Evidence-Based Anti-Diabetic Properties of Plant from the Occitan Valleys of the Piedmont Alps
Abstract
1. Introduction
2. Plants from the Occitan Valleys of the Piedmont Alps and Their Traditional Use
2.1. Rubus idaeus L. (Raspberry)
2.2. Rubus fructicosus L./Rubus ulmifolius (Blackberry)
2.3. Fragraria vesca L. (Wild Strawberry)
2.4. Rosa canina L. (Rosehip)
2.5. Vaccinum myrtillus L. (Bilberry)
2.6. Sambucus nigra L. (Black Elderberry)
2.7. Achillea millefolium L. (Yarrow)
2.8. Urtica dioica L. (Stinging Nettle)
2.9. Cornus mas L. (Cornelian Cherry)
3. Active Ingredients of Plants from the Occitan Valleys of the Piedmont Alps Recognized to Have Anti-Diabetic Properties: An Overview
3.1. Poliphenols
3.2. Oligosaccharides
4. Mechanistic Interpretation of the Anti-Diabetic Effects of Plants from the Occitan Valleys of the Piedmont Alps: Evidence from Experimental Studies
4.1. Stinging Nettle
4.2. Yarrow
4.3. Bilberry
4.4. Raspberry
4.5. Blackberry
4.6. Wild Strawberry
4.7. Elderberry
4.8. Rosehip
4.9. Cornelian Cherry
5. The Anti-Diabetic Effects of Plants from the Occitan Valleys of the Piedmont Alps: Clinical Evidence of Efficacy
5.1. Stinging Nettle
5.2. Wild Strawberry
5.3. Cornelian Cherry
5.4. Raspberry
5.5. Blackberry
5.6. Bilberry
5.7. Rosehip
6. Discussion
6.1. Herbal Remedies for Diabetes: The Bench-to-Bedside Challenge
6.2. Herbal Remedies for Diabetes: The Standardization of Titration Challenge
6.3. Herbal Remedies for Diabetes: The Bioavailability Challenge
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- IDF. IDF Diabetes Atlas. Available online: https://www.diabetesatlas.org (accessed on 15 January 2022).
- Lankatillake, C.; Huynh, T.; Dias, D.A. Understanding glycaemic control and current approaches for screening antidiabetic natural products from evidence-based medicinal plants. Plant Methods 2019, 15, 105. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Introduction: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S1–S2. [Google Scholar] [CrossRef] [PubMed]
- Blahova, J.; Martiniakova, M.; Babikova, M.; Kovacova, V.; Mondockova, V.; Omelka, R. Pharmaceutical Drugs and Natural Therapeutic Products for the Treatment of Type 2 Diabetes Mellitus. Pharmaceuticals 2021, 14, 806. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef]
- Malviya, N.; Jain, S.; Malviya, S. Antidiabetic potential of medicinal plants. Acta Pol. Pharm. 2010, 67, 113–118. [Google Scholar]
- Patel, D.K.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antaidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- Silveira, D.; Prieto-Garcia, J.M.; Boylan, F.; Estrada, O.; Fonseca-Bazzo, Y.M.; Jamal, C.M.; Magalhães, P.O.; Pereira, E.O.; Tomczyk, M.; Heinrich, M. COVID-19: Is There Evidence for the Use of Herbal Medicines as Adjuvant Symptomatic Therapy? Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Eggers, M.; Jungke, P.; Wolkinger, V.; Bauer, R.; Kessler, U.; Frank, B. Antiviral activity of plant juices and green tea against SARS-CoV-2 and influenza virus. Phytother. Res. 2022, 36, 2109–2115. [Google Scholar] [CrossRef]
- Sabzian-Molaei, F.; Nasiri Khalili, M.A.; Sabzian-Molaei, M.; Shahsavarani, H.; Fattah Pour, A.; Molaei Rad, A.; Hadi, A. Urtica dioica Agglutinin: A plant protein candidate for inhibition of SARS-COV-2 receptor-binding domain for control of Covid19 Infection. PLoS ONE 2022, 17, e0268156. [Google Scholar] [CrossRef]
- Vilhelmova-Ilieva, N.; Petrova, Z.; Georgieva, A.; Tzvetanova, E.; Trepechova, M.; Mileva, M. Anti-Coronavirus Efficiency and Redox-Modulating Capacity of Polyphenol-Rich Extracts from Traditional Bulgarian Medicinal Plants. Life 2022, 12, 1088. [Google Scholar] [CrossRef]
- Xu, J.; Gao, L.; Liang, H.; Chen, S.-d. In silico screening of potential anti–COVID-19 bioactive natural constituents from food sources by molecular docking. Nutrition 2021, 82, 111049. [Google Scholar] [CrossRef]
- Tilwani, K.; Patel, A.; Parikh, H.; Thakker, D.J.; Dave, G. Investigation on anti-Corona viral potential of Yarrow tea. J. Biomol. Struct. Dyn. 2022, 1–13. [Google Scholar] [CrossRef]
- Alqathama, A.; Alluhiabi, G.; Baghdadi, H.; Aljahani, L.; Khan, O.; Jabal, S.; Makkawi, S.; Alhomoud, F. Herbal medicine from the perspective of type II diabetic patients and physicians: What is the relationship? BMC Complement. Med. Ther. 2020, 20, 65. [Google Scholar] [CrossRef]
- Medagama, A.B.; Bandara, R. The use of complementary and alternative medicines (CAMs) in the treatment of diabetes mellitus: Is continued use safe and effective? Nutr. J. 2014, 13, 102. [Google Scholar] [CrossRef]
- Gruppo di lavoro Diabetologia e Futuro. Focus Diabete alcuni esempi regionali. Il Sole 24 Ore, 24 July 2018. [Google Scholar]
- Gnavi, R.; Picariello, R.; Pilutti, S.; Di Monaco, R.; Oleandri, S.; Costa, G. Epidemiology in support of intervention priorities: The case of diabetes in Turin (Piedmont Region, Northern Italy). Epidemiol. Prev. 2020, 44, 172–178. [Google Scholar] [CrossRef]
- Camoletto, S.; Bellandi, M. Forms of «hybrid» local development: A case study on the province of Cuneo. Stato Mercato 2021, 3, 389–420. [Google Scholar]
- Acharya, K.P.; Prajapati, D.; Sherpa, K.; Khanal, A.; Poudel, P.; Hirachan, A.; Bogati, A.; Adhikari, C. Prevalence and determinants of diabetes mellitus in high altitude: A cross sectional study in mountainous region of Nepal. Asian J. Med. Sci. 2020, 11, 44–48. [Google Scholar] [CrossRef]
- Regis, R. On this side of the Alps: A sociolinguistic overview of Francoprovençal in northwestern Italy. Int. J. Sociol. Lang. 2018, 249, 119–133. [Google Scholar] [CrossRef]
- Pieroni, A.; Giusti, M.E. Alpine ethnobotany in Italy: Traditional knowledge of gastronomic and medicinal plants among the Occitans of the upper Varaita valley, Piedmont. J. Ethnobiol. Ethnomed. 2009, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Mattalia, G.; Quave, C.; Pieroni, A. Traditional uses of wild food and medicinal plants among Brigasc, Kyé, and Provençal communities on the Western Italian Alps. Genet. Resour. Crop Evol. 2012, 60, 587–603. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional foods: Benefits, concerns and challenges-a position paper from the american council on science and health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Shaoib, M.; Shah, S.W.; Shah, I.; Shuaib, M. Pharmacological profile of the aerial parts of Rubus ulmifolius Schott. BMC Complement. Altern. Med. 2017, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Hummer, K.E. Rubus Pharmacology: Antiquity to the Present. HortScience 2010, 45, 1587–1591. [Google Scholar] [CrossRef]
- Schulz, M.; Seraglio, S.K.T.; Della Betta, F.; Nehring, P.; Valese, A.C.; Daguer, H.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Blackberry (Rubus ulmifolius Schott): Chemical composition, phenolic compounds and antioxidant capacity in two edible stages. Food Res. Int. 2019, 122, 627–634. [Google Scholar] [CrossRef]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.Z.; Moga, M. Rubus fruticosus L.: Constituents, biological activities and health related uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef]
- Jaberg, K.; Jud, J.; Scheuermeier, P. Prach-und Sachatlas Italiens und der Südschweiz (AIS) (Linguistic and Ethnographic Atlas of Italy and Southern Switzerland) Electronic Version NavigAIS from Tisato, G.; Verlagsanstalt Ringier & Co.: Zofingen, Switzerland, 1928–1940; pp. 606, 609, 610, 611, 622. [Google Scholar]
- Canobbio, S.; Telmon, T. Atlante Linguistico ed Etnografico del Piemonte Occidentale: Il Mondo Vegetale. Alberi e Arbusti. (ALEPO); Priuli & Verlucca: Pavone Canavese-Scarmagno, Italy, 2009; Volume 1, pp. 65, 69, 89, 165, 169. [Google Scholar]
- Cusan, F. Parola alle piante. Saggio di Fitotoponomastica di una Valle Alpina; Edizioni dell’Orso: Alessandria, Italy, 2020; p. 208. [Google Scholar]
- Acta Plantarum-Flora delle Regioni Italiane. Available online: https://www.actaplantarum.org/ (accessed on 1 June 2022).
- European Directorate for the Quality of Medicines & HealthCare. Council of Europe, 10th ed.; European Pharmacopoeia: Strasbourg Cedex, France; Available online: https://pheur.edqm.eu/home (accessed on 1 June 2022).
- Committee on Herbal Medicinal Products. European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en (accessed on 1 June 2022).
- Calvano, A.; Izuora, K.; Oh, E.C.; Ebersole, J.L.; Lyons, T.J.; Basu, A. Dietary berries, insulin resistance and type 2 diabetes: An overview of human feeding trials. Food Funct. 2019, 10, 6227–6243. [Google Scholar] [CrossRef]
- Bellia, G.; Martina, P.A.; Pons, A.; Richard, F. Available online: http://www.coltivareparole.it/ (accessed on 1 May 2022).
- Pons, A. Coltivare parole. Un Piccolo Atlante Fitonimico del Pinerolese e delle Valli Valdesi. Boll. Atlante Linguist. Ital. 2016, III, 153–162. [Google Scholar]
- Burton-Freeman, B.M.; Sandhu, A.K.; Edirisinghe, I. Red Raspberries and Their Bioactive Polyphenols: Cardiometabolic and Neuronal Health Links. Adv. Nutr. 2016, 7, 44–65. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Glod, D.; Kula, M.; Majdan, M.; Halasa, R.; Matkowski, A.; Kozlowska, W.; Kawiak, A. Chemical composition and biological activity of Rubus idaeus shoots—A traditional herbal remedy of Eastern Europe. BMC Complement. Altern. Med. 2014, 14, 480. [Google Scholar] [CrossRef]
- Gesek, J.; Jakimiuk, K.; Atanasov, A.G.; Tomczyk, M. Sanguiins-Promising Molecules with Broad Biological Potential. Int. J. Mol. Sci. 2021, 22, 12972. [Google Scholar] [CrossRef]
- CABI. Invasive Species Compendium. Available online: www.cabi.org/isc. (accessed on 15 January 2022).
- de Souza, V.R.; Pereira, P.A.; da Silva, T.L.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Uncini Manganelli, R.E.; Tomei, P.E. Ethnopharmacobotanical studies of the Tuscan Archipelago. J. Ethnopharmacol. 1999, 65, 181–202. [Google Scholar] [CrossRef]
- Gowd, V.; Bao, T.; Wang, L.; Huang, Y.; Chen, S.; Zheng, X.; Cui, S.; Chen, W. Antioxidant and antidiabetic activity of blackberry after gastrointestinal digestion and human gut microbiota fermentation. Food Chem. 2018, 269, 618–627. [Google Scholar] [CrossRef]
- Riaz, M.; Mansoor, A.; Najmur, R. Antimicrobial screening of fruit, leaves, root and stem of Rubus fruticosus. J. Med. Plants Res. 2011, 5, 5920–5924. [Google Scholar] [CrossRef]
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, C. Rubus fruticosus (blackberry) use as an herbal medicine. Pharm. Rev. 2014, 8, 101–104. [Google Scholar] [CrossRef]
- Maugini, E.; Maleci Bini, L.; Mariotti Lippi, M. Botanica Farmaceutica, IX ed.; Piccin-Nuova Libraria: Padua, Italy, 2014. [Google Scholar]
- Canobbio, S.; Telmon, T. Atlante Linguistico ed Etnografico del Piemonte Occidentale: Il Mondo Vegetale. Erbaceei. (ALEPO); Priuli & Verlucca: Pavone Canavese-Scarmagno, Italy, 2008; Volume 1, pp. 220, 259. [Google Scholar]
- Liberal, J.; Francisco, V.; Costa, G.; Figueirinha, A.; Amaral, M.T.; Marques, C.; Girao, H.; Lopes, M.C.; Cruz, M.T.; Batista, M.T. Bioactivity of Fragaria vesca leaves through inflammation, proteasome and autophagy modulation. J. Ethnopharmacol. 2014, 158 Pt A, 113–122. [Google Scholar] [CrossRef]
- Mudnic, I.; Modun, D.; Brizic, I.; Vukovic, J.; Generalic, I.; Katalinic, V.; Bilusic, T.; Ljubenkov, I.; Boban, M. Cardiovascular effects in vitro of aqueous extract of wild strawberry (Fragaria vesca, L.) leaves. Phytomedicine 2009, 16, 462–469. [Google Scholar] [CrossRef]
- Couto, J.; Figueirinha, A.; Batista, M.T.; Paranhos, A.; Nunes, C.; Goncalves, L.M.; Marto, J.; Fitas, M.; Pinto, P.; Ribeiro, H.M.; et al. Fragaria vesca L. Extract: A Promising Cosmetic Ingredient with Antioxidant Properties. Antioxidants 2020, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- A.A.V.V. Piante e Fiori delle Nostre Montagne: Plantes et Fleurs de Nos Montagnes; Valchisone, C.S., Ed.; Standard Perosa Argentina: Perosa Argentina, Italy, 2019; p. 224. [Google Scholar]
- Bernard, G. Lou Saber; Vivo, O., Ed.; dizionario enciclopedico dell’occitano di Blins: Venasca, Italy, 1996; pp. 24, 62, 368. [Google Scholar]
- Winther, K.; Campbell-Tofte, J.; Hansen, A. Bioactive ingredients of rose hips (Rosa canina L.) with special reference to antioxidative and anti-inflammatory properties: In vitro studies. Bot. Targets Ther. 2016, 6, 11–23. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Exotic fruits as a source of important phytochemicals: Improving the traditional use of rosa canina fruits in portugal. Food Res. Int. 2011, 44, 2233–2236. [Google Scholar] [CrossRef]
- Marmol, I.; Sanchez-de-Diego, C.; Jimenez-Moreno, N.; Ancin-Azpilicueta, C.; Rodriguez-Yoldi, M.J. Therapeutic Applications of Rose Hips from Different Rosa Species. Int. J. Mol. Sci. 2017, 18, 1137. [Google Scholar] [CrossRef] [PubMed]
- Pekacar, S.; Bulut, S.; Özüpek, B.; Orhan, D.D. Anti-Inflammatory and Analgesic Effects of Rosehip in Inflammatory Musculoskeletal Disorders and Its Active Molecules. Curr. Mol. Pharm. 2021, 14, 731–745. [Google Scholar] [CrossRef]
- Chu, W.K.; Cheung, S.C.; Lau, R.A.; Benzie, I.F. Bilberry (Vaccinium myrtillus L.). In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Mlynarczyk, K.; Walkowiak-Tomczak, D.; Lysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Baser, K.H.; Demirci, B.; Demirci, F.; Kocak, S.; Akinci, C.; Malyer, H.; Guleryuz, G. Composition and antimicrobial activity of the essential oil of Achillea multifida. Planta Med. 2002, 68, 941–943. [Google Scholar] [CrossRef]
- Benedek, B.; Rothwangl-Wiltschnigg, K.; Rozema, E.; Gjoncaj, N.; Reznicek, G.; Jurenitsch, J.; Kopp, B.; Glasl, S. Yarrow (Achillea millefolium L. s.l.): Pharmaceutical quality of commercial samples. Die Pharm. 2008, 63, 23–26. [Google Scholar] [CrossRef]
- Guarrera, P.M. Usi e Tradizioni della Flora Italiana. Medicina Popolare ed Etnobotanica; Aracne: Rome, Italy, 2007; p. 436. [Google Scholar]
- Applequist, W.L.; Moerman, D.E. Yarrow (Achillea millefolium L.): A Neglected Panacea? A Review of Ethnobotany, Bioactivity, and Biomedical Research1. Econ. Bot. 2011, 65, 209–225. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Sharon, D.; Vandebroek, I.; Jones, A.; Revene, Z. Health for sale: The medicinal plant markets in Trujillo and Chiclayo, Northern Peru. J. Ethnobiol. Ethnomed. 2007, 3, 37. [Google Scholar] [CrossRef]
- Ali, S.I.; Gopalakrishnan, B.; Venkatesalu, V. Pharmacognosy, Phytochemistry and Pharmacological Properties of Achillea millefolium L.: A Review. Phytother. Res. 2017, 31, 1140–1161. [Google Scholar] [CrossRef]
- Karamenderes, C.; Apaydin, S. Antispasmodic effect of Achillea nobilis L. subsp. sipylea (O. Schwarz) Bassler on the rat isolated duodenum. J. Ethnopharmacol. 2003, 84, 175–179. [Google Scholar] [CrossRef]
- Stojanovic, G.; Radulovic, N.; Hashimoto, T.; Palic, R. In vitro antimicrobial activity of extracts of four Achillea species: The composition of Achillea clavennae L. (Asteraceae) extract. J. Ethnopharmacol. 2005, 101, 185–190. [Google Scholar] [CrossRef]
- Cavalcanti, A.M.; Baggio, C.H.; Freitas, C.S.; Rieck, L.; de Sousa, R.S.; Da Silva-Santos, J.E.; Mesia-Vela, S.; Marques, M.C. Safety and antiulcer efficacy studies of Achillea millefolium L. after chronic treatment in Wistar rats. J. Ethnopharmacol. 2006, 107, 277–284. [Google Scholar] [CrossRef]
- Si, X.T.; Zhang, M.L.; Shi, Q.W.; Kiyota, H. Chemical constituents of the plants in the genus Achillea. Chem. Biodivers 2006, 3, 1163–1180. [Google Scholar] [CrossRef]
- Lazarevic, J.; Radulovic, N.; Zlatkovic, B.; Palic, R. Composition of Achillea distans Willd. subsp. distans root essential oil. Nat. Prod. Res. 2010, 24, 718–731. [Google Scholar] [CrossRef]
- Falconieri, D.; Piras, A.; Porcedda, S.; Marongiu, B.; Goncalves, M.J.; Cabral, C.; Cavaleiro, C.; Salgueiro, L. Chemical composition and biological activity of the volatile extracts of Achillea millefolium. Nat. Prod. Commun. 2011, 6, 1527–1530. [Google Scholar] [CrossRef]
- Akram, M. Minireview on Achillea millefolium Linn. J. Membr. Biol. 2013, 246, 661–663. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.; Santos-Buelga, C.; Ferreira, I.C. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef]
- Benedek, B.; Geisz, N.; Jäger, W.; Thalhammer, T.; Kopp, B. Choleretic effects of yarrow (Achillea millefolium s.l.) in the isolated perfused rat liver. Phytomedicine 2006, 13, 702–706. [Google Scholar] [CrossRef]
- Zolghadri, Y.; Fazeli, M.; Kooshki, M.; Shomali, T.; Karimaghayee, N.; Dehghani, M. Achillea Millefolium, L. Hydro-Alcoholic Extract Protects Pancreatic Cells by Down Regulating IL-1β and iNOS Gene Expression in Diabetic Rats. Int. J. Mol. Cell. Med. 2014, 3, 255–262. [Google Scholar] [PubMed]
- Gadgoli, C.; Mishra, S. Antihepatotoxic activity of 5-hydroxy 3, 40, 6, 7-tetramethoxy flavone from Achillea millefolium. Pharmacology 2007, 1, 391–399. [Google Scholar]
- Yaeesh, S.; Jamal, Q.; Khan, A.-u.; Gilani, A.H. Studies on hepatoprotective, antispasmodic and calcium antagonist activities of the aqueous-methanol extract of Achillea millefolium. Phytother. Res. 2006, 20, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.M.; Bajracharya, A.; Shrestha, A.K. Comparison of nutritional properties of Stinging nettle (Urtica dioica) flour with wheat and barley flours. Food Sci. Nutr. 2016, 4, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Rutto, L.K.; Xu, Y.; Ramirez, E.; Brandt, M. Mineral Properties and Dietary Value of Raw and Processed Stinging Nettle (Urtica dioica L.). Int. J. Food Sci. 2013, 2013, 857120. [Google Scholar] [CrossRef]
- Kregiel, D.; Pawlikowska, E.; Antolak, H. Urtica spp.: Ordinary Plants with Extraordinary Properties. Molecules 2018, 23, 1664. [Google Scholar] [CrossRef]
- Gul, S.; Demirci, B.; Baser, K.H.; Akpulat, H.A.; Aksu, P. Chemical composition and in vitro cytotoxic, genotoxic effects of essential oil from Urtica dioica L. Bull. Environ. Contam. Toxicol. 2012, 88, 666–671. [Google Scholar] [CrossRef]
- Orcic, D.; Franciskovic, M.; Bekvalac, K.; Svircev, E.; Beara, I.; Lesjak, M.; Mimica-Dukic, N. Quantitative determination of plant phenolics in Urtica dioica extracts by high-performance liquid chromatography coupled with tandem mass spectrometric detection. Food Chem. 2014, 143, 48–53. [Google Scholar] [CrossRef]
- Otles, S.; Yalcin, B. Phenolic compounds analysis of root, stalk, and leaves of nettle. Sci. World J. 2012, 2012, 564367. [Google Scholar] [CrossRef]
- Pinelli, P.; Ieri, F.; Vignolini, P.; Bacci, L.; Baronti, S.; Romani, A. Extraction and HPLC analysis of phenolic compounds in leaves, stalks, and textile fibers of Urtica dioica L. J. Agric. Food Chem. 2008, 56, 9127–9132. [Google Scholar] [CrossRef]
- Said, A.A.H.; Otmani, I.S.E.; Derfoufi, S.; Benmoussa, A. Highlights on nutritional and therapeutic value of stinging nettle (Urtica dioica). Int. J. Pharm. Pharm. Sci. 2015, 7, 8–14. [Google Scholar]
- Upton, R. Stinging nettles leaf (Urtica dioica L.): Extraordinary vegetable medicine. J. Herb. Med. 2013, 3, 9–36. [Google Scholar] [CrossRef]
- El Haouari, M.; Rosado, J.A. Phytochemical, Anti-diabetic and Cardiovascular Properties of Urtica dioica L. (Urticaceae): A Review. Mini Rev. Med. Chem. 2019, 19, 63–71. [Google Scholar] [CrossRef]
- De Biaggi, M.; Donno, D.; Mellano, M.G.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. Cornus mas (L.) Fruit as a Potential Source of Natural Health-Promoting Compounds: Physico-Chemical Characterisation of Bioactive Components. Plant Foods Hum. Nutr. 2018, 73, 89–94. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Mohammadian, M.; Dianat, M. Antidiabetic effect of hydroalcholic urticadioica leaf extract in male rats with fructose-induced insulin resistance. Iran. J. Med. Sci. 2012, 37, 181–186. [Google Scholar]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.U.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef]
- Kadan, S.; Saad, B.; Sasson, Y.; Zaid, H. In Vitro Evaluations of Cytotoxicity of Eight Antidiabetic Medicinal Plants and Their Effect on GLUT4 Translocation. Evid.-Based Complement. Altern. Med. 2013, 2013, 549345. [Google Scholar] [CrossRef]
- Qujeq, D.; Tatar, M.; Feizi, F.; Parsian, H.; Sohan Faraji, A.; Halalkhor, S. Effect of Urtica dioica Leaf Alcoholic and Aqueous Extracts on the Number and the Diameter of the Islets in Diabetic Rats. Int. J. Mol. Cell. Med. 2013, 2, 21–26. [Google Scholar]
- Rahimzadeh, M.; Jahanshahi, S.; Moein, S.; Moein, M.R. Evaluation of alpha- amylase inhibition by Urtica dioica and Juglans regia extracts. Iran. J. Basic Med. Sci. 2014, 17, 465–469. [Google Scholar]
- Ranjbari, A.; Azarbayjani, M.A.; Yusof, A.; Halim Mokhtar, A.; Akbarzadeh, S.; Ibrahim, M.Y.; Tarverdizadeh, B.; Farzadinia, P.; Hajiaghaee, R.; Dehghan, F. In vivo and in vitro evaluation of the effects of Urtica dioica and swimming activity on diabetic factors and pancreatic beta cells. BMC Complement. Altern. Med. 2016, 16, 101. [Google Scholar] [CrossRef]
- Obanda, D.N.; Zhao, P.; Richard, A.J.; Ribnicky, D.; Cefalu, W.T.; Stephens, J.M. Stinging Nettle (Urtica dioica L.) Attenuates FFA Induced Ceramide Accumulation in 3T3-L1 Adipocytes in an Adiponectin Dependent Manner. PLoS ONE 2016, 11, e0150252. [Google Scholar] [CrossRef] [PubMed]
- Obanda, D.N.; Ribnicky, D.; Yu, Y.; Stephens, J.; Cefalu, W.T. An extract of Urtica dioica L. mitigates obesity induced insulin resistance in mice skeletal muscle via protein phosphatase 2A (PP2A). Sci. Rep. 2016, 6, 22222. [Google Scholar] [CrossRef] [PubMed]
- Gohari, A.; Noorafshan, A.; Akmali, M.; Zamani-Garmsiri, F.; Seghatoleslam, A. Urtica dioica Distillate Regenerates Pancreatic Beta Cells in Streptozotocin-Induced Diabetic Rats. Iran. J. Med. Sci. 2018, 43, 174–183. [Google Scholar] [PubMed]
- Abedinzade, M.; Rostampour, M.; Mirzajani, E.; Khalesi, Z.B.; Pourmirzaee, T.; Khanaki, K. Urtica dioica and Lamium Album Decrease Glycogen Synthase Kinase-3 beta and Increase K-Ras in Diabetic Rats. J. Pharmacopunct. 2019, 22, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Raychaudhuri, S.; Kraus, O.; Shahinozzaman, M.; Lofti, L.; Obanda, D.N. Urtica dioica Whole Vegetable as a Functional Food Targeting Fat Accumulation and Insulin Resistance—A Preliminary Study in a Mouse Pre-Diabetic Model. Nutrients 2020, 12, 1059. [Google Scholar] [CrossRef]
- Salim, B.; Said, G.; Kambouche, N.; Kress, S. Identification of Phenolic Compounds from Nettle as New Candidate Inhibitors of Main Enzymes Responsible on Type-II Diabetes. Curr. Drug Discov. Technol. 2020, 17, 197–202. [Google Scholar] [CrossRef]
- Mustafa, K.G.; Ganai, B.A.; Akbar, S.; Dar, M.Y.; Masood, A. ß-Cell protective efficacy, hypoglycemic and hypolipidemic effects of extracts of Achillea millifolium in diabetic rats. Chin. J. Nat. Med. 2012, 10, 0185–0189. [Google Scholar] [CrossRef]
- Ramirez, G.; Zavala, M.; Perez, J.; Zamilpa, A. In vitro screening of medicinal plants used in Mexico as antidiabetics with glucosidase and lipase inhibitory activities. Evid.-Based Complement. Altern. Med. 2012, 2012, 701261. [Google Scholar] [CrossRef]
- Chavez-Silva, F.; Ceron-Romero, L.; Arias-Duran, L.; Navarrete-Vazquez, G.; Almanza-Perez, J.; Roman-Ramos, R.; Ramirez-Avila, G.; Perea-Arango, I.; Villalobos-Molina, R.; Estrada-Soto, S. Antidiabetic effect of Achillea millefollium through multitarget interactions: Alpha-glucosidases inhibition, insulin sensitization and insulin secretagogue activities. J. Ethnopharmacol. 2018, 212, 1–7. [Google Scholar] [CrossRef]
- Brader, L.; Overgaard, A.; Christensen, L.P.; Jeppesen, P.B.; Hermansen, K. Polyphenol-rich bilberry ameliorates total cholesterol and LDL-cholesterol when implemented in the diet of Zucker diabetic fatty rats. Rev. Diabet. Stud. RDS 2013, 10, 270–282. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.S.; Lee, Y.M.; Sohn, E.; Jo, K.; Kim, J.S. Vaccinium myrtillus extract prevents or delays the onset of diabetes—Induced blood-retinal barrier breakdown. Int. J. Food Sci. Nutr. 2015, 66, 236–242. [Google Scholar] [CrossRef]
- Asgary, S.; RafieianKopaei, M.; Sahebkar, A.; Shamsi, F.; Goli-malekabadi, N. Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium myrtillus fruit). J. Sci. Food Agric. 2016, 96, 764–768. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Medicinal Plants Traditionally Used for Treatment of Obesity and Diabetes Mellitus—Screening for Pancreatic Lipase and alpha-Amylase Inhibition. Phytother. Res. 2016, 30, 260–266. [Google Scholar] [CrossRef]
- Bljajic, K.; Petlevski, R.; Vujic, L.; Cacic, A.; Sostaric, N.; Jablan, J.; Saraiva de Carvalho, I.; Zovko Koncic, M. Chemical Composition, Antioxidant and alpha-Glucosidase-Inhibiting Activities of the Aqueous and Hydroethanolic Extracts of Vaccinium myrtillus Leaves. Molecules 2017, 22, 703. [Google Scholar] [CrossRef]
- Karcheva-Bahchevanska, D.P.; Lukova, P.K.; Nikolova, M.M.; Mladenov, R.D.; Iliev, I.N. Effect of Extracts of Bilberries (Vaccinium myrtillus L.) on Amyloglucosidase and alpha-Glucosidase Activity. Folia Med. 2017, 59, 197–202. [Google Scholar] [CrossRef]
- Xiao, T.; Guo, Z.; Sun, B.; Zhao, Y. Identification of Anthocyanins from Four Kinds of Berries and Their Inhibition Activity to alpha-Glycosidase and Protein Tyrosine Phosphatase 1B by HPLC-FT-ICR MS/MS. J. Agric. Food Chem. 2017, 65, 6211–6221. [Google Scholar] [CrossRef]
- Schreck, K.; Melzig, M.F. Traditionally Used Plants in the Treatment of Diabetes Mellitus: Screening for Uptake Inhibition of Glucose and Fructose in the Caco2-Cell Model. Front. Pharm. 2021, 12, 692566. [Google Scholar] [CrossRef]
- Zhu, M.J.; Kang, Y.; Xue, Y.; Liang, X.; Garcia, M.P.G.; Rodgers, D.; Kagel, D.R.; Du, M. Red raspberries suppress NLRP3 inflammasome and attenuate metabolic abnormalities in diet-induced obese mice. J. Nutr. Biochem. 2018, 53, 96–103. [Google Scholar] [CrossRef]
- Xiong, S.-L.; Yue, L.-M.; Lim, G.T.; Yang, J.-M.; Lee, J.; Park, Y.-D. Inhibitory effect of raspberry ketone on α-glucosidase: Docking simulation integrating inhibition kinetics. Int. J. Biol. Macromol. 2018, 113, 212–218. [Google Scholar] [CrossRef]
- Zhao, L.; Zou, T.; Gomez, N.A.; Wang, B.; Zhu, M.J.; Du, M. Raspberry alleviates obesity-induced inflammation and insulin resistance in skeletal muscle through activation of AMP-activated protein kinase (AMPK) alpha1. Nutr. Diabetes 2018, 8, 39. [Google Scholar] [CrossRef]
- Xing, T.; Kang, Y.; Xu, X.; Wang, B.; Du, M.; Zhu, M.J. Raspberry Supplementation Improves Insulin Signaling and Promotes Brown-Like Adipocyte Development in White Adipose Tissue of Obese Mice. Mol. Nutr. Food Res. 2018, 62, 1701035. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Albanchez, E.; Kirakosyan, A.; Bolling, S.F.; Garcia-Villaraco, A.; Gutierrez-Manero, J.; Ramos-Solano, B. Biotic elicitation as a tool to improve strawberry and raspberry extract potential on metabolic syndrome-related enzymes in vitro. J. Sci. Food Agric. 2019, 99, 2939–2946. [Google Scholar] [CrossRef] [PubMed]
- Bispo, K.; Amusquivar, E.; Garcia-Seco, D.; Ramos-Solano, B.; Gutierrez-Manero, J.; Herrera, E. Supplementing diet with blackberry extract causes a catabolic response with increments in insulin sensitivity in rats. Plant Foods Hum. Nutr. 2015, 70, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Takács, I.; Szekeres, A.; Takács, Á.; Rakk, D.; Mézes, M.; Polyák, Á.; Lakatos, L.; Gyémánt, G.; Csupor, D.; Kovács, K.J. Wild strawberry, blackberry, and blueberry leaf extracts alleviate starch-induced hyperglycemia in prediabetic and diabetic mice. Planta Med. 2020, 86, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Schrader, E.; Wein, S.; Kristiansen, K.; Christensen, L.P.; Rimbach, G.; Wolffram, S. Plant extracts of winter savory, purple coneflower, buckwheat and black elder activate PPAR-γ in COS-1 cells but do not lower blood glucose in db/db mice in vivo. Plant Foods Hum. Nutr. 2012, 67, 377–383. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Christensen, K.B.; Olsen, L.C.; Christensen, L.P.; Grevsen, K.; Faergeman, N.J.; Kristiansen, K.; Young, J.F.; Oksbjerg, N. Bioactive components from flowers of Sambucus nigra L. increase glucose uptake in primary porcine myotube cultures and reduce fat accumulation in Caenorhabditis elegans. J. Agric. Food Chem. 2013, 61, 11033–11040. [Google Scholar] [CrossRef]
- Farrell, N.J.; Norris, G.H.; Ryan, J.; Porter, C.M.; Jiang, C.; Blesso, C.N. Black elderberry extract attenuates inflammation and metabolic dysfunction in diet-induced obese mice. Br. J. Nutr. 2015, 114, 1123–1131. [Google Scholar] [CrossRef]
- Salvador, A.C.; Krol, E.; Lemos, V.C.; Santos, S.A.; Bento, F.P.; Costa, C.P.; Almeida, A.; Szczepankiewicz, D.; Kulczynski, B.; Krejpcio, Z.; et al. Effect of Elderberry (Sambucus nigra L.) Extract Supplementation in STZ-Induced Diabetic Rats Fed with a High-Fat Diet. Int. J. Mol. Sci. 2016, 18, 13. [Google Scholar] [CrossRef]
- Ho, G.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Phenolic Elderberry Extracts, Anthocyanins, Procyanidins, and Metabolites Influence Glucose and Fatty Acid Uptake in Human Skeletal Muscle Cells. J. Agric. Food Chem. 2017, 65, 2677–2685. [Google Scholar] [CrossRef]
- Ho, G.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Effect of Phenolic Compounds from Elderflowers on Glucose- and Fatty Acid Uptake in Human Myotubes and HepG2-Cells. Molecules 2017, 22, 90. [Google Scholar] [CrossRef]
- Zielinska-Wasielica, J.; Olejnik, A.; Kowalska, K.; Olkowicz, M.; Dembczynski, R. Elderberry (Sambucus nigra L.) Fruit Extract Alleviates Oxidative Stress, Insulin Resistance, and Inflammation in Hypertrophied 3T3-L1 Adipocytes and Activated RAW 264.7 Macrophages. Foods 2019, 8, 326. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Rashidi, A.A.; Taherian, A.A.; Vakili, Z.; Sajad Sajadian, M.; Ghardashi, M. Antidiabetic and Antihyperlipidemic Effects of Ethanol Extract of Rosa canina L. fruit on Diabetic Rats: An Experimental Study With Histopathological Evaluations. J. Evid.-Based Complement. Altern. Med. 2016, 21, NP25–NP30. [Google Scholar] [CrossRef]
- Fattahi, A.; Niyazi, F.; Shahbazi, B.; Farzaei, M.H.; Bahrami, G. Antidiabetic Mechanisms of Rosa canina Fruits: An In Vitro Evaluation. J. Evid.-Based Complement. Altern. Med. 2017, 22, 127–133. [Google Scholar] [CrossRef]
- Jemaa, H.B.; Jemia, A.B.; Khlifi, S.; Ahmed, H.B.; Slama, F.B.; Benzarti, A.; Elati, J.; Aouidet, A. Antioxidant Activity and a-Amylase Inhibitory Potential of Rosa Canina, L. Afr. J. Tradit. Complement. Altern. Med. AJTCAM 2017, 14, 1–8. [Google Scholar] [CrossRef]
- Chen, S.J.; Aikawa, C.; Yoshida, R.; Kawaguchi, T.; Matsui, T. Anti-prediabetic effect of rose hip (Rosa canina) extract in spontaneously diabetic Torii rats. J. Sci. Food Agric. 2017, 97, 3923–3928. [Google Scholar] [CrossRef]
- Bahrami, G.; Miraghaee, S.S.; Mohammadi, B.; Bahrami, M.T.; Taheripak, G.; Keshavarzi, S.; Babaei, A.; Sajadimajd, S.; Hatami, R. Molecular mechanism of the anti-diabetic activity of an identified oligosaccharide from Rosa canina. Res. Pharm. Sci. 2020, 15, 36–47. [Google Scholar] [CrossRef]
- Rahimi, M.; Sajadimajd, S.; Mahdian, Z.; Hemmati, M.; Malekkhatabi, P.; Bahrami, G.; Mohammadi, B.; Miraghaee, S.; Hatami, R.; Mansouri, K.; et al. Characterization and anti-diabetic effects of the oligosaccharide fraction isolated from Rosa canina in STZ-Induced diabetic rats. Carbohydr. Res. 2020, 489, 107927. [Google Scholar] [CrossRef]
- Capcarova, M.; Kalafova, A.; Schwarzova, M.; Schneidgenova, M.; Svik, K.; Prnova, M.S.; Slovak, L.; Kovacik, A.; Lory, V.; Zorad, S.; et al. Cornelian cherry fruit improves glycaemia and manifestations of diabetes in obese Zucker diabetic fatty rats. Res. Vet. Sci. 2019, 126, 118–123. [Google Scholar] [CrossRef]
- Dzydzan, O.; Bila, I.; Kucharska, A.Z.; Brodyak, I.; Sybirna, N. Antidiabetic effects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct. 2019, 10, 6459–6472. [Google Scholar] [CrossRef]
- Dzydzan, O.; Brodyak, I.; Sokol-Letowska, A.; Kucharska, A.Z.; Sybirna, N. Loganic Acid, an Iridoid Glycoside Extracted from Cornus mas L. Fruits, Reduces of Carbonyl/Oxidative Stress Biomarkers in Plasma and Restores Antioxidant Balance in Leukocytes of Rats with Streptozotocin-Induced Diabetes Mellitus. Life 2020, 10, 349. [Google Scholar] [CrossRef]
- Blagojevic, B.; Agic, D.; Serra, A.T.; Matic, S.; Matovina, M.; Bijelic, S.; Popovic, B.M. An in vitro and in silico evaluation of bioactive potential of cornelian cherry (Cornus mas L.) extracts rich in polyphenols and iridoids. Food Chem. 2021, 335, 127619. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating Type 2 Diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitam. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Iwai, K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-A(y) mice. Plant Foods Hum. Nutr. 2008, 63, 163–169. [Google Scholar] [CrossRef]
- Dao, T.M.; Waget, A.; Klopp, P.; Serino, M.; Vachoux, C.; Pechere, L.; Drucker, D.J.; Champion, S.; Barthelemy, S.; Barra, Y.; et al. Resveratrol increases glucose induced GLP-1 secretion in mice: A mechanism which contributes to the glycemic control. PLoS ONE 2011, 6, e20700. [Google Scholar] [CrossRef]
- Johnston, K.L.; Clifford, M.N.; Morgan, L.M. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: Glycemic effects of chlorogenic acid and caffeine. Am. J. Clin. Nutr. 2003, 78, 728–733. [Google Scholar] [CrossRef]
- Jung, U.J.; Lee, M.K.; Jeong, K.S.; Choi, M.S. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004, 134, 2499–2503. [Google Scholar] [CrossRef]
- Wolfram, S.; Raederstorff, D.; Preller, M.; Wang, Y.; Teixeira, S.R.; Riegger, C.; Weber, P. Epigallocatechin gallate supplementation alleviates diabetes in rodents. J. Nutr. 2006, 136, 2512–2518. [Google Scholar] [CrossRef]
- Zhang, B.; Kang, M.; Xie, Q.; Xu, B.; Sun, C.; Chen, K.; Wu, Y. Anthocyanins from Chinese bayberry extract protect beta cells from oxidative stress-mediated injury via HO-1 upregulation. J. Agric. Food Chem. 2011, 59, 537–545. [Google Scholar] [CrossRef]
- Vetterli. Resveratrol potentiates glucose-stimulated insulin secretion in INS-1E β-cells and human islets through a SIRT1-dependent mechanism. J. Biol. Chem. 2011, 286, 6049–6060. [Google Scholar] [CrossRef]
- Rowley, T.J.t.; Bitner, B.F.; Ray, J.D.; Lathen, D.R.; Smithson, A.T.; Dallon, B.W.; Plowman, C.J.; Bikman, B.T.; Hansen, J.M.; Dorenkott, M.R.; et al. Monomeric cocoa catechins enhance beta-cell function by increasing mitochondrial respiration. J. Nutr. Biochem. 2017, 49, 30–41. [Google Scholar] [CrossRef]
- Clifford, M.N. Anthocyanins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1063–1072. [Google Scholar] [CrossRef]
- Ghosh, D.; Konishi, T. Anthocyanins and anthocyanin-rich extracts: Role in diabetes and eye function. Asia Pac. J. Clin. Nutr. 2007, 16, 200–208. [Google Scholar]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef]
- Upton, R. Bilberry Fruit Vaccinium myrtillus L. Standards of Analysis, Quality Control, and Therapeutics. In American Herbal Pharmacopoeia and Therapeutic Compendium; Santa Cruz: Santa Cruz, CA, USA, 2001. [Google Scholar]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radic. Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef]
- Zoratti, L.; Jaakola, L.; Haggman, H.; Giongo, L. Anthocyanin Profile in Berries of Wild and Cultivated Vaccinium spp. along Altitudinal Gradients in the Alps. J. Agric. Food Chem. 2015, 63, 8641–8650. [Google Scholar] [CrossRef]
- Wilkes, K.; Howard, L.R.; Brownmiller, C.; Prior, R.L. Changes in chokeberry (Aronia melanocarpa L.) polyphenols during juice processing and storage. J. Agric. Food Chem. 2014, 62, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Wojdylo, A.; Figiel, A.; Oszmianski, J. Effect of drying methods with the application of vacuum microwaves on the bioactive compounds, color, and antioxidant activity of strawberry fruits. J. Agric. Food Chem. 2009, 57, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Rozanska, D.; Regulska-Ilow, B. The significance of anthocyanins in the prevention and treatment of type 2 diabetes. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2018, 27, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Geelen, A.; Kromhout, D. Dietary flavonol intake may lower stroke risk in men and women. J. Nutr. 2010, 140, 600–604. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef]
- Inaguma, T.; Han, J.; Isoda, H. Improvement of insulin resistance by Cyanidin 3-glucoside, anthocyanin from black beans through the up-regulation of GLUT4 gene expression. BMC Proc. 2011, 5 (Suppl. 8), 21. [Google Scholar] [CrossRef]
- Takikawa, M.; Inoue, S.; Horio, F.; Tsuda, T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J. Nutr. 2010, 140, 527–533. [Google Scholar] [CrossRef]
- Kurimoto, Y.; Shibayama, Y.; Inoue, S.; Soga, M.; Takikawa, M.; Ito, C.; Nanba, F.; Yoshida, T.; Yamashita, Y.; Ashida, H.; et al. Black soybean seed coat extract ameliorates hyperglycemia and insulin sensitivity via the activation of AMP-activated protein kinase in diabetic mice. J. Agric. Food Chem. 2013, 61, 5558–5564. [Google Scholar] [CrossRef]
- Rios, J.L.; Francini, F.; Schinella, G.R. Natural Products for the Treatment of Type 2 Diabetes Mellitus. Planta Med. 2015, 81, 975–994. [Google Scholar] [CrossRef]
- Bedekar, A.; Shah, K.; Koffas, M. Natural products for type II diabetes treatment. Adv. Appl. Microbiol. 2010, 71, 21–73. [Google Scholar] [CrossRef]
- McDougall, G.J.; Stewart, D. The inhibitory effects of berry polyphenols on digestive enzymes. BioFactors 2005, 23, 189–195. [Google Scholar] [CrossRef]
- Mussatto, S.; Mancilha, I. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Zhu, D.; Yan, Q.; Liu, J.; Wu, X.; Jiang, Z. Can functional oligosaccharides reduce the risk of diabetes mellitus? FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2019, 33, 11655–11667. [Google Scholar] [CrossRef]
- Cheng, W.; Lu, J.; Li, B.; Lin, W.; Zhang, Z.; Wei, X.; Sun, C.; Chi, M.; Bi, W.; Yang, B.; et al. Effect of Functional Oligosaccharides and Ordinary Dietary Fiber on Intestinal Microbiota Diversity. Front. Microbiol. 2017, 8, 1750. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, G.; Dan, C.; Ye, H. The antidiabetic effect and potential mechanisms of natural polysaccharides based on the regulation of gut microbiota. J. Funct. Foods 2020, 75, 104222. [Google Scholar] [CrossRef]
- Courtois, J. Oligosaccharides from land plants and algae: Production and applications in therapeutics and biotechnology. Curr. Opin. Microbiol. 2009, 12, 261–273. [Google Scholar] [CrossRef]
- Dhouibi, R.; Affes, H.; Ben Salem, M.; Hammami, S.; Sahnoun, Z.; Zeghal, K.M.; Ksouda, K. Screening of pharmacological uses of Urtica dioica and others benefits. Prog. Biophys. Mol. Biol. 2020, 150, 67–77. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.J.; Shahabimajd, N. Antioxidant activity of phenol and flavonoid contents of some Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5, 1142–1145. [Google Scholar]
- Bnouham, M.; Merhfour, F.Z.; Ziyyat, A.; Mekhfi, H.; Aziz, M.; Legssyer, A. Antihyperglycemic activity of the aqueous extract of Urtica dioica. Fitoterapia 2003, 74, 677–681. [Google Scholar] [CrossRef]
- Onal, S.; Timur, S.; Okutucu, B.; Zihnioglu, F. Inhibition of alpha-glucosidase by aqueous extracts of some potent antidiabetic medicinal herbs. Prep. Biochem. Biotechnol. 2005, 35, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Farzami, B.; Ahmadvand, D.; Vardasbi, S.; Majin, F.J.; Khaghani, S. Induction of insulin secretion by a component of Urtica dioica leave extract in perifused Islets of Langerhans and its in vivo effects in normal and streptozotocin diabetic rats. J. Ethnopharmacol. 2003, 89, 47–53. [Google Scholar] [CrossRef]
- Obanda, D.N.; Hernandez, A.; Ribnicky, D.; Yu, Y.; Zhang, X.H.; Wang, Z.Q.; Cefalu, W.T. Bioactives of Artemisia dracunculus L. mitigate the role of ceramides in attenuating insulin signaling in rat skeletal muscle cells. Diabetes 2012, 61, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Kraegen, E.W.; Cooney, G.J.; Ye, J.M.; Thompson, A.L.; Furler, S.M. The role of lipids in the pathogenesis of muscle insulin resistance and beta cell failure in type II diabetes and obesity. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2001, 109 (Suppl. 2), S189–S201. [Google Scholar] [CrossRef]
- Blouin, C.M.; Prado, C.; Takane, K.K.; Lasnier, F.; Garcia-Ocana, A.; Ferre, P.; Dugail, I.; Hajduch, E. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes 2010, 59, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Jope, R.S.; Yuskaitis, C.J.; Beurel, E. Glycogen synthase kinase-3 (GSK3): Inflammation, diseases, and therapeutics. Neurochem. Res. 2007, 32, 577–595. [Google Scholar] [CrossRef]
- Kusminski, C.M.; Scherer, P.E. Lowering ceramides to overcome diabetes. Science 2019, 365, 319–320. [Google Scholar] [CrossRef]
- Patel, S.S.; Udayabanu, M. Effect of Urtica dioica on memory dysfunction and hypoalgesia in an experimental model of diabetic neuropathy. Neurosci. Lett. 2013, 552, 114–119. [Google Scholar] [CrossRef]
- Golalipour, M.J.; Kabiri Balajadeh, B.; Ghafari, S.; Azarhosh, R.; Khori, V. Protective Effect of Urtica dioica L. (Urticaceae) on Morphometric and Morphologic Alterations of Seminiferous Tubules in STZ Diabetic Rats. Iran. J. Basic Med. Sci. 2011, 14, 472–477. [Google Scholar]
- Shokrzadeh, M.; Sadat-Hosseini, S.; Fallah, M.; Shaki, F. Synergism effects of pioglitazone and Urtica dioica extract in streptozotocin-induced nephropathy via attenuation of oxidative stress. Iran. J. Basic Med. Sci. 2017, 20, 497–502. [Google Scholar] [CrossRef]
- Tesch, G.H.; Allen, T.J. Rodent models of streptozotocin-induced diabetic nephropathy. Nephrology 2007, 12, 261–266. [Google Scholar] [CrossRef]
- Seyydi, S.M.; Tofighi, A.; Rahmati, M.; Tolouei Azar, J. Exercise and Urtica dioica extract ameliorate mitochondrial function and the expression of cardiac muscle Nuclear Respiratory Factor 2 and Peroxisome proliferator-activated receptor Gamma Coactivator 1-alpha in STZ-induced diabetic rats. Gene 2022, 822, 146351. [Google Scholar] [CrossRef]
- Rabinovitch, A.; Suarez-Pinzon, W.L. Cytokines and their roles in pancreatic islet beta-cell destruction and insulin-dependent diabetes mellitus. Biochem. Pharmacol. 1998, 55, 1139–1149. [Google Scholar] [CrossRef]
- Clark, R.B. The role of PPARs in inflammation and immunity. J. Leukoc. Biol. 2002, 71, 388–400. [Google Scholar] [CrossRef]
- Rezaei, S.; Ashkar, F.; Koohpeyma, F.; Mahmoodi, M.; Gholamalizadeh, M.; Mazloom, Z.; Doaei, S. Hydroalcoholic extract of Achillea millefolium improved blood glucose, liver enzymes and lipid profile compared to metformin in streptozotocin-induced diabetic rats. Lipids Health Dis. 2020, 19, 81. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-Induced Diabetic Models in Mice and Rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Pini, A.; Grange, C.; Veglia, E.; Argenziano, M.; Cavalli, R.; Guasti, D.; Calosi, L.; Ghe, C.; Solarino, R.; Thurmond, R.L.; et al. Histamine H4 receptor antagonism prevents the progression of diabetic nephropathy in male DBA2/J mice. Pharm. Res. 2018, 128, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Verta, R.; Grange, C.; Gurrieri, M.; Borga, S.; Nardini, P.; Argenziano, M.; Ghe, C.; Cavalli, R.; Benetti, E.; Miglio, G.; et al. Effect of Bilastine on Diabetic Nephropathy in DBA2/J Mice. Int. J. Mol. Sci. 2019, 20, 2554. [Google Scholar] [CrossRef] [PubMed]
- Mouhid, L.; Gomez de Cedron, M.; Quijada-Freire, A.; Fernandez-Marcos, P.J.; Reglero, G.; Fornari, T.; Ramirez de Molina, A. Yarrow Supercritical Extract Ameliorates the Metabolic Stress in a Model of Obesity Induced by High-Fat Diet. Nutrients 2019, 12, 72. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.; Saeidi, G.; Goli, S.A. Effect of Drought Stress on Total Phenolic, Lipid Peroxidation, and Antioxidant Activity of Achillea Species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Nematy, M.; Mazidi, M.; Jafari, A.; Baghban, S.; Rakhshandeh, H.; Norouzy, A.; Esmaily, H.; Etemad, L.; Patterson, M.; Mohammadpour, A.H. The effect of hydro-alcoholic extract of Achillea millefolium on appetite hormone in rats. Avicenna J. Phytomed. 2017, 7, 10–15. [Google Scholar] [PubMed]
- Sidorova, Y.; Shipelin, V.; Mazo, V.; Zorin, S.; Petrov, N.; Kochetkova, A. Hypoglycemic and hypolipidemic effect of Vaccinium myrtillus L. leaf and Phaseolus vulgaris L. seed coat extracts in diabetic rats. Nutrition 2017, 41, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wei, X.; Zhang, J.; Pariyani, R.; Jokioja, J.; Kortesniemi, M.; Linderborg, K.M.; Heinonen, J.; Sainio, T.; Zhang, Y.; et al. Effects of Anthocyanin Extracts from Bilberry (Vaccinium myrtillus L.) and Purple Potato (Solanum tuberosum L. var. ‘Synkea Sakari’) on the Plasma Metabolomic Profile of Zucker Diabetic Fatty Rats. J. Agric. Food Chem. 2020, 68, 9436–9450. [Google Scholar] [CrossRef] [PubMed]
- Shiota, M.; Printz, R.L. Diabetes in Zucker diabetic fatty rat. Methods Mol. Biol. 2012, 933, 103–123. [Google Scholar] [CrossRef] [PubMed]
- Mykkanen, O.T.; Kalesnykas, G.; Adriaens, M.; Evelo, C.T.; Torronen, R.; Kaarniranta, K. Bilberries potentially alleviate stress-related retinal gene expression induced by a high-fat diet in mice. Mol. Vis. 2012, 18, 2338–2351. [Google Scholar]
- Matysek, M.; Borowiec, K.; Szwajgier, D.; Szalak, R.; Arciszewski, M.B. Insulin receptors in the CA1 field of hippocampus and selected blood parameters in diabetic rats fed with bilberry fruit. Ann. Agric. Environ. Med. 2021, 28, 430–436. [Google Scholar] [CrossRef]
- Velickov, A.; Mitrovic, O.; Djordjevic, B.; Sokolovic, D.; Zivkovic, V.; Velickov, A.; Pantovic, V.; Urlih, N.P.; Radenkovic, G. The effect of bilberries on diabetes-related alterations of interstitial cells of Cajal in the lower oesophageal sphincter in rats. Histol. Histopathol. 2017, 32, 639–647. [Google Scholar] [CrossRef]
- Cho, H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitam. Horm. 2013, 91, 405–424. [Google Scholar] [CrossRef]
- Nieto-Vazquez, I.; Fernandez-Veledo, S.; de Alvaro, C.; Rondinone, C.M.; Valverde, A.M.; Lorenzo, M. Protein-tyrosine phosphatase 1B-deficient myocytes show increased insulin sensitivity and protection against tumor necrosis factor-alpha-induced insulin resistance. Diabetes 2007, 56, 404–413. [Google Scholar] [CrossRef]
- Delibegovic, M.; Bence, K.K.; Mody, N.; Hong, E.G.; Ko, H.J.; Kim, J.K.; Kahn, B.B.; Neel, B.G. Improved glucose homeostasis in mice with muscle-specific deletion of protein-tyrosine phosphatase 1B. Mol. Cell. Biol. 2007, 27, 7727–7734. [Google Scholar] [CrossRef]
- Silva, F.M.; Kramer, C.K.; de Almeida, J.C.; Steemburgo, T.; Gross, J.L.; Azevedo, M.J. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: A systematic review with meta-analysis of randomized controlled trials. Nutr. Rev. 2013, 71, 790–801. [Google Scholar] [CrossRef]
- Derrick, S.A.; Kristo, A.S.; Reaves, S.K.; Sikalidis, A.K. Effects of Dietary Red Raspberry Consumption on Pre-Diabetes and Type 2 Diabetes Mellitus Parameters. Int. J. Environ. Res. Public Health 2021, 18, 9364. [Google Scholar] [CrossRef]
- Spinola, V.; Pinto, J.; Llorent-Martinez, E.J.; Tomas, H.; Castilho, P.C. Evaluation of Rubus grandifolius L. (wild blackberries) activities targeting management of type-2 diabetes and obesity using in vitro models. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 123, 443–452. [Google Scholar] [CrossRef]
- Wedick, N.M.; Pan, A.; Cassidy, A.; Rimm, E.B.; Sampson, L.; Rosner, B.; Willett, W.; Hu, F.B.; Sun, Q.; van Dam, R.M. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am. J. Clin. Nutr. 2012, 95, 925–933. [Google Scholar] [CrossRef]
- Luo, T.; Miranda-Garcia, O.; Sasaki, G.; Shay, N.F. Consumption of a single serving of red raspberries per day reduces metabolic syndrome parameters in high-fat fed mice. Food Funct. 2017, 8, 4081–4088. [Google Scholar] [CrossRef]
- Fan, R.; You, M.; Toney, A.M.; Kim, J.; Giraud, D.; Xian, Y.; Ye, F.; Gu, L.; Ramer-Tait, A.E.; Chung, S. Red Raspberry Polyphenols Attenuate High-Fat Diet-Driven Activation of NLRP3 Inflammasome and its Paracrine Suppression of Adipogenesis via Histone Modifications. Mol. Nutr. Food Res. 2020, 64, e1900995. [Google Scholar] [CrossRef]
- Stefanut, M.N.; Cata, A.; Pop, R.; Tanasie, C.; Boc, D.; Ienascu, I.; Ordodi, V. Anti-hyperglycemic effect of bilberry, blackberry and mulberry ultrasonic extracts on diabetic rats. Plant Foods Hum. Nutr. 2013, 68, 378–384. [Google Scholar] [CrossRef]
- Jouad, H.; Maghrani, M.; Eddouks, M. Hypoglycaemic effect of Rubus fructicosis L. and Globularia alypum L. in normal and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2002, 81, 351–356. [Google Scholar] [CrossRef]
- Lalanza, J.F.; Snoeren, E.M. The cafeteria diet: A standardized protocol and its effects on behavior. Neurosci. Biobehav. Rev. 2021, 122, 92–119. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Temocico, G.; Fierascu, I.; Ortan, A.; Babeanu, N.E. Fragaria Genus: Chemical Composition and Biological Activities. Molecules 2020, 25, 498. [Google Scholar] [CrossRef]
- Roy, S.; Wu, B.; Liu, W.; Archbold, D.D. Comparative analyses of polyphenolic composition of Fragaria spp. color mutants. Plant Physiol. Biochem. PPB 2018, 125, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Abdulazeez, S.S. Effects of freeze-dried Fragaria x ananassa powder on alloxan-induced diabetic complications in Wistar rats. J. Taibah Univ. Med. Sci. 2014, 9, 268–273. [Google Scholar] [CrossRef]
- Yella, V.; Dass, A. Effect of Ethanolic Extract of Fragaria Vesca on serum glucose levels and body weight in diet induced obese rats. Int. J. Pharmacol. Res. 2015, 5, 236–238. [Google Scholar] [CrossRef]
- Bailey, C.J.; Flatt, P.R. Development of antidiabetic drugs. In Drugs, Diet and Disease; Ioannides, C., Flatt, P.R., Eds.; Mechanistic Approaches to Diabetes; Ellis Horwood Ltd.: Chichester, UK, 1995; Volume 2, pp. 279–326. [Google Scholar]
- Gray, A.M.; Abdel-Wahab, Y.H.; Flatt, P.R. The traditional plant treatment, Sambucus nigra (elder), exhibits insulin-like and insulin-releasing actions in vitro. J. Nutr. 2000, 130, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Gray, A.M.; Flatt, P.R. Pancreatic and extra-pancreatic effects of the traditional anti-diabetic plant, Medicago sativa (lucerne). Br. J. Nutr. 1997, 78, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef]
- Raafat, K.; El-Lakany, A. Acute and subchronic in-vivo effects of Ferula hermonis L. and Sambucus nigra L. and their potential active isolates in a diabetic mouse model of neuropathic pain. BMC Complement. Altern. Med. 2015, 15, 257. [Google Scholar] [CrossRef]
- Badescu, M.; Badulescu, O.; Badescu, L.; Ciocoiu, M. Effects of Sambucus nigra and Aronia melanocarpa extracts on immune system disorders within diabetes mellitus. Pharm. Biol. 2015, 53, 533–539. [Google Scholar] [CrossRef]
- Orhan, N.; Aslan, M.; Hosbas, C.S.; Didem, D.D. Antidiabetic Effect and Antioxidant Potential of Rosa canina Fruits. Pharmacogn. Mag. 2009, 20, 309–315. [Google Scholar] [CrossRef]
- Orhan, D.; Hartevioglu, A.; Kupeli, E.; Yesilada, E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits. J. Ethnopharmacol. 2007, 112, 394–400. [Google Scholar] [CrossRef]
- Wenzig, E.M.; Widowitz, U.; Kunert, O.; Chrubasik, S.; Bucar, F.; Knauder, E.; Bauer, R. Phytochemical composition and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine 2008, 15, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Hashem Dabaghian, F.; Abdollahifard, M.; Khalighi, S.F.; Taghavi, S.M.; Shojaee, A.; Sabet, Z.; Fallah, H.H. Effects of Rosa canina L. Fruit on Glycemia and Lipid Profile in Type 2 Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Med. Plants 2015, 14, 95–104. [Google Scholar]
- Michau, A.; Guillemain, G.; Grosfeld, A.; Vuillaumier-Barrot, S.; Grand, T.; Keck, M.; L’Hoste, S.; Chateau, D.; Serradas, P.; Teulon, J.; et al. Mutations in SLC2A2 gene reveal hGLUT2 function in pancreatic beta cell development. J. Biol. Chem. 2013, 288, 31080–31092. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, G.; Sajadimajd, S.; Mohammadi, B.; Hatami, R.; Miraghaee, S.; Keshavarzi, S.; Khazaei, M.; Madani, S.H. Anti-diabetic effect of a novel oligosaccharide isolated from Rosa canina via modulation of DNA methylation in Streptozotocin-diabetic rats. DARU J. Fac. Pharm. Tehran Univ. Med. Sci. 2020, 28, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.B.; Ji, T.F.; Zhou, H.W.; Yu, J.L. Effects of microRNA-21 on Nerve Cell Regeneration and Neural Function Recovery in Diabetes Mellitus Combined with Cerebral Infarction Rats by Targeting PDCD4. Mol. Neurobiol. 2018, 55, 2494–2505. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Wu, D.; Liu, X.; Shi, M.; Wang, Y.; Zhang, F.; Ding, J.; Xiao, Y.; Guo, B. MicroRNA-22 Promotes Renal Tubulointerstitial Fibrosis by Targeting PTEN and Suppressing Autophagy in Diabetic Nephropathy. J. Diabetes Res. 2018, 2018, 4728645. [Google Scholar] [CrossRef]
- Qadir, M.M.F.; Klein, D.; Alvarez-Cubela, S.; Dominguez-Bendala, J.; Pastori, R.L. The Role of MicroRNAs in Diabetes-Related Oxidative Stress. Int. J. Mol. Sci. 2019, 20, 5423. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahrami, G.; Mohammadi, B.; Nouri, Z.; Farzaei, M.H.; Chen, J.T. Protective effect of the isolated oligosaccharide from Rosa canina in STZ-treated cells through modulation of the autophagy pathway. J. Food Biochem. 2020, 44, e13404. [Google Scholar] [CrossRef]
- Seeram, N.P.; Schutzki, R.; Chandra, A.; Nair, M.G. Characterization, quantification, and bioactivities of anthocyanins in Cornus species. J. Agric. Food Chem. 2002, 50, 2519–2523. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M.G. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef]
- Tural, S.; Koca, I. Physico-chemical and antioxidant properties of cornelian cherry fruits (Cornus mas L.) grown in Turkey. Sci. Hortic. 2008, 116, 362–366. [Google Scholar] [CrossRef]
- Asgary, S.; Rafieian-Kopaei, M.; Shamsi, F.; Najafi, S.; Sahebkar, A. Biochemical and histopathological study of the anti-hyperglycemic and anti-hyperlipidemic effects of cornelian cherry (Cornus mas L.) in alloxan-induced diabetic rats. J. Complement. Integr. Med. 2014, 11, 63–69. [Google Scholar] [CrossRef]
- Ziaei, R.; Foshati, S.; Hadi, A.; Kermani, M.A.H.; Ghavami, A.; Clark, C.C.T.; Tarrahi, M.J. The effect of nettle (Urtica dioica) supplementation on the glycemic control of patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Phytother. Res. 2020, 34, 282–294. [Google Scholar] [CrossRef]
- Korani, B.; Mirzapour, A.; Moghadamnia, A.; Khafri, S.; Neamati, N.; Parsian, H. The Effect of Urtica dioica Hydro-Alcoholic Extract on Glycemic Index and AMP-Activated Protein Kinase Levels in Diabetic Patients: A Randomized Single-Blind Clinical Trial. Iran. Red Crescent Med. J. 2017, 19, e40572. [Google Scholar] [CrossRef]
- Khajeh-Mehrizi, R.; Mozaffari-khosravi, H.; Ghadiri-anari, A.; Dehghani, A. The Effect of Urtica dioica Extract on Glycemic Control and Insulin Resistance Indices in Patients with Type2 Diabetes: A Randomized, Double-Blind Clinical Trial. Iran. J. Diabetes Obes. 2014, 6, 149–155. [Google Scholar]
- Dabagh, S.; Nikbakht, M. Glycemic Control by Exercise and Urtica dioica Supplements in Men with Type 2 Diabetes. Jundishapur J. Chronic Dis. Care 2016, 5, e31745. [Google Scholar] [CrossRef]
- Ghalavand, A.; Motamedi, P.; Delaramnasab, M.; Khodadoust, M. The Effect of Interval Training and Nettle Supplement on Glycemic Control and Blood Pressure in Men WithType 2 Diabetes. Int. J. Basic Sci. Med. 2017, 2, 33–40. [Google Scholar] [CrossRef][Green Version]
- Kianbakht, S.; Khalighi-Sigaroodi, F.; Dabaghian, F.H. Improved glycemic control in patients with advanced type 2 diabetes mellitus taking Urtica dioica leaf extract: A randomized double-blind placebo-controlled clinical trial. Clin. Lab. 2013, 59, 1071–1076. [Google Scholar] [CrossRef]
- Tarighat, E.A.; Namazi, N.; Bahrami, A.M.E. Effect of Hydroalcoholic Extract Of Nettle (Urtica Dioica) On Glycemic Index And Insulin Resistance Index In Type 2 Diabetic Patients. Iran. J. Endocrinol. Metab. (IJEM) 2012, 13, 561–568. [Google Scholar]
- Hassani, A.; Maryam, E.; Reza, R. Survey on the Effect of Eight Weeks of Regular Aerobic Eexercise with Consumption of Nettle Extract on Blood Glucose and Insulin Resistance Index among women with Type II Diabetes. J. Knowl. Health 2016, 10, 57–64. [Google Scholar]
- Dadvar, N.; Ghalavand, K.; Zakerkish, M.; Hojat, S.; Alijani, E.; Mahmoodkhani, K.R. The Effect Of Aerobic Training And Urtica dioica On Lipid Profile And Fasting Blood Glucose In Middle Age Female With Type Ii Diabetes. Jundishapur Sci. Med. J. (JSMJ) 2016, 15, 707–716. [Google Scholar]
- Namazi, N.; Esfanjani, A.T.; Heshmati, J.; Bahrami, A. The effect of hydro alcoholic Nettle (Urtica dioica) extracts on insulin sensitivity and some inflammatory indicators in patients with type 2 diabetes: A randomized double-blind control trial. Pak. J. Biol. Sci. PJBS 2011, 14, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Namazi, N.; Tarighat, A.; Bahrami, A. The effect of hydro alcoholic nettle (Urtica dioica) extract on oxidative stress in patients with type 2 diabetes: A randomized double-blind clinical trial. Pak. J. Biol. Sci. PJBS 2012, 15, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Amiri Behzadi, A.; Kalalian-Moghaddam, H.; Ahmadi, A.H. Effects of Urtica dioica supplementation on blood lipids, hepatic enzymes and nitric oxide levels in type 2 diabetic patients: A double blind, randomized clinical trial. Avicenna J. Phytomed. 2016, 6, 686–695. [Google Scholar]
- Cohen, R.A. Role of nitric oxide in diabetic complications. Am J. Ther. 2005, 12, 499–502. [Google Scholar] [CrossRef]
- Zhu, Y.; Miao, Y.; Meng, Z.; Zhong, Y. Effects of Vaccinium Berries on Serum Lipids: A Meta-Analysis of Randomized Controlled Trials. Evid.-Based Complement. Altern. Med. 2015, 2015, 790329. [Google Scholar] [CrossRef]
- Basu, A.; Lyons, T.J. Strawberries, blueberries, and cranberries in the metabolic syndrome: Clinical perspectives. J. Agric. Food Chem. 2012, 60, 5687–5692. [Google Scholar] [CrossRef]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, e285–e350. [Google Scholar] [CrossRef]
- Mattiuzzi, C.; Sanchis-Gomar, F.; Lippi, G. Worldwide burden of LDL cholesterol: Implications in cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 241–244. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; Linares, A.; Hyson, D.; Kappagoda, T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J. Am. Coll. Nutr. 2010, 29, 46–54. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Wei, H.; Vijayakumar, L.P.; Banaszewski, K.; Cappozzo, J.C.; Burton-Freeman, B. A dose-response evaluation of freeze-dried strawberries independent of fiber content on metabolic indices in abdominally obese individuals with insulin resistance in a randomized, single-blinded, diet-controlled crossover trial. Mol. Nutr. Food Res. 2016, 60, 1099–1109. [Google Scholar] [CrossRef]
- Huang, Y.; Park, E.; Edirisinghe, I.; Burton-Freeman, B.M. Maximizing the health effects of strawberry anthocyanins: Understanding the influence of the consumption timing variable. Food Funct. 2016, 7, 4745–4752. [Google Scholar] [CrossRef]
- Basu, A.; Betts, N.M.; Nguyen, A.; Newman, E.D.; Fu, D.; Lyons, T.J. Freeze-dried strawberries lower serum cholesterol and lipid peroxidation in adults with abdominal adiposity and elevated serum lipids. J. Nutr. 2014, 144, 830–837. [Google Scholar] [CrossRef]
- Basu, A.; Izuora, K.; Betts, N.M.; Kinney, J.W.; Salazar, A.M.; Ebersole, J.L.; Scofield, R.H. Dietary Strawberries Improve Cardiometabolic Risks in Adults with Obesity and Elevated Serum LDL Cholesterol in a Randomized Controlled Crossover Trial. Nutrients 2021, 13, 1421. [Google Scholar] [CrossRef]
- Schell, J.; Scofield, R.H.; Barrett, J.R.; Kurien, B.T.; Betts, N.; Lyons, T.J.; Zhao, Y.D.; Basu, A. Strawberries Improve Pain and Inflammation in Obese Adults with Radiographic Evidence of Knee Osteoarthritis. Nutrients 2017, 9, 949. [Google Scholar] [CrossRef]
- Soltani, R.; Gorji, A.; Asgary, S.; Sarrafzadegan, N.; Siavash, M. Evaluation of the Effects of Cornus mas L. Fruit Extract on Glycemic Control and Insulin Level in Type 2 Diabetic Adult Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Evid.-Based Complement. Altern. Med. 2015, 2015, 740954. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, A.; Sandhu, A.K.; Edirisinghe, I.; Burton-Freeman, B.M. Red Raspberry and Fructo-Oligosaccharide Supplementation, Metabolic Biomarkers, and the Gut Microbiota in Adults with Prediabetes: A Randomized Crossover Clinical Trial. J. Nutr. 2022, 152, 1438–1449. [Google Scholar] [CrossRef]
- Xiao, D.; Zhu, L.; Edirisinghe, I.; Fareed, J.; Brailovsky, Y.; Burton-Freeman, B. Attenuation of Postmeal Metabolic Indices with Red Raspberries in Individuals at Risk for Diabetes: A Randomized Controlled Trial. Obesity 2019, 27, 542–550. [Google Scholar] [CrossRef]
- Franck, M.; de Toro-Martin, J.; Varin, T.V.; Garneau, V.; Pilon, G.; Roy, D.; Couture, P.; Couillard, C.; Marette, A.; Vohl, M.C. Raspberry consumption: Identification of distinct immune-metabolic response profiles by whole blood transcriptome profiling. J. Nutr. Biochem. 2022, 101, 108946. [Google Scholar] [CrossRef]
- Puupponen-Pimia, R.; Seppanen-Laakso, T.; Kankainen, M.; Maukonen, J.; Torronen, R.; Kolehmainen, M.; Leppanen, T.; Moilanen, E.; Nohynek, L.; Aura, A.M.; et al. Effects of ellagitannin-rich berries on blood lipids, gut microbiota, and urolithin production in human subjects with symptoms of metabolic syndrome. Mol. Nutr. Food Res. 2013, 57, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Kolehmainen, M.; Mykkanen, O.; Kirjavainen, P.V.; Leppanen, T.; Moilanen, E.; Adriaens, M.; Laaksonen, D.E.; Hallikainen, M.; Puupponen-Pimia, R.; Pulkkinen, L.; et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol. Nutr. Food Res. 2012, 56, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- de Mello, V.D.; Lankinen, M.A.; Lindstrom, J.; Puupponen-Pimia, R.; Laaksonen, D.E.; Pihlajamaki, J.; Lehtonen, M.; Uusitupa, M.; Tuomilehto, J.; Kolehmainen, M.; et al. Fasting serum hippuric acid is elevated after bilberry (Vaccinium myrtillus) consumption and associates with improvement of fasting glucose levels and insulin secretion in persons at high risk of developing type 2 diabetes. Mol. Nutr. Food Res. 2017, 61, 1700019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, J.; Xiao, D.; Edirisinghe, I.; Burton-Freeman, B.M.; Sandhu, A.K. Pharmacokinetic Evaluation of Red Raspberry (Poly)phenols from Two Doses and Association with Metabolic Indices in Adults with Prediabetes and Insulin Resistance. J. Agric. Food Chem. 2021, 69, 9238–9248. [Google Scholar] [CrossRef]
- Schell, J.; Betts, N.M.; Lyons, T.J.; Basu, A. Raspberries Improve Postprandial Glucose and Acute and Chronic Inflammation in Adults with Type 2 Diabetes. Ann. Nutr. Metab. 2019, 74, 165–174. [Google Scholar] [CrossRef]
- Solverson, P.M.; Rumpler, W.V.; Leger, J.L.; Redan, B.W.; Ferruzzi, M.G.; Baer, D.J.; Castonguay, T.W.; Novotny, J.A. Blackberry Feeding Increases Fat Oxidation and Improves Insulin Sensitivity in Overweight and Obese Males. Nutrients 2018, 10, 1048. [Google Scholar] [CrossRef]
- Gallicchio, M.; Rosa, A.C.; Dianzani, C.; Brucato, L.; Benetti, E.; Collino, M.; Fantozzi, R. Celecoxib decreases expression of the adhesion molecules ICAM-1 and VCAM-1 in a colon cancer cell line (HT29). Br. J. Pharm. 2008, 153, 870–878. [Google Scholar] [CrossRef]
- Lehtonen, H.M.; Suomela, J.P.; Tahvonen, R.; Yang, B.; Venojarvi, M.; Viikari, J.; Kallio, H. Different berries and berry fractions have various but slightly positive effects on the associated variables of metabolic diseases on overweight and obese women. Eur. J. Clin. Nutr. 2011, 65, 394–401. [Google Scholar] [CrossRef]
- Yang, L.; Ling, W.; Qiu, Y.; Liu, Y.; Wang, L.; Yang, J.; Wang, C.; Ma, J. Anthocyanins increase serum adiponectin in newly diagnosed diabetes but not in prediabetes: A randomized controlled trial. Nutr. Metab. 2020, 17, 78. [Google Scholar] [CrossRef]
- Chan, S.W.; Chu, T.T.; Choi, S.W.; Benzie, I.F.; Tomlinson, B. Impact of short-term bilberry supplementation on glycemic control, cardiovascular disease risk factors, and antioxidant status in Chinese patients with type 2 diabetes. Phytother. Res. 2021, 35, 3236–3245. [Google Scholar] [CrossRef]
- Yang, L.; Ling, W.; Yang, Y.; Chen, Y.; Tian, Z.; Du, Z.; Chen, J.; Xie, Y.; Liu, Z.; Yang, L. Role of Purified Anthocyanins in Improving Cardiometabolic Risk Factors in Chinese Men and Women with Prediabetes or Early Untreated Diabetes—A Randomized Controlled Trial. Nutrients 2017, 9, 1104. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z.; Ling, W.; Wang, L.; Wang, C.; Ma, J.; Peng, X.; Chen, J. Effect of Anthocyanins Supplementation on Serum IGFBP-4 Fragments and Glycemic Control in Patients with Fasting Hyperglycemia: A Randomized Controlled Trial. Diabetes Metab. Syndr Obes 2020, 13, 3395–3404. [Google Scholar] [CrossRef]
- Yang, L.; Qiu, Y.; Ling, W.; Liu, Z.; Yang, L.; Wang, C.; Peng, X.; Wang, L.; Chen, J. Anthocyanins regulate serum adipsin and visfatin in patients with prediabetes or newly diagnosed diabetes: A randomized controlled trial. Eur. J. Nutr. 2021, 60, 1935–1944. [Google Scholar] [CrossRef]
- Lo, J.C.; Ljubicic, S.; Leibiger, B.; Kern, M.; Leibiger, I.B.; Moede, T.; Kelly, M.E.; Chatterjee Bhowmick, D.; Murano, I.; Cohen, P.; et al. Adipsin is an adipokine that improves beta cell function in diabetes. Cell 2014, 158, 41–53. [Google Scholar] [CrossRef]
- Chen, M.P.; Chung, F.M.; Chang, D.M.; Tsai, J.C.; Huang, H.F.; Shin, S.J.; Lee, Y.J. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2006, 91, 295–299. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Liu, Y.; Sun, R.; Xia, M. Purified anthocyanin supplementation reduces dyslipidemia, enhances antioxidant capacity, and prevents insulin resistance in diabetic patients. J. Nutr. 2015, 145, 742–748. [Google Scholar] [CrossRef]
- Hoggard, N.; Cruickshank, M.; Moar, K.M.; Bestwick, C.; Holst, J.J.; Russell, W.; Horgan, G. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36% wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J. Nutr. Sci. 2013, 2, e22. [Google Scholar] [CrossRef]
- Xu, J.; Jonsson, T.; Plaza, M.; Hakansson, A.; Antonsson, M.; Ahren, I.L.; Turner, C.; Spegel, P.; Granfeldt, Y. Probiotic fruit beverages with different polyphenol profiles attenuated early insulin response. Nutr. J. 2018, 17, 34. [Google Scholar] [CrossRef]
- Mazzolani, F.; Togni, S.; Franceschi, F.; Eggenhoffner, R.; Giacomelli, L. The effect of oral supplementation with standardized bilberry extract (Mirtoselect®) on retino-cortical bioelectrical activity in severe diabetic retinopathy. Minerva Oftalmol 2017, 59, 38–41. [Google Scholar] [CrossRef]
- Gizzi, C.; Belcaro, G.; Gizzi, G.; Feragalli, B.; Dugall, M.; Luzzi, R.; Cornelli, U. Bilberry extracts are not created equal: The role of non anthocyanin fraction. Discovering the “dark side of the force” in a preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 2418–2424. [Google Scholar]
- Mehrzadi, S.; Mirzaei, R.; Heydari, M.; Sasani, M.; Yaqoobvand, B.; Huseini, H.F. Efficacy and Safety of a Traditional Herbal Combination in Patients with Type II Diabetes Mellitus: A Randomized Controlled Trial. J. Diet. Suppl. 2021, 18, 31–43. [Google Scholar] [CrossRef]
- Roman, I.; Stanila, A.; Stanila, S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013, 7, 73. [Google Scholar] [CrossRef]
- Andersson, U.; Berger, K.; Hogberg, A.; Landin-Olsson, M.; Holm, C. Effects of rose hip intake on risk markers of type 2 diabetes and cardiovascular disease: A randomized, double-blind, cross-over investigation in obese persons. Eur. J. Clin. Nutr. 2012, 66, 585–590. [Google Scholar] [CrossRef]
- Sanchez, M.; Gonzalez-Burgos, E.; Iglesias, I.; Lozano, R.; Gomez-Serranillos, M.P. Current uses and knowledge of medicinal plants in the Autonomous Community of Madrid (Spain): A descriptive cross-sectional study. BMC Complement. Med. Ther. 2020, 20, 306. [Google Scholar] [CrossRef] [PubMed]
- Li, F.S.; Weng, J.K. Demystifying traditional herbal medicine with modern approach. Nat. Plants 2017, 3, 17109. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Narkevich, I.A.; Akamova, A.V.; Nemyatykh, O.D.; Flisyuk, E.V.; Luzhanin, V.G.; Povydysh, M.N.; Mikhailova, I.V.; Pozharitskaya, O.N. Medical Species Used in Russia for the Management of Diabetes and Related Disorders. Front. Pharm. 2021, 12, 697411. [Google Scholar] [CrossRef] [PubMed]
- Zunino, S.J.; Parelman, M.A.; Freytag, T.L.; Stephensen, C.B.; Kelley, D.S.; Mackey, B.E.; Woodhouse, L.R.; Bonnel, E.L. Effects of dietary strawberry powder on blood lipids and inflammatory markers in obese human subjects. Br. J. Nutr. 2012, 108, 900–909. [Google Scholar] [CrossRef]
- Wu, J.; Yan, L.J. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic beta cell glucotoxicity. Diabetes Metab. Syndr. Obes. 2015, 8, 181–188. [Google Scholar] [CrossRef]
- Grange, C.; Gurrieri, M.; Verta, R.; Fantozzi, R.; Pini, A.; Rosa, A.C. Histamine in the kidneys: What is its role in renal pathophysiology? Br. J. Pharm. 2020, 177, 503–515. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Adeosun, A.M.; Akinloye, O.A. Alloxan-induced diabetes, a common model for evaluating the glycemic-control potential of therapeutic compounds and plants extracts in experimental studies. Medicina 2017, 53, 365–374. [Google Scholar] [CrossRef]
- Heydemann, A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2902351. [Google Scholar] [CrossRef]
- Benetti, E.; Mastrocola, R.; Vitarelli, G.; Cutrin, J.C.; Nigro, D.; Chiazza, F.; Mayoux, E.; Collino, M.; Fantozzi, R. Empagliflozin Protects against Diet-Induced NLRP-3 Inflammasome Activation and Lipid Accumulation. J. Pharm. Exp. Ther. 2016, 359, 45–53. [Google Scholar] [CrossRef]
- Doumett, S.; Fibbi, D.; Cincinelli, A.; Giordani, E.; Nin, S.; Del Bubba, M. Comparison of nutritional and nutraceutical properties in cultivated fruits of Fragaria vesca L. produced in Italy. Food Res. Intern. 2011, 44, 1209–1216. [Google Scholar] [CrossRef]
- Peñarrieta, J.M.; Alvarado, J.A.; Bergenståhl, B.; Ákesson, B. Total antioxidant capacity and content of phenolic compounds in wild strawberries (Fragaria vesca) collected in Bolivia. Int. J. Fruit Sci. 2009, 9, 344–359. [Google Scholar] [CrossRef]
- Larskaya, I.A.; Gorshkova, T.A. Plant oligosaccharides—Outsiders among elicitors? Biochemistry 2015, 80, 881–900. [Google Scholar] [CrossRef]
- De Natale, C.; Annuzzi, G.; Bozzetto, L.; Mazzarella, R.; Costabile, G.; Ciano, O.; Riccardi, G.; Rivellese, A.A. Effects of a plant-based high-carbohydrate/high-fiber diet versus high-monounsaturated fat/low-carbohydrate diet on postprandial lipids in type 2 diabetic patients. Diabetes Care 2009, 32, 2168–2173. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, F.; Ding, X.; Wu, G.; Lam, Y.Y.; Wang, X.; Fu, H.; Xue, X.; Lu, C.; Ma, J.; et al. Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 2018, 359, 1151–1156. [Google Scholar] [CrossRef]
- Annunziata, G.; Jimenez-Garcia, M.; Capo, X.; Moranta, D.; Arnone, A.; Tenore, G.C.; Sureda, A.; Tejada, S. Microencapsulation as a tool to counteract the typical low bioavailability of polyphenols in the management of diabetes. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 139, 111248. [Google Scholar] [CrossRef]
- Cerda, B.; Periago, P.; Espin, J.C.; Tomas-Barberan, F.A. Identification of urolithin a as a metabolite produced by human colon microflora from ellagic acid and related compounds. J. Agric. Food Chem. 2005, 53, 5571–5576. [Google Scholar] [CrossRef]
- Cerda, B.; Tomas-Barberan, F.A.; Espin, J.C. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: Identification of biomarkers and individual variability. J. Agric. Food Chem. 2005, 53, 227–235. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Sip, S.; Szymanowska, D.; Chanaj-Kaczmarek, J.; Skalicka-Wozniak, K.; Budzynska, B.; Wronikowska-Denysiuk, O.; Slowik, T.; Szulc, P.; Cielecka-Piontek, J. Potential for Prebiotic Stabilized Cornus mas L. Lyophilized Extract in the Prophylaxis of Diabetes Mellitus in Streptozotocin Diabetic Rats. Antioxidants 2022, 11, 380. [Google Scholar] [CrossRef]
- Oledzka, A.; Cichocka, K.; Wolinski, K.; Melzig, M.F.; Czerwinska, M.E. Potentially Bio-Accessible Metabolites from an Extract of Cornus mas Fruit after Gastrointestinal Digestion In Vitro and Gut Microbiota Ex Vivo Treatment. Nutrients 2022, 14, 2287. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal. Transduct. Target Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Healey, G.R.; Murphy, R.; Brough, L.; Butts, C.A.; Coad, J. Interindividual variability in gut microbiota and host response to dietary interventions. Nutr. Rev. 2017, 75, 1059–1080. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Paoletti, A.; Gallo, E.; Benemei, S.; Vietri, M.; Lapi, F.; Volpi, R.; Menniti-Ippolito, F.; Gori, L.; Mugelli, A.; Firenzuoli, F.; et al. Interactions between Natural Health Products and Oral Anticoagulants: Spontaneous Reports in the Italian Surveillance System of Natural Health Products. Evid.-Based Complement. Altern. Med. 2011, 2011, 612150. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to enhance the bioavailability and physiological functions of polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef]
- Opris, R.; Tatomir, C.; Olteanu, D.; Moldovan, R.; Moldovan, B.; David, L.; Nagy, A.; Decea, N.; Kiss, M.L.; Filip, G.A. The effect of Sambucus nigra L. extract and phytosinthesized gold nanoparticles on diabetic rats. Colloids Surf. B Biointerfaces 2017, 150, 192–200. [Google Scholar] [CrossRef]
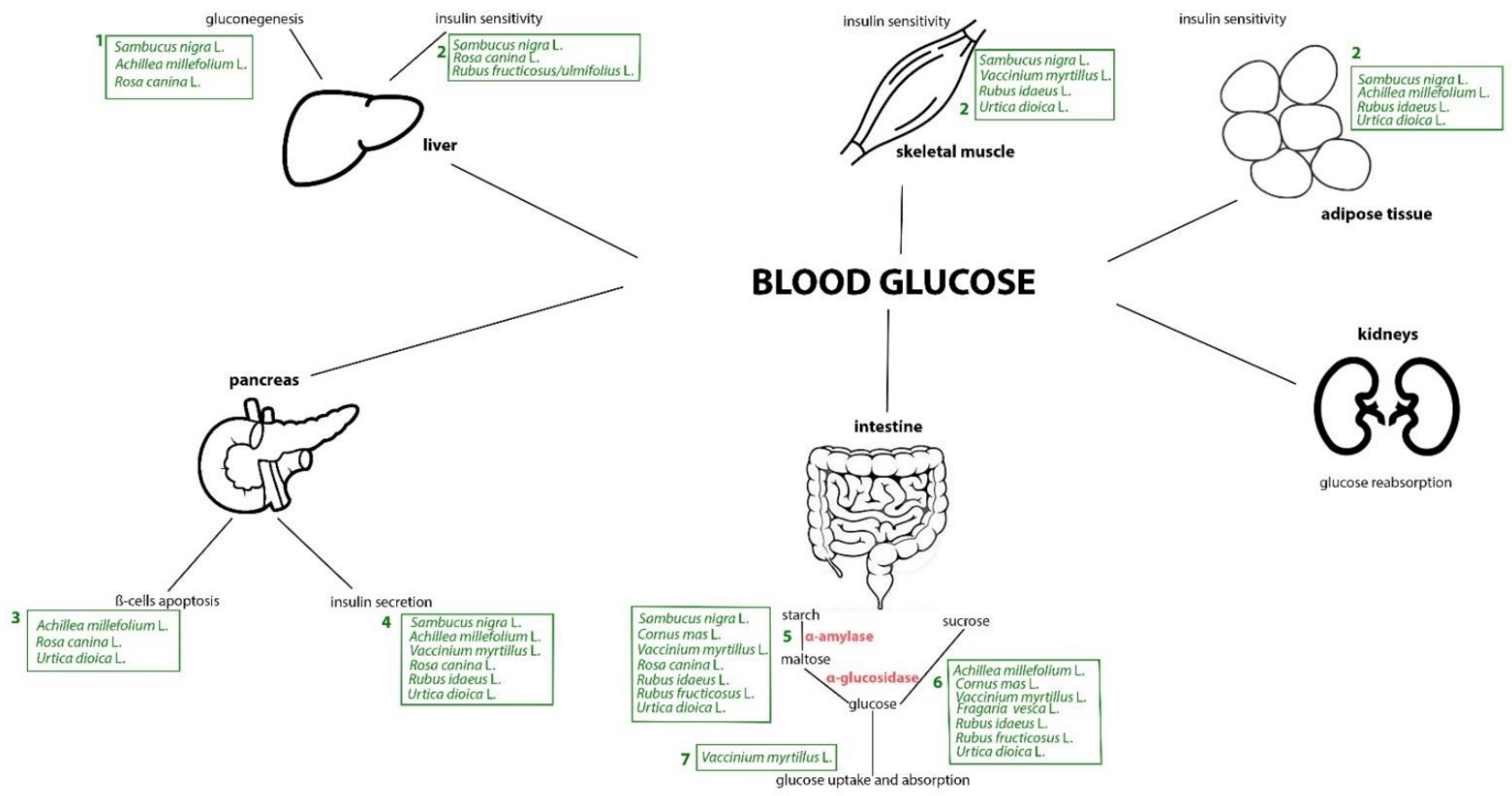
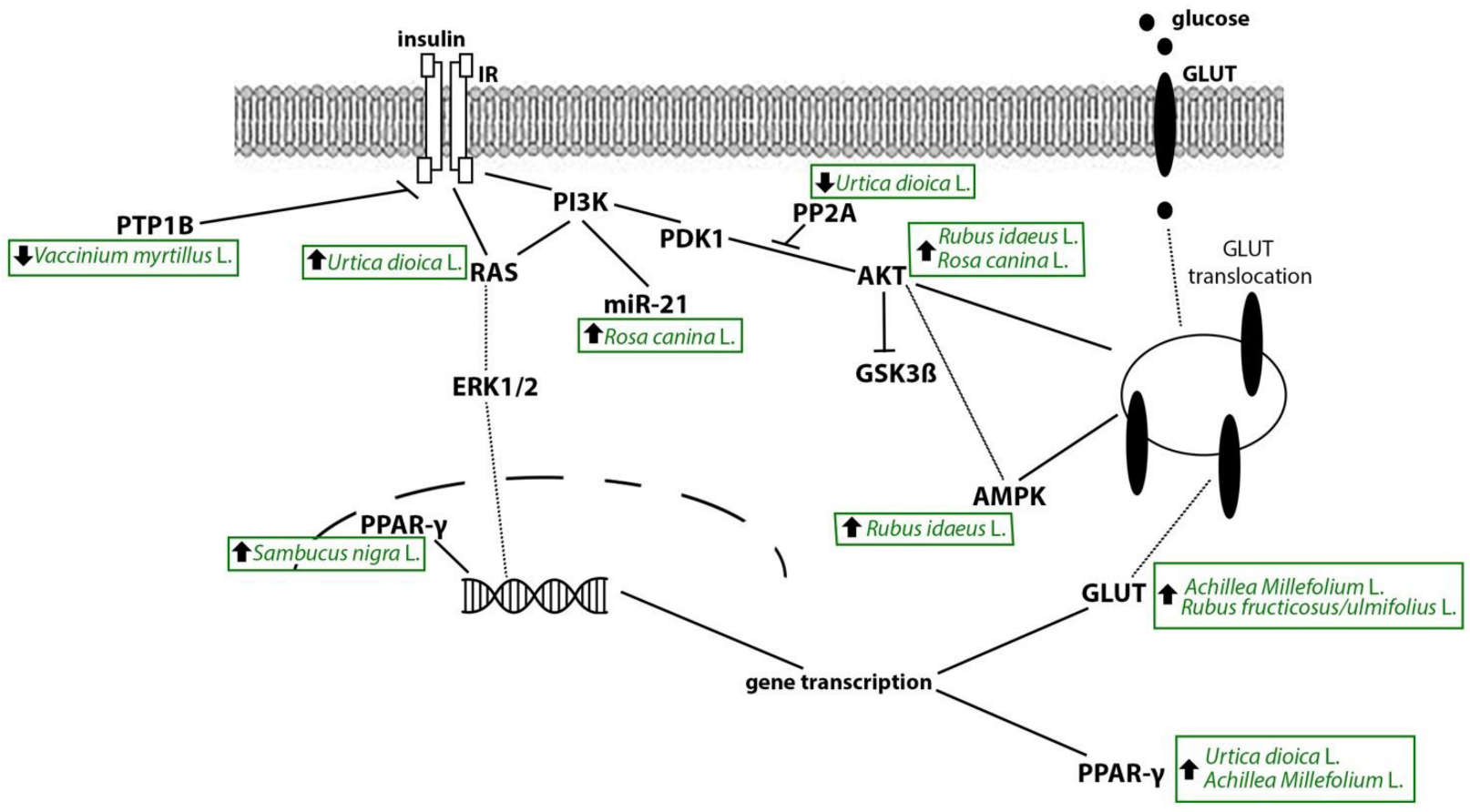
| Family | Botanical Name | Plant | Altitude (msl) |
|---|---|---|---|
| Adoxaceae | Sambucus nigra L. | Black elderberry | 0–1400 |
| Asteraceae | Achillea millefolium L. | Yarrow | 0–2500 |
| Cornaceae | Cornus mas L. | Cornelian cherry or dogwoods | 0–1500 |
| Ericaceae | Vaccinium myrtillus L. | Bilberry | 1200–2000 (rarely 300–2800) |
| Rosaceae | Fragaria vesca L. | Wild strawberry | 200–1900 (rarely up to 2400) |
| Rosaceae | Rosa canina L. | Rosehip or dog rose | 0–1900 |
| Rosaceae | Rubus fruticosus L., Rubus ulmifolius L. | Blackberry | 0–1400 |
| Rosaceae | Rubus idaeus L. | Raspberry | 200–2000 |
| Urticaceae | Urtica dioica L. | Stinging nettle | 0–1800 |
| Plant | Part of the Plant Tested | Mechanisms (Experimental Evidence) | References | |
|---|---|---|---|---|
| Formulation | Identified Active Ingredients | |||
| Urtica dioica L. | Leaves | insulin secretion (++) insulin sensitivity (++) α-glucosidase/α-amylase inhibition (+) | Ahangarpour et al., 2012 [91] Dar et al., 2013 [92] Kadan et al., 2013 [93] Qujeq et al., 2013 [94] Rahimzadeh et al., 2014 [95] Ranjbari et al., 2016 [96] Obanda et al., 2016 [97] Obanda et al., 2016 [98] Gohari et al., 2018 [99] Abedinzade et al., 2019 [100] Fan et al., 2020 [101] Salim et al., 2020 [102] | |
| H2O extract [92,94,95,96] | polyphenols (phenolic acids, flavonoids and anthocyanins) | |||
| Hexane extract [92] | terpenes, fatty acids [92] | |||
| Chloroform extract [92] | ||||
| Ethyl-acetate [92] | ||||
| MeOH [92] | ||||
| EtOH extracts [94] | ||||
| Hydroalcoholic extract [91] | ||||
| Leaves and stem | ||||
| Hydroalcoholic extract [93] | ||||
| Aerial parts | ||||
| Hydroalcoholic extract [100] | ||||
| powder [101] | ||||
| distillate [99] | ||||
| H2O extract [97,98] | ||||
| Achillea millefolium L. | Aerial parts | insulin secretion (++) insulin sensitivity (++) α-glucosidase inhibition (+) | Mustafa et al., 2012 [103] Ramirez et al., 2012 [104] Zolghadri et al., 2014 [77] Chávez-Silva et al., 2018 [105] | |
| H2O extract [103] | Tannins, glycosides, terpenoids, flavonoids and phenolics [103] | |||
| MeOH extract [103] | ||||
| Hydroalcoholic extract [77,104,105] | ||||
| Vaccinium myrtillus L. | Leaves with stems | insulin secretion (+/-) α-glucosidase inhibition (+) α-amylase inhibition (+/-) reduction of glucose absorption (+) | Brader et al., 2013 [106] Kim et al., 2015 [107] Asgary et al., 2016 [108] Buchholz & Melzig, 2016 [109] Bljajić et al., 2017 [110] Karcheva-Bahchevanska et al., 2017 [111] Xiao et al., 2017 [112] Schreck & Melzig, 2021 [113] | |
| H2O extract [110] | Polyphenols: phenolic acids > flavonoids [110] | |||
| Hydroalcoholic extract [110] | Polyphenols: flavonoids > phenolic acids [110] | |||
| Fruits | ||||
| MeOH:H2O:trifluoroacetic acid extract [106] | Polyphenols (anthocyanins, phenolic acids, flavonols) [106] | |||
| Hydroalcoholic extract [107,112] | Polyphenols (anthocyanins, flavonoids and phenolic acids) [112] Anthocyanins [107] | |||
| MeOH:H2O: HCl [111] | Polyphenols [111] | |||
| Acetone: H2O: HCl [111] | ||||
| H2O extract [109,111,113] | ||||
| MeOH extract [109,113] | ||||
| Powder [108] | ||||
| Rubus idaeus L. | Fruits | insulin sensitivity (+) α-glucosidases/α-amylase inhibition (+) | Zhu et al., 2018 [114] Xiong et al., 2018 [115] Zhao et al., 2018 [116] Xing et al., 2018 [117] Gutierrez-Albanchez et al., 2019 [118] | |
| Powder [114,116,117] | Polyphenols [114,116] | |||
| MeOH extract [118] | Polyphenols (anthocyanins) [118] | |||
| Rubus fruticosus L., Rubus ulmifolius L. | Fruits | insulin sensitivity (+/-) | Bispo et al., 2015 [119] Gowd et al., 2018 [46] | |
| MeOH extract [119] | ||||
| EtOH extract [46] | ||||
| Fragaria vesca L. | Leaves | α-glucosidase/α-amylase inhibition (+) | Takács et al., 2020 [120] | |
| H2O extract [120] | Flavonoids [120] | |||
| Sambucus nigra L. | Flowers | insulin sensitivity (+/-) α-glucosidase/α-amylase inhibition (+) | Schrader et al., 2012 [121] Bhattacharya et al., 2013 [122] Farrell et al., 2015 [123] Salvador et al., 2016 [124] Ho et al., 2017 [125] Ho et al., 2017 [126] Zielinska-Wasielica et al., 2019 [127] | |
| MeOH extract [121,122] | Polyphenols (phenolic acids, flavonoids) [122] Polyphenols [126] | |||
| DCM extract [122,126] | ||||
| EtOH extract [126] | ||||
| Hydroalcoholic extract [126] | ||||
| H2O extract [126] | ||||
| Fruits | ||||
| Anthocyanin-rich extract [123] | Anthocyanins [123] | |||
| DCM extract [125] | Polyphenols (anthocyanins and procyanidins) [125] | |||
| EtOH extract [125] | ||||
| Hydroalcoholic extract [125] | ||||
| H2O extract [125] | ||||
| pressed juice [125] | ||||
| H2O extract [127] | Polyphenols (anthocyanins, flavonoids, phenolic acids) [127] | |||
| Rose canina L. | Pseudo fruits and flowers | insulin secretion (++) insulin sensitivity (+/-) α-amylase inhibition (+) | Taghizadeh et al., 2016 [128] Fattahi et al., 2017 [129] Jemaa et al., 2017 [130] Chen et al., 2017 [131] Bahrami et al., 2020 [132] Rahimi et al., 2020 [133] | |
| MeOH extracts [130] | Flavonoids [130] | |||
| Pseudo fruits | ||||
| Oligosaccharide fraction of extract [132,133] | Oligosaccharides [132,133] | |||
| H2O extract [129,131] | ||||
| Hydroethanolic extract [128] | ||||
| Cornus mas L. | Fruits | insulin sensitivity (+/-) α-glucosidase/α-amylase inhibition (+) | Capcarova et al., 2019 [134] Dzydzan et al., 2019 [135] Dzydzan et al., 2020 [136] Blagojevic et al., 2021 [137] | |
| Pressed juice [25,136] | Polyphenols (anthocyanins, phenolic acids, flavonols) and iridoids (loganic acid) [135] Iridoids [136] | |||
| Hydroalcoholic extract [137] | Iridoids and anthocyanins [137] | |||
| Homogenized [134] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscaro, V.; Rivoira, M.; Sgorbini, B.; Bordano, V.; Dadone, F.; Gallicchio, M.; Pons, A.; Benetti, E.; Rosa, A.C. Evidence-Based Anti-Diabetic Properties of Plant from the Occitan Valleys of the Piedmont Alps. Pharmaceutics 2022, 14, 2371. https://doi.org/10.3390/pharmaceutics14112371
Boscaro V, Rivoira M, Sgorbini B, Bordano V, Dadone F, Gallicchio M, Pons A, Benetti E, Rosa AC. Evidence-Based Anti-Diabetic Properties of Plant from the Occitan Valleys of the Piedmont Alps. Pharmaceutics. 2022; 14(11):2371. https://doi.org/10.3390/pharmaceutics14112371
Chicago/Turabian StyleBoscaro, Valentina, Matteo Rivoira, Barbara Sgorbini, Valentina Bordano, Francesca Dadone, Margherita Gallicchio, Aline Pons, Elisa Benetti, and Arianna Carolina Rosa. 2022. "Evidence-Based Anti-Diabetic Properties of Plant from the Occitan Valleys of the Piedmont Alps" Pharmaceutics 14, no. 11: 2371. https://doi.org/10.3390/pharmaceutics14112371
APA StyleBoscaro, V., Rivoira, M., Sgorbini, B., Bordano, V., Dadone, F., Gallicchio, M., Pons, A., Benetti, E., & Rosa, A. C. (2022). Evidence-Based Anti-Diabetic Properties of Plant from the Occitan Valleys of the Piedmont Alps. Pharmaceutics, 14(11), 2371. https://doi.org/10.3390/pharmaceutics14112371








