Harnessing Ultrasound for Targeting Drug Delivery to the Brain and Breaching the Blood–Brain Tumour Barrier
Abstract
1. Introduction
1.1. Therapeutic Challenges in Brain Cancer
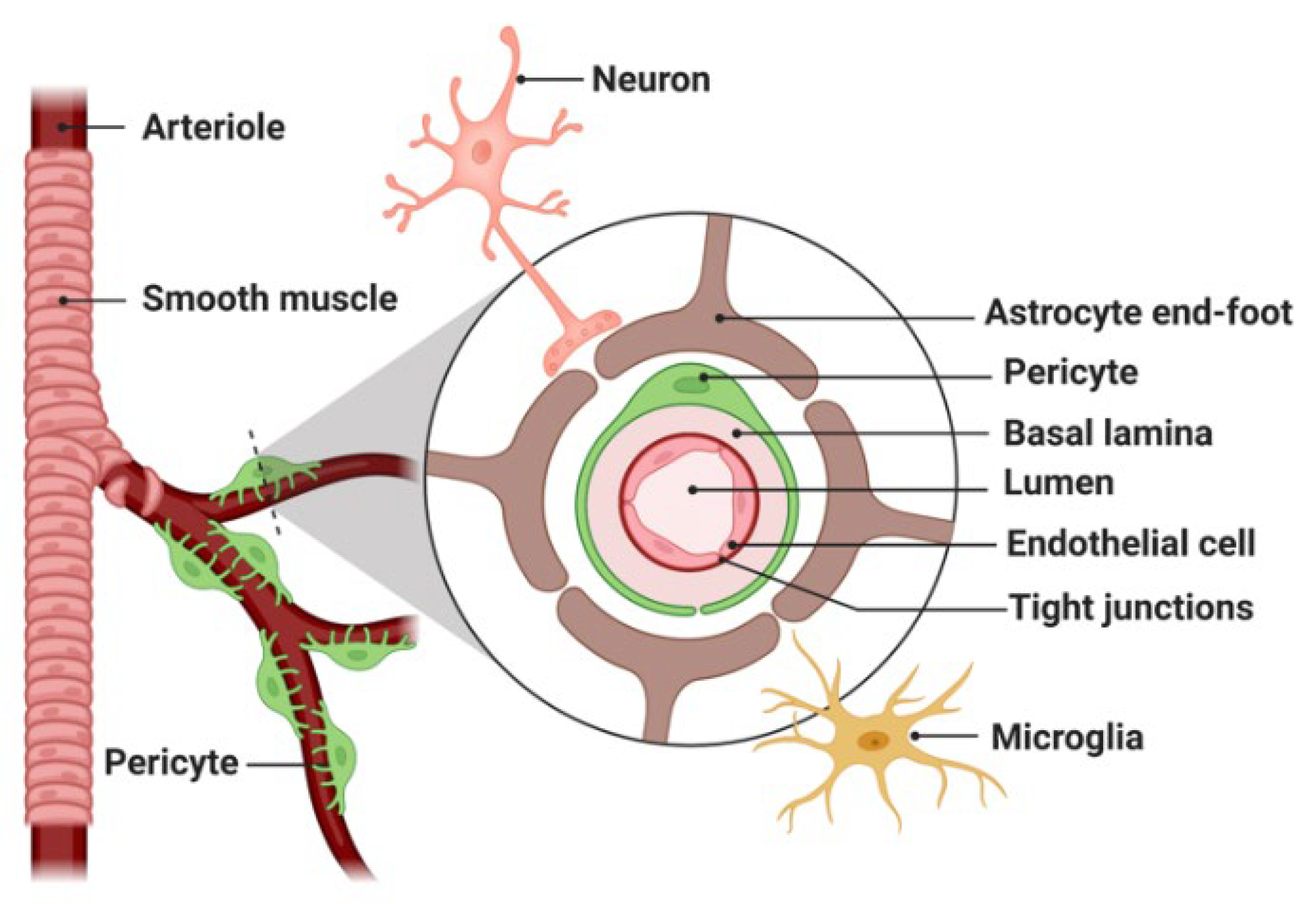
1.2. Nanomedicine and Limitations in Brain Drug Delivery
1.3. Strategies to Enhance Drug Delivery across the BBB/BTB
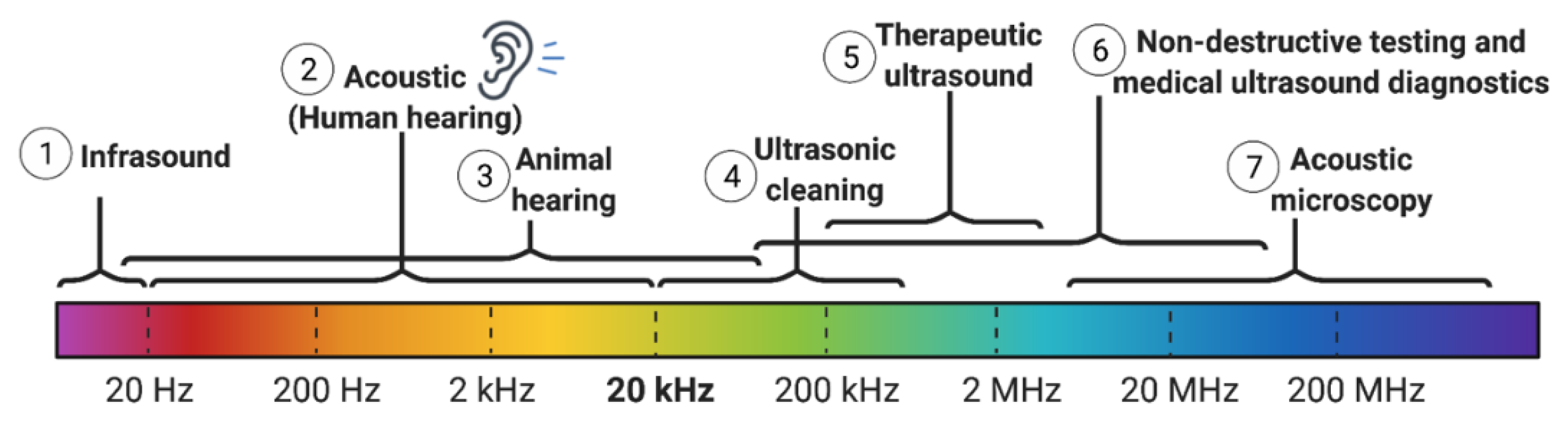
1.4. Physics of Ultrasound
1.5. Historical Perspective of Diagnostic and Therapeutic Applications of Ultrasound in Brain
1.5.1. Thermal Effects of Ultrasound
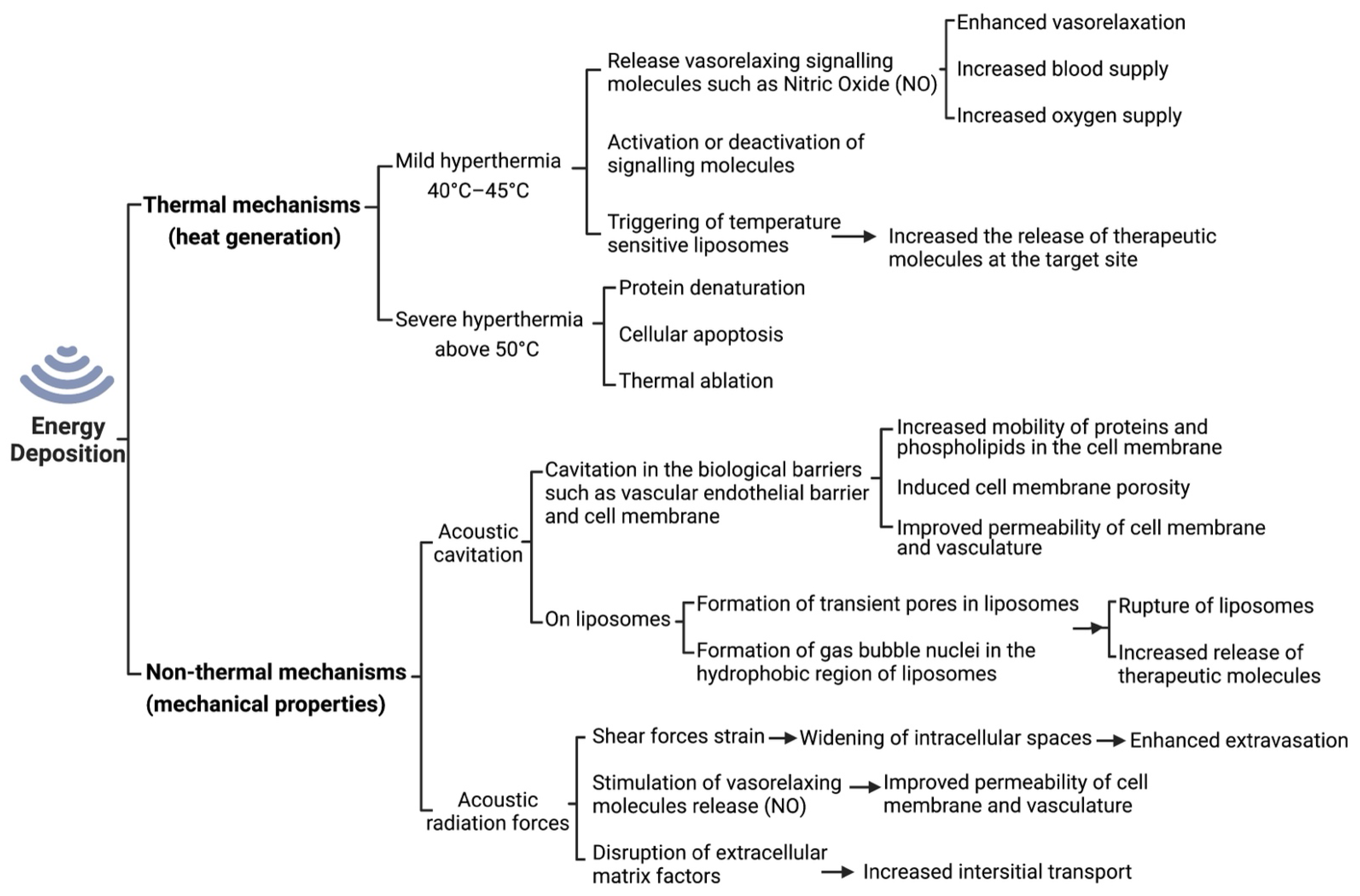
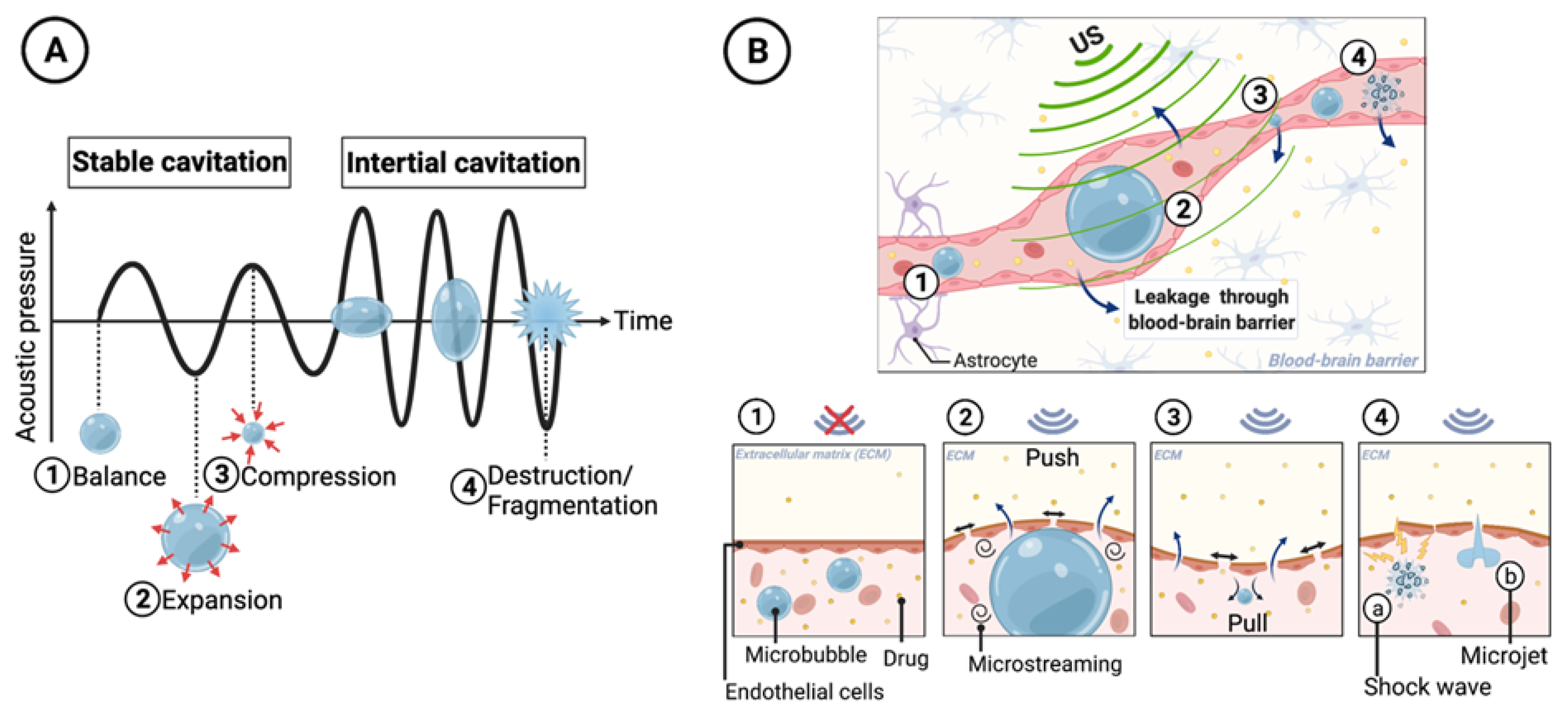
1.5.2. Non-Thermal Effects of Ultrasound
1.5.3. Ultrasound-Induced Vasorelaxation
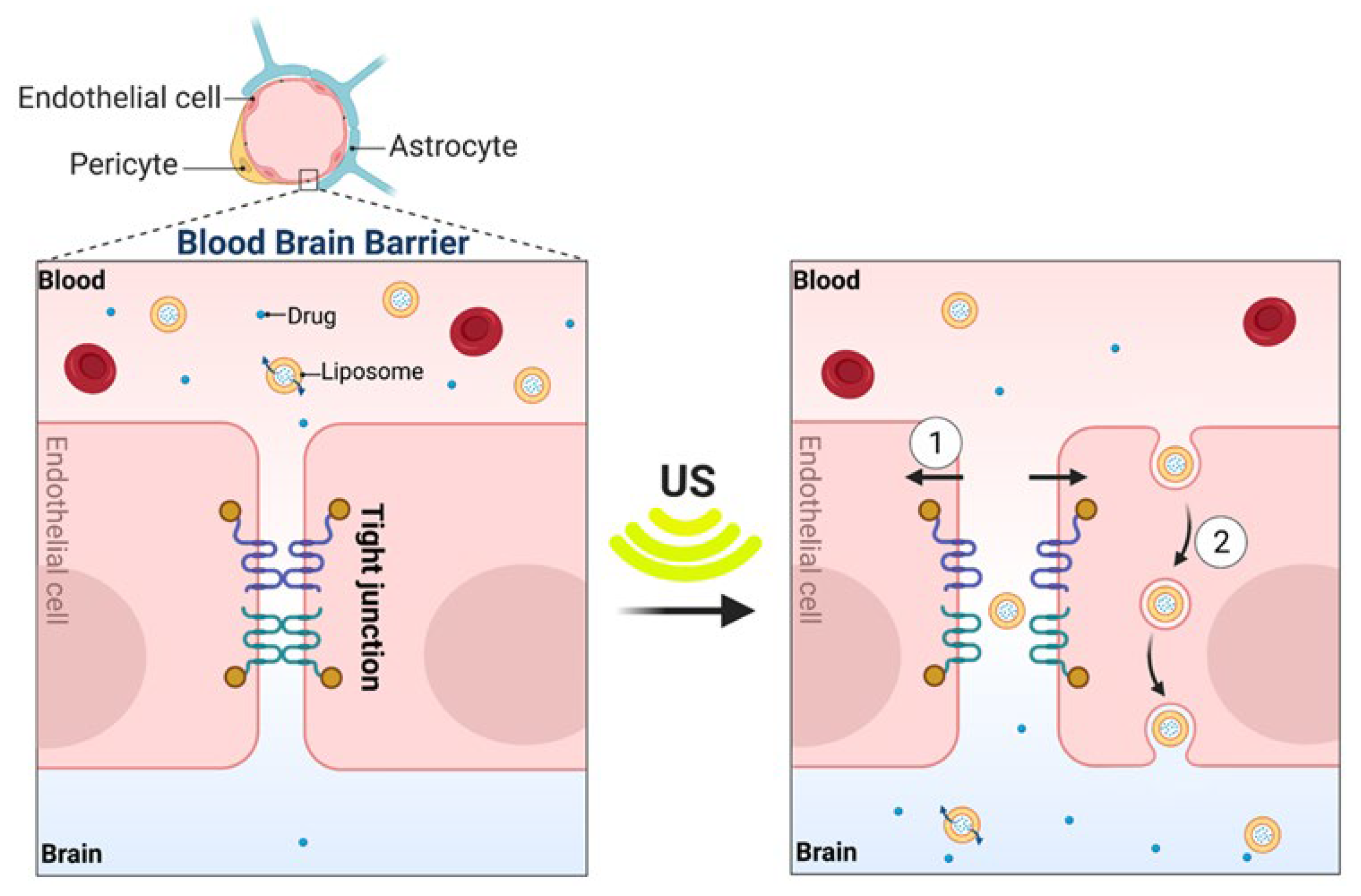
1.5.4. Ultrasound-Induced Permeability
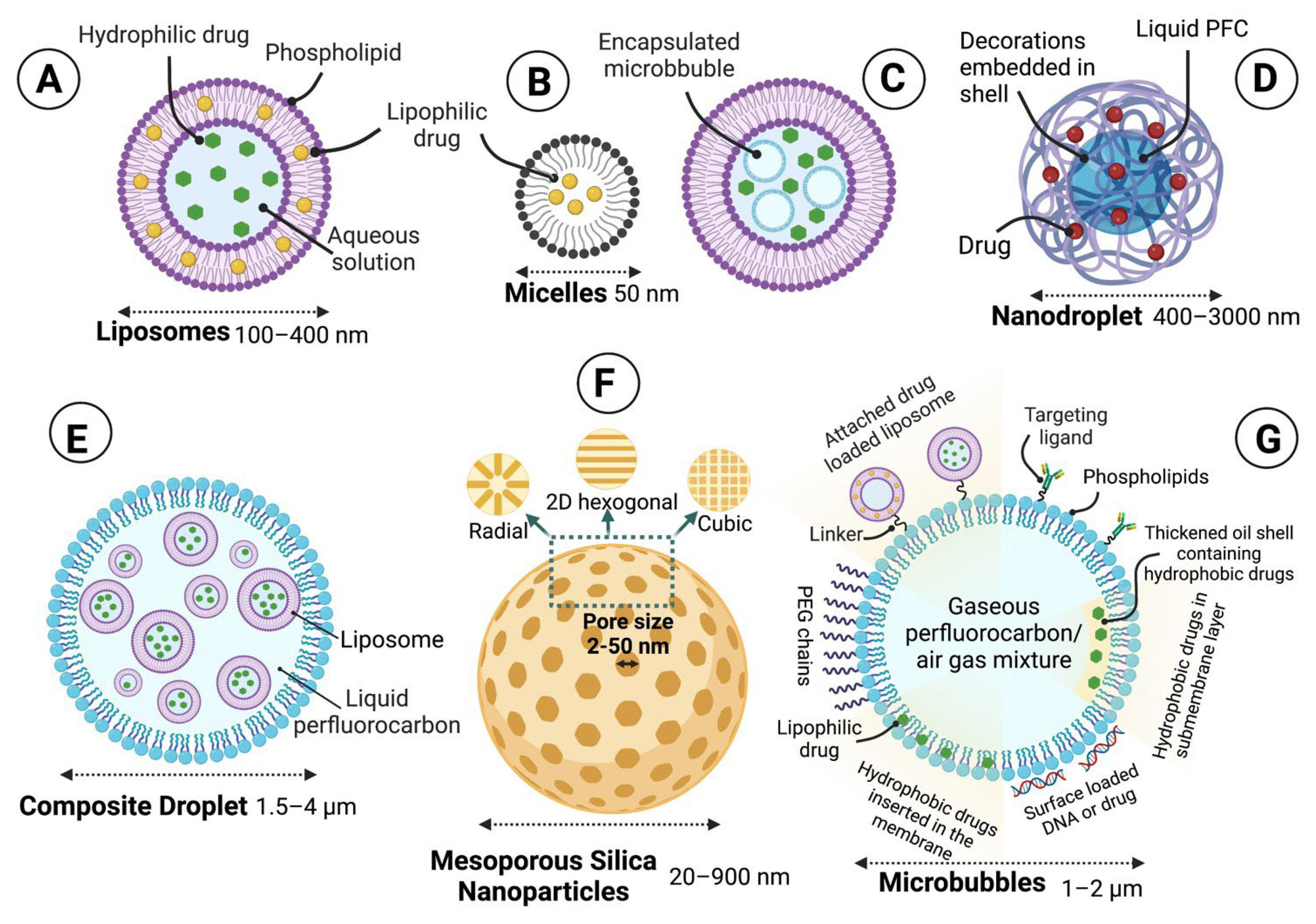
1.6. Ultrasound-Responsive Drug Release Systems
1.6.1. Thermal Effects of Ultrasound on Increased Drug Release from Nano/Micro-Particles
1.6.2. Mechanical Effects of Ultrasound on Increased Drug Release from Nano/micro-particles
1.6.3. Sono-Responsive Carriers
1.7. Pre-Clinical Models for Ultrasound-Mediated BBB Opening
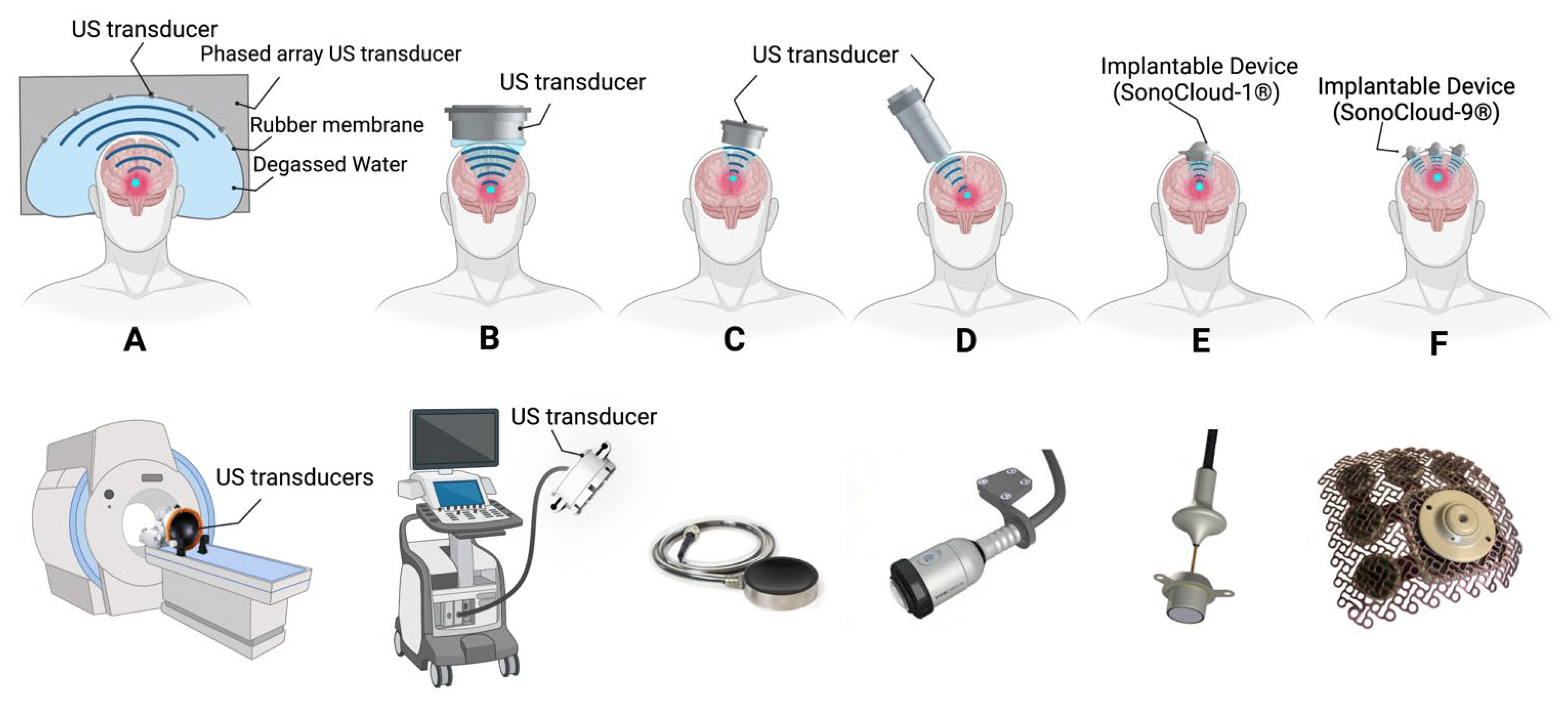
1.8. Ultrasound Devices Developed for Clinical Application of BBB Opening with Low-Intensity Pulsed Ultrasound
1.9. Ultrasound-Mediated Therapies in Clinical Trials
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misra, A.; Ganesh, S.; Shahiwala, A.; Shah, S.P. Drug delivery to the central nervous system: A review. J. Pharm. Pharm. Sci. 2003, 6, 252–273. [Google Scholar] [PubMed]
- Jain, R.K.; Di Tomaso, E.; Duda, D.G.; Loeffler, J.S.; Sorensen, A.G.; Batchelor, T.T. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 2007, 8, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Brem, S.; Abdullah, K.G. Glioblastoma E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, S.K.; Monsky, W.L.; Yuan, F.; Roberts, W.G.; Griffith, L.; Torchilin, V.P.; Jain, R.K. Regulation of transport pathways in tumor vessels: Role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 1998, 95, 4607–4612. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Barriers to drug delivery in solid tumors. Sci. Am. 1994, 271, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Sarkaria, J.N.; Hu, L.S.; Parney, I.F.; Pafundi, D.H.; Brinkmann, D.H.; Laack, N.N.; Giannini, C.; Burns, T.C.; Kizilbash, S.H.; Laramy, J.K. Is the blood–brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018, 20, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.A.; Cloughesy, T.F. Brain tumor treatment: Chemotherapy and other new developments. Semin. Oncol. Nurs. 2004, 20, 260–272. [Google Scholar] [CrossRef]
- Nhan, T.Q. Drug Delivery to the Brain by Focused Ultrasound and Microbubble Mediated Blood-Brain Barrier Disruption: Vascular-Level Investigation Using Two-Photon Fluorescent Microscopy; University of Toronto (Canada): Toronto, ON, USA, 2015. [Google Scholar]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A. Blood–brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Safaei, M. Application of various types of liposomes in drug delivery systems. Adv. Pharm. Bull. 2017, 7, 3. [Google Scholar] [CrossRef]
- Jagannathan, J.; Sanghvi, N.T.; Crum, L.A.; Yen, C.-P.; Medel, R.; Dumont, A.S.; Sheehan, J.P.; Steiner, L.; Jolesz, F.; Kassell, N.F. High-intensity focused ultrasound surgery of the brain: Part 1—A historical perspective with modern applications. Neurosurgery 2009, 64, 201–211. [Google Scholar] [CrossRef]
- Schroeder, A.; Kost, J.; Barenholz, Y. Ultrasound, liposomes, and drug delivery: Principles for using ultrasound to control the release of drugs from liposomes. Chem. Phys. Lipids 2009, 162, 1–16. [Google Scholar] [CrossRef]
- Huse, J.T.; Holland, E.C. Targeting brain cancer: Advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer 2010, 10, 319–331. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Marie, S.K.N.; Shinjo, S.M.O. Metabolism and brain cancer. Clinics 2011, 66, 33–43. [Google Scholar] [CrossRef]
- Olson, J.J.; Kalkanis, S.N.; Ryken, T.C. Congress of neurological surgeons systematic review and evidence-based guidelines for the treatment of adults with metastatic brain tumors: Executive summary. Neurosurgery 2019, 84, 550–552. [Google Scholar] [CrossRef]
- Walker, M.D.; Alexander, E.; Hunt, W.E.; MacCarty, C.S.; Mahaley, M.S.; Mealey, J.; Norrell, H.A.; Owens, G.; Ransohoff, J.; Wilson, C.B. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas: A cooperative clinical trial. J. Neurosurg. 1978, 49, 333–343. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Wen, P.Y.; Weller, M.; Lee, E.Q.; Alexander, B.M.; Barnholtz-Sloan, J.S.; Barthel, F.P.; Batchelor, T.T.; Bindra, R.S.; Chang, S.M.; Chiocca, E.A.; et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020, 22, 1073–1113. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015, 17, iv1–iv62. [Google Scholar] [CrossRef]
- McFaline-Figueroa, J.R.; Lee, E.Q. Brain tumors. Am. J. Med. 2018, 131, 874–882. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. The microenvironmental landscape of brain tumors. Cancer Cell 2017, 31, 326–341. [Google Scholar] [CrossRef]
- Lah, T.T.; Novak, M.; Breznik, B. Brain malignancies: Glioblastoma and brain metastases. In Proceedings of the Semin. Cancer Biol. 2020; pp. 262–273. [Google Scholar]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Barichello, T.; Collodel, A.; Hasbun, R.; Morales, R. An overview of the blood-brain barrier. In Blood-Brain Barrier; Humana Press: New York, NY, USA, 2019; pp. 1–8. [Google Scholar]
- Yang, F.Y.; Wong, T.T.; Teng, M.C.; Liu, R.S.; Lu, M.; Liang, H.F.; Wei, M.C. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control. Release 2012, 160, 652–658. [Google Scholar] [CrossRef]
- Agarwal, S.; Mittapalli, R.K.; Zellmer, D.M.; Gallardo, J.L.; Donelson, R.; Seiler, C.; Decker, S.A.; Santacruz, K.S.; Pokorny, J.L.; Sarkaria, J.N.; et al. Active efflux of Dasatinib from the brain limits efficacy against murine glioblastoma: Broad implications for the clinical use of molecularly targeted agents. Mol. Cancer Ther. 2012, 11, 2183–2192. [Google Scholar] [CrossRef]
- Wu, M.; Frieboes, H.B.; Chaplain, M.A.; McDougall, S.R.; Cristini, V.; Lowengrub, J.S. The effect of interstitial pressure on therapeutic agent transport: Coupling with the tumor blood and lymphatic vascular systems. J. Theor. Biol. 2014, 355, 194–207. [Google Scholar] [CrossRef]
- Pirzkall, A.; McGue, C.; Saraswathy, S.; Cha, S.; Liu, R.; Vandenberg, S.; Lamborn, K.R.; Berger, M.S.; Chang, S.M.; Nelson, S.J. Tumor regrowth between surgery and initiation of adjuvant therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2009, 11, 842–852. [Google Scholar] [CrossRef]
- Bode, U.; Zimmermann, M.; Moser, O.; Rutkowski, S.; Warmuth-Metz, M.; Pietsch, T.; Kortmann, R.; Faldum, A.; Fleischhack, G. Treatment of recurrent primitive neuroectodermal tumors (PNET) in children and adolescents with high-dose chemotherapy (HDC) and stem cell support: Results of the HITREZ 97 multicentre trial. J. Neurooncol. 2014, 120, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Merisko-Liversidge, E.M.; Liversidge, G.G. Drug nanoparticles: Formulating poorly water-soluble compounds. Toxicol. Pathol. 2008, 36, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lillard, J.W., Jr. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009, 86, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Tardi, P.; Boman, N.; Cullis, P. Liposomal doxorubicin. J. Drug Target. 1996, 4, 129–140. [Google Scholar] [CrossRef]
- Barenholz, Y.C. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941. [Google Scholar] [CrossRef]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef]
- Bhattacharyya, J.; Bellucci, J.J.; Weitzhandler, I.; McDaniel, J.R.; Spasojevic, I.; Li, X.; Lin, C.-C.; Chi, J.-T.A.; Chilkoti, A. A paclitaxel-loaded recombinant polypeptide nanoparticle outperforms Abraxane in multiple murine cancer models. Nat. Commun. 2015, 6, 7939. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Aboti, P.; Shah, P.; Patel, D.; Dalwadi, S. Quetiapine fumarate loaded solid lipid nanoparticles for improved oral bioavailability. Drug Deliv. Lett. 2014, 4, 170–184. [Google Scholar] [CrossRef]
- Sun, Y.; Kang, C.; Liu, F.; Song, L. Delivery of antipsychotics with nanoparticles. Drug Dev. Res. 2016, 77, 393–399. [Google Scholar] [CrossRef]
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendsen, C.N.; Heywood, P. Direct brain infusion of glial cell line–derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595. [Google Scholar] [CrossRef]
- Yin, D.; Forsayeth, J.; Bankiewicz, K.S. Optimized cannula design and placement for convection-enhanced delivery in rat striatum. J. Neurosci. Methods 2010, 187, 46–51. [Google Scholar] [CrossRef]
- Bobo, R.H.; Laske, D.W.; Akbasak, A.; Morrison, P.F.; Dedrick, R.L.; Oldfield, E.H. Convection-enhanced delivery of macromolecules in the brain. Proc. Natl. Acad. Sci. USA 1994, 91, 2076–2080. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Westphal, M.; Ram, Z.; Riddle, V.; Hilt, D.; Bortey, E. Gliadel® wafer in initial surgery for malignant glioma: Long-term follow-up of a multicenter controlled trial. Acta Neurochir. 2006, 148, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Kemper, E.M.; Boogerd, W.; Thuis, I.; Beijnen, J.H.; van Tellingen, O. Modulation of the blood–brain barrier in oncology: Therapeutic opportunities for the treatment of brain tumours? Cancer Treat. Rev. 2004, 30, 415–423. [Google Scholar] [CrossRef]
- Haluska, M.; Anthony, M.L. Osmotic blood-brain barrier modification for the treatment of malignant brain tumors. Clin. J. Oncol. Nurs. 2004, 8, 263–267. [Google Scholar] [CrossRef]
- Thiel, V.E.; Audus, K.L. Nitric oxide and blood–brain barrier integrity. Antioxid. Redox Signal. 2001, 3, 273–278. [Google Scholar] [CrossRef]
- Mayhan, W.G. Nitric oxide donor-induced increase in permeability of the blood–brain barrier. Brain Res. 2000, 866, 101–108. [Google Scholar]
- Treat, L.H.; McDannold, N.; Vykhodtseva, N.; Zhang, Y.; Tam, K.; Hynynen, K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using MRI-guided focused ultrasound. Int. J. Cancer 2007, 121, 901–907. [Google Scholar] [CrossRef]
- Mesiwala, A.H.; Farrell, L.; Wenzel, H.J.; Silbergeld, D.L.; Crum, L.A.; Winn, H.R.; Mourad, P.D. High-intensity focused ultrasound selectively disrupts the blood-brain barrier in vivo. Ultrasound Med. Biol. 2002, 28, 389–400. [Google Scholar] [CrossRef]
- Gandhi, K.; Barzegar-Fallah, A.; Banstola, A.; Rizwan, S.B.; Reynolds, J.N. Ultrasound-mediated blood–brain barrier disruption for drug delivery: A systematic review of protocols, efficacy, and safety outcomes from preclinical and clinical studies. Pharmaceutics 2022, 14, 833. [Google Scholar] [CrossRef]
- Wang, S.; Hossack, J.A.; Klibanov, A.L. From anatomy to functional and molecular biomarker imaging and therapy: Ultrasound is safe, ultrafast, portable, and inexpensive. Investig. Radiol. 2020, 55, 559–572. [Google Scholar] [CrossRef]
- Li, L.; ten Hagen, T.L.; Bolkestein, M.; Gasselhuber, A.; Yatvin, J.; van Rhoon, G.C.; Eggermont, A.M.; Haemmerich, D.; Koning, G.A. Improved intratumoral nanoparticle extravasation and penetration by mild hyperthermia. J. Control. Release 2013, 167, 130–137. [Google Scholar] [CrossRef]
- Hashizume, H.; Baluk, P.; Morikawa, S.; McLean, J.W.; Thurston, G.; Roberge, S.; Jain, R.K.; McDonald, D.M. Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 2000, 156, 1363–1380. [Google Scholar] [CrossRef]
- Böhmer, M.; Chlon, C.; Raju, B.; Chin, C.; Shevchenko, T.; Klibanov, A. Focused ultrasound and microbubbles for enhanced extravasation. J. Control. Release 2010, 148, 18–24. [Google Scholar] [CrossRef]
- Aryal, M.; Vykhodtseva, N.; Zhang, Y.-Z.; Park, J.; McDannold, N. Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood–tumor and blood–brain barriers improve outcomes in a rat glioma model. J. Control. Release 2013, 169, 103–111. [Google Scholar] [CrossRef]
- Aldrich, J.E. Basic physics of ultrasound imaging. Crit. Care Med. 2007, 35, S131–S137. [Google Scholar] [CrossRef]
- Borden, M.A.; Kruse, D.E.; Caskey, C.F.; Zhao, S.; Dayton, P.A.; Ferrara, K.W. Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 1992–2002. [Google Scholar] [CrossRef]
- Shibaguchi, H.; Tsuru, H.; Kuroki, M.; Kuroki, M. Sonodynamic cancer therapy: A non-invasive and repeatable approach using low-intensity ultrasound with a sonosensitizer. Anticancer Res. 2011, 31, 2425–2429. [Google Scholar]
- Hall, D.O.; Selfridge, A.R. Multi-Frequency Ultrasound Therapy Systems and Methods. Patent Cooperation Treaty Application No. PCT/US1995/002592, 3 March 1995. [Google Scholar]
- Draper, D.O.; Castel, J.C.; Castel, D. Rate of temperature increase in human muscle during 1 MHz and 3 MHz continuous ultrasound. J. Orthop. Sport. Phys. Ther. 1995, 22, 142–150. [Google Scholar] [CrossRef]
- Huber, P.E.; Jenne, J.W.; Rastert, R.; Simiantonakis, I.; Sinn, H.-P.; Strittmatter, H.-J.; von Fournier, D.; Wannenmacher, M.F.; Debus, J. A new noninvasive approach in breast cancer therapy using magnetic resonance imaging-guided focused ultrasound surgery. Cancer Res. 2001, 61, 8441–8447. [Google Scholar]
- Singh, V.; Shriwastava, M. Ultrasonic Hyperthermia for Cancer Treatment. Def. Sci. J. 1993, 43, 235. [Google Scholar] [CrossRef][Green Version]
- Warden, S.J.; Fuchs, R.K.; Kessler, C.K.; Avin, K.G.; Cardinal, R.E.; Stewart, R.L. Ultrasound produced by a conventional therapeutic ultrasound unit accelerates fracture repair. Phys. Ther. 2006, 86, 1118–1127. [Google Scholar] [CrossRef]
- Kennedy, J.E. High-intensity focused ultrasound in the treatment of solid tumours. Nat. Rev. Cancer 2005, 5, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Lynn, J.G.; Zwemer, R.L.; Chick, A.J.; Miller, A.E. A new method for the generation and use of focused ultrasound in experimental biology. J. Gen. Physiol. 1942, 26, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Leksell, L. Echo-encephalography. 1. Detection of intracranial complications following brain injury. Acta Chir. Scand. 1956, 110, 301–315. [Google Scholar] [PubMed]
- Meyers, R.; Fry, W.J.; Fry, F.J.; Dreyer, L.L.; Schultz, D.F.; Noyes, R.F. Early experiences with ultrasonic irradiation of the pallidofugal and nigral complexes in hyperkinetic and hypertonic disorders. J. Neurosurg. 1959, 16, 32–54. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.; Lindstrom, P.A.; Haymaker, W. Pathological effects of ultrasound on the human brain: A study of 25 cases in which ultrasonic irradiation was used as a lobotomy procedure. J. Neuropathol. Exp. Neurol. 1959, 18, 489–508. [Google Scholar] [CrossRef]
- Fry, F.J. Precision high intensity focusing ultrasonic machines for surgery. Am. J. Phys. Med. Rehabil. 1958, 37, 152–156. [Google Scholar] [CrossRef]
- Kremkau, F.W. Cancer therapy with ultrasound: A historical review. J. Clin. Ultrasound 1979, 7, 287–300. [Google Scholar] [CrossRef]
- Oka, M.; Okumura, T.; Yokoi, H.; Murao, T.; Miyashita, Y.; Oka, K.; Yoshitatsu, S.; Yoshioka, K.; Hirano, H.; Kawashima, Y. Surgical application of high intensity focused ultrasound. Med. J. Osaka Univ. 1960, 10, 42. [Google Scholar]
- Heimburger, R.; Fry, F.; Franklin, T.; Eggleton, R. Ultrasound potentiation of chemotherapy for brain malignancy. In Ultrasound in Medicine; Springer: Boston, MA, USA, 1975; pp. 273–281. [Google Scholar]
- Heimburger, R. Ultrasound augmentation of central nervous system tumor therapy. Indiana Med. 1985, 78, 469–476. [Google Scholar]
- Newell, J. Ultrasonics in medicine. Phys. Med. Biol. 1963, 8, 241. [Google Scholar] [CrossRef]
- Clement, G.T.; Hynynen, K. A non-invasive method for focusing ultrasound through the human skull. Phys. Med. Biol. 2002, 47, 1219. [Google Scholar] [CrossRef]
- Aubry, J.-F.; Tanter, M. MR-guided transcranial focused ultrasound. In Therapeutic Ultrasound; Springer: Cham, Switzerland, 2016; pp. 97–111. [Google Scholar]
- Hynynen, K.; Darkazanli, A.; Unger, E.; Schenck, J. MRI-guided noninvasive ultrasound surgery. Med. Phys. 1993, 20, 107–115. [Google Scholar] [CrossRef]
- Jeanmonod, D.; Werner, B.; Morel, A.; Michels, L.; Zadicario, E.; Schiff, G.; Martin, E. Transcranial magnetic resonance imaging–guided focused ultrasound: Noninvasive central lateral thalamotomy for chronic neuropathic pain. Neurosurg. Focus 2012, 32, E1. [Google Scholar] [CrossRef]
- Carpentier, A.; Canney, M.; Vignot, A.; Reina, V.; Beccaria, K.; Horodyckid, C.; Karachi, C.; Leclercq, D.; Lafon, C.; Chapelon, J.-Y. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci. Transl. Med. 2016, 8, 343re2. [Google Scholar] [CrossRef]
- Maimbourg, G.; Houdouin, A.; Deffieux, T.; Tanter, M.; Aubry, J.-F. 3D-printed adaptive acoustic lens as a disruptive technology for transcranial ultrasound therapy using single-element transducers. Phys. Med. Biol. 2018, 63, 025026. [Google Scholar] [CrossRef]
- Robertson, V.J.; Baker, K.G. A review of therapeutic ultrasound: Effectiveness studies. Phys. Ther. 2001, 81, 1339–1350. [Google Scholar] [CrossRef]
- Miller, D.L.; Smith, N.B.; Bailey, M.R.; Czarnota, G.J.; Hynynen, K.; Makin, I.R.S.; Bioeffects Committee of the American Institute of Ultrasound in Medicine. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012, 31, 623–634. [Google Scholar] [CrossRef]
- Mitragotri, S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef]
- Frenkel, V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv. Drug Deliv. Rev. 2008, 60, 1193–1208. [Google Scholar] [CrossRef]
- Dickson, J.; Calderwood, S. Temperature range and selective sensitivity of tumors to hyperthermia: A critical review. Ann. N. Y. Acad. Sci. 1980, 335, 180–205. [Google Scholar] [CrossRef]
- Silisteanu, S.C.; Antonescu, E.; Szakacs, J.; Totan, M.; ROMAN FILIP, C.; Serb, B.H.; Cernusca Mitariu, M.; Grigore, N.; Cernusca Mitariu, S. Study on changes in some physiological parameters under the action of therapeutic ultrasound. Rev. Chim. 2017, 68, 1306–1311. [Google Scholar] [CrossRef]
- Jones, E.L.; Oleson, J.R.; Prosnitz, L.R.; Samulski, T.V.; Vujaskovic, Z.; Yu, D.; Sanders, L.L.; Dewhirst, M.W. Randomized trial of hyperthermia and radiation for superficial tumors. J. Clin. Oncol. 2005, 23, 3079–3085. [Google Scholar] [CrossRef]
- Moros, E.G.; Peñagaricano, J.; Novàk, P.; Straube, W.L.; Myerson, R.J. Present and future technology for simultaneous superficial thermoradiotherapy of breast cancer. Int. J. Hyperth. 2010, 26, 699–709. [Google Scholar] [CrossRef]
- Frazier, N.; Ghandehari, H. Hyperthermia approaches for enhanced delivery of nanomedicines to solid tumors. Biotechnol. Bioeng. 2015, 112, 1967–1983. [Google Scholar] [CrossRef]
- Wu, S.-K.; Chiang, C.-F.; Hsu, Y.-H.; Lin, T.-H.; Liou, H.-C.; Fu, W.-M.; Lin, W.-L. Short-time focused ultrasound hyperthermia enhances liposomal doxorubicin delivery and antitumor efficacy for brain metastasis of breast cancer. Int. J. Nanomed. 2014, 9, 4485. [Google Scholar]
- Hynynen, K. MRI-guided focused ultrasound treatments. Ultrasonics 2010, 50, 221–229. [Google Scholar] [CrossRef]
- O’Brien, W.D., Jr. Ultrasound–biophysics mechanisms. Prog. Biophys. Mol. Biol. 2007, 93, 212–255. [Google Scholar] [CrossRef]
- Baker, K.G.; Robertson, V.J.; Duck, F.A. A review of therapeutic ultrasound: Biophysical effects. Phys. Ther. 2001, 81, 1351–1358. [Google Scholar] [CrossRef]
- Dalecki, D. Mechanical bioeffects of ultrasound. Annu. Rev. Biomed. Eng. 2004, 6, 229–248. [Google Scholar] [CrossRef] [PubMed]
- Ter Haar, G. Therapeutic applications of ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Herr, H.W. ‘Crushing the stone’: A brief history of lithotripsy, the first minimally invasive surgery. BJU Int. 2008, 102, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Eggers, J. Sonothrombolysis for treatment of acute ischemic stroke: Current evidence and new developments. Perspect. Med. 2012, 1, 14–20. [Google Scholar] [CrossRef]
- Rubiera, M.; Alexandrov, A.V. Sonothrombolysis in the management of acute ischemic stroke. Am. J. Cardiovasc. Drugs 2010, 10, 5–10. [Google Scholar] [CrossRef]
- Barnett, S.; Ter Haar, G.; Ziskin, M.; Nyborg, W.; Maeda, K.; Bang, J. Current status of research on biophysical effects of ultrasound. Ultrasound Med. Biol. 1994, 20, 205–218. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Vykhodtseva, N.; Jolesz, F.A. Noninvasive MR imaging–guided focal opening of the blood-brain barrier in rabbits. Radiology 2001, 220, 640–646. [Google Scholar] [CrossRef]
- Deng, C.X. Targeted drug delivery across the blood–brain barrier using ultrasound technique. Ther. Deliv. 2010, 1, 819–848. [Google Scholar] [CrossRef]
- Liu, H.-L.; Hua, M.-Y.; Chen, P.-Y.; Chu, P.-C.; Pan, C.-H.; Yang, H.-W.; Huang, C.-Y.; Wang, J.-J.; Yen, T.-C.; Wei, K.-C. Blood-brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 2010, 255, 415–425. [Google Scholar] [CrossRef]
- Maruo, A.; Hamner, C.E.; Rodrigues, A.J.; Higami, T.; Greenleaf, J.F.; Schaff, H.V. Nitric oxide and prostacyclin in ultrasonic vasodilatation of the canine internal mammary artery. Ann. Thorac. Surg. 2004, 77, 126–132. [Google Scholar] [CrossRef]
- Iida, K.; Luo, H.; Hagisawa, K.; Akima, T.; Shah, P.K.; Naqvi, T.Z.; Siegel, R.J. Noninvasive low-frequency ultrasound energy causes vasodilation in humans. J. Am. Coll. Cardiol. 2006, 48, 532–537. [Google Scholar] [CrossRef]
- Bakay, L.; Hueter, T.; Ballantine, H.; Sosa, D. Ultrasonically produced changes in the blood-brain barrier. AMA Arch. Neurol. 1956, 76, 457–467. [Google Scholar] [CrossRef]
- Ballantine, H.; Bell, E.; Manlapaz, J. Progress and problems in the neurological applications of focused ultrasound. J. Neurosurg. 1960, 17, 858–876. [Google Scholar] [CrossRef]
- Patrick, J.T.; Nolting, M.N.; Goss, S.A.; Dines, K.A.; Clendenon, J.L.; Rea, M.A.; Heimburger, R.F. Ultrasound and the blood-brain barrier. In Consensus on Hyperthermia for the 1990s; Springer: Berlin/Heidelberg, Germany, 1990; pp. 369–381. [Google Scholar]
- Vykhodtseva, N.; Hynynen, K.; Damianou, C. Histologic effects of high intensity pulsed ultrasound exposure with subharmonic emission in rabbit brain in vivo. Ultrasound Med. Biol. 1995, 21, 969–979. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Lin, M.I.; Murata, T.; Landskroner-Eiger, S.; Schleicher, M.; Kothiya, M.; Iwakiri, Y.; Yu, J.; Huang, P.L.; Sessa, W.C. eNOS-derived nitric oxide regulates endothelial barrier function through VE-cadherin and Rho GTPases. J. Cell Sci. 2013, 126, 5541–5552. [Google Scholar] [CrossRef][Green Version]
- Fyfe, M.C.; Bullock, M.I. Therapeutic ultrasound: Some historical background and development in knowledge of its effect on healing. Aust. J. Physiother. 1985, 31, 220–224. [Google Scholar] [CrossRef][Green Version]
- Stringham, S.B.; Viskovska, M.A.; Richardson, E.S.; Ohmine, S.; Husseini, G.A.; Murray, B.K.; Pitt, W.G. Over-pressure suppresses ultrasonic-induced drug uptake. Ultrasound Med. Biol. 2009, 35, 409–415. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Jones, R.M.; Barrett, E.; Schwab, A.; Head, E.; Hynynen, K. Investigation of the safety of focused ultrasound-induced blood-brain barrier opening in a natural canine model of aging. Theranostics 2017, 7, 3573. [Google Scholar] [CrossRef]
- Sheikov, N.; McDannold, N.; Sharma, S.; Hynynen, K. Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med. Biol. 2008, 34, 1093–1104. [Google Scholar] [CrossRef]
- Choi, H.; Lee, E.-H.; Han, M.; An, S.-H.; Park, J. Diminished expression of P-glycoprotein using focused ultrasound is associated with JNK-dependent signaling pathway in cerebral blood vessels. Front. Neurosci. 2019, 13, 1350. [Google Scholar] [CrossRef]
- Cho, H.; Lee, H.-Y.; Han, M.; Choi, J.-r.; Ahn, S.; Lee, T.; Chang, Y.; Park, J. Localized down-regulation of P-glycoprotein by focused ultrasound and microbubbles induced blood-brain barrier disruption in rat brain. Sci. Rep. 2016, 6, 31201. [Google Scholar] [CrossRef]
- Aryal, M.; Fischer, K.; Gentile, C.; Gitto, S.; Zhang, Y.-Z.; McDannold, N. Effects on P-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles. PLoS ONE 2017, 12, e0166061. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, H.; Mayer, M.; Deng, C.X. Spatiotemporally controlled single cell sonoporation. Proc. Natl. Acad. Sci. USA 2012, 109, 16486–16491. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Chen, D.; Deng, C. Improving ultrasound gene transfection efficiency by controlling ultrasound excitation of microbubbles. J. Control. Release 2013, 170, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.X.; Sieling, F.; Pan, H.; Cui, J. Ultrasound-induced cell membrane porosity. Ultrasound Med. Biol. 2004, 30, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, K.; Cui, J.; Ye, J.; Deng, C. Controlled permeation of cell membrane by single bubble acoustic cavitation. J. Control. Release 2012, 157, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Pitt, W.G.; Husseini, G.A.; Staples, B.J. Ultrasonic drug delivery–a general review. Expert Opin. Drug Deliv. 2004, 1, 37–56. [Google Scholar] [CrossRef]
- Liu, H.-L.; Fan, C.-H.; Ting, C.-Y.; Yeh, C.-K. Combining microbubbles and ultrasound for drug delivery to brain tumors: Current progress and overview. Theranostics 2014, 4, 432. [Google Scholar] [CrossRef]
- Wasielewska, J.M.; White, A.R. Focused ultrasound-mediated drug delivery in humans—A path towards translation in neurodegenerative diseases. Pharm. Res. 2022, 39, 427–439. [Google Scholar] [CrossRef]
- Loverock, P.; ter Haar, G.; Ormerod, M.; Imrie, P. The effect of ultrasound on the cytoxicity of adriamycin. Br. J. Radiol. 1990, 63, 542–546. [Google Scholar] [CrossRef]
- Tachibana, K.; Uchida, T.; Tamura, K.; Eguchi, H.; Yamashita, N.; Ogawa, K. Enhanced cytotoxic effect of Ara-C by low intensity ultrasound to HL-60 cells. Cancer Lett. 2000, 149, 189–194. [Google Scholar] [CrossRef]
- Connor, J.; Yatvin, M.B.; Huang, L. pH-sensitive liposomes: Acid-induced liposome fusion. Proc. Natl. Acad. Sci. USA 1984, 81, 1715–1718. [Google Scholar] [CrossRef]
- Ishida, T.; Okada, Y.; Kobayashi, T.; Kiwada, H. Development of pH-sensitive liposomes that efficiently retain encapsulated doxorubicin (DXR) in blood. Int. J. Pharm. 2006, 309, 94–100. [Google Scholar] [CrossRef]
- Simões, S.; Moreira, J.N.; Fonseca, C.; Düzgüneş, N.; de Lima, M.C.P. On the formulation of pH-sensitive liposomes with long circulation times. Adv. Drug Deliv. Rev. 2004, 56, 947–965. [Google Scholar] [CrossRef]
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A new temperature-sensitive liposome for use with mild hyperthermia: Characterization and testing in a human tumor xenograft model. Cancer Res. 2000, 60, 1197–1201. [Google Scholar]
- Ding, C.; Tong, L.; Feng, J.; Fu, J. Recent advances in stimuli-responsive release function drug delivery systems for tumor treatment. Molecules 2016, 21, 1715. [Google Scholar] [CrossRef]
- Meers, P. Enzyme-activated targeting of liposomes. Adv. Drug Deliv. Rev. 2001, 53, 265–272. [Google Scholar] [CrossRef]
- Fischel-Ghodsian, F.; Brown, L.; Mathiowitz, E.; Brandenburg, D.; Langer, R. Enzymatically controlled drug delivery. Proc. Natl. Acad. Sci. USA 1988, 85, 2403–2406. [Google Scholar] [CrossRef]
- Ghadiali, J.E.; Stevens, M.M. Enzyme-responsive nanoparticle systems. Adv. Mater. 2008, 20, 4359–4363. [Google Scholar] [CrossRef]
- Goldbart, R.; Traitel, T.; Lapidot, S.A.; Kost, J. Enzymatically controlled responsive drug delivery systems. Polym. Adv. Technol. 2002, 13, 1006–1018. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Proc. Natl. Acad. Sci. USA 2006, 103, 11719–11723. [Google Scholar] [CrossRef] [PubMed]
- Kost, J.; Leong, K.; Langer, R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc. Natl. Acad. Sci. USA 1989, 86, 7663–7666. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Prog. Polym. Sci. 2007, 32, 962–990. [Google Scholar] [CrossRef]
- Nakano, T.; Rizwan, S.B.; Myint, D.M.A.; Gray, J.; Mackay, S.M.; Harris, P.; Perk, C.G.; Hyland, B.I.; Empson, R.; Tan, E.W.; et al. An on-demand drug delivery system for control of epileptiform seizures. Pharmaceutics 2022, 14, 468. [Google Scholar] [CrossRef] [PubMed]
- Mackay, S.M.; Myint, D.M.A.; Easingwood, R.A.; Hegh, D.Y.; Wickens, J.R.; Hyland, B.I.; Jameson, G.N.; Reynolds, J.N.; Tan, E.W. Dynamic control of neurochemical release with ultrasonically-sensitive nanoshell-tethered liposomes. Commun. Chem. 2019, 2, 122. [Google Scholar] [CrossRef]
- Nakano, T.; Chin, C.; Myint, D.M.; Tan, E.W.; Hale, P.J.; Krishna, M.B.; Reynolds, J.N.; Wickens, J.; Dani, K.M. Mimicking subsecond neurotransmitter dynamics with femtosecond laser stimulated nanosystems. Sci. Rep. 2014, 4, 5398. [Google Scholar] [CrossRef][Green Version]
- Mannaris, C.; Efthymiou, E.; Meyre, M.-E.; Averkiou, M.A. In vitro localized release of thermosensitive liposomes with ultrasound-induced hyperthermia. Ultrasound Med. Biol. 2013, 39, 2011–2020. [Google Scholar] [CrossRef]
- Hijnen, N.; Langereis, S.; Grüll, H. Magnetic resonance guided high-intensity focused ultrasound for image-guided temperature-induced drug delivery. Adv. Drug Deliv. Rev. 2014, 72, 65–81. [Google Scholar] [CrossRef]
- Salomir, R.; Palussière, J.; Fossheim, S.L.; Rogstad, A.; Wiggen, U.N.; Grenier, N.; Moonen, C.T. Local delivery of magnetic resonance (MR) contrast agent in kidney using thermosensitive liposomes and MR imaging-guided local hyperthermia: A feasibility study in vivo. J. Magn. Reson. Imaging 2005, 22, 534–540. [Google Scholar] [CrossRef]
- Gray, M.D.; Lyon, P.C.; Mannaris, C.; Folkes, L.K.; Stratford, M.; Campo, L.; Chung, D.Y.; Scott, S.; Anderson, M.; Goldin, R. Focused ultrasound hyperthermia for targeted drug release from thermosensitive liposomes: Results from a phase I trial. Radiology 2019, 291, 232–238. [Google Scholar] [CrossRef]
- Jørgensen, K.; Mouritsen, O.G. Phase separation dynamics and lateral organization of two-component lipid membranes. Biophys. J. 1995, 69, 942–954. [Google Scholar] [CrossRef]
- Leidy, C.; Kaasgaard, T.; Crowe, J.H.; Mouritsen, O.G.; Jørgensen, K. Ripples and the formation of anisotropic lipid domains: Imaging two-component supported double bilayers by atomic force microscopy. Biophys. J. 2002, 83, 2625–2633. [Google Scholar] [CrossRef]
- Yatvin, M.B.; Weinstein, J.N.; Dennis, W.H.; Blumenthal, R. Design of liposomes for enhanced local release of drugs by hyperthermia. Science 1978, 202, 1290–1293. [Google Scholar] [CrossRef]
- Tagami, T.; Ernsting, M.J.; Li, S.-D. Efficient tumor regression by a single and low dose treatment with a novel and enhanced formulation of thermosensitive liposomal doxorubicin. J. Control. Release 2011, 152, 303–309. [Google Scholar] [CrossRef]
- Dou, Y.N.; Zheng, J.; Foltz, W.D.; Weersink, R.; Chaudary, N.; Jaffray, D.A.; Allen, C. Heat-activated thermosensitive liposomal cisplatin (HTLC) results in effective growth delay of cervical carcinoma in mice. J. Control. Release 2014, 178, 69–78. [Google Scholar] [CrossRef]
- Kneidl, B.; Peller, M.; Winter, G.; Lindner, L.H.; Hossann, M. Thermosensitive liposomal drug delivery systems: State of the art review. Int. J. Nanomed. 2014, 9, 4387. [Google Scholar]
- Wang, Z.-Y.; Zhang, H.; Yang, Y.; Xie, X.-Y.; Yang, Y.-F.; Li, Z.; Li, Y.; Gong, W.; Yu, F.-L.; Yang, Z. Preparation, characterization, and efficacy of thermosensitive liposomes containing paclitaxel. Drug Deliv. 2016, 23, 1222–1231. [Google Scholar] [CrossRef]
- Nardecchia, S.; Sánchez-Moreno, P.; de Vicente, J.; Marchal, J.A.; Boulaiz, H. Clinical trials of thermosensitive nanomaterials: An overview. Nanomaterials 2019, 9, 191. [Google Scholar] [CrossRef]
- Patrucco, D.; Terreno, E. MR-guided drug release from liposomes triggered by thermal and mechanical ultrasound-induced effects. Front. Phys. 2020, 8, 325. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Thomas, J.L. Factors affecting responsivity of unilamellar liposomes to 20 kHz ultrasound. Langmuir 2004, 20, 6100–6106. [Google Scholar] [CrossRef]
- Schroeder, A.; Avnir, Y.; Weisman, S.; Najajreh, Y.; Gabizon, A.; Talmon, Y.; Kost, J.; Barenholz, Y. Controlling liposomal drug release with low frequency ultrasound: Mechanism and feasibility. Langmuir 2007, 23, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Pong, M.; Umchid, S.; Guarino, A.J.; Lewin, P.A.; Litniewski, J.; Nowicki, A.; Wrenn, S.P. In vitro ultrasound-mediated leakage from phospholipid vesicles. Ultrasonics 2006, 45, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Thomas, J.L. Peg− Lipids and Oligo (ethylene Glycol) surfactants enhance the ultrasonic permeabilizability of liposomes. Langmuir 2003, 19, 1098–1105. [Google Scholar] [CrossRef]
- Barenholz, Y. Liposome application: Problems and prospects. Curr. Opin. Colloid Interface Sci. 2001, 6, 66–77. [Google Scholar] [CrossRef]
- Couture, O.; Foley, J.; Kassell, N.F.; Larrat, B.; Aubry, J.-F. Review of ultrasound mediated drug delivery for cancer treatment: Updates from pre-clinical studies. Transl Cancer Res 2014, 3, 494–511. [Google Scholar]
- Faez, T.; Emmer, M.; Kooiman, K.; Versluis, M.; van der Steen, A.F.; de Jong, N. 20 years of ultrasound contrast agent modeling. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 60, 7–20. [Google Scholar] [CrossRef]
- Tinkov, S.; Bekeredjian, R.; Winter, G.; Coester, C. Microbubbles as ultrasound triggered drug carriers. J. Pharm. Sci. 2009, 98, 1935–1961. [Google Scholar] [CrossRef]
- Ferrara, K.; Pollard, R.; Borden, M. Ultrasound microbubble contrast agents: Fundamentals and application to gene and drug delivery. Annu. Rev. Biomed. Eng. 2007, 9, 415–447. [Google Scholar] [CrossRef]
- Torchilin, V.P. Fluorescence microscopy to follow the targeting of liposomes and micelles to cells and their intracellular fate. Adv. Drug Deliv. Rev. 2005, 57, 95–109. [Google Scholar] [CrossRef]
- Husseini, G.A.; Pitt, W.G. Ultrasonic-activated micellar drug delivery for cancer treatment. J. Pharm. Sci. 2009, 98, 795–811. [Google Scholar] [CrossRef]
- Wood, A.K.; Sehgal, C.M. A review of low-intensity ultrasound for cancer therapy. Ultrasound Med. Biol. 2015, 41, 905–928. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Magin, R.; Yatvin, M.; Zaharko, D. Liposomes and local hyperthermia: Selective delivery of methotrexate to heated tumors. Science 1979, 204, 188–191. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Allen, T.; Chonn, A. Large unilamellar liposomes with low uptake into the reticuloendothelial system. FEBS Lett. 1987, 223, 42–46. [Google Scholar] [CrossRef]
- Drummond, D.C.; Meyer, O.; Hong, K.; Kirpotin, D.B.; Papahadjopoulos, D. Optimizing liposomes for delivery of chemotherapeutic agents to solid tumors. Pharmacol. Rev. 1999, 51, 691–744. [Google Scholar]
- Drummond, D.C.; Zignani, M.; Leroux, J. Current status of pH-sensitive liposomes in drug delivery. Prog. Lipid Res. 2000, 39, 409–460. [Google Scholar] [CrossRef]
- Allen, T.; Hansen, C.; Martin, F.; Redemann, C.; Yau-Young, A. Liposomes containing synthetic lipid derivatives of poly (ethylene glycol) show prolonged circulation half-lives in vivo. Biochim. Biophys. Acta (BBA)-Biomembr. 1991, 1066, 29–36. [Google Scholar] [CrossRef]
- Woodle, M.C. Controlling liposome blood clearance by surface-grafted polymers. Adv. Drug Deliv. Rev. 1998, 32, 139–152. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Xing, M.; Yan, F.; Yu, S.; Shen, P. Efficacy and cardiotoxicity of liposomal doxorubicin-based chemotherapy in advanced breast cancer: A meta-analysis of ten randomized controlled trials. PLoS ONE 2015, 10, e0133569. [Google Scholar] [CrossRef]
- Sachdeva, M.S. Drug targeting systems for cancer chemotherapy. Expert Opin. Investig. Drugs 1998, 7, 1849–1864. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Catane, R.; Uziely, B.; Kaufman, B.; Safra, T.; Cohen, R.; Martin, F.; Huang, A.; Barenholz, Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994, 54, 987–992. [Google Scholar] [PubMed]
- Lian, T.; Ho, R.J. Trends and developments in liposome drug delivery systems. J. Pharm. Sci. 2001, 90, 667–680. [Google Scholar] [CrossRef] [PubMed]
- American Chemical Society (CAS). Top 5 COVID-19 Vaccine Questions Answered; American Chemical Society (CAS): Columbus, OH, USA, 2020. [Google Scholar]
- American Chemical Society (CAS). Understanding the Nanotechnology in COVID-19 Vaccines. Available online: https://www.cas.org/resource/blog/understanding-nanotechnology-covid-19-vaccines (accessed on 21 January 2022).
- Turki, R. Preparation of Albumin-Targeted Liposomes and the Study of Their Release Characteristics Using Ultrasound. Master’s Thesis, American University of Sharjah, University City, United Arab Emirates, 2016. Available online: http://hdl.handle.net/11073/8107 (accessed on 21 January 2022).
- Evjen, T.J.; Hupfeld, S.; Barnert, S.; Fossheim, S.; Schubert, R.; Brandl, M. Physicochemical characterization of liposomes after ultrasound exposure–mechanisms of drug release. J. Pharm. Biomed. Anal. 2013, 78, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Miranda, D.; Lovell, J.F. Mechanisms of light-induced liposome permeabilization. Bioeng. Transl. Med. 2016, 1, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Viitala, L.; Pajari, S.; Lajunen, T.; Kontturi, L.-S.; Laaksonen, T.; Kuosmanen, P.i.; Viitala, T.; Urtti, A.; Murtomäki, L. Photothermally triggered lipid bilayer phase transition and drug release from gold nanorod and indocyanine green encapsulated liposomes. Langmuir 2016, 32, 4554–4563. [Google Scholar] [CrossRef]
- Wu, G.; Mikhailovsky, A.; Khant, H.A.; Zasadzinski, J.A. Synthesis, characterization, and optical response of gold nanoshells used to trigger release from liposomes. Methods Enzymol. 2009, 464, 279–307. [Google Scholar]
- Kwon, H.J.; Byeon, Y.; Jeon, H.N.; Cho, S.H.; Han, H.D.; Shin, B.C. Gold cluster-labeled thermosensitive liposmes enhance triggered drug release in the tumor microenvironment by a photothermal effect. J. Control. Release 2015, 216, 132–139. [Google Scholar] [CrossRef]
- Wu, G.; Mikhailovsky, A.; Khant, H.A.; Fu, C.; Chiu, W.; Zasadzinski, J.A. Remotely triggered liposome release by near-infrared light absorption via hollow gold nanoshells. J. Am. Chem. Soc. 2008, 130, 8175–8177. [Google Scholar] [CrossRef]
- An, X.; Zhan, F.; Zhu, Y. Smart photothermal-triggered bilayer phase transition in AuNPs–liposomes to release drug. Langmuir 2013, 29, 1061–1068. [Google Scholar] [CrossRef]
- Guo, H.; Kim, J.-C. Photothermally induced release from liposome suspended in mixture solution of gold nanoparticle and thermo-sensitive polymer. Colloids Surf. Physicochem. Eng. Asp. 2015, 469, 73–82. [Google Scholar] [CrossRef]
- Volodkin, D.V.; Skirtach, A.G.; Möhwald, H. Near-IR remote release from assemblies of liposomes and nanoparticles. Angew. Chem. 2009, 121, 1839–1841. [Google Scholar] [CrossRef]
- Marmottant, P.; Hilgenfeldt, S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature 2003, 423, 153–156. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Abd Shukor, S.; Vijayakumaran, A.; Vlatakis, S.; Wright, M.; Thanou, M. Phase-shift nanodroplets as an emerging sonoresponsive nanomaterial for imaging and drug delivery applications. Nanoscale 2022, 14, 2943–2965. [Google Scholar] [CrossRef]
- Kumar, K.N. Acoustic Studies on Nanodroplets, Microbubbles and Liposomes. Ph.D. Thesis, The George Washington University, Washington, DC, USA, 2018. [Google Scholar]
- Moyer, L.C.; Timbie, K.F.; Sheeran, P.S.; Price, R.J.; Miller, G.W.; Dayton, P.A. High-intensity focused ultrasound ablation enhancement in vivo via phase-shift nanodroplets compared to microbubbles. J. Ther. Ultrasound 2015, 3, 7. [Google Scholar] [CrossRef]
- Pelekanos, M.; Leinenga, G.; Odabaee, M.; Odabaee, M.; Saifzadeh, S.; Steck, R.; Götz, J. Establishing sheep as an experimental species to validate ultrasound-mediated blood-brain barrier opening for potential therapeutic interventions. Theranostics 2018, 8, 2583. [Google Scholar] [CrossRef]
- Dasgupta, A.; Liu, M.; Ojha, T.; Storm, G.; Kiessling, F.; Lammers, T. Ultrasound-mediated drug delivery to the brain: Principles, progress and prospects. Drug Discov. Today Technol. 2016, 20, 41–48. [Google Scholar] [CrossRef]
- Park, J.; Zhang, Y.; Vykhodtseva, N.; Jolesz, F.A.; McDannold, N.J. The kinetics of blood brain barrier permeability and targeted doxorubicin delivery into brain induced by focused ultrasound. J. Control. Release 2012, 162, 134–142. [Google Scholar] [CrossRef]
- Aryal, M.; Arvanitis, C.D.; Alexander, P.M.; McDannold, N. Ultrasound-mediated blood–brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 2014, 72, 94–109. [Google Scholar] [CrossRef]
- Brighi, C.; Salimova, E.; de Veer, M.; Puttick, S.; Egan, G. Translation of focused ultrasound for blood-brain barrier opening in glioma. J. Control. Release 2022, 345, 443–463. [Google Scholar] [CrossRef]
- Kinoshita, M.; McDannold, N.; Jolesz, F.A.; Hynynen, K. Targeted delivery of antibodies through the blood–brain barrier by MRI-guided focused ultrasound. Biochem. Biophys. Res. Commun. 2006, 340, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K. Ultrasound for drug and gene delivery to the brain. Adv. Drug Deliv. Rev. 2008, 60, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Hsieh, H.-Y.; Pitt, W.G.; Huang, C.-Y.; Tseng, I.-C.; Yeh, C.-K.; Wei, K.-C.; Liu, H.-L. Focused ultrasound-induced blood-brain barrier opening for non-viral, non-invasive, and targeted gene delivery. J. Control. Release 2015, 212, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Szablowski, J.O.; Lee-Gosselin, A.; Lue, B.; Malounda, D.; Shapiro, M.G. Acoustically targeted chemogenetics for the non-invasive control of neural circuits. Nat. Biomed. Eng. 2018, 2, 475–484. [Google Scholar] [CrossRef]
- Burgess, A.; Ayala-Grosso, C.A.; Ganguly, M.; Jordão, J.F.; Aubert, I.; Hynynen, K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS ONE 2011, 6, e27877. [Google Scholar] [CrossRef]
- Alkins, R.; Burgess, A.; Ganguly, M.; Francia, G.; Kerbel, R.; Wels, W.S.; Hynynen, K. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 2013, 73, 1892–1899. [Google Scholar] [CrossRef]
- Marquet, F.; Teichert, T.; Wu, S.-Y.; Tung, Y.-S.; Downs, M.; Wang, S.; Chen, C.; Ferrera, V.; Konofagou, E.E. Real-time, transcranial monitoring of safe blood-brain barrier opening in non-human primates. PLoS ONE 2014, 9, e84310. [Google Scholar] [CrossRef]
- McDannold, N.; Vykhodtseva, N.; Raymond, S.; Jolesz, F.A.; Hynynen, K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med. Biol. 2005, 31, 1527–1537. [Google Scholar] [CrossRef]
- Wang, J.B.; Di Ianni, T.; Vyas, D.B.; Huang, Z.; Park, S.; Hosseini-Nassab, N.; Aryal, M.; Airan, R.D. Focused ultrasound for noninvasive, focal pharmacologic neurointervention. Front. Neurosci. 2020, 14, 675. [Google Scholar] [CrossRef]
- Meng, Y.; Pople, C.B.; Lea-Banks, H.; Abrahao, A.; Davidson, B.; Suppiah, S.; Vecchio, L.M.; Samuel, N.; Mahmud, F.; Hynynen, K. Safety and efficacy of focused ultrasound induced blood-brain barrier opening, an integrative review of animal and human studies. J. Control. Release 2019, 309, 25–36. [Google Scholar] [CrossRef]
- Brighi, C.; Reid, L.; Genovesi, L.A.; Kojic, M.; Millar, A.; Bruce, Z.; White, A.L.; Day, B.W.; Rose, S.; Whittaker, A.K. Comparative study of preclinical mouse models of high-grade glioma for nanomedicine research: The importance of reproducing blood-brain barrier heterogeneity. Theranostics 2020, 10, 6361. [Google Scholar] [CrossRef]
- Karmur, B.S.; Philteos, J.; Abbasian, A.; Zacharia, B.E.; Lipsman, N.; Levin, V.; Grossman, S.; Mansouri, A. Blood-brain barrier disruption in neuro-oncology: Strategies, failures, and challenges to overcome. Front. Oncol. 2020, 10, 1811. [Google Scholar] [CrossRef]
- Deffieux, T.; Konofagou, E.E. Numerical study of a simple transcranial focused ultrasound system applied to blood-brain barrier opening. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 2637–2653. [Google Scholar] [CrossRef]
- Yoon, K.; Lee, W.; Lee, J.E.; Xu, L.; Croce, P.; Foley, L.; Yoo, S.-S. Effects of sonication parameters on transcranial focused ultrasound brain stimulation in an ovine model. PLoS ONE 2019, 14, e0224311. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.D.; Park, M.Y.; Foley, L.; Purcell-Estabrook, E.; Kim, H.; Fischer, K.; Maeng, L.-S.; Yoo, S.-S. Image-guided focused ultrasound-mediated regional brain stimulation in sheep. Ultrasound Med. Biol. 2016, 42, 459–470. [Google Scholar] [CrossRef]
- Huang, Y.; Alkins, R.; Schwartz, M.L.; Hynynen, K. Opening the blood-brain barrier with MR imaging–guided focused ultrasound: Preclinical testing on a trans–human skull porcine model. Radiology 2017, 282, 123–130. [Google Scholar] [CrossRef]
- McDannold, N.; Arvanitis, C.D.; Vykhodtseva, N.; Livingstone, M.S. Temporary disruption of the blood–brain barrier by use of ultrasound and microbubbles: Safety and efficacy evaluation in rhesus macaques. Cancer Res. 2012, 72, 3652–3663. [Google Scholar] [CrossRef]
- Pernot, M.; Aubry, J.-F.; Tanter, M.; Boch, A.-L.; Marquet, F.; Kujas, M.; Seilhean, D.; Fink, M. In vivo transcranial brain surgery with an ultrasonic time reversal mirror. J. Neurosurg. 2007, 106, 1061–1066. [Google Scholar] [CrossRef]
- Banstola, A.; Reynolds, J.N. Mapping sheep to human brain: The need for a sheep brain atlas. Front. Vet. Sci. 2022, 9, 1152. [Google Scholar] [CrossRef]
- McBride, S.D.; Morton, A.J. Indices of comparative cognition: Assessing animal models of human brain function. Exp. Brain Res. 2018, 236, 3379–3390. [Google Scholar] [CrossRef]
- Laure, B.; Petraud, A.; Sury, F.; Tranquart, F.; Goga, D. Resistance of the sheep skull after a monocortical cranial graft harvest. J. Craniomaxillofac. Surg. 2012, 40, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.-Q.; Lü, L.; Wang, F.; Luo, Y.; Lou, S.-F. Focused ultrasound-induced blood–brain barrier disruption enhances the delivery of cytarabine to the rat brain. J. Chemother. 2012, 24, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Wei, K.-C.; Chu, P.-C.; Wang, H.-Y.J.; Huang, C.-Y.; Chen, P.-Y.; Tsai, H.-C.; Lu, Y.-J.; Lee, P.-Y.; Tseng, I.-C.; Feng, L.-Y. Focused ultrasound-induced blood–brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: A preclinical study. PLoS ONE 2013, 8, e58995. [Google Scholar] [CrossRef]
- Mei, J.; Cheng, Y.; Song, Y.; Yang, Y.; Wang, F.; Liu, Y.; Wang, Z. Experimental study on targeted methotrexate delivery to the rabbit brain via magnetic resonance imaging–guided focused ultrasound. J. Ultrasound Med. 2009, 28, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Deng, J.; Wang, F.; Chen, S.; Liu, Y.; Wang, Z.; Wang, Z.; Cheng, Y. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood–brain barrier disruption. Exp. Neurol. 2012, 233, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.; Jordão, J.F.; O’Reilly, M.A.; Markham, K.; Weng, Y.-Q.; Foust, K.D.; Kaspar, B.K.; Hynynen, K.; Aubert, I. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum. Gene Ther. 2012, 23, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Reinz, E.; Leuchs, B.; Kleinschmidt, J.; Fatar, M.; Geers, B.; Lentacker, I.; Hennerici, M.G.; De Smedt, S.C.; Meairs, S. Focal delivery of AAV2/1-transgenes into the rat brain by localized ultrasound-induced BBB opening. Mol. Ther. Nucleic Acids 2013, 2, e73. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-H.; Wei, K.-C.; Huang, C.-Y.; Wen, C.-J.; Yen, T.-C.; Liu, C.-L.; Lin, Y.-T.; Chen, J.-C.; Shen, C.-R.; Liu, H.-L. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS ONE 2013, 8, e57682. [Google Scholar] [CrossRef]
- Hakimova, H.; Kim, S.; Chu, K.; Lee, S.K.; Jeong, B.; Jeon, D. Ultrasound stimulation inhibits recurrent seizures and improves behavioral outcome in an experimental model of mesial temporal lobe epilepsy. Epilepsy Behav. 2015, 49, 26–32. [Google Scholar] [CrossRef]
- Li, X.; Yang, H.; Yan, J.; Wang, X.; Li, X.; Yuan, Y. Low-intensity pulsed ultrasound stimulation modulates the nonlinear dynamics of local field potentials in temporal lobe epilepsy. Front. Neurosci. 2019, 13, 287. [Google Scholar] [CrossRef]
- Min, B.-K.; Bystritsky, A.; Jung, K.-I.; Fischer, K.; Zhang, Y.; Maeng, L.-S.; Park, S.I.; Chung, Y.-A.; Jolesz, F.A.; Yoo, S.-S. Focused ultrasound-mediated suppression of chemically-induced acute epileptic EEG activity. BMC Neurosci. 2011, 12, 23. [Google Scholar] [CrossRef]
- Zou, J.; Meng, L.; Lin, Z.; Qiao, Y.; Tie, C.; Wang, Y.; Huang, X.; Yuan, T.; Chi, Y.; Meng, W. Ultrasound neuromodulation inhibits seizures in acute epileptic monkeys. Iscience 2020, 23, 101066. [Google Scholar] [CrossRef]
- Kyriakou, A.; Neufeld, E.; Werner, B.; Paulides, M.M.; Szekely, G.; Kuster, N. A review of numerical and experimental compensation techniques for skull-induced phase aberrations in transcranial focused ultrasound. Int. J. Hyperth. 2014, 30, 36–46. [Google Scholar] [CrossRef]
- Fry, F. Transkull transmission of an intense focused ultrasonic beam. Ultrasound Med. Biol. 1977, 3, 179–184. [Google Scholar] [CrossRef]
- Clement, G.T.; Sun, J.; Giesecke, T.; Hynynen, K. A hemisphere array for non-invasive ultrasound brain therapy and surgery. Phys. Med. Biol. 2000, 45, 3707. [Google Scholar] [CrossRef]
- Beccaria, K.; Canney, M.; Bouchoux, G.; Puget, S.; Grill, J.; Carpentier, A. Blood-brain barrier disruption with low-intensity pulsed ultrasound for the treatment of pediatric brain tumors: A review and perspectives. Neurosurg. Focus 2020, 48, E10. [Google Scholar] [CrossRef]
- Meng, Y.; Hynynen, K.; Lipsman, N. Applications of focused ultrasound in the brain: From thermoablation to drug delivery. Nat. Rev. Neurol. 2020, 17, 7–22. [Google Scholar] [CrossRef]
- Hynynen, K.; Jones, R.M. Image-guided ultrasound phased arrays are a disruptive technology for non-invasive therapy. Phys. Med. Biol. 2016, 61, R206. [Google Scholar] [CrossRef]
- Tanter, M.; Pernot, M.; Aubry, J.-F.; Montaldo, G.; Marquet, F.; Fink, M. Compensating for bone interfaces and respiratory motion in high-intensity focused ultrasound. Int. J. Hyperth. 2007, 23, 141–151. [Google Scholar] [CrossRef]
- Raymond, S.B.; Hynynen, K. Acoustic transmission losses and field alterations due to human scalp hair. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2005, 52, 1415–1419. [Google Scholar] [CrossRef]
- McDannold, N.; Clement, G.T.; Black, P.; Jolesz, F.; Hynynen, K. Transcranial magnetic resonance imaging–guided focused ultrasound surgery of brain tumors: Initial findings in 3 patients. Neurosurgery 2010, 66, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-T.; Chai, W.-Y.; Lin, Y.-J.; Lin, C.-J.; Chen, P.-Y.; Tsai, H.-C.; Huang, C.-Y.; Kuo, J.S.; Liu, H.-L.; Wei, K.-C. Neuronavigation-guided focused ultrasound for transcranial blood-brain barrier opening and immunostimulation in brain tumors. Sci. Adv. 2021, 7, eabd0772. [Google Scholar] [CrossRef] [PubMed]
- Beccaria, K.; Canney, M.; Bouchoux, G.; Desseaux, C.; Grill, J.; Heimberger, A.B.; Carpentier, A. Ultrasound-induced blood-brain barrier disruption for the treatment of gliomas and other primary CNS tumors. Cancer Lett. 2020, 479, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Beisteiner, R.; Matt, E.; Fan, C.; Baldysiak, H.; Schönfeld, M.; Philippi Novak, T.; Amini, A.; Aslan, T.; Reinecke, R.; Lehrner, J. Transcranial pulse stimulation with ultrasound in Alzheimer’s disease—A new navigated focal brain therapy. Adv. Sci. 2020, 7, 1902583. [Google Scholar] [CrossRef]
- Idbaih, A.; Canney, M.; Belin, L.; Desseaux, C.; Vignot, A.; Bouchoux, G.; Asquier, N.; Law-Ye, B.; Leclercq, D.; Bissery, A. Safety and feasibility of repeated and transient blood–brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin. Cancer. Res. 2019, 25, 3793–3801. [Google Scholar] [CrossRef]
- Beccaria, K.; Canney, M.; Bouchoux, G.; Zohar, S.; Boddaert, N.; Bourdeaut, F.; Doz, F.; Dufour, C.; Grill, J.; Carpentier, A. NSRG-05. Safety of ultrasound-induced blood-brain barrier opening in pediatric patients with refractory sus-tentorial malignant brain tumors before chemotherapy administration–the Sonokid clinical trial. Neuro Oncol. 2018, 20, i146. [Google Scholar] [CrossRef]
- Idbaih, A.; Ducray, F.; Stupp, R.; Baize, N.; Chinot, O.; de Groot, J.; Guyotat, J.; Sonabend, A.; Menei, P.; Dufour, H. CTNI-31. Interim results of a Phase I/IIa study to evaluate the safety and efficacy of BBB opening with the Sonocloud-9 implantable ultrasound device in recurrent glioblastoma patients prior to IV carboplatin. Neuro Oncol. 2020, 22, ii49. [Google Scholar] [CrossRef]
- Pouliopoulos, A.N.; Wu, S.-Y.; Burgess, M.T.; Karakatsani, M.E.; Kamimura, H.A.; Konofagou, E.E. A clinical system for non-invasive blood–brain barrier opening using a neuronavigation-guided single-element focused ultrasound transducer. Ultrasound Med. Biol. 2020, 46, 73–89. [Google Scholar] [CrossRef]
- Downs, M.E.; Buch, A.; Sierra, C.; Karakatsani, M.E.; Chen, S.; Konofagou, E.E.; Ferrera, V.P. Long-term safety of repeated blood-brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS ONE 2015, 10, e0125911. [Google Scholar]
- Medel, R.; Monteith, S.J.; Elias, W.J.; Eames, M.; Snell, J.; Sheehan, J.P.; Wintermark, M.; Jolesz, F.A.; Kassell, N.F. Magnetic resonance–guided focused ultrasound surgery: Part 2: A review of current and future applications. Neurosurgery 2012, 71, 755–763. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Hynynen, K. Blood-brain barrier: Real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions–based controller. Radiology 2012, 263, 96–106. [Google Scholar] [CrossRef]
- Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S.E.; Hynynen, K. First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019, 10, 4373. [Google Scholar] [CrossRef]
- Jordão, J.F.; Thévenot, E.; Markham-Coultes, K.; Scarcelli, T.; Weng, Y.-Q.; Xhima, K.; O’Reilly, M.; Huang, Y.; McLaurin, J.; Hynynen, K. Amyloid-β plaque reduction, endogenous antibody delivery and glial activation by brain-targeted, transcranial focused ultrasound. Exp. Neurol. 2013, 248, 16–29. [Google Scholar] [CrossRef]
- Burgess, A.; Dubey, S.; Yeung, S.; Hough, O.; Eterman, N.; Aubert, I.; Hynynen, K. Alzheimer disease in a mouse model: MR imaging–guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology 2014, 273, 736–745. [Google Scholar] [CrossRef]
- Leinenga, G.; Götz, J. Scanning ultrasound removes amyloid-β and restores memory in an Alzheimer’s disease mouse model. Sci. Transl. Med. 2015, 7, 278ra233. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hsieh, H.-Y.; Chen, C.-M.; Wu, S.-R.; Tsai, C.-H.; Huang, C.-Y.; Hua, M.-Y.; Wei, K.-C.; Yeh, C.-K.; Liu, H.-L. Non-invasive, neuron-specific gene therapy by focused ultrasound-induced blood-brain barrier opening in Parkinson’s disease mouse model. J. Control. Release 2016, 235, 72–81. [Google Scholar] [CrossRef]
- LeWitt, P.A.; Lipsman, N.; Kordower, J.H. Focused ultrasound opening of the blood–brain barrier for treatment of Parkinson’s disease. Mov. Disord. 2019, 34, 1274–1278. [Google Scholar] [CrossRef]
| Trial Number | Study Title, Date | Condition | Interventions | Number of Participants | Therapeutic Protocol | Location | Status | |
|---|---|---|---|---|---|---|---|---|
| Brain tumours | ||||||||
| 1 | NCT02253212 | Safety of BBB Opening With the SonoCloud (SONOCLOUD), 2014 | Glioma or GBM | Device: SonoCloud® Drug: Carboplatin and SonoVue® microbubble | 27 | The US (0.5–1.1 Mpa) was activated monthly before IV administration of carboplatin and microbbuble (0.1 mL/kg) (min. 6 cycles) [83]. | France | Completed |
| 2 | NCT02343991 | Blood–Brain Barrier Disruption Using Transcranial MRI-Guided Focused US, 2014 | Primary brain tumours | Device: ExAblate MRgFUS Drug: Liposomal doxorubicin or Temozolomide with Definity® microbubbles | 10 | Five patients underwent the MRgFUS in conjunction with administration of chemotherapy (n = 1 liposomal doxorubicin, n = 4 temozolomide) one day prior to surgical resection. Samples of “sonicated” and “unsonicated” tissue were collected during surgery [242]. | Canada | Active, not recruiting |
| 3 | NCT03712293 | ExAblate Blood–Brain Barrier Disruption for Glioblastoma in Patients Undergoing Standard Chemotherapy, 2018 | GBM | Device: Transcranial ExAblate 4000 Type 2.0 MRgFUS Drug: Temozolomide | 10 | The ExAblate BBB disruption will coincide with one of three first days of each planned temozolomide adjuvant therapy cycle as one procedure per cycle. | South Korea | Recruiting |
| 4 | NCT03626896 | Safety of BBB Disruption Using NaviFUS System in Recurrent Glioblastoma Multiforme (GBM) Patients, 2018 | Glioma or GBM | Device: NaviFUS® System Drug: None. | 6 | The study will be carried out in patients with recurrent GBM who will undergo surgery within 2 weeks to evaluate the safety and the tolerated US dose (escalated exposure average 10–16 W). | Taiwan | Completed |
| 5 | NCT03616860 | Assessment of Safety and Feasibility of ExAblate Blood–Brain Barrier (BBB) Disruption for Treatment of Glioma, 2018 | GBM | Device: ExAblate 4000 Type 2.0 MRgFUS Drug: Temozolomide and Definity® microbubbles | 20 | Patients will undergo up to 6 treatments with FUS coincident with their standard temozolomide cycles. | Canada | Recruiting |
| 6 | NCT03714243 | Blood–Brain Barrier Disruption (BBBD) Using MRgFUS in the Treatment of Her2-positive Breast Cancer Brain Metastases (BBBD), 2019 | Metastatic HER-2 positive breast cancer | Device: ExAblate 4000 Type 2.0 MRgFUS Drug: Trastuzumab | 10 | Six study ExAblate BBB opening treatment cycles, every 2–3 weeks based on their trastuzumab regimen. | Canada | Recruiting |
| 7 | NCT03744026 | Safety and Efficacy of Transient Opening of the Blood–Brain Barrier (BBB) With the SonoCloud-9 (SC9-GBM-01), 2019 | GBM | Device: SonoCloud-9 Drug: Carboplatin | 33 | Patients will undergo 6 cycles of carboplatin treatments every 4 weeks coincident with BBB opening in resection area and surrounding tissues using SonoCloud-9 system [238]. | France | Recruiting |
| 8 | NCT03551249 | Assessment of Safety and Feasibility of ExAblate Blood–Brain Barrier (BBB) Disruption, 2019 | Glioma or GBM | Device: ExAblate 4000 Type 2.0 MRgFUS Drug: Temozolomide | 20 | BBB will be disturbed along the periphery of tumour resection cavity prior to beginning the planned adjuvant temozolomide chemotherapy phase of treatment. | USA | Recruiting |
| 9 | NCT04021420 | Safety and Efficacy of Sonocloud Device Combined With Nivolumab in Brain Metastases From Patients With Melanoma (SONIMEL01), 2019 | Metastatic melanoma | Device: SonoCloud® Drug: Nivolumab Injection alone or with Ipilimumab | 21 | Along with systemic injection of an US resonator and prior to beginning the chemo-treatment, SonoCloud® delivers US for a duration of 120–270 s. A total of 3 US dose levels will be evaluated (0.78, 0.9 and 1.03 MPa). | France | Recruiting |
| 10 | NCT04528680 | US-based Blood–Brain Barrier Opening and Albumin-bound Paclitaxel for Recurrent Glioblastoma (SC9/ABX), 2020 | GBM | Device: SonoCloud-9 Drug: Albumin-bound paclitaxel (Abraxane®), microbubbles | 39 | The device will be implanted at the time of surgical resection of the recurrent tumour. During that procedure, a first test dose of the chemotherapy will be administered in the operating room after sonication and tissue concentrations in different parts of the resected tumour will be measured. In select patients, the sonication procedure will occur immediately after the test dose of chemotherapy is administered. | USA | Recruiting |
| 11 | NCT04614493 | Innovative SonoCloud-9 Device for Blood–Brain Barrier Opening in First Line Temozolomide Glioblastoma Patients. (SonoFIRST), 2020 | GBM | Device: SonoCloud-9 Drug: Temozolomide | 66 | The patients will receive daily temozolomide during Radiation, followed by 6 months of adjuvant temozolomide 5 days/months) with 6 concomitant BBB opening sessions by US + 9 BBB opening sessions by US without any associated drug. | Belgium/France | Not yet recruiting |
| 12 | NCT04440358 | ExAblate Blood–Brain Barrier Disruption With Carboplatin for the Treatment of rGBM, 2020 | GBM | Device: ExAblate 4000 Type 2.0 MRgFUS Drug: Carboplatin with microbubble | 50 | Patients will undergo up to 6 cycles of ExAblate BBBD procedures in conjunction with carboplatin chemotherapy about every 4 weeks. | South Korea | Recruiting |
| 13 | NCT04417088 | ExAblate Blood–Brain Barrier Disruption for the Treatment of rGBM in Subjects Undergoing Carboplatin Monotherapy, 2020 | GBM | Device: ExAblate 4000 Type 2.0 MRgFUS Drug: Carboplatin with microbubble | 30 | Patients will undergo up to 6 cycles of ExAblate BBBD procedures in conjunction with carboplatin chemotherapy about every 4 weeks. | USA | Recruiting |
| 14 | NCT04804709 | Non-Invasive Focused US (FUS) With Oral Panobinostat in Children With Progressive Diffuse Midline Glioma (DMG), 2021 | Diffuse Midline Glioma | Device: FUS treatment with neuro-navigator-controlled sonication Drug: Panobinostat, microbubbles | 15 | After each instance of opening the BBB using specific parameters of FUS in the specific number of tumour sites (one, two, or three), the subjects will receive oral Panobinostat. | USA | Recruiting |
| 15 | NCT04063514 | The Use of Focused US and DCE K-trans Imaging to Evaluate Permeability of the Blood–Brain Barrier, 2025 | Glioma | Device: Brainsonix FUS and DWL Doppler system Drug: Definity® microbubbles | 15 | Not mentioned. | USA | Not yet recruiting |
| Alzheimer’s Disease | ||||||||
| 1 | NCT02986932 | Blood–Brain Barrier Opening Using Focused US With IV Contrast Agents in Patients With Early Alzheimer’s Disease (BBB-Alzheimers), 2016 | Alzheimer’s Disease | Device: ExAblate MRgFUS Drug: Definity® microbubbles | 6 | In the first stage, patients will undergo a small area BBB opening (9 × 9 mm) with multiple sonications to establish the minimum required sonication parameters. In stage II, a larger volume (2.5–3.0 cm) will be targeted. | Canada | Completed |
| 2 | NCT03119961 | Blood–Brain Barrier Opening in Alzheimer’s Disease (BOREAL1), 2017 | Alzheimer’s Disease | Device: SonoCloud®, CarThéra Drug: anti- Alzheimer’s Disease drugs | 10 | Not mentioned. | France | Completed |
| 3 | NCT03671889 | ExAblate Blood–Brain Barrier (BBB) Disruption for the Treatment of Alzheimer’s Disease, 2018 | Alzheimer’s Disease | Device: ExAblate 4000 Type 2.0 MRgFUS Drug: Not mentioned | 20 | Three serial ExAblate BBB disruption procedures in specific areas in the brain will be carried out. | USA | Recruiting |
| 4 | NCT03739905 | ExAblate Blood–Brain Barrier Opening for Treatment of Alzheimer’s Disease, 2018 | Alzheimer’s Disease | Device: ExAblate 4000 Type 2.0 MRgFUS Drug: Alzheimer’s medication | 30 | Three serial ExAblate BBB disruption procedures in specific areas in the brain will be carried out. | Canada | Recruiting |
| 5 | NCT04118764 | Non-invasive Blood–Brain Barrier Opening in Alzheimer’s Disease Patients Using Focused US, 2020 | Alzheimer’s Disease | Device: Neuronavigation-guided single-element focused US transducer Drug: Definity® microbubbles | 6 | Patients will undergo a FUS treatment to the brain, along with Magnetic Resonance Imagine [128] with or without gadolinium contrast agents and Positron Emission Tomography (PET) scans. | USA | Recruiting |
| 6 | NCT04526262 | Assessment of Initial Efficacy and Safety of High Intensity Focused US ‘ExAblate 4000 Type 2′ for Blood–Brain Barrier Disruption in Patients With Alzheimer’s Disease, 2020 | Alzheimer’s Disease | Device: ExAblate 4000 Type 2.0 MRgFUS Drug: Alzheimer’s medication | 6 | Two sessions of transcranial magnetic resonance-guided focused US blood–brain barrier disruption every 3 months. | South Korea | Active, not recruiting |
| Other | ||||||||
| 1 | NCT03608553 | A Study to Evaluate Temporary Blood–Brain Barrier Disruption in Patients With Parkinson’s Disease Dementia, 2018 | Parkinson’s Disease with dementia | Device: ExAblate 4000 Type 2.0 MRgFUS Drug: Luminity® | 10 | In the first stage, patients will undergo a small area BBB opening (9 × 9 mm) with multiple sonications to establish the minimum required sonication parameters. In stage II, a larger volume (2.5–3.0 cm) will be targeted. | Spain | Active, not recruiting |
| 2 | NCT03321487 | Blood–Brain Barrier Opening Using MR-Guided Focused US in Patients With Amyotrophic Lateral Sclerosis, 2018 | Amyotrophic Lateral Sclerosis (ALS) | Device: ExAblate MRgFUS Drug: None | 8 | BBB will be disturbed using US in conjunction with an intravenous US contrast agent. | Canada | Active, not recruiting |
| 3 | NCT04370665 | Blood–Brain Barrier Disruption With Cerezyme in Patients With Parkinson’s Disease, 2020 | Parkinson’s Disease | Device: ExAblate MRgFUS Drug: Cerezyme® (an analogue of the human enzyme beta-glucocerebrosidase) | 4 | Patients will undergo three biweekly delivery of Cerezyme® via MRgFUS induced BBB opening to unilateral putamen. | Canada | Active, not recruiting |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barzegar-Fallah, A.; Gandhi, K.; Rizwan, S.B.; Slatter, T.L.; Reynolds, J.N.J. Harnessing Ultrasound for Targeting Drug Delivery to the Brain and Breaching the Blood–Brain Tumour Barrier. Pharmaceutics 2022, 14, 2231. https://doi.org/10.3390/pharmaceutics14102231
Barzegar-Fallah A, Gandhi K, Rizwan SB, Slatter TL, Reynolds JNJ. Harnessing Ultrasound for Targeting Drug Delivery to the Brain and Breaching the Blood–Brain Tumour Barrier. Pharmaceutics. 2022; 14(10):2231. https://doi.org/10.3390/pharmaceutics14102231
Chicago/Turabian StyleBarzegar-Fallah, Anita, Kushan Gandhi, Shakila B. Rizwan, Tania L. Slatter, and John N. J. Reynolds. 2022. "Harnessing Ultrasound for Targeting Drug Delivery to the Brain and Breaching the Blood–Brain Tumour Barrier" Pharmaceutics 14, no. 10: 2231. https://doi.org/10.3390/pharmaceutics14102231
APA StyleBarzegar-Fallah, A., Gandhi, K., Rizwan, S. B., Slatter, T. L., & Reynolds, J. N. J. (2022). Harnessing Ultrasound for Targeting Drug Delivery to the Brain and Breaching the Blood–Brain Tumour Barrier. Pharmaceutics, 14(10), 2231. https://doi.org/10.3390/pharmaceutics14102231






