Real-World Comparative Effectiveness of Nivolumab versus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma
Abstract
1. Introduction
2. Material and Methods
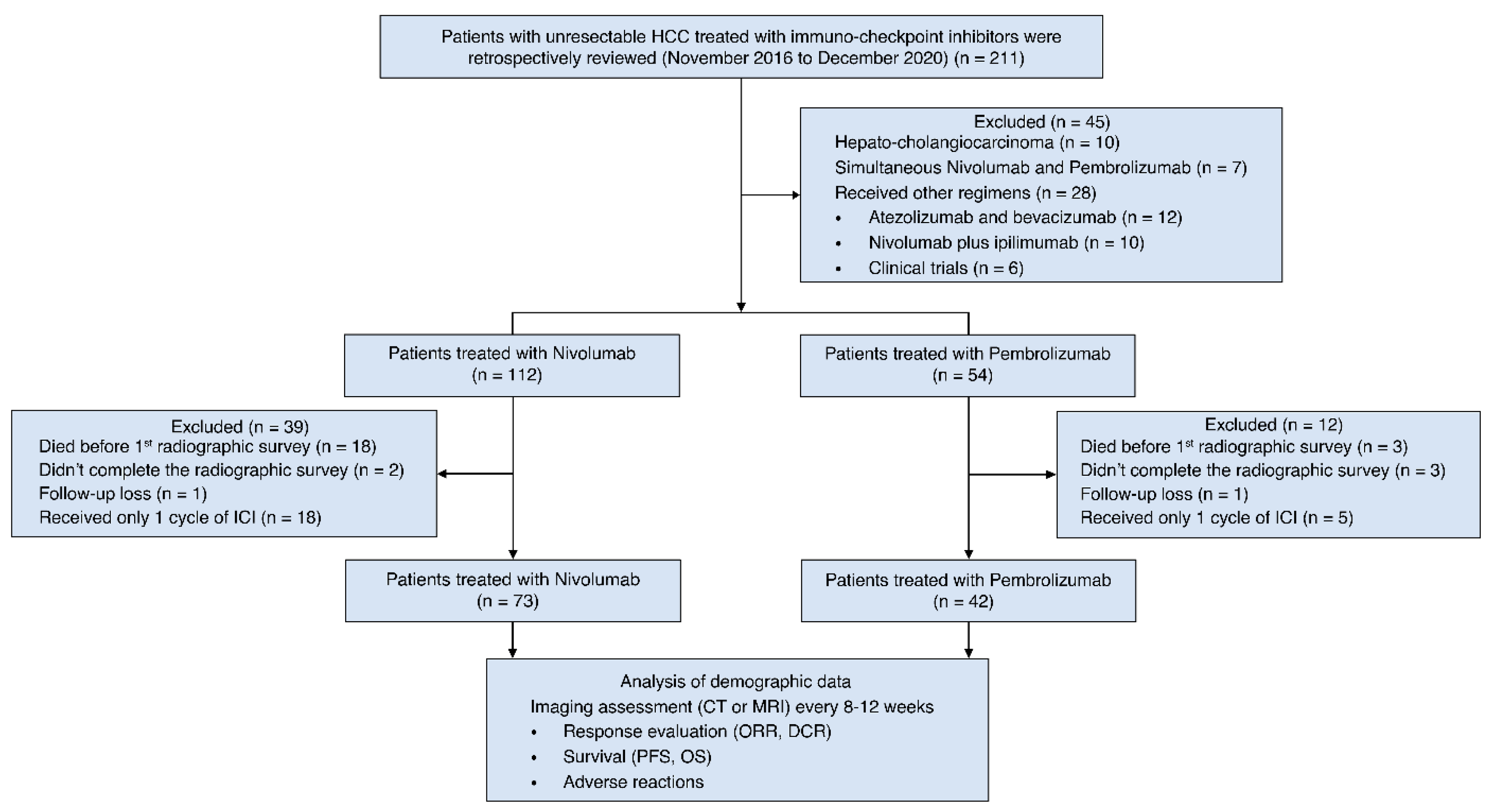
2.1. Study Subjects
2.2. Treatment and Response Evaluation
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Subjects
3.2. Tumor Response
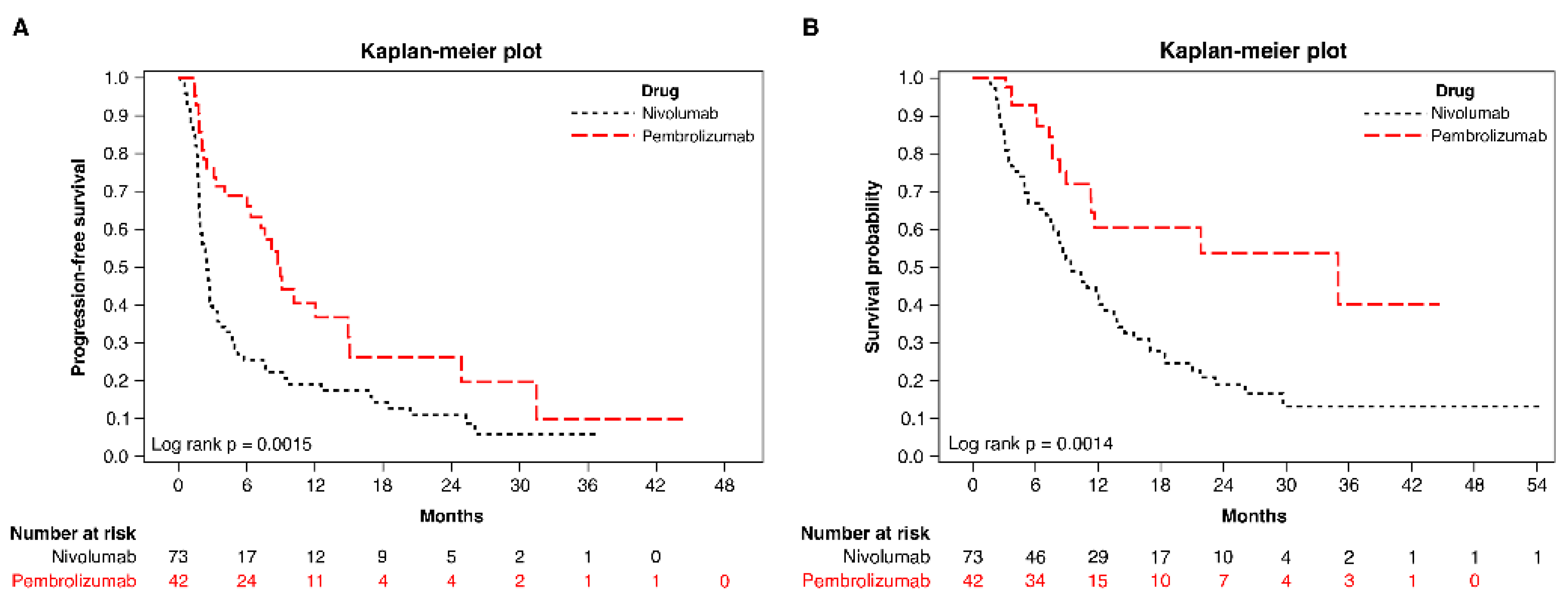
3.3. Survival
3.4. Subgroup Analyses of Survival between Nivolumab- and Pembrolizumab-Treated Patients
3.5. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef]
- Lin, L.; Yan, L.; Liu, Y.; Qu, C.; Ni, J.; Li, H. The burden and trends of primary liver cancer caused by specific etiologies from 1990 to 2017 at the global, regional, national, age, and sex level results from the global burden of disease Study 2017. Liver Cancer 2020, 9, 563–582. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Sia, D.; Finn, R.S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 2018, 15, 599–616. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Casak, S.J.; Donoghue, M.; Fashoyin-Aje, L.; Jiang, X.; Rodriguez, L.; Shen, Y.-L.; Xu, Y.; Jiang, X.; Liu, J.; Zhao, H.; et al. FDA approval summary: Atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin. Cancer Res. 2021, 27, 1836–1841. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Trojan, J.; Welling, T.H., 3rd; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–25502. [Google Scholar] [CrossRef]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- Pinato, D.J.; Guerra, N.; Fessas, P.; Murphy, R.; Mineo, T.; Mauri, F.A.; Mukherjee, S.K.; Thursz, M.; Wong, C.N.; Sharma, R.; et al. Immune-based therapies for hepatocellular carcinoma. Oncogene 2020, 39, 3620–3637. [Google Scholar] [CrossRef]
- Chen, Y.; Pei, Y.; Luo, J.; Huang, Z.; Yu, J.; Meng, X. Looking for the optimal PD-1/PD-L1 inhibitor in cancer treatment: A comparison in basic structure, function, and clinical practice. Front. Immunol. 2020, 11, 1088. [Google Scholar] [CrossRef]
- Fessas, P.; Lee, H.; Ikemizu, S.; Janowitz, T. A molecular and preclinical comparison of the PD-1-targeted T-cell checkpoint inhibitors nivolumab and pembrolizumab. Semin. Oncol. 2017, 44, 136–140. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef]
- Lu, L.-C.; Hsu, C.; Shao, Y.-Y.; Chao, Y.; Yen, C.-J.; Shih, I.-L.; Hung, Y.-P.; Chang, C.-J.; Shen, Y.-C.; Guo, J.-C.; et al. Differential organ-specific tumor response to immune checkpoint inhibitors in hepatocellular carcinoma. Liver Cancer 2019, 8, 480–490. [Google Scholar] [CrossRef]
- Lee, P.-C.; Chao, Y.; Chen, M.-H.; Lan, K.-H.; Lee, C.-J.; Lee, I.-C.; Chen, S.-C.; Hou, M.-C.; Huang, Y.-H. Predictors of response and survival in immune checkpoint inhibitor-treated unresectable hepatocellular carcinoma. Cancers 2020, 12, 182. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Lencioni, R.; Llovet, J.M. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin. Liver Dis. 2010, 30, 52–60. [Google Scholar] [CrossRef]
- Lv, J.W.; Li, J.Y.; Luo, L.N.; Wang, Z.X.; Chen, Y.P. Comparative safety and efficacy of anti-PD-1 monotherapy, chemotherapy alone, and their combination therapy in advanced nasopharyngeal carcinoma: Findings from recent advances in landmark trials. J. Immunother. Cancer 2019, 7, 159. [Google Scholar] [CrossRef]
- Moser, J.C.; Wei, G.; Colonna, S.V.; Grossmann, K.F.; Patel, S.; Hyngstrom, J.R. Comparative-effectiveness of pembrolizumab vs. nivolumab for patients with metastatic melanoma. Acta Oncol. 2020, 59, 434–437. [Google Scholar] [CrossRef]
- Cui, P.; Li, R.; Huang, Z.; Wu, Z.; Tao, H.; Zhang, S.; Hu, Y. Comparative effectiveness of pembrolizumab vs. nivolumab in patients with recurrent or advanced NSCLC. Sci. Rep. 2020, 10, 13160. [Google Scholar]
- Wang, R.; Lin, N.; Mao, B.; Wu, Q. The efficacy of immune checkpoint inhibitors in advanced hepatocellular carcinoma: A meta-analysis based on 40 cohorts incorporating 3697 individuals. J. Cancer Res. Clin. Oncol. 2022, 148, 1195–1210. [Google Scholar] [CrossRef]
- Gordan, J.D.; Kennedy, E.B.; Abou-Alfa, G.K.; Beg, M.S.; Brower, S.T.; Gade, T.P. Systemic therapy for advanced hepatocellular carcinoma: ASCO Guideline [ASCO guideline:4317-45]. J. Clin. Oncol. 2020, 38, 4317–4345. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, L.; Weng, L. Comparisons of underlying mechanisms, clinical efficacy and safety Between anti-PD-1 and anti-PD-L1 immunotherapy: The state-of-the-art review and future perspectives. Front. Pharmacol. 2021, 12, 714483. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.; Chan, S.L.; Choo, S.P.; Kudo, M. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J. Hepatol. 2020, 72, 307–319. [Google Scholar] [CrossRef]
- Finn, R.S.; Ryoo, B.-Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. KEYNOTE-240 investigators. Pembrolizumab as second-line therapy in patients with advanced hepatocellular carcinoma in KEYNOTE-240: A randomized, double-blind, Phase III trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef]
- Yau, T.; Kang, Y.; Kim, T.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.; Matilla, A.; et al. Efficacy and safety of Nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: The CheckMate 040 randomized clinical trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef]
- Kudo, M.; Lim, H.Y.; Cheng, A.-L.; Chao, Y.; Yau, T.; Ogasawara, S.; Kurosaki, M.; Morimoto, N.; Ohkawa, K.; Yamashita, T.; et al. Pembrolizumab as second-line therapy for advanced hepatocellular carcinoma: A subgroup analysis of Asian patients in the Phase 3 KEYNOTE-240 Trial. Liver Cancer 2021, 10, 275–284. [Google Scholar] [CrossRef]
- Scheiner, B.; Kirstein, M.M.; Hucke, F.; Finkelmeier, F.; Schulze, K.; Von Felden, J.; Koch, S.; Schwabl, P.; Hinrichs, J.B.; Waneck, F.; et al. Programmed cell death protein-1 (PD-1)-targeted immunotherapy in advanced hepatocellular carcinoma: Efficacy and safety data from an international multicentre real-world cohort. Aliment. Pharmacol. Ther. 2019, 49, 1323–1333. [Google Scholar] [CrossRef]
- Yau, T.; Hsu, C.; Kim, T.-Y.; Choo, S.-P.; Kang, Y.-K.; Hou, M.-M.; Numata, K.; Yeo, W.; Chopra, A.; Ikeda, M.; et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J. Hepatol. 2019, 71, 543–552. [Google Scholar] [CrossRef]
- Obi, S.; Sato, T.; Sato, S. Immune checkpoint inhibitor in liver cancer-unique regional differences. Ann. Transl. Med. 2020, 8, 1336. [Google Scholar] [CrossRef]

| Characteristic | Nivolumab (n = 73) | Pembrolizumab (n = 42) | p |
|---|---|---|---|
| Number (%) | Number (%) | ||
| Gender | |||
| Female | 55 (75.3) | 34 (81.0) | 0.645 |
| Male | 18 (24.7) | 8 (19.1) | |
| Age, years—Mean ± SD | 62.7 ± 11.4 | 63.0 ± 11.8 | 0.893 |
| <55 | 15 (20.6) | 12 (28.6) | 0.454 |
| ≥55 | 58 (79.5) | 30 (71.4) | |
| ECOG | |||
| 0 | 47 (64.4) | 21 (50.0) | 0.263 |
| 1 | 23 (31.5) | 18 (42.9) | |
| 2 | 3 (4.1) | 2 (4.8) | |
| 3 | 0 (0.0) | 1 (2.4) | |
| α-Fetoprotein, ng/mL | |||
| <400 | 44 (60.3) | 23 (54.8) | 0.703 |
| ≥400 | 29 (39.7) | 19 (45.2) | |
| Etiology of chronic liver disease | |||
| No liver disease | 3 (4.1) | 4 (9.5) | 0.257 |
| Liver disease present | 70 (95.9) | 38 (90.5) | |
| Chronic hepatitis B | 51 (72.9) | 30 (79.0) | 0.642 |
| Chronic hepatitis C | 20 (28.6) | 8 (21.1) | 0.534 |
| Alcoholic hepatitis | 4 (5.7) | 5 (13.2) | 0.273 |
| Nonalcoholic steatohepatitis | 1 (1.4) | 1 (2.6) | 1.000 |
| Child–Pugh class | |||
| A | 55 (75.3) | 32 (76.2) | 1.000 |
| B | 18 (24.7) | 10 (23.8) | |
| BCLC stage | |||
| B | 12 (16.4) | 4 (9.5) | 0.452 |
| C–D | 61 (83.6) | 38 (90.5) | |
| CLIP | |||
| 0–1 | 33 (45.2) | 21 (50.0) | 0.763 |
| 2–5 | 40 (54.8) | 21 (50.0) | |
| Distant metastases | |||
| No | 33 (45.2) | 15 (35.7) | 0.425 |
| Yes | 40 (54.8) | 27 (64.3) | |
| Lung | 27 (67.5) | 16 (59.3) | 0.667 |
| Bone | 7 (17.5) | 2 (7.4) | 0.295 |
| Brain | 1 (2.5) | 0 (0.0) | 1.000 |
| Lymph node | 11 (27.5) | 10 (37.0) | 0.433 |
| Other a | 18 (45.0) | 7 (25.9) | 0.185 |
| Prior treatment | |||
| No | 10 (13.7) | 5 (11.9) | 1.000 |
| Yes | 63 (86.3) | 37 (88.1) | |
| Surgical resection | 33 (52.4) | 16 (43.2) | 0.499 |
| TACE | 43 (68.3) | 20 (54.1) | 0.228 |
| RFA/PEI | 25 (39.7) | 10 (27.0) | 0.287 |
| HAIC | 23 (36.5) | 19 (51.4) | 0.214 |
| TKI | 55 (87.3) | 28 (75.7) | 0.223 |
| Systemic chemotherapy | 2 (3.2) | 0 (0.0) | 0.529 |
| Radioembolization | 1 (1.6) | 3 (8.1) | 0.142 |
| Radiotherapy | 15 (23.8) | 6 (16.2) | 0.518 |
| Clinical trial | 2 (3.2) | 2 (5.4) | 0.625 |
| Liver transplantation | 1 (1.6) | 0 (0.0) | 1.000 |
| Macrovascular invasion | |||
| No | 46 (63.0) | 26 (61.9) | 1.000 |
| Yes | 27 (37.0) | 16 (38.1) | |
| PD-1 inhibitors combined with TKI | |||
| No | 34 (46.6) | 12 (28.6) | 0.089 |
| Yes | 39 (53.4) | 30 (71.4) | |
| Sorafenib | 22 (56.4) | 6 (20.0) | 0.003 |
| Regorafenib | 4 (10.3) | 5 (16.7) | 0.488 |
| Lenvatinib | 13 (33.3) | 19 (63.3) | 0.016 |
| PD-1 inhibitors as systemic therapy | |||
| First line | 18 (24.7) | 14 (33.3) | 0.261 |
| Second line | 49 (67.1) | 22 (52.4) | |
| Third line | 5 (6.9) | 6 (14.3) | |
| Fourth line | 1 (1.4) | 0 (0.0) | |
| Median cycles received (range) | 6 (2–54) | 6 (2–55) | 0.835 |
| Median cumulative dose, mg (range) | 940 (200–8680) | 600 (200–5550) | 0.008 |
| Total (n = 115) | |||
|---|---|---|---|
| Objective Response a | Nivolumab (n = 73) | Pembrolizumab (n = 42) | p |
| n (%) | 11 (15.07) | 16 (38.10) | 0.010 |
| Unadjusted OR (95% CI) | 1 | 3.47 (1.42–8.48) | 0.006 |
| Adjusted model 1 b | 1 | 3.61 (1.40–9.29) | 0.008 |
| Adjusted model 2 c | 1 | 4.18 (1.53–11.44) | 0.005 |
| PFS | p | OS | p | |
|---|---|---|---|---|
| Unadjusted Cox Model | 0.49 (0.31–0.77) | 0.002 | 0.41 (0.23–0.72) | 0.002 |
| Adjusted Cox model 1 a | 0.44 (0.27–0.71) | 0.001 | 0.23 (0.12–0.44) | 0.001 |
| Adjusted Cox model 2 b | 0.48 (0.29–0.79) | 0.004 | 0.39 (0.21–0.74) | 0.004 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, H.-Y.; Han, M.-Z.; Liao, C.-H.; Lin, Y.-J.; Wang, C.-T.; Chen, S.-H.; Chang, T.-T.; Chen, P.-J.; Lin, S.-H.; Chen, C.-Y.; et al. Real-World Comparative Effectiveness of Nivolumab versus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. Pharmaceutics 2022, 14, 2263. https://doi.org/10.3390/pharmaceutics14112263
Kuo H-Y, Han M-Z, Liao C-H, Lin Y-J, Wang C-T, Chen S-H, Chang T-T, Chen P-J, Lin S-H, Chen C-Y, et al. Real-World Comparative Effectiveness of Nivolumab versus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. Pharmaceutics. 2022; 14(11):2263. https://doi.org/10.3390/pharmaceutics14112263
Chicago/Turabian StyleKuo, Hsin-Yu, Meng-Zhi Han, Chih-Hsiang Liao, Yih-Jyh Lin, Chung-Teng Wang, Shang-Hung Chen, Ting-Tsung Chang, Po-Jun Chen, Sheng-Hsiang Lin, Chiung-Yu Chen, and et al. 2022. "Real-World Comparative Effectiveness of Nivolumab versus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma" Pharmaceutics 14, no. 11: 2263. https://doi.org/10.3390/pharmaceutics14112263
APA StyleKuo, H.-Y., Han, M.-Z., Liao, C.-H., Lin, Y.-J., Wang, C.-T., Chen, S.-H., Chang, T.-T., Chen, P.-J., Lin, S.-H., Chen, C.-Y., Chuang, C.-H., Wu, I.-C., Wu, J.-S., Hong, T.-C., Hsieh, M.-T., Lee, Y.-C., Wu, H.-T., & Tsai, H.-M. (2022). Real-World Comparative Effectiveness of Nivolumab versus Pembrolizumab in Patients with Unresectable Hepatocellular Carcinoma. Pharmaceutics, 14(11), 2263. https://doi.org/10.3390/pharmaceutics14112263






