Analgesic and Anti-Inflammatory Properties of Ethanolic Extract of Piper vicosanum Leaves
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Ethanolic Extract of P. vicosanum Leaves (EEPV)
2.2. Determination of the Content of Phenolic Compounds
2.3. Determination of Flavonoid Content
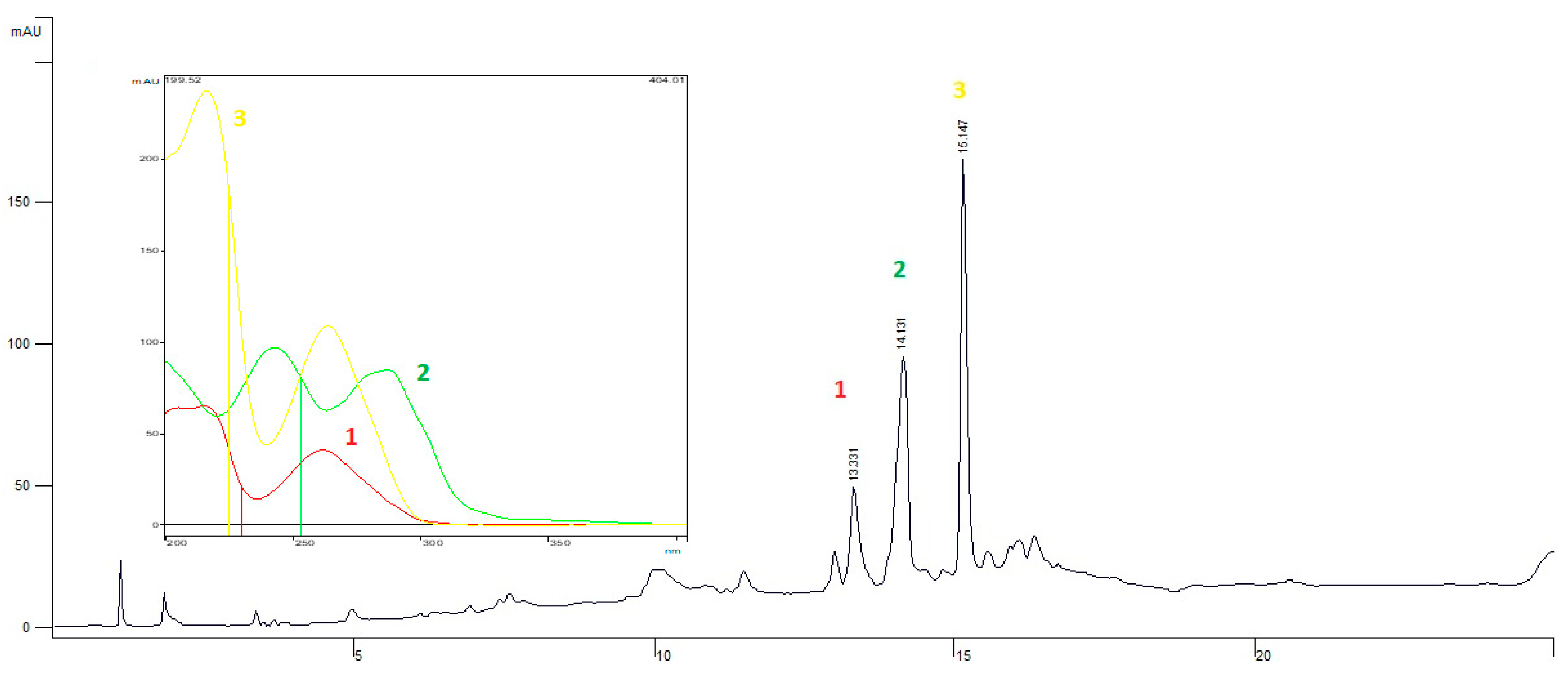
2.4. Phytochemical Analysis by HPLC-DAD
2.5. Animals
2.6. Formalin-Induced Nociception Test
2.7. Acetic Acid-Induced Abdominal Writhing Test
2.8. Carrageenan-Induced Pleurisy
2.9. Zymosan-Induced Arthritis Model
2.10. In Situ Intravital Microscopy Analysis for Rolling and Adhesion Events of Leukocytes in the Mesenteric Microcirculation
2.11. Paw Edema, Mechanical Hyperalgesia, Cold Response, Myeloperoxidase (MPO), and Enzyme N-acetilglucosaminidase (NAG) Analysis in CFA-Induced Paw Inflammation for 24 h
2.12. Rota Rod Test
2.13. Statistical Analysis
3. Results
3.1. Phytochemical Analysis
3.2. Analysis of EEPV by HPLC-DAD
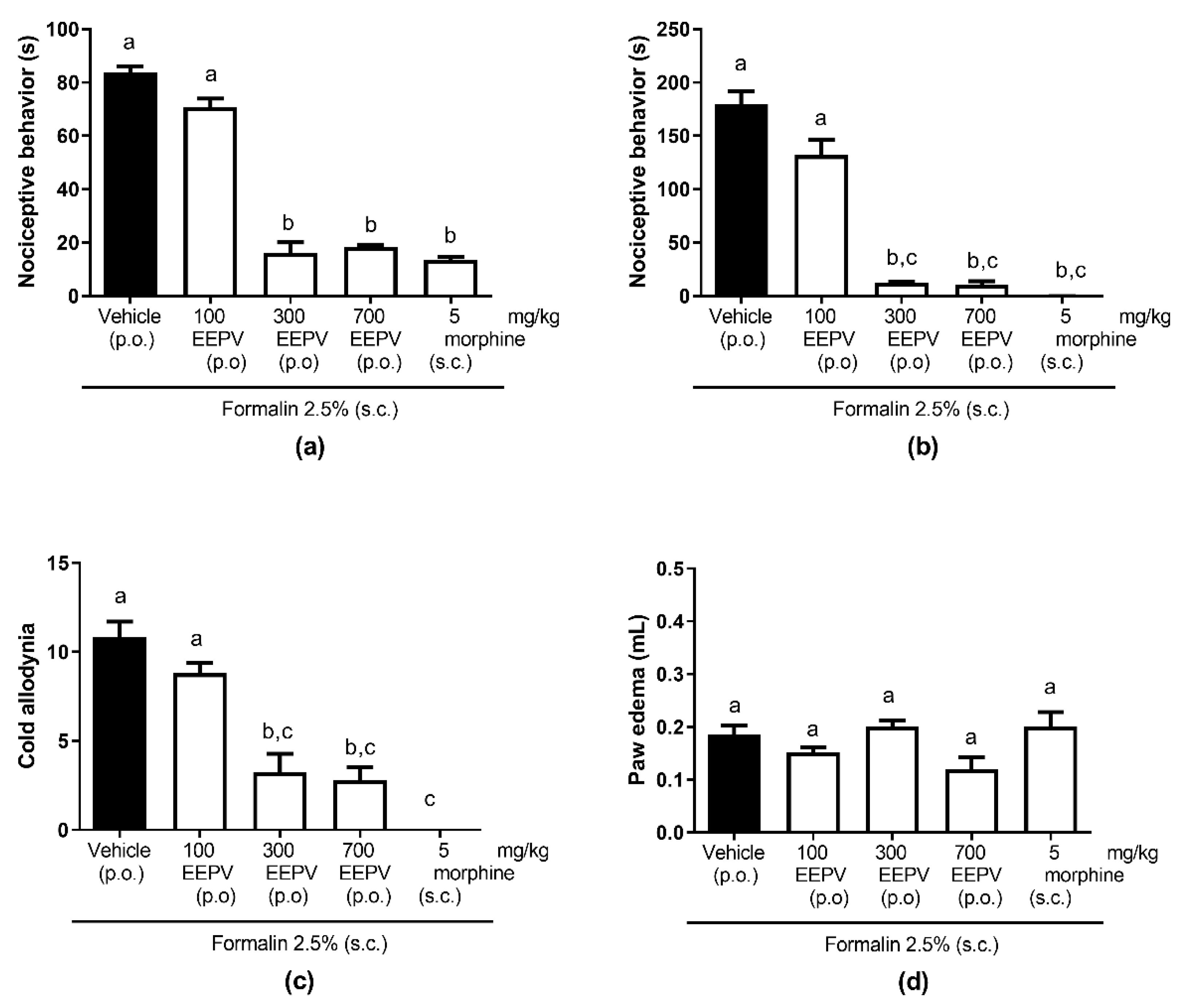
3.3. EEPV Inhibited Nociceptive Response in Phase 1 and 2 and Cold Allodynia in Formalin Model
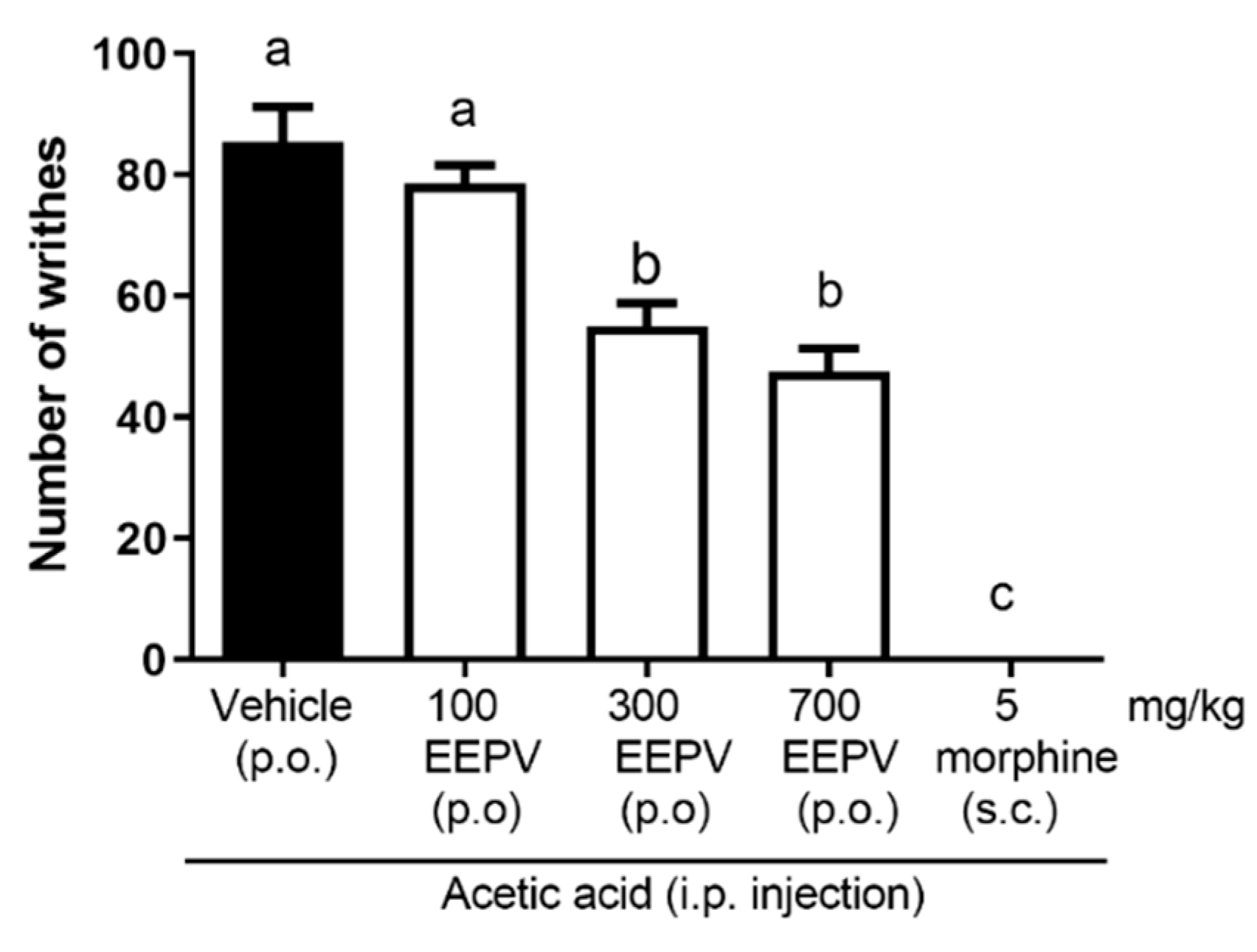
3.4. EEPV Inhibited Abdominal Writhing Induced by Acetic Acid
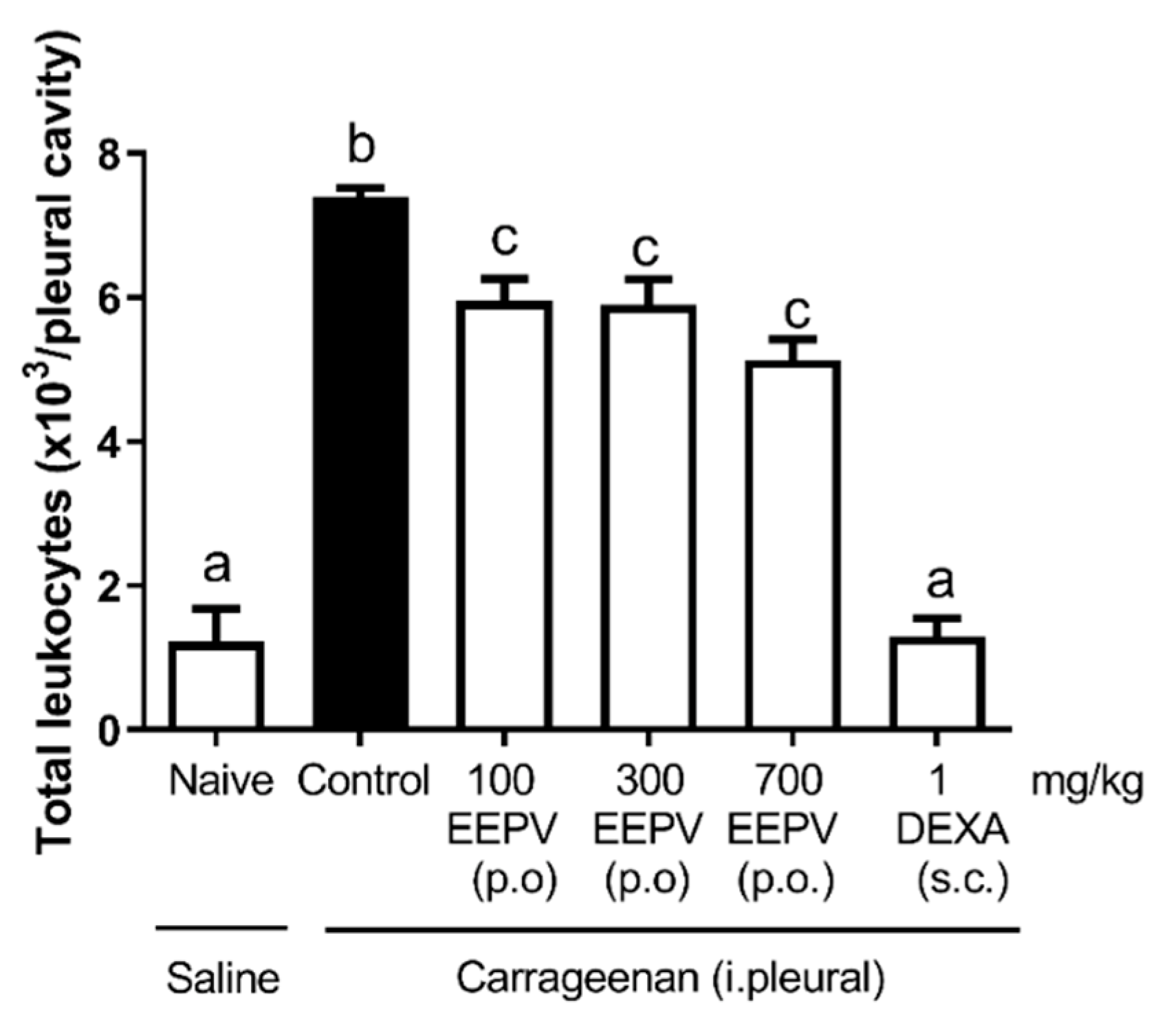
3.5. EEPV Reduced Leukocyte Migration in Carrageenan-Induced Pleurisy Model
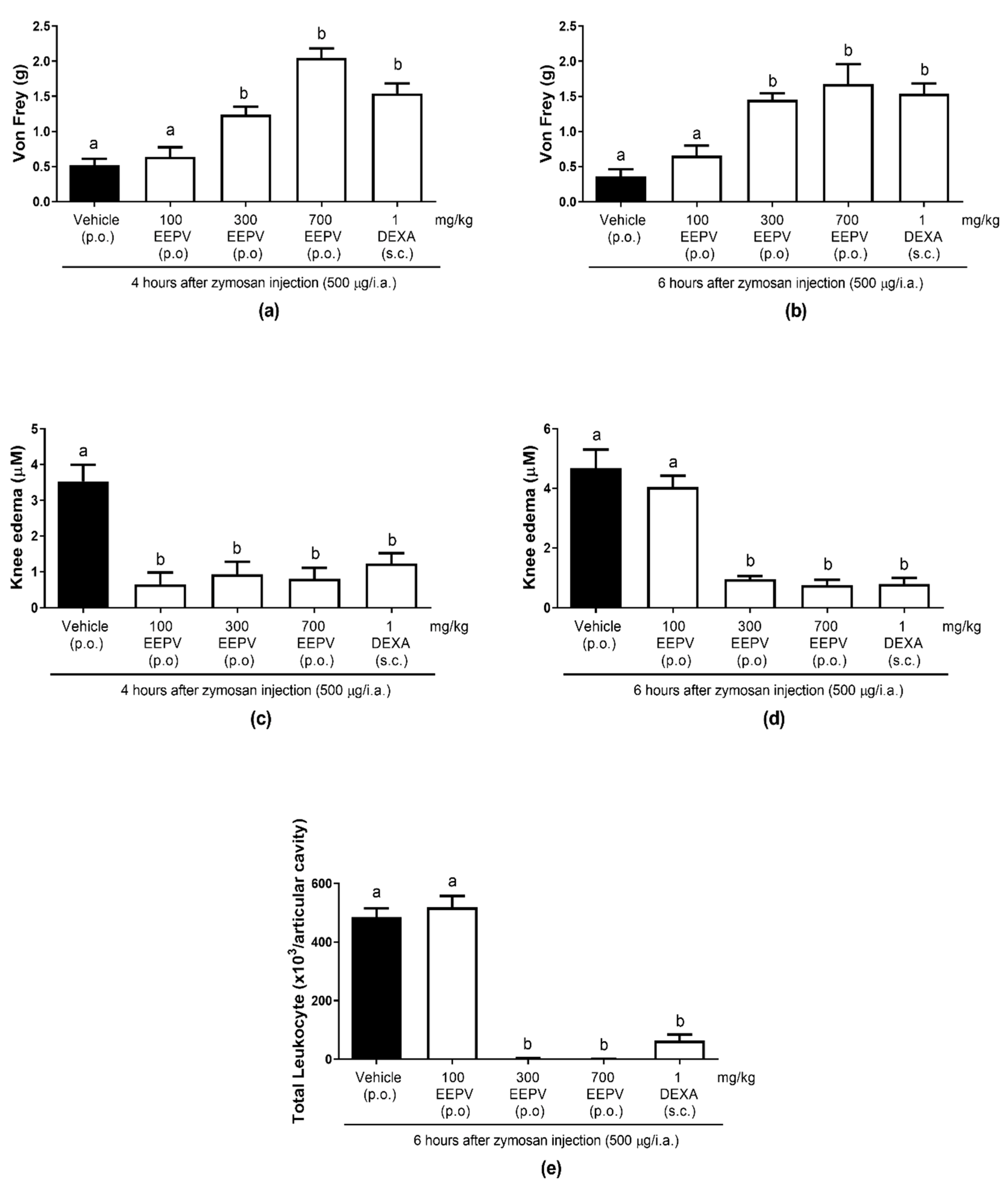
3.6. EEPV Reduced Knee Edema, Mechanical Hyperalgesia, Leukocyte Migration in Zymosan-Induced Articular Inflammation
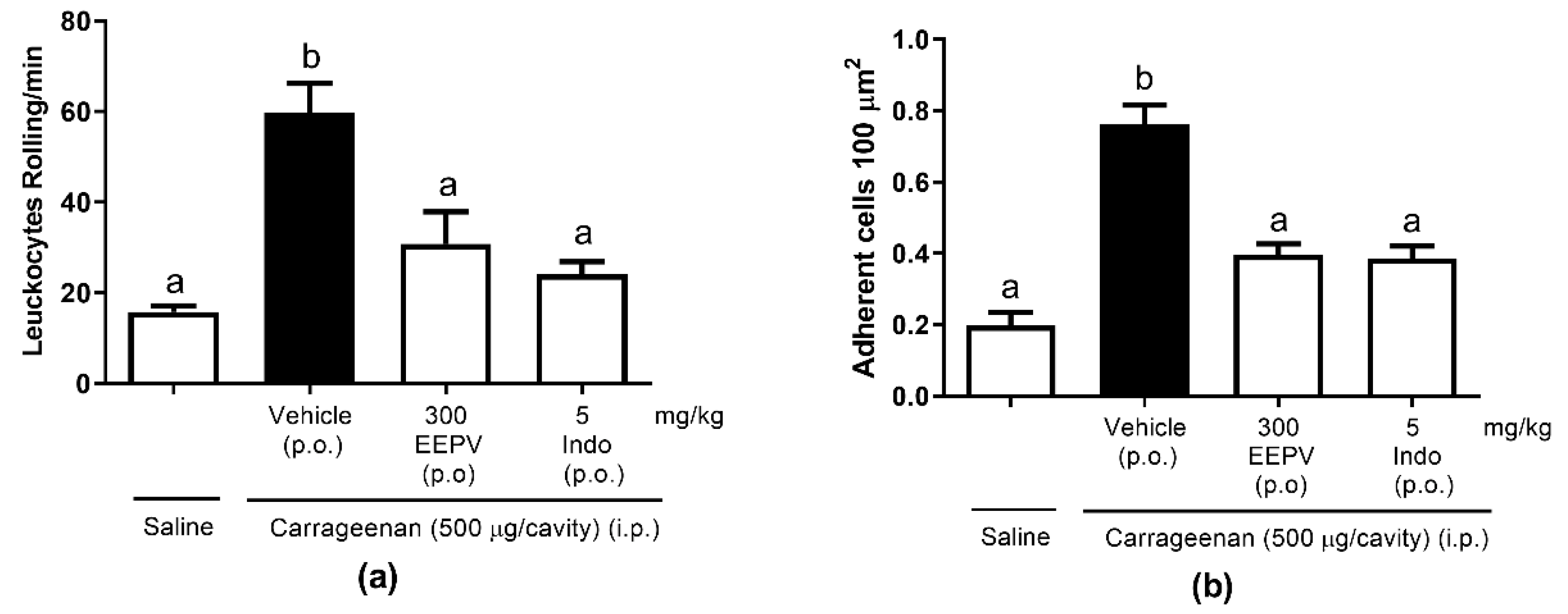
3.7. EEPV Reduces Rolling Leukocytes and Leukocyte Adhesion on Mesenteric In Situ Microcirculation
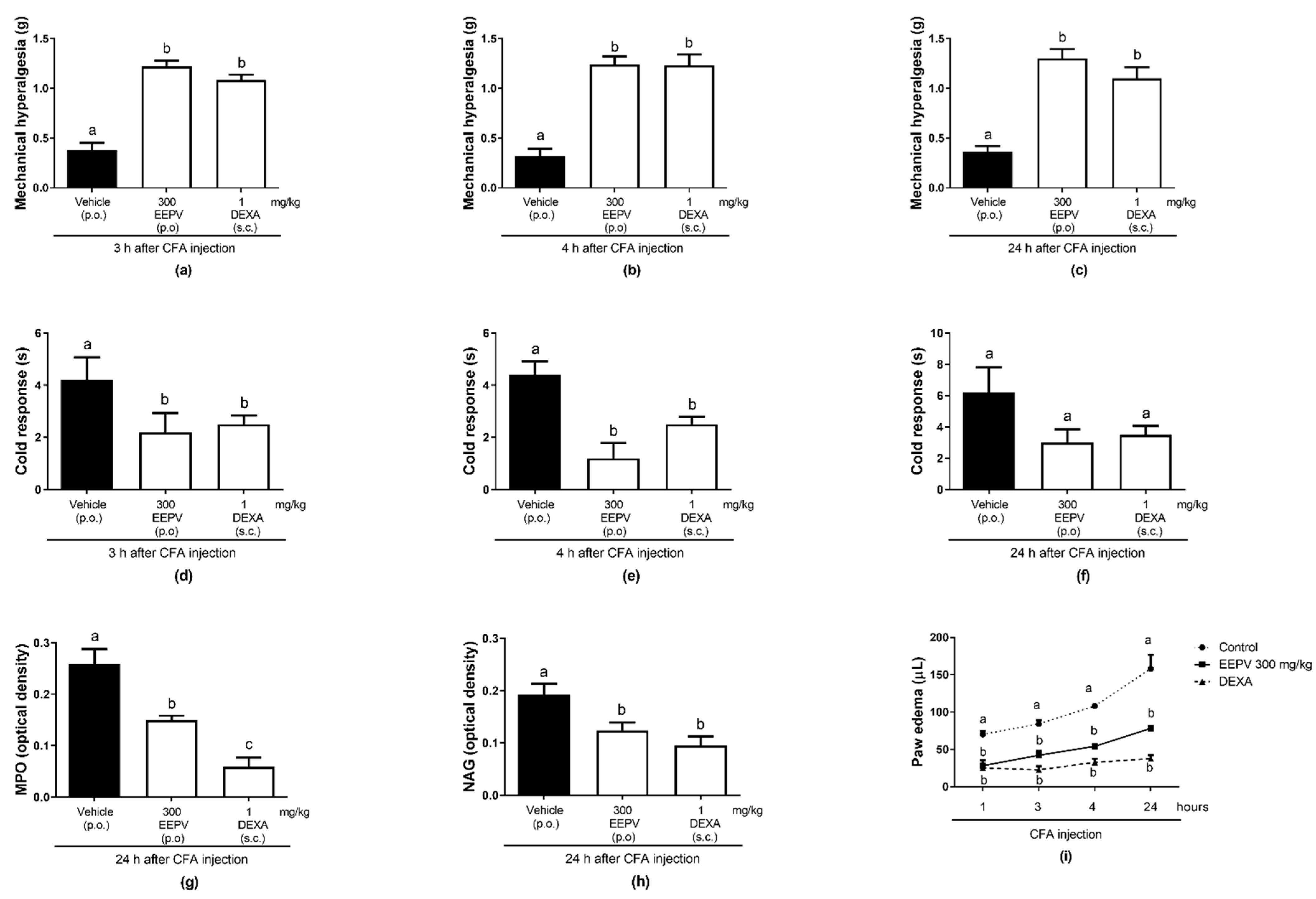
3.8. EEPV Reduces Mechanical Hyperalgesia, Cold Sensitivity, Edema Formation, Myeloperoxidase (MPO), and Enzyme N-acetilglucosaminidase (NAG) Activity in CFA-Induced Inflammation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bezerra Carvalho, A.C.; Ramalho, L.S.; de Oliveira Marques, R.F.; Silvério Perfeito, J.P. Regulation of Herbal Medicines in Brazil. J. Ethnopharmacol. 2014, 158, 503–506. [Google Scholar] [CrossRef]
- Carvalho, A.C.B.; Lana, T.N.; Perfeito, J.P.S.; Silveira, D. The Brazilian Market of Herbal Medicinal Products and the Impacts of the New Legislation on Traditional Medicines. J. Ethnopharmacol. 2018, 212, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.L.; Bento, A.F.; Cavalli, J.; Oliveira, S.K.; Schwanke, R.C.; Siqueira, J.M.; Freitas, C.S.; Marcon, R.; Calixto, J.B. Non-Clinical Studies in the Process of New Drug Development—Part II: Good Laboratory Practice, Metabolism, Pharmacokinetics, Safety and Dose Translation to Clinical Studies. Braz. J. Med. Biol. Res. 2016, 49, e5646. [Google Scholar] [CrossRef] [PubMed]
- Jorge, B.C.; Júnior, A.J.; Stein, J.; Reis, A.C.C.; da Silva Moreira, S.; de Matos Manoel, B.; da Silva Mota, J.; Kassuya, C.A.L.; Arena, A.C. Safety Assessment of the Ethanolic Extract from Piper Vicosanum Yunck Leaves in Male Rats. Phytomedicine Plus 2022, 2, 100165. [Google Scholar] [CrossRef]
- Mesquita, J.M.O.; Oliveira, A.B.; Braga, F.C.; Lombardi, J.A.; da Cunha, A.P.; Salgueiro, L.; Cavaleiro, C. Essential Oil Constituents of Piper Vicosanum Yunker from the Brazilian Atlantic Forest. J. Essent. Oil Res. 2006, 18, 392–395. [Google Scholar] [CrossRef]
- Hoff Brait, D.R.; Mattos Vaz, M.S.; da Silva Arrigo, J.; Borges de Carvalho, L.N.; Souza de Araújo, F.H.; Vani, J.M.; da Silva Mota, J.; Cardoso, C.A.L.; Oliveira, R.J.; Negrão, F.J.; et al. Toxicological Analysis and Anti-Inflammatory Effects of Essential Oil from Piper Vicosanum Leaves. Regul. Toxicol. Pharmacol. 2015, 73, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Oesterreich, S.A.; Traesel, G.K.; Piccinelli, A.C.; Aquino, D.F.S.; Mota, J.d.S.; Estanislau, C.; Kassuya, C.A.L. Antidepressant and Anxiolytic Effects Of Ethanol Extracts from Four Piper Species. SaBios 2015, 10, 34–42. [Google Scholar]
- Hunskaar, S.; Hole, K. The Formalin Test in Mice: Dissociation between Inflammatory and Non-Inflammatory Pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Collier, H.O.J.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The Abdominal Constriction Response and Its Suppression by Analgesic Drugs in the Mouse. Br. J. Pharmacol. Chemother. 1968, 32, 295–310. [Google Scholar] [CrossRef]
- Penido, C.; Conte, F.P.; Chagas, M.S.S.; Rodrigues, C.A.B.; Pereira, J.F.G.; Henriques, M.G.M.O. Antiinflammatory Effects of Natural Tetranortriterpenoids Isolated from Carapa Guianensis Aublet on Zymosan-Induced Arthritis in Mice. Inflamm. Res. 2006, 55, 457–464. [Google Scholar] [CrossRef]
- Di Rosa, M.; Giroud, J.P.; Willoughby, D.A. Studies of the Mediators of the Acute Inflammatory Response Induced in Rats in Different Sites by Carrageenan and Turpentine. J. Pathol. 1971, 104, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Conte, F.d.P.; Barja-Fidalgo, C.; Verri, W.A.; Cunha, F.Q.; Rae, G.A.; Penido, C.; Henriques, M.d.G.O. Endothelins Modulate Inflammatory Reaction in Zymosan-Induced Arthritis: Participation of LTB 4, TNF-α, and CXCL-1. J. Leukoc. Biol. 2008, 84, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Formagio, A.S.N.; Vilegas, W.; Volobuff, C.R.F.; Kassuya, C.A.L.; Cardoso, C.A.L.; Pereira, Z.V.; Silva, R.M.M.F.; dos Santos Yamazaki, D.A.; de Freitas Gauze, G.; Manfron, J.; et al. Exploration of Essential Oil from Psychotria Poeppigiana as an Anti-Hyperalgesic and Anti-Acetylcholinesterase Agent: Chemical Composition, Biological Activity and Molecular Docking. J. Ethnopharmacol. 2022, 296, 115220. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Measuring Motor Coordination in Mice. J. Vis. Exp. 2013, 75, e2609. [Google Scholar] [CrossRef]
- Dourado, R.S.; Ladeira, A.M. Identificação de flavonóides em Hypericum cordatum (Vell.) N. Robson (Clusiaceae). Braz. J. Bot. 2008, 31, 611–620. [Google Scholar] [CrossRef]
- da Silva Arrigo, J.; Balem, E.; Júnior, U.L.; Mota, J.S.; Iwamoto, R.D.; Barison, A.; Sugizaki, M.M.; Kassuya, C.A.L. Anti-nociceptive, anti-hyperalgesic and anti-arthritic activity of amides and extract obtained from Piper amalago in rodents. J. Ethnopharmacol. 2016, 179, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Jorge, B.C.; Casali Reis, A.C.; Santos Radai, J.A.; da Silva Moreira, S.; Fraga, T.L.; da Silva Mota, J.; Oliveira, R.J.; Kassuya, C.A.L.; Arena, A.C. Evaluation of the Safety of Ethanolic Extract from Piper Amalago L. (Piperaceae) Leaves in Vivo: Subacute Toxicity and Genotoxicity Studies. Regul. Toxicol. Pharmacol. 2022, 129, 105118. [Google Scholar] [CrossRef] [PubMed]
- Kaserer, T.; Steinacher, T.; Kainhofer, R.; Erli, F.; Sturm, S.; Waltenberger, B.; Schuster, D.; Spetea, M. Identification and Characterization of Plant-Derived Alkaloids, Corydine and Corydaline, as Novel Mu Opioid Receptor Agonists. Sci. Rep. 2020, 10, 13804. [Google Scholar] [CrossRef] [PubMed]
- Oriola, A.O.; Aladesanmi, A.J.; Idowu, T.O.; Akinwumi, F.O.; Obuotor, E.M.; Idowu, T.; Oyedeji, A.O. Ursane-Type Triterpenes, Phenolics and Phenolic Derivatives from Globimetula Braunii Leaf. Molecules 2021, 26, 6528. [Google Scholar] [CrossRef]
- Omar, N.; Ismail, C.A.N.; Long, I. Tannins in the Treatment of Diabetic Neuropathic Pain: Research Progress and Future Challenges. Front. Pharmacol. 2022, 12, 805854. [Google Scholar] [CrossRef]
- Previtali, D.; Di Laura Frattura, G.; Filardo, G.; Delcogliano, M.; Deabate, L.; Candrian, C. Peri-Operative Steroids Reduce Pain, Inflammatory Response and Hospitalisation Length Following Knee Arthroplasty without Increased Risk of Acute Complications: A Meta-Analysis. Knee Surg. Sport. Traumatol. Arthrosc. 2021, 29, 59–81. [Google Scholar] [CrossRef]
- Khakpour Taleghani, B.; Ghaderi, B.; Rostampour, M.; Fekjur, E.M.; Hasannejad, F.; Ansar, M.M. Involvement of Opioidergic and GABAergic Systems in the Anti-Nociceptive Activity of the Methanolic Extract of Cuscuta Epithymum Murr. in Mice. J. Ethnopharmacol. 2021, 273, 113826. [Google Scholar] [CrossRef]
- Akter, S.; Jahan, I.; Khatun, M.R.; Khan, M.F.; Arshad, L.; Jakaria, M.; Haque, M.A. Pharmacological Insights into Merremia Vitifolia (Burm.f.) Hallier f. Leaf for Its Antioxidant, Thrombolytic, Anti-Arthritic and Anti-Nociceptive Potential. Biosci. Rep. 2021, 41, BSR20203022. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.R.; da Fonseca, A.G.; de Sena Fernandes, L.L.; Furtado, A.A.; da Silva, V.C.; da Veiga Júnior, V.F.; Sant’Ana, A.E.G.; Oliveira, C.N.; Guerra, G.C.B.; de Freitas Fernandes-Pedrosa, M.; et al. Toxicological and Pharmacological Effects of Pentacyclic Triterpenes Rich Fraction Obtained from the Leaves of Mansoa Hirsuta. Biomed. Pharmacother. 2022, 145, 112478. [Google Scholar] [CrossRef]
- Pacheco, A.G.M.; Pacheco, E.J.; Macedo, L.A.R.O.; Silva, J.C.; Lima-Saraiva, S.R.G.; Barros, V.P.; Oliveira-Junior, R.G.; Branco, A.; Quintans, J.S.S.; Quintans-Junior, L.J.; et al. Antinociceptive and Anti-Inflammatory Activities of Hymenaea Martiana Hayne (Fabaceae) in Mice. Braz. J. Biol. 2022, 82, e240359. [Google Scholar] [CrossRef]
- Griffioen, M.A.; Dernetz, V.H.; Yang, G.S.; Griffith, K.A.; Dorsey, S.G.; Renn, C.L. Evaluation of Dynamic Weight Bearing for Measuring Nonevoked Inflammatory Hyperalgesia in Mice. Nurs. Res. 2015, 64, 81–87. [Google Scholar] [CrossRef]
- Branquinho, L.S.; Santos, J.A.; Cardoso, C.A.L.; Mota, J.d.S.; Junior, U.L.; Kassuya, C.A.L.; Arena, A.C. Anti-Inflammatory and Toxicological Evaluation of Essential Oil from Piper Glabratum Leaves. J. Ethnopharmacol. 2017, 198, 372–378. [Google Scholar] [CrossRef]
- Bassi, G.S.; do Malvar, D.; Cunha, T.M.; Cunha, F.Q.; Kanashiro, A. Spinal GABA-B Receptor Modulates Neutrophil Recruitment to the Knee Joint in Zymosan-Induced Arthritis. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 851–861. [Google Scholar] [CrossRef]
- Wipke, B.T.; Allen, P.M. Essential Role of Neutrophils in the Initiation and Progression of a Murine Model of Rheumatoid Arthritis. J. Immunol. 2001, 167, 1601–1608. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Junior, A.J.; Lencina, J.d.S.; Santos, E.d.; da Silva Mota, J.; Cuman, R.K.N.; Konkiewitz, E.C.; Kassuya, C.A.L.; Silva-Filho, S.E. Analgesic and Anti-Inflammatory Properties of Ethanolic Extract of Piper vicosanum Leaves. Pharmaceutics 2022, 14, 2455. https://doi.org/10.3390/pharmaceutics14112455
Junior AJ, Lencina JdS, Santos Ed, da Silva Mota J, Cuman RKN, Konkiewitz EC, Kassuya CAL, Silva-Filho SE. Analgesic and Anti-Inflammatory Properties of Ethanolic Extract of Piper vicosanum Leaves. Pharmaceutics. 2022; 14(11):2455. https://doi.org/10.3390/pharmaceutics14112455
Chicago/Turabian StyleJunior, Armando Jorge, Joyce dos Santos Lencina, Elisangela dos Santos, Jonas da Silva Mota, Roberto Kenji Nakamura Cuman, Elisabete Castelon Konkiewitz, Cândida Aparecida Leite Kassuya, and Saulo Euclides Silva-Filho. 2022. "Analgesic and Anti-Inflammatory Properties of Ethanolic Extract of Piper vicosanum Leaves" Pharmaceutics 14, no. 11: 2455. https://doi.org/10.3390/pharmaceutics14112455
APA StyleJunior, A. J., Lencina, J. d. S., Santos, E. d., da Silva Mota, J., Cuman, R. K. N., Konkiewitz, E. C., Kassuya, C. A. L., & Silva-Filho, S. E. (2022). Analgesic and Anti-Inflammatory Properties of Ethanolic Extract of Piper vicosanum Leaves. Pharmaceutics, 14(11), 2455. https://doi.org/10.3390/pharmaceutics14112455





