Plug and Pop: A 3D-Printed, Modular Platform for Drug Delivery Using Clinical Ultrasound and Microbubbles
Abstract
1. Introduction
2. Materials and Methods
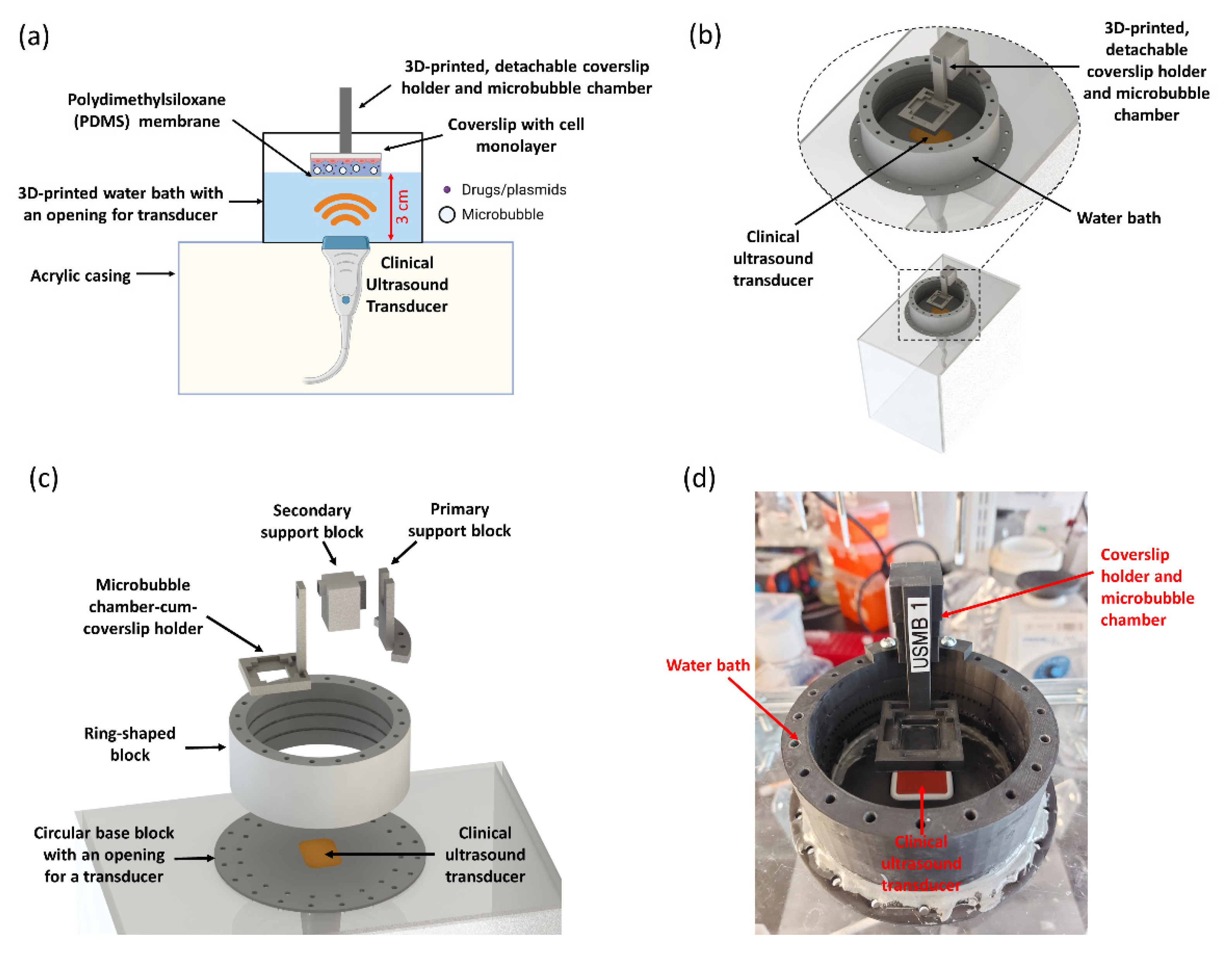
2.1. Engineering UMCC: A 3D-Printed, Modular Platform for USMB-Delivery Testing
2.2. Clinical Ultrasound System and Transducer
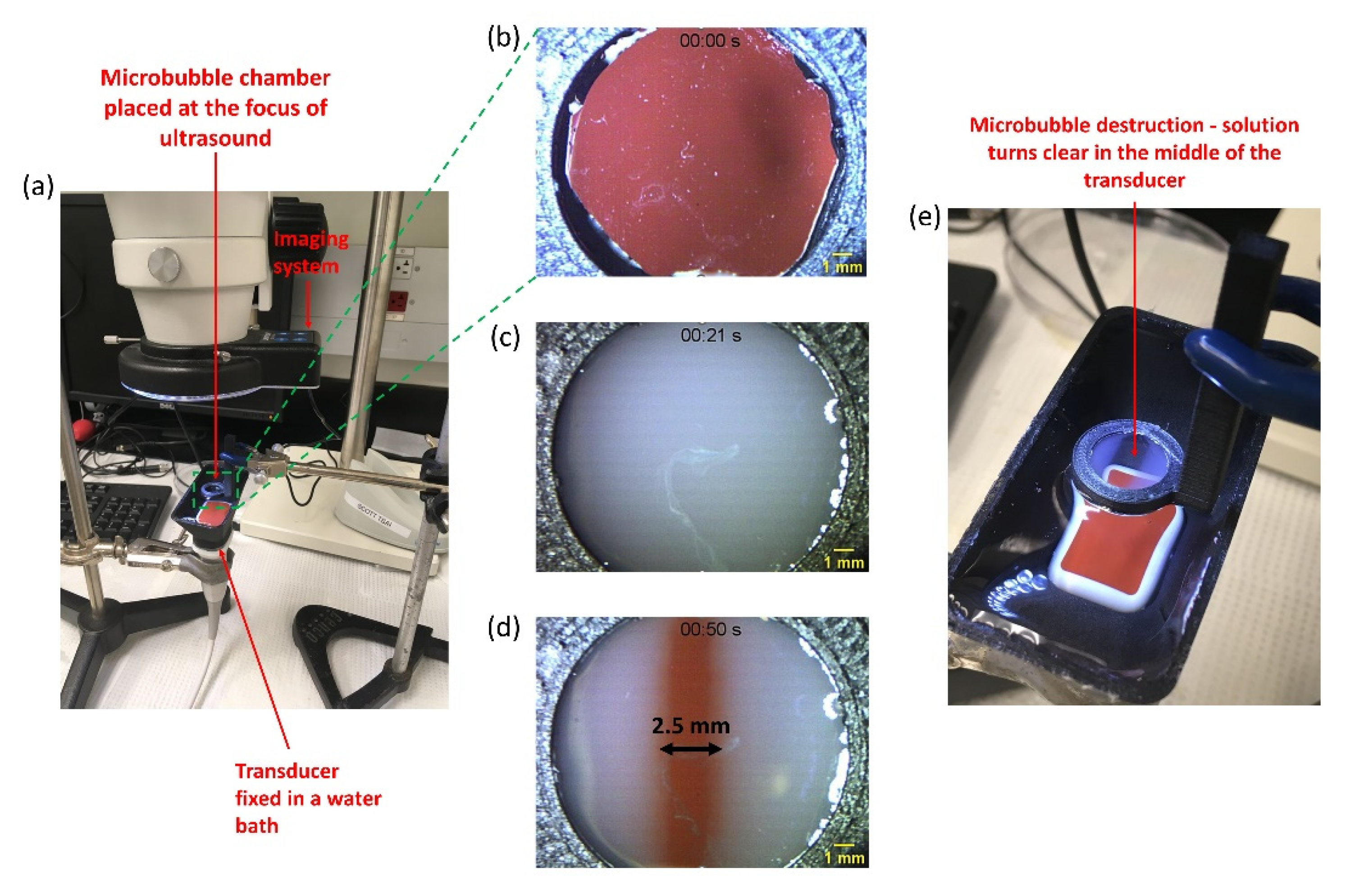
2.3. Experimental Setup for Real-Time Visualization of Ultrasound-Mediated Microbubble Cavitation
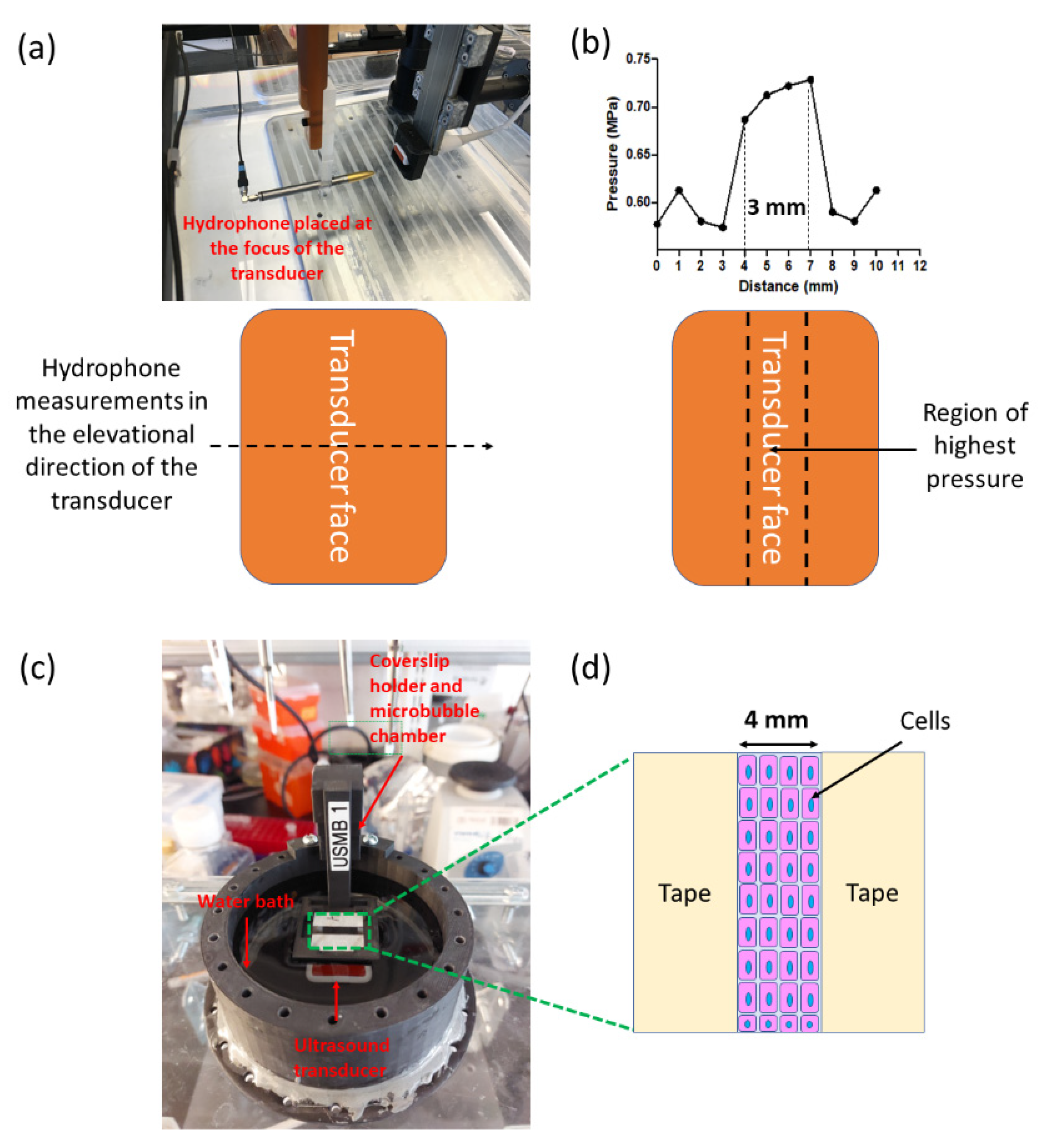
2.4. Transducer Characterization Using Hydrophone Measurements
2.5. Cell Culturing
2.6. Experimental Protocol for Optimizing Delivery of 70 kDa and 4 kDa Dextran
2.7. Quantifying Dextran Delivery by Flow Cytometry
2.8. Statistics
3. Results
3.1. Characterization of a Clinical Ultrasound Transducer for USMB
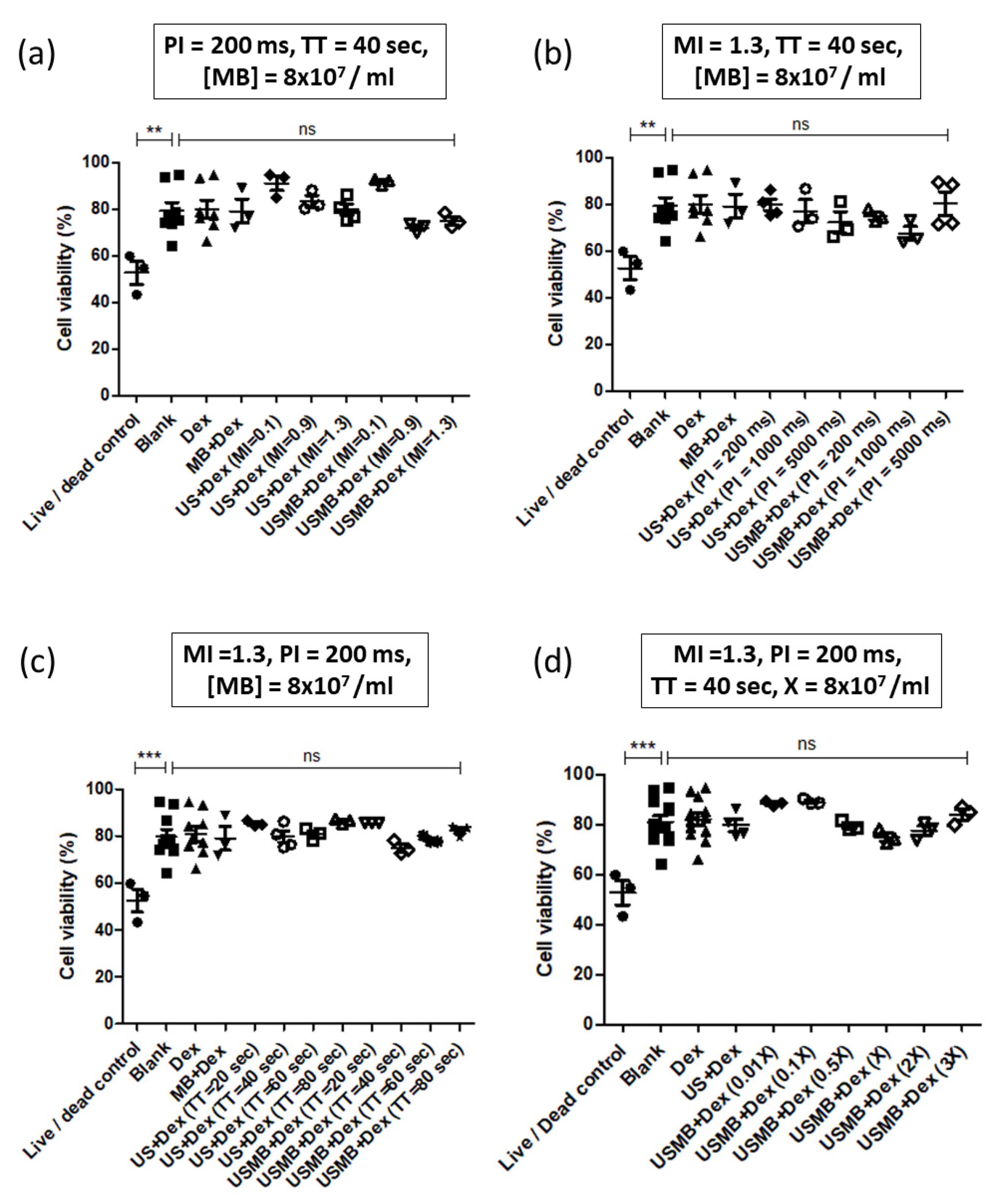
3.2. Optimization of 70 kDa Dextran Delivery in HEK293 Cells Using UMCC
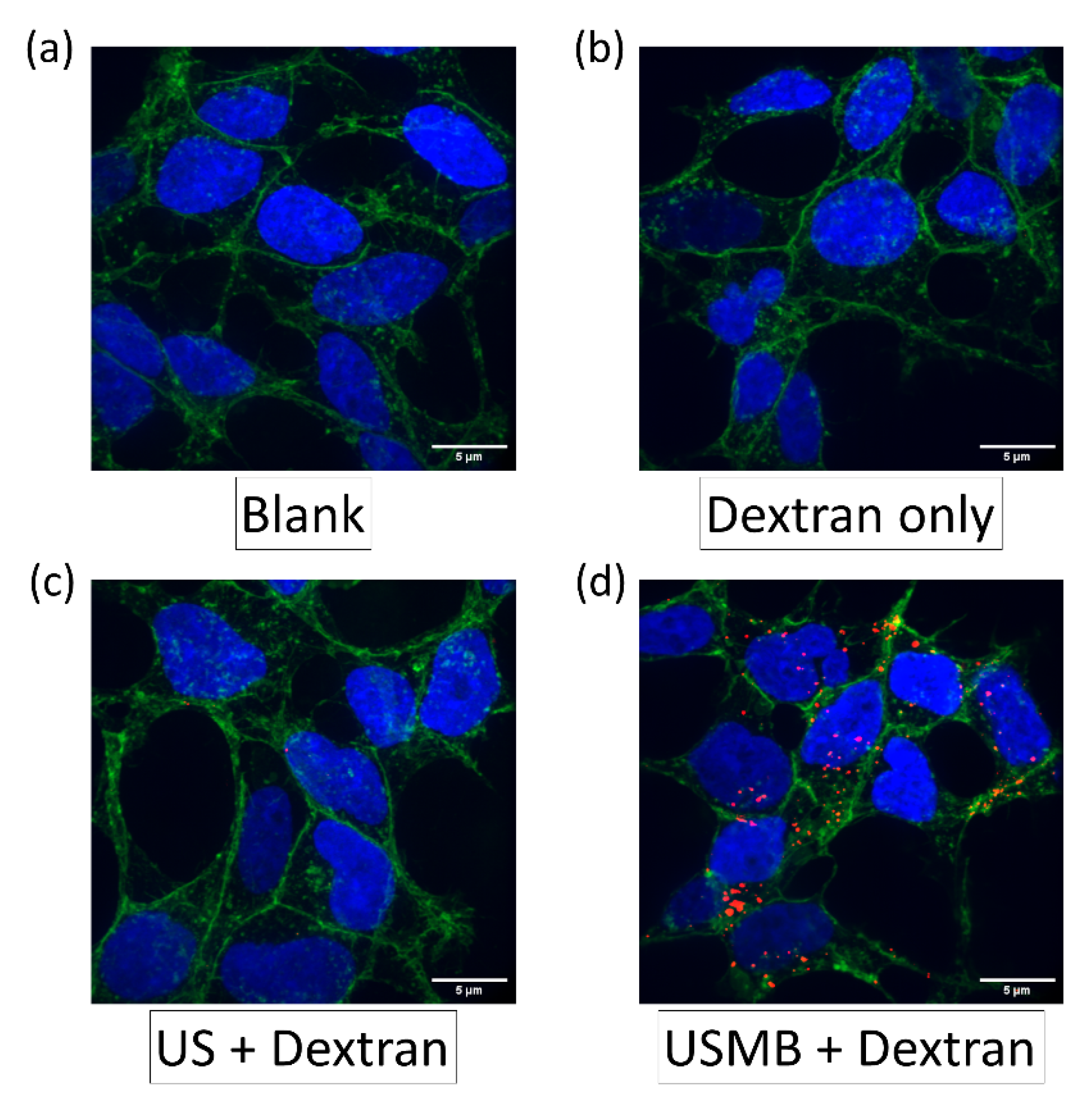
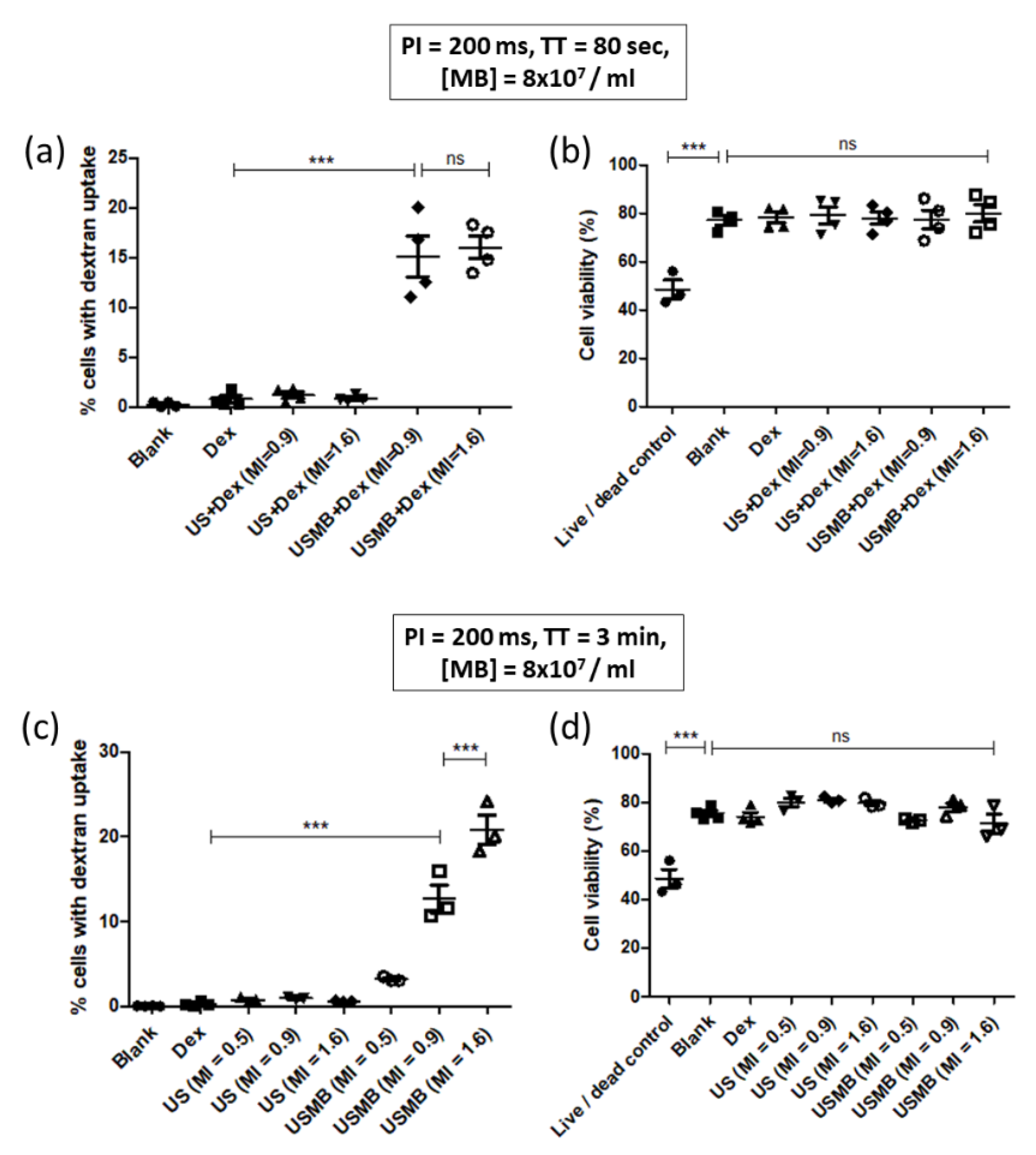
3.3. Demonstration of 70 kDa and 4 kDa Dextran Delivery in CMT167 Lung Cancer Cells
4. Discussion
4.1. Proportion of Dextran Positive Cells Increases with Increasing MI
4.2. Proportion of Dextran Positive Cells Decreases with Increasing PI
4.3. Proportion of Dextran Positive Cells Increases with Ultrasound Exposure Time
4.4. Proportion of Dextran Positive Cells Peaks at a Range of Microbubble Concentrations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sugiyama, M.G.; Mintsopoulos, V.; Raheel, H.; Goldenberg, N.M.; Batt, J.E.; Brochard, L.; Kuebler, W.M.; Leong-Poi, H.; Karshafian, R.; Lee, W.L. Lung Ultrasound and Microbubbles Enhance Aminoglycoside Efficacy and Delivery to the Lung in Escherichia Coli-Induced Pneumonia and Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2018, 198, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Han, Z.; Shao, L.; Zhao, Y. Evaluation of In Vivo Antitumor Effects of Low-Frequency Ultrasound-Mediated MiRNA-133a Microbubble Delivery in Breast Cancer. Cancer Med. 2016, 5, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, L.; Folliero, V.; Palomba, L.; Zannella, C.; Isticato, R.; Di Francia, R.; Berretta, M.; de Sio, I.; Adinolfi, L.E.; Morelli, G.; et al. Sonoporation by Microbubbles as Gene Therapy Approach against Liver Cancer. Oncotarget 2018, 9, 32182–32190. [Google Scholar] [CrossRef] [PubMed]
- Yusefi, H.; Helfield, B. Ultrasound Contrast Imaging: Fundamentals and Emerging Technology. Front. Phys. 2022, 10, 100. [Google Scholar] [CrossRef]
- Fabiilli, M.L.; Haworth, K.J.; Fakhri, N.H.; Kripfgans, O.D.; Carson, P.L.; Fowlkes, J.B. The Role of Inertial Cavitation in Acoustic Droplet Vaporization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Sanwal, R.; Joshi, K.; Ditmans, M.; Tsai, S.S.H.; Lee, W.L. Ultrasound and Microbubbles for Targeted Drug Delivery to the Lung Endothelium in ARDS: Cellular Mechanisms and Therapeutic Opportunities. Biomedicines 2021, 9, 803. [Google Scholar] [CrossRef]
- Doinikov, A.A.; Bouakaz, A. Acoustic Microstreaming around a Gas Bubble. J. Acoust. Soc. Am. 2010, 127, 703–709. [Google Scholar] [CrossRef]
- Brujan, E.A.; Ikeda, T.; Matsumoto, Y. Jet Formation and Shock Wave Emission during Collapse of Ultrasound-Induced Cavitation Bubbles and Their Role in the Therapeutic Applications of High-Intensity Focused Ultrasound. Phys. Med. Biol. 2005, 50, 4797–4809. [Google Scholar] [CrossRef]
- Fekri, F.; Abousawan, J.; Bautista, S.; Orofiamma, L.; Dayam, R.M.; Antonescu, C.N.; Karshafian, R. Targeted Enhancement of Flotillin-Dependent Endocytosis Augments Cellular Uptake and Impact of Cytotoxic Drugs. Sci. Rep. 2019, 9, 17768. [Google Scholar] [CrossRef]
- Rahim, A.; Taylor, S.L.; Bush, N.L.; ter Haar, G.R.; Bamber, J.C.; Porter, C.D. Physical Parameters Affecting Ultrasound/Microbubble-Mediated Gene Delivery Efficiency In Vitro. Ultrasound Med. Biol. 2006, 32, 1269–1279. [Google Scholar] [CrossRef]
- Li, H.L.; Zheng, X.Z.; Wang, H.P.; Li, F.; Wu, Y.; Du, L.F. Ultrasound-Targeted Microbubble Destruction Enhances AAV-Mediated Gene Transfection in Human RPE Cells in Vitro and Rat Retina in Vivo. Gene Ther. 2009, 16, 1146–1153. [Google Scholar] [CrossRef]
- Choi, J.J.; Feshitan, J.A.; Baseri, B.; Wang, S.; Tung, Y.-S.; Borden, M.A.; Konofagou, E.E. Microbubble-Size Dependence of Focused Ultrasound-Induced Blood-Brain Barrier Opening in Mice in Vivo. IEEE Trans. Biomed. Eng. 2010, 57, 145–154. [Google Scholar] [CrossRef]
- Shapiro, G.; Wong, A.W.; Bez, M.; Yang, F.; Tam, S.; Even, L.; Sheyn, D.; Ben-David, S.; Tawackoli, W.; Pelled, G.; et al. Multiparameter Evaluation of In Vivo Gene Delivery Using Ultrasound-Guided, Microbubble-Enhanced Sonoporation. J. Control. Release 2016, 223, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Zhang, C.; Tu, J.; Zhang, D. Microbubble-Induced Sonoporation Involved in Ultrasound-Mediated DNA Transfection In Vitro at Low Acoustic Pressures. J. Biomech. 2012, 45, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Lucas, V.S.; Burk, R.S.; Creehan, S.; Grap, M.J. Utility of High-Frequency Ultrasound: Moving beyond the Surface to Detect Changes in Skin Integrity. Plast. Surg. Nurs. 2014, 34, 34–38. [Google Scholar] [CrossRef] [PubMed]
- De Maar, J.S.; Rousou, C.; van Elburg, B.; Vos, H.J.; Lajoinie, G.P.R.; Bos, C.; Moonen, C.T.W.; Deckers, R. Ultrasound-Mediated Drug Delivery with a Clinical Ultrasound System: In Vitro Evaluation. Front. Pharmacol. 2021, 12, 768436. [Google Scholar] [CrossRef] [PubMed]
- Meijering, B.D.; Juffermans, L.J.; van Wamel, A.; Henning, R.H.; Zuhorn, I.S.; Emmer, M.; Versteilen, A.M.; Paulus, W.J.; van Gilst, W.H.; Kooiman, K.; et al. Ultrasound and Microbubble-Targeted Delivery of Macromolecules Is Regulated by Induction of Endocytosis and Pore Formation. Circ. Res. 2009, 104, 679–687. [Google Scholar] [CrossRef]
- Karshafian, R.; Bevan, P.D.; Williams, R.; Samac, S.; Burns, P.N. Sonoporation by Ultrasound-Activated Microbubble Contrast Agents: Effect of Acoustic Exposure Parameters on Cell Membrane Permeability and Cell Viability. Ultrasound Med. Biol. 2009, 35, 847–860. [Google Scholar] [CrossRef]
- Escoffre, J.M.; Piron, J.; Novell, A.; Bouakaz, A. Doxorubicin Delivery into Tumor Cells with Ultrasound and Microbubbles. Mol. Pharm. 2011, 8, 799–806. [Google Scholar] [CrossRef]
- De Jong, N.; Bouakaz, A.; Frinking, P. Basic Acoustic Properties of Microbubbles. Echocardiography 2002, 19, 229–240. [Google Scholar] [CrossRef]
- Bhutto, D.F.; Murphy, E.M.; Priddy, M.C.; Centner, C.C.; Moore, J.B., IV; Bolli, R.; Kopechek, J.A. Effect of Molecular Weight on Sonoporation-Mediated Uptake in Human Cells. Ultrasound Med. Biol. 2018, 44, 2662–2672. [Google Scholar] [CrossRef]
- Zarnitsyn, V.; Rostad, C.A.; Prausnitz, M.R. Modeling Transmembrane Transport through Cell Membrane Wounds Created by Acoustic Cavitation. Biophys. J. 2008, 95, 4124–4138. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-K.; Su, S.-Y. Effects of Acoustic Insonation Parameters on Ultrasound Contrast Agent Destruction. Ultrasound Med. Biol. 2008, 34, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.T.; Forsberg, F.; Vaidyanathan, P.; Tornes, A.; Østensen, J.; Goldberg, B.B. The Influence of Acoustic Transmit Parameters on the Destruction of Contrast Microbubbles In Vitro. Phys. Med. Biol. 2006, 51, 4031. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.T.; Nguyen, T.P.; Duong, N.H.T.; Truong, D.H.; Nguyen, B.X.; Bui, C.K.; Nguyen, L.T. Effect of Ultrasonic Parameters on Gene Transfection Efficiency and Cell Viability of the Multifunctional Microbubble In Vitro. J. Drug Deliv. Sci. Technol. 2022, 77, 103882. [Google Scholar] [CrossRef]
- Wei, K.; Skyba, D.M.; Firschke, C.; Jayaweera, A.R.; Lindner, J.R.; Kaul, S. Interactions Between Microbubbles and Ultrasound: In Vitro and In Vivo Observations. J. Am. Coll. Cardiol. 1997, 29, 1081–1088. [Google Scholar] [CrossRef]
- Keller, S.; Bruce, M.; Averkiou, M.A. Ultrasound Imaging of Microbubble Activity during Sonoporation Pulse Sequences. Ultrasound Med. Biol. 2019, 45, 833–845. [Google Scholar] [CrossRef]
- Chan, M.C.M.; Soetanto, K.S.K. Study on Contrast Effect of Microbubbles as Ultrasound Contrast Agents. Jpn. J. Appl. Phys. 1998, 37, 3078. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joshi, K.; Sanwal, R.; Thu, K.L.; Tsai, S.S.H.; Lee, W.L. Plug and Pop: A 3D-Printed, Modular Platform for Drug Delivery Using Clinical Ultrasound and Microbubbles. Pharmaceutics 2022, 14, 2516. https://doi.org/10.3390/pharmaceutics14112516
Joshi K, Sanwal R, Thu KL, Tsai SSH, Lee WL. Plug and Pop: A 3D-Printed, Modular Platform for Drug Delivery Using Clinical Ultrasound and Microbubbles. Pharmaceutics. 2022; 14(11):2516. https://doi.org/10.3390/pharmaceutics14112516
Chicago/Turabian StyleJoshi, Kushal, Rajiv Sanwal, Kelsie L. Thu, Scott S. H. Tsai, and Warren L. Lee. 2022. "Plug and Pop: A 3D-Printed, Modular Platform for Drug Delivery Using Clinical Ultrasound and Microbubbles" Pharmaceutics 14, no. 11: 2516. https://doi.org/10.3390/pharmaceutics14112516
APA StyleJoshi, K., Sanwal, R., Thu, K. L., Tsai, S. S. H., & Lee, W. L. (2022). Plug and Pop: A 3D-Printed, Modular Platform for Drug Delivery Using Clinical Ultrasound and Microbubbles. Pharmaceutics, 14(11), 2516. https://doi.org/10.3390/pharmaceutics14112516





