Searching for the Optimal Donor for Allogenic Adipose-Derived Stem Cells: A Comprehensive Review
Abstract
1. Introduction
- Donor-to-recipient critical steps
- Harvesting
- Processing
- ○
- Initial manipulation
- ○
- Culture and expansion
- ○
- Storage
- ○
- Package and transportation
- Handling and application
- Donor factors
- Donor characteristics
- Donor tissue type and site
2. Donor-to-Recipient Critical Steps
3. Donor Factors
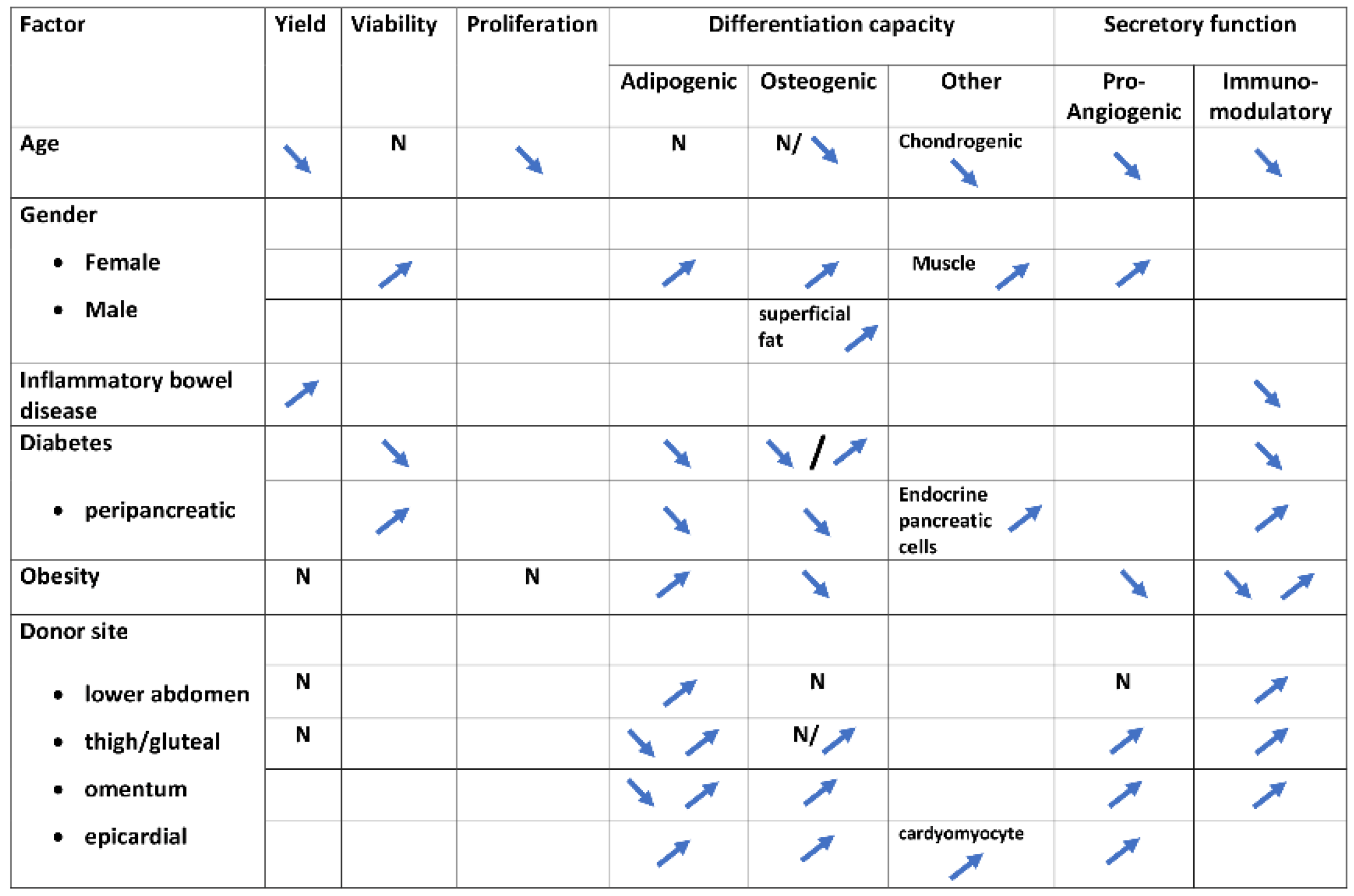
3.1. Age
3.2. Gender
3.3. Immune Conditions
3.4. Diabetes
3.5. Obesity
3.6. Lifestyle Habits
3.7. Donor Site
4. Discussion
4.1. Donor Selection
4.2. Preconditioning
5. Limitations of the Study
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, K.; Zvonic, S.; Garrett, S.; Mitchell, J.B.; Floyd, Z.E.; Hammill, L.; Kloster, A.; Di Halvorsen, Y.; Ting, J.P.; Storms, R.W.; et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 2006, 24, 1246–1253. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell. Biosci. 2021, 11, 187. [Google Scholar] [CrossRef]
- Harris, D.T. Banking of Adipose- and Cord Tissue-Derived Stem Cells: Technical and Regulatory Issues. Adv. Exp. Med. Biol. 2016, 951, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Ezzoubi, M.; Malka, G. Overview of current adipose-derived stem cell (ADSCs) processing involved in therapeutic advancements: Flow chart and regulation updates before and after COVID-19. Stem Cell Res. Ther. 2021, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santos, M.E.; Garcia-Arranz, M.; Andreu, E.J.; García-Hernández, A.M.; López-Parra, M.; Villarón, E.; Sepúlveda, P.; Fernández-Avilés, F.; García-Olmo, D.; Prosper, F.; et al. Optimization of Mesenchymal Stromal Cell (MSC) Manufacturing Processes for a Better Therapeutic Outcome. Front. Immunol. 2022, 13, 918565. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.C.; Semon, J.A.; Kaushal, D.; O’Sullivan, R.P.; Glowacki, J.; Gimble, J.M.; Bunnell, B.A. MicroRNA profiling reveals age-dependent differential expression of nuclear factor κB and mitogen-activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Res. Ther. 2011, 2, 49. [Google Scholar] [CrossRef] [PubMed]
- Scruggs, B.A.; Semon, J.A.; Zhang, X.; Zhang, S.; Bowles, A.C.; Pandey, A.C.; Imhof, K.M.; Kalueff, A.V.; Gimble, J.M.; Bunnell, B.A. Age of the donor reduces the ability of human adipose-derived stem cells to alleviate symptoms in the experimental autoimmune encephalomyelitis mouse model. Stem Cells Transl. Med. 2013, 2, 797–807. [Google Scholar] [CrossRef]
- Efimenko, A.; Dzhoyashvili, N.; Kalinina, N.; Kochegura, T.; Akchurin, R.; Tkachuk, V.; Parfyonova, Y. Adipose-derived mesenchymal stromal cells from aged patients with coronary artery disease keep mesenchymal stromal cell properties but exhibit characteristics of aging and have impaired angiogenic potential. Stem Cells Transl. Med. 2014, 3, 32–41. [Google Scholar] [CrossRef]
- Madonna, R.; Renna, F.V.; Cellini, C.; Cotellese, R.; Picardi, N.; Francomano, F.; Innocenti, P.; De Caterina, R. Age-dependent impairment of number and angiogenic potential of adipose tissue-derived progenitor cells. Eur. J. Clin. Investig. 2011, 41, 126–133. [Google Scholar] [CrossRef]
- Kornicka, K.; Marycz, K.; Tomaszewski, K.A.; Marędziak, M.; Śmieszek, A. The Effect of Age on Osteogenic and Adipogenic Differentiation Potential of Human Adipose Derived Stromal Stem Cells (hASCs) and the Impact of Stress Factors in the Course of the Differentiation Process. Oxid. Med. Cell. Longev. 2015, 2015, 309169. [Google Scholar] [CrossRef] [PubMed]
- Choudhery, M.S.; Badowski, M.; Muise, A.; Pierce, J.; Harris, D.T. Donor age negatively impacts adipose tissue-derived mesenchymal stem cell expansion and differentiation. J. Transl. Med. 2014, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lei, H.; Dong, P.; Fu, X.; Yang, Z.; Yang, Y.; Ma, J.; Liu, X.; Cao, Y.; Xiao, R. Adipose-Derived Mesenchymal Stem Cells from the Elderly Exhibit Decreased Migration and Differentiation Abilities with Senescent Properties. Cell. Transplant. 2017, 26, 1505–1519. [Google Scholar] [CrossRef] [PubMed]
- de Girolamo, L.; Lopa, S.; Arrigoni, E.; Sartori, M.F.; Baruffaldi Preis, F.W.; Brini, A.T. Human adipose-derived stem cells isolated from young and elderly women: Their differentiation potential and scaffold interaction during in vitro osteoblastic differentiation. Cytotherapy 2009, 11, 793–803. [Google Scholar] [CrossRef]
- Horinouchi, C.D.; Barisón, M.J.; Robert, A.W.; Kuligovski, C.; Aguiar, A.M.; Dallagiovanna, B. Influence of donor age on the differentiation and division capacity of human adipose-derived stem cells. World J. Stem Cells 2020, 12, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Niklason, L.; Steinbacher, D.M. The effect of age on human adipose-derived stem cells. Plast. Reconstr. Surg. 2013, 131, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Z.; Zhao, Y.; Zhang, L.; Xu, L.; Cao, L.; He, W. The Effect of Age on the Regenerative Potential of Human Eyelid Adipose-Derived Stem Cells. Stem Cells Int. 2018, 2018, 5654917. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, G.; Hong, H.S. Age affects the paracrine activity and differentiation potential of human adipose-derived stem cells. Mol. Med. Rep. 2021, 23, 160. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, E.; Casadei, R.; Frabetti, F.; Ventura, C.; Facchin, F.; Canaider, S. Sex-Specific Transcriptome Differences in Human Adipose Mesenchymal Stem Cells. Genes 2020, 11, 909. [Google Scholar] [CrossRef] [PubMed]
- McKinnirey, F.; Herbert, B.; Vesey, G.; McCracken, S. Immune modulation via adipose derived Mesenchymal Stem cells is driven by donor sex in vitro. Sci. Rep. 2021, 11, 12454. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Mizuno, H.; Watanabe, A.; Migita, M.; Hyakusoku, H.; Shimada, T. Adipogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice-including relationship of sex differences. Biochem. Biophys. Res. Commun. 2004, 319, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Shu, Y.T.; Dai, C.Y.; Zhen, Q.Z. Comparing the biological characteristics of adipose tissue-derived stem cells of different persons. J. Cell. Biochem. 2012, 113, 2020–2026. [Google Scholar] [CrossRef]
- Aksu, A.E.; Rubin, J.P.; Dudas, J.R.; Marra, K.G. Role of gender and anatomical region on induction of osteogenic differentiation of human adipose-derived stem cells. Ann. Plast. Surg. 2008, 60, 306–322. [Google Scholar] [CrossRef]
- Mizushima, T.; Fukata, T.; Takeyama, H.; Takahashi, H.; Haraguchi, N.; Nishimura, J.; Hata, T.; Matsuda, C.; Yamamoto, H.; Doki, Y.; et al. The features of adipose-derived stem cells in patients with inflammatory bowel diseases. Surg. Today 2018, 48, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Keiran, N.; Madeira, A.; Maymó-Masip, E.; Ejarque, M.; Terrón-Puig, M.; Espin, E.; Martí, M.; Borruel, N.; Guarner, F.; et al. Crohn’s Disease Disturbs the Immune Properties of Human Adipose-Derived Stem Cells Related to Inflammasome Activation. Stem Cell Rep. 2017, 9, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Mu, Y.; Yao, J.; Lin, F.; Wu, D.; Ma, Z. Adipose-Derived Stem Cells From Patients With Ulcerative Colitis Exhibit Impaired Immunosuppressive Function. Front. Cell Dev. Biol. 2022, 10, 822772. [Google Scholar] [CrossRef] [PubMed]
- Serena, C.; Keiran, N.; Ceperuelo-Mallafre, V.; Ejarque, M.; Fradera, R.; Roche, K.; Nuñez-Roa, C.; Vendrell, J.; Fernández-Veledo, S. Obesity and Type 2 Diabetes Alters the Immune Properties of Human Adipose Derived Stem Cells. Stem Cells 2016, 34, 2559–2573. [Google Scholar] [CrossRef] [PubMed]
- Alicka, M.; Major, P.; Wysocki, M.; Marycz, K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced "Stemness" through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. J. Clin. Med. 2019, 8, 765. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, L.; Liang, X.; Zou, J.; Liu, N.; Liu, T.; Wang, G.; Ding, X.; Liu, Y.; Zhang, B.; et al. Adipose Tissue-Derived Stem Cells from Type 2 Diabetics Reveal Conservative Alterations in Multidimensional Characteristics. Int. J. Stem Cells 2020, 13, 268–278. [Google Scholar] [CrossRef]
- Skubis-Sikora, A.; Sikora, B.; Witkowska, A.; Mazurek, U.; Gola, J. Osteogenesis of adipose-derived stem cells from patients with glucose metabolism disorders. Mol. Med. 2020, 26, 67. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Faustini, M.; Bucco, M.; Chlapanidas, T.; Lucconi, G.; Marazzi, M.; Tosca, M.C.; Gaetani, P.; Klinger, M.; Villani, S.; Ferretti, V.V.; et al. Nonexpanded mesenchymal stem cells for regenerative medicine: Yield in stromal vascular fraction from adipose tissues. Tissue Eng. Part C Methods 2010, 16, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Mojallal, A.; Lequeux, C.; Shipkov, C.; Duclos, A.; Braye, F.; Rohrich, R.; Brown, S.; Damour, O. Influence of age and body mass index on the yield and proliferation capacity of adipose-derived stem cells. Aesthetic Plast. Surg. 2011, 35, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Cubedo, J. Adipose tissue depots and inflammation: Effects on plasticity and resident mesenchymal stem cell function. Cardiovasc. Res. 2017, 113, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Roldan, M.; Macias-Gonzalez, M.; Garcia, R.; Tinahones, F.J.; Martin, M. Obesity short-circuits stemness gene network in human adipose multipotent stem cells. FASEB J. 2011, 25, 4111–4126. [Google Scholar] [CrossRef] [PubMed]
- Oñate, B.; Vilahur, G.; Ferrer-Lorente, R.; Ybarra, J.; Díez-Caballero, A.; Ballesta-López, C.; Moscatiello, F.; Herrero, J.; Badimon, L. The subcutaneous adipose tissue reservoir of functionally active stem cells is reduced in obese patients. FASEB J. 2012, 26, 4327–4336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Klomjit, N.; Conley, S.M.; Ostlie, M.M.; Jordan, K.L.; Lerman, A.; Lerman, L.O. Impaired immunomodulatory capacity in adipose tissue-derived mesenchymal stem/stromal cells isolated from obese patients. J. Cell. Mol. Med. 2021, 25, 9051–9059. [Google Scholar] [CrossRef] [PubMed]
- Juntunen, M.; Heinonen, S.; Huhtala, H.; Rissanen, A.; Kaprio, J.; Kuismanen, K.; Pietiläinen, K.H.; Miettinen, S.; Patrikoski, M. Evaluation of the effect of donor weight on adipose stromal/stem cell characteristics by using weight-discordant monozygotic twin pairs. Stem Cell Res. Ther. 2021, 12, 516. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.A.A.; Wise, R.M.; Benjamin, B.P.; Hochreiner, E.M.; Mohiuddin, O.A.; Bunnell, B.A. Adipose-Derived Stem Cells from Obese Donors Polarize Macrophages and Microglia toward a Pro-Inflammatory Phenotype. Cells 2020, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Meng, Y.; Zhu, X.Y.; Li, Y.; Saadiq, I.M.; Jordan, K.L.; Tang, H.; Lerman, A.; van Wijnen, A.J.; Lerman, L.O. The Micro-RNA Cargo of Extracellular Vesicles Released by Human Adipose Tissue-Derived Mesenchymal Stem Cells Is Modified by Obesity. Front. Cell. Dev. Biol. 2021, 9, 660851. [Google Scholar] [CrossRef] [PubMed]
- De Girolamo, L.; Stanco, D.; Salvatori, L.; Coroniti, G.; Arrigoni, E.; Silecchia, G.; Russo, M.A.; Niada, S.; Petrangeli, E.; Brini, A.T. Stemness and osteogenic and adipogenic potential are differently impaired in subcutaneous and visceral adipose derived stem cells (ASCs) isolated from obese donors. Int. J. Immunopathol. Pharmacol. 2013, 26, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Oliva-Olivera, W.; Leiva Gea, A.; Lhamyani, S.; Coín-Aragüez, L.; Alcaide Torres, J.; Bernal-López, M.R.; García-Luna, P.P.; Morales Conde, S.; Fernández-Veledo, S.; El Bekay, R.; et al. Differences in the Osteogenic Differentiation Capacity of Omental Adipose-Derived Stem Cells in Obese Patients With and Without Metabolic Syndrome. Endocrinology 2015, 156, 4492–4501. [Google Scholar] [CrossRef] [PubMed]
- Strong, A.L.; Hunter, R.S.; Jones, R.B.; Bowles, A.C.; Dutreil, M.F.; Gaupp, D.; Hayes, D.J.; Gimble, J.M.; Levi, B.; McNulty, M.A.; et al. Obesity inhibits the osteogenic differentiation of human adipose-derived stem cells. J. Transl. Med. 2016, 14, 27. [Google Scholar] [CrossRef]
- Shaito, A.; Saliba, J.; Husari, A.; El-Harakeh, M.; Chhouri, H.; Hashem, Y.; Shihadeh, A.; El-Sabban, M. Electronic Cigarette Smoke Impairs Normal Mesenchymal Stem Cell Differentiation. Sci. Rep. 2017, 7, 14281. [Google Scholar] [CrossRef] [PubMed]
- Aspera-Werz, R.H.; Ehnert, S.; Müller, M.; Zhu, S.; Chen, T.; Weng, W.; Jacoby, J.; Nussler, A.K. Assessment of tobacco heating system 2.4 on osteogenic differentiation of mesenchymal stem cells and primary human osteoblasts compared to conventional cigarettes. World. J. Stem Cells 2020, 12, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Alpagot, T.; Oh, H.; Ojcius, D.; Xiao, N. Comparison of the effect of cigarette smoke on mesenchymal stem cells and dental stem cells. Am. J. Physiol. Cell. Physiol. 2021, 320, C175–C181. [Google Scholar] [CrossRef]
- Aspera-Werz, R.H.; Chen, T.; Ehnert, S.; Zhu, S.; Fröhlich, T.; Nussler, A.K. Cigarette Smoke Induces the Risk of Metabolic Bone Diseases: Transforming Growth Factor Beta Signaling Impairment via Dysfunctional Primary Cilia Affects Migration, Proliferation, and Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2019, 20, 2915. [Google Scholar] [CrossRef]
- Di Rocco, G.; Baldari, S.; Pani, G.; Toietta, G. Stem cells under the influence of alcohol: Effects of ethanol consumption on stem/progenitor cells. Cell. Mol. Life. Sci. 2019, 76, 231–244. [Google Scholar] [CrossRef]
- Varlamov, O.; Bucher, M.; Myatt, L.; Newman, N.; Grant, K.A. Daily Ethanol Drinking Followed by an Abstinence Period Impairs Bone Marrow Niche and Mitochondrial Function of Hematopoietic Stem/Progenitor Cells in Rhesus Macaques. Alcohol. Clin. Exp. Res. 2020, 44, 1088–1098. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Li, Y.; Sun, J.; Zhao, G. The effect of combined regulation of the expression of peroxisome proliferator-activated receptor-γ and calcitonin gene-related peptide on alcohol-induced adipogenic differentiation of bone marrow mesenchymal stem cells. Mol. Cell. Biochem. 2014, 392, 39–48. [Google Scholar] [CrossRef]
- Padoin, A.V.; Braga-Silva, J.; Martins, P.; Rezende, K.; Rezende, A.; Grechi, B.; Gehlen, D.; Machado, D.C. Sources of processed lipoaspirate cells: Influence of donor site on cell concentration. Plast. Reconstr. Surg. 2008, 122, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Geissler, P.J.; Davis, K.; Roostaeian, J.; Unger, J.; Huang, J.; Rohrich, R.J. Improving fat transfer viability: The role of aging, body mass index, and harvest site. Plast. Reconstr. Surg. 2014, 134, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, W.J.; Oedayrajsingh-Varma, M.J.; Helder, M.N.; Zandiehdoulabi, B.; Schouten, T.E.; Kuik, D.J.; Ritt, M.J.; van Milligen, F.J. Effect of tissue-harvesting site on yield of stem cells derived from adipose tissue: Implications for cell-based therapies. Cell Tissue Res. 2008, 332, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Tsekouras, A.; Mantas, D.; Tsilimigras, D.I.; Moris, D.; Kontos, M.; Zografos, G.C. Comparison of the Viability and Yield of Adipose-Derived Stem Cells (ASCs) from Different Donor Areas. In Vivo 2017, 31, 1229–1234. [Google Scholar] [CrossRef]
- Levi, B.; James, A.W.; Glotzbach, J.P.; Wan, D.C.; Commons, G.W.; Longaker, M.T. Depot-specific variation in the osteogenic and adipogenic potential of human adipose-derived stromal cells. Plast. Reconstr. Surg. 2010, 126, 822–834. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Chen, C.; Tian, W.; Wang, H. Increased Angiogenic and Adipogenic Differentiation Potentials in Adipose-Derived Stromal Cells from Thigh Subcutaneous Adipose Depots Compared with Cells from the Abdomen. Aesthet. Surg. J. 2019, 39, NP140–NP149. [Google Scholar] [CrossRef]
- Iwen, K.A.; Priewe, A.C.; Winnefeld, M.; Rose, C.; Siemers, F.; Rohwedel, J.; Cakiroglu, F.; Lehnert, H.; Schepky, A.; Klein, J.; et al. Gluteal and abdominal subcutaneous adipose tissue depots as stroma cell source: Gluteal cells display increased adipogenic and osteogenic differentiation potentials. Exp. Dermatol. 2014, 23, 395–400. [Google Scholar] [CrossRef]
- Di Taranto, G.; Cicione, C.; Visconti, G.; Isgrò, M.A.; Barba, M.; Di Stasio, E.; Stigliano, E.; Bernardini, C.; Michetti, F.; Salgarello, M.; et al. Qualitative and quantitative differences of adipose-derived stromal cells from superficial and deep subcutaneous lipoaspirates: A matter of fat. Cytotherapy 2015, 17, 1076–1089. [Google Scholar] [CrossRef]
- Schipper, B.M.; Marra, K.G.; Zhang, W.; Donnenberg, A.D.; Rubin, J.P. Regional anatomic and age effects on cell function of human adipose-derived stem cells. Ann. Plast. Surg. 2008, 60, 538–544. [Google Scholar] [CrossRef]
- Siciliano, C.; Bordin, A.; Ibrahim, M.; Chimenti, I.; Cassiano, F.; Gatto, I.; Mangino, G.; Coccia, A.; Miglietta, S.; Bastianelli, D.; et al. The adipose tissue of origin influences the biological potential of human adipose stromal cells isolated from mediastinal and subcutaneous fat depots. Stem Cell Res. 2016, 17, 342–351. [Google Scholar] [CrossRef][Green Version]
- Wystrychowski, W.; Patlolla, B.; Zhuge, Y.; Neofytou, E.; Robbins, R.C.; Beygui, R.E. Multipotency and cardiomyogenic potential of human adipose-derived stem cells from epicardium, pericardium, and omentum. Stem Cell Res. Ther. 2016, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Yu, C.; Belliveau, P.; Hamilton, A.; Flynn, L.E. Comparison of human adipose-derived stem cells isolated from subcutaneous, omental, and intrathoracic adipose tissue depots for regenerative applications. Stem Cells Transl. Med. 2014, 3, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Lee, B.; Kim, M.K.; Gong, S.P.; Park, N.H.; Chung, H.H.; Kim, H.S.; No, J.H.; Park, W.Y.; Park, A.K.; et al. Gene expression profiles of human subcutaneous and visceral adipose-derived stem cells. Cell Biochem. Funct. 2016, 34, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.S.; Li, J.; Dietrich, M.; Wu, X.; Hausmann, M.G.; LeBlanc, K.A.; Wade, J.W.; Gimble, J.M. Comparison of Stromal/Stem Cells Isolated from Human Omental and Subcutaneous Adipose Depots: Differentiation and Immunophenotypic Characterization. Cells Tissues Organs 2014, 200, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Tencerova, M.; Lundby, L.; Buntzen, S.; Norderval, S.; Hougaard, H.T.; Pedersen, B.G.; Kassem, M. Molecular differences of adipose-derived mesenchymal stem cells between non-responders and responders in treatment of transphincteric perianal fistulas. Stem Cell Res. Ther. 2021, 12, 586. [Google Scholar] [CrossRef]
- Widholz, B.; Tsitlakidis, S.; Reible, B.; Moghaddam, A.; Westhauser, F. Pooling of Patient-Derived Mesenchymal Stromal Cells Reduces Inter-Individual Confounder-Associated Variation without Negative Impact on Cell Viability, Proliferation and Osteogenic Differentiation. Cells 2019, 8, 633. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Hou, Q.; Zhao, Y.; Zhong, L.; Fu, X. Potential pre-activation strategies for improving therapeutic efficacy of mesenchymal stem cells: Current status and future prospects. Stem Cell Res. Ther. 2022, 13, 146. [Google Scholar] [CrossRef]
- Mitterberger, M.C.; Mattesich, M.; Zwerschke, W. Bariatric surgery and diet-induced long-term caloric restriction protect subcutaneous adipose-derived stromal/progenitor cells and prolong their life span in formerly obese humans. Exp. Gerontol. 2014, 56, 106–113. [Google Scholar] [CrossRef]
|
|
|
|
|
|
| Rules and Guidelines | Webpage/Link |
|---|---|
| https://health.ec.europa.eu/system/files/2017-11/2017_11_22_guidelines_gmp_for_atmps_0.pdf (accessed on 1 September 2022) |
| https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-5-d-derivation-characterisation-cell-substrates-used-production-biotechnological/biological-products-step-5_en.pdf (accessed on 1 September 2022) |
| https://www.ema.europa.eu/en/documents/scientific-guideline/ich-topic-q-5-c-quality-biotechnological-products-stability-testing-biotechnological/biological-products_en.pdf (accessed on 1 September 2022) |
| https://permanent.fdlp.gov/LPS111884/LPS111884_gtindcmc.pdf (accessed on 1 September 2022) |
| https://www.fda.gov/media/73624/download (accessed on 1 September 2022) |
| https://www.fda.gov/media/79856/download (accessed on 1 September 2022) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgiev-Hristov, T.; García-Arranz, M.; Trébol-López, J.; Barba-Recreo, P.; García-Olmo, D. Searching for the Optimal Donor for Allogenic Adipose-Derived Stem Cells: A Comprehensive Review. Pharmaceutics 2022, 14, 2338. https://doi.org/10.3390/pharmaceutics14112338
Georgiev-Hristov T, García-Arranz M, Trébol-López J, Barba-Recreo P, García-Olmo D. Searching for the Optimal Donor for Allogenic Adipose-Derived Stem Cells: A Comprehensive Review. Pharmaceutics. 2022; 14(11):2338. https://doi.org/10.3390/pharmaceutics14112338
Chicago/Turabian StyleGeorgiev-Hristov, Tihomir, Mariano García-Arranz, Jacobo Trébol-López, Paula Barba-Recreo, and Damián García-Olmo. 2022. "Searching for the Optimal Donor for Allogenic Adipose-Derived Stem Cells: A Comprehensive Review" Pharmaceutics 14, no. 11: 2338. https://doi.org/10.3390/pharmaceutics14112338
APA StyleGeorgiev-Hristov, T., García-Arranz, M., Trébol-López, J., Barba-Recreo, P., & García-Olmo, D. (2022). Searching for the Optimal Donor for Allogenic Adipose-Derived Stem Cells: A Comprehensive Review. Pharmaceutics, 14(11), 2338. https://doi.org/10.3390/pharmaceutics14112338







