Besifloxacin Nanocrystal: Towards an Innovative Ophthalmic Preparation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drug Analysis
2.2.1. Particle Size Determination by Laser Diffraction
2.2.2. Determination of Minimum Inhibitory Concentration (MIC)
2.3. Preparation of Besifloxacin Nanocrystals Using Small-Scale Wet Bead Milling
2.3.1. Exploratory Tests for Stabilizer and Statistical Variables Selection
2.3.2. Nanosuspension Optimization Process Applying Box–Behnken Experiment Design
2.3.3. Mathematical Model Verification
2.4. Dynamic Light Scattering and Zeta Potential
2.5. Distribution and Particle Size by Laser Diffraction (LD)
2.6. Density, Viscosity, and pH
2.7. Osmolality
2.8. Validation of the Method by High-Performance Liquid Chromatography (HPLC)
2.8.1. HPLC System and Apparatus
2.8.2. Solutions
2.9. Scanning Electron Microscopy (SEM)
2.10. Lyophilization
2.11. Thermal Analysis
2.12. X-ray Diffraction (XRD)
2.13. Saturation Solubility
2.14. In Vivo Toxicity
2.15. Determination of Minimum Inhibitory Concentration (MIC)
2.16. Stability Study
3. Results and Discussion
3.1. Drug Analysis
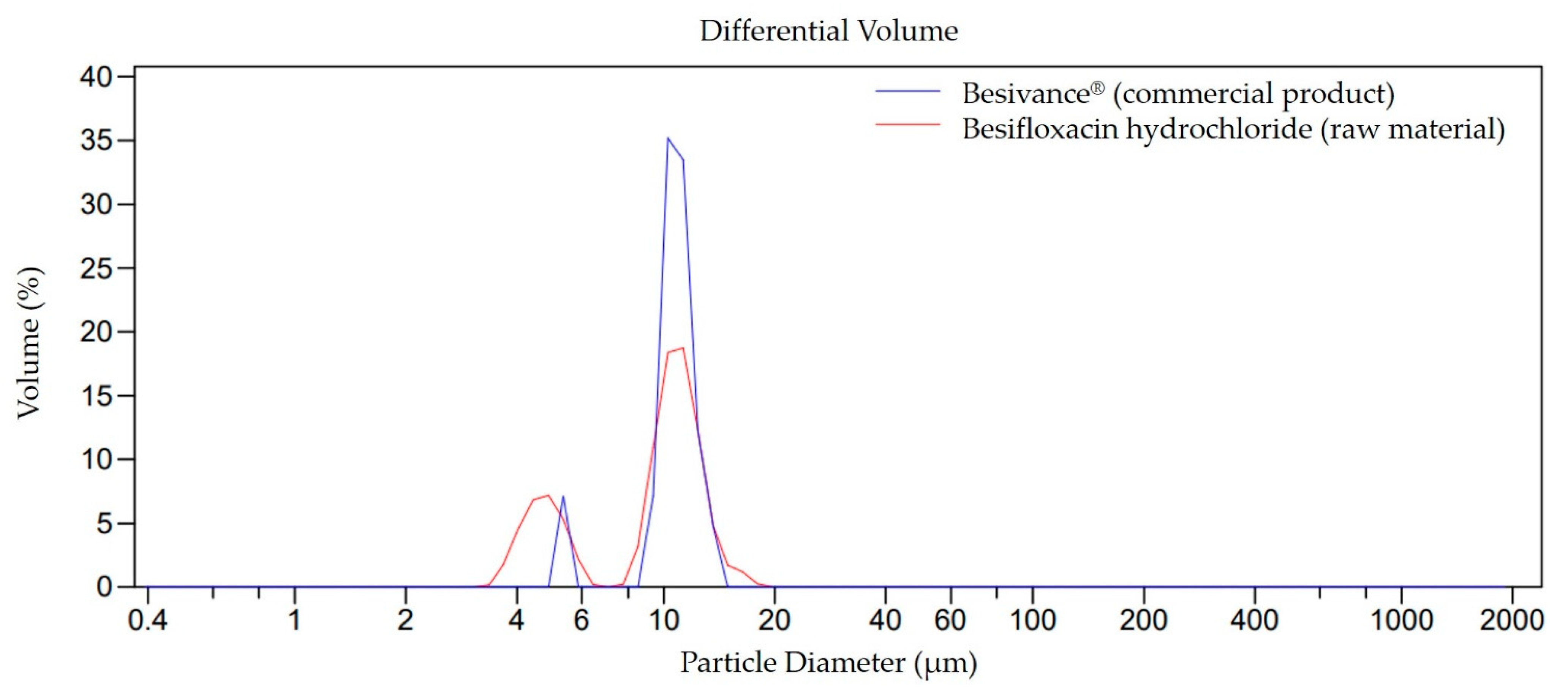
3.1.1. Particle Size Determination by Laser Diffraction
3.1.2. Determination of Minimum Inhibitory Concentration (MIC)
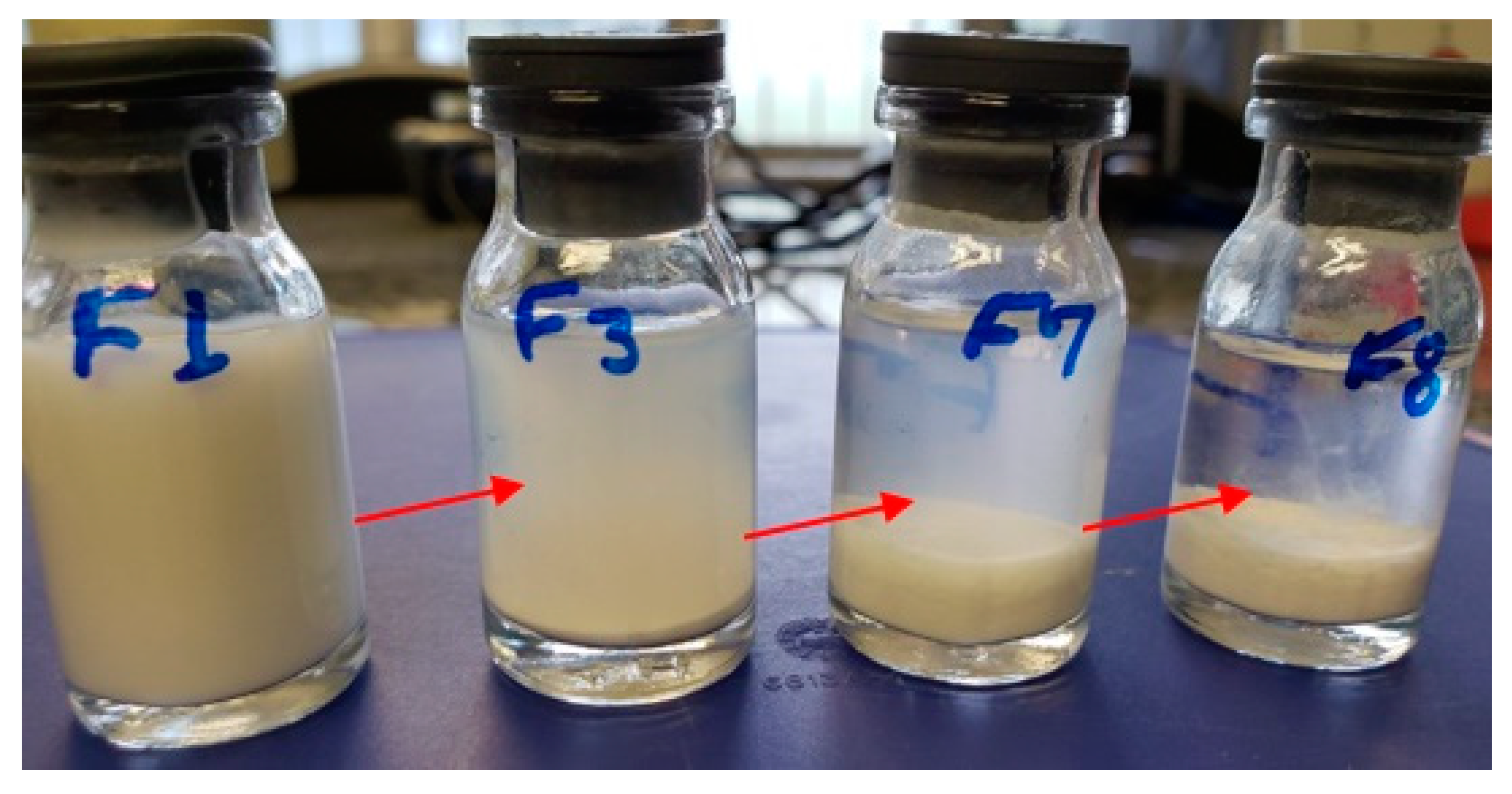
3.2. Preparation of Besifloxacin Nanocrystals Using Small-Scale Wet Bead Milling
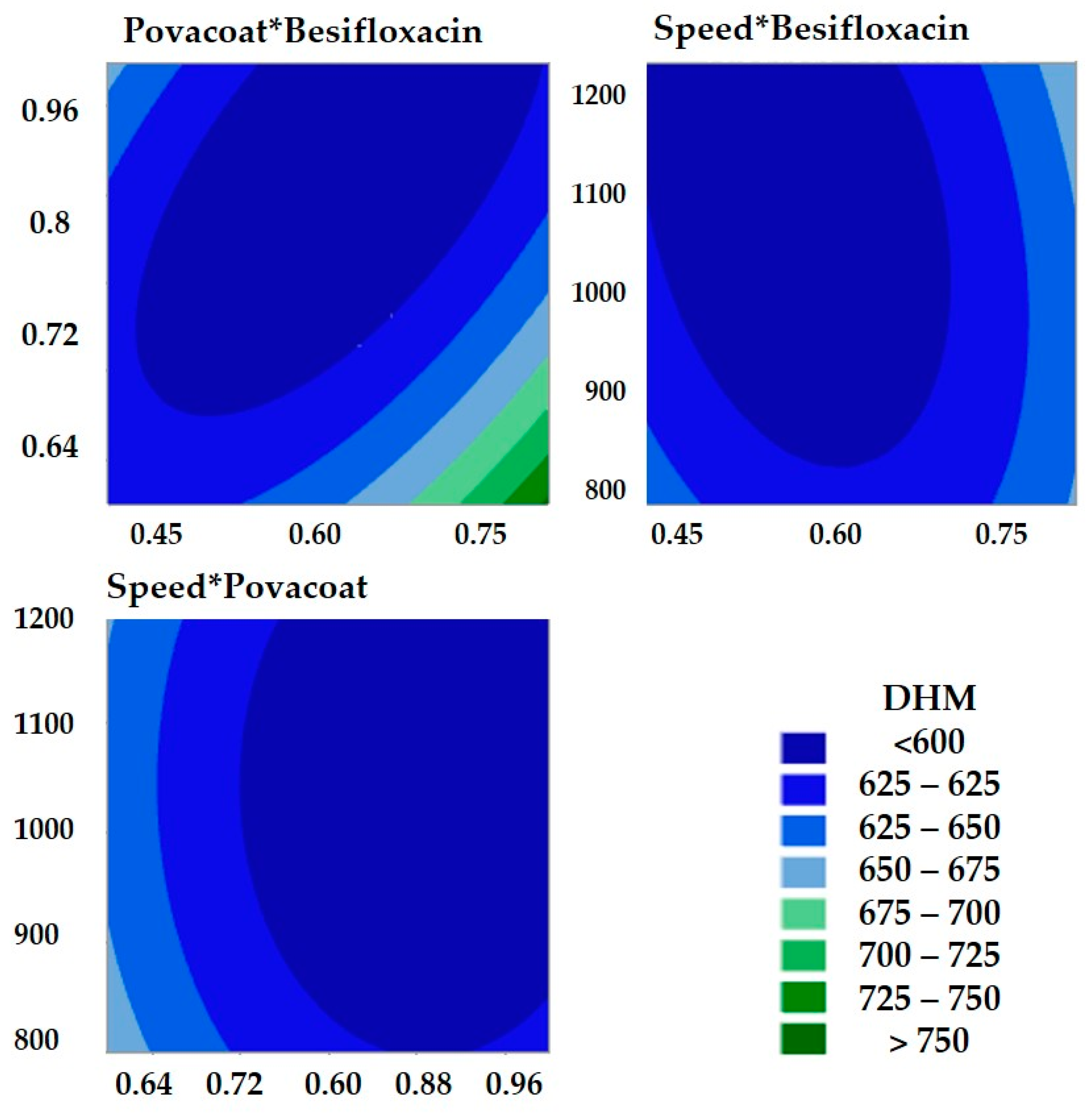
3.2.1. Exploratory Tests for Stabilizer and Nanosuspension Optimization Process Applying Box–Behnken Experiment Design
3.2.2. Mathematical Model Verification
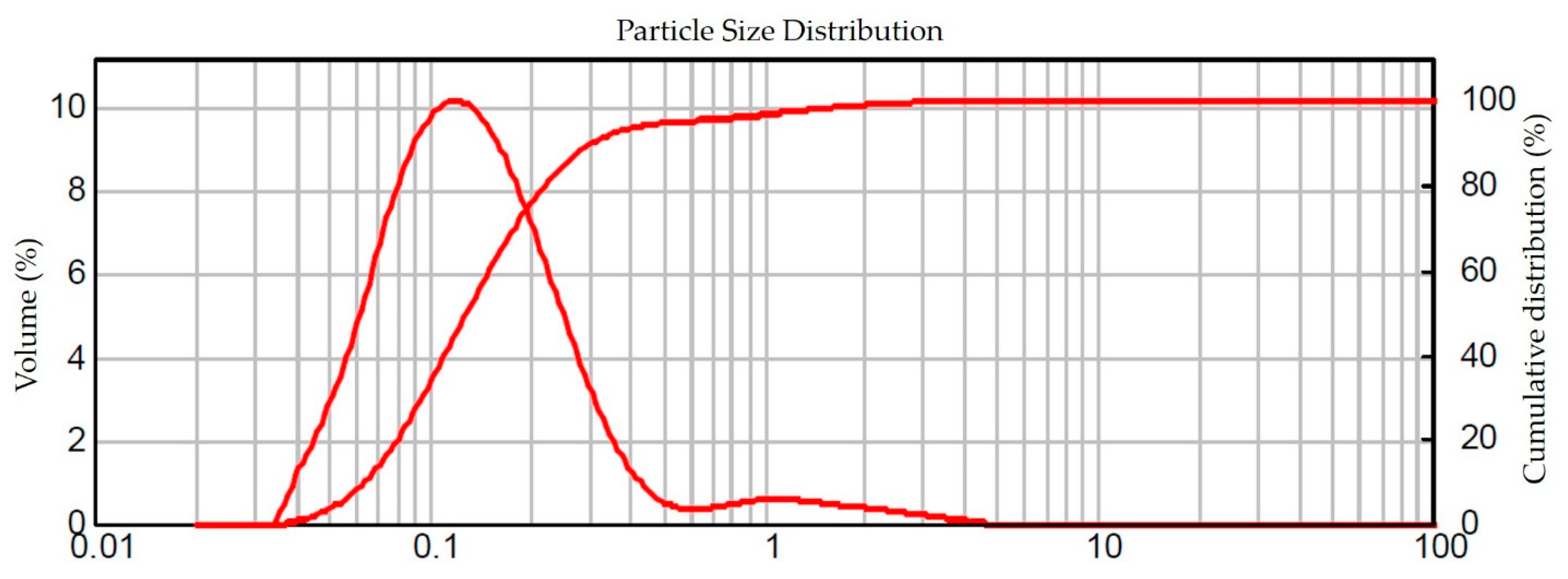
3.3. Distribution and Particle Size by Laser Diffraction (LD)
3.4. pH, Density, and Viscosity
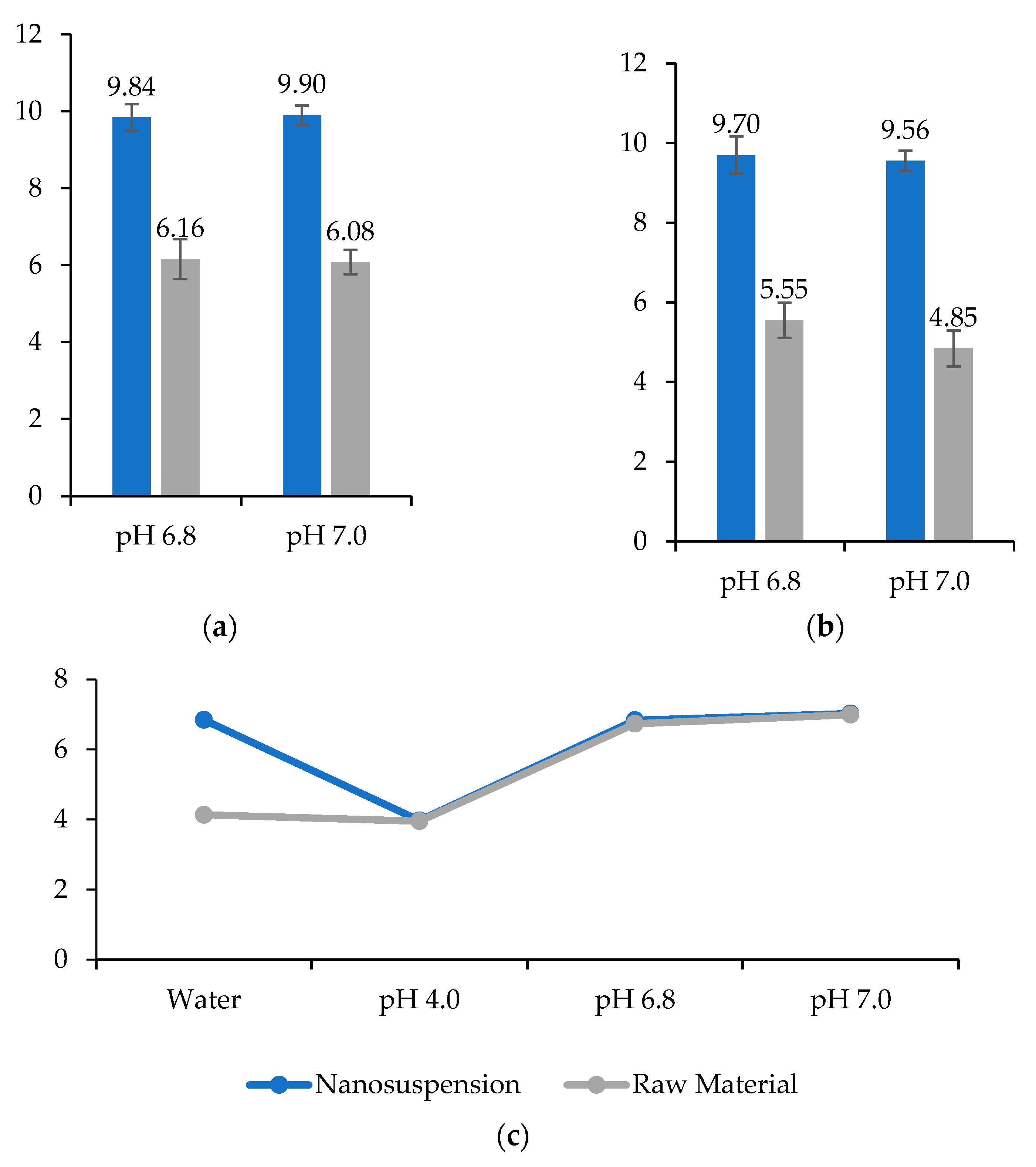
3.5. Osmolality
3.6. Validation of the Method by High Performance Liquid Chromatography (HPLC)
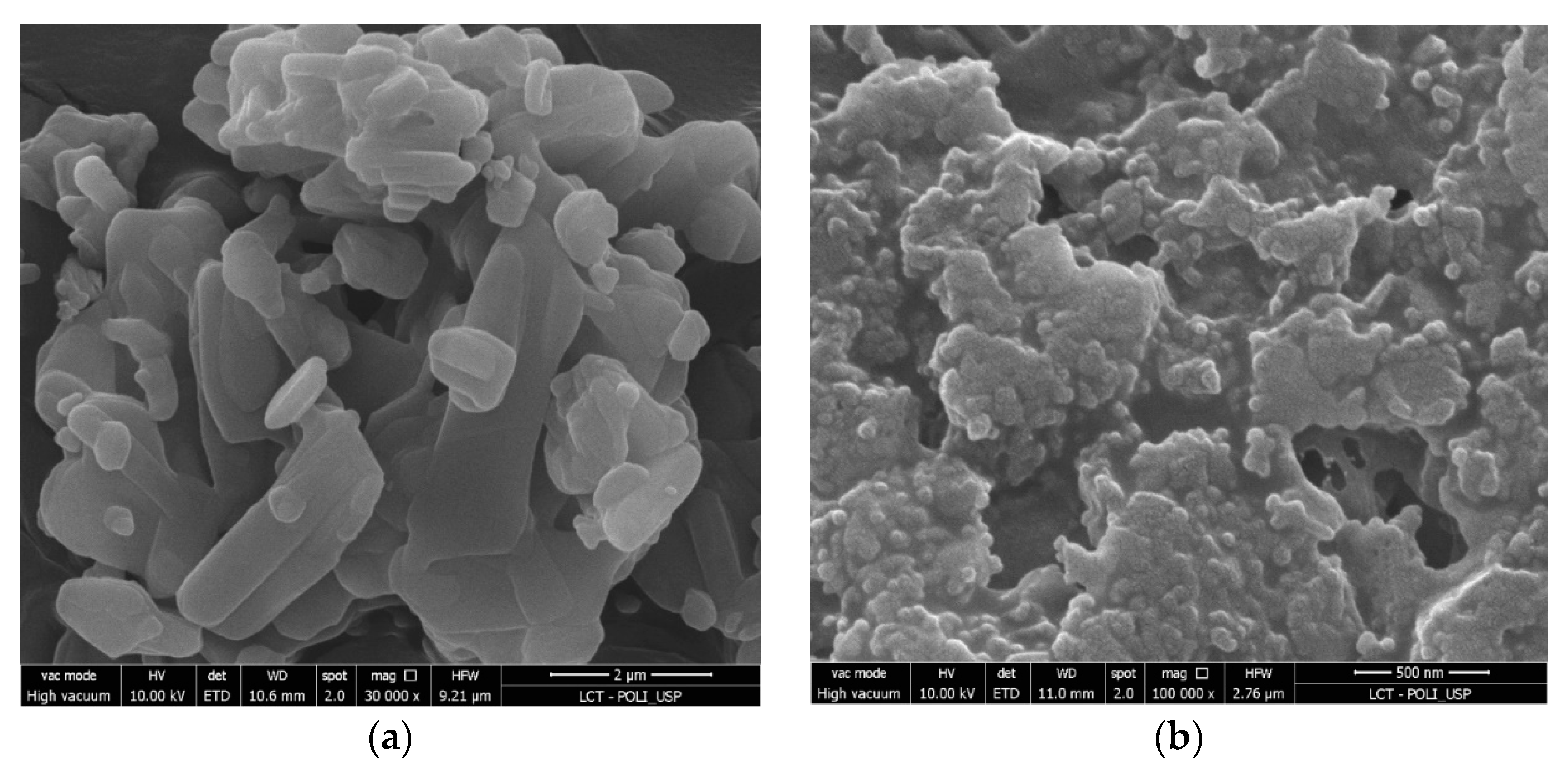
3.7. Scanning Electron Microscopy (SEM)
3.8. Lyophilization
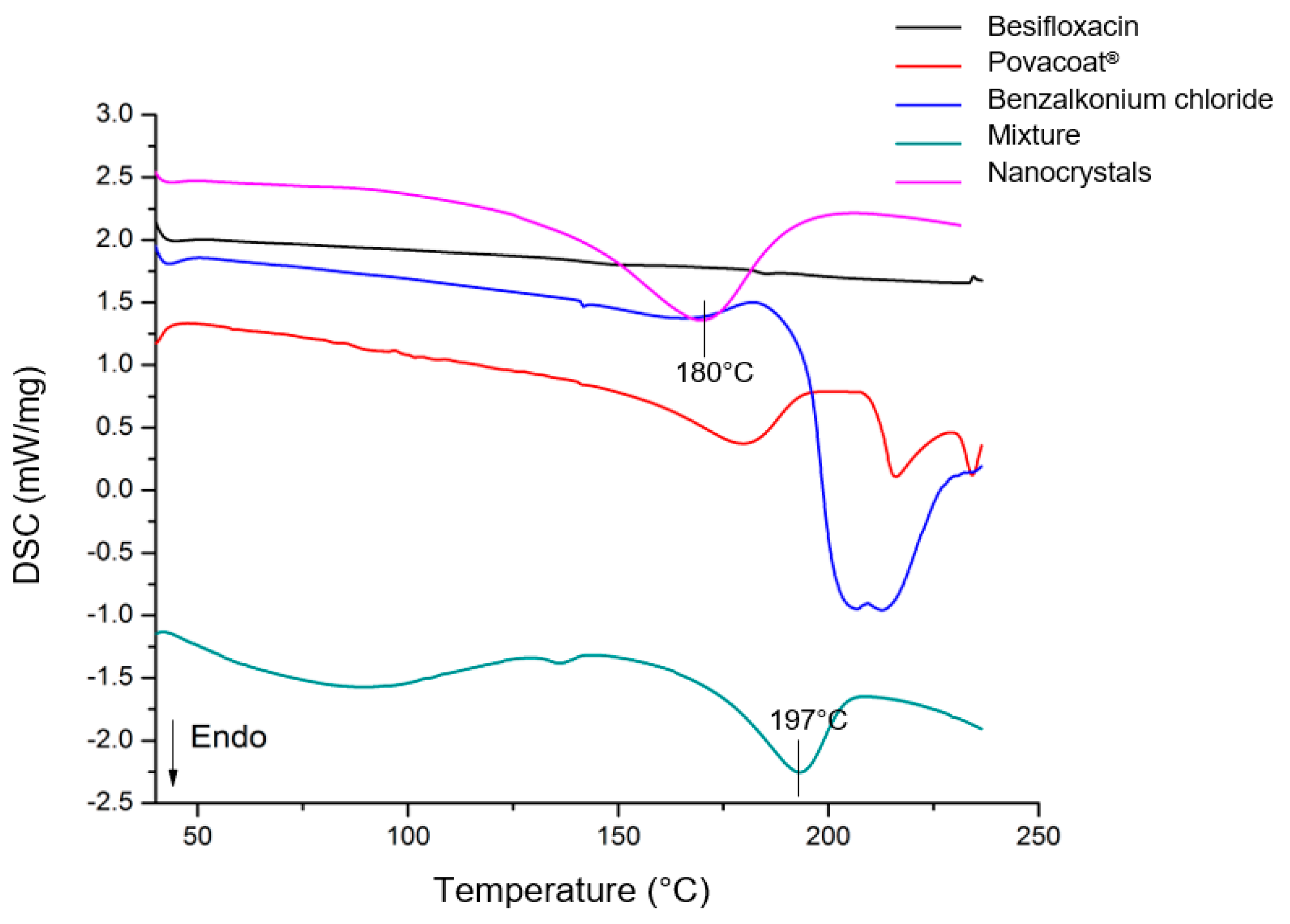
3.9. Thermal Analysis
3.10. X-ray Diffraction (XRD)
3.11. Saturation Solubility
3.12. Determination of Minimum Inhibitory Concentration (MIC)
3.13. In Vivo Toxicity
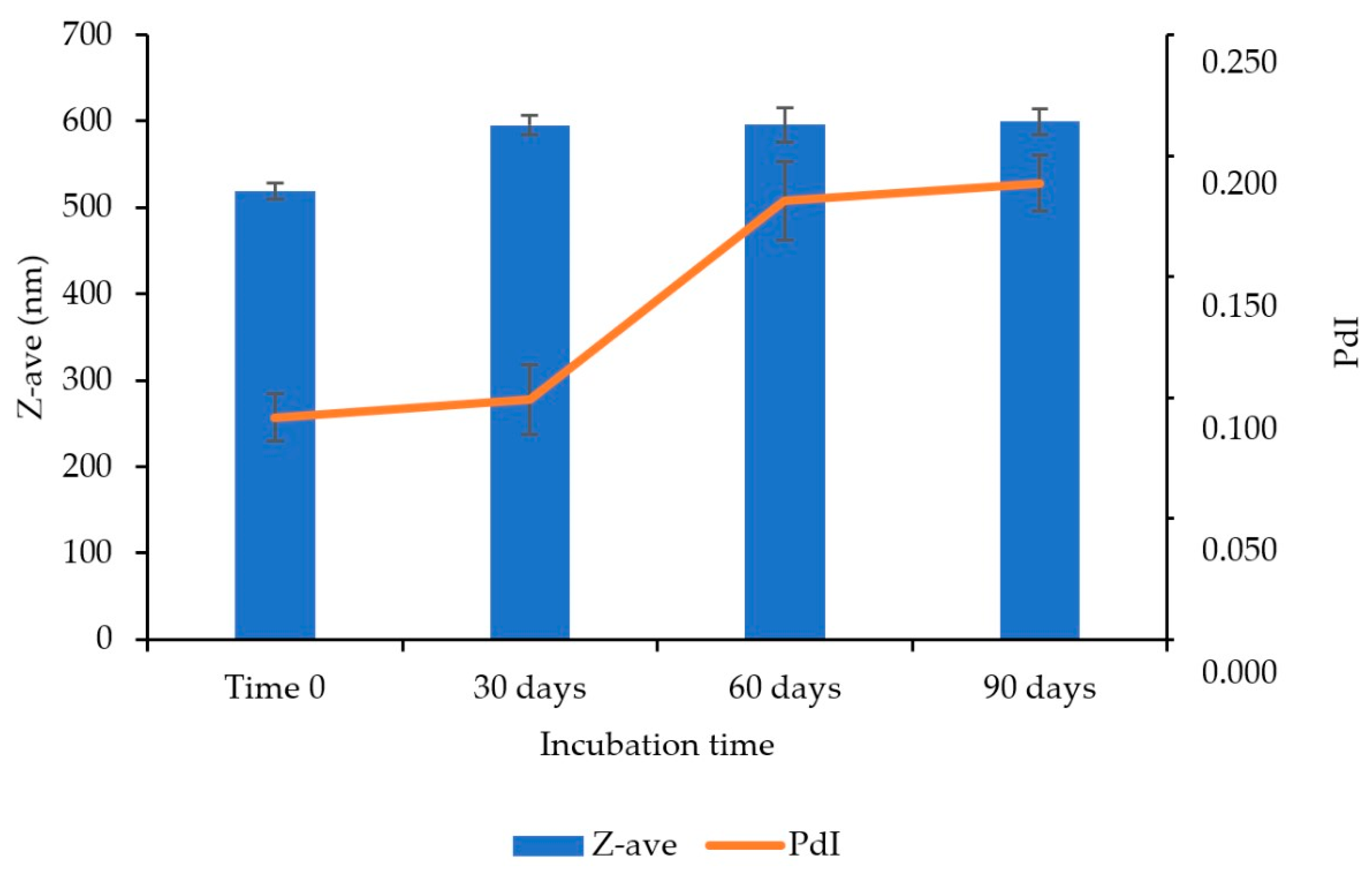
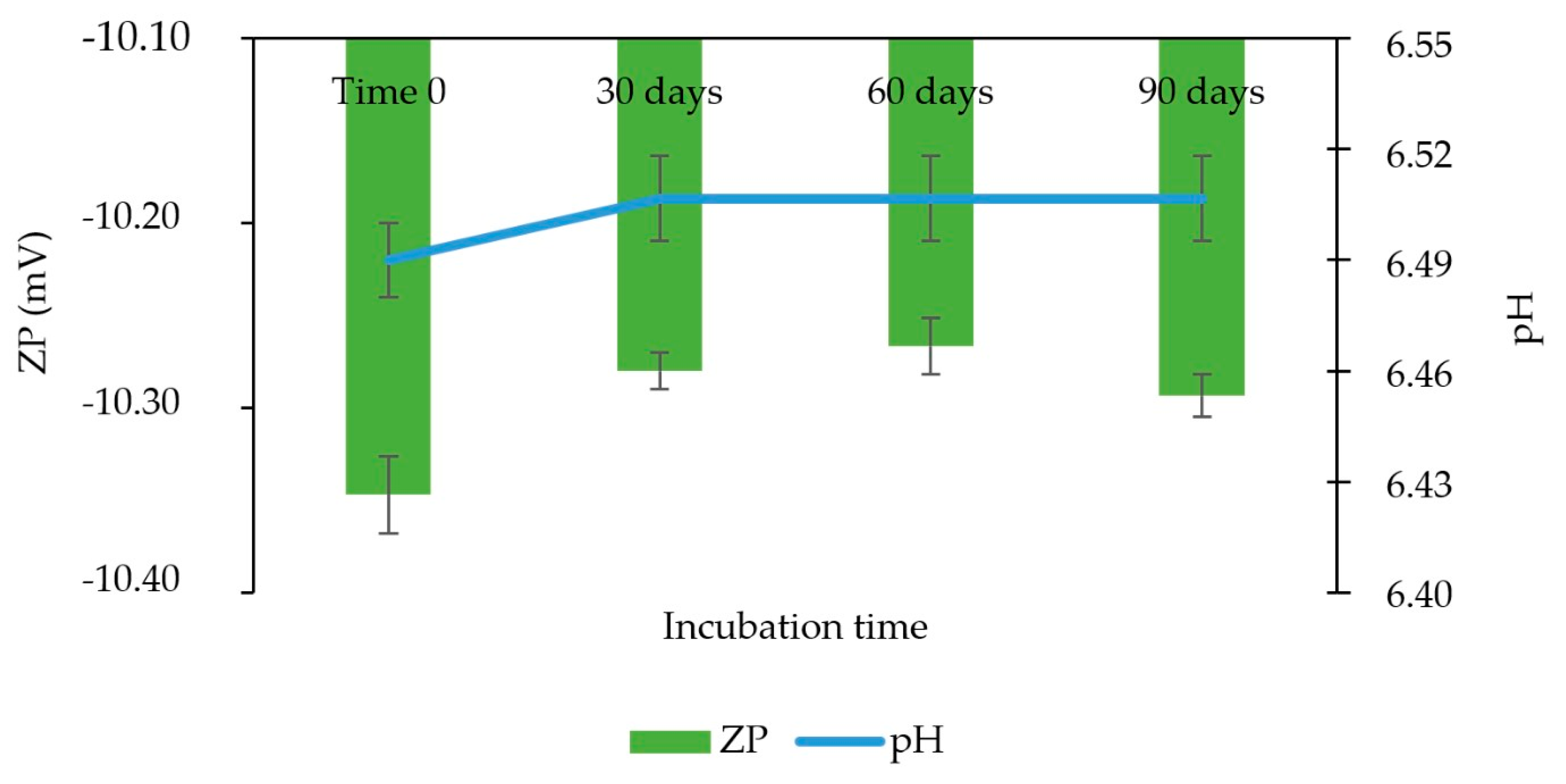
3.14. Stability Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azari, A.A.; Barney, N.P. Conjunctivitis: A Systematic Review of Diagnosis and Treatment. JAMA 2013, 310, 1721–1729. [Google Scholar] [CrossRef] [PubMed]
- Khimdas, S.; Visscher, K.L.; Hutnik, C.M.L. Besifloxacin Ophthalmic Suspension: Emerging Evidence of Its Therapeutic Value in Bacterial Conjunctivitis. Ophthalmol. Eye Dis. 2011, 3, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Mejia-Lopez, H.; Alberto, C.; Climent-Flores, A.; Bautista-de Lucio, V.M. Epidemiological Aspects of Infectious Conjunctivitis. In Conjunctivitis—A Complex and Multifaceted Disorder; Intech Open: London, UK, 2011. [Google Scholar]
- Saher, O.; Ghorab, D.M.; Mursi, N.M. Levofloxacin Hemihydrate Ocular Semi-Sponges for Topical Treatment of Bacterial Conjunctivitis: Formulation and in-Vitro/in-Vivo Characterization. J. Drug Deliv. Sci. Technol. 2016, 31, 22–34. [Google Scholar] [CrossRef]
- Tótoli, E.G.; Salgado, H.R.N. Besifloxacin: A Critical Review of Its Characteristics, Properties, and Analytical Methods. Crit. Rev. Anal. Chem. 2018, 48, 132–142. [Google Scholar] [CrossRef]
- Alfonso, S.A.; Fawley, J.D.; Lu, X.A. Conjunctivitis. Prim. Care Clin. Off. Pract. 2015, 42, 325–345. [Google Scholar] [CrossRef]
- Asbell, P.A.; Sanfilippo, C.M.; Pillar, C.M.; DeCory, H.H.; Sahm, D.F.; Morris, T.W. Antibiotic Resistance among Ocular Pathogens in the United States Five-Year Results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) Surveillance Study. JAMA Ophthalmol. 2015, 133, 1445–1454. [Google Scholar] [CrossRef]
- Comstock, T.L.; Karpecki, P.M.; Morris, T.W.; Zhang, J.Z. Besifloxacin: A Novel Anti-Infective for the Treatment of Bacterial Conjunctivitis. Clin. Ophthalmol. 2010, 4, 215–225. [Google Scholar] [CrossRef][Green Version]
- Kirstahler, P.; Bjerrum, S.S.; Friis-Møller, A.; La Cour, M.; Aarestrup, F.M.; Westh, H.; Pamp, S.J. Genomics-Based Identification of Microorganisms in Human Ocular Body Fluid. Sci. Rep. 2018, 8, 4126. [Google Scholar] [CrossRef]
- Lipsky, L.; Barrett, G. Intracameral Antibiotics for Prophylaxis of Postoperative Endophthalmitis in Australia: A Review. Clin. Exp. Ophthalmol. 2019, 47, 537–541. [Google Scholar] [CrossRef]
- Seiple, I.B.; Zhang, Z.; Jakubec, P.; Langlois-Mercier, A.; Wright, P.M.; Hog, D.T.; Yabu, K.; Allu, S.R.; Fukuzaki, T.; Carlsen, P.N.; et al. A Platform for the Discovery of New Macrolide Antibiotics. Nature 2016, 533, 338–345. [Google Scholar] [CrossRef]
- Costa, M.C.N.; Barden, A.T.; Andrade, J.M.M.; Oppe, T.P.; Schapoval, E.E.S. Quantitative Evaluation of Besifloxacin Ophthalmic Suspension by HPLC, Application to Bioassay Method and Cytotoxicity Studies. Talanta 2014, 119, 367–374. [Google Scholar] [CrossRef] [PubMed]
- Hughes, B. 2009 FDA Drug Approvals. Nat. Rev. Drug Discov. 2010, 9, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Bausch & Lomb Incorporated BesivanceTM Material Safety Data Sheet. Available online: //s3-us-west-2.amazonaws.com/drugbank/msds/DB06771.pdf?1365982762 (accessed on 6 September 2022).
- Achouri, D.; Alhanout, K.; Piccerelle, P.; Andrieu, V. Recent Advances in Ocular Drug Delivery. Drug Dev. Ind. Pharm. 2013, 39, 1599–1617. [Google Scholar] [CrossRef]
- Gao, L.; Liu, G.; Ma, J.; Wang, X.; Zhou, L.; Li, X. Drug Nanocrystals: In Vivo Performances. J. Control. Release 2012, 160, 418–430. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.A.; Ferreira-Nunes, R.; Dalmolin, L.F.; dos Santos Ré, A.C.; Anjos, J.L.V.; Mendanha, S.A.; Aires, C.P.; Lopez, R.F.V.; Cunha-Filho, M.; Gelfuso, G.M.; et al. Besifloxacin Liposomes with Positively Charged Additives for an Improved Topical Ocular Delivery. Sci. Rep. 2020, 10, 19285. [Google Scholar] [CrossRef] [PubMed]
- Kassaee, S.N.; Mahboobian, M.M. Besifloxacin-Loaded Ocular Nanoemulsions: Design, Formulation and Efficacy Evaluation. Drug Deliv. Transl. Res. 2022, 12, 229–239. [Google Scholar] [CrossRef]
- Polat, H.K.; Kurt, N.; Aytekin, E.; Akdağ Çaylı, Y.; Bozdağ Pehlivan, S.; Çalış, S. Design of Besifloxacin HCl-Loaded Nanostructured Lipid Carriers: In Vitro and Ex Vivo Evaluation. J. Ocul. Pharmacol. Ther. 2022, 38, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Polat, H.K.; Bozdağ Pehlivan, S.; Özkul, C.; Çalamak, S.; Öztürk, N.; Aytekin, E.; Fırat, A.; Ulubayram, K.; Kocabeyoğlu, S.; İrkeç, M.; et al. Development of Besifloxacin HCl Loaded Nanofibrous Ocular Inserts for the Treatment of Bacterial Keratitis: In Vitro, Ex Vivo and in Vivo Evaluation. Int. J. Pharm. 2020, 585, 119552. [Google Scholar] [CrossRef]
- Barbosa, S.F.; Takatsuka, T.; Tavares, G.D.; Araújo, G.L.B.; Wang, H.; Vehring, R.; Löbenberg, R.; Bou-Chacra, N.A. Physical–Chemical Properties of Furosemide Nanocrystals Developed Using Rotation Revolution Mixer. Pharm. Dev. Technol. 2016, 21, 812–822. [Google Scholar] [CrossRef]
- Chen, M.-L.; John, M.; Lee, S.L.; Tyner, K.M. Development Considerations for Nanocrystal Drug Products. AAPS J. 2017, 19, 642–651. [Google Scholar] [CrossRef]
- Davis, B.M.; Pahlitzsch, M.; Guo, L.; Balendra, S.; Shah, P.; Ravindran, N.; Malaguarnera, G.; Sisa, C.; Shamsher, E.; Hamze, H.; et al. Topical Curcumin Nanocarriers Are Neuroprotective in Eye Disease. Sci. Rep. 2018, 8, 6712. [Google Scholar] [CrossRef] [PubMed]
- Tyner, K.M.; Zheng, N.; Choi, S.; Xu, X.; Zou, P.; Jiang, W.; Guo, C.; Cruz, C.N. How Has CDER Prepared for the Nano Revolution? A Review of Risk Assessment, Regulatory Research, and Guidance Activities. AAPS J. 2017, 19, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Williams, H.D.; Trevaskis, N.L.; Charman, S.A.; Shanker, R.M.; Charman, W.N.; Pouton, C.W.; Porter, C.J.H. Strategies to Address Low Drug Solubility in Discovery and Development. Pharmacol. Rev. 2013, 65, 315–499. [Google Scholar] [CrossRef] [PubMed]
- Markets, R. Nanotechnology—Global Market Trajectory & Analytics; Global Industry Analysts, Inc.: San Jose, CA, USA, 2020. [Google Scholar]
- Peters, M.C.C.; de Oliveira, I.F.; Machado, M.G.M.; Ferreira, D.C.; Zanin, M.H.A.; Bou-Chacra, N. The Glucocorticoid Derivative with the Phthalimide Group Cationic Nanocrystal for Ophthalmic Application: A Design Space Development Approach. Mater. Today Chem. 2021, 19, 100396. [Google Scholar] [CrossRef]
- Zuo, J.; de Araujo, G.L.B.; Stephano, M.A.; Zuo, Z.; Bou-Chacra, N.A.; Löbenberg, R. Design Space Approach in the Development of Esculetin Nanocrystals by a Small-Scale Wet-Bead Milling Process. J. Drug Deliv. Sci. Technol. 2020, 55, 101486. [Google Scholar] [CrossRef]
- Haas, W.; Pillar, C.M.; Hesje, C.K.; Sanfilippo, C.M.; Morris, T.W. Bactericidal Activity of Besifloxacin against Staphylococci, Streptococcus Pneumoniae and Haemophilus Influenzae. J. Antimicrob. Chemother. 2010, 65, 1441–1447. [Google Scholar] [CrossRef]
- Romero, G.B.; Keck, C.M.; Müller, R.H.; Bou-Chacra, N.A. Development of Cationic Nanocrystals for Ocular Delivery. Eur. J. Pharm. Biopharm. 2016, 107, 215–222. [Google Scholar] [CrossRef] [PubMed]
- MALVERN Zetasizer Nano Series—Performance, Simplicity, Versatility. Malvern Instruments Ltd. 2014. Available online: https://www.malvernpanalytical.com/en/assets/MRK1839_tcm50-17228.pdf (accessed on 10 August 2022).
- VICH ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. 2005. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-2-r1-validation-analytical-procedures-text-methodology-step-5_en.pdf (accessed on 6 September 2022).
- United States Pharmacopeia. 〈1225〉 Validation of Compendial Procedures; USP 43-NF 38; Pharmacopeial: Rockville Convention, MD, USA, 2021. [Google Scholar]
- Brazilian Health Surveillance Agency (ANVISA). Resolução da Diretoria Colegiada RDC No 166, de 24 de Julho de 2017. Dispõe sobre a validação de métodos analíticos e dá outras providências. Available online: https://www.in.gov.br/materia/-/asset_publisher/Kujrw0TZC2Mb/content/id/19194581/do1-2017-07-25-resolucao-rdc-n-166-de-24-de-julho-de-2017-19194412 (accessed on 6 September 2022).
- Brazilian Health Surveillance Agency (ANVISA). Resolução da Diretoria Colegiada RDC No 37, de 03 de Agosto de 2011. Dispõe Sobre o Guia Para Isenção e Substituição de Estudos de Biodisponibilidade Relativa/Bioequivalência e Dá Outras Providências. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/anvisa/2011/res0037_03_08_2011.html (accessed on 6 September 2022).
- de Spadari, C.; de Bastiani, F.W.M.d.S.; Lopes, L.B.; Ishida, K. Alginate Nanoparticles as Non-Toxic Delivery System for Miltefosine in the Treatment of Candidiasis and Cryptococcosis. Int. J. Nanomed. 2019, 14, 5187–5199. [Google Scholar] [CrossRef]
- ICH Q1—Evaluation of Stability Data. Guideline 2003. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-q-1-r2-stability-testing-new-drug-substances-products-step-5_en.pdf (accessed on 6 September 2022).
- Brazilian Health Surveillance Agency (ANVISA). Resolução da Diretoria Colegiada RDC N° 318, de 6 de Novembro de 2019. Estabelece os critérios para a realização de Estudos de Estabilidade de insumos farmacêuticos ativos e medicamentos, exceto biológicos, e dá outras providências. Available online: https://www.in.gov.br/web/dou/-/resolucao-rdc-n-318-de-6-de-novembro-de-2019-226513805 (accessed on 26 August 2022).
- Silverstein, B.E.; Morris, T.W.; Gearinger, L.S.; DeCory, H.H.; Comstock, T.L. Besifloxacin Ophthalmic Suspension 0.6% in the Treatment of Bacterial Conjunctivitis Patients with Pseudomonas Aeruginosa Infections. Clin. Ophthalmol. 2012, 6, 1987–1996. [Google Scholar] [CrossRef][Green Version]
- de Cássia Zaghi Compri, J.; Andres Felli, V.M.; Lourenço, F.R.; Takatsuka, T.; Fotaki, N.; Löbenberg, R.; Bou-Chacra, N.A.; Barros de Araujo, G.L. Highly Water-Soluble Orotic Acid Nanocrystals Produced by High-Energy Milling. J. Pharm. Sci. 2019, 108, 1848–1856. [Google Scholar] [CrossRef]
- Lin, S.Y.; Lin, H.L.; Chi, Y.T.; Hung, R.Y.; Huang, Y.T.; Kao, C.Y.; Hsieh, W.H. Povacoat Affecting Solid-State Polymorphic Changes of Indomethacin after Co-Evaporation from Different Types of Solvents via Conventional and Microwave Drying Techniques. Asian J. Pharm. Sci. 2016, 11, 376–384. [Google Scholar] [CrossRef]
- Melo, K.J.C.; Henostroza, M.A.B.; Löbenberg, R.; Bou-Chacra, N.A. Rifampicin Nanocrystals: Towards an Innovative Approach to Treat Tuberculosis. Mater. Sci. Eng. C 2020, 112, 110895. [Google Scholar] [CrossRef] [PubMed]
- Ochi, M.; Kawachi, T.; Toita, E.; Hashimoto, I.; Yuminoki, K.; Onoue, S.; Hashimoto, N. Development of Nanocrystal Formulation of Meloxicam with Improved Dissolution and Pharmacokinetic Behaviors. Int. J. Pharm. 2014, 474, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Aikawa, S.; Kashima, Y.; Kikuchi, J.; Ida, Y.; Tanino, T.; Kadota, K.; Tozuka, Y. Anti-Plasticizing Effect of Amorphous Indomethacin Induced by Specific Intermolecular Interactions with PVA Copolymer. J. Pharm. Sci. 2014, 103, 2829–2838. [Google Scholar] [CrossRef] [PubMed]
- Yuminoki, K.; Seko, F.; Horii, S.; Takeuchi, H.; Teramoto, K.; Nakada, Y.; Hashimoto, N. Preparation and Evaluation of High Dispersion Stable Nanocrystal Formulation of Poorly Water-Soluble Compounds by Using Povacoat. J. Pharm. Sci. 2014, 103, 3772–3781. [Google Scholar] [CrossRef] [PubMed]
- Yuminoki, K.; Seko, F.; Horii, S.; Takeuchi, H.; Teramoto, K.; Nakada, Y.; Hashimoto, N. Application of Povacoat as Dispersion Stabilizer of Nanocrystal Formulation. Asian J. Pharm. Sci. 2016, 11, 48–49. [Google Scholar] [CrossRef][Green Version]
- ICH Q8(R2). Pharmaceutical Development. Guideline. 2009. Available online: https://www.ema.europa.eu/en/ich-q8-r2-pharmaceutical-development (accessed on 6 September 2022).
- Peters, M.C.C.; dos Santos Neto, E.; Monteiro, L.M.; Yukuyama, M.N.; Machado, M.G.M.; de Oliveira, I.F.; Zanin, M.H.A.; Löbenberg, R.; Bou-Chacra, N. Advances in Ophthalmic Preparation: The Role of Drug Nanocrystals and Lipid-Based Nanosystems. J. Drug Target. 2020, 28, 259–270. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration Food and Drug Administration—Guidance for Industry Considering Whether an FDA-Regulated Product Involves the Application of Nanotechnology. Biotechnol. Law Rep. 2014, 30, 613–616. [CrossRef]
- Kornecki, M.; Strube, J. Process Analytical Technology for Advanced Process Control in Biologics Manufacturing with the Aid of Macroscopic Kinetic Modeling. Bioengineering 2018, 5, 25. [Google Scholar] [CrossRef]
- HORIBA A Guidebook to Particle Size Analysis. Available online: https://static.horiba.com/fileadmin/Horiba/Products/Scientific/Particle_Characterization/Particle_Guidebook_06-2021.pdf (accessed on 2 August 2022).
- Australian Government Department of Health and Ageing AusPAR: Besifloxacin Hydrochloride. Available online: https://www.tga.gov.au/resources/auspar/auspar-besifloxacin-hydrochloride (accessed on 2 August 2022).
- Pharmacopeia, U.S. 〈771〉 Ophthalmic Products—Quality Tests; USP 43-NF 38; Pharmacopeial: Rockville Convention, MD, USA, 2021. [Google Scholar]
- Abelson, M.B.; Udell, I.J.; Weston, J.H. Normal Human Tear Ph by Direct Measurement. Arch. Ophthalmol. 1981, 99, 301. [Google Scholar] [CrossRef]
- Aldrich, D.S.; Bach, C.M.; Brown, W.; Chambers, W.; Fleitman, J.; Hunt, D.; Marques, M.R.C.; Mille, Y.; Mitra, A.K.; Platzer, S.M.; et al. Ophthalmic Preparations. Pharmacop. Forum 2013, 39, 1–21. [Google Scholar]
- Skrdla, P.J.; Yang, H. On the Stability of Nano-Formulations Prepared by Direct Synthesis: Simulated Ostwald Ripening of a Typical Nanocrystal Distribution Post-Nucleation. AAPS PharmSciTech 2019, 20, 34. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia. 〈785〉 Osmolality and Osmolarity; USP 43-NF 38; Pharmacopeial: Rockville Convention, MD, USA, 2021. [Google Scholar]
- Bazán Henostroza, M.A.; Curo Melo, K.J.; Nishitani Yukuyama, M.; Löbenberg, R.; Araci Bou-Chacra, N. Cationic Rifampicin Nanoemulsion for the Treatment of Ocular Tuberculosis. Colloids Surf. A: Physicochem. Eng. Asp. 2020, 597, 124755. [Google Scholar] [CrossRef]
- Food and Drug Administration 2018 NEW DRUG THERAPY APPROVALS. Available online: https://www.fda.gov/files/drugs/published/New-Drug-Therapy-Approvals-2018_3.pdf (accessed on 2 August 2022).
- Harry, M.; King, J. Fluoroquinolone Carboxylic Acid Molecular Crystals. U.S. Patent No. 8,481,526, 9 July 2013. [Google Scholar]
- Piatek, M.; Sheehan, G.; Kavanagh, K. Galleria Mellonella: The Versatile Host for Drug Discovery, in Vivo Toxicity Testing and Characterising Host-Pathogen Interactions. Antibiotics 2021, 10, 1545. [Google Scholar] [CrossRef] [PubMed]
- Moya-Andérico, L.; Vukomanovic, M.; del Cendra, M.; Segura-Feliu, M.; Gil, V.; del Río, J.A.; Torrents, E. Utility of Galleria Mellonella Larvae for Evaluating Nanoparticle Toxicology. Chemosphere 2021, 266, 129235. [Google Scholar] [CrossRef]
- Mikulak, E.; Gliniewicz, A.; Przygodzka, M.; Solecka, J. Galleria Mellonella L. as Model Organism Used in Biomedical and Other Studies. Prz. Epidemiol. 2018, 72, 57–73. [Google Scholar]

| Level | Besifloxacin (wt%) | Stabilizer (Povacoat®) (wt%) | Stirring Speed (rpm) |
|---|---|---|---|
| Minimum (−1) | 0.4 | 0.6 | 800 |
| Central point (0) | 0.6 | 0.8 | 1000 |
| Maximum (+1) | 0.8 | 1.0 | 1200 |
| Formula | Order | Central Point | Besifloxacin (wt%) | Povacoat® (wt%) | Speed (rpm) | Z-ave (nm) | PdI |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 2 | 0.4 | 0.6 | 1000 | 641.2 ± 45.2 | 0.110 ± 0.123 |
| 2 | 12 | 2 | 0.6 | 1.0 | 1200 | 596.7 ± 27.6 | 0.199 ± 0.137 |
| 3 | 11 | 2 | 0.6 | 0.6 | 1200 | 651.7 ± 61.8 | 0.131 ± 0.174 |
| 4 | 8 | 2 | 0.8 | 0.8 | 1200 | 677.5 ± 78.4 | 0.145 ± 0.137 |
| 5 | 5 | 2 | 0.4 | 0.8 | 800 | 638.6 ± 33.0 | 0.204 ± 0.166 |
| 6 | 13 | 0 | 0.6 | 0.8 | 1000 | 585.3 ± 21.7 | 0.168 ± 0.069 |
| 7 | 4 | 2 | 0.8 | 1.0 | 1000 | 592.9 ± 28.1 | 0.199 ± 0.187 |
| 8 | 10 | 2 | 0.6 | 1.0 | 800 | 608.3 ± 18.6 | 0.198 ± 0.186 |
| 9 | 2 | 2 | 0.8 | 0.6 | 1000 | 748.4 ± 47.6 | 0.183 ± 0.107 |
| 10 | 7 | 2 | 0.4 | 0.8 | 1200 | 595.4 ± 35.2 | 0.139 ± 0.070 |
| 11 | 3 | 2 | 0.4 | 1.0 | 1000 | 666.7 ± 37.6 | 0.310 ± 0.217 |
| 12 | 9 | 2 | 0.6 | 0.6 | 800 | 666.7 ± 51.6 | 0.197 ± 0.141 |
| 13 | 6 | 2 | 0.8 | 0.8 | 800 | 661.5 ± 35.4 | 0.162 ± 0.104 |
| 14 | 15 | 0 | 0.6 | 0.8 | 1000 | 587.6 ± 30.1 | 0.110 ± 0.103 |
| 15 | 14 | 0 | 0.6 | 0.8 | 1000 | 578.9 ± 12.3 | 0.158 ± 0.039 |
| Source | DF | Adj. SS | Adj. MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 8 | 30,471.9 | 3808.99 | 45.16 | 0.0001 |
| Linear | 3 | 9902.4 | 3300.82 | 39.14 | 0.0001 |
| Besifloxacin | 1 | 2791.2 | 2791.17 | 33.09 | 0.001 |
| Povacoat® | 1 | 6749.5 | 6749.48 | 80.03 | 0.0001 |
| Speed | 1 | 361.8 | 361.81 | 4.29 | 0.084 |
| Square | 3 | 10,474.4 | 3491.47 | 41.40 | 0.0001 |
| Besifloxacin * Besifloxacin | 1 | 7149.8 | 7149.80 | 84.77 | 0.0001 |
| Povacoat® * Povacoat® | 1 | 3688.1 | 3688.06 | 43.73 | 0.001 |
| Speed * Speed | 1 | 865.7 | 865.70 | 10.26 | 0.019 |
| Interaction between 2 factors | 2 | 10,095.0 | 5047.52 | 59.85 | 0.0001 |
| Besifloxacin * Povacoat® | 1 | 9218.9 | 9218.88 | 109.30 | 0.0001 |
| Besifloxacin * Speed | 1 | 876.2 | 876.16 | 10.39 | 0.018 |
| Error | 6 | 506.1 | 84.34 | ||
| Lack-of-fit | 4 | 465.4 | 116.35 | 5.72 | 0.154 |
| Pure error | 2 | 40.6 | 20.32 | * | * |
| Total | 14 | 30,977.9 |
| Term | Coef | SE Coef | t-Value | p-Value | VIF |
|---|---|---|---|---|---|
| Constant | 583.93 | 5.30 | 110.13 | 0.0001 | |
| Besifloxacin | 18.68 | 3.25 | 5.75 | 0.001 | 1.00 |
| Povacoat® | −29.05 | 3.25 | −8.95 | 0.000 | 1.00 |
| Speed | −6.72 | 3.25 | −2.07 | 0.084 | 1.00 |
| Besifloxacin * Besifloxacin | 44.00 | 4.78 | 9.21 | 0.0001 | 1.01 |
| Povacoat® * Povacoat® | 31.60 | 4.78 | 6.61 | 0.001 | 1.01 |
| Speed * Speed | 15.31 | 4.78 | 3.20 | 0.019 | 1.01 |
| Besifloxacin * Povacoat® | −48.01 | 4.59 | −10.45 | 0.0001 | 1.00 |
| Besifloxacin * Speed | 14.80 | 4.59 | 3.22 | 0.018 | 1.00 |
| S: 9.18% R2: 98.37% R2 (adj.): 96.16% R2 (pred): 75.85% | |||||
| Formula | Besifloxacin (wt%) | Povacoat® (wt%) | Speed (rpm) | Theoretical Z-ave (nm) | Experimental Z-ave (nm) | PdI | ZP (mV) |
|---|---|---|---|---|---|---|---|
| F1 | 0.6 | 0.8 | 834 | 600 | 639.9 ± 30.3 | 0.101 ± 0.056 | −10.10 ± 0.15 |
| F2 | 0.6 | 0.9 | 1044 | 576 | 560.3 ± 4.2 | 0.098 ± 0.067 | −10.20 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, J.I.S.d.S.d.; Lourenço, F.R.; Ishida, K.; Barreto, T.L.; Avino, V.C.; Neto, E.d.S.; Bou-Chacra, N.A. Besifloxacin Nanocrystal: Towards an Innovative Ophthalmic Preparation. Pharmaceutics 2022, 14, 2221. https://doi.org/10.3390/pharmaceutics14102221
Jesus JISdSd, Lourenço FR, Ishida K, Barreto TL, Avino VC, Neto EdS, Bou-Chacra NA. Besifloxacin Nanocrystal: Towards an Innovative Ophthalmic Preparation. Pharmaceutics. 2022; 14(10):2221. https://doi.org/10.3390/pharmaceutics14102221
Chicago/Turabian StyleJesus, José Izo Santana da Silva de, Felipe Rebello Lourenço, Kelly Ishida, Thayná Lopes Barreto, Valdir Carlos Avino, Edson dos Santos Neto, and Nádia Araci Bou-Chacra. 2022. "Besifloxacin Nanocrystal: Towards an Innovative Ophthalmic Preparation" Pharmaceutics 14, no. 10: 2221. https://doi.org/10.3390/pharmaceutics14102221
APA StyleJesus, J. I. S. d. S. d., Lourenço, F. R., Ishida, K., Barreto, T. L., Avino, V. C., Neto, E. d. S., & Bou-Chacra, N. A. (2022). Besifloxacin Nanocrystal: Towards an Innovative Ophthalmic Preparation. Pharmaceutics, 14(10), 2221. https://doi.org/10.3390/pharmaceutics14102221









