Microemulsions Enhance the In Vitro Antioxidant Activity of Oleanolic Acid in RAW 264.7 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagentds
2.2. Cromatography Conditions and Instruments
2.3. Preparation of Microemulsions
2.3.1. Solubility Study
2.3.2. Pseudoternary Phase Diagram
2.3.3. Preparation of MEs
2.4. Characterization of Microemulsions
2.4.1. Particle Size and ζ-Potential Measurements
2.4.2. Morphological Characterization
2.4.3. Chemical and Physical Stability during Storage
2.4.4. In Vitro Release Studies
2.4.5. In Vitro Parallel Artificial Membrane Permeability Assay (PAMPA)
2.5. Cell-Based In Vitro Experiments
2.5.1. Cell line and Culture Conditions
2.5.2. MTT Assay
2.5.3. Intracellular ROS Production Detection
2.6. Statistical Analysis
3. Results
3.1. Preparation of Microemulsions
3.1.1. Solubility Studies
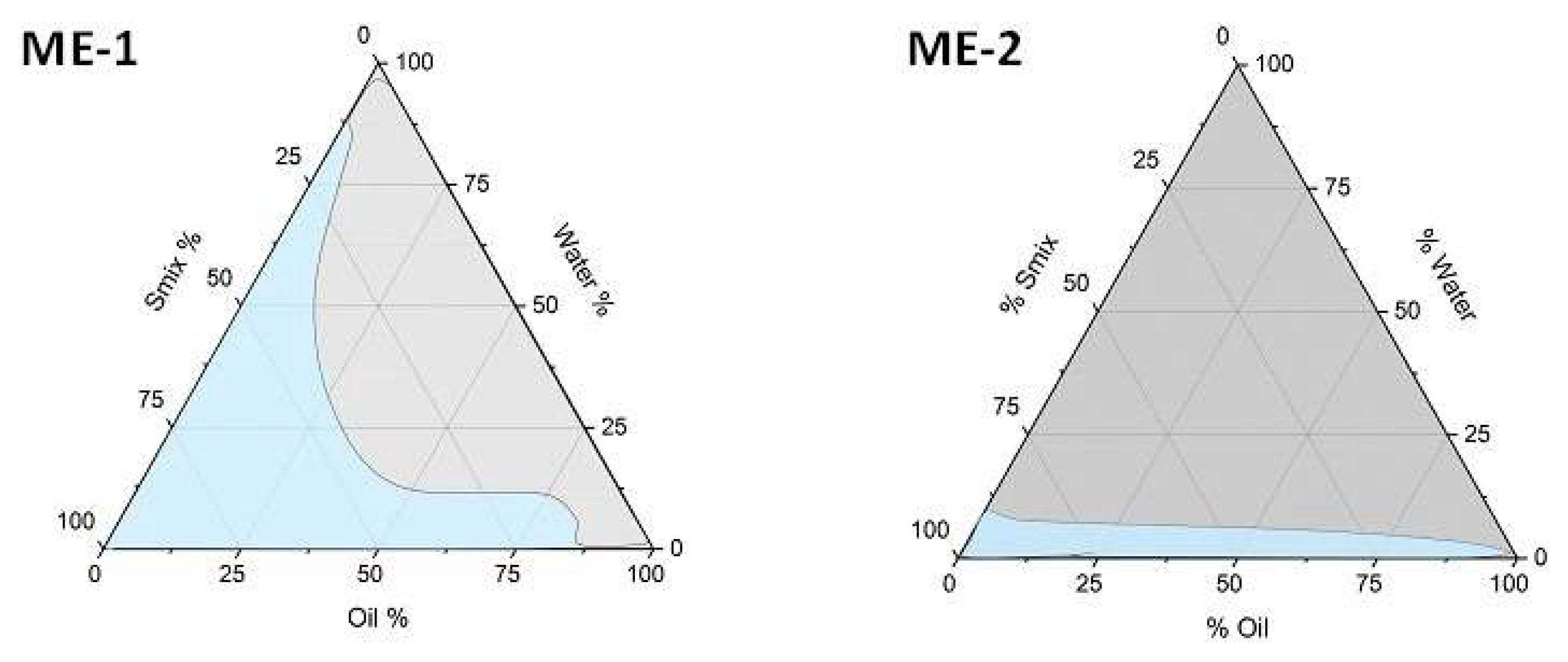
3.1.2. Pseudoternary Phase Diagrams
3.2. Characterization of Microemulsions
3.2.1. Particle Size and ζ-Potential Measurements
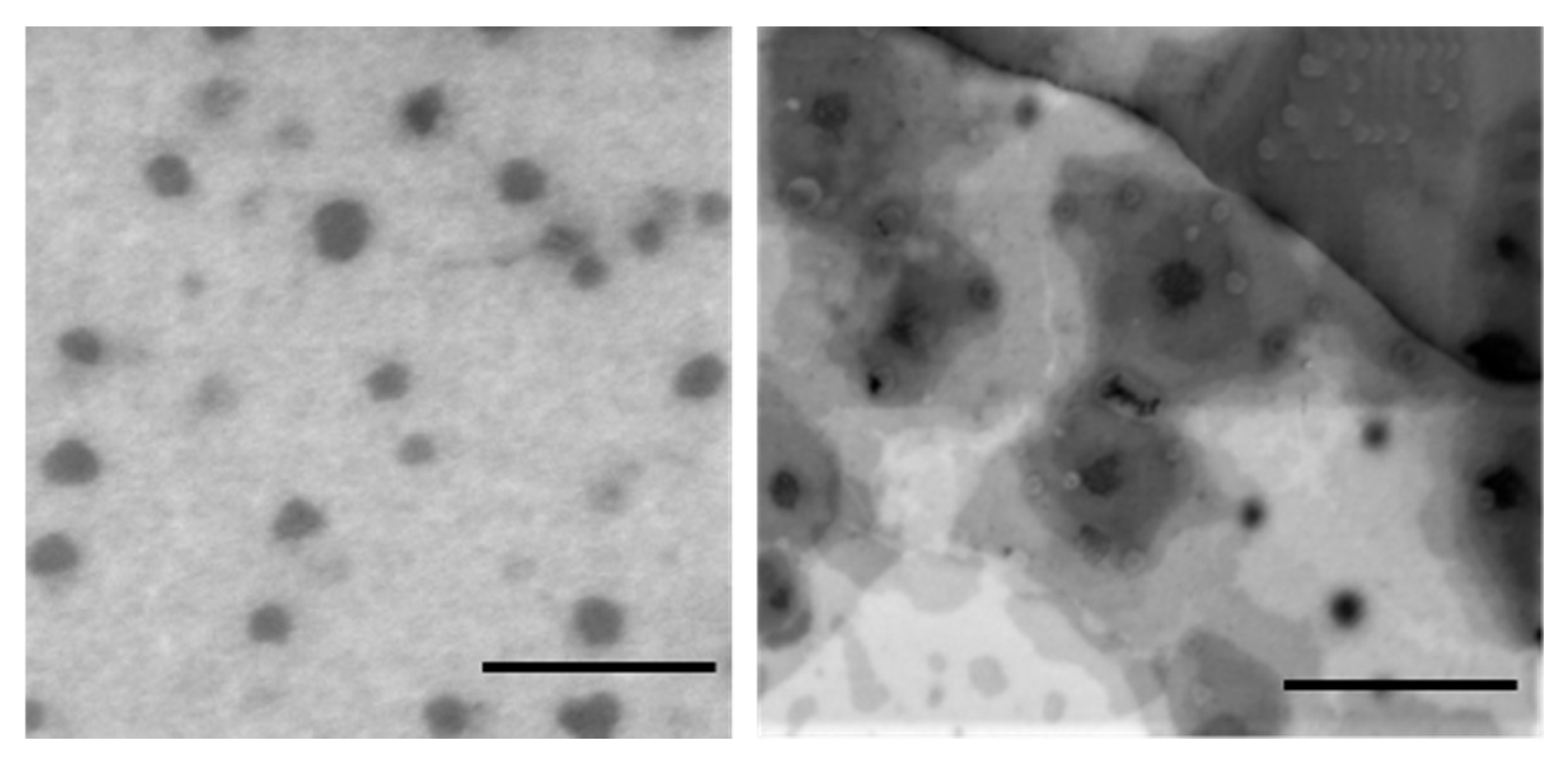
3.2.2. TEM Analysis
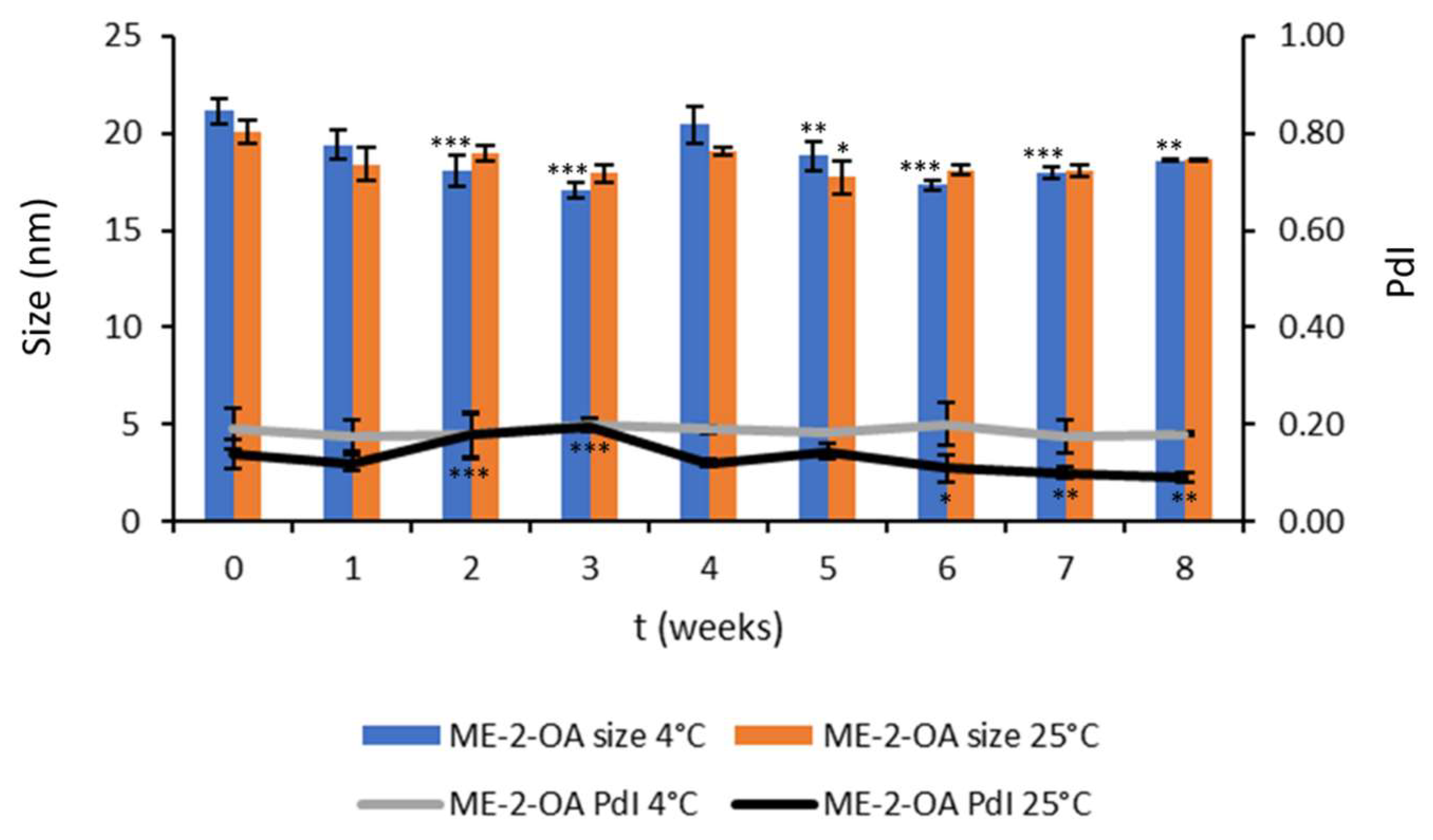
3.2.3. Chemical and Physical Stability during Storage
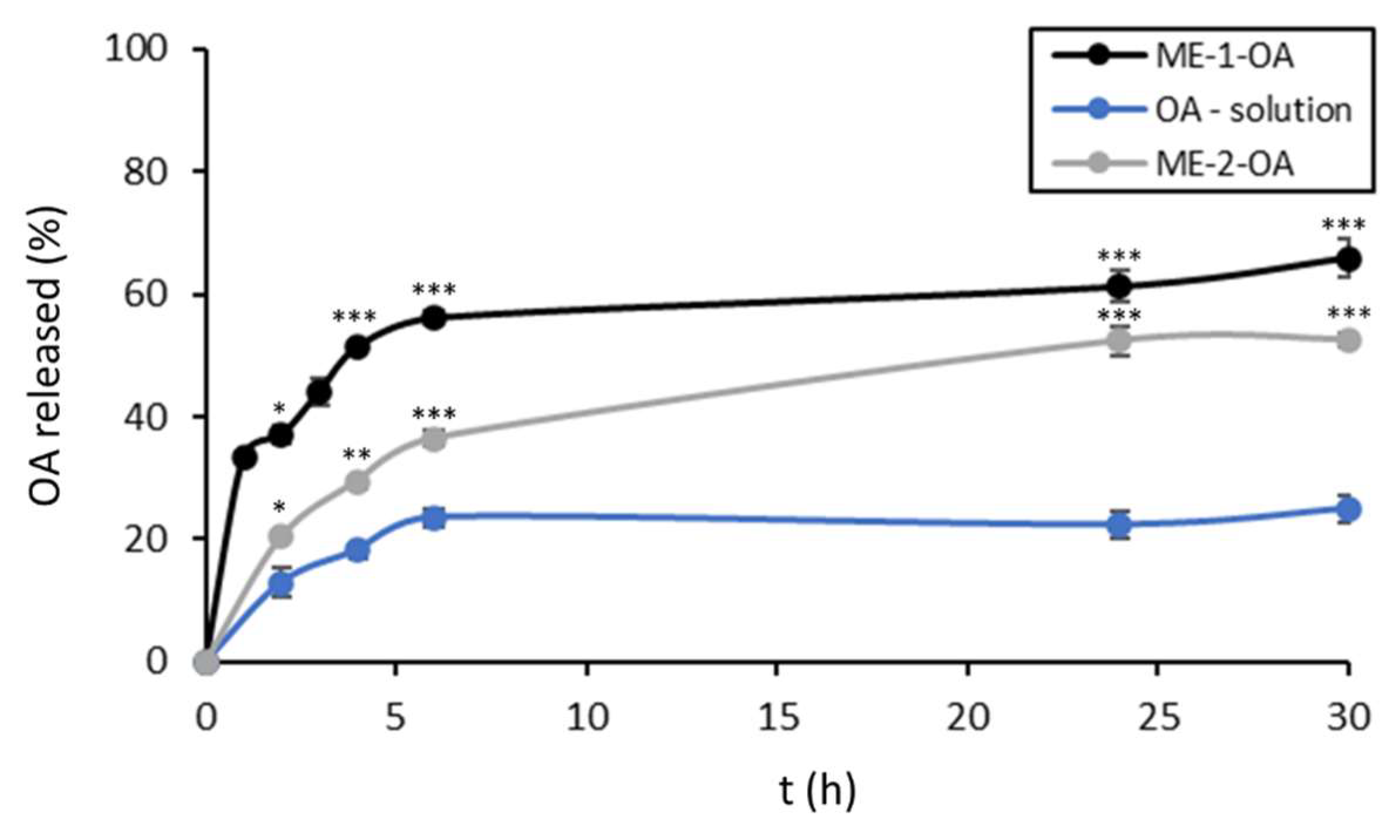
3.2.4. In Vitro Release Studies
3.2.5. PAMPA Assay
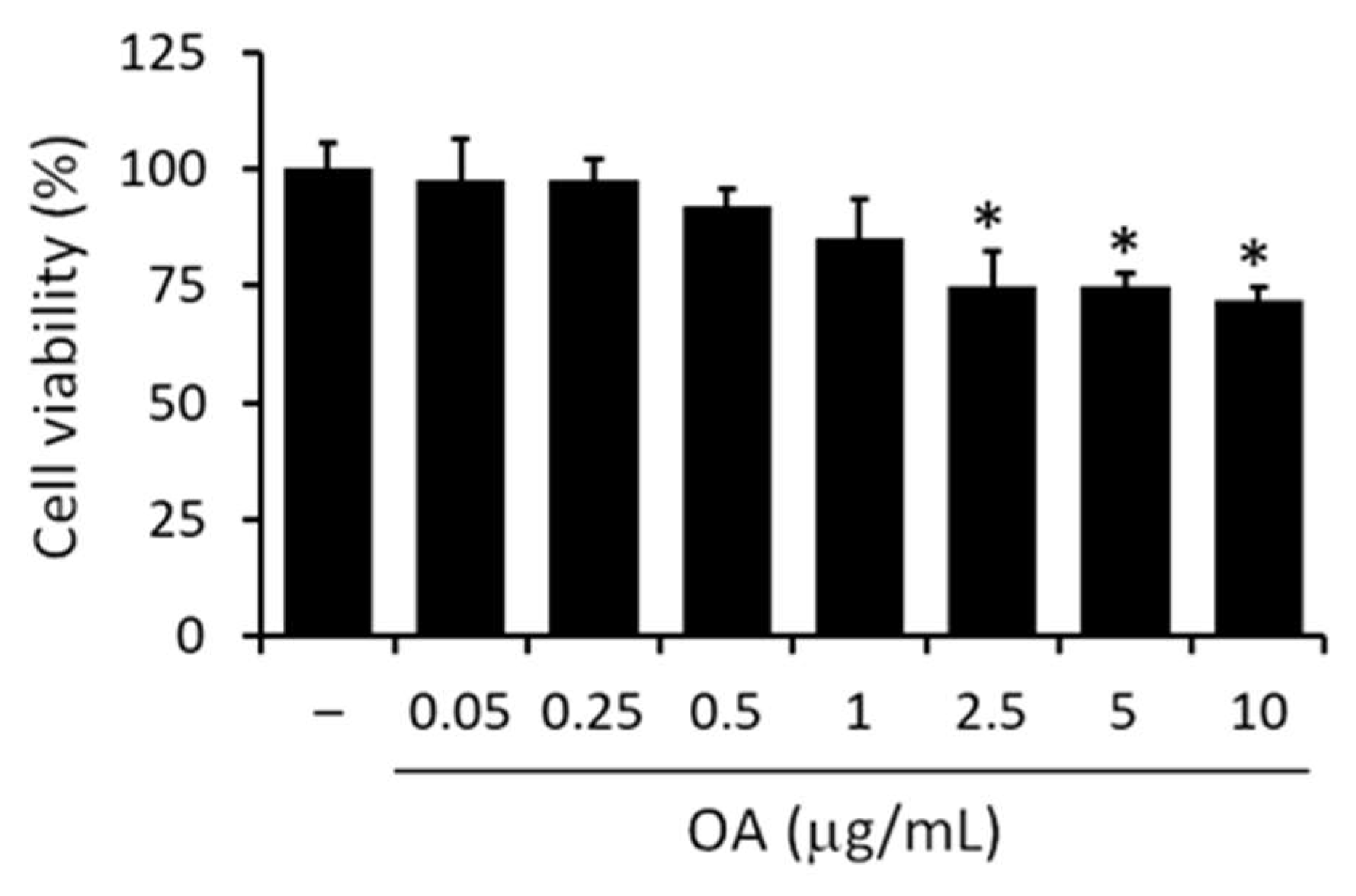
3.3. Effect of OA on Macrophages Cell Viability
3.4. Effect of OA on LPS-Induced Harmful Effects on Macrophages
3.5. Bio-Enhancement of OA Activity Once Loaded into Microemulsions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Matschke, V.; Theiss, C.; Matschke, J. Oxidative Stress: The Lowest Common Denominator of Multiple Diseases. Neural Regen. Res. 2019, 14, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Iles, K.E.; Forman, H.J. Macrophage signaling and respiratory burst. Immunol. Res. 2002, 26, 95–105. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B.; Kitts, D.D. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Singh, K.; Khedkar, R. Phytochemicals. In Functional and Preservative Properties of Phytochemicals; Elsevier: Amsterdam, The Netherlands, 2020; pp. 341–361. [Google Scholar]
- Bilia, A.R.; Isacchi, B.; Righeschi, C.; Guccione, C.; Bergonzi, M.C. Flavonoids Loaded in Nanocarriers: An Opportunity to Increase Oral Bioavailability and Bioefficacy. Food Sci. Nutr. 2014, 5, 1212–1327. [Google Scholar] [CrossRef]
- Bilia, A.R.; Guccione, C.; Isacchi, B.; Righeschi, C.; Firenzuoli, F.; Bergonzi, M.C. Essential Oils Loaded in Nanosystems: A Developing Strategy for a Successful Therapeutic Approach. Evid.-Based Complement. Altern. Med. 2014, 2014, 651593. [Google Scholar] [CrossRef]
- Puglia, C.; Lauro, M.R.; Tirendi, G.G.; Fassari, G.E.; Carbone, C.; Bonina, F.; Puglisi, G. Modern drug delivery strategies applied to natural active compounds. Expert. Opin. Drug Deliv. 2017, 14, 755–768. [Google Scholar] [CrossRef]
- Shanmugam, H.; Rengarajan, C.; Nataraj, S.; Sharma, A. Interactions of plant food bioactives-loaded nano delivery systems at the nano-bio interface and its pharmacokinetics: An overview. Food Front. 2022, 3, 256–275. [Google Scholar] [CrossRef]
- Bergonzi, M.C.; Bilia, A.R.; Landucci, E. Applications of innovative technologies to the delivery of antipsychotics. Drug Discov. Today 2022, 27, 401–421. [Google Scholar] [CrossRef]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. Emerging Frontiers in Drug Delivery. J. Am. Chem. Soc. 2016, 138, 704–717. [Google Scholar] [CrossRef]
- Rahdar, A.; Hasanein, P.; Bilal, M.; Beyzaei, H.; Kyzas, G.Z. Quercetin-loaded F127 nanomicelles: Antioxidant activity and protection against renal injury induced by gentamicin in rats. Life Sci. 2021, 276, 119420. [Google Scholar] [CrossRef]
- Verma, R.; Mittal, V.; Pandey, P.; Bhatia, S.; Bhatia, M.; Karavasili, C.; Behl, T.; Al-Harrasi, A.; Tagde, P.; Kumar, M.; et al. Exploring the Role of Self-Nanoemulsifying Systems in Drug Delivery: Challenges, Issues, Applications and Recent Advances. Curr Drug Deliv. 2022. [Google Scholar] [CrossRef]
- Jeon, S.-W.; Jin, H.-S.; Park, Y.-J. Formation of Self-Assembled Liquid Crystalline Nanoparticles and Absorption Enhancement of Ω-3s by Phospholipids and Oleic Acids. Pharmaceutics 2022, 14, 68. [Google Scholar] [CrossRef]
- Razzaq, S.; Rauf, A.; Raza, A.; Akhtar, S.; Tabish, T.A.; Sandhu, M.A.; Zaman, M.; Ibrahim, I.M.; Shahnaz, G.; Rahdar, A.; et al. A Multifunctional Polymeric Micelle for Targeted Delivery of Paclitaxel by the Inhibition of the P-Glycoprotein Transporters. Nanomaterials 2021, 11, 2858. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Farmany, A.; Alikhani, M.Y.; Taheri, M.; Asl, S.S.; Alamian, S.; Arabestani, M.R. Co-Delivery of Doxycycline and Hydroxychloroquine Using CdTe-Labeled Solid Lipid Nanoparticles for Treatment of Acute and Chronic Brucellosis. Front. Chem. 2022, 10, 890252. [Google Scholar] [CrossRef]
- Rehman, F.U.; Shah, K.U.; Shah, S.U.; Khan, I.U.; Khan, G.M.; Khan, A. From nanoemulsions to self-nanoemulsions, with recent advances in self-nanoemulsifying drug delivery systems (SNEDDS). Expert Opin. Drug Deliv. 2017, 14, 1325–1340. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants - Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic Acid and Its Derivatives: Biological Activities and Therapeutic Potential in Chronic Diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Sen, A. Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases. World J. Clin. Cases 2020, 8, 1767–1792. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2020, 14, 623. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Feng, A.; Yang, S.; Sun, Y.; Zhang, L.; Bo, F.; Li, L. Development and Evaluation of Oleanolic Acid Dosage Forms and Its Derivatives. Biomed Res. Int. 2020, 2020, 1308749. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: Structure, Preparation and Application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef]
- Castaneda, O.A.; Lee, S.C.; Ho, C.T.; Huang, T.C. Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds. J. Food Drug. Anal. 2017, 25, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Wen, M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002, 277, 22131–22139. [Google Scholar] [CrossRef] [PubMed]
- Freemerman, A.J.; Johnson, A.R.; Sacks, G.N.; Milner, J.J.; Kirk, E.L.; Troester, M.A.; Macintyre, A.N.; Goraksha-Hicks, P.; Rathmell, J.C.; Makowski, L. Metabolic reprogramming of macrophages: Glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J. Biol. Chem. 2014, 289, 7884–7896. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, L.; Piazzini, V.; D’Ambrosio, M.; Luceri, C.; Rocco, F.; Innocenti, M.; Vanti, G.; Mulinacci, N.; Bergonzi, M.C. Formulation of a Phenol-Rich Extract from Unripe Olives (Olea Europaea l.) in Microemulsion to Improve Its Solubility and Intestinal Permeability. Molecules 2020, 25, 3198. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Murthy, R.S. Microemulsions: A potential drug delivery system. Curr Drug Deliv. 2006, 3, 167–180. [Google Scholar] [CrossRef]
- USP 25 the United States Pharmacopeia. Revision; NF 20 The National Formulary, 20th ed.; Supplement; United States Pharmacopeial Convention: Rockville, MD, USA, 2002; print. [Google Scholar]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl’Innocenti, D. Anti-Inflammatory Properties of the Marine Plant Posidonia Oceanica (L.) Delile. J. Ethnopharmacol. 2020, 247, 112252. [Google Scholar] [CrossRef]
- De Stefani, C.; Lodovichi, J.; Albonetti, L.; Salvatici, M.C.; Quintela, J.C.; Bilia, A.R.; Bergonzi, M.C. Solubility and Permeability Enhancement of Oleanolic Acid by Solid Dispersion in Poloxamers and γ-CD. Molecules 2022, 27, 3042. [Google Scholar] [CrossRef]
- Piazzini, V.; Bigagli, E.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Enhanced Solubility and Permeability of Salicis cortex Extract by Formulating as a Microemulsion. Planta Med. 2018, 84, 976–984. [Google Scholar] [CrossRef]
- Lodovichi, J.; Landucci, E.; Pitto, L.; Gisone, I.; D′Ambrosio, M.; Luceri, C.; Salvatici, M.C.; Bergonzi, M.C. Evaluation of the increase of the thymoquinone permeability formulated in polymeric micelles: In vitro test and in vivo toxicity assessment in Zebrafish embryos. Eur. J. Pharm. Sci. 2022, 169, 106090. [Google Scholar] [CrossRef]
- Piazzini, V.; Cinci, L.; D′Ambrosio, M.; Luceri, C.; Bilia, A.R.; Bergonzi, M.C. Solid Lipid Nanoparticles and Chitosan-coated Solid Lipid Nanoparticles as Promising Tool for Silybin Delivery: Formulation, Characterization, and In vitro Evaluation. Curr. Drug. Deliv. 2019, 16, 142–152. [Google Scholar] [CrossRef]
- Hussain, A.; Altamimi, M.A.; Alshehri, S.; Imam, S.S. Assessment of Solubility and Hansen Solubility Parameters of Rifampicin in Various Permeation Enhancers: Experimental and Computational Approach. J. Mol. Liq. 2021, 328, 115432. [Google Scholar] [CrossRef]
- Legen, I.; Kračun, M.; Salobir, M.; Kerč, J. The Evaluation of Some Pharmaceutically Acceptable Excipients as Permeation Enhancers for Amoxicillin. Int. J. Pharm. 2006, 308, 84–89. [Google Scholar] [CrossRef]
- Cencioni, C.; Spallotta, F.; Martelli, F.; Valente, S.; Mai, A.; Zeiher, A.M.; Gaetano, C. Oxidative Stress and Epigenetic Regulation in Ageing and Age-Related Diseases. Int. J. Mol. Sci. 2013, 14, 17643–17663. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.H.; Wang, H.; Ji, Y.L.; Ning, H.; Wang, S.F.; Zhang, C.; Lu, J.W.; Duan, Z.H.; Xu, D.X. Reactive Oxygen Species Contribute to Lipopolysaccharide-Induced Teratogenesis in Mice. Toxicol. Sci. 2008, 103, 149–157. [Google Scholar] [CrossRef]
- Kallapura, G.; Pumford, N.R.; Hernandez-velasco, X.; Hargis, B.M.; Tellez, G. Mechanisms involved in lipopolysaccharide derived ROS and RNS oxidative stress and septic shock. J. Microbiol. Res. Rev. 2014, 2, 6–11. [Google Scholar]
- Salehi, B.; Azzini, E.; Zucca, P.; Maria Varoni, E.; Anil Kumar, N.V.; Dini, L.; Panzarini, E.; Rajkovic, J.; Valere Tsouh Fokou, P.; Peluso, I.; et al. Plant-Derived Bioactives and Oxidative Stress-Related Disorders: A Key Trend towards Healthy Aging and Longevity Promotion. Appl. Sci. 2020, 10, 947. [Google Scholar] [CrossRef]
- Wang, X.; Ye, X.L.; Liu, R.; Chen, H.L.; Bai, H.; Liang, X.; Zhang, X.D.; Wang, Z.; Li, W.L.; Hai, C.X. Antioxidant Activities of Oleanolic Acid in Vitro: Possible Role of Nrf2 and MAP Kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef]
- Kim, C.-K.; Cho, Y.-J.; Gao, Z.-G. Preparation and Evaluation of Biphenyl Dimethyl Dicarboxylate Microemulsions for Oral Delivery. J. Control. Release 2001, 70, 149–155. [Google Scholar] [CrossRef]
- Constantinides, P.P. Lipid microemulsions for improving drug dissolution and oral absorption: Physical and biopharmaceutical aspects. Pharm. Res. 1995, 12, 1561–1572. [Google Scholar] [CrossRef]
- Pouton, C.W. Formulation of Poorly Water-Soluble Drugs for Oral Administration: Physicochemical and Physiological Issues and the Lipid Formulation Classification System. Eur. J. Pharm. Sci. 2006, 29, 278–287. [Google Scholar] [CrossRef]
- Djekic, L.; Primorac, M. The Influence of Cosurfactants and Oils on the Formation of Pharmaceutical Microemulsions Based on PEG-8 Caprylic/Capric Glycerides. Int. J. Pharm. 2008, 352, 231–239. [Google Scholar] [CrossRef]
- Mahdi, E.S.; Sakeena, M.H.; Abdulkarim, M.F.; Abdullah, G.Z.; Sattar, M.A.; Noor, A.M. Effect of Surfactant and Surfactant Blends on Pseudoternary Phase Diagram Behavior of Newly Synthesized Palm Kernel Oil Esters. Drug Des. Dev. Ther. 2011, 5, 311–323. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Nie, S.; Guo, H.; Pan, W. Effects of Transcutol P on the corneal permeability of drugs and evaluation of its ocular irritation of rabbit eyes. J. Pharm. Pharmacol. 2006, 58, 45–50. [Google Scholar] [CrossRef]
- Khoddami, A.; Ghazali, H.M.; Yassoralipour, A. Physicochemical Characteristics of Nigella Seed (Nigella sativa L.) Oil as Affected by Different Extraction Methods. J. Am. Oil Chem. Soc. 2011, 88, 533–540. [Google Scholar] [CrossRef]
- Ramya, A.R.; Sudheer, P.; Mohameid, A.S.; Das, K. Design and Evaluation of a Self-Emulsifying Drug Delivery System of Aripiprazole. Indian J. Pharm. Sci. 2019, 81, 1089–1098. [Google Scholar] [CrossRef]
- Lavanya, N.; Aparna, C.; Umamahesh, B. Formulation and evaluation of glipizide microemulsion. Int. J. Pharm. Sci. 2016, 8, 171–176. [Google Scholar]
- Pankaj, L.; Vrunda, S.; Shital, B. Development and optimization of self microemulsifying drug delivery of domperidone. Braz. J. Pharm. Sci. 2014, 50, 91–100. [Google Scholar] [CrossRef]
- Kommuru, T.R.; Gurley, B.; Khan, M.A.; Reddy, I.K. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: Formulation development and bioavailability assessment. Int. J. Pharm. 2001, 212, 233–246. [Google Scholar] [CrossRef]
- Xi, J.; Chang, Q.; Chan, C.K.; Meng, Z.Y.; Wang, G.N.; Sun, J.B.; Wang, Y.T.; Tong, H.H.; Zheng, Y. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech 2009, 10, 172–182. [Google Scholar] [CrossRef]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduño-Ramirez, M.L.; Clares, B.; García, M.L.; Calpena, A.C. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef]
- He, C.X.; He, Z.G.; Gao, J.Q. Microemulsions as drug delivery systems to improve the solubility and the bioavailability of poorly water-soluble drugs. Expert Opin. Drug Deliv. 2010, 7, 445–460. [Google Scholar] [CrossRef]
- Yang, R.; Huang, X.; Dou, J.; Zhai, G.; Su, L. Self-microemulsifying drug delivery system for improved oral bioavailability of oleanolic acid: Design and evaluation. Int. J. Nanomed. 2013, 8, 2917–2926. [Google Scholar] [CrossRef]
- Wu, H.; Zhong, Q.X.; Zhong, R.L.; Huang, H.C.; Xia, Z.; Ke, Z.C.; Zhang, Z.H.; Song, J.; Jia, X.B. Preparation and antitumor evaluation of self-assembling oleanolic acid-loaded Pluronic P105/D-α-tocopheryl polyethylene glycol succinate mixed micelles for non-small-cell lung cancer treatment. Int. J. Nanomed. 2016, 11, 6337–6352. [Google Scholar] [CrossRef]
- An, J.Y.; Yang, H.S.; Park, N.R.; Koo, T.S.; Shin, B.; Lee, E.H.; Cho, S.H. Development of Polymeric Micelles of Oleanolic Acid and Evaluation of Their Clinical Efficacy. Nanoscale Res. Lett. 2020, 15, 133. [Google Scholar] [CrossRef]
- Djekic, L.; Primorac, M.; Filipic, S.; Agbaba, D. Investigation of surfactant/cosurfactant synergism impact on ibuprofen solubilization capacity and drug release characteristics of nonionic microemulsions. Int. J. Pharm. 2012, 433, 25–33. [Google Scholar] [CrossRef]
- Ei Hlaing, N.H.; Pakpayat, N.; Boonme, P. Stability and Release Kinetics of Natural Oil Microemulsions Containing Nicotinamide. J. Cosmet. Sci. 2020, 71, 23–36. [Google Scholar]
- Rege, B.D.; Kao, J.P.; Polli, J.E. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur. J. Pharm. Sci. 2002, 16, 237–246. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kondo, H.; Yasuda, T.; Watanabe, T.; Kobayashi, S.; Yokohama, S. Common solubilizers to estimate the Caco-2 transport of poorly water-soluble drugs. Int. J. Pharm. 2002, 246, 85–94. [Google Scholar] [CrossRef]
- Seljak, K.B.; Berginc, K.; Trontelj, J.; Zvonar, A.; Kristl, A.; Gašperlin, M. A self-microemulsifying drug delivery system to overcome intestinal resveratrol toxicity and presystemic metabolism. J. Pharm. Sci. 2014, 103, 3491–3500. [Google Scholar] [CrossRef]
- Sha, X.; Yan, G.; Wu, Y.; Li, J.; Fang, X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur. J. Pharm. Sci. 2005, 24, 477–486. [Google Scholar] [CrossRef]
- Yin, Y.M.; Cui, F.D.; Mu, C.F.; Choi, M.K.; Kim, J.S.; Chung, S.J.; Shim, C.K.; Kim, D.D. Docetaxel microemulsion for enhanced oral bioavailability: Preparation and in vitro and in vivo evaluation. J. Control. Release 2009, 140, 86–94. [Google Scholar] [CrossRef]
- Zheng, Y.; Xu, G.; Ni, Q.; Wang, Y.; Gao, Q.; Zhang, Y. Microemulsion Delivery System Improves Cellular Uptake of Genipin and Its Protective Effect against Aβ1-42-Induced PC12 Cell Cytotoxicity. Pharmaceutics 2022, 14, 617. [Google Scholar] [CrossRef]



| Solvent | Solubility | Solvent | Solubility |
|---|---|---|---|
| Capmul PG-8/NF | 19.72 ± 0.13 | Isopropil myristate | 9.44 ± 1.05 |
| Capmul PG-12/NF | 16.42 ± 1.04 | Lauroglycole 90 | 10.75 ± 0.56 |
| Captex 300 | 3.74 ± 0.10 | Nigella oil | 35.40 ± 1.41 |
| Captex 355 | 3.19 ± 0.24 | Sunflower oil | 11.75 ± 0.46 |
| Capryol 90 | 24.95 ± 0.90 | Soybean oil | 1.06 ± 0.17 |
| Cremophor EL | 28.32 ± 0.93 | PEG 400 | 8.49 ± 0.58 |
| Labrafac Lipophile | 13.29 ± 0.87 | Transcutol HP | 41.02 ± 2.41 |
| Labrasol | 13.27 ± 0.21 | Triacetine | 3.09 ± 0.17 |
| Labrasol ALF | 11.98 ± 0.84 | Tween 20 | 6.19 ± 0.58 |
| Etyloleate | 2.13 ± 0.21 | Tween 80 | 4.56 ± 0.36 |
| Formulation | Capmul | Isopropyl Myristate/ Nigella Oil (1:1) | Tween 20 | Transcutol HP | Cremophor EL | Water |
|---|---|---|---|---|---|---|
| ME-1 | 6 | 17 | 37 | 40 | ||
| ME-2 | 4 | 30 | 16 | 50 |
| Sample | Size (nm) ± ds | PdI ± ds | ζ-Pot ± ds |
|---|---|---|---|
| ME-1 | 94.51 ± 2.17 | 0.21 ± 0.03 | −4.15 ± 0.05 |
| ME-2 | 15.62 ± 0.19 | 0.20 ± 0.07 | −9.87 ± 0.09 |
| ME-1-OA | 93.01 ± 3.37 | 0.20 ± 0.04 | −3.32 ± 0.02 |
| ME-2-OA | 17.62 ± 0.23 | 0.20 ± 0.07 | −11.63 ± 0.01 |
| Release Kinetics | ME-1-OA | ME-2-OA |
|---|---|---|
| Zero order | 0.4812 | 0.7518 |
| First order | 0.6326 | 0.8402 |
| Korsmeyer–Peppas | 0.4527 | 0.6329 |
| Hixson | 0.5822 | 0.8129 |
| Higuchi | 0.7194 | 0.9320 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Stefani, C.; Vasarri, M.; Salvatici, M.C.; Grifoni, L.; Quintela, J.C.; Bilia, A.R.; Degl’Innocenti, D.; Bergonzi, M.C. Microemulsions Enhance the In Vitro Antioxidant Activity of Oleanolic Acid in RAW 264.7 Cells. Pharmaceutics 2022, 14, 2232. https://doi.org/10.3390/pharmaceutics14102232
De Stefani C, Vasarri M, Salvatici MC, Grifoni L, Quintela JC, Bilia AR, Degl’Innocenti D, Bergonzi MC. Microemulsions Enhance the In Vitro Antioxidant Activity of Oleanolic Acid in RAW 264.7 Cells. Pharmaceutics. 2022; 14(10):2232. https://doi.org/10.3390/pharmaceutics14102232
Chicago/Turabian StyleDe Stefani, Chiara, Marzia Vasarri, Maria Cristina Salvatici, Lucia Grifoni, Jose Carlos Quintela, Anna Rita Bilia, Donatella Degl’Innocenti, and Maria Camilla Bergonzi. 2022. "Microemulsions Enhance the In Vitro Antioxidant Activity of Oleanolic Acid in RAW 264.7 Cells" Pharmaceutics 14, no. 10: 2232. https://doi.org/10.3390/pharmaceutics14102232
APA StyleDe Stefani, C., Vasarri, M., Salvatici, M. C., Grifoni, L., Quintela, J. C., Bilia, A. R., Degl’Innocenti, D., & Bergonzi, M. C. (2022). Microemulsions Enhance the In Vitro Antioxidant Activity of Oleanolic Acid in RAW 264.7 Cells. Pharmaceutics, 14(10), 2232. https://doi.org/10.3390/pharmaceutics14102232








