Cancer-Associated Membrane Protein as Targeted Therapy for Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
3. Stages of Bladder Cancer
4. Current Status of Biomarker in Bladder Cancer
5. Targeted Therapy for Bladder Cancer
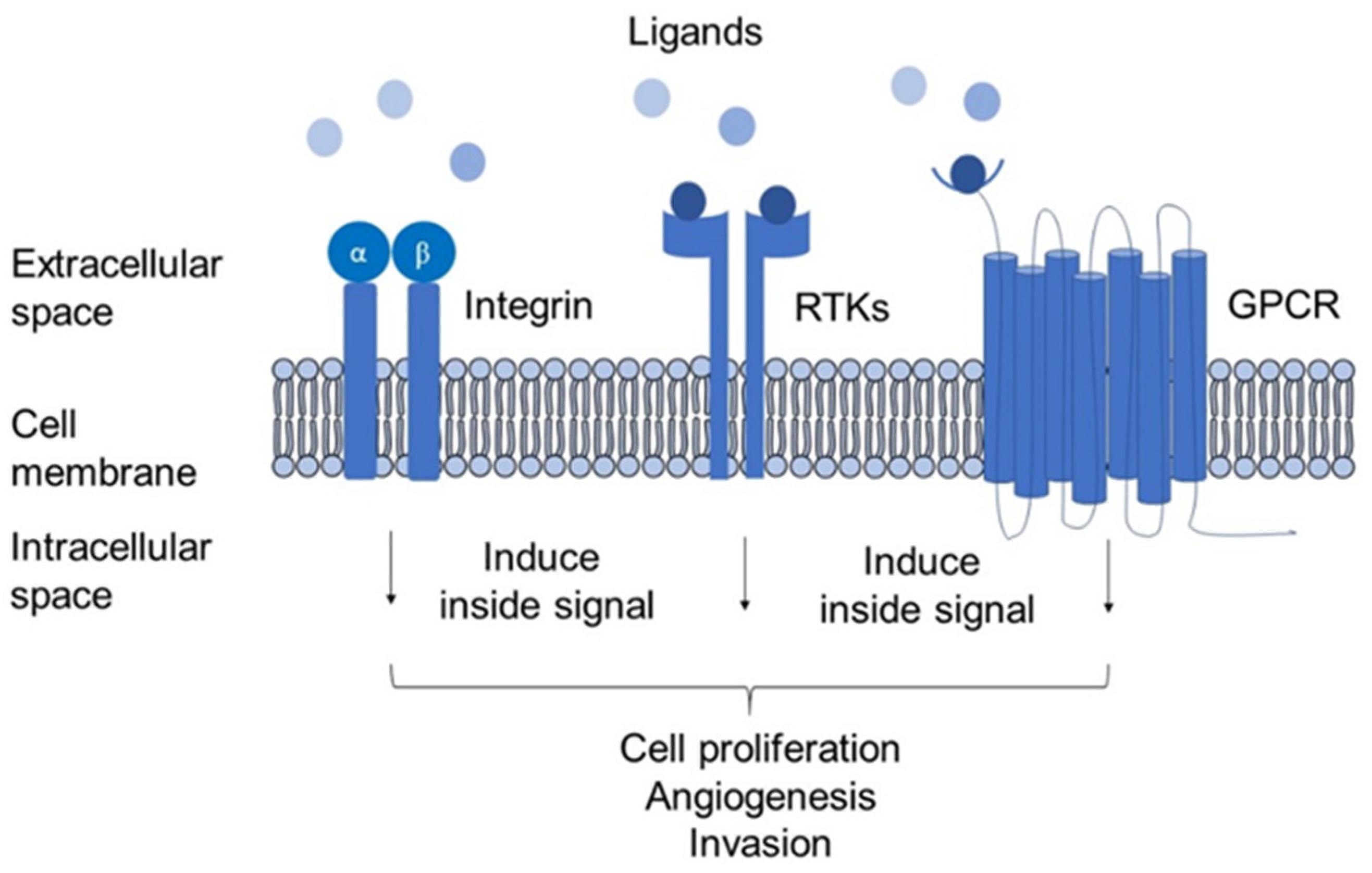
6. Membrane Proteins: Structural and Biological Role in Cancer
6.1. Integrins
6.2. Receptor Tyrosine Kinases (RTKs)
6.3. G-Protein Coupled Receptor-Chemokine Receptor
7. Mechanism of Bladder Cancer Recurrence
7.1. Modification of Drug Efflux
7.2. Induction of Drug-Detoxifying Mechanism
7.3. Modification of Drug Targets
7.4. DNA-Damage Repair
7.5. Activation of Prosurvival Signalling Pathways
8. Membrane Proteins as Targeted Therapy for Cancer
8.1. Pgp-1
8.2. Her2
8.3. TACSTD2
8.4. VEFGR1/2
8.5. Integrin β8
8.6. FGFR3
8.7. CXCR7
| No. | Protein Biomarkers | Types of Proteins/Receptors | In Vitro | Clinical Study | ||
|---|---|---|---|---|---|---|
| Method of Detection | Expression in Cell Lines | Method of Detection | Expression in Specimens | |||
| 1. | Pgp-1 | Transporter | WB | Highly expressed in 253J and J82 cells [63]. | IHC | Highly expressed in 39 of 55 BC specimens (71%) (China) [63]. |
| 2. | Her2 | RTK | WB | Expressed in BC cell lines but 10-fold lower in the breast cancer cell, SKBR3 cells [64]. | IHC | Overexpressed more in NMIBC patients (21%) (China) [65] |
| 3. | TCSTD2 | RTK | RT-PCR | Highly expressed in multiple BC cell lines [50]. | IHC | Highly expressed in 27.3% of the 99 patients (Japan) [66]. |
| 4. | VEGFR1 | RTK | Immunoblot | Higher expression in TCCSUP [67]. | IHC | Increased 2-fold in BC specimens compared with the normal (USA) [67]. |
| 5. | VEGFR2 | RTK | Immunoblot | Highly expressed in J82 and HT1376 BC cells [67]. | IHC | Increased 55% in BC specimens compared with the normal (USA) [67]. |
| 6. | Integrin β8 | Integrin | Immunofluorescence assay | Overexpressed in Biu87 and T24 BC cells [24]. | IHC | Increased 2-fold higher in highly malignant BC (China) [24]. |
| 7. | FGFR3 | RTK | WB | Highly expressed in RT4, RT112, and SW780 cells [68]. | RT-qPCR | Highly expressed (40.0%) in patients with pT1 BC (Korea) [69]. |
| 8. | CXCR7 | GPCR | Q-PCR | Highly expressed (3-10-fold) in 5637 and HT1197 cell lines than in other cell lines [62]. | IHC | Highly expressed (5–10-fold) in BC tissues than in normal tissues (Florida, USA) [62]. |
| No. | Protein Biomarkers | Types of Proteins/Receptors | Chemotherapy Resistance | Expression in Other Cancer | In Vitro | Clinical Study | ||
|---|---|---|---|---|---|---|---|---|
| Method of Detection | Expression in Cell Lines | Method of Detection | Expression in Specimens | |||||
| 1. | Pgp-1 | Transporter | Reported with paclitaxel and docetaxel resistance [70]. Increased chemotherapeutic drug outflow in cell culture, which promotes multidrug resistance [71]. | NSCLC | Immunofluorescence assay | Expressed in SPCA1, lung cancer cell line and downregulated by Verapamil [72]. | IHC | Expressed in 52/60 NSCLC patients treated with Docetaxel and 46/60 patients in the docetaxel + tamoxifen group (China) [73]. |
| Breast | WB and immunofluorescence | Highly expressed in MCF-7R and MCF-7 breast cancer cell lines [74]. | IHC | Expressed in 9/49 patients in the pretreatment group and increased to 29/49 after chemotherapy (India) [75]. | ||||
| Stomach (gastric) | WB | Expressed in SGC-790, gastric cancer cell line, increased when stimulated with Paclitaxel [76]. | RT-PCR, IHC | Expressed in 54 gastric patients (61.3%) with low IRF-1 expression (China) [77]. | ||||
| 2. | Her2 | RTK | Reported with cisplatin-based regimens resistance [78]. | Ovarian | WB | Overexpressed in Caov-3 ovarian cancer cells. | IHC | Highly expressed (79%) in samples of recurrent ovarian cancer (Taiwan) [79]. |
| 3. | TACSTD2 | RTK | Reported with tamoxifen and trastuzumab resistance [57,80]. | NSCLC | WB | Upregulated in human NSCLC, A549, NCI-H520, NCIH441, and NCI-H226 cell lines, compared to normal HBE cell lines [28]. | RT-PCR, IHC | 1.5-fold up-regulated in 58/107 NSCLC patients and increased in the advanced cancer stage (China) [28]. |
| Thyroid | WB | Overexpressed and promoted the invasion and migration of K1, FTC-133, and 8505C thyroid cancer cell lines [29]. | RT-PCR, IHC | Highly expressed (53.1%) in malignant thyroid tissues of 51/96 patients (China) [29]. | ||||
| Breast | WB | Expressed higher in the breast cancer cell line (MCF-7 and MDA-MB-231 cells) than normal breast cell line (MCF-10A) [81]. | RT-PCR, IHC | Expressed with 1.55 ± 0.78 fold higher in 20 pairs of breast cancer tissues than in adjacent tissues (China) [81]. | ||||
| 4. | VEGFR1 | RTK | Reported with bortezomib resistance [82]. Alterations of VEGFR1 contributed to resistance to anti-VEGF therapy [83]. | Ovarian and Cervical | RT-PCR | Expressed low in an ovarian carcinoma cell line (DOV13) [84]. | IHC | Significantly expressed in 8 patients with post-radiotherapy relapsed/persistent cervical cancer (Japan) [85]. |
| Colon | WB | Express in human colon cancer (HCT116 cell line) [86]. | RT-PCR | Expressed in 39 metastatic colorectal cancer (Italy) [87]. | ||||
| NSCLC | RT-PCR and WB | Induced expression at higher concentrations in NSCLC cell lines (A549 and SKMES1), when treated with Trichostatin A (TSA), compared to untreated [88]. | RT-PCR | Expressed in 23 (85.2%) of 27 malignant specimens (Greece) [89]. | ||||
| 5. | VEGFR2 | RTK | VEGF-C/VEGFR2 signalling promotes tumorigenicity and potentially contributes to bevacizumab resistance [90]. | Ovarian | RT-PCR | Expressed in an ovarian carcinoma cell line (DOV13) but low in levels [84]. | WB | Express in 70 (92.1%) of 76 ovarian cancer tissues (Europe) [91]. |
| NSCLC | RT-PCR and WB | Expression increased in NSCLC cell lines (A549 and SKMES1) when treated with Trichostatin A (TSA) compared to untreated [89]. | RT-PCR | Expressed in 24 of 27 (88.9%) malignant specimens (Greece) [89]. | ||||
| Colon | WB | Weakly expressed in human colon cancer (HCT116 cell line) [86]. | RT-PCR | Expressed in 72 metastatic colorectal cancer patients (Italy) [87]. | ||||
| 6. | Integrin β8 | Integrin | Correlated with resistance to gefitinib [92]. | Pancreatic | WB | Highly expressed in a pancreatic cancer cell line (Panc-1) [93]. | IHC | 3.1-fold upregulated in pancreatic cancer patients (78 patients) [93]. |
| Colon | WB | Weakly expressed in human colon cancer (HCT116 cell line) [86]. | RT-PCR | Expressed in 72 metastatic colorectal cancer patients (Italy) [87]. | ||||
| 7. | FGFR3 | RTK | Overexpressed FGFR3-S isoform was reported to be resistant to docetaxel [94]. | NSCLC | RT-PCR & WB | Increased in A549 and NCIH460 NSCLC cell lines with exposure to nicotine [95]. | IHC | Highly expressed FGFR3 in 3.3% NSCLC tumour samples (Netherlands) [96]. |
| 8. | CXCR7 | GPCR | Overexpression of CXCR7 constitutes a mechanism of resistance to EGFR tyrosine kinase inhibitors [97]. | Bladder | RT-PCR & WB | Expressed higher in RT4 compared to J82 and T24 cells [32]. | IHC | Expressed highly in high-grade 2 BC specimens (33 of 78) (CA) [32] |
| Breast | Flow cytometry | Expressed in breast cancer cell lines (4T1) [98]. | IHC | Highly expressed in 106 of 109 human breast cancer specimens (97%) (CA) [98]. | ||||
9. Proteomic Platforms for Biomarker Discovery
| No. | Platforms | Principle | Advantages | Drawbacks | Utilisation in BC Studies |
|---|---|---|---|---|---|
| 1. | Liquid chromatography-tandem mass spectrometry (LC-MS/MS) | Ionise, fragmented molecules, and analyse the ions produced based on the mass-to-charge ratio (m/z). | Specific, sensitive, no antibody required. | Complexity | [120] |
| 2. | Enzyme-linked Immunosorbent assay (ELISA) | Immobilise antigen to a plate-based surface and interact with an enzyme-linked antibody. | Cost-effective and easy to use. | Require high-quality antibody, possible unspecific binding. | [121] |
| 3. | Western blot | Proteins are separated based on molecular weight, transferred onto a membrane, and detected with antibodies. | Separate based on molecular weight. | Require a large amount of protein, high-quality antibody, and possible unspecific binding. | [81] |
| 4. | Immunohistochemistry (IHC) | Antibodies bind specifically to proteins. | The localised protein of interest. | The semi-quantitative assay requires high-quality antibodies and possible unspecific binding. | [81] |
| 5. | Surface-enhanced Raman spectroscopy (SERS) | Vibrational optical spectroscopic technique based on strong interactions between proteins and metal nanoparticles. | Little sample preparation. | Unspecific interaction, less sensitive. | [122] |
| 6. | Colorimetric assay | Change of colour due to an enzymatic or chemical interaction between spotted reagents and the analyte. | Fast, inexpensive. | Stability/shelf-life, possible unspecific interaction | [123] |
| 7. | Electrochemical ELISA-based assay/ biosensor | Requires specific markers to promote selective binding and detection. This biomolecular recognition takes place close to the functionalised surface of an electrode. | Versatile | Long optimisation, possible unspecific binding. | [121] |
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Konety, B.; Isharwal, S. Non-Muscle Invasive Bladder Cancer Risk Stratification. Indian J. Urol. 2015, 31, 289. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Marks, P.; Soave, A.; Shariat, S.F.; Fajkovic, H.; Fisch, M.; Rink, M. Female with Bladder Cancer: What and Why Is There a Difference? Transl. Androl. Urol. 2016, 5, 668–682. [Google Scholar] [CrossRef]
- Bhindi, B.; Kool, R.; Kulkarni, G.S.; Siemens, D.R.; Aprikian, A.G.; Breau, R.H.; Brimo, F.; Fairey, A.; French, C.; Hanna, N.; et al. Canadian Urological Association Guideline on the Management of Non-Muscle-Invasive Bladder Cancer–Abridged Version. Can. Urol. Assoc. J. 2021, 15, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Akagashi, K.; Tanda, H.; Kato, S.; Ohnishi, S.; Nakajima, H.; Nanbu, A.; Nitta, T.; Koroku, M.; Sato, Y.; Hanzawa, T. Recurrence Pattern for Superficial Bladder Cancer. Int. J. Urol. 2006, 13, 686–691. [Google Scholar] [CrossRef]
- Breau, R.H.; Karnes, R.J.; Farmer, S.A.; Thapa, P.; Cagiannos, I.; Morash, C.; Frank, I. Progression to Detrusor Muscle Invasion during Urothelial Carcinoma Surveillance Is Associated with Poor Prognosis. Br. J. Urol. 2014, 113, 900–906. [Google Scholar] [CrossRef]
- Quek, M.L.; Stein, J.P.; Clark, P.E.; Daneshmand, S.; Miranda, G.; Cai, J.; Groshen, S.; Lieskovsky, G.; Quinn, D.I.; Raghavan, D.; et al. Natural History of Surgically Treated Bladder Carcinoma with Extravesical Tumor Extension. Cancer 2003, 98, 955–961. [Google Scholar] [CrossRef]
- Chamie, K.; Litwin, M.S.; Bassett, J.C.; Daskivich, T.J.; Lai, J.; Hanley, J.M.; Konety, B.R.; Saigal, C.S. Recurrence of High-Risk Bladder Cancer: A Population-Based Analysis. Cancer 2013, 119, 3219–3227. [Google Scholar] [CrossRef]
- Tada, Y.; Wada, M.; Migita, T.; Nagayama, J.; Hinoshita, E.; Mochida, Y.; Maehara, Y.; Tsuneyoshi, M.; Kuwano, M.; Naito, S. Increased Expression of Multidrug Resistance-Associated Proteins in Bladder Cancer during Clinical Course and Drug Resistance to Doxorubicin. Int. J. Cancer 2002, 98, 630–635. [Google Scholar] [CrossRef]
- Kilari, D.; Iczkowski, K.A.; Pandya, C.; Robin, A.J.; Messing, E.M.; Guancial, E.; Kim, E.S. Copper Transporter-CTR1 Expression and Pathological Outcomes in Platinum-Treated Muscle-Invasive Bladder Cancer Patients. Anticancer Res. 2016, 36, 495–501. [Google Scholar] [PubMed]
- Loktionov, A. Biomarkers for Detecting Colorectal Cancer Non-Invasively: DNA, RNA or Proteins? World J. Gastrointest. Oncol. 2020, 12, 124–148. [Google Scholar] [CrossRef] [PubMed]
- Magers, M.J.; Lopez-Beltran, A.; Montironi, R.; Williamson, S.R.; Kaimakliotis, H.Z.; Cheng, L. Staging of Bladder Cancer. Histopathology 2019, 74, 112–134. [Google Scholar] [CrossRef] [PubMed]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.E.; Lotan, Y. Bladder Cancer. Nat. Rev. Dis. Primers 2017, 3, 17022. [Google Scholar] [CrossRef]
- DeGeorge, K.C.; Holt, H.R.; Hodges, S.C. Bladder Cancer: Diagnosis and Treatment. Am. Fam. Physician 2017, 96, 507–514. [Google Scholar]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Padma, V.V. An Overview of Targeted Cancer Therapy. BioMedicine 2015, 5, 19. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Qoronfleh, M.W.; Benton, B.; Ignacio, R.; Kaboord, B. Selective Enrichment of Membrane Proteins by Partition Phase Separation for Proteomic Studies. J. Biomed. Biotechnol. 2003, 2003, 249–255. [Google Scholar] [CrossRef]
- Rucevic, M.; Hixson, D.; Josic, D. Mammalian Plasma Membrane Proteins as Potential Biomarkers and Drug Targets. Electrophoresis 2011, 32, 1549–1564. [Google Scholar] [CrossRef]
- Várady, G.; Cserepes, J.; Németh, A.; Szabó, E.; Sarkadi, B. Cell Surface Membrane Proteins as Personalized Biomarkers: Where We Stand and Where We Are Headed. Biomarkers Med. 2013, 7, 803–819. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Ivaska, J. Every Step of the Way: Integrins in Cancer Progression and Metastasis. Nat. Cancer 2018, 18, 533–548. [Google Scholar] [CrossRef] [PubMed]
- Cresswell, J.; Wong, W.; Henry, M.; Robertson, H.; Neal, D.; Kirby, J. Adhesion of Lymphocytes to Bladder Cancer Cells: The Role of the α E β 7 Integrin. Cancer Immunol. Immunother. 2002, 51, 483–491. [Google Scholar] [CrossRef]
- Liu, S.; Chen, L.; Zhao, H.; Li, Q.; Hu, R.; Wang, H. Integrin Β8 Facilitates Tumor Growth and Drug Resistance through a Y-box Binding Protein 1-dependent Signaling Pathway in Bladder Cancer. Cancer Sci. 2020, 111, 2423–2430. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, K.E.M.; Zuiverloon, T.C.M.; Alberts, A.R.; Boormans, J.L.; Zwarthoff, E.C. Targeted Therapies in Bladder Cancer: An Overview of in Vivo Research. Nat. Rev. Urol. 2015, 12, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Xia, G.; Kumar, S.R.; Hawes, D.; Cai, J.; Hassanieh, L.; Groshen, S.; Zhu, S.; Masood, R.; Quinn, D.I.; Broek, D.; et al. Expression and Significance of Vascular Endothelial Growth Factor Receptor 2 in Bladder Cancer. J. Urol. 2006, 175, 1245–1252. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, M.; Dong, J.; Han, G.; Jiang, X.; Wu, R.; Zou, H. Specific Phosphopeptide Enrichment with Immobilized Titanium Ion Affinity Chromatography Adsorbent for Phosphoproteome Analysis. J. Proteome Res. 2008, 7, 3957–3967. [Google Scholar] [CrossRef]
- Guo, X.; Zhu, X.; Zhao, L.; Li, X.; Cheng, D.; Feng, K. Tumor-Associated Calcium Signal Transducer 2 Regulates Neovascularization of Non-Small-Cell Lung Cancer via Activating ERK1/2 Signaling Pathway. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef]
- Guan, H.; Guo, Z.; Liang, W.; Li, H.; Wei, G.; Xu, L.; Xiao, H.; Li, Y. Trop2 Enhances Invasion of Thyroid Cancer by Inducing MMP2 through ERK and JNK Pathways. BMC Cancer 2017, 17, 486. [Google Scholar] [CrossRef]
- Sriram, K.; Insel, P.A. G Protein-Coupled Receptors as Targets for Approved Drugs: How Many Targets and How Many Drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef]
- Lodowski, D.T.; Palczewski, K. Chemokine Receptors and Other G Protein-Coupled Receptors. Curr. Opin. HIV AIDS 2009, 4, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Zheng, J.; Hou, K.; Wang, J.; Chen, X.; Lu, X.; Bo, J.; Xu, C.; Shen, K.; Wang, J. Role of Chemokine Receptor CXCR7 in Bladder Cancer Progression. Biochem. Pharmacol. 2012, 84, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Long, L.; Wang, J.; Wang, Y.; Liu, Y.; Wang, L.; Luo, F. Insights on CXC Chemokine Receptor 2 in Breast Cancer: An Emerging Target for Oncotherapy (Review). Oncol. Lett. 2019, 18, 5699–5708. [Google Scholar] [CrossRef] [PubMed]
- Kazanietz, M.G.; Durando, M.; Cooke, M. CXCL13 and Its Receptor CXCR5 in Cancer: Inflammation, Immune Response, and Beyond. Front. Endocrinol. 2019, 10, 471. [Google Scholar] [CrossRef] [PubMed]
- Takanami, I. Overexpression of CCR7 MRNA in Nonsmall Cell Lung Cancer: Correlation with Lymph Node Metastasis. Int. J. Cancer 2003, 105, 186–189. [Google Scholar] [CrossRef]
- Avril, T.; Vauléon, E.; Chevet, E. Endoplasmic Reticulum Stress Signaling and Chemotherapy Resistance in Solid Cancers. Oncogenesis 2017, 6, e373. [Google Scholar] [CrossRef]
- Gottesman, M.M. Mechanisms of Cancer Drug Resistance. Annu. Rev. Med. 2002, 53, 615–627. [Google Scholar] [CrossRef]
- Tsuruo, T.; Naito, M.; Tomida, A.; Fujita, N.; Mashima, T.; Sakamoto, H.; Haga, N. Molecular Targeting Therapy of Cancer: Drug Resistance, Apoptosis and Survival Signal. Cancer Sci. 2003, 94, 15–21. [Google Scholar] [CrossRef]
- Agarwal, R.; Kaye, S.B. Ovarian Cancer: Strategies for Overcoming Resistance to Chemotherapy. Nat. Rev. Cancer 2003, 3, 502–516. [Google Scholar] [CrossRef]
- Borst, P.; Evers, R.; Kool, M.; Wijnholds, J. A Family of Drug Transporters: The Multidrug Resistance-Associated Proteins. JNCI: J. Natl. Cancer Inst. 2000, 92, 1295–1302. [Google Scholar] [CrossRef]
- Holohan, C.; van Schaeybroeck, S.; Longley, D.B.; Johnston, P.G. Cancer Drug Resistance: An Evolving Paradigm. Nat. Rev. Cancer 2013, 13, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.; Johnston, P. Molecular Mechanisms of Drug Resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, A.J. Epidermal Growth Factor Receptor and Bladder Cancer. Postgrad. Med. J. 2002, 78, 584–589. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mann, M.J.; Hendershot, L.M. UPR Activation Alters Chemosensitivity of Tumor Cells. Cancer Biol. Ther. 2006, 5, 736–740. [Google Scholar] [CrossRef]
- Davies, M.P.A.; Barraclough, D.L.; Stewart, C.; Joyce, K.A.; Eccles, R.M.; Barraclough, R.; Rudland, P.S.; Sibson, D.R. Expression and Splicing of the Unfolded Protein Response Gene XBP-1 Are Significantly Associated with Clinical Outcome of Endocrine-Treated Breast Cancer. Int. J. Cancer 2008, 123, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, P.; Jonkers, J. The Effects of Deregulated DNA Damage Signalling on Cancer Chemotherapy Response and Resistance. Nat. Cancer 2012, 12, 587–598. [Google Scholar] [CrossRef]
- Dejeans, N.; Barroso, K.; Fernandez-Zapico, M.E.; Samali, A.; Chevet, E. Novel Roles of the Unfolded Protein Response in the Control of Tumor Development and Aggressiveness. Semin. Cancer Biol. 2015, 33, 67–73. [Google Scholar] [CrossRef]
- Hetz, C.; Chevet, E.; Oakes, S.A. Proteostasis Control by the Unfolded Protein Response. Nat. Cell Biol. 2015, 17, 829–838. [Google Scholar] [CrossRef]
- Gschwind, A.; Fischer, O.M.; Ullrich, A. The Discovery of Receptor Tyrosine Kinases: Targets for Cancer Therapy. Nat. Rev. Cancer 2004, 4, 361–370. [Google Scholar] [CrossRef]
- Chou, J.; Trepka, K.; Sjöström, M.; Egusa, E.A.; Chu, C.E.; Zhu, J.; Chan, E.; Gibb, E.A.; Badura, M.L.; Contreras-Sanz, A.; et al. TROP2 Expression Across Molecular Subtypes of Urothelial Carcinoma and Enfortumab Vedotin-resistant Cells. Eur. Urol. Oncol. 2022. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- Fukuokaya, W.; Kimura, T.; Miki, J.; Kimura, S.; Watanabe, H.; Bo, F.; Okada, D.; Aikawa, K.; Ochi, A.; Suzuki, K.; et al. Effectiveness of Intravesical Doxorubicin Immediately Following Resection of Primary Non–Muscle-Invasive Bladder Cancer: A Propensity Score-Matched Analysis. Clin. Genitourinary Cancer 2020, 18, e55–e61. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Flynn, A.D. Drugging Membrane Protein Interactions. Annu. Rev. Biomed. Eng. 2016, 18, 51–76. [Google Scholar] [CrossRef]
- Chen, D.; Ye, Y.; Guo, S.; Yao, K. Progress in the Research and Targeted Therapy of ErbB/HER Receptors in Urothelial Bladder Cancer. Front. Mol. Biosci. 2021, 8, 800945. [Google Scholar] [CrossRef] [PubMed]
- Ecke, T.H.; Schlechte, H.H.; Schulze, G.; Lenk, S.V.; Loening, S.A. Four Tumour Markers for Urinary Bladder Cancer--Tissue Polypeptide Antigen (TPA), HER-2/Neu (ERB B2), Urokinase-Type Plasminogen Activator Receptor (UPAR) and TP53 Mutation. Anticancer Res. 2005, 25, 635–641. [Google Scholar]
- Su, H.; Jiang, H.; Tao, T.; Kang, X.; Zhang, X.; Kang, D.; Li, S.; Li, C.; Wang, H.; Yang, Z.; et al. Hope and challenge: Precision medicine in bladder cancer. Cancer Med. 2019, 8, 1806–1816. [Google Scholar] [CrossRef]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: Regulation and clinical/ therapeutic implications. Genes Cancer 2014, 6, 84–105. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Fléchon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients with Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef]
- Cheetham, P.J.; Petrylak, D.P. New Agents for the Treatment of Advanced Bladder Cancer. Oncology 2016, 30, 571–588, 571–579, 588. [Google Scholar]
- Tan, T.Z.; Rouanne, M.; Tan, K.T.; Huang, R.Y.-J.; Thiery, J.-P. Molecular Subtypes of Urothelial Bladder Cancer: Results from a Meta-Cohort Analysis of 2411 Tumors. Eur. Urol. 2019, 75, 423–432. [Google Scholar] [CrossRef]
- Casadei, C.; Dizman, N.; Schepisi, G.; Cursano, M.C.; Basso, U.; Santini, D.; Pal, S.K.; de Giorgi, U. Targeted Therapies for Advanced Bladder Cancer: New Strategies with FGFR Inhibitors. Ther. Adv. Med. Oncol. 2019, 11, 1758835919890285. [Google Scholar] [CrossRef] [PubMed]
- Yates, T.J.; Knapp, J.; Gosalbez, M.; Lokeshwar, S.D.; Gomez, C.S.; Benitez, A.; Ekwenna, O.O.; Young, E.E.; Manoharan, M.; Lokeshwar, V.B. C-X-C Chemokine Receptor 7. Cancer 2013, 119, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.G.; Sikic, B.I. Molecular Pathways: Regulation and Therapeutic Implications of Multidrug Resistance. Clin. Cancer Res. 2012, 18, 1863–1869. [Google Scholar] [CrossRef] [PubMed]
- Nini, A.; Hoffmann, M.J.; Lampignano, R.; Siemer, R.G.; Van Dalum, G.; Szarvas, T.; Cotarelo, C.L.; Schulz, W.A.; Niederacher, D.; Neubauer, H.; et al. Evaluation of HER2 expression in urothelial carcinoma cells as a biomarker for circulating tumor cells. Cytom. Part B: Clin. Cytom. 2020, 98, 355–367. [Google Scholar] [CrossRef]
- Ding, W.; Tong, S.; Gou, Y.; Sun, C.; Wang, H.; Chen, Z.; Tan, J.; Xu, K.; Xia, G.; Ding, Q. Human Epidermal Growth Factor Receptor 2: A Significant Indicator for Predicting Progression in Non-Muscle-Invasive Bladder Cancer Especially in High-Risk Groups. World J. Urol. 2015, 33, 1951–1957. [Google Scholar] [CrossRef]
- Tomiyama, E.; Fujita, K.; Nakano, K.; Kuwahara, K.; Minami, T.; Kato, T.; Hatano, K.; Kawashima, A.; Uemura, M.; Takao, T.; et al. Trop-2 in Upper Tract Urothelial Carcinoma. Curr. Oncol. 2022, 29, 3911–3921. [Google Scholar] [CrossRef]
- Kopparapu, P.K.; Boorjian, S.A.; Robinson, B.D.; Downes, M.; Gudas, L.J.; Mongan, N.P.; Persson, J.L. Expression of VEGF and Its Receptors VEGFR1/VEGFR2 Is Associated with Invasiveness of Bladder Cancer. Anticancer. Res. 2013, 33, 2381–2390. [Google Scholar]
- Williams, S.v.; Hurst, C.D.; Knowles, M.A. Oncogenic FGFR3 Gene Fusions in Bladder Cancer. Hum. Mol. Genet. 2013, 22, 795–803. [Google Scholar] [CrossRef]
- Kang, H.W.; Kim, Y.-H.; Jeong, P.; Park, C.; Kim, W.T.; Ryu, D.H.; Cha, E.-J.; Ha, Y.-S.; Kim, T.-H.; Kwon, T.G.; et al. Expression levels of FGFR3 as a prognostic marker for the progression of primary pT1 bladder cancer and its association with mutation status. Oncol. Lett. 2017, 14, 3817–3824. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a Target for Anticancer Drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Márián, T.; Szabó, G.; Goda, K.; Nagy, H.; Szincsák, N.; Juhász, I.; Galuska, L.; Balkay, L.; Mikecz, P.; Trón, L.; et al. In Vivo and in Vitro Multitracer Analyses of P-Glycoprotein Expression-Related Multidrug Resistance. Eur. J. Nuclear Med. Mol. Imaging 2003, 30, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Zhao, L.; Meng, Q.; Wang, Q. Analysis of Chemoresistance in Lung Cancer with a Simple Microfluidic Device. Electrophoresis 2010, 31, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Fu, X.; Li, G.; He, L.; Zhao, C.; Hu, X.; Pan, R.; Guo, C.; Zhang, X.; Hu, X. Efficacy of Tamoxifen in Combination with Docetaxel in Patients with Advanced Non-Small-Cell Lung Cancer Pretreated with Platinum-Based Chemotherapy. Anti-Cancer Drugs 2016, 27, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Bai, J.; Jin, L.; Kang, X.; Zhang, H.; Wang, Z. Pluronic P123 Modified Nano Micelles Loaded with Doxorubicin Enhanced Tumor-Suppressing Effect on Drug-Resistant Breast Cancer Cells. Aging 2020, 12, 8289–8300. [Google Scholar] [CrossRef]
- Mehrotra, M.; Anand, A.; Singh, K.R.; Kumar, S.; Husain, N.; Sonkar, A.A. P-Glycoprotein Expression in Indian Breast Cancer Patients with Reference to Molecular Subtypes and Response to Anthracycline-Based Chemotherapy—A Prospective Clinical Study from a Developing Country. Indian J. Surg. Oncol. 2018, 9, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Gu, K.-S.; Chen, Y. Mechanism of P-Glycoprotein Expression in the SGC7901 Human Gastric Adenocarcinoma Cell Line Induced by Cyclooxygenase-2. Asian Pac. J. Cancer Prev. 2012, 13, 2379–2383. [Google Scholar] [CrossRef][Green Version]
- Yuan, J.; Yin, Z.; Tan, L.; Zhu, W.; Tao, K.; Wang, G.; Shi, W.; Gao, J. Interferon Regulatory Factor-1 Reverses Chemoresistance by Downregulating the Expression of P-Glycoprotein in Gastric Cancer. Cancer Lett. 2019, 457, 28–39. [Google Scholar] [CrossRef]
- Tsai, C.-M.; Yu, D.; Chang, K.-T.; Wu, L.-H.; Perng, R.-P.; Ibrahim, N.K.; Hung, M.-C. Enhanced Chemoresistance by Elevation of P185neu Levels in HER-2/Neu-Transfected Human Lung Cancer Cells. JNCI J. Natl. Cancer Inst. 1995, 87, 682–684. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Chen, C.-A.; Chou, C.-H.; Lai, K.-P.; Jeng, Y.-M.; Kuo, M.-L.; Wei, L.-H. Overexpression of Her-2/Neu in Epithelial Ovarian Carcinoma Induces Vascular Endothelial Growth Factor C by Activating NF-ΚB: Implications for Malignant Ascites Formation and Tumor Lymphangiogenesis. J. Biomed. Sci. 2004, 11, 249–259. [Google Scholar] [CrossRef]
- Zimmers, S.M.; Browne, E.P.; Williams, K.E.; Jawale, R.M.; Otis, C.N.; Schneider, S.S.; Arcaro, K.F. TROP2 Methylation and Expression in Tamoxifen-Resistant Breast Cancer. Cancer Cell Int. 2018, 18, 94. [Google Scholar] [CrossRef]
- Zhao, W.; Kuai, X.; Zhou, X.; Jia, L.; Wang, J.; Yang, X.; Tian, Z.; Wang, X.; Lv, Q.; Wang, B.; et al. Trop2 is a potential biomarker for the promotion of EMT in human breast cancer. Oncol. Rep. 2018, 40, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Kalitin, N.N.; Kostyukova, M.N.; Kakpakova, E.S.; Tupitsyn, N.N.; Karamysheva, A.F. Expression of Vascular Endothelial Growth Factor Receptors VEGFR1 in Cultured Multiple Myeloma Cells: Correlation with Immunophenotype and Drug Resistance. Bull. Exp. Biol. Med. 2012, 153, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Sainson, R.C.A.; Oon, C.E.; Turley, H.; Leek, R.; Sheldon, H.; Bridges, E.; Shi, W.; Snell, C.; Bowden, E.T.; et al. DLL4-Notch Signaling Mediates Tumor Resistance to Anti-VEGF Therapy. In Vivo Cancer Res. 2011, 71, 6073–6083. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; So, J.; Reierstad, S.; Fishman, D.A. Vascular Endothelial Growth Factor-Regulated Ovarian Cancer Invasion and Migration Involves Expression and Activation of Matrix Metalloproteinases. Int. J. Cancer 2006, 118, 879–888. [Google Scholar] [CrossRef]
- Yoshida, K.; Suzuki, S.; Sakata, J.; Utsumi, F.; Niimi, K.; Yoshikawa, N.; Nishino, K.; Shibata, K.; Kikkawa, F.; Kajiyama, H. The Upregulated Expression of Vascular Endothelial Growth Factor in Surgically Treated Patients with Recurrent/Radioresistant Cervical Cancer of the Uterus. Ncol. Lett. 2018, 16, 515–521. [Google Scholar] [CrossRef]
- Nagano, H.; Tomida, C.; Yamagishi, N.; Teshima-Kondo, S. VEGFR-1 Regulates EGF-R to Promote Proliferation in Colon Cancer Cells. Int. J. Mol. Sci. 2019, 20, 5608. [Google Scholar] [CrossRef]
- Hanna, D.L.; Loupakis, F.; Yang, D.; Cremolini, C.; Schirripa, M.; Li, M.; Matsusaka, S.; Berger, M.D.; Miyamoto, Y.; Zhang, W.; et al. Prognostic Value of ACVRL1 Expression in Metastatic Colorectal Cancer Patients Receiving First-line Chemotherapy with Bevacizumab: Results from the Triplet Plus Bevacizumab (TRIBE) Study. Clin. Color. Cancer 2018, 17, 471–488. [Google Scholar] [CrossRef]
- Barr, M.P.; O’Byrne, K.J.; Al-Sarraf, N.; Gray, S.G. VEGF-mediated cell survival in non-small-cell lung cancer: Implications for epigenetic targeting of VEGF receptors as a therapeutic approach. Epigenomics 2015, 7, 897–910. [Google Scholar] [CrossRef]
- Zygalaki, E.; Tsaroucha, E.G.; Kaklamanis, L.; Lianidou, E.S. Quantitative Real-Time Reverse Transcription–PCR Study of the Expression of Vascular Endothelial Growth Factor (VEGF) Splice Variants and VEGF Receptors (VEGFR-1 and VEGFR-2) in Non–Small Cell Lung Cancer. Clin. Chem. 2007, 53, 1433–1439. [Google Scholar] [CrossRef]
- Michaelsen, S.R.; Staberg, M.; Pedersen, H.; E Jensen, K.; Majewski, W.; Broholm, H.; Nedergaard, M.K.; Meulengracht, C.; Urup, T.; Villingshøj, M.; et al. VEGF-C sustains VEGFR2 activation under bevacizumab therapy and promotes glioblastoma maintenance. Neuro-Oncology 2018, 20, 1462–1474. [Google Scholar] [CrossRef]
- Klasa-Mazurkiewicz, D.; Jarząb, M.; Milczek, T.; Lipińska, B.; Emerich, J. Clinical Significance of VEGFR-2 and VEGFR-3 Expression in Ovarian Cancer Patients. Pol. J. Pathol. 2011, 62, 31–40. [Google Scholar] [PubMed]
- Wang, W.-W.; Wang, Y.-B.; Wang, D.-Q.; Lin, Z.; Sun, R.-J. Integrin Beta-8 (ITGB8) Silencing Reverses Gefitinib Resistance of Human Hepatic Cancer HepG2/G Cell Line. Int. J. Clin. Exp. Med. 2015, 8, 3063–3071. [Google Scholar]
- Jin, S.; Lee, W.-C.; Aust, D.; Pilarsky, C.; Cordes, N. Β8 Integrin Mediates Pancreatic Cancer Cell Radiochemoresistance. Mol. Cancer Res. 2019, 17, 2126–2138. [Google Scholar] [CrossRef]
- Olender, J.; Wang, B.-D.; Ching, T.; Garmire, L.X.; Garofano, K.; Ji, Y.; Knox, T.; Latham, P.; Nguyen, K.; Rhim, J.; et al. A Novel FGFR3 Splice Variant Preferentially Expressed in African American Prostate Cancer Drives Aggressive Phenotypes and Docetaxel Resistance. Mol. Cancer Res. 2019, 17, 2115–2125. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Qi, F.; Lu, S.; Li, Y.; Han, W. Nicotine Upregulates FGFR3 and RB1 Expression and Promotes Non-Small Cell Lung Cancer Cell Proliferation and Epithelial-to-Mesenchymal Transition via Downregulation of MiR-99b and MiR-192. Biomed. Pharmacother. 2018, 101, 656–662. [Google Scholar] [CrossRef]
- Theelen, W.S.M.E.; Mittempergher, L.; Willems, S.M.; Bosma, A.J.; Peters, D.D.G.C.; Van Der Noort, V.; Japenga, E.J.; Peeters, T.; Koole, K.; Šuštić, T.; et al. FGFR1, 2 and 3 protein overexpression and molecular aberrations of FGFR3 in early stage non-small cell lung cancer. J. Pathol. Clin. Res. 2016, 2, 223–233. [Google Scholar] [CrossRef]
- Becker, J.H.; Gao, Y.; Soucheray, M.; Pulido, I.; Kikuchi, E.; Rodríguez, M.L.; Gandhi, R.; Lafuente-Sanchis, A.; Aupí, M.; Fernández-Coronado, J.A.; et al. CXCR7 Reactivates ERK Signaling to Promote Resistance to EGFR Kinase Inhibitors in NSCLC. Cancer Res. 2019, 79, 4439–4452. [Google Scholar] [CrossRef]
- Miao, Z.; Luker, K.E.; Summers, B.C.; Berahovich, R.; Bhojani, M.S.; Rehemtulla, A.; Kleer, C.G.; Essner, J.J.; Nasevicius, A.; Luker, G.D.; et al. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc. Natl. Acad. Sci. USA 2007, 104, 15735–15740. [Google Scholar] [CrossRef]
- Faria, S.S.; Morris, C.F.M.; Silva, A.R.; Fonseca, M.P.; Forget, P.; Castro, M.S.; Fontes, W. A Timely Shift from Shotgun to Targeted Proteomics and How It Can Be Groundbreaking for Cancer Research. Front. Oncol. 2017, 7, 13. [Google Scholar] [CrossRef][Green Version]
- Nwabo Kamdje, A.H.; Seke Etet, P.F.; Vecchio, L.; Muller, J.M.; Krampera, M.; Lukong, K.E. Signaling Pathways in Breast Cancer: Therapeutic Targeting of the Microenvironment. Cell. Signal. 2014, 26, 2843–2856. [Google Scholar] [CrossRef]
- Tan, H.T.; Lee, Y.H.; Chung, M.C.M. Cancer Proteomics. Mass Spectrom. Rev. 2012, 31, 583–605. [Google Scholar] [CrossRef] [PubMed]
- Boja, E.S.; Rodriguez, H. Proteogenomic Convergence for Understanding Cancer Pathways and Networks. Clin. Proteom. 2014, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.-E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable Isotope Labeling by Amino Acids in Cell Culture, SILAC, as a Simple and Accurate Approach to Expression Proteomics. Mol. Cell. Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, S.; Ji, Y.; Guo, X.; Yang, F. Quantitative Proteomics Using SILAC: Principles, Applications, and Developments. Proteomics 2015, 15, 3175–3192. [Google Scholar] [CrossRef] [PubMed]
- Gygi, S.P.; Rist, B.; Gerber, S.A.; Turecek, F.; Gelb, M.H.; Aebersold, R. Quantitative Analysis of Complex Protein Mixtures Using Isotope-Coded Affinity Tags. Nat. Biotechnol. 1999, 17, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Steen, H.; Gygi, S.P. Protein Profiling with Cleavable Isotope-Coded Affinity Tag (CICAT) Reagents. Mol. Cell. Proteom. 2003, 2, 1198–1204. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.C.; Schmitt-Ulms, G.; Chalkley, R.J.; Hirsch, J.; Baldwin, M.A.; Burlingame, A.L. Mass Spectrometric Analysis of Protein Mixtures at Low Levels Using Cleavable 13C-Isotope-Coded Affinity Tag and Multidimensional Chromatography. Mol. Cell. Proteom. 2003, 2, 299–314. [Google Scholar] [CrossRef]
- Boersema, P.J.; Raijmakers, R.; Lemeer, S.; Mohammed, S.; Heck, A.J.R. Multiplex Peptide Stable Isotope Dimethyl Labeling for Quantitative Proteomics. Nat. Protoc. 2009, 4, 484–494. [Google Scholar] [CrossRef]
- Hsu, J.-L.; Huang, S.-Y.; Chow, N.-H.; Chen, S.-H. Stable-Isotope Dimethyl Labeling for Quantitative Proteomics. Anal. Chem. 2003, 75, 6843–6852. [Google Scholar] [CrossRef]
- Ross, P.L.; Huang, Y.N.; Marchese, J.N.; Williamson, B.; Parker, K.; Hattan, S.; Khainovski, N.; Pillai, S.; Dey, S.; Daniels, S.; et al. Multiplexed Protein Quantitation in Saccharomyces cerevisiae Using Amine-reactive Isobaric Tagging Reagents. Mol. Cell. Proteom. 2004, 3, 1154–1169. [Google Scholar] [CrossRef]
- Choe, L.; D’Ascenzo, M.; Relkin, N.R.; Pappin, D.; Ross, P.; Williamson, B.; Guertin, S.; Pribil, P.; Lee, K.H. 8-Plex Quantitation of Changes in Cerebrospinal Fluid Protein Expression in Subjects Undergoing Intravenous Immunoglobulin Treatment for Alzheimer’s Disease. Proteomics 2007, 7, 3651–3660. [Google Scholar] [CrossRef] [PubMed]
- Bantscheff, M.; Lemeer, S.; Savitski, M.M.; Kuster, B. Quantitative Mass Spectrometry in Proteomics: Critical Review Update from 2007 to the Present. Anal. Bioanal. Chem. 2012, 404, 939–965. [Google Scholar] [CrossRef] [PubMed]
- Neilson, K.A.; Ali, N.A.; Muralidharan, S.; Mirzaei, M.; Mariani, M.; Assadourian, G.; Lee, A.; van Sluyter, S.C.; Haynes, P.A. Less Label, More Free: Approaches in Label-Free Quantitative Mass Spectrometry. Proteomics 2011, 11, 535–553. [Google Scholar] [CrossRef] [PubMed]
- Bantscheff, M.; Schirle, M.; Sweetman, G.; Rick, J.; Kuster, B. Quantitative Mass Spectrometry in Proteomics: A Critical Review. Anal. Bioanal. Chem. 2007, 389, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Collier, T.S.; Sarkar, P.; Franck, W.L.; Rao, B.M.; Dean, R.A.; Muddiman, D.C. Direct Comparison of Stable Isotope Labeling by Amino Acids in Cell Culture and Spectral Counting for Quantitative Proteomics. Anal. Chem. 2010, 82, 8696–8702. [Google Scholar] [CrossRef]
- Adeola, H.A.; Calder, B.; Soares, N.C.; Kaestner, L.; Blackburn, J.M.; Zerbini, L.F. In Silico Verification and Parallel Reaction Monitoring Prevalidation of Potential Prostate Cancer Biomarkers. Futur. Oncol. 2016, 12, 43–57. [Google Scholar] [CrossRef]
- Gallien, S.; Duriez, E.; Domon, B. Selected Reaction Monitoring Applied to Proteomics. J. Mass Spectrom. 2011, 46, 298–312. [Google Scholar] [CrossRef]
- Domon, B.; Gallien, S. Recent Advances in Targeted Proteomics for Clinical Applications. Proteom. Clin. Appl. 2015, 9, 423–431. [Google Scholar] [CrossRef]
- Ebhardt, H.A.; Root, A.; Sander, C.; Aebersold, R. Applications of Targeted Proteomics in Systems Biology and Translational Medicine. Proteomics 2015, 15, 3193–3208. [Google Scholar] [CrossRef]
- Yang, N.; Feng, S.; Shedden, K.; Xie, X.; Liu, Y.; Rosser, C.J.; Lubman, D.M.; Goodison, S. Urinary Glycoprotein Biomarker Discovery for Bladder Cancer Detection Using LC/MS-MS and Label-Free Quantification. Clin. Cancer Res. 2011, 17, 3349–3359. [Google Scholar] [CrossRef]
- Arya, S.K.; Estrela, P. Electrochemical ELISA-Based Platform for Bladder Cancer Protein Biomarker Detection in Urine. Biosens. Bioelectron. 2018, 117, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Moisoiu, T.; Dragomir, M.P.; Iancu, S.D.; Schallenberg, S.; Birolo, G.; Ferrero, G.; Burghelea, D.; Stefancu, A.; Cozan, R.G.; Licarete, E.; et al. Combined miRNA and SERS urine liquid biopsy for the point-of-care diagnosis and molecular stratification of bladder cancer. Mol. Med. 2022, 28, 39. [Google Scholar] [CrossRef] [PubMed]
- Gogalic, S.; Sauer, U.; Doppler, S.; Preininger, C. Investigating Colorimetric Protein Array Assay Schemes for Detection of Recurrence of Bladder Cancer. Biosensors 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Vogenberg, F.R.; Isaacson Barash, C.; Pursel, M. Personalized Medicine: Part 1: Evolution and Development into Theranostics. P T 2010, 35, 560–576. [Google Scholar] [PubMed]
| No. | Hallmarks of Cancer | Relation with Membrane Proteins |
|---|---|---|
| 1. | Sustaining proliferative signalling | The cancer cells convey the signals by binding the growth factors to cell-surface receptors (tyrosine kinase domains, a membrane protein). |
| 2. | Evading growth suppressors | In normal cells, the tumour suppressor further suppresses proliferation by coupling cell-surface adhesion molecules (e.g., E-cadherin) to transmembrane receptor tyrosine kinases (e.g., the EGF receptor). Sequestration of growth factor receptors restricts cell division signals. In cancer patients, tumour formation occurs due to the loss of the tumour suppressor. |
| 3. | Resisting cell death | Programmed cell death was resisted by upstream regulators involving the Fas ligand/Fas receptor. |
| 4. | Inducing angiogenesis | Regulators of angiogenesis involve signalling proteins that bind to cell surface receptors. |
| 5. | Activating invasion and metastasis | The expression of the key molecule for cell adhesion, E-cadherin (one type of transmembrane protein), increased and became an antagonist of invasion and metastasis. |
| 6. | Enabling replicative immortality | No membrane proteins were found to be associated, but telomerase, a ribonucleoprotein, was reported to be involved in unlimited proliferation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roslan, A.; Sulaiman, N.; Mohd Ghani, K.A.; Nurdin, A. Cancer-Associated Membrane Protein as Targeted Therapy for Bladder Cancer. Pharmaceutics 2022, 14, 2218. https://doi.org/10.3390/pharmaceutics14102218
Roslan A, Sulaiman N, Mohd Ghani KA, Nurdin A. Cancer-Associated Membrane Protein as Targeted Therapy for Bladder Cancer. Pharmaceutics. 2022; 14(10):2218. https://doi.org/10.3390/pharmaceutics14102218
Chicago/Turabian StyleRoslan, Adlina, Nurshahira Sulaiman, Khairul Asri Mohd Ghani, and Armania Nurdin. 2022. "Cancer-Associated Membrane Protein as Targeted Therapy for Bladder Cancer" Pharmaceutics 14, no. 10: 2218. https://doi.org/10.3390/pharmaceutics14102218
APA StyleRoslan, A., Sulaiman, N., Mohd Ghani, K. A., & Nurdin, A. (2022). Cancer-Associated Membrane Protein as Targeted Therapy for Bladder Cancer. Pharmaceutics, 14(10), 2218. https://doi.org/10.3390/pharmaceutics14102218






