Evaluation of Adjuvant Activity and Bio-Distribution of Archaeosomes Prepared Using Microfluidic Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Archaeosomes Preparation by Thin-Film Hydration Method
2.2. Orthogonal Array Experimental Design on Preparation of Archaeosomes by Microfluidic Mixing
2.3. Archaeosome Characterization
2.4. Animals
2.5. Vaccine Immunization
2.6. Anti-OVA Antibody ELISA
2.7. ELISpot Assay
2.8. In Vivo Cytolytic Activity
2.9. Fluorescence Imaging
2.10. Statistical Analysis
3. Results
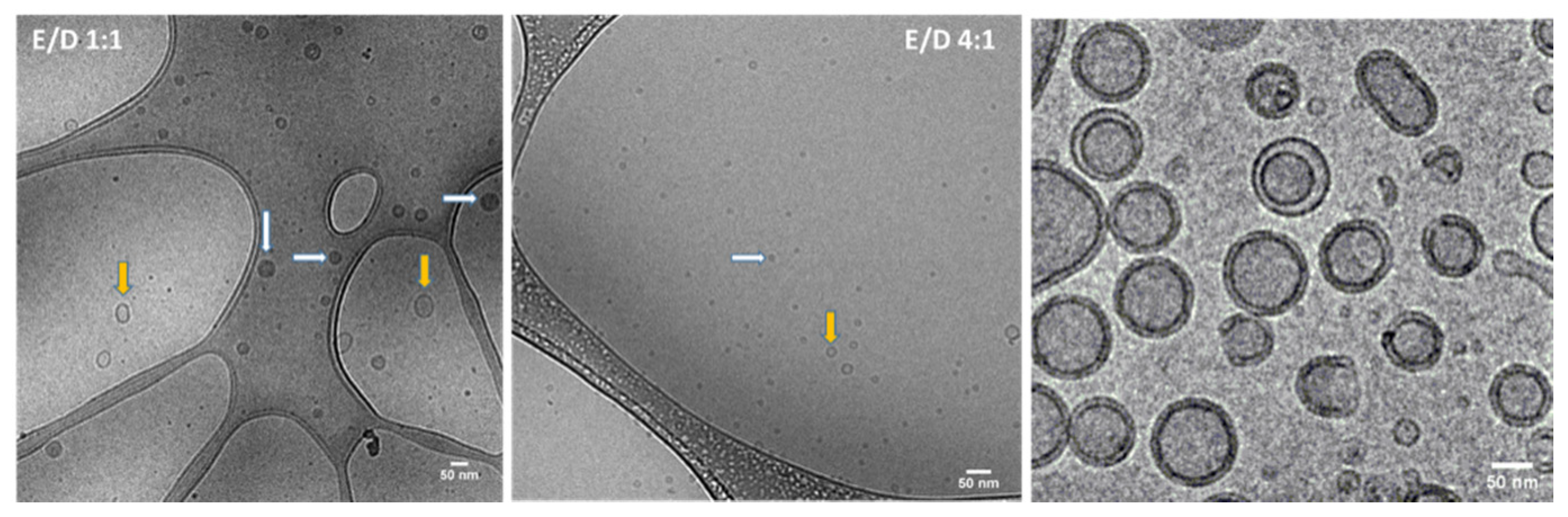
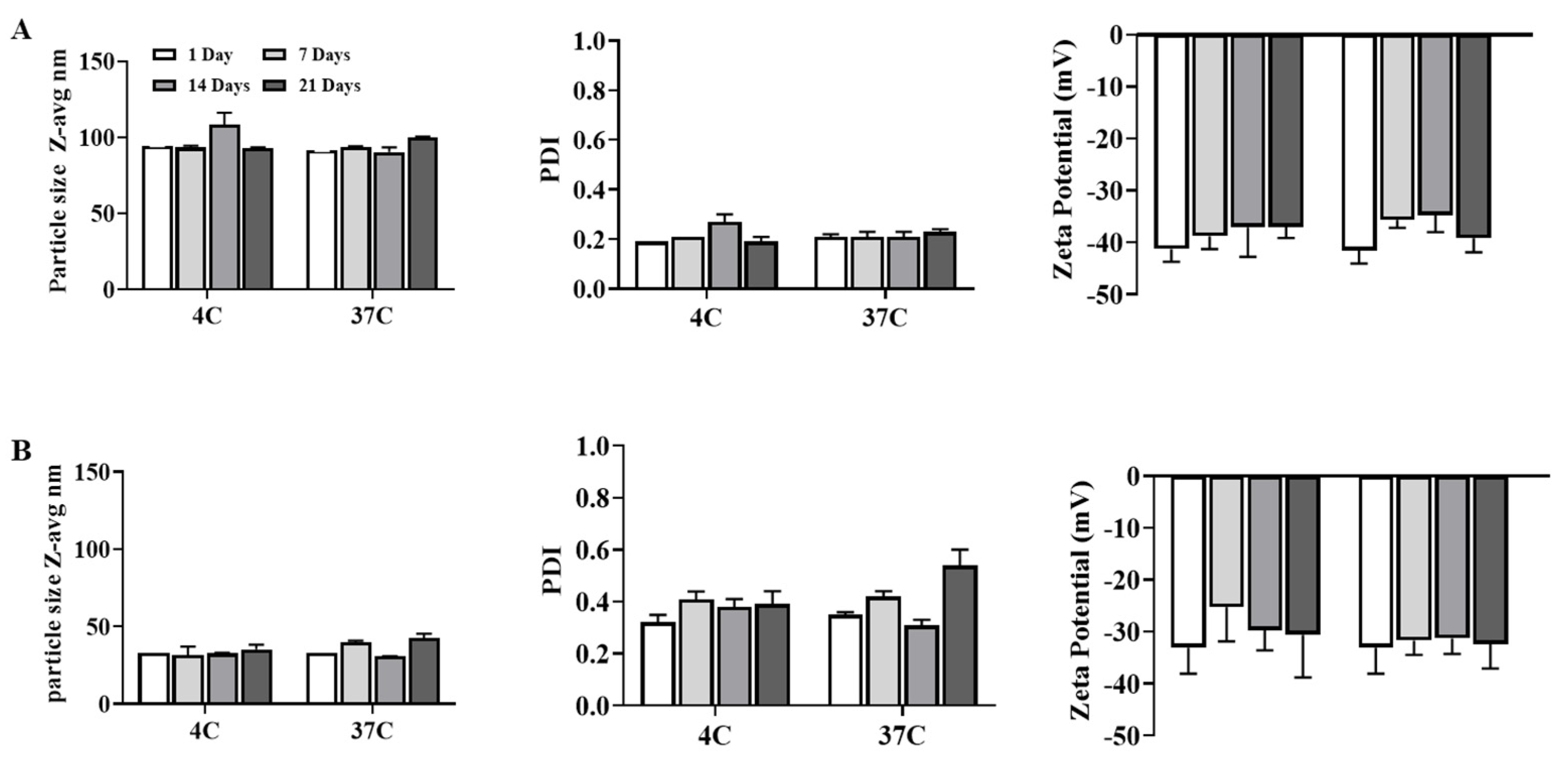
3.1. Generation and Characterization of Archaeosomes Utilizing an Orthogonal Experimental Design Approach
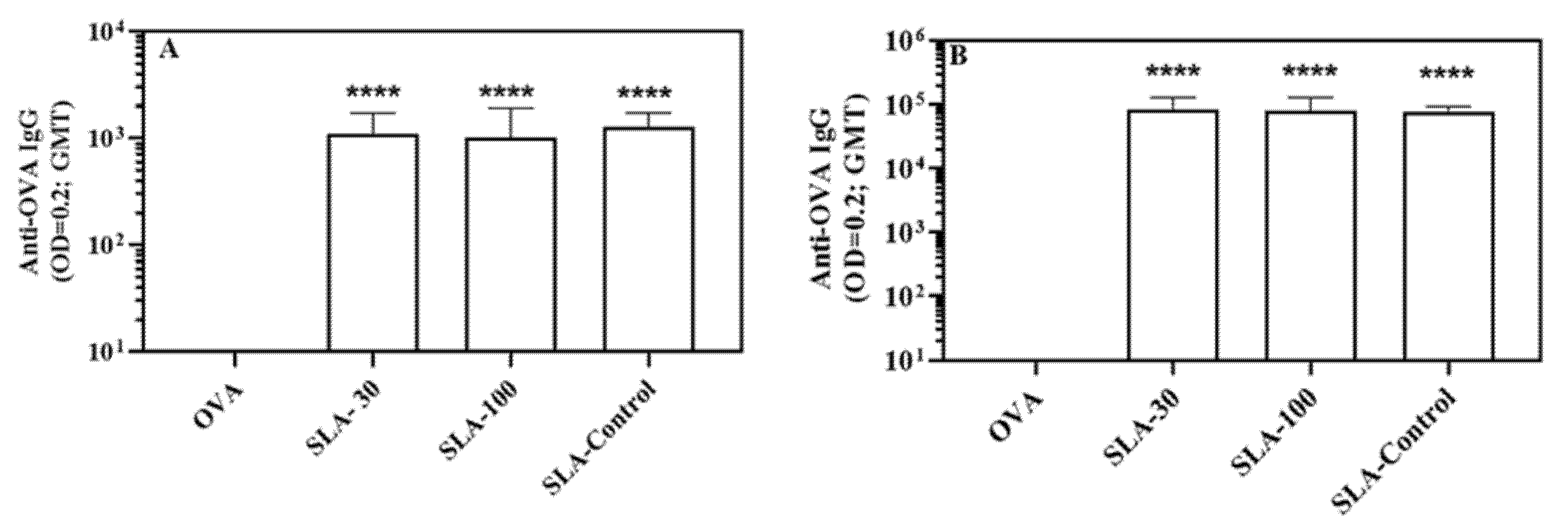
3.2. Induction of Antigen-Specific Antibody Responses by SLA Archaeosomes
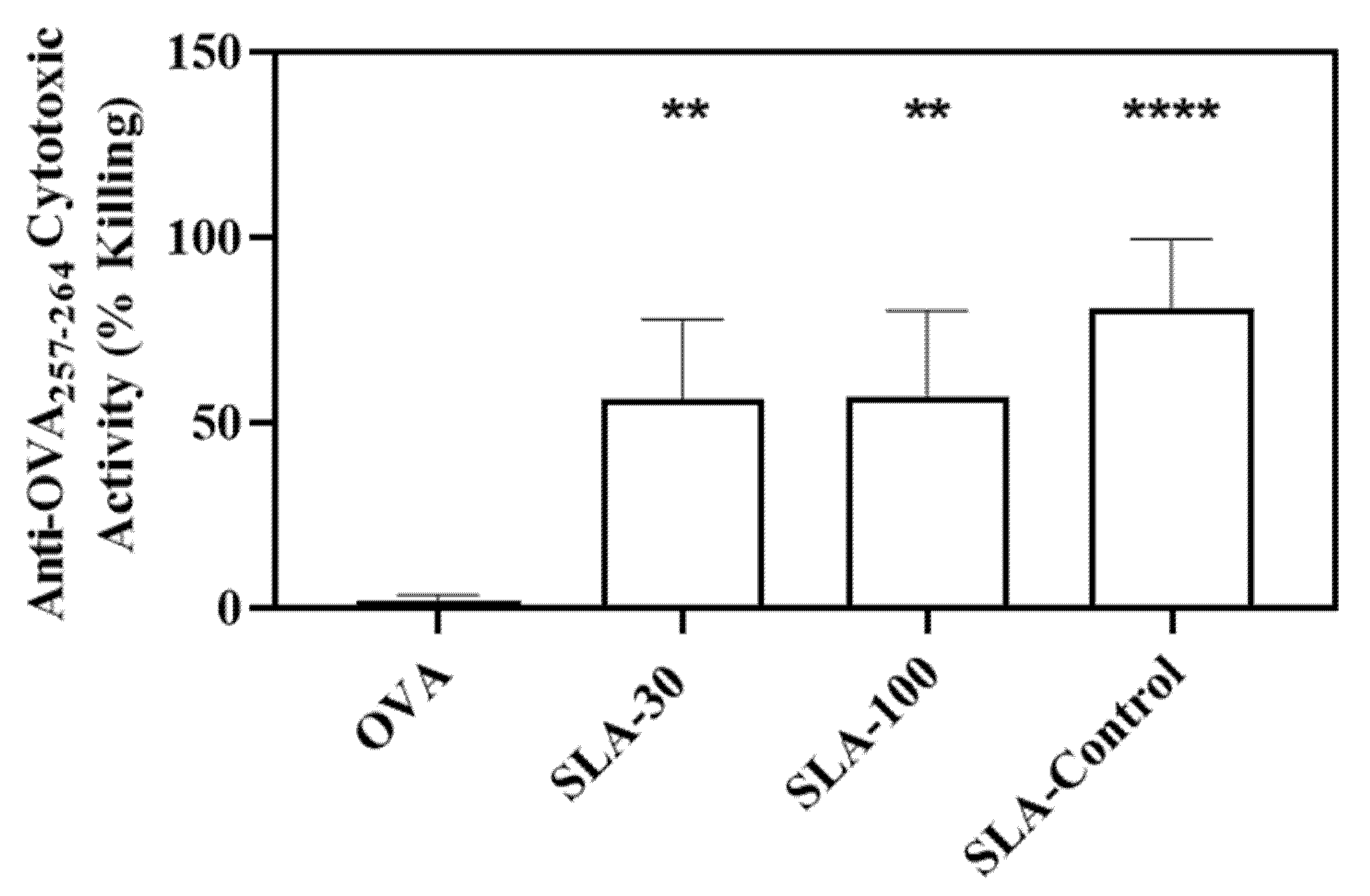
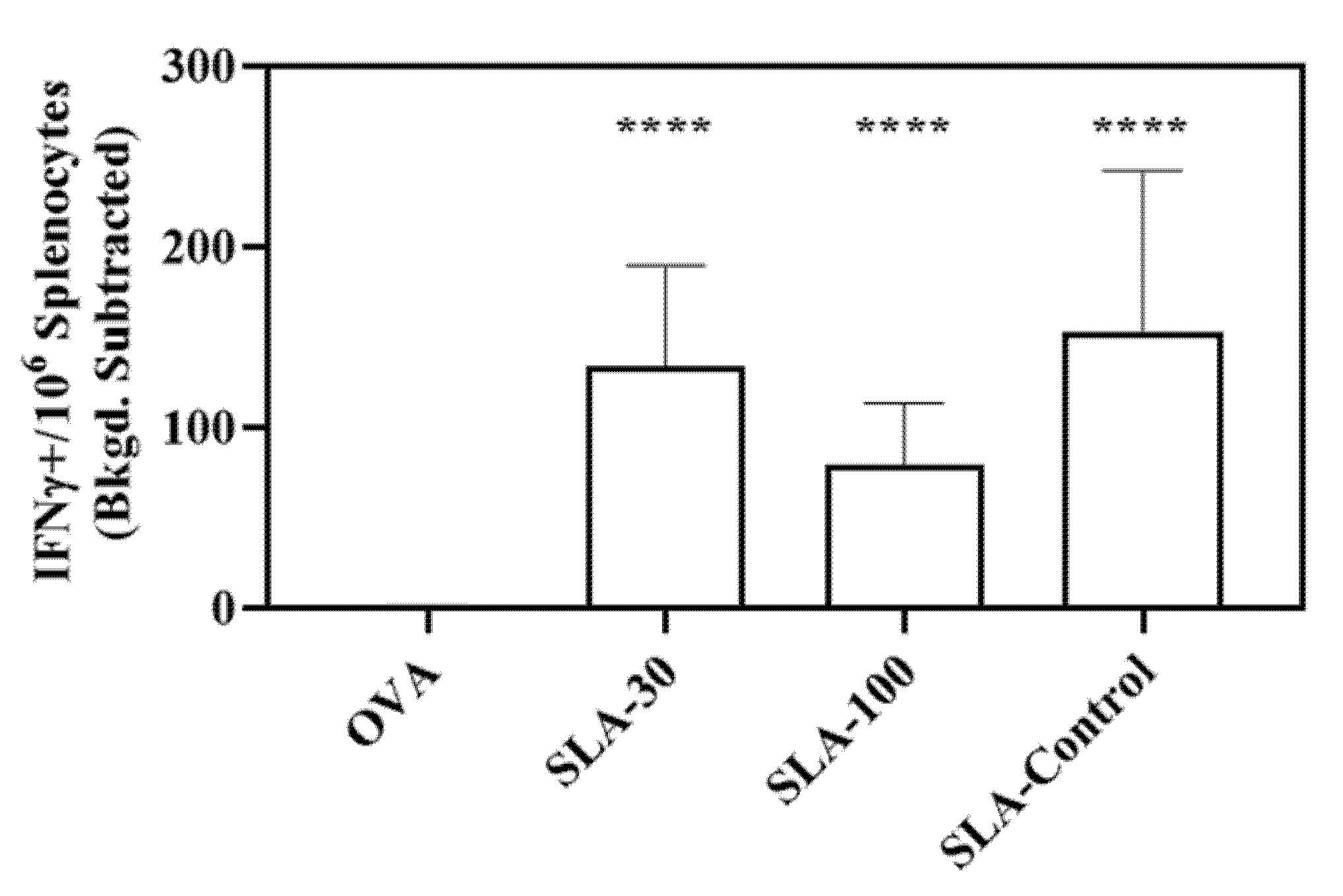
3.3. Induction of Antigen-Specific Cell Mediated Immunity by SLA Archaeosomes
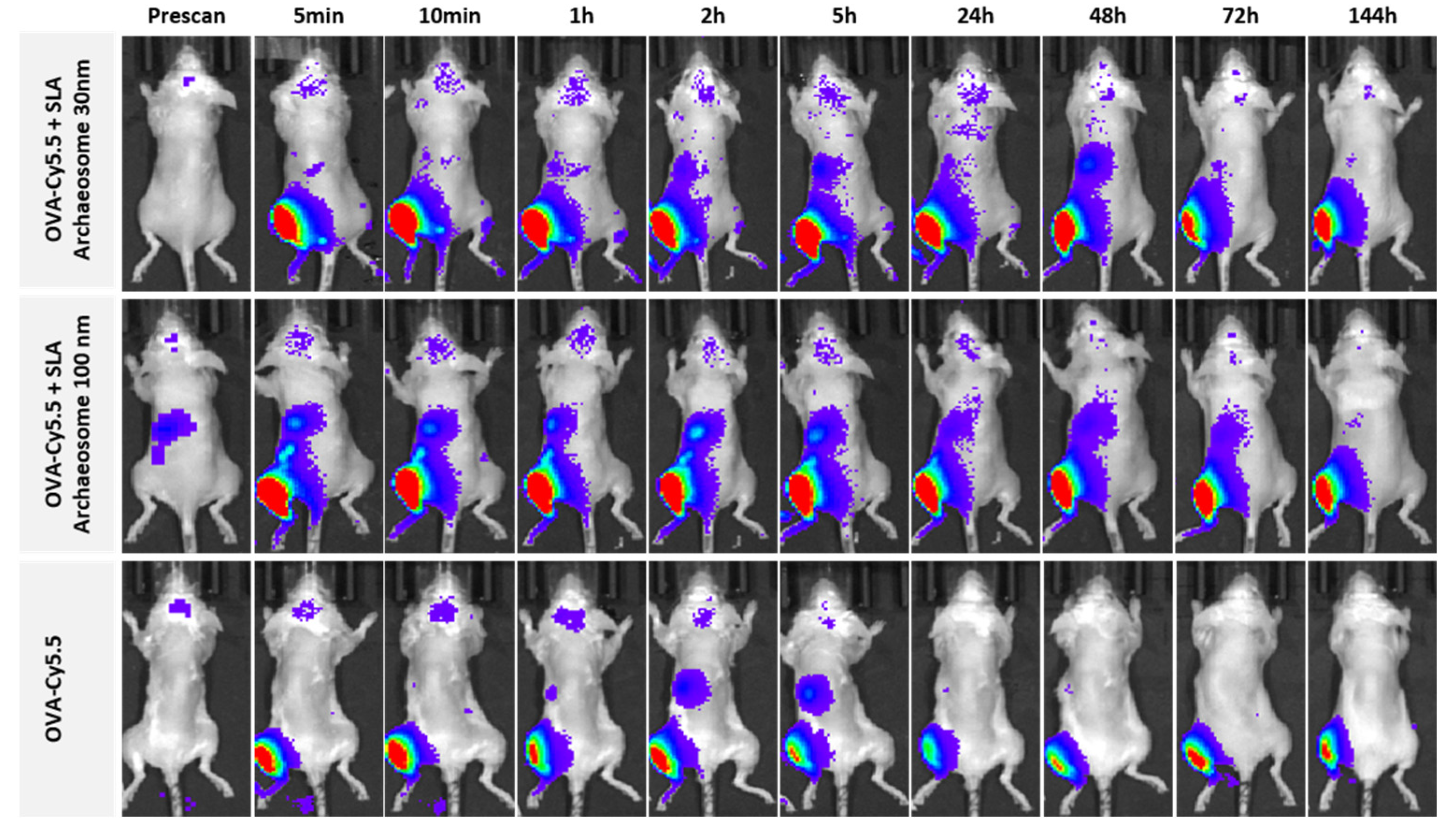
3.4. Bio-Distribution of Archaeosomes and Effect on Antigen Retention
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitfield, D.M.; Yu, S.H.; Dicaire, C.J.; Sprott, G.D. Development of new glycosylation methodologies for the synthesis of archaeal-derived glycolipid adjuvants. Carbohydr. Res. 2010, 345, 214–229. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Deschatelets, L.; Krishnan, L. Sulfated archaeal glycolipid archaeosomes as a safe and effective vaccine adjuvant for induction of cell-mediated immunity. Hum. Vaccin. Immunother. 2017, 13, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; Stark, F.C.; Jia, Y.; Deschatelets, L.; Dudani, R.; Harrison, B.A.; Agbayani, G.; Williams, D.; Jamshidi, M.P.; Krishnan, L.; et al. Sulfated archaeol glycolipids: Comparison with other immunological adjuvants in mice. PLoS ONE 2018, 13, e0208067. [Google Scholar] [CrossRef]
- Perera, D.J.; Hassan, A.S.; Jia, Y.; Ricciardi, A.; McCluskie, M.J.; Weeratna, R.D.; Ndao, M. Adjuvanted Schistosoma mansoni-Cathepsin B with Sulfated Lactosyl Archaeol Archaeosomes or AddaVax™ Provide Protection in a Pre-Clinical Schistosomiasis Model. Front. Immunol. 2020, 11, 605288. [Google Scholar] [CrossRef]
- Akache, B.; Deschatelets, L.; Harrison, B.A.; Dudani, R.; Stark, F.C.; Jia, Y.; Landi, A.; Law, J.L.M.; Logan, M.; Hockman, D.; et al. Effect of Different Adjuvants on the Longevity and Strength of Humoral and Cellular Immune Responses to the HCV Envelope Glycoproteins. Vaccines 2019, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Stark, F.C.; Akache, B.; Ponce, A.; Dudani, R.; Deschatelets, L.; Jia, Y.; Sauvageau, J.; Williams, D.; Jamshidi, M.P.; Agbayani, G.; et al. Archaeal glycolipid adjuvanted vaccines induce strong influenza-specific immune responses through direct immunization in young and aged mice or through passive maternal immunization. Vaccine 2019, 37, 7108–7116. [Google Scholar] [CrossRef] [PubMed]
- Akache, B.; Renner, T.M.; Tran, A.; Deschatelets, L.; Dudani, R.; Harrison, B.A.; Duque, D.; Haukenfrers, J.; Rossotti, M.A.; Gaudreault, F.; et al. Immunogenic and efficacious SARS-CoV-2 vaccine based on resistin-trimerized spike antigen SmT1 and SLA archaeosome adjuvant. Sci. Rep. 2021, 11, 21849. [Google Scholar] [CrossRef] [PubMed]
- Stark, F.C.; Agbayani, G.; Sandhu, J.K.; Akache, B.; McPherson, C.; Deschatelets, L.; Dudani, R.; Hewitt, M.; Jia, Y.; Krishnan, L.; et al. Simplified Admix Archaeal Glycolipid Adjuvanted Vaccine and Checkpoint Inhibitor Therapy Combination Enhances Protection from Murine Melanoma. Biomedicines 2019, 7, 91. [Google Scholar] [CrossRef]
- Haq, K.; Jia, Y.; Elahi, S.M.; MacLean, S.; Akache, B.; Gurnani, K.; Chattopadhyay, A.; Nazemi-Moghaddam, N.; Gilbert, R.; McCluskie, M.J.; et al. Evaluation of recombinant adenovirus vectors and adjuvanted protein as a heterologous prime-boost strategy using HER2 as a model antigen. Vaccine 2019, 37, 7029–7040. [Google Scholar] [CrossRef]
- Jia, Y.; Akache, B.; Deschatelets, L.; Qian, H.; Dudani, R.; Harrison, B.A.; Stark, F.C.; Chandan, V.; Jamshidi, M.P.; Krishnan, L.; et al. A comparison of the immune responses induced by antigens in three different archaeosome-based vaccine formulations. Int. J. Pharm. 2019, 561, 187–196. [Google Scholar] [CrossRef]
- Belliveau, N.M.; Huft, J.; Lin, P.J.; Chen, S.; Leung, A.K.; Leaver, T.J.; Wild, A.W.; Lee, J.B.; Taylor, R.J.; Tam, Y.K.; et al. Microfluidic Synthesis of Highly Potent Limit-size Lipid Nanoparticles for In Vivo Delivery of siRNA. Mol. Ther. Nucleic Acids 2012, 1, e37. [Google Scholar] [CrossRef] [PubMed]
- Kotoucek, J.; Hubatka, F.; Masek, J.; Kulich, P.; Velinska, K.; Bezdekova, J.; Fojtikova, M.; Bartheldyova, E.; Tomeckova, A.; Straska, J.; et al. Preparation of nanoliposomes by microfluidic mixing in herring-bone channel and the role of membrane fluidity in liposomes formation. Sci. Rep. 2020, 10, 5595. [Google Scholar] [CrossRef] [PubMed]
- Arduino, I.; Liu, Z.; Iacobazzi, R.M.; Lopedota, A.A.; Lopalco, A.; Cutrignelli, A.; Laquintana, V.; Porcelli, L.; Azzariti, A.; Franco, M.; et al. Microfluidic preparation and in vitro evaluation of iRGD-functionalized solid lipid nanoparticles for targeted delivery of paclitaxel to tumor cells. Int. J. Pharm. 2021, 610, 121246. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hardie, J.; Zhang, X.; Rotello, V.M. Effects of engineered nanoparticles on the innate immune system. Semin. Immunol. 2017, 34, 25–32. [Google Scholar] [CrossRef]
- Pelkmans, L. Secrets of caveolae- and lipid raft-mediated endocytosis revealed by mammalian viruses. Biochim. Biophys. Acta 2005, 1746, 295–304. [Google Scholar] [CrossRef]
- Manolova, V.; Flace, A.; Bauer, M.; Schwarz, K.; Saudan, P.; Bachmann, M.F. Nanoparticles target distinct dendritic cell populations according to their size. Eur. J. Immunol. 2008, 38, 1404–1413. [Google Scholar] [CrossRef]
- Shah, R.R.; Dodd, S.; Schaefer, M.; Ugozzoli, M.; Singh, M.; Otten, G.R.; Amiji, M.M.; O’Hagan, D.T.; Brito, L.A. The development of self-emulsifying oil-in-water emulsion adjuvant and an evaluation of the impact of droplet size on performance. J. Pharm. Sci. 2015, 104, 1352–1361. [Google Scholar] [CrossRef]
- Fifis, T.; Gamvrellis, A.; Crimeen-Irwin, B.; Pietersz, G.A.; Li, J.; Mottram, P.L.; McKenzie, I.F.; Plebanski, M. Size-dependent immunogenicity: Therapeutic and protective properties of nano-vaccines against tumors. J. Immunol. 2004, 173, 3148–3154. [Google Scholar] [CrossRef]
- Whitfield, D.; Sprott, G.D.; Krishnan, L. Sulfated-Glycolipids as Adjuvants for Vaccines. WO2016004512A1, 14 January 2016. [Google Scholar]
- Jia, Y.; Chandan, V.; Akache, B.; Qian, H.; Jakubek, Z.J.; Vinogradov, E.; Dudani, R.; Harrison, B.A.; Jamshidi, M.P.; Stark, F.C.; et al. Assessment of stability of sulphated lactosyl archaeol archaeosomes for use as a vaccine adjuvant. J. Liposome Res. 2021, 31, 237–245. [Google Scholar] [CrossRef]
- Rebollo, R.; Oyoun, F.; Corvis, Y.; El-Hammadi, M.M.; Saubamea, B.; Andrieux, K.; Mignet, N.; Alhareth, K. Microfluidic Manufacturing of Liposomes: Development and Optimization by Design of Experiment and Machine Learning. ACS Appl. Mater. Interfaces 2022, 14, 39736–39745. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Jia, Y.; McCluskie, M.J. Application of Cryogenic Transmission Electron Microscopy for Evaluation of Vaccine Delivery Carriers. Methods Mol. Biol. 2021, 2183, 499–511. [Google Scholar] [PubMed]
- Akache, B.; Stark, F.C.; McCluskie, M.J. Measurement of Antigen-Specific IgG Titers by Direct ELISA. Methods Mol. Biol. 2021, 2183, 537–547. [Google Scholar]
- Akache, B.; McCluskie, M.J. The Quantification of Antigen-Specific T Cells by IFN-gamma ELISpot. Methods Mol. Biol. 2021, 2183, 525–536. [Google Scholar]
- Barber, D.L.; Wherry, E.J.; Ahmed, R. Cutting edge: Rapid in vivo killing by memory CD8 T cells. J. Immunol. 2003, 171, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Stark, F.C.; Dudani, R.; Agbayani, G.; McCluskie, M.J. A Method to Evaluate In Vivo CD8(+) T Cell Cytotoxicity in a Murine Model. Methods Mol. Biol. 2021, 2183, 549–558. [Google Scholar] [PubMed]
- Xia, H.M.; Seah, Y.P.; Liu, Y.C.; Wang, W.; Toh, A.G.G.; Wang, Z.P. Anti-solvent precipitation of solid lipid nanoparticles using a microfluidic oscillator mixer. Microfluid. Nanofluidics 2015, 19, 283–290. [Google Scholar] [CrossRef]
- Anderluzzi, G.; Lou, G.; Su, Y.; Perrie, Y. Scalable Manufacturing Processes for Solid Lipid Nanoparticles. Pharm. Nanotechnol. 2019, 7, 444–459. [Google Scholar] [CrossRef]
- Sturm, L.; Poklar Ulrih, N. Basic Methods for Preparation of Liposomes and Studying Their Interactions with Different Compounds, with the Emphasis on Polyphenols. Int. J. Mol. Sci. 2021, 22, 6547. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, J. Lipid in Chips: A Brief Review of Liposomes Formation by Microfluidics. Int. J. Nanomed. 2021, 16, 7391–7416. [Google Scholar] [CrossRef]
- Perrett, S.; Golding, M.; Williams, W.P. A simple method for the preparation of liposomes for pharmaceutical applications: Characterization of the liposomes. J. Pharm. Pharmacol. 1991, 43, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Tam, Y.Y.; Chen, S.; Hafez, I.M.; Cullis, P.R. Microfluidic Mixing: A General Method for Encapsulating Macromolecules in Lipid Nanoparticle Systems. J. Phys. Chem. B 2015, 119, 8698–8706. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.K.; Hafez, I.M.; Baoukina, S.; Belliveau, N.M.; Zhigaltsev, I.V.; Afshinmanesh, E.; Tieleman, D.P.; Hansen, C.L.; Hope, M.J.; Cullis, P.R. Lipid Nanoparticles Containing siRNA Synthesized by Microfluidic Mixing Exhibit an Electron-Dense Nanostructured Core. J. Phys. Chem. C Nanomater. Interfaces 2012, 116, 18440–18450. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.; Shakir, E.; Carter, K.C.; Mullen, A.B.; Alexander, J.; Ferro, V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine 2009, 27, 3643–3649. [Google Scholar] [CrossRef] [PubMed]
- Agbayani, G.; Jia, Y.; Akache, B.; Chandan, V.; Iqbal, U.; Stark, F.C.; Deschatelets, L.; Lam, E.; Hemraz, U.D.; Regnier, S.; et al. Mechanistic insight into the induction of cellular immune responses by encapsulated and admixed archaeosome-based vaccine formulations. Hum. Vaccin. Immunother. 2020, 16, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Allegra, A.; Tonacci, A.; Musolino, C.; Ricciardi, L.; Gangemi, S. Mast Cells and Vitamin D Status: A Clinical and Biological Link in the Onset of Allergy and Bone Diseases. Biomedicines 2022, 10, 1877. [Google Scholar] [CrossRef] [PubMed]
- Murdaca, G.; Paladin, F.; Tonacci, A.; Borro, M.; Greco, M.; Gerosa, A.; Isola, S.; Allegra, A.; Gangemi, S. Involvement of Il-33 in the Pathogenesis and Prognosis of Major Respiratory Viral Infections: Future Perspectives for Personalized Therapy. Biomedicines 2022, 10, 715. [Google Scholar] [CrossRef]

| Response Parameter/Variable | Level-1 | Level-2 | Level-3 |
|---|---|---|---|
| Co-solvent (v:v) | E/M (3:1) | E/D (39:1) | E/A (39:1) |
| FRR (aqueous:lipid; v:v) | 1:1 | 2:1 | 4:1 |
| TFR (mL/min) | 8 | 12 | 18 |
| Experimental Runs | Control Parameters | Experimental Values | |||||
|---|---|---|---|---|---|---|---|
| FRR | TFR | Solvent | Z-Avg nm | Percentage of Main Peak | PDI | Zeta Potential mV | |
| 1 | 1:1 | 8 | E/A | 94.24 ± 1.95 | 94.8 ± 3.26 | 0.27 ± 0.01 | −35.73 ± 2.98 |
| 2 | 1:1 | 12 | E/M | 112.13 ± 4.45 | 100.0 ± 0.21 | 0.23 ± 0.05 | −37.83 ± 2.60 |
| 3 | 1:1 | 18 | E/D | 94.61 ± 0.29 | 98.0 ± 2.89 | 0.19 ± 0.01 | −41.27 ± 2.40 |
| 4 | 2:1 | 8 | E/M | 153.0 ± 6.58 | 91.1 ± 5.21 | 0.51 ± 0.02 | −19.83 ± 9.70 |
| 5 | 2:1 | 12 | E/D | 346.4 ± 178.74 | 84.0 ± 10.71 | 0.68 ± 0.01 | −42.03 ± 2.32 |
| 6 | 2:1 | 18 | E/A | 59.01 ± 0.96 | 98.5 ± 1.43 | 0.39 ± 0.01 | −35.6 ± 3.70 |
| 7 | 4:1 | 8 | E/D | 32.84 ± 0.04 | 96.0 ± 2.64 | 0.29 ± 0.01 | −33.07 ± 3.01 |
| 8 | 4:1 | 12 | E/A | 30.93 ± 0.95 | 57.6 ± 12.71 | 0.64 ± 0.02 | −21.64 ± 8.70 |
| 9 | 4:1 | 18 | E/M | 212.27 ± 16.9 | 81.4 ± 5.32 | 0.40 ± 0.05 | −29.43 ± 7.80 |
| Thin-film hydration | 94.56 ± 0.36 | 96.7 ± 2.56 | 0.27 ± 0.00 | −48.00 ±3.48 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Y.; Agbayani, G.; Chandan, V.; Iqbal, U.; Dudani, R.; Qian, H.; Jakubek, Z.; Chan, K.; Harrison, B.; Deschatelets, L.; et al. Evaluation of Adjuvant Activity and Bio-Distribution of Archaeosomes Prepared Using Microfluidic Technology. Pharmaceutics 2022, 14, 2291. https://doi.org/10.3390/pharmaceutics14112291
Jia Y, Agbayani G, Chandan V, Iqbal U, Dudani R, Qian H, Jakubek Z, Chan K, Harrison B, Deschatelets L, et al. Evaluation of Adjuvant Activity and Bio-Distribution of Archaeosomes Prepared Using Microfluidic Technology. Pharmaceutics. 2022; 14(11):2291. https://doi.org/10.3390/pharmaceutics14112291
Chicago/Turabian StyleJia, Yimei, Gerard Agbayani, Vandana Chandan, Umar Iqbal, Renu Dudani, Hui Qian, Zygmunt Jakubek, Kenneth Chan, Blair Harrison, Lise Deschatelets, and et al. 2022. "Evaluation of Adjuvant Activity and Bio-Distribution of Archaeosomes Prepared Using Microfluidic Technology" Pharmaceutics 14, no. 11: 2291. https://doi.org/10.3390/pharmaceutics14112291
APA StyleJia, Y., Agbayani, G., Chandan, V., Iqbal, U., Dudani, R., Qian, H., Jakubek, Z., Chan, K., Harrison, B., Deschatelets, L., Akache, B., & McCluskie, M. J. (2022). Evaluation of Adjuvant Activity and Bio-Distribution of Archaeosomes Prepared Using Microfluidic Technology. Pharmaceutics, 14(11), 2291. https://doi.org/10.3390/pharmaceutics14112291






