Abstract
(1) Background: With the massive demand for the use and commercialization of medicinal cannabidiol (CBD) products, new randomized clinical trials (RCTs) are being published worldwide, with a constant need for safety and efficacy evaluation. (2) Methods: We performed an update on a systematic review published in 2020 that focused on analyzing the serious adverse effects (SAEs) of CBD in RCTs and its possible association with drug interactions. We also updated the report of the most prevalent CBD adverse effects (AEs). We systematically searched EMBASE, MEDLINE/PubMed, and Web of Science without language restriction for RCTs that reported adverse effects after repeated oral CBD administration for at least one week in healthy volunteers or clinical samples published from January 2019 to May 2022. The included studies were assessed for methodological quality by the Quality Assessment of Controlled Intervention Studies tool. The present review is registered on PROSPERO, number CRD42022334399. (3) Results: Twelve studies involving 745 randomized subjects analyzed were included (range 1.1–56.8 y). A total of 454 participants used CBD in the trials. The most common AEs of CBD were mild or moderate and included gastrointestinal symptoms (59.5%), somnolence (16.7%), loss of appetite (16.5%), and hypertransaminasemia (ALT/AST) (12.8%). Serious adverse effects include mainly hypertransaminasemia with serum levels elevations greater than three times the upper limit of the normal (6.4%), seizures (1.3%), and rash (1.1%). All SAEs reported in the studies were observed on CBD as an add-on therapy to anticonvulsant medications, including clobazam and valproate. (4) Conclusion: Recent RCTs involving oral CBD administration for at least a week suggest that CBD has a good safety and tolerability profile, confirming previous data. However, it can potentially interact with other drugs and its use should be monitored, especially at the beginning of treatment.
1. Introduction
Cannabis has been used for centuries for medicinal purposes. In the last two decades, decriminalization policies and new scientific evidence have significantly increased interest in the therapeutic potential of cannabis and its derivatives [1]. As a result, in several countries, there was a continuous process of authorizations for the marketing of cannabis-based products (CBMPs). CBMPs can range from purified single compounds, commonly cannabidiol (CBD) or delta-9-tetrahydrocannabinol (THC), to complex mixtures of hundreds of components in multiple formulations (e.g., oils, solutions, capsules, sublingual sprays), various routes of administration (e.g., oral, inhalation, topical), and differ in their manufacturing processes and quality control [2].
Cannabidiol (CBD) is a non-psychotomimetic phytocannabinoid with potential therapeutic properties across diverse physical and neuropsychiatric conditions due to its anti-inflammatory, analgesic, neuroprotective, anticonvulsant, and anxiolytic actions, among others [3]. There is increasing social pressure in many countries for patients to legally use this cannabinoid to treat anxiety, depression, sleep disorders, pain, and more extensively, promote quality of life and well-being [4,5]. However, in the United States and Europe, only the oral purified CBD preparation Epidiolex® (GW-Pharm, United Kingdom) has been licensed to treat seizures associated with Lennox-Gastaut syndrome, Dravet syndrome, and Tuberous Sclerosis Complex. Some of the most common side effects of Epidiolex® include sleepiness, decreased appetite, diarrhea, increased liver enzymes, sleep problems, and rash [6]. Thus, previous data suggest that oral cannabidiol is well tolerated and has relatively few serious adverse effects. Two recent systematic reviews [7,8] evaluating the safety of oral CBD in RCTs in healthy volunteers or clinical samples (a meta-analysis on general AEs and a systematic review focused on drug interactions and SAEs) reported AEs such as decreased appetite, diarrhea, somnolence, sedation, dizziness, headache, nausea, vomiting, abdominal discomfort, and rash. In addition, SAEs were elevated transaminases, convulsion, sedation, lethargy, and upper respiratory tract infections. For SAEs, both reviews reported being limited to childhood epilepsy studies that indicate that CBD may have interacted with other medications such as clobazam or sodium valproate.
Thus, with the increase in demand for the medicinal use of CBD, the emergence of new products, and recently published clinical studies, it is essential to follow the data on CBD effectiveness and its safety and tolerability. Therefore, the present review aims to address this issue by updating our previous systematic review on the SAEs of CBD and its interactions with other drugs in randomized clinical trials published between January 2019 to May 2022; we also extracted from the previous review and updated the report of the most prevalent AEs of CBD and evaluated the quality of the methodology used in the studies.
2. Methods
This systematic review was prospectively registered on PROSPERO (CRD42022334399) and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The PRISMA flowchart is shown in Figure 1. As an update of a systematic review, the methodology used was similar to the previous study [8], with minimal adaptations when necessary (see Supplement).
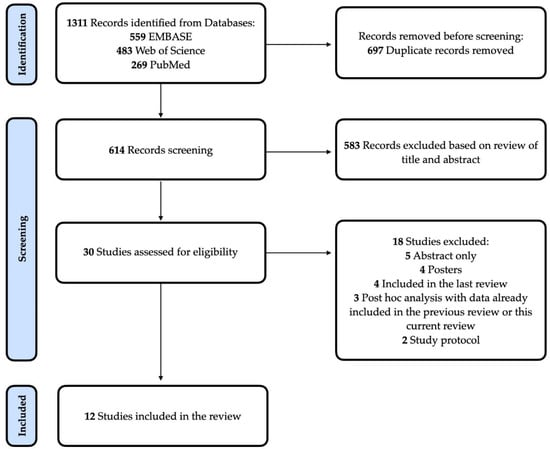
Figure 1.
PRISMA Flow Diagram. Flowchart describing the systematic search strategy, including the identification, screening, and inclusion of relevant studies.
2.1. Inclusion and Exclusion Criteria
We included double-blind, randomized controlled clinical trials that reported data on adverse effects from controlled trials using repeated oral administration in humans with formulations containing purified CBD (≥98% CBD). Open-label studies that did not include a placebo or drug comparison or lasted less than seven days were excluded. There were no restrictions concerning participant characteristics or disease indication.
2.2. Search Strategy and Study Eligibility
Randomized clinical trials on CBD published in peer-reviewed journals between January 2019 to May 2022 reported adverse effects identified using EMBASE, MEDLINE/PubMed, and Web of Science (independently performed by JS, JP, and GR); by screening the reference lists of articles identified and by correspondence with study investigators using the approach recommended by the PRISMA guidelines. The computer-based searches combined the words “(cannabidiol OR CBD) AND (randomized clinical trial OR double-blind OR placebo-controlled)” without language restriction.
2.3. Data Extraction and Quality Assessment
The following information was independently extracted from each article by three trained investigators (JS, JP, and GR) using a standardized form: study design, year of the publication, geographic location, sample size, the average age of participants, number and percentage of female participants, the condition being studied, cannabidiol treatment, control (placebo or other drugs), number and percentage of CBD users, CBD product formulation, safety assessment, adverse effects, serious adverse effects, and concomitant therapy. Adverse effects were classified into mild, moderate, and severe according to the reported categories. All discrepancies were resolved by consensus and, if necessary, by a research supervisor (RS) adjudication. The risk of bias and the methodological quality of the studies were assessed using the Quality Assessment of Controlled Intervention Studies tool from the National Heart, Lung, and Blood Institute [9]. Studies were considered methodologically poor, fair, or good based on the percentage of reported items < 50%, 50–75%, or >75%, respectively (see Supplement); low risk of bias translates to a rating of good quality.
3. Results
3.1. Study Characteristics
Twelve RCTs [10,11,12,13,14,15,16,17,18,19,20,21] involving 745 randomized subjects analyzed were included. The median number of participants per study was 62 (range 1.1–56.8 y). Eleven RCTs were placebo-controlled, and one was active-controlled [19]. A total of 454 participants used oral CBD in the trials, and 303 participants were female (40.7%). Treatment duration ranged from 1 to 16 weeks. Daily CBD doses included fixed doses (300 mg/day to 800 mg/day) and doses adjusted by weight (from 20 mg/kg/day to 50 mg/kg/day). All RCTs evaluated the effects of CBD on clinical populations and most used CBD concomitant with other drugs (9/12). Two studies evaluated the effects of cannabidiol on epilepsy, two on cocaine use disorder, one on psychosis, one on the high risk of psychosis, one on drug-resistant seizures in tuberous sclerosis complex, one on cannabis use disorder, one on COVID-19 patients, one on rapid eye movement sleep behavior disorder, one on behavioral problems in children and adolescents with intellectual disability, and one on functional dyspepsia. Three studies took place in Brazil, three in multiple countries, two in the United Kingdom, one in Australia, one in Canada, one in Germany, and one in the United States. All this information, in addition to concomitant therapy and investigational product formulations, are reported in Table 1.

Table 1.
A visual summary of the characteristics from included studies from the systematic review.
3.2. Safety Assessment
The safety of oral CBD isolate use was assessed differently across studies. Four studies evaluated laboratory parameters [11,12,15,21], two employed participants’ self-reports [13,18], two studies used the UKU Side Effects Scale [14,17], two did not apply a questionnaire or adverse event scale [10,19], one used the Systematic Assessment for Treatment Emergent Events (SAFTEE) tool [20], one used the Medical Dictionary for Regulatory Activities (MedDRA) [16], one used the Monitoring of Side Effects Scale (MOSES) [12], and one employed the Drug abuse liability [11].
3.3. Adverse Effects of CBD
CBD showed mild and moderate AEs in most studies (9/12). The most common AEs (≥10% incidence) included gastrointestinal symptoms (59.5%), somnolence (16.7%), loss of appetite (16.5%), increased ALT/AST (12.8%), and fatigue (11.4%). All AEs reported with an incidence ≥1% are described in Table 2. In most studies, participants used CBD concomitantly with another medication (see Table 1). Statistical analysis with Fisher’s exact test revealed significance (p ≤ 0.05) in using CBD for the increase in gastrointestinal symptoms, ALT/AST, rash, as well as change in appetite (both increasing and decreasing). In contrast, there was statistical significance for fever, with fewer cases for individuals who used CBD in the trials.

Table 2.
The most common adverse effects reported in clinical trials on Cannabidiol.
3.4. Serious Adverse Effects of CBD
Serious adverse effects of cannabidiol were reported in only three studies, all on epilepsy [11,15,16]. All SAEs reported in these studies were in the context of using CBD at doses between 20 mg/kg/day to 50 mg/kg/day as an add-on treatment to anticonvulsant medications, including clobazam and valproate. The most common SAEs were hypertransaminasemia (ALT/AST) with serum levels elevations greater than three times the upper limit (6.4%), seizures (1.3%), and rash (1.1%) (Table 3). Dosages greater than 25 mg/kg/day were associated with higher incidences of SAEs. For complete data on serious adverse effects (≥1 patient) see Table S2. Statistical analysis with Fisher’s exact test revealed significance (p ≤ 0.05) in using CBD for ALT/AST increase.

Table 3.
The most common serious adverse effects reported in RCTs on CBD.
3.5. Evaluation of the Studies’ Methodology and Bias Assessment
All studies showed an adequate to a good level of description. Half of the RCTs had fair methodological quality, while the other half had good quality. Thus, no study showed poor methodological quality. Two RCTs [13,17] reported 100% of items from the Quality Assessment of Controlled Intervention Studies tool (see Table 4), indicating a tendency towards a low bias risk.

Table 4.
Summary of the analysis of the studies’ methodologies.
4. Discussion
The present paper is an updated systematic review of the adverse effects of orally administered purified cannabidiol for different clinical conditions published in the last three years. The previous review [8] reported data from 18 RCTs published between 1980 and early 2020, involving 1127 subjects from clinical samples and healthy volunteers, with daily CBD treatment at doses ranging from 20 to 1500 mg/day and a duration ranging from one to 18 weeks. The results showed that CBD had mild and moderate adverse effects in most studies, the most common being drowsiness, sedation, fatigue, dizziness, headache, diarrhea, nausea, decreased appetite, and abdominal discomfort. Regarding SAEs, the highest incidence was reported in RCTs on epilepsy. Some of the most relevant effects were severe drowsiness, lethargy, hypertransaminasemia, rash, and pneumonia with or without respiratory failure. In the present review, we identified 12 trials with a total of 745 participants. In most studies, CBD showed mild and moderate adverse effects. The most common adverse effects were gastrointestinal symptoms (59.5%), which are probably related to the endocannabinoid system’s role in regulating gastrointestinal motility [22] and or by the product formulations (drug vehicle); other common AEs were somnolence (16.7%), loss of appetite (16.5%), increased ALT/AST (12.8%), and fatigue (11.4%). Although CBD was associated with a probability of SAEs, these events only occurred in epilepsy studies that used CBD as an add-on anticonvulsant treatment. SAEs with an incidence ≥1% were hypertransaminasemia (6.4%), seizures (1.3%), and rash (1.1%). Statistical analysis revealed a significant effect of CBD treatment for the increase in gastrointestinal symptoms, ALT/AST, rash, as well as change in appetite (both increasing and decreasing). In contrast, there was statistical significance for fever, with fewer cases for subjects who used CBD in the trials, possibly reflecting its anti-inflammatory effects. With the increase in the use of CBD in several countries and several RCTs being carried out, the strengths of our study included updating the evaluation of the most recent published studies and assessing their methodological qualities, resulting in a trend towards a low level of bias between the RCTs included. However, our analysis and conclusions are limited by the small sample size of the RCTs and the absence of studies with low doses of purified CBD.
Since cannabidiol is typically added to existing medicine treatments, interactions can occur between CBD and other co-administered medications. Pharmacokinetic drug-drug interactions can occur at the absorption, distribution, and elimination stages, resulting in changes in drug plasma concentrations. When cannabidiol is used with another medication, the pharmacokinetics of the CBD or the other drug may change [23]. CYP2C19 and, to a lesser extent, CYP3A4 are the main cytochrome P450 (CYP) enzymes that metabolize CBD, converting it to its primary active metabolite 7-hydroxy CBD [24,25]. These hepatic enzymes are also involved in the metabolism of several other drugs, including the anticonvulsants clobazam and valproate. CBD has been reported to inhibit CYP2C19 at doses as low as 5 mg/kg [26]. CYP2C19 inhibition increases the levels of N-desmethylclobazam, the main active metabolite in clobazam [26], and has the potential to interact with a broad range of other generally prescribed medications. The clinical impact of these interactions, particularly at the usual therapeutic doses of CBD, must be carefully investigated. Hypertransaminasemia has also been reported after CBD’s clinical use. Even if most cases occurred in epileptic patients using valproate, increases in hepatic transaminases were reported in open-label studies employing 300 mg/day CBD as a single medication [27]. The precise mechanism of valproate-CBD interaction is not fully understood. The co-administration of CBD and valproate does not significantly alter their plasma levels or metabolites [28]. However, 7-COOH-CBD, valproate and its metabolite 4-ene-valproic acid may affect hepatic mitochondrial function [26].
As SAEs, in addition to hypertransaminasemia, seizures and rash have also been reported, resulting in treatment discontinuation. The three studies reporting severe seizures were in the context of patients diagnosed with epilepsy, and it is not simple to differentiate the worsening of the baseline pathology (epilepsy) from an adverse effect of the treatment. Rash is one of the most common adverse drug reactions. It can present in a variety of ways, from mild rashes to extensive lesions with epidermal detachment associated with severe systemic involvement, as seen, for example, in Stevens-Johnson syndrome and toxic epidermal necrolysis. However, the rash is very non-specific and can often be found in other conditions, such as viral infections; in isolated CBD oil, the rash may be related to the medication vehicle, for example. Thus, rash diagnosis secondary to medication requires extensive laboratory investigation and histopathological confirmation. A case series [29] from an open-label clinical trial [30] involving CBD and rash were reported in four participants who were not using other medications. An extensive laboratory investigation, including histopathological analysis, was performed, in addition to re-exposure to the medication vehicle only, showing no new reactions, suggesting that the skin reactions were related to CBD.
While numerous countries have a growing demand for legal cannabidiol use to treat various mental and physical health problems, determining the best regulatory response to these requests is a substantial challenge. CBD products can come in a variety of formulations (e.g., purified CBD, CBD:THC ratios, CBD enriched products), routes of administration (e.g., oral [oil, tablets, capsules], sublingual spray, inhaled, topical) [2], and many are prepared without adequate manufacturing quality control, pharmaceutical grade production and labeling. There is a common perception that CBD is a “natural medicine”, even though its derivatives are commonly extracted from cannabis and synthetically modified. Furthermore, to date, there needs to be more robust data to support the indications of its therapeutic use for many of these conditions required by global demand. In some countries, CBD is marketed as a supplement, food, or pet product, which becomes a problem as no robust regulatory standards are required. Recently, the Science Advisory Committee of Health Canada reported some recommendations on Health Products Containing Cannabis—products containing cannabidiol can be purchased without needing a prescription from a medical professional, similar to other over-the-counter products [31]; At the same time, the European Food Safety Authority made a statement on the safety of cannabidiol as a novel food and, considering the significant uncertainties and data gaps, concluded that the safety of CBD as a novel food cannot currently be established [32].
With the increase in demand for the medicinal use of CBD, new high-quality studies are essential, with dose variations, particularly smaller doses, contemplating real-world studies with larger samples, and reporting adverse events, to better assess the effectiveness, safety, and tolerability of CBD. The clinical use of CBD must be aligned with many other pharmaceutical products, ensuring its effectiveness and safety for patients, and informing them of its potential adverse effects and interactions with other drugs. As new research emerges, it may become evident that there is a range of CBD doses at which clinically relevant adverse effects and drug interactions are likely to occur [23]. Therefore, as quality data is built, it is urgently necessary to inform and protect consumers, ensuring the quality and safety of CBD products.
5. Conclusions
The data from the present systematic review agree with previous data on the safety of purified CBD. The most common adverse effects are mild and moderate, and serious adverse effects are rare and have been only reported in epilepsy studies, with concomitant use of CBD with antiepileptic drugs. Physicians must carry out the indication for the use of CBD through prescription, and its use must be monitored, especially at the beginning of the treatment. Due to the high global demand for CBD in various conditions, additional safety data from clinical trials with larger samples, different dosages, and different products are needed (e.g., evaluating the effects in the comparison of purified CBD with broad-spectrum and full-spectrum formulations).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics14122598/s1, Methodological adaptations compared to the previous review; Table S1: Quality Assessment of Controlled Intervention Studies; Quality Assessment of Controlled Intervention Studies toll—Items reported in each study; Table S2: Serious Adverse Events in ≥1 Patient.
Author Contributions
All authors contributed to drafting and revising the manuscript and approved its final version. J.D.R.S. and J.C.P. contributed equally and were involved in the study’s concept, design, registration, systematic search, extraction of data, and analysis. G.N.R. contributed to the systematic search, extraction of data, and analysis. B.O.d.-P. contributed to data extraction and analysis. J.A.C. and R.G.D.S. were involved in the design of the study. J.A.C., A.W.Z., F.S.G., J.E.C.H. and R.G.D.S. were supervisors of the present review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in Supplementary Materials.
Conflicts of Interest
JC was a consultant and/or has received speaker fees and/or sits on the advisory board and/or receives research funding and/or receives speaker fees from Janssen-Cilag, Torrent Pharm, Prati-Donaduzzi, Mantecorp, ArtMed, PurMed Global, and BSPG Pharm over the past 3 years. JC, JH, and AZ are coinventors of the patent “Fluorinated CBD compounds, compositions and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023,” Def. US number Reg. 62193296; 29 July 2015; INPI on 19 August 2015 (BR1120150164927; Mechoulam R, Zuardi AW, Kapczinski F, Hallak JEC, Guimarães FS, Crippa JAS, Breuer A). Universidade de São Paulo (USP) has licensed this patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1) and has an agreement with Prati-Donaduzzi to “develop a pharmaceutical product containing synthetic CBD and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson’s disease, and anxiety disorders”. JC, JH, and AZ are coinventors of the patent “Cannabinoid-containing oral pharmaceutical composition, method for preparing and using same”, INPI on 16 September 2016 (BR 112018005423-2). The other authors report no conflict of interest.
References
- Brunetti, P.; Pichini, S.; Pacifici, R.; Busardò, F.P.; Del Rio, A. Herbal Preparations of Medical Cannabis: A Vademecum for Prescribing Doctors. Medicina 2020, 56, 237. [Google Scholar] [CrossRef] [PubMed]
- Schlag, A.K.; O’Sullivan, S.E.; Zafar, R.R.; Nutt, D.J. Current controversies in medical cannabis: Recent developments in human clinical applications and potential therapeutics. Neuropharmacology 2021, 191, 108586. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.; Guimarães, F.S.; Campos, A.C.; Zuardi, A.W. Translational Investigation of the Therapeutic Potential of Cannabidiol (CBD): Toward a New Age. Front. Immunol. 2018, 9, 2009. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Naveed, S.; Mian, N.; Fida, A.; Raafey, M.A.; Aedma, K.K. The therapeutic role of Cannabidiol in mental health: A systematic review. J. Cannabis Res. 2020, 2, 2. [Google Scholar] [CrossRef]
- Wieckiewicz, G.; Stokłosa, I.; Stokłosa, M.; Gorczyca, P.; Pudlo, R. Cannabidiol (CBD) in the Self-Treatment of Depression-Exploratory Study and a New Phenomenon of Concern for Psychiatrists. Front. Psychiatry 2022, 13, 837946. [Google Scholar] [CrossRef] [PubMed]
- EPIDIOLEX. EPIDIOLEX® (Cannabidiol). Available online: https://www.epidiolex.com/ (accessed on 13 September 2022).
- Chesney, E.; Oliver, D.; Green, A.; Sovi, S.; Wilson, J.; Englund, A.; Freeman, T.; McGuire, P. Adverse effects of cannabidiol: A systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology 2020, 45, 1799–1806. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Guimarães, F.S.; Crippa, J.A.S.; Hallak, J.E.; Rossi, G.N.; Rocha, J.M.; Zuardi, A.W. Serious adverse effects of cannabidiol (CBD): A review of randomized controlled trials. Expert. Opin. Drug Metab. Toxicol. 2020, 16, 517–526. [Google Scholar] [CrossRef]
- NHLBI; NIH. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 13 September 2022).
- Appiah-Kusi, E.; Petros, N.; Wilson, R.; Colizzi, M.; Bossong, M.G.; Valmaggia, L.; Mondelli, V.; McGuire, P.; Bhattacharyya, S. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high risk of developing psychosis. Psychopharmacology 2020, 237, 1121–1130. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Gunning, B.; Cabrera, C.M.A.; VanLandingham, K.; Crockett, J.; Critchley, D.; Wray, L.; Tayo, B.; Morrison, G.; Toledo, M. A Phase II Randomized Trial to Explore the Potential for Pharmacokinetic Drug-Drug Interactions with Stiripentol or Valproate when Combined with Cannabidiol in Patients with Epilepsy. CNS Drugs 2020, 34, 661–672. [Google Scholar] [CrossRef]
- Efron, D.; Freeman, J.L.; Cranswick, N.; Payne, J.; Mulraney, M.; Prakash, C.; Lee, K.J.; Taylor, K.; Williams, K. A pilot randomised placebo-controlled trial of cannabidiol to reduce severe behavioural problems in children and adolescents with intellectual disability. Br. J. Clin. Pharmacol. 2021, 87, 436–446. [Google Scholar] [CrossRef]
- Freeman, T.P.; Hindocha, C.; Baio, G.; Shaban, N.D.C.; Thomas, E.M.; Astbury, D.; Freeman, A.M.; Lees, R.; Craft, S.; Morrison, P.D.; et al. Cannabidiol for the treatment of cannabis use disorder: A phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry 2020, 7, 865–874. [Google Scholar] [CrossRef]
- De Meneses-Gaya, C.; Crippa, J.A.; Hallak, J.E.; Miguel, A.Q.; Laranjeira, R.; Bressan, R.A.; Zuardi, A.W.; Lacerda, A.L. Cannabidiol for the treatment of crack-cocaine craving: An exploratory double-blind study. Braz. J. Psychiatry 2021, 43, 467–476. [Google Scholar] [CrossRef]
- Thiele, E.A.; Bebin, E.M.; Bhathal, H.; Jansen, F.E.; Kotulska, K.; Lawson, J.A.; O’Callaghan, F.J.; Wong, M.; Sahebkar, F.; Checketts, D.; et al. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurol. 2021, 78, 285–292. [Google Scholar] [CrossRef]
- VanLandingham, K.E.; Crockett, J.; Taylor, L.; Morrison, G. A Phase 2, Double-Blind, Placebo-Controlled Trial to Investigate Potential Drug-Drug Interactions Between Cannabidiol and Clobazam. J. Clin. Pharmacol. 2020, 60, 1304–1313. [Google Scholar] [CrossRef]
- Crippa, J.A.S.; Pacheco, J.C.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; Osório, F.D.L.; Loureiro, S.R.; dos Santos, R.G.; Souza, J.D.S.; Ushirohira, J.M.; et al. Cannabidiol for COVID-19 Patients with Mild to Moderate Symptoms (CANDIDATE Study): A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Cannabis Cannabinoid Res. 2021, 7, 658–669. [Google Scholar] [CrossRef]
- De Almeida, C.M.; Brito, M.M.; Bosaipo, N.B.; Pimentel, A.V.; Tumas, V.; Zuardi, A.W.; Crippa, J.A.; Hallak, J.E.; Eckeli, A.L. Cannabidiol for Rapid Eye Movement Sleep Behavior Disorder. Mov. Disord. 2021, 36, 1711–1715. [Google Scholar] [CrossRef]
- Leweke, F.M.; Rohleder, C.; Gerth, C.W.; Hellmich, M.; Pukrop, R.; Koethe, D. Cannabidiol and Amisulpride Improve Cognition in Acute Schizophrenia in an Explorative, Double-Blind, Active-Controlled, Randomized Clinical Trial. Front. Pharmacol. 2021, 12, 614811. [Google Scholar] [CrossRef]
- Mongeau-Pérusse, V.; Brissette, S.; Bruneau, J.; Conrod, P.; Dubreucq, S.; Gazil, G.; Stip, E.; Jutras-Aswad, D. Cannabidiol as a treatment for craving and relapse in individuals with cocaine use disorder: A randomized placebo-controlled trial. Addiction 2021, 116, 2431–2442. [Google Scholar] [CrossRef]
- Atieh, J.; Maselli, D.; Breen-Lyles, M.; Torres, M.; Katzka, D.; Ryks, M.; Busciglio, I.; Burton, D.; Carlson, P.; Harmsen, W.S.; et al. Cannabidiol for Functional Dyspepsia with Normal Gastric Emptying: A Randomized Controlled Trial. Am. J. Gastroenterol. 2022, 117, 1296–1304. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoids and the gastrointestinal tract. Gut 2001, 48, 859–867. [Google Scholar] [CrossRef]
- Graham, M.; Martin, J.; Lucas, C.; Murnion, B.; Schneider, J. Cannabidiol drug interaction considerations for prescribers and pharmacists. Expert Rev. Clin. Pharmacol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Watanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Zendulka, O.; Dovrtělová, G.; Nosková, K.; Turjap, M.; Šulcová, A.; Hanuš, L.; Jurica, J. Cannabinoids and Cytochrome P450 Interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/210365lbl.pdf (accessed on 14 September 2022).
- Crippa, J.A.S.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; de Lima Osório, F.; Loureiro, S.R.; Dos Santos, R.G.; Souza, J.D.S.; Ushirohira, J.M.; Pacheco, J.C.; et al. Efficacy and Safety of Cannabidiol Plus Standard Care vs Standard Care Alone for the Treatment of Emotional Exhaustion and Burnout Among Frontline Health Care Workers During the COVID-19 Pandemic: A Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e2120603. [Google Scholar] [CrossRef]
- Morrison, G.; Crockett, J.; Blakey, G.; Sommerville, K. A Phase 1, Open-Label, Pharmacokinetic Trial to Investigate Possible Drug-Drug Interactions Between Clobazam, Stiripentol, or Valproate and Cannabidiol in Healthy Subjects. Clin. Pharmacol. Drug Dev. 2019, 8, 1009–1031. [Google Scholar] [CrossRef]
- Souza, J.D.S.; Fassoni-Ribeiro, M.; Batista, R.M.; Ushirohira, J.M.; Zuardi, A.W.; Guimarães, F.S.; Campos, A.C.; Osório, F.D.L.; Elias, D.; Souza, C.S.; et al. Case Report: Cannabidiol-Induced Skin Rash: A Case Series and Key Recommendations. Front. Pharmacol. 2022, 13, 881617. [Google Scholar] [CrossRef]
- Souza, J.D.S.; Zuardi, A.W.; Guimarães, F.S.; Osório, F.D.L.; Loureiro, S.R.; Campos, A.C.; Hallak, J.E.C.; Dos Santos, R.G.; Silveira, I.L.M.; Pereira-Lima, K.; et al. Maintained anxiolytic effects of cannabidiol after treatment discontinuation in healthcare workers during the COVID-19 pandemic. Front. Pharmacol. 2022, 13, 856846. [Google Scholar] [CrossRef]
- Health Canada. Review of Cannabidiol: Report of the Science Advisory Committee on Health Products Containing Cannabis. 2022. Available online: https://www.canada.ca/en/health-canada/corporate/about-health-canada/public-engagement/external-advisory-bodies/health-products-containing-cannabis/review-cannabidiol-health-products-containing-cannabis.html (accessed on 14 September 2022).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Statement on safety of cannabidiol as a novel food: Data gaps and uncertainties. EFSA J. 2022, 20, e07322. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).