giacomelli, C.; Gonçalves, R.S.; Spinelli, A. Adsorption behavior of caffeine as a green corrosion inhibitor for copper. Mater. Sci. Eng. C 2012, 32, 2436–2444. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Ahmed, M.H.O.; Abdullah, T.A.; Gaaz, T.S.; Kadhum, A.A.H. Electrochemical studies of novel corrosion inhibitor for mild steel in 1 M hydrochloric acid. Results Phys. 2018, 9, 978–981. [Google Scholar] [CrossRef]
- de Souza, F.S.; Gonçalves, R.S.; Spinelli, A. Assessment of caffeine adsorption onto mild steel surface as an eco-friendly corrosion inhibitor. J. Braz. Chem. Soc. 2014, 25, 81–90. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2008; Available online: http://w.electrochem.org (accessed on 10 August 2022).
- Nyikos, L.; Pajkossy, T. Fractal Dimension and Fractional Power Frequency-Dependent Impedance of Blocking Electrodes. Electrochim. Acta 1985, 30, 1533–1540. [Google Scholar] [CrossRef]
- de Motte, R.; Basilico, E.; Mingant, R.; Kittel, J.; Ropital, F.; Combrade, P.; Necib, S.; Deydier, V.; Crusset, D.; Marcelin, S. A study by electrochemical impedance spectroscopy and surface analysis of corrosion product layers formed during CO2 corrosion of low alloy steel. Corros. Sci. 2020, 172, 108666. [Google Scholar] [CrossRef]
- Garcia-Ochoa, E.; Guzmán-Jiménez, S.J.; Hernández, J.G.; Pandiyan, T.; Vásquez-Pérez, J.M.; Cruz-Borbolla, J. Benzimidazole ligands in the corrosion inhibition for carbon steel in acid medium: DFT study of its interaction on Fe30 surface. J. Mol. Struct. 2016, 1119, 314–324. [Google Scholar] [CrossRef]
- Bland, L.G.; King, A.D.; Birbilis, N.; Scully, J.R. Assessing the Corrosion of Commercially Pure Magnesium and Commercial AZ31B by Electrochemical Impedance, Mass-Loss, Hydrogen Collection, and Inductively Coupled Plasma Optical Emission Spectrometry Solution Analysis. Corrosion 2015, 71, 128–145. [Google Scholar] [CrossRef]
- King, A.D.; Birbilis, N.; Scully, J.R. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Impedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Delgado, M.C.; García-Galvan, F.R.; Barranco, V.; Batlle, S.F. A Measuring Approach to Assess the Corrosion Rate of Magnesium Alloys Using Electrochemical Impedance Spectroscopy. Magnes. Alloy. 2017, 129–159. [Google Scholar] [CrossRef]
- Risović, D.; Poljaček, S.M.; Furić, K.; Gojo, M. Inferring fractal dimension of rough/porous surfaces-A comparison of SEM image analysis and electrochemical impedance spectroscopy methods. Appl. Surf. Sci. 2008, 255, 3063–3070. [Google Scholar] [CrossRef]
- Solehudin, A.; Berman, E.T.; Nurdin, I. Study of caffeine as corrosion inhibitors of carbon steel in chloride solution containing hydrogen sulfide using electrochemical impedance spectroscopy (EIS). In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2015; Volume 1677, p. 070025. [Google Scholar] [CrossRef]




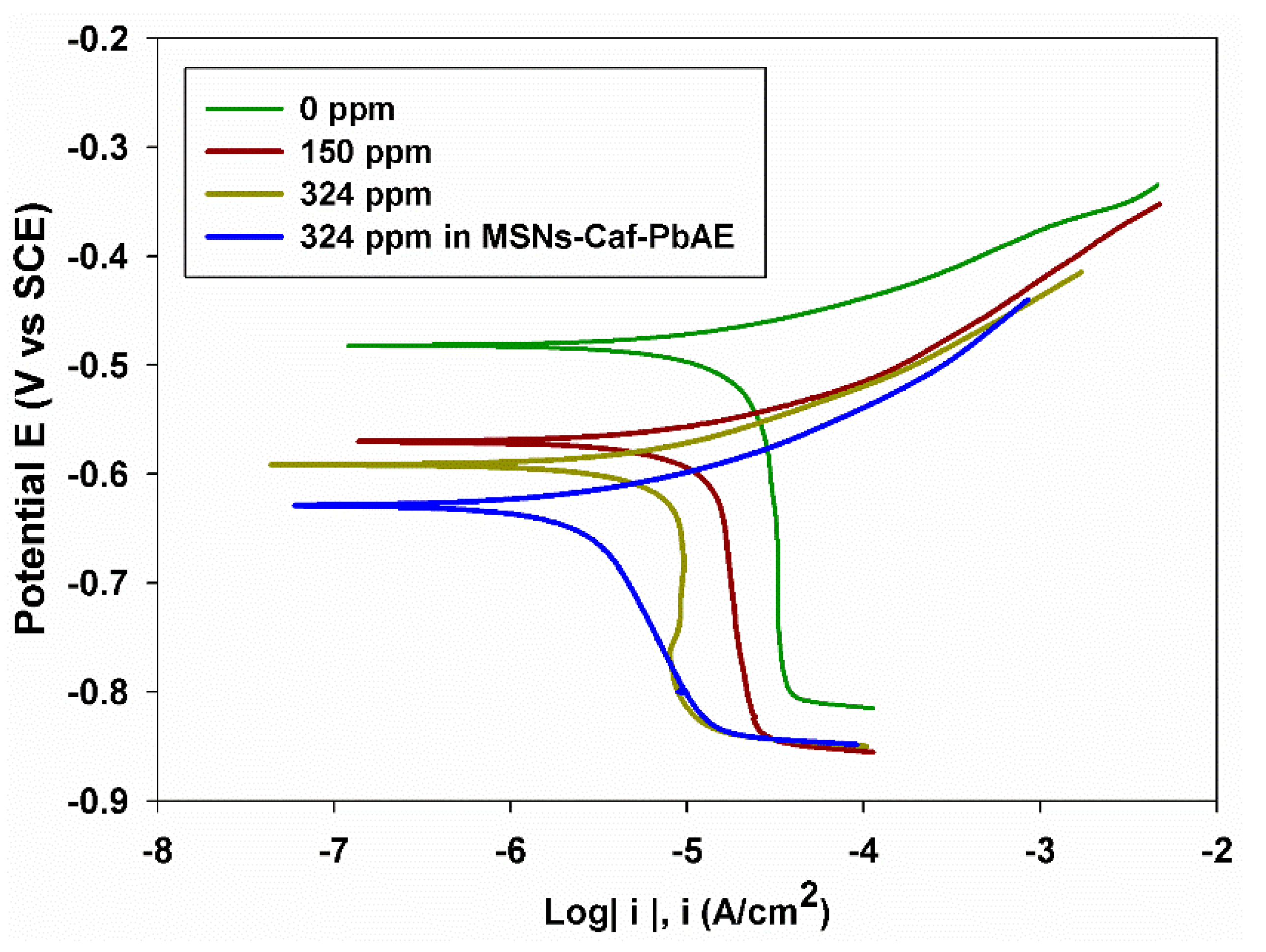
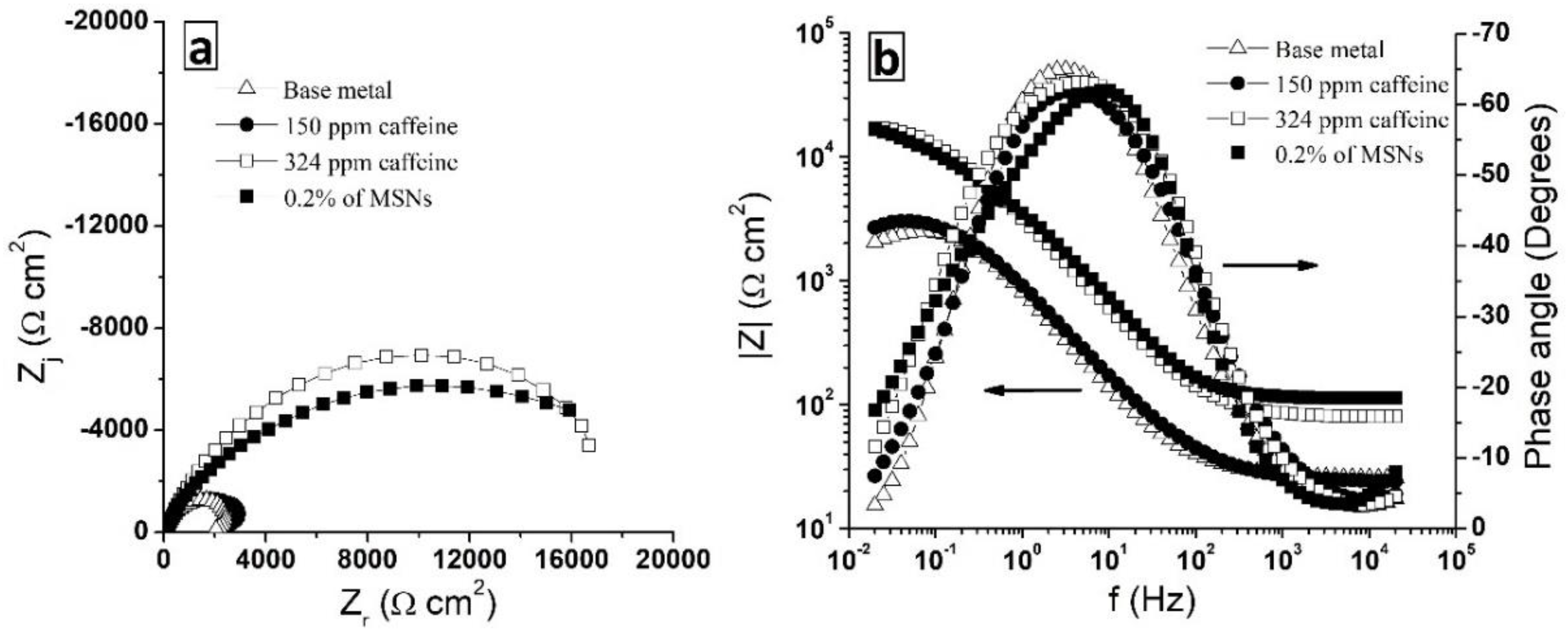
| Caffeine Concentration ppm | Rp Ω cm2 | icorr μA cm−2 | ϴ | %IE |
|---|---|---|---|---|
| 0 | 3135 | 8.293 | - | - |
| 150 | 3495 | 7.439 | 0.103 | 10.30 |
| 324 | 19,435 | 1.338 | 0.838 | 83.87 |
| MSNs-Caf-PbAE | 21,175 | 1.228 | 0.851 | 85.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguirre-Pulido, M.; González-Sánchez, J.A.; Dzib-Pérez, L.R.; Soria-Castro, M.; Ávila-Ortega, A.; Talavera-Pech, W.A. Synthesis and Electrochemical Evaluation of MSNs-PbAE Nanocontainers for the Controlled Release of Caffeine as a Corrosion Inhibitor. Pharmaceutics 2022, 14, 2670. https://doi.org/10.3390/pharmaceutics14122670
Aguirre-Pulido M, González-Sánchez JA, Dzib-Pérez LR, Soria-Castro M, Ávila-Ortega A, Talavera-Pech WA. Synthesis and Electrochemical Evaluation of MSNs-PbAE Nanocontainers for the Controlled Release of Caffeine as a Corrosion Inhibitor. Pharmaceutics. 2022; 14(12):2670. https://doi.org/10.3390/pharmaceutics14122670
Chicago/Turabian StyleAguirre-Pulido, Martín, Jorge A. González-Sánchez, Luis R. Dzib-Pérez, Montserrat Soria-Castro, Alejandro Ávila-Ortega, and William A. Talavera-Pech. 2022. "Synthesis and Electrochemical Evaluation of MSNs-PbAE Nanocontainers for the Controlled Release of Caffeine as a Corrosion Inhibitor" Pharmaceutics 14, no. 12: 2670. https://doi.org/10.3390/pharmaceutics14122670
APA StyleAguirre-Pulido, M., González-Sánchez, J. A., Dzib-Pérez, L. R., Soria-Castro, M., Ávila-Ortega, A., & Talavera-Pech, W. A. (2022). Synthesis and Electrochemical Evaluation of MSNs-PbAE Nanocontainers for the Controlled Release of Caffeine as a Corrosion Inhibitor. Pharmaceutics, 14(12), 2670. https://doi.org/10.3390/pharmaceutics14122670