Natural Herbal Non-Opioid Topical Pain Relievers—Comparison with Traditional Therapy
Abstract
1. Introduction
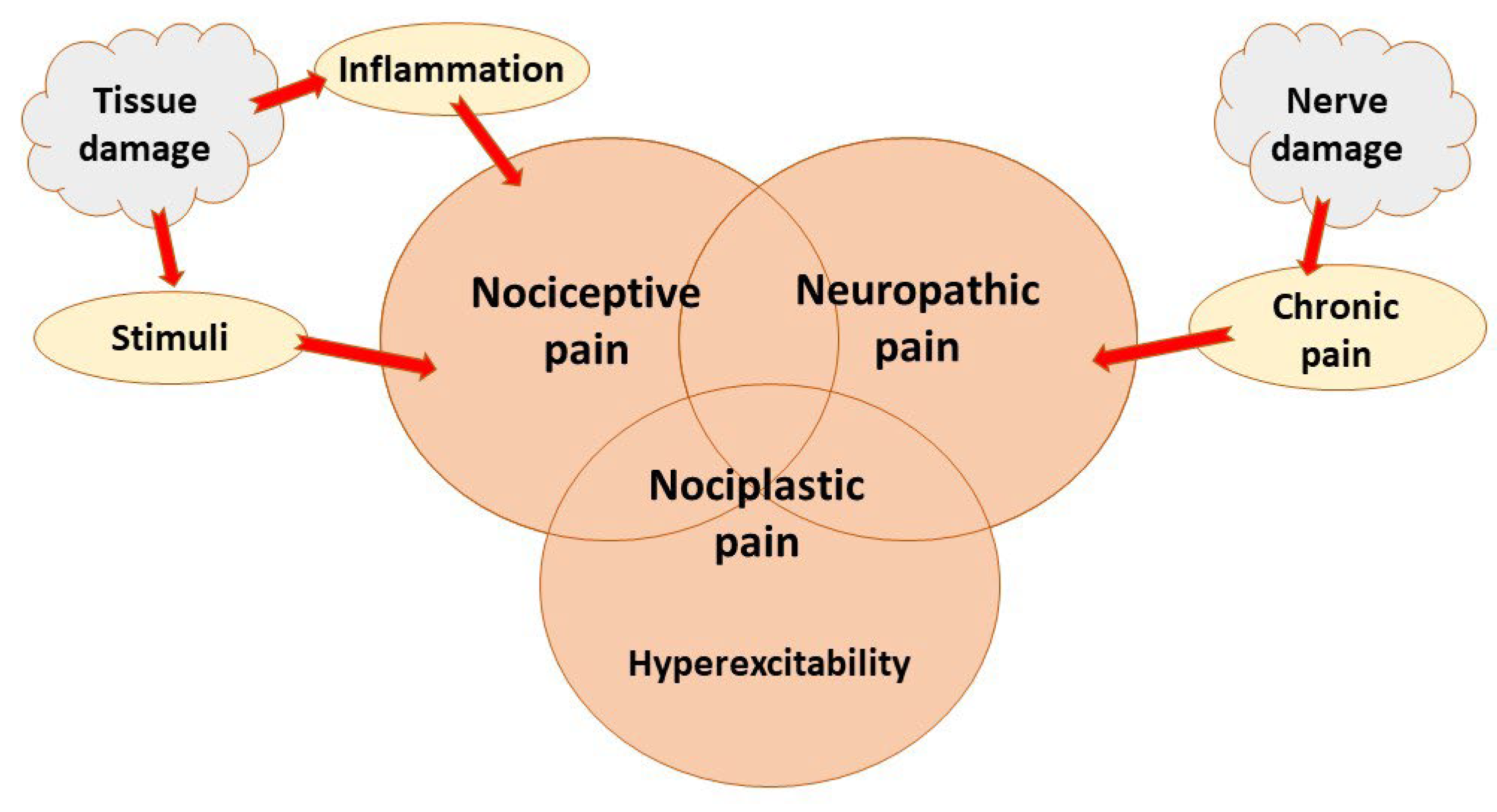
2. Pain Mechanisms and Control
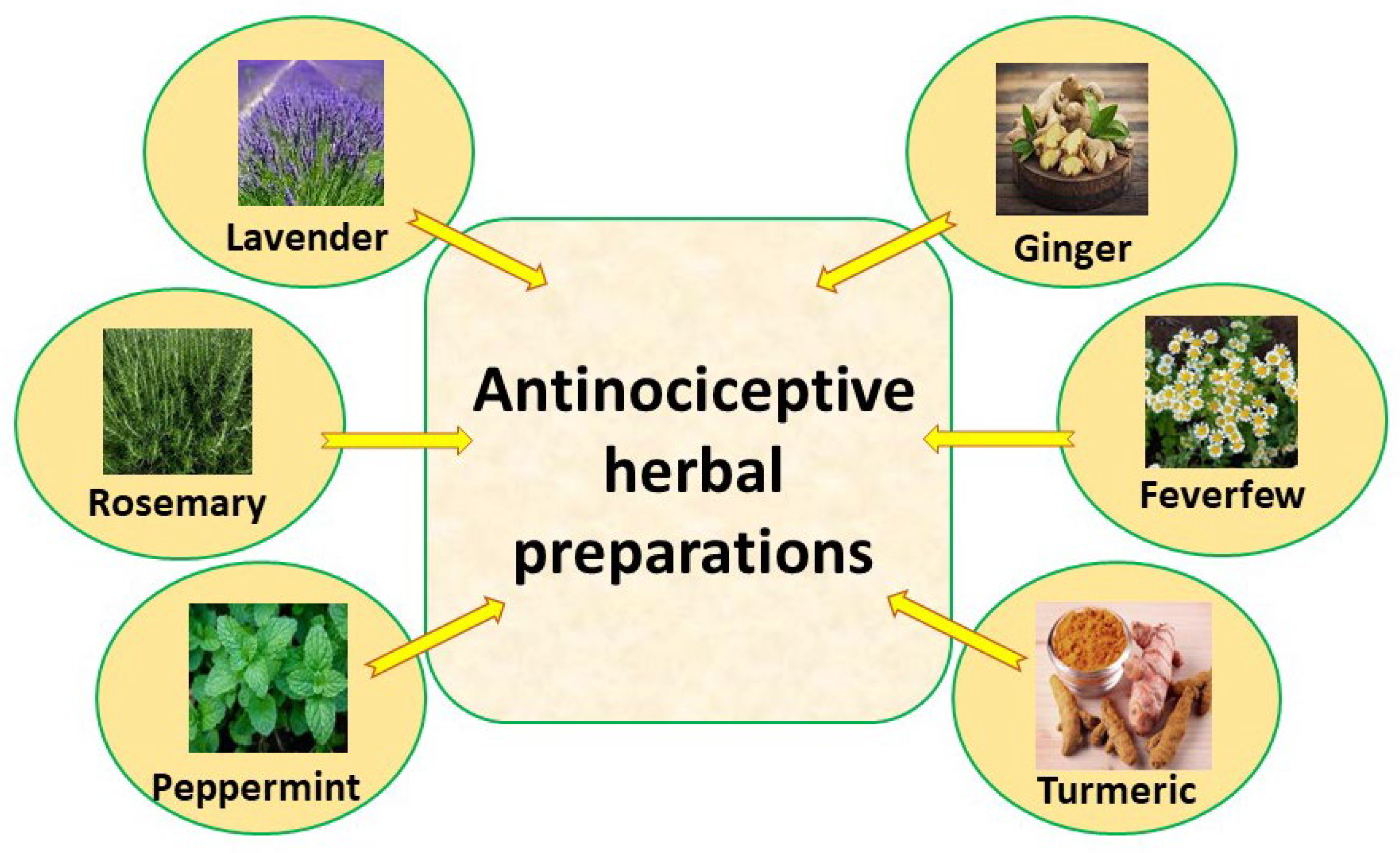
3. Natural Herbal Analgesic Substances
3.1. Lavender (Lavandula angustifolia Mill.)
3.2. Rosemary (Rosmarinus officinalis L.)
3.3. Peppermint (Mentha piperita L.)
3.4. Ginger (Zingiber officinale Roscoe)
3.5. Feverfew (Tanacetum parthenium L.)
3.6. Turmeric (Curcuma longa L.)
4. Emerging Herbal Therapies
4.1. Clove (Syzygium aromaticum L.)
4.2. Clover (Trifolium Species)
5. Pros and Cons of Natural Painkillers: Their Efficacy vs. Traditional Therapy
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Fernandes, V.; Sharma, D.; Vaidya, S.; PA, S.; Guan, Y.; Kalia, K.; Tiwari, V. Cellular and molecular mechanisms driving neuropathic pain: Recent advancements and challenges. Expert Opin. Ther. Targets 2018, 22, 131–142. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic Pain: From Mechanisms to Treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Luchting, B.; Azad, S.C. Pain therapy for the elderly patient: Is opioid-free an option? Curr. Opin. Anaesthesiol. 2019, 32, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Raymond, T.J.; Tobin, K.A.; Rogers, T.S. Nonopioid Pharmacologic Treatments for Chronic Pain. Am. Fam. Physician 2021, 103, 561–565. [Google Scholar]
- Boyd, A.; Bleakley, C.; Hurley, D.A.; Gill, C.; Hannon-Fletcher, M.; Bell, P.; McDonough, S. Herbal medicinal products or preparations for neuropathic pain. Cochrane. Database Syst. Rev. 2019, 4, Cd010528. [Google Scholar] [CrossRef]
- Jahromi, B.; Pirvulescu, I.; Candido, K.D.; Knezevic, N.N. Herbal Medicine for Pain Management: Efficacy and Drug Interactions. Pharmaceutics 2021, 13, 251. [Google Scholar] [CrossRef]
- Casale, R.; Symeonidou, Z.; Ferfeli, S.; Micheli, F.; Scarsella, P.; Paladini, A. Food for Special Medical Purposes and Nutraceuticals for Pain: A Narrative Review. Pain Ther. 2021, 10, 225–242. [Google Scholar] [CrossRef]
- Casale, R.; Symeonidou, Z.; Bartolo, M. Topical Treatments for Localized Neuropathic Pain. Curr. Pain Headache Rep. 2017, 21, 15. [Google Scholar] [CrossRef]
- Kocot-Kępska, M.; Zajączkowska, R.; Mika, J.; Kopsky, D.J.; Wordliczek, J.; Dobrogowski, J.; Przeklasa-Muszyńska, A. Topical Treatments and Their Molecular/Cellular Mechanisms in Patients with Peripheral Neuropathic Pain-Narrative Review. Pharmaceutics 2021, 13, 450. [Google Scholar] [CrossRef]
- Treede, R.D.; Jensen, T.S.; Campbell, J.N.; Cruccu, G.; Dostrovsky, J.O.; Griffin, J.W.; Hansson, P.; Hughes, R.; Nurmikko, T.; Serra, J. Neuropathic pain: Redefinition and a grading system for clinical and research purposes. Neurology 2008, 70, 1630–1635. [Google Scholar] [CrossRef]
- Sacerdote, P.; Franchi, S.; Moretti, S.; Castelli, M.; Procacci, P.; Magnaghi, V.; Panerai, A.E. Cytokine modulation is necessary for efficacious treatment of experimental neuropathic pain. J. Neuroimmune Pharmacol. 2013, 8, 202–211. [Google Scholar] [CrossRef]
- Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Rossi, A.; Sacerdote, P.; Colleoni, M. The soy isoflavone genistein reverses oxidative and inflammatory state, neuropathic pain, neurotrophic and vasculature deficits in diabetes mouse model. Eur. J. Pharmacol. 2011, 650, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.J.; Ji, R.R. Targeting astrocyte signaling for chronic pain. Neurotherapeutics 2010, 7, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Hingtgen, C.M.; Waite, K.J.; Vasko, M.R. Prostaglandins facilitate peptide release from rat sensory neurons by activating the adenosine 3’,5’-cyclic monophosphate transduction cascade. J. NeuroSci. 1995, 15, 5411–5419. [Google Scholar] [CrossRef] [PubMed]
- Page-McCaw, A.; Ewald, A.J.; Werb, Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat. Rev. Mol. Cell Biol. 2007, 8, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Xu, Z.Z.; Wang, X.; Lo, E.H. Matrix metalloprotease regulation of neuropathic pain. Trends Pharmacol. Sci. 2009, 30, 336–340. [Google Scholar] [CrossRef]
- Rahbardar, M.G.; Amin, B.; Mehri, S.; Mirnajafi-Zadeh, S.J.; Hosseinzadeh, H. Rosmarinic acid attenuates development and existing pain in a rat model of neuropathic pain: An evidence of anti-oxidative and anti-inflammatory effects. Phytomedicine 2018, 40, 59–67. [Google Scholar] [CrossRef]
- Thorn, C.F.; Whirl-Carrillo, M.; Leeder, J.S.; Klein, T.E.; Altman, R.B. PharmGKB summary: Phenytoin pathway. Pharma. Genom. 2012, 22, 466–470. [Google Scholar] [CrossRef]
- Zhu, W.; Li, T.; Silva, J.R.; Chen, J. Conservation and divergence in NaChBac and Na(V)1.7 pharmacology reveals novel drug interaction mechanisms. Sci. Rep. 2020, 10, 10730. [Google Scholar] [CrossRef]
- Sunkari, S.; Thatikonda, S.; Pooladanda, V.; Challa, V.S.; Godugu, C. Protective effects of ambroxol in psoriasis like skin inflammation: Exploration of possible mechanisms. Int. Immunopharmacol. 2019, 71, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Gerner, P.; Kuo Wang, G. Amitriptyline for prolonged cutaneous analgesia in the rat. Anesthesiology 2002, 96, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Dworsky, Z.D.; Bennett, R.; Kim, J.M.; Kuo, D.J. Severe medication-induced peripheral neuropathy treated with topical doxepin cream in a paediatric patient with leukaemia. BMJ Case Rep. 2017, 2017, 219900. [Google Scholar] [CrossRef] [PubMed]
- Price, N.; Namdari, R.; Neville, J.; Proctor, K.J.; Kaber, S.; Vest, J.; Fetell, M.; Malamut, R.; Sherrington, R.P.; Pimstone, S.N.; et al. Safety and Efficacy of a Topical Sodium Channel Inhibitor (TV-45070) in Patients With Postherpetic Neuralgia (PHN): A Randomized, Controlled, Proof-of-Concept, Crossover Study, With a Subgroup Analysis of the Nav1.7 R1150W Genotype. Clin. J. Pain 2017, 33, 310–318. [Google Scholar] [CrossRef]
- Kumamoto, E. Inhibition of Fast Nerve Conduction Produced by Analgesics and Analgesic Adjuvants-Possible Involvement in Pain Alleviation. Pharmaceuticals 2020, 13, 62. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vij, A.S.; Sharma, M. Mechanisms and clinical uses of capsaicin. Eur. J. Pharmacol. 2013, 720, 55–62. [Google Scholar] [CrossRef]
- Starowicz, K.; Nigam, S.; Di Marzo, V. Biochemistry and pharmacology of endovanilloids. Pharmacol. Ther. 2007, 114, 13–33. [Google Scholar] [CrossRef]
- Nozadze, I.; Tsiklauri, N.; Gurtskaia, G.; Tsagareli, M.G. NSAIDs attenuate hyperalgesia induced by TRP channel activation. Data Brief 2016, 6, 668–673. [Google Scholar] [CrossRef]
- Liu, S.; Li, Q.; Zhang, M.T.; Mao-Ying, Q.L.; Hu, L.Y.; Wu, G.C.; Mi, W.L.; Wang, Y.Q. Curcumin ameliorates neuropathic pain by down-regulating spinal IL-1β via suppressing astroglial NALP1 inflammasome and JAK2-STAT3 signalling. Sci. Rep. 2016, 6, 28956. [Google Scholar] [CrossRef]
- Bannister, K.; Qu, C.; Navratilova, E.; Oyarzo, J.; Xie, J.Y.; King, T.; Dickenson, A.H.; Porreca, F. Multiple sites and actions of gabapentin-induced relief of ongoing experimental neuropathic pain. Pain 2017, 158, 2386–2395. [Google Scholar] [CrossRef]
- Warncke, T.; Jørum, E.; Stubhaug, A. Local treatment with the N-methyl-D-aspartate receptor antagonist ketamine, inhibit development of secondary hyperalgesia in man by a peripheral action. NeuroSci. Lett. 1997, 227, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kopsky, D.J.; Keppel Hesselink, J.M.; Bhaskar, A.; Hariton, G.; Romanenko, V.; Casale, R. Analgesic effects of topical ketamine. Minerva Anestesiol. 2015, 81, 440–449. [Google Scholar] [PubMed]
- Barygin, O.I.; Nagaeva, E.I.; Tikhonov, D.B.; Belinskaya, D.A.; Vanchakova, N.P.; Shestakova, N.N. Inhibition of the NMDA and AMPA receptor channels by antidepressants and antipsychotics. Brain Res. 2017, 1660, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.D.; Svensson, P.; Cairns, B.E. The analgesic action of topical diclofenac may be mediated through peripheral NMDA receptor antagonism. Pain 2009, 147, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Uchida, I.; Mashimo, T. Local anaesthetics have different mechanisms and sites of action at the recombinant N-methyl-D-aspartate (NMDA) receptors. Br. J. Pharmacol. 2003, 138, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Magnaghi, V.; Ballabio, M.; Consoli, A.; Lambert, J.J.; Roglio, I.; Melcangi, R.C. GABA receptor-mediated effects in the peripheral nervous system: A cross-interaction with neuroactive steroids. J. Mol. NeuroSci. 2006, 28, 89–102. [Google Scholar] [CrossRef]
- Whitehead, R.A.; Puil, E.; Ries, C.R.; Schwarz, S.K.; Wall, R.A.; Cooke, J.E.; Putrenko, I.; Sallam, N.A.; MacLeod, B.A. GABA(B) receptor-mediated selective peripheral analgesia by the non-proteinogenic amino acid, isovaline. Neuroscience 2012, 213, 154–160. [Google Scholar] [CrossRef]
- Wu, C.; Qin, X.; Du, H.; Li, N.; Ren, W.; Peng, Y. The immunological function of GABAergic system. Front. BioSci. 2017, 22, 1162–1172. [Google Scholar] [CrossRef]
- Dogrul, A.; Uzbay, T.I. Topical clonidine antinociception. Pain 2004, 111, 385–391. [Google Scholar] [CrossRef]
- Drummond, P.D. Neuronal changes resulting in up-regulation of alpha-1 adrenoceptors after peripheral nerve injury. Neural. Regen. Res. 2014, 9, 1337–1340. [Google Scholar] [CrossRef]
- Cui, M.; Khanijou, S.; Rubino, J.; Aoki, K.R. Subcutaneous administration of botulinum toxin A reduces formalin-induced pain. Pain 2004, 107, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, J.; Lawrence, G.; Dolly, J.O. Synaptobrevin I mediates exocytosis of CGRP from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J. Cell Sci. 2007, 120, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Welch, M.J.; Purkiss, J.R.; Foster, K.A. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 2000, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Lavender. In Drugs and Lactation Database (LactMed); National Library of Medicine: Bethesda, MD, USA, 2006.
- Silva, G.L.; Luft, C.; Lunardelli, A.; Amaral, R.H.; Melo, D.A.; Donadio, M.V.; Nunes, F.B.; de Azambuja, M.S.; Santana, J.C.; Moraes, C.M.; et al. Antioxidant, analgesic and anti-inflammatory effects of lavender essential oil. Ann. Acad. Bras. Cienc. 2015, 87, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.D.; Les, F.; Lopez, V.; Galeotti, N. Lavender (Lavandula angustifolia Mill.) Essential Oil Alleviates Neuropathic Pain in Mice With Spared Nerve Injury. Front. Pharmacol. 2019, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Donatello, N.N.; Emer, A.A.; Salm, D.C.; Ludtke, D.D.; Bordignon, S.; Ferreira, J.K.; Salgado, A.S.I.; Venzke, D.; Bretanha, L.C.; Micke, G.A.; et al. Lavandula angustifolia essential oil inhalation reduces mechanical hyperalgesia in a model of inflammatory and neuropathic pain: The involvement of opioid and cannabinoid receptors. J. Neuroimmunol. 2020, 340, 577145. [Google Scholar] [CrossRef]
- Papotto, N.; Reithofer, S.; Baumert, K.; Carr, R.; Möhrlen, F.; Frings, S. Olfactory stimulation Inhibits Nociceptive Signal Processing at the Input Stage of the Central Trigeminal System. Neuroscience 2021, 479, 35–47. [Google Scholar] [CrossRef]
- Qadeer, S.; Emad, S.; Perveen, T.; Yousuf, S.; Sheikh, S.; Sarfaraz, Y.; Sadaf, S.; Haider, S. Role of ibuprofen and lavender oil to alter the stress induced psychological disorders: A comparative study. Pak. J. Pharm. Sci. 2018, 31, 1603–1608. [Google Scholar]
- Nasiri, A.; Mahmodi, M.A.; Nobakht, Z. Effect of aromatherapy massage with lavender essential oil on pain in patients with osteoarthritis of the knee: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2016, 25, 75–80. [Google Scholar] [CrossRef]
- Tabatabaeichehr, M.; Mortazavi, H. The Effectiveness of Aromatherapy in the Management of Labor Pain and Anxiety: A Systematic Review. Ethiop. J. Health Sci. 2020, 30, 449–458. [Google Scholar] [CrossRef]
- Abedian, S.; Abedi, P.; Jahanfar, S.; Iravani, M.; Zahedian, M. The effect of Lavender on pain and healing of episiotomy: A systematic review. Complement. Ther. Med. 2020, 53, 102510. [Google Scholar] [CrossRef] [PubMed]
- Boehm, K.; Büssing, A.; Ostermann, T. Aromatherapy as an adjuvant treatment in cancer care--a descriptive systematic review. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 503–518. [Google Scholar] [CrossRef] [PubMed]
- Tüzün Özdemir, S.; Akyol, A. Effect of inhaler and topical lavender oil on pain management of arteriovenous fistula cannulation. J. Vasc. Access 2021. [Google Scholar] [CrossRef] [PubMed]
- Gok Metin, Z.; Arikan Donmez, A.; Izgu, N.; Ozdemir, L.; Arslan, I.E. Aromatherapy Massage for Neuropathic Pain and Quality of Life in Diabetic Patients. J. Nurs. Scholarsh. 2017, 49, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Mizrak Sahin, B.; Culha, I.; Gursoy, E.; Yalcin, O.T. Effect of Massage With Lavender Oil on Postoperative Pain Level of Patients Who Underwent Gynecologic Surgery: A Randomized, Placebo-Controlled Study. Holist Nurs. Pract. 2021, 35, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Stallings Welden, L.M.; Leatherland, P.; Schitter, M.B.; Givens, A.; Stallings, J.D. Abdominal Surgical Patients Randomized to Aromatherapy for Pain Management. J. Perianesth. Nurs. 2021, 36, 291–299.e3. [Google Scholar] [CrossRef] [PubMed]
- Benli, M.; Olson, J.; Huck, O.; Özcan, M. A novel treatment modality for myogenous temporomandibular disorders using aromatherapy massage with lavender oil: A randomized controlled clinical trial. Cranio 2020, 1–11. [Google Scholar] [CrossRef]
- Kim, J.T.; Ren, C.J.; Fielding, G.A.; Pitti, A.; Kasumi, T.; Wajda, M.; Lebovits, A.; Bekker, A. Treatment with lavender aromatherapy in the post-anesthesia care unit reduces opioid requirements of morbidly obese patients undergoing laparoscopic adjustable gastric banding. Obes. Surg. 2007, 17, 920–925. [Google Scholar] [CrossRef]
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Sagorchev, P.; Lukanov, J.; Beer, A.M. Investigations into the specific effects of rosemary oil at the receptor level. Phytomedicine 2010, 17, 693–697. [Google Scholar] [CrossRef]
- Martínez, A.L.; González-Trujano, M.E.; Pellicer, F.; López-Muñoz, F.J.; Navarrete, A. Antinociceptive effect and GC/MS analysis of Rosmarinus officinalis L. essential oil from its aerial parts. Planta Med. 2009, 75, 508–511. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Peña, E.I.; Martínez, A.L.; Moreno, J.; Guevara-Fefer, P.; Déciga-Campos, M.; López-Muñoz, F.J. Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents. J. Ethnopharmacol. 2007, 111, 476–482. [Google Scholar] [CrossRef]
- Raskovic, A.; Milanovic, I.; Pavlovic, N.; Milijasevic, B.; Ubavic, M.; Mikov, M. Analgesic effects of rosemary essential oil and its interactions with codeine and paracetamol in mice. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 165–172. [Google Scholar]
- Akbari, J.; Saeedi, M.; Farzin, D.; Morteza-Semnani, K.; Esmaili, Z. Transdermal absorption enhancing effect of the essential oil of Rosmarinus officinalis on percutaneous absorption of Na diclofenac from topical gel. Pharm. Biol. 2015, 53, 1442–1447. [Google Scholar] [CrossRef]
- Mohammadifar, M.; Aarabi, M.H.; Aghighi, F.; Kazemi, M.; Vakili, Z.; Memarzadeh, M.R.; Talaei, S.A. Anti-osteoarthritis potential of peppermint and rosemary essential oils in a nanoemulsion form: Behavioral, biochemical, and histopathological evidence. BMC Complement. Med. Ther. 2021, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Maresca, M.; Cravotto, G.; Bellumori, M.; Innocenti, M.; Mulinacci, N.; Ghelardini, C. Anti-neuropathic effects of Rosmarinus officinalis L. terpenoid fraction: Relevance of nicotinic receptors. Sci. Rep. 2016, 6, 34832. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.L.; González-Trujano, M.E.; Chávez, M.; Pellicer, F. Antinociceptive effectiveness of triterpenes from rosemary in visceral nociception. J. Ethnopharmacol. 2012, 142, 28–34. [Google Scholar] [CrossRef]
- Abdelhalim, A.; Karim, N.; Chebib, M.; Aburjai, T.; Khan, I.; Johnston, G.A.; Hanrahan, J. Antidepressant, Anxiolytic and Antinociceptive Activities of Constituents from Rosmarinus officinalis. J. Pharm. Pharm. Sci. 2015, 18, 448–459. [Google Scholar] [CrossRef]
- Keshavarzian, S.; Shahgholian, N. Comparison of the Effect of Topical Application of Rosemary and Menthol for Musculoskeletal Pain in Hemodialysis Patients. Iran. J. Nurs. Midwifery Res. 2017, 22, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. 2018, 4, Fso283. [Google Scholar] [CrossRef] [PubMed]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Peppermint. In Drugs and Lactation Database (LactMed); National Library of Medicine: Bethesda, MD, USA, 2006.
- Liu, B.; Fan, L.; Balakrishna, S.; Sui, A.; Morris, J.B.; Jordt, S.E. TRPM8 is the principal mediator of menthol-induced analgesia of acute and inflammatory pain. Pain 2013, 154, 2169–2177. [Google Scholar] [CrossRef]
- Chumpitazi, B.P.; Kearns, G.L.; Shulman, R.J. Review article: The physiological effects and safety of peppermint oil and its efficacy in irritable bowel syndrome and other functional disorders. Aliment. Pharmacol. Ther. 2018, 47, 738–752. [Google Scholar] [CrossRef] [PubMed]
- Hawthorn, M.; Ferrante, J.; Luchowski, E.; Rutledge, A.; Wei, X.Y.; Triggle, D.J. The actions of peppermint oil and menthol on calcium channel dependent processes in intestinal, neuronal and cardiac preparations. Aliment. Pharmacol. Ther. 1988, 2, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Liotta, R.; Mulè, F. Effects of menthol on circular smooth muscle of human colon: Analysis of the mechanism of action. Eur. J. Pharmacol. 2014, 740, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Wie, J.; So, I.; Jung, M.H.; Ha, K.T.; Kim, B.J. Menthol Modulates Pacemaker Potentials through TRPA1 Channels in Cultured Interstitial Cells of Cajal from Murine Small Intestine. Cell. Physiol. Biochem. 2016, 38, 1869–1882. [Google Scholar] [CrossRef]
- Harrington, A.M.; Hughes, P.A.; Martin, C.M.; Yang, J.; Castro, J.; Isaacs, N.J.; Blackshaw, A.L.; Brierley, S.M. A novel role for TRPM8 in visceral afferent function. Pain 2011, 152, 1459–1468. [Google Scholar] [CrossRef]
- Alammar, N.; Wang, L.; Saberi, B.; Nanavati, J.; Holtmann, G.; Shinohara, R.T.; Mullin, G.E. The impact of peppermint oil on the irritable bowel syndrome: A meta-analysis of the pooled clinical data. BMC Complement. Altern. Med. 2019, 19, 21. [Google Scholar] [CrossRef]
- Korterink, J.J.; Rutten, J.M.; Venmans, L.; Benninga, M.A.; Tabbers, M.M. Pharmacologic treatment in pediatric functional abdominal pain disorders: A systematic review. J. Pediatr. 2015, 166, 424–431.e6. [Google Scholar] [CrossRef]
- Li, J.; Lv, L.; Zhang, J.; Xu, L.; Zeng, E.; Zhang, Z.; Wang, F.; Tang, X. A Combination of Peppermint Oil and Caraway Oil for the Treatment of Functional Dyspepsia: A Systematic Review and Meta-Analysis. Evid. Based Complement. Alternat. Med. 2019, 2019, 7654947. [Google Scholar] [CrossRef]
- Shavakhi, A.; Ardestani, S.K.; Taki, M.; Goli, M.; Keshteli, A.H. Premedication with peppermint oil capsules in colonoscopy: A double blind placebo-controlled randomized trial study. Acta Gastroenterol. Belg. 2012, 75, 349–353. [Google Scholar]
- Khalaf, M.H.G.; Chowdhary, S.; Elmunzer, B.J.; Elias, P.S.; Castell, D. Impact of Peppermint Therapy on Dysphagia and Non-cardiac Chest Pain: A Pilot Study. Dig. Dis. Sci. 2019, 64, 2214–2218. [Google Scholar] [CrossRef]
- Akbari, F.; Rezaei, M.; Khatony, A. Effect Of Peppermint Essence On The Pain And Anxiety Caused By Intravenous Catheterization In Cardiac Patients: A Randomized Controlled Trial. J. Pain Res. 2019, 12, 2933–2939. [Google Scholar] [CrossRef]
- Borhani Haghighi, A.; Motazedian, S.; Rezaii, R.; Mohammadi, F.; Salarian, L.; Pourmokhtari, M.; Khodaei, S.; Vossoughi, M.; Miri, R. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: A randomised, double-blind, placebo-controlled, crossed-over study. Int. J. Clin. Pract. 2010, 64, 451–456. [Google Scholar] [CrossRef]
- Rafieian-Kopaei, M.; Hasanpour-Dehkordi, A.; Lorigooini, Z.; Deris, F.; Solati, K.; Mahdiyeh, F. Comparing the Effect of Intranasal Lidocaine 4% with Peppermint Essential Oil Drop 1.5% on Migraine Attacks: A Double-Blind Clinical Trial. Int. J. Prev. Med. 2019, 10, 121. [Google Scholar] [CrossRef]
- Göbel, H.; Heinze, A.; Heinze-Kuhn, K.; Göbel, A.; Göbel, C. Peppermint oil in the acute treatment of tension-type headache. Schmerz 2016, 30, 295–310. [Google Scholar] [CrossRef]
- Davies, S.J.; Harding, L.M.; Baranowski, A.P. A novel treatment of postherpetic neuralgia using peppermint oil. Clin. J. Pain 2002, 18, 200–202. [Google Scholar] [CrossRef]
- Akbari, S.A.; Alamolhoda, S.H.; Baghban, A.A.; Mirabi, P. Effects of menthol essence and breast milk on the improvement of nipple fissures in breastfeeding women. J. Res. Med. Sci. 2014, 19, 629–633. [Google Scholar]
- Shanazi, M.; Farshbaf Khalili, A.; Kamalifard, M.; Asghari Jafarabadi, M.; Masoudin, K.; Esmaeli, F. Comparison of the Effects of Lanolin, Peppermint, and Dexpanthenol Creams on Treatment of Traumatic Nipples in Breastfeeding Mothers. J. Caring Sci. 2015, 4, 297–307. [Google Scholar] [CrossRef]
- Hoffman, T. Ginger: An ancient remedy and modern miracle drug. Hawaii Med. J. 2007, 66, 326–327. [Google Scholar]
- Pagano, E.; Souto, E.B.; Durazzo, A.; Sharifi-Rad, J.; Lucarini, M.; Souto, S.B.; Salehi, B.; Zam, W.; Montanaro, V.; Lucariello, G.; et al. Ginger (Zingiber officinale Roscoe) as a nutraceutical: Focus on the metabolic, analgesic, and antiinflammatory effects. Phytother. Res. 2020, 35, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Unuofin, J.O.; Masuku, N.P.; Paimo, O.K.; Lebelo, S.L. Ginger from Farmyard to Town: Nutritional and Pharmacological Applications. Front. Pharmacol. 2021, 12, 779352. [Google Scholar] [CrossRef]
- Kiuchi, F.; Iwakami, S.; Shibuya, M.; Hanaoka, F.; Sankawa, U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem. Pharm. Bull. 1992, 40, 387–391. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Tao, Y.; Li, W. Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale). Fitoterapia 2011, 82, 38–43. [Google Scholar] [CrossRef]
- Gopalsamy, B.; Farouk, A.A.O.; Tengku Mohamad, T.A.S.; Sulaiman, M.R.; Perimal, E.K. Antiallodynic and antihyperalgesic activities of zerumbone via the suppression of IL-1β, IL-6, and TNF-α in a mouse model of neuropathic pain. J. Pain Res. 2017, 10, 2605–2619. [Google Scholar] [CrossRef]
- Hitomi, S.; Ono, K.; Terawaki, K.; Matsumoto, C.; Mizuno, K.; Yamaguchi, K.; Imai, R.; Omiya, Y.; Hattori, T.; Kase, Y.; et al. [6]-gingerol and [6]-shogaol, active ingredients of the traditional Japanese medicine hangeshashinto, relief oral ulcerative mucositis-induced pain via action on Na(+) channels. Pharmacol. Res. 2017, 117, 288–302. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Kuttan, R. Antioxidant, anti-inflammatory and antinociceptive activities of essential oil from ginger. Indian J. Physiol. Pharmacol. 2013, 57, 51–62. [Google Scholar]
- Mozafari, S.; Esmaeili, S.; Momenyan, S.; Zadeh Modarres, S.; Ozgoli, G. Effect of Zingiber officinale Roscoe rhizome (ginger) capsule on postpartum pain: Double-blind randomized clinical trial. J. Res. Med. Sci. 2021, 26, 105. [Google Scholar] [CrossRef]
- Ozgoli, G.; Goli, M.; Moattar, F. Comparison of effects of ginger, mefenamic acid, and ibuprofen on pain in women with primary dysmenorrhea. J. Altern. Complement. Med. 2009, 15, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Daily, J.W.; Zhang, X.; Kim, D.S.; Park, S. Efficacy of Ginger for Alleviating the Symptoms of Primary Dysmenorrhea: A Systematic Review and Meta-analysis of Randomized Clinical Trials. Pain Med. 2015, 16, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
- Cady, R.K.; Goldstein, J.; Nett, R.; Mitchell, R.; Beach, M.E.; Browning, R. A double-blind placebo-controlled pilot study of sublingual feverfew and ginger (LipiGesic™ M) in the treatment of migraine. Headache 2011, 51, 1078–1086. [Google Scholar] [CrossRef]
- Therkleson, T. Ginger Therapy for Osteoarthritis: A Typical Case. J. Holist Nurs. 2014, 32, 232–239. [Google Scholar] [CrossRef]
- Al-Nahain, A.; Jahan, R.; Rahmatullah, M. Zingiber officinale: A Potential Plant against Rheumatoid Arthritis. Arthritis 2014, 2014, 159089. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Tenci, B.; Zanardelli, M.; Maidecchi, A.; Lugli, A.; Mattoli, L.; Ghelardini, C. Widespread pain reliever profile of a flower extract of Tanacetum parthenium. Phytomedicine 2015, 22, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Suthar, M.; Rathore, G.S.; Bansal, V. Feverfew (Tanacetum parthenium L.): A systematic review. Pharmacogn Rev. 2011, 5, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.K.; Kulkarni, S.K. Antinociceptive and anti-inflammatory effects of Tanacetum parthenium L. extract in mice and rats. J. Ethnopharmacol. 1999, 68, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, N.; Maidecchi, A.; Mattoli, L.; Burico, M.; Ghelardini, C. St. John’s Wort seed and feverfew flower extracts relieve painful diabetic neuropathy in a rat model of diabetes. Fitoterapia 2014, 92, 23–33. [Google Scholar] [CrossRef]
- Johnson, E.S.; Kadam, N.P.; Hylands, D.M.; Hylands, P.J. Efficacy of feverfew as prophylactic treatment of migraine. Br. Med. J. 1985, 291, 569–573. [Google Scholar] [CrossRef]
- Murphy, J.J.; Heptinstall, S.; Mitchell, J.R. Randomised double-blind placebo-controlled trial of feverfew in migraine prevention. Lancet 1988, 2, 189–192. [Google Scholar] [CrossRef]
- Saranitzky, E.; White, C.M.; Baker, E.L.; Baker, W.L.; Coleman, C.I. Feverfew for migraine prophylaxis: A systematic review. J. Diet. Suppl. 2009, 6, 91–103. [Google Scholar] [CrossRef]
- Urošević, M.; Nikolić, L.; Gajić, I.; Nikolić, V.; Dinić, A.; Miljković, V. Curcumin: Biological Activities and Modern Pharmaceutical Forms. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, F.; Braun, C.; Zhou, Y.Q.; Rittner, H.; Tian, Y.K.; Cai, X.Y.; Ye, D.W. Role of curcumin in the management of pathological pain. Phytomedicine 2018, 48, 129–140. [Google Scholar] [CrossRef]
- Razavi, B.M.; Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother. Res. 2021, 35, 6489–6513. [Google Scholar] [CrossRef] [PubMed]
- Lubbad, A.; Oriowo, M.A.; Khan, I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol. Cell. Biochem. 2009, 322, 127–135. [Google Scholar] [CrossRef]
- Srivastava, S.; Saksena, A.K.; Khattri, S.; Kumar, S.; Dagur, R.S. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: A four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology 2016, 24, 377–388. [Google Scholar] [CrossRef]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and efficacy of curcumin versus diclofenac in knee osteoarthritis: A randomized open-label parallel-arm study. Trials 2019, 20, 214. [Google Scholar] [CrossRef]
- Jamali, N.; Adib-Hajbaghery, M.; Soleimani, A. The effect of curcumin ointment on knee pain in older adults with osteoarthritis: A randomized placebo trial. BMC Complement. Med. Ther. 2020, 20, 305. [Google Scholar] [CrossRef]
- Esmaeili, F.; Zahmatkeshan, M.; Yousefpoor, Y.; Alipanah, H.; Safari, E.; Osanloo, M. Anti-inflammatory and anti-nociceptive effects of Cinnamon and Clove essential oils nanogels: An in vivo study. BMC Complement. Med. Ther. 2022, 22, 143. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Booth, N.L.; Overk, C.R.; Yao, P.; Burdette, J.E.; Nikolic, D.; Chen, S.N.; Bolton, J.L.; van Breemen, R.B.; Pauli, G.F.; Farnsworth, N.R. The chemical and biologic profile of a red clover (Trifolium pratense L.) phase II clinical extract. J. Altern. Complement. Med. 2006, 12, 133–139. [Google Scholar] [CrossRef]
- Sabudak, T.; Guler, N. Trifolium L.— review on its phytochemical and pharmacological profile. Phytother. Res. 2009, 23, 439–446. [Google Scholar] [CrossRef]
- Booth, N.L.; Piersen, C.E.; Banuvar, S.; Geller, S.E.; Shulman, L.P.; Farnsworth, N.R. Clinical studies of red clover (Trifolium pratense) dietary supplements in menopause: A literature review. Menopause 2006, 13, 251–264. [Google Scholar] [CrossRef]
- Ferraris, C.; Ballestra, B.; Listorti, C.; Cappelletti, V.; Reduzzi, C.; Scaperrotta, G.P.; Pulice, I.; Ferrari, E.G.A.; Folli, S.; Mariani, L.; et al. Red clover and lifestyle changes to contrast menopausal symptoms in premenopausal patients with hormone-sensitive breast cancer receiving tamoxifen. Breast Cancer Res. Treat. 2020, 180, 157–165. [Google Scholar] [CrossRef]
- Ghazanfarpour, M.; Sadeghi, R.; Roudsari, R.L.; Khorsand, I.; Khadivzadeh, T.; Muoio, B. Red clover for treatment of hot flashes and menopausal symptoms: A systematic review.w and meta-analysis. J. Obstet. Gynaecol. 2016, 36, 301–311. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Pereira, L. Effects of red clover on perimenopausal and postmenopausal women’s blood lipid profile: A meta-analysis. Climacteric 2018, 21, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Vishali, N.; Kamakshi, K.; Suresh, S.; Prakash, S. Red clover Trifolium pratense (Linn.) isoflavones extract on the pain threshold of normal and ovariectomized rats—A long-term study. Phytother. Res. 2011, 25, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.S.; Guan, S.Y.; Liu, A.; Yue, J.; Hu, L.N.; Zhang, K.; Yang, L.K.; Lu, L.; Tian, Z.; Zhao, M.G.; et al. Anxiolytic effects of Formononetin in an inflammatory pain mouse model. Mol. Brain 2019, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Gartoulla, P.; Han, M.M. Red clover extract for alleviating hot flushes in postmenopausal women: A meta-analysis. Maturitas 2014, 79, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Berardino, K.; Carroll, A.H.; Popovsky, D.; Ricotti, R.; Civilette, M.D.; Sherman, W.F.; Kaye, A.D. Opioid Use Consequences, Governmental Strategies, and Alternative Pain Control Techniques Following Total Hip Arthroplasties. Orthop Rev. 2022, 14, 35318. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopustinskiene, D.M.; Bernatonyte, U.; Maslii, Y.; Herbina, N.; Bernatoniene, J. Natural Herbal Non-Opioid Topical Pain Relievers—Comparison with Traditional Therapy. Pharmaceutics 2022, 14, 2648. https://doi.org/10.3390/pharmaceutics14122648
Kopustinskiene DM, Bernatonyte U, Maslii Y, Herbina N, Bernatoniene J. Natural Herbal Non-Opioid Topical Pain Relievers—Comparison with Traditional Therapy. Pharmaceutics. 2022; 14(12):2648. https://doi.org/10.3390/pharmaceutics14122648
Chicago/Turabian StyleKopustinskiene, Dalia M., Urte Bernatonyte, Yuliia Maslii, Nataliia Herbina, and Jurga Bernatoniene. 2022. "Natural Herbal Non-Opioid Topical Pain Relievers—Comparison with Traditional Therapy" Pharmaceutics 14, no. 12: 2648. https://doi.org/10.3390/pharmaceutics14122648
APA StyleKopustinskiene, D. M., Bernatonyte, U., Maslii, Y., Herbina, N., & Bernatoniene, J. (2022). Natural Herbal Non-Opioid Topical Pain Relievers—Comparison with Traditional Therapy. Pharmaceutics, 14(12), 2648. https://doi.org/10.3390/pharmaceutics14122648






