Fused Deposition Modeling (FDM) 3D Printing of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
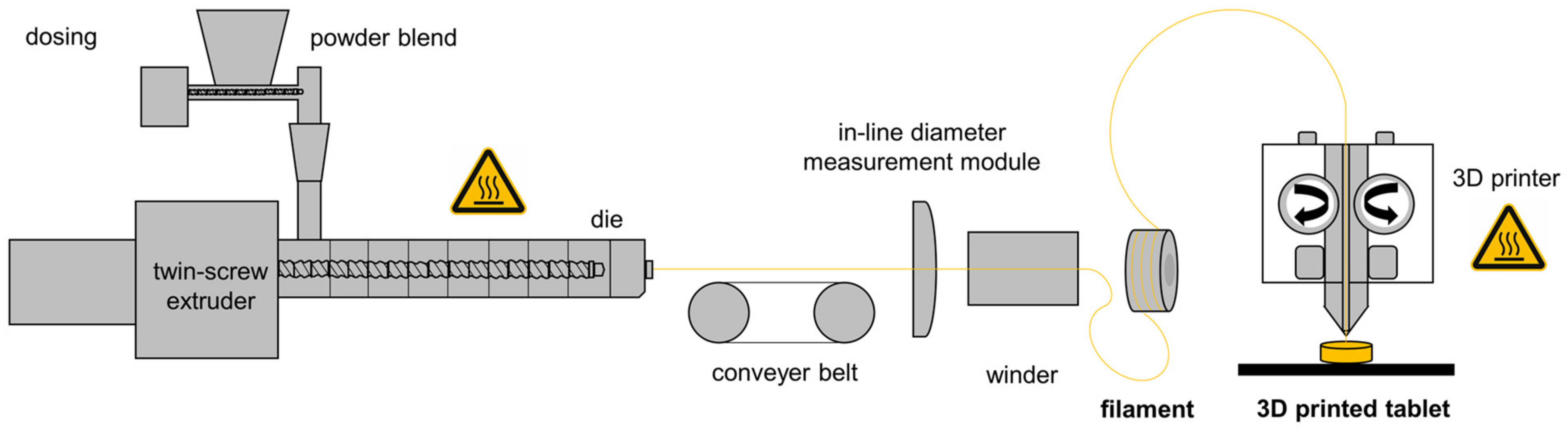
2.2. Preparation of Drug Loaded Extrudates
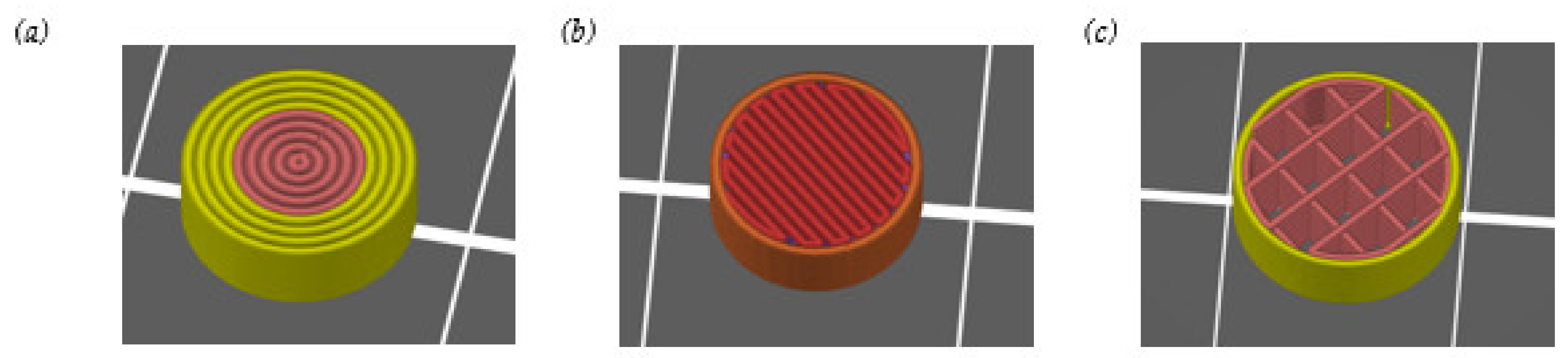
2.3. 3D Printing of Enalapril Maleate Tablets
2.4. Scanning Electron Microscopy (SEM) Imaging
2.5. Solid State Analysis
2.5.1. DSC Analysis
2.5.2. X-ray Diffractometer (XRD)
2.6. Drug Content of Extrudates and 3D Printed Tablets
2.7. Dissolution
3. Results
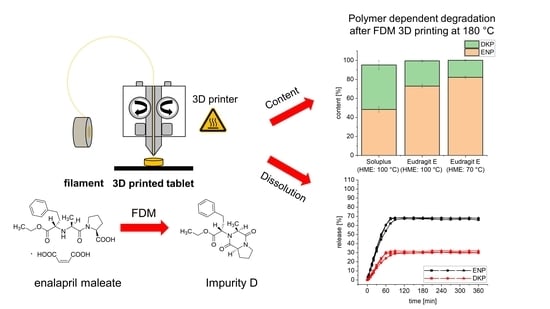
3.1. Hot-Melt Extrusion and 3D Printing of Tablets
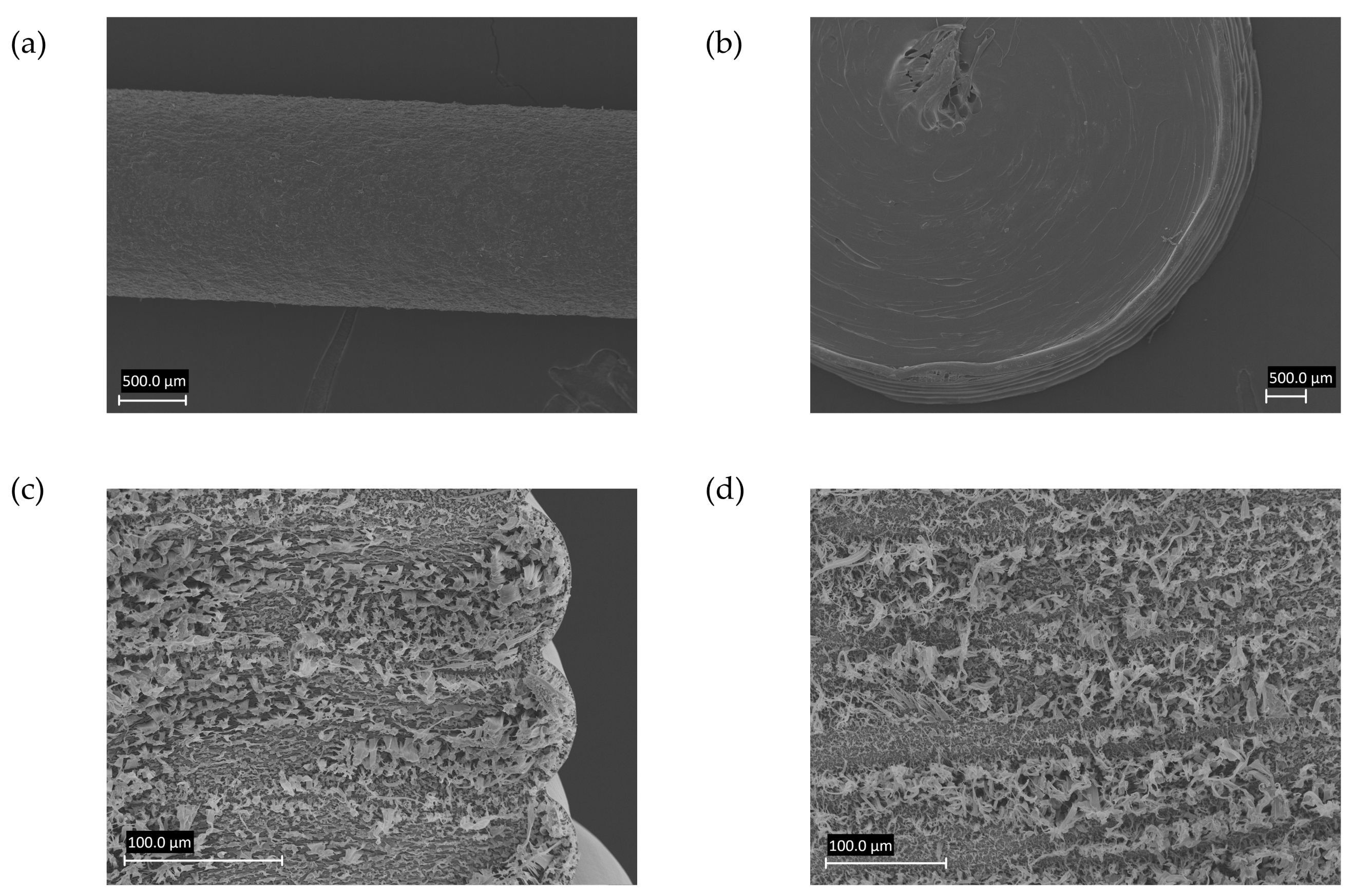
3.2. Scanning Electron Microscopy (SEM) Imaging
3.3. Solid State Analysis
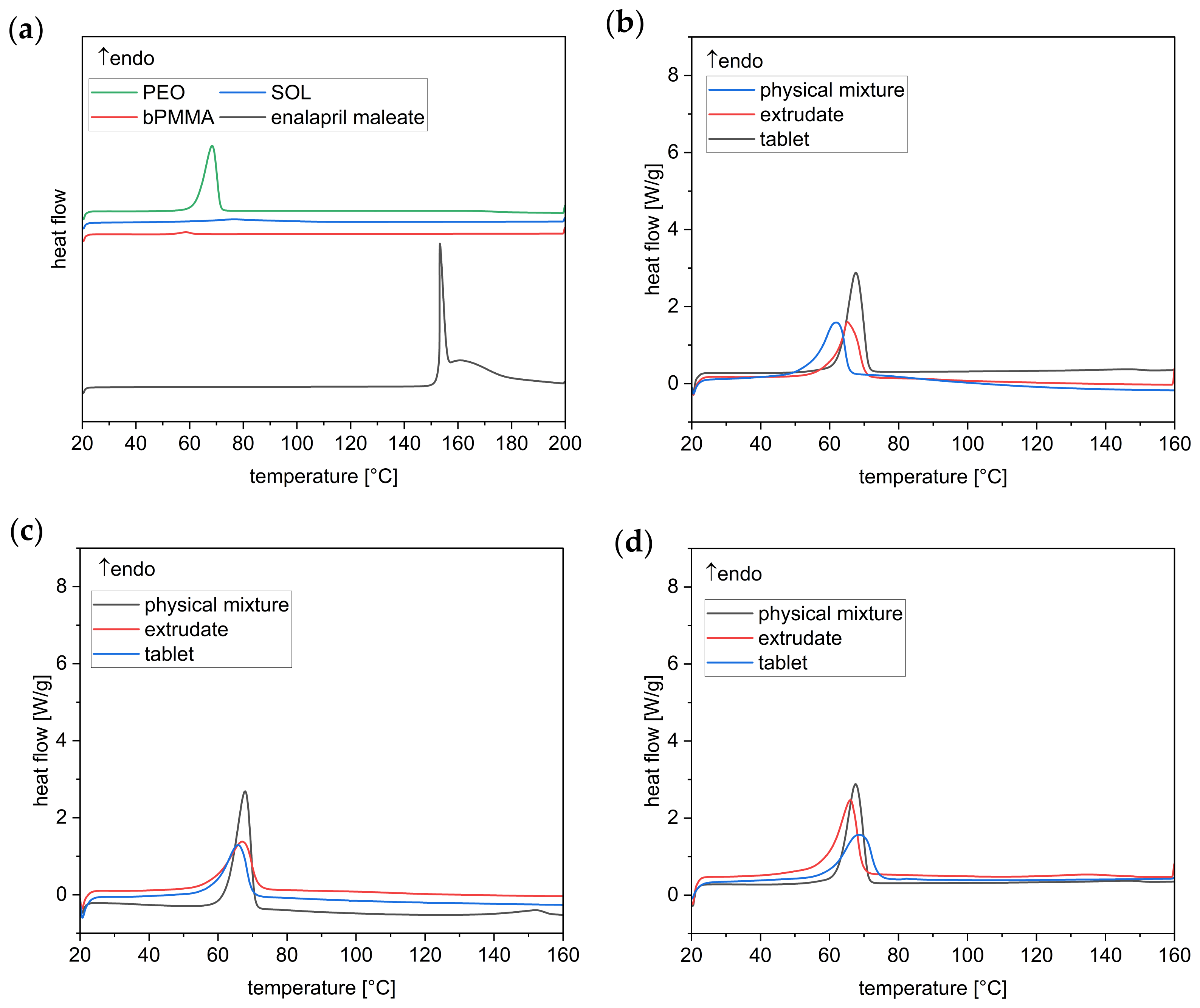
3.3.1. Thermo Analysis
3.3.2. X-ray Diffractometer (XRD)
3.4. Drug Content of Extrudates and 3D Printed Tablets
3.5. Dissolution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACEI | angiotensin-converting enzyme inhibitor |
| BJ | binder jetting |
| bPMMA | basic butylated methacrylate copolymer |
| CV | coefficient of variation |
| DKP | diketopiperazine derivative |
| DSC | Differential Scanning Calorimetry |
| EM | enalapril maleate |
| ENP | enalapril |
| EVA | ethylene-vinyl acetate |
| HME | hot melt extrusion |
| HPLC | High Performance Liquid Chromatography |
| FDM | fused deposition modeling |
| GMP | good manufacturing practice |
| Imp | impurity |
| LOD | limit of detection |
| LOQ | limit of quantification |
| PEG | polyethylene glycol |
| PEO | polyethylene oxide |
| PF | Pyrogen-free |
| PLA | polylactic acid |
| PVP | polyvinylpyrrolidone |
| SD | standard deviation |
| SEM | Scanning Electron Microscopy |
| SiO2 | fumed silica |
| SLA | stereolithography |
| SLS | selective laser sintering |
| SOL | Soluplus |
| SSE | semi-solid extrusion |
| TTSPP | time- and temperature-sensitive pharmaceutical product |
| USP | United States Pharmacopeia |
| UV | ultraviolet |
| VA | poly(vinylpyrrolidone-vinyl acetate)-copolymer |
| WHO | World Health Organization |
| XRD | X-ray diffractometer |
References
- Litman, T. Personalized medicine—Concepts, technologies, and applications in inflammatory skin diseases. Apmis 2019, 127, 386–424. [Google Scholar] [PubMed]
- Beer, N.; Heggerm, I.; Kaae, S.; De Bruin, M.L.; Genina, N.; Alves, T.L.; Hoebert, J.; Kälvemark Sporrong, S. Scenarios for 3D printing of personalized medicines—A case study. Explor. Res. Clin. Soc. Pharm. 2021, 4, 100073. [Google Scholar] [CrossRef] [PubMed]
- Basit, A. Recent innovations in 3D-printed personalized medicines: An interview with Abdul Basit. J. 3D Print. Med. 2020, 4, 5–7. [Google Scholar] [CrossRef]
- Vaz, V.M.; Kumar, L. 3D Printing as a Promising Tool in Personalized Medicine. AAPS PharmSciTech 2021, 22, 49. [Google Scholar] [CrossRef]
- El Aita, I.; Ponsar, H.; Quodbach, J. A critical review on 3D-printed dosage forms. Curr. Pharm. 2018, 24, 4957–4978. [Google Scholar] [CrossRef]
- Manini, G.; Benali, S.; Mathew, A.; Napolitano, S.; Raquez, J.-M.; Goole, J. Paliperidone palmitate as model of heat-sensitive drug for long-acting 3D printing application. Int. J. Pharm. 2022, 618, 121662. [Google Scholar] [CrossRef]
- Abdella, S.; Youssef, S.H.; Afinjuomo, F.; Song, Y.; Fouladian, P.; Upton, R.; Garg, S. 3D Printing of thermo-sensitive drugs. Pharmaceutics 2021, 13, 1524. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for extrusion-based 3D printing of pharmaceuticals: A holistic materials–process perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.-W.; Ahmed, W.; Arafat, B. Emergence of 3D printed dosage forms: Opportunities and challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef]
- Quodbach, J.; Bogdahn, M.; Breitkreutz, J.; Chamberlain, R.; Eggenreich, K.; Elia, A.G.; Gottschalk, N.; Gunkel-Grabole, G.; Hoffmann, L.; Kapote, D. Quality of FDM 3D printed medicines for pediatrics: Considerations for formulation development, filament extrusion, printing process and printer design. Ther. Innov. Regul. Sci. 2021, 56, 1–19. [Google Scholar] [CrossRef]
- World Health Organization. WHO Technical Report Series, No. 961, 2011. Annex 9 Model Guidance for the Storage and Transport of Time- and Temperature-Sensitive Pharmaceutical Products. 2011. Available online: https://www.who.int/docs/default-source/medicines/norms-and-standards/guidelines/distribution/trs961-annex9-modelguidanceforstoragetransport.pdf?sfvrsn=b80e925f_2 (accessed on 27 July 2022).
- do Pazo-Oubiña, F.; Alorda-Ladaria, B.; Gomez-Lobon, A.; Boyeras-Vallespir, B.; Santandreu-Estelrich, M.M.; Martorell-Puigserver, C.; Gomez-Zamora, M.; Ventayol-Bosch, P.; Delgado-Sanchez, O. Thermolabile drug storage in an ambulatory setting. Sci. Rep. 2021, 11, 5959. [Google Scholar] [CrossRef] [PubMed]
- Okwuosa, T.C.; Stefaniak, D.; Arafat, B.; Isreb, A.; Wan, K.-W.; Alhnan, M.A. A Lower temperature FDM 3D printing for the manufacture of patient-specific immediate release tablets. Pharm. Res. 2016, 33, 2704–2712. [Google Scholar] [CrossRef] [PubMed]
- Kempin, W.; Domsta, V.; Grathoff, G.; Brecht, I.; Semmling, B.; Tillmann, S.; Weitschies, W.; Seidlitz, A. Immediate release 3D-printed tablets produced via fused deposition modeling of a thermo-sensitive drug. Pharm. Res. 2018, 35, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef]
- Sadia, M.; Sośnicka, A.; Arafat, B.; Isreb, A.; Ahmed, W.; Kelarakis, A.; Alhnan, M.A. Adaptation of pharmaceutical excipients to FDM 3D printing for the fabrication of patient-tailored immediate release tablets. Int. J. Pharm. 2016, 513, 659–668. [Google Scholar] [CrossRef]
- Neubeck, M. Commentary on Ph. Eur. 9.0, 2017. 57th Supply, enalapril maleate. In Commentary on the European Pharmacopoeia; Wissenschaftliche Verlagsgesellschaft mbH: Stuttgart, Germany; Govi-Verlag: Eschborn, Germany, 2017. [Google Scholar]
- Brambilla, C.R.; Okafor-Muo, O.L.; Hassanin, H.; ElShaer, A. 3DP printing of oral solid formulations: A systematic review. Pharmaceutics 2021, 13, 358. [Google Scholar] [CrossRef]
- Hoffmann, L.; Breitkreutz, J.; Quodbach, J. Hot-Melt Extrusion of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate. Pharmaceutics 2022, 14, 2091. [Google Scholar] [CrossRef]
- Tidau, M.; Kwade, A.; Finke, J.H. Influence of high, disperse API load on properties along the fused-layer modeling process chain of solid dosage forms. Pharmaceutics 2019, 11, 194. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tadokoro, H. Structural studies of polyethers, (-(CH2)m-O-)n. X. Crystal structure of poly(ethylene oxide). Macromolecules 1973, 6, 672–675. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Wang, S.-L.; Chen, T.-F.; Hu, T.-C. Intramolecular cyclization of diketopiperazine formation in solid-state enalapril maleate studied by thermal FT-IR microscopic system. Eur. J. Pharm. Biopharm. 2002, 54, 249–254. [Google Scholar] [CrossRef]
- Gómez Pineda, E.A.; Martins Ferrarezi, A.D.; Ferrarezi, J.G.; Winkler Hechenleitner, A.A. Thermal decomposition of enalapril maleate studied by dynamic isoconversional method. J. Therm. Anal. Calorim. 2005, 79, 259–262. [Google Scholar] [CrossRef]
- Ramírez-Rigo, M.V.; Olivera, M.E.; Rubio, M.; Manzo, R.H. Enhanced intestinal permeability and oral bioavailability of enalapril maleate upon complexation with the cationic polymethacrylate Eudragit E100. Eur. J. Pharm. Sci. 2014, 55, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jiang, L.; Zhou, Z.; Wu, X.-F.; Wang, Y. Preparation and properties of electrospun soy protein isolate/polyethylene oxide nanofiber membranes. ACS Appl. Mater. Interfaces 2012, 4, 4331–4337. [Google Scholar] [CrossRef] [PubMed]
- Ilyés, K.; Kovács, N.K.; Balogh, A.; Borbás, E.; Farkas, B.; Casian, T.; Marosi, G.; Tomuță, I.; Nagy, Z.K. The applicability of pharmaceutical polymeric blends for the fused deposition modelling (FDM) 3D technique: Material considerations–printability–process modulation, with consecutive effects on in vitro release, stability and degradation. Eur. J. Pharm. Sci. 2019, 129, 110–123. [Google Scholar] [CrossRef]
- Lima, D.M.; dos Santos, L.D.; Lima, E.M. Stability and in vitro release profile of enalapril maleate from different commercially available tablets: Possible therapeutic implications. J. Pharm. Biomed. Anal. 2008, 47, 934–937. [Google Scholar] [CrossRef]

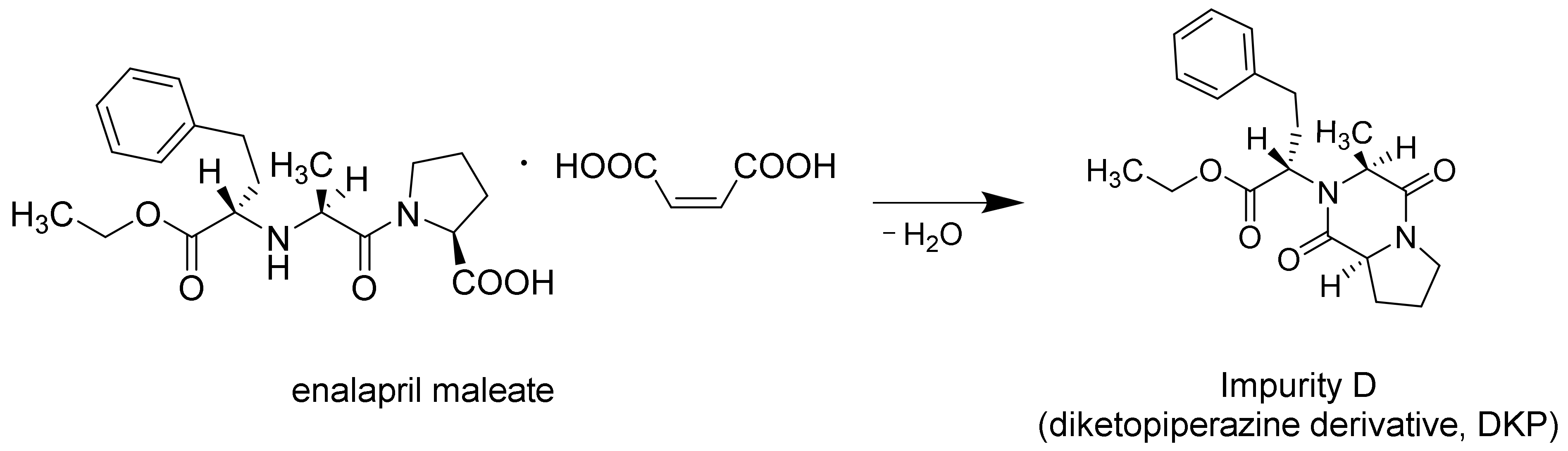
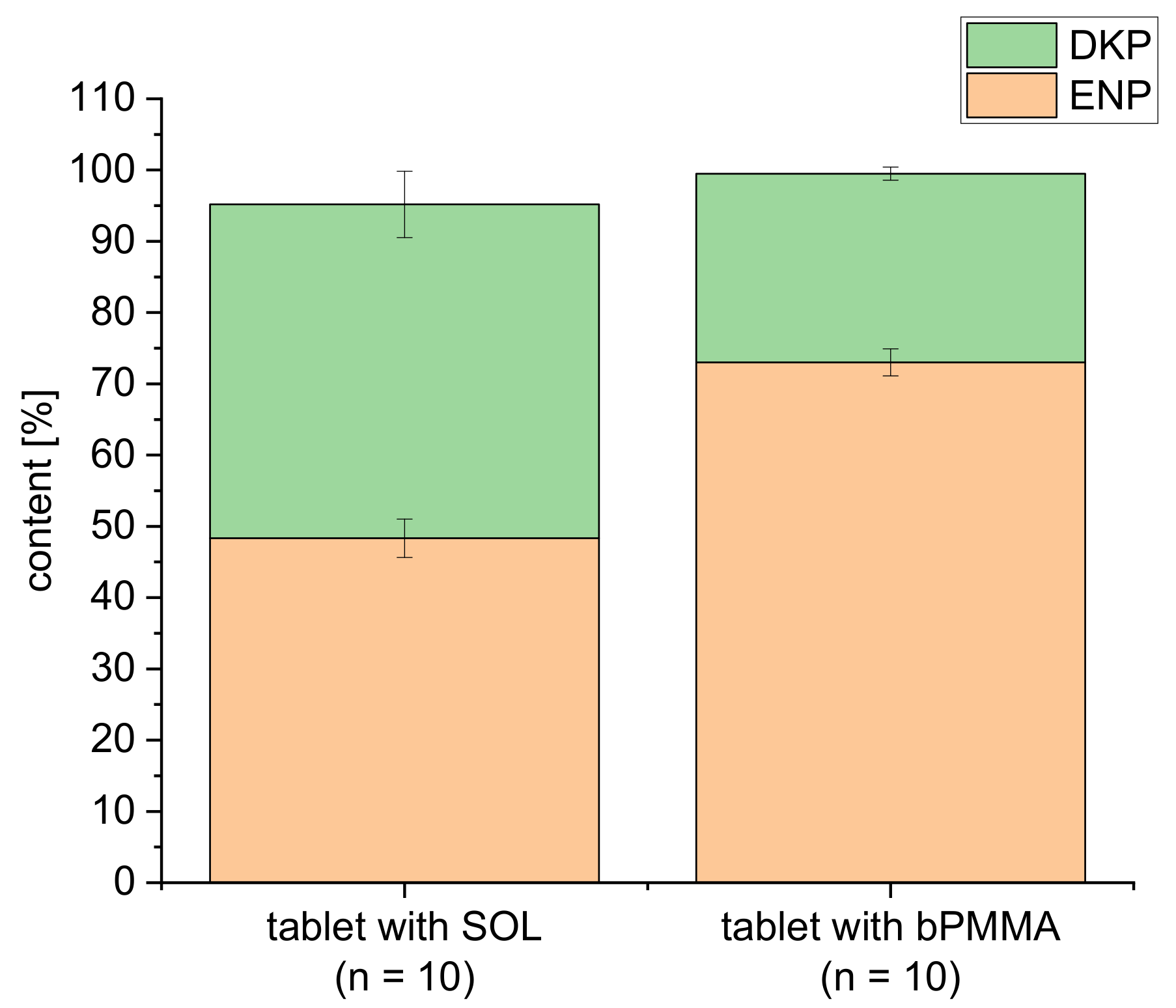
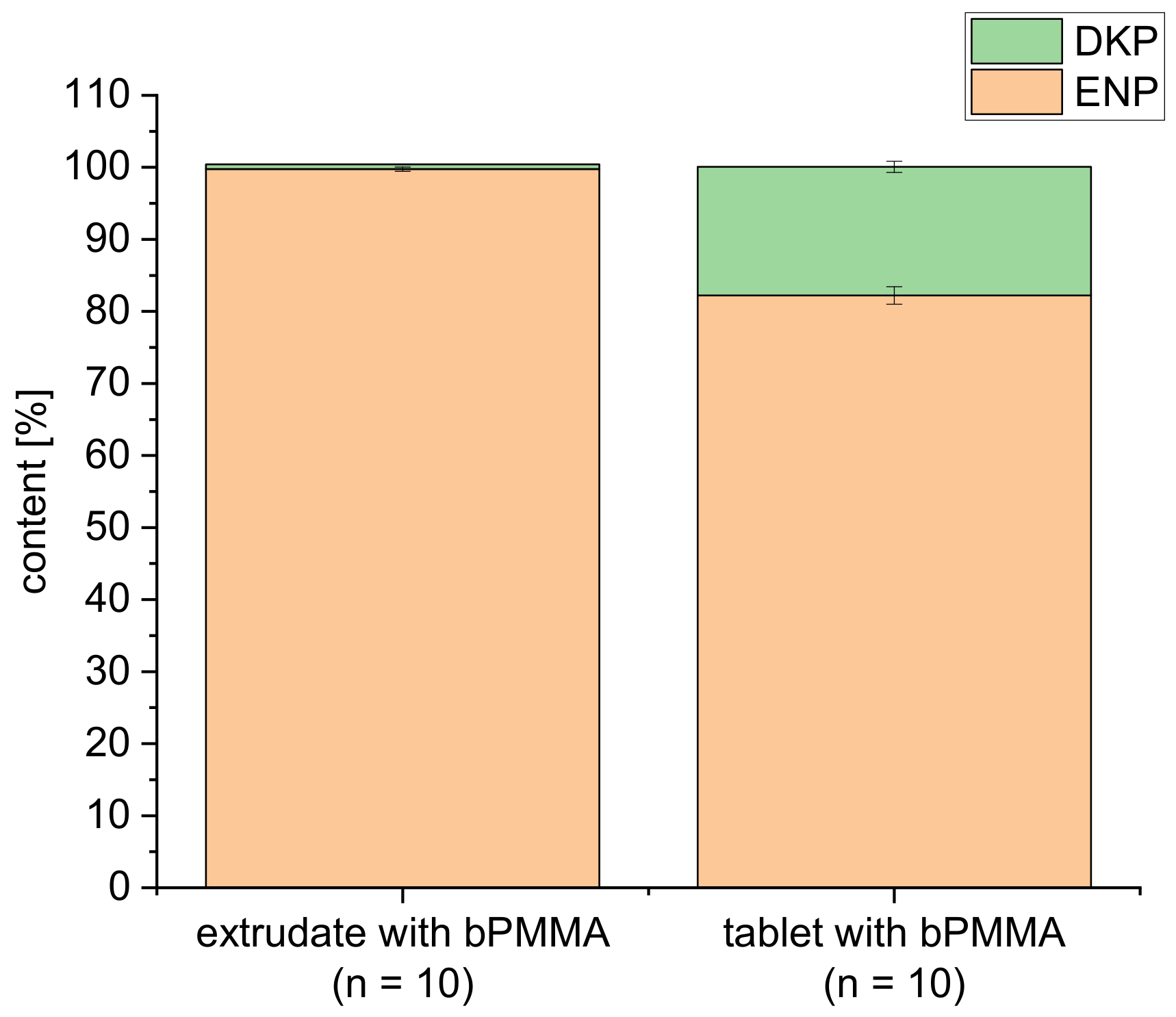

| Formulations | API (%) | Matrix (%) | Plasticizer (%) | Glidant (%) | ||||
|---|---|---|---|---|---|---|---|---|
| F1 | EM | 10 | SOL | 44.75 | PEO | 44.75 | SiO2 | 0.5 |
| F2 | EM | 10 | bPMMA | 44 | PEO | 44 | SiO2 | 2.0 |
| Time (min) | Acetonitrile (% v/v) | Buffer (% v/v) |
|---|---|---|
| 0–1.0 | 2 | 98 |
| 1.0–1.2 | 2 → 25 | 98 → 75 |
| 1.2–5.0 | 25 | 75 |
| 5.0–7.5 | 25 → 40 | 75 → 60 |
| 7.5–9.0 | 40 → 75 | 60 → 25 |
| 9.0–11.0 | 75 → 95 | 25 → 5 |
| 11.0–12.5 | 95 | 5 |
| 12.5–12.6 | 95 → 2 | 5 → 98 |
| 12.6–15.0 | 2 | 98 |
| Temperature [°C] | Printing Speed [mm/s] | |||||
|---|---|---|---|---|---|---|
| 30 | 60 | 90 | ||||
| ENP | DKP | ENP | DKP | ENP | DKP | |
| 180 | 85.55 ± 1.48% | 15.55 ± 0.87% | 82.83 ± 1.58% | 16.33 ± 0.94% | 79.97 ± 1.80% | 19.89 ± 1.12% |
| 190 | 78.47 ± 0.77% | 21.55 ± 0.75% | 77.02 ± 0.89% | 22.11 ± 0.59% | 74.98 ± 1.47% | 24.37 ± 0.99% |
| Temperature [°C] | Printing Speed [mm/s] | |||||
|---|---|---|---|---|---|---|
| 30 | 60 | 90 | ||||
| ENP | DKP | ENP | DKP | ENP | DKP | |
| 180 | 79.40 ± 0.64% | 18.39 ± 0.54% | 79.91 ± 0.81% | 18.69 ± 0.60% | 84.41 ± 1.30% | 15.10 ± 0.71% |
| 190 | 79.27 ± 1.36% | 21.43 ± 0.66% | 75.32 ± 1.53% | 24.66 ± 1.28% | 79.24 ± 0.98% | 21.63 ± 0.54% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, L.; Breitkreutz, J.; Quodbach, J. Fused Deposition Modeling (FDM) 3D Printing of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate. Pharmaceutics 2022, 14, 2411. https://doi.org/10.3390/pharmaceutics14112411
Hoffmann L, Breitkreutz J, Quodbach J. Fused Deposition Modeling (FDM) 3D Printing of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate. Pharmaceutics. 2022; 14(11):2411. https://doi.org/10.3390/pharmaceutics14112411
Chicago/Turabian StyleHoffmann, Lena, Jörg Breitkreutz, and Julian Quodbach. 2022. "Fused Deposition Modeling (FDM) 3D Printing of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate" Pharmaceutics 14, no. 11: 2411. https://doi.org/10.3390/pharmaceutics14112411
APA StyleHoffmann, L., Breitkreutz, J., & Quodbach, J. (2022). Fused Deposition Modeling (FDM) 3D Printing of the Thermo-Sensitive Peptidomimetic Drug Enalapril Maleate. Pharmaceutics, 14(11), 2411. https://doi.org/10.3390/pharmaceutics14112411