Surface Modifiers on Composite Particles for Direct Compaction
Abstract
1. Introduction
2. HPMC (Hydroxypropyl Methylcellulose)
2.1. Unitary Modifier
2.1.1. Co-Spray Drying
2.1.2. Co-Freeze Drying
2.1.3. Fluid-Bed Coating
2.1.4. Co-Milling
2.1.5. Crystallo-Co-Agglomeration
2.2. Binary Modifiers
3. PVP (Polyvinylpyrrolidone)
3.1. Unitary Modifier
3.1.1. Co-Spray Drying
3.1.2. Co-Freeze Drying
3.1.3. Fluid-Bed Coating
3.2. Binary Modifiers
3.2.1. Co-Spray Drying
3.2.2. Fluid-Bed Coating
3.2.3. Dry Coating
4. SiO2
4.1. Unitary Modifier
4.1.1. Dry Coating
4.1.2. Liquid Dispersion
4.1.3. Co-Milling
4.2. Binary Modifiers
5. MCC
5.1. Unitary Modifier
5.1.1. Co-Spray Drying
5.1.2. Liquid Dispersion
5.2. Binary Modifiers
6. Mannitol
6.1. Co-Spray Drying
6.2. Adsorption
7. Others
7.1. Polyvinylpolypyrrolidone
7.2. Ammonium Bicarbonate
7.3. Sodium Lauryl Sulphate
7.4. Magnesium Stearate
7.5. Hydroxypropyl Cellulose
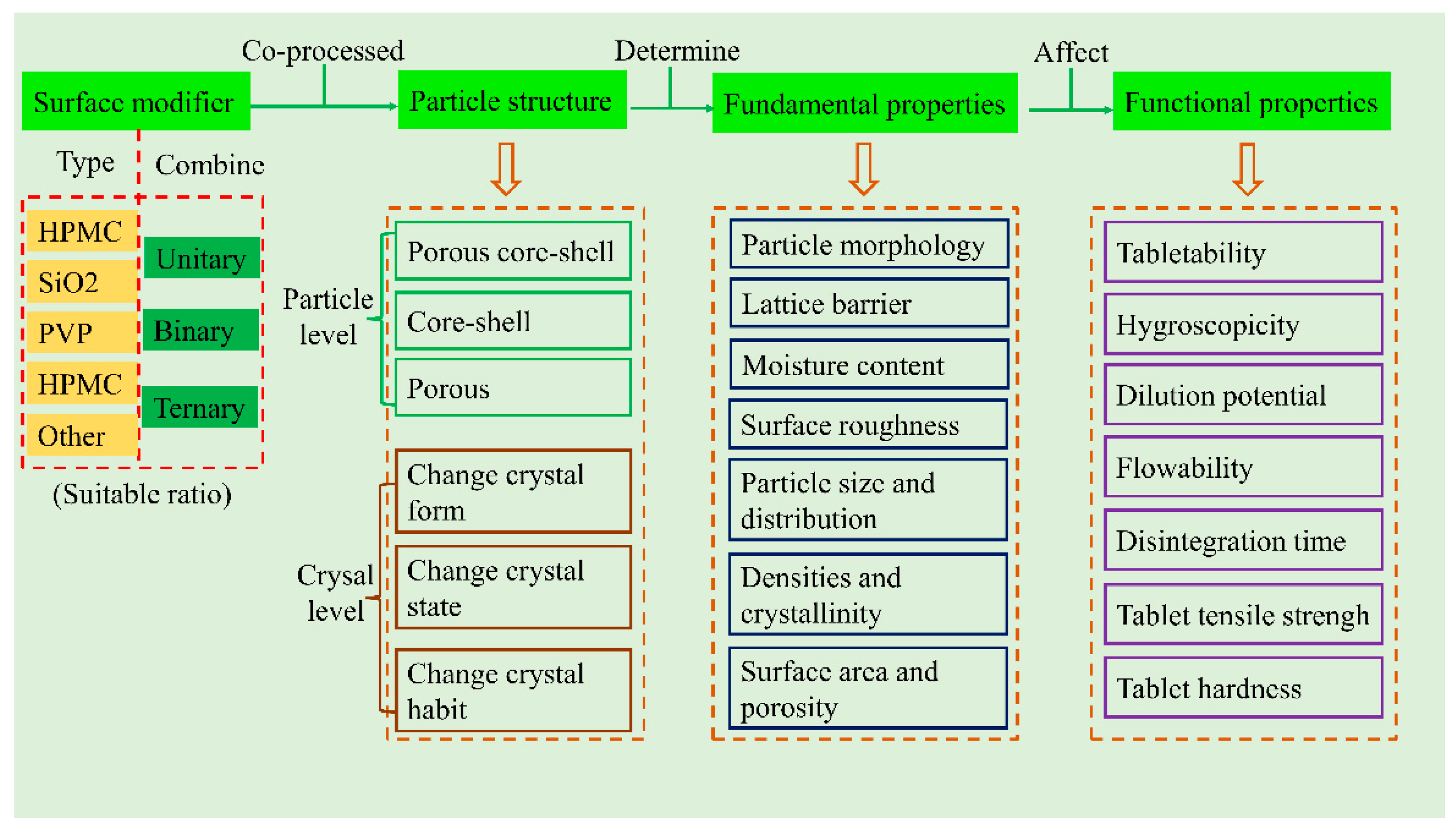
8. General Modification Mechanisms
8.1. The Functional Properties Improved through Modifying the Structure
8.1.1. Particle Structure
8.1.2. Crystal Forms and Habits
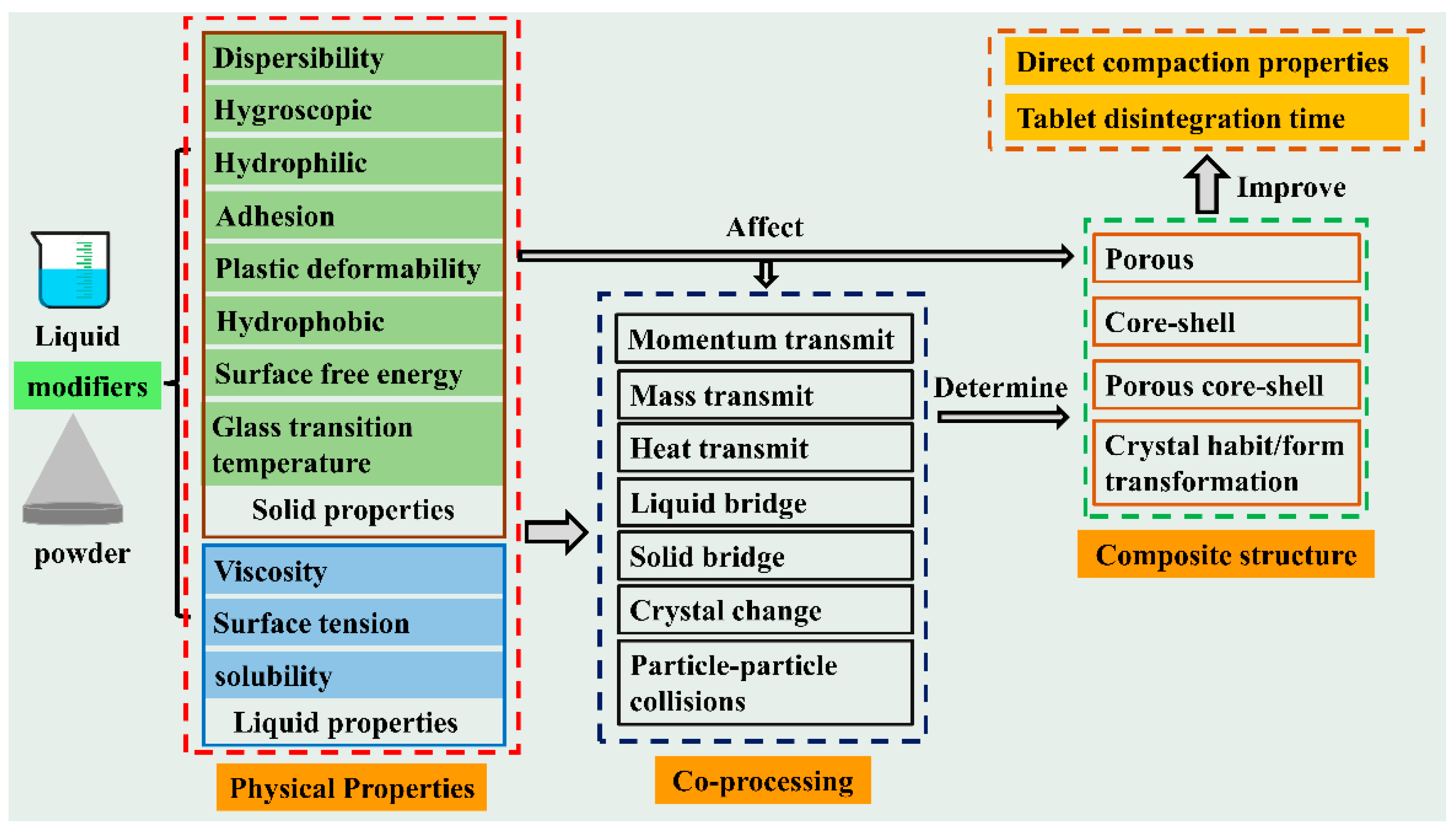
8.2. The Synergistic Effect of Co-Processing Methods
8.3. Others
9. Future Perspectives and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azad, M.A.; Osorio, J.G.; Wang, A.; Klee, D.M.; Eccles, M.E.; Grela, E.; Sloan, R.; Hammersmith, G.; Rapp, K.; Brancazio, D.; et al. On-demand manufacturing of direct compressible tablets: Can formulation be simplified? Pharm. Res. 2019, 36, 14. [Google Scholar] [CrossRef] [PubMed]
- Mazel, V.; Busignies, V.; Diarra, H.; Tchoreloff, P. Lamination of pharmaceutical tablets due to air entrapment: Direct visualization and influence of the compact thickness. Int. J. Pharm. 2015, 478, 702–704. [Google Scholar] [CrossRef] [PubMed]
- Roopwani, R.; Buckner, I.S. Co-processed particles: An approach to transform poor tableting properties. J. Pharm. Sci. 2019, 108, 3209–3217. [Google Scholar] [CrossRef]
- Kirchengast, M.; Celikovic, S.; Rehrl, J.; Sacher, S.; Kruisz, J.; Khinast, J.G.; Horn, M. Ensuring tablet quality via model-based control of a continuous direct compaction process. Int. J. Pharm. 2019, 567, 118457. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, L.; Lin, X.; Shen, L.; Feng, Y. Direct compaction: An update of materials, trouble-shooting, and application. Int. J. Pharm. 2017, 529, 543–556. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.; Wu, F.; Shen, L.; Lin, X.; Feng, Y. Direct compaction properties of Zingiberis rhizoma extracted powders coated with various shell materials: Improvements and mechanism analysis. Int. J. Pharm. 2019, 564, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, A.; Leong, K.H.; Urata, E.; Hayashi, Y.; Kumada, S.; Okada, K.; Onuki, Y. Effect of different direct compaction grades of mannitol on the storage stability of tablet properties investigated using a kohonen self-organizing map and elastic net regression model. Pharmaceutics 2020, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; He, Z.; Kunnath, K.T.; Fan, S.; Wei, Y.; Ding, X.; Zheng, K.; Dave, R.N. Surface engineered excipients: III. Facilitating direct compaction tableting of binary blends containing fine cohesive poorly-compactable APIs. Int. J. Pharm. 2019, 557, 354–365. [Google Scholar] [CrossRef]
- Li, Z.; Lin, X.; Shen, L.; Hong, Y.; Feng, Y. Composite particles based on particle engineering for direct compaction. Int. J. Pharm. 2017, 519, 272–286. [Google Scholar] [CrossRef]
- Schaller, B.E.; Moroney, K.M.; Castro-Dominguez, B.; Cronin, P.; Belen-Girona, J.; Ruane, P.; Croker, D.M.; Walker, G. Systematic development of a high dosage formulation to enable direct compression of a poorly flowing API: A case study. Int. J. Pharm. 2019, 566, 615–630. [Google Scholar] [CrossRef]
- Sun, C.C. A classification system for tableting behaviors of binary powder mixtures. Asian J. Pharm. Sci. 2016, 11, 486–491. [Google Scholar] [CrossRef]
- Singh, R.; Sahay, A.; Karry, K.M.; Muzzio, F.J.; Ierapetritou, M.G.; Ramachandran, R. Implementation of an advanced hybrid MPC-PID control system using PAT tools into a direct compaction continuous pharmaceutical tablet manufacturing pilot plant. Int. J. Pharm. 2014, 473, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, G.K.; Armstrong, N.A. Excipients for direct compaction—An update. Pharm. Dev. Technol. 2006, 11, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ding, X.; He, Z.; Fan, S.; Kunnath, K.T.; Zheng, K.; Dave, R.N. Surface engineered excipients: II. simultaneous milling and dry coating for preparation of fine-grade microcrystalline cellulose with enhanced properties. Int. J. Pharm. 2018, 546, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ding, X.; He, Z.; Huang, Z.; Kunnath, K.T.; Zheng, K.; Dave, R.N. Surface engineered excipients: I. Improved functional properties of fine grade microcrystalline cellulose. Int. J. Pharm. 2018, 536, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Hurychova, H.; Ondrejcek, P.; Sklubalova, Z.; Vranikova, B.; Sverak, T. The influence of stevia on the flow, shear and compression behavior of sorbitol, a pharmaceutical excipient for direct compression. Pharm. Dev. Technol. 2018, 23, 125–131. [Google Scholar] [CrossRef]
- Bolhuis, G.K.; Rexwinkel, E.G.; Zuurman, K. Polyols as filler-binders for disintegrating tablets prepared by direct compaction. Drug Dev. Ind. Pharm. 2009, 35, 671–677. [Google Scholar] [CrossRef]
- Li, Z.; Xian, J.; Wu, F.; Lin, X.; Shen, L.; Feng, Y. Development of TCM-based composite particles for direct compaction by particle design. Powder Technol. 2018, 338, 481–492. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Lin, X.; Shen, L.; Feng, Y. Co-spray drying with HPMC as a platform to improve direct compaction properties of various tablet fillers. AAPS PharmSciTech 2017, 18, 3105–3115. [Google Scholar] [CrossRef]
- Dudhat, S.M.; Kettler, C.N.; Dave, R.H. To study capping or lamination tendency of tablets through evaluation of powder rheological properties and tablet mechanical properties of directly compressible blends. AAPS PharmSciTech 2017, 18, 1177–1189. [Google Scholar] [CrossRef]
- Belic, A.; Škrjanc, I.; Bozic, D.Z.; Vrečer, F. Tableting process optimisation with the application of fuzzy models. Int. J. Pharm. 2010, 389, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Rojas, J.; Buckner, I.; Kumar, V. Co-proccessed excipients with enhanced direct compression functionality for improved tableting performance. Drug Dev. Ind. Pharm. 2012, 38, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Parmar, P.K.; Rao, S.G.; Bansal, A.K. Co-processing of small molecule excipients with polymers to improve functionality. Expert Opin. Drug Deliv. 2021, 18, 907–928. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Dhingra, A.; Chopra, B.; Guarve, K. Co-processed excipients: Recent advances and future perspective. J. Drug Deliv. Sci. Technol. 2022, 71, 103316. [Google Scholar] [CrossRef]
- Han, X.; Ghoroi, C.; Dave, R. Dry coating of micronized API powders for improved dissolution of directly compacted tablets with high drug loading. Int. J. Pharm. 2013, 442, 74–85. [Google Scholar] [CrossRef]
- Gao, Y.; Li, J.; Zhao, L.; Hong, Y.; Shen, L.; Wang, Y.; Lin, X. Distribution pattern and surface nature-mediated differential effects of hydrophilic and hydrophobic nano-silica on key direct compaction properties of Citri Reticulatae Pericarpium powder by co-processing. Powder Technol. 2022, 404, 117442. [Google Scholar] [CrossRef]
- Draskovic, M.; Djuris, J.; Ibric, S.; Parojcic, J. Functionality and performance evaluation of directly compressible co-processed excipients based on dynamic compaction analysis and percolation theory. Powder Technol. 2018, 326, 292–301. [Google Scholar] [CrossRef]
- Al-Zoubi, N.; Odeh, F.; Nikolakakis, I. Co-spray drying of metformin hydrochloride with polymers to improve compaction behavior. Powder Technol. 2017, 307, 163–174. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical Particle Engineering via Spray Drying. Pharm. Res. 2007, 25, 999–1022. [Google Scholar] [CrossRef]
- Dong, Q.Q.; Zhou, M.M.; Lin, X.; Shen, L.; Feng, Y. Differences in fundamental and functional properties of HPMC co-processed fillers prepared by fluid-bed coating and spray drying. Eur. J. Pharm. Sci. 2018, 119, 147–158. [Google Scholar] [CrossRef]
- Kaialy, W.; Maniruzzaman, M.; Shojaee, S.; Nokhodchi, A. Antisolvent precipitation of novel xylitol-additive crystals to engineer tablets with improved pharmaceutical performance. Int. J. Pharm. 2014, 477, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, F.; Zhao, L.; Lin, X.; Shen, L.; Feng, Y. Evaluation of fundamental and functional properties of natural plant product powders for direct compaction based on multivariate statistical analysis. Adv. Powder Technol. 2018, 29, 2881–2894. [Google Scholar] [CrossRef]
- Yuan, J.L.X.; Shi, L.; Sun, W.-J.; Chen, J.; Zhou, Q.; Sun, C.C. Enabling direct compression of formulated Danshen powder by surface engineering. Powder Technol. 2013, 241, 211–218. [Google Scholar] [CrossRef]
- Capece, M.; Huang, Z.H.; Dave, R. Insight into a novel strategy for the design of tablet formulations intended for direct compression. J. Pharm. Sci. 2017, 106, 1608–1617. [Google Scholar] [CrossRef]
- Huang, Z.G.; Scicolone, J.V.; Han, X.; Dave, R.N. Improved blend and tablet properties of fine pharmaceutical powders via dry particle coating. Int. J. Pharm. 2015, 478, 447–455. [Google Scholar] [CrossRef]
- Qu, L.; Zhou, Q.; Gengenbach, T.; Denman, J.A.; Stewart, P.J.; Hapgood, K.P.; Gamlen, M.; Morton, D.A.V. Investigation of the potential for direct compaction of a fine ibuprofen powder dry-coated with magnesium stearate. Drug Dev. Ind. Pharm. 2015, 41, 825–837. [Google Scholar] [CrossRef]
- Mitchell, W.R.; Forny, L.; Althaus, T.O.; Dopfer, D.J.; Niederreiter, G.; Palzer, S. Compaction of food powders: The influence of material properties and process parameters on product structure, strength, and dissolution. Chem. Eng. Sci. 2017, 167, 29–41. [Google Scholar] [CrossRef]
- Šantl, M.; Ilič, I.G.; Vrečer, F.; Baumgartner, S. A compressibility and compactibility study of real tableting mixtures: The impact of wet and dry granulation versus a direct tableting mixture. Int. J. Pharm. 2011, 414, 131–139. [Google Scholar] [CrossRef]
- Sharma, R.; Setia, G. Mechanical dry particle coating on cohesive pharmaceutical powders for improving flowability—A review. Powder Technol. 2019, 356, 458–479. [Google Scholar] [CrossRef]
- Dai, S.; Xu, B.; Shi, G.; Liu, J.; Zhang, Z.; Shi, X.; Qiao, Y. SeDeM expert system for directly compressed tablet formulation: A review and new perspectives. Powder Technol. 2019, 342, 517–527. [Google Scholar] [CrossRef]
- Su, Q.; Bommireddy, Y.; Shah, Y.; Ganesh, S.; Moreno, M.; Liu, J.; Gonzalez, M.; Yazdanpanah, N.; O’Connor, T.F.; Reklaitis, G.V.; et al. Data reconciliation in the quality-by-design (QbD) implementation of pharmaceutical continuous tablet manufacturing. Int. J. Pharm. 2019, 563, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gernaey, K.V.; De Beer, T.; Nopens, I. Model-based analysis of high shear wet granulation from batch to continuous processes in pharmaceutical production—A critical review. Eur. J. Pharm. Biopharm. 2013, 85 Pt B, 814–832. [Google Scholar] [CrossRef]
- Rehrl, J.; Kruisz, J.; Sacher, S.; Khinast, J.G.; Horn, M. Optimized continuous pharmaceutical manufacturing via model-predictive control. Int. J. Pharm. 2016, 510, 100–115. [Google Scholar] [CrossRef] [PubMed]
- Siow, C.R.S.; Heng, P.W.S.; Chan, L.W. Bulk freeze-drying milling: A versatile method of developing highly porous cushioning excipients for compacted multiple-unit pellet systems (MUPS). AAPS PharmSciTech 2018, 19, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Bowles, B.J.; Dziemidowicz, K.; Lopez, F.L.; Orlu, M.; Tuleu, C.; Edwards, A.J.; Ernest, T.B. Co-processed excipients for dispersible tablets-part 1: Manufacturability. AAPS PharmSciTech 2018, 19, 2598–2609. [Google Scholar] [CrossRef]
- Gupta, J.; Papadikis, K.; Kozhevnikov, I.V.; Konysheva, E.Y. Exploring the potential of red mud and beechwood co-processing for the upgrading of fast pyrolysis vapours. J. Anal. Appl. Pyrolysis 2017, 128, 35–43. [Google Scholar] [CrossRef]
- Sierra-Vega, N.O.; Karry, K.M.; Romañach, R.J.; Méndez, R. Monitoring of high-load dose formulations based on co-processed and non co-processed excipients. Int. J. Pharm. 2021, 606, 120910. [Google Scholar] [CrossRef]
- Erdemir, D.; Daftary, V.; Lindrud, M.; Buckley, D.; Lane, G.; Malsbury, A.; Tao, J.; Kopp, N.; Hsieh, D.S.; Nikitczuk, W.; et al. Design and scale-up of a co-processing Technology to improve powder properties of drug substances. Org. Process Res. Dev. 2019, 23, 2685–2698. [Google Scholar] [CrossRef]
- Dziemidowicz, K.; Lopez, F.L.; Bowles, B.J.; Edwards, A.J.; Ernest, T.B.; Orlu, M.; Tuleu, C. Co-processed excipients for dispersible tablets-part 2: Patient acceptability. AAPS PharmSciTech 2018, 19, 2646–2657. [Google Scholar] [CrossRef]
- Slow, C.R.S.; Tang, D.S.; Heng, P.W.S.; Chan, L.W. Probing the impact of HPMC viscosity grade and proportion on the physical properties of co-freeze-dried mannitol-HPMC tableting excipients using multivariate analysis methods. Int. J. Pharm. 2019, 556, 246–262. [Google Scholar]
- Mullarney, M.P.; Beach, L.; Davé, R.N.; Langdon, B.A.; Polizzi, M.A.; Blackwood, D.O. Applying dry powder coatings to pharmaceutical powders using a comil for improving powder flow and bulk density. Powder Technol. 2011, 212, 397–402. [Google Scholar] [CrossRef]
- Chen, Y.; Jallo, L.J.; Quintanilla, M.A.S.; Davé, R.N. Characterization of particle and bulk level cohesion reduction of surface modified fine aluminum powders. Colloids Surf. A 2010, 361, 66–80. [Google Scholar] [CrossRef]
- Han, X.; Ghoroi, C.; To, D.; Chen, Y.; Davé, R.N. Simultaneous micronization and surface modification for improvement of flow and dissolution of drug particles. Int. J. Pharm. 2011, 415, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Kaialy, W.; Khan, U.; Mawlud, S. Influence of mannitol concentration on the physicochemical, mechanical and pharmaceutical properties of lyophilised mannitol. Int. J. Pharm. 2016, 510, 73–85. [Google Scholar] [CrossRef]
- Karde, V.; Ghoroi, C. Influence of surface modification on wettability and surface energy characteristics of pharmaceutical excipient powders. Int. J. Pharm. 2014, 475, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Rowe, C. Handbook of Pharmaceutical Excipients, 6th ed.; Rowe, R.C., Ed.; Pharmaceutical Press: London, UK, 2012. [Google Scholar]
- Tundisi, L.L.; Mostaco, G.B.; Carricondo, P.C.; Petri, D.F.S. Hydroxypropyl methylcellulose: Physicochemical properties and ocular drug delivery formulations. Eur. J. Pharm. Sci. 2021, 159, 12. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Peppas, N.A. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC). Adv. Drug Deliv. Rev. 2012, 64, 163–174. [Google Scholar] [CrossRef]
- Caraballo, I. Factors affecting drug release from hydroxypropyl methylcellulose matrix systems in the light of classical and percolation theories. Expert Opin. Drug Deliv. 2010, 7, 1291–1301. [Google Scholar] [CrossRef]
- Guarve, K.; Kriplani, P. HPMC- a marvel polymer for pharmaceutical industry-patent review. Recent Adv. Drug Deliv. Formul. 2021, 15, 46–58. [Google Scholar] [CrossRef]
- Al-Tabakha, M.M. HPMC capsules: Current status and future prospects. J. Pharm. Pharm. Sci. 2010, 13, 428–442. [Google Scholar] [CrossRef]
- Li, J.; Wu, F.; Lin, X.; Shen, L.; Wang, Y.; Feng, Y. Novel application of hydroxypropyl methylcellulose to improving direct compaction properties of tablet fillers by co-spray drying. RSC Adv. 2015, 5, 69289–69298. [Google Scholar] [CrossRef]
- Li, J.; Lin, X.; Wu, F.; Shen, L.; Wang, Y.; Feng, Y. Application of the central composite design to optimize the calcium carbonate-HPMC co-processed excipient prepared by co-spray drying. RSC Adv. 2015, 5, 94105–94114. [Google Scholar] [CrossRef]
- Siow, C.R.S.; Heng, P.W.S.; Chan, L.W. A study on the impact of HPMC viscosity grade and proportion on the functional properties of co-freeze-dried mannitol-HPMC cushioning excipients for compacted MUPS. Eur. J. Pharm. Biopharm. 2020, 151, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Asai, R.; Takeuchi, T.; Kondo, K.; Niwa, T. Design of xerogel pill with good swallowing performance through wet milling and drop freeze-drying processes. Int. J. Pharm. 2022, 621, 11. [Google Scholar] [CrossRef] [PubMed]
- Ayenew, Z.; Puri, V.; Kumar, L.; Bansal, A.K. Trends in pharmaceutical taste masking technologies: A patent review. Recent Pat. Drug Deliv. Formul. 2009, 3, 26–39. [Google Scholar] [CrossRef]
- Abbaspour, M.R.; Sadeghi, F.; Garekani, H.A. Design and study of ibuprofen disintegrating sustained-release tablets comprising coated pellets. Eur. J. Pharm. Biopharm. 2008, 68, 747–759. [Google Scholar] [CrossRef]
- Sibanc, R.; Turk, M.; Dreu, R. An analysis of the mini-tablet fluidized bed coating process. Chem. Eng. Res. Des. 2018, 134, 15–25. [Google Scholar] [CrossRef]
- To, D.; Dave, R.N. Fluid bed film coating of fine ibuprofen particles. Powder Technol. 2016, 290, 102–113. [Google Scholar] [CrossRef]
- Werner, S.R.L.; Jones, J.R.; Paterson, A.H.J.; Archer, R.H.; Pearce, D.L. Air-suspension particle coating in the food industry: Part I—State of the art. Powder Technol. 2007, 171, 25–33. [Google Scholar] [CrossRef]
- Horisawa, E.; Komura, A.; Danjo, K.; Otsuka, A. Effect of granule strength on compressed tablet strength. Chem. Pharm. Bull. 1995, 43, 2261–2263. [Google Scholar] [CrossRef]
- Khlibsuwan, R.; Pongjanyakul, T. Particle agglomeration of chitosan-magnesium aluminum silicate nanocomposites for direct compression tablets. Int. J. Pharm. 2018, 535, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.H.; Zhang, Q.Y.; Wang, P.X. Hydrochlorothiazide/losartan potassium tablet prepared by direct compression. Pharmaceutics 2022, 14, 1741. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Smith, G.; Khan, K.A.; Bukhari, N.I.; Pedge, N.I.; Ermolina, I. Solubility and dissolution rate enhancement of ibuprofen by co-milling with polymeric excipients. Eur. J. Pharm. Sci. 2018, 123, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Jagadish, B.; Yelchuri, R.; Bindu, K.; Tangi, H.; Maroju, S.; Rao, V.U. Enhanced dissolution and bioavailability of raloxifene hydrochloride by co-grinding with different superdisintegrants. Chem. Pharm. Bull. 2010, 58, 293–300. [Google Scholar] [CrossRef]
- Ibraheem, B.; Wagner, K.G. Influence of high pressure compaction on solubility and intrinsic dissolution of ibuprofen binary mixtures employing standard excipients. Int. J. Pharm. X 2021, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Varghese, S.; Ghoroi, C. Improving the wetting and dissolution of ibuprofen using solventless co-milling. Int. J. Pharm. 2017, 533, 145–155. [Google Scholar] [CrossRef]
- Kawabata, Y.; Wada, K.; Nakatani, M.; Yamada, S.; Onoue, S. Formulation design for poorly water-soluble drugs based on biopharmaceutics classification system: Basic approaches and practical applications. Int. J. Pharm. 2011, 420, 1–10. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.-I.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J. Pharm. Sci. 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Han, X.; Jallo, L.; To, D.; Ghoroi, C.; Dave, R. Passivation of high-surface-energy sites of milled ibuprofen crystals via dry coating for reduced cohesion and improved flowability. J. Pharm. Sci. 2013, 102, 2282–2296. [Google Scholar] [CrossRef]
- Barzegar-Jalali, M.; Valizadeh, H.; Shadbad, M.R.S.; Adibkia, K.; Mohammadi, G.; Farahani, A.; Arash, Z.; Nokhodchi, A. Cogrinding as an approach to enhance dissolution rate of a poorly water-soluble drug (gliclazide). Powder Technol. 2010, 197, 150–158. [Google Scholar] [CrossRef]
- Lim, R.T.Y.; Ng, W.K.; Widjaja, E.; Tan, R.B.H. Comparison of the physical stability and physicochemical properties of amorphous indomethacin prepared by co-milling and supercritical anti-solvent co-precipitation. J. Supercrit. Fluids 2013, 79, 186–201. [Google Scholar] [CrossRef]
- Lin, S.Y.; Hsu, C.H.; Ke, W.T. Solid-state transformation of different gabapentin polymorphs upon milling and co-milling. Int. J. Pharm. 2010, 396, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Grassi, G.; Grassi, M. Drug mechanochemical activation. J. Pharm. Sci. 2009, 98, 3961–3986. [Google Scholar] [CrossRef] [PubMed]
- Slamova, M.; Prausova, K.; Epikaridisova, J.; Brokesova, J.; Kuentz, M.; Patera, J.; Zamostny, P. Effect of co-milling on dissolution rate of poorly soluble drugs. Int. J. Pharm. 2021, 597, 10. [Google Scholar] [CrossRef]
- Wikarsa, S.; Durand, D.; Delarbre, J.L.; Baylac, G.; Bataille, B. The improvement of ibuprofen dissolution rate through microparticles spray drying processed in an aqueous system. Drug Dev. Ind. Pharm. 2008, 34, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Zhang, M.; Hou, Q.; Tang, P.; Suo, Z.; Zhu, Y.; Li, H. Solid dispersions of telaprevir with improved solubility prepared by co-milling: Formulation, physicochemical characterization, and cytotoxicity evaluation. Mater. Sci. Eng. 2019, 105, 110012. [Google Scholar] [CrossRef]
- Choi, I.; Park, S.Y.; Lee, S.W.; Kang, Z.; Jin, Y.S.; Kim, I.W. Dissolution enhancement of sorafenib tosylate by co-milling with tetradecanol post-extracted using supercritical carbon dioxide. Pharmazie 2020, 75, 13–17. [Google Scholar]
- Chen, X.; Partheniadis, I.; Nikolakakis, I.; Al-Obaidi, H. Solubility improvement of progesterone from solid dispersions prepared by solvent evaporation and co-milling. Polymers 2020, 12, 854. [Google Scholar] [CrossRef]
- Shaikh, R.; Shirazian, S.; Guerin, S.; Sheehan, E.; Thompson, D.; Walker, G.M.; Croker, D.M. Understanding solid-state processing of pharmaceutical cocrystals via milling: Role of tablet excipients. Int. J. Pharm. 2021, 601, 13. [Google Scholar] [CrossRef]
- Pawar, A.; Paradkar, A.; Kadam, S.; Mahadik, K. Agglomeration of Ibuprofen with talc by novel crystallo-co-agglomeration technique. AAPS PharmSciTech 2004, 5, e55. [Google Scholar] [CrossRef]
- Paradkar, A.; Pawar, A. Crystallo-co-agglomeration: A novel particle engineering technique. Asian J. Pharm. 2010, 4, 4. [Google Scholar] [CrossRef]
- Garala, K.; Patel, J.; Patel, A.; Raval, M.; Dharamsi, A. Influence of excipients and processing conditions on the development of agglomerates of racecadotril by crystallo-co-agglomeration. Int. J. Pharm. Investig. 2012, 2, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, T.; Erdemir, D.; Chang, S.Y.; Kientzler, D.; Wang, S.; Chan, S.H.; Brown, J.; Hanley, S.; Kiang, S. A novel co-processing method to manufacture an API for extended release formulation via formation of agglomerates of active ingredient and hydroxypropyl methylcellulose during crystallization. Drug Dev. Ind. Pharm. 2018, 44, 1606–1612. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Lin, X.; Feng, Y.; Kou, X.; Babu, S.; Panicucci, R. Novel coprocessed excipients composed of lactose, HPMC, and PVPP for tableting and its application. Int. J. Pharm. 2015, 486, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, H.; Raikkonen, H.; Antikainen, O.; Heinamaki, J.; Yliruusi, J. Improving flow properties of ibuprofen by fluidized bed particle thin-coating. Int. J. Pharm. 2009, 368, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, F.; Hong, Y.; Shen, L.; Lin, X.; Feng, Y. The fundamental and functional property differences between HPMC and PVP co-processed herbal particles prepared by fluid bed coating. AAPS PharmSciTech 2020, 21, 1–17. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, Y.; Wu, F.; Shen, L.; Lin, X.; Feng, Y. Development on porous particles of Pueraria lobatae radix for improving its compactibility and dissolution. RSC Adv. 2018, 8, 24250–24260. [Google Scholar] [CrossRef]
- Košir, D.; Vrečer, F. The performance of HPMC matrix tablets using various agglomeration manufacturing processes. Drug Dev. Ind. Pharm. 2017, 43, 329–337. [Google Scholar] [CrossRef]
- Jain, A.K.; Söderlind, E.; Viridén, A.; Schug, B.S.; Abrahamsson, B.; Knopke, C.; Tajarobi, F.; Blume, H.; Anschütz, M.; Welinder, A.; et al. The influence of hydroxypropyl methylcellulose (HPMC) molecular weight, concentration and effect of food on in vivo erosion behavior of HPMC matrix tablets. J. Control. Release 2014, 187, 50–58. [Google Scholar] [CrossRef]
- Luo, Y.; Hong, Y.L.; Shen, L.; Wu, F.; Lin, X. Multifunctional role of polyvinylpyrrolidone in pharmaceutical formulations. AAPS PharmSciTech 2021, 22, 16. [Google Scholar] [CrossRef]
- Husain, M.S.B.; Gupta, A.; Alashwal, B.Y.; Sharma, S. Synthesis of PVA/PVP based hydrogel for biomedical applications: A review. Energy Sources Part A-Recovery Util. Environ. Eff. 2018, 40, 2388–2393. [Google Scholar] [CrossRef]
- Chinatangkul, N.; Tubtimsri, S.; Panchapornpon, D.; Akkaramongkolporn, P.; Limmatvapirat, C.; Limmatvapirat, S. Design and characterisation of electrospun shellac-polyvinylpyrrolidone blended micro/nanofibres loaded with monolaurin for application in wound healing. Int. J. Pharm. 2019, 562, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Swei, J.; Talbot, J.B. Viscosity correlation for aqueous polyvinylpyrrolidone (PVP) solutions. J. Appl. Polym. Sci. 2003, 90, 1153–1155. [Google Scholar] [CrossRef]
- Morkhade, D.M. Comparative impact of different binder addition methods, binders and diluents on resulting granule and tablet attributes via high shear wet granulation. Powder Technol. 2017, 320, 114–124. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A versatile polymer for biomedical and beyond medical applications. Polym.-Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Chavan, R.B.; Thipparaboina, R.; Kumar, D.; Shastri, N.R. Evaluation of the inhibitory potential of HPMC, PVP and HPC polymers on nucleation and crystal growth. RSC Adv. 2016, 6, 77569–77576. [Google Scholar] [CrossRef]
- Sadeghi, F.; Torab, M.; Khattab, M.; Homayouni, A.; Garekani, H.A. Improvement of Physico-mechanical properties of partially amorphous acetaminophen developed from hydroalcoholic solution using spray drying technique. Iran. J. Basic Med. Sci. 2013, 16, 1100–1108. [Google Scholar]
- Berggren, J.; Frenning, G.; Alderborn, G. Compression behaviour and tablet-forming ability of spray-dried amorphous composite particles. Eur. J. Pharm. Sci. 2004, 22, 191–200. [Google Scholar] [CrossRef]
- Corrigan, D.O.; Healy, A.M.; Corrigan, O.I. The effect of spray drying solutions of polyethylene glycol (PEG) and lactose/PEG on their physicochemical properties. Int. J. Pharm. 2002, 235, 193–205. [Google Scholar] [CrossRef]
- Jinapong, N.; Suphantharika, M.; Jamnong, P. Production of instant soymilk powders by ultrafiltration, spray drying and fluidized bed agglomeration. J. Food Eng. 2008, 84, 194–205. [Google Scholar] [CrossRef]
- Billon, A.; Bataille, B.; Cassanas, G.; Jacob, M. Development of spray-dried acetaminophen microparticles using experimental designs. Int. J. Pharm. 2000, 203, 159–168. [Google Scholar] [CrossRef]
- Zhu, W.F.; Zhu, L.; Li, Z.; Wu, W.T.; Guan, Y.M.; Chen, L.H.; Mao, Z.X.; Ming, L.S. The novel use of PVP K30 as templating agent in production of porous lactose. Pharmaceutics 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Volkova, T.V.; Perlovich, G.L.; Terekhova, I.V. Enhancement of dissolution behavior of antiarthritic drug leflunomide using solid dispersion methods. Thermochim. Acta 2017, 656, 123–128. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Peeters, E.; Van Snick, B.; Remon, J.P.; Vervaet, C. Crystal coating via spray drying to improve powder tabletability. Eur. J. Pharm. Biopharm. 2014, 88, 939–944. [Google Scholar] [CrossRef]
- Shi, L.M.; Sun, C.C. Transforming powder mechanical properties by core/shell structure: Compressible sand. J. Pharm. Sci. 2010, 99, 4458–4462. [Google Scholar] [CrossRef]
- Vanhoorne, V.; Van Bockstal, P.J.; Van Snick, B.; Peeters, E.; Monteyne, T.; Gomes, P.; De Beer, T.; Remon, J.P.; Vervaet, C. Continuous manufacturing of delta mannitol by cospray drying with PVP. Int. J. Pharm. 2016, 501, 139–147. [Google Scholar] [CrossRef]
- Kolašinac, N.; Kachrimanis, K.; Djuriš, J.; Homšek, I.; Grujić, B.; Ibrić, S. Spray coating as a powerful technique in preparation of solid dispersions with enhanced desloratadine dissolution rate. Drug Dev. Ind. Pharm. 2013, 39, 1020–1027. [Google Scholar] [CrossRef]
- Vlachou, M.; Geraniou, E.; Siamidi, A. Modified release of furosemide from eudragits (R) and poly(ethylene oxide)-based matrices and dry-coated tablets. Acta Pharm. 2020, 70, 49–61. [Google Scholar] [CrossRef]
- Jamadar, S.; Pore, Y.; Sayyad, F. Formation of amorphous telmisartan polymeric microparticles for improvement of physicochemical characteristics. Part. Sci. Technol. 2014, 32, 512–519. [Google Scholar] [CrossRef]
- Frizon, F.; Eloy, J.D.; Donaduzzi, C.M.; Mitsui, M.L.; Marchetti, J.M. Dissolution rate enhancement of loratadine in polyvinylpyrrolidone K-30 solid dispersions by solvent methods. Powder Technol. 2013, 235, 532–539. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Le, Y.; Wang, J.X.; Zhao, H.; Chen, J.F. Irbesartan drug formulated as nanocomposite particles for the enhancement of the dissolution rate. Particuology 2012, 10, 462–467. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, F.; Hong, Y.L.; Shen, L.; Lin, X.; Feng, Y. Improvements in sticking, hygroscopicity, and compactibility of effervescent systems by fluid-bed coating. RSC Adv. 2019, 9, 31594–31608. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.T.; Zhang, Y.; Hong, Y.L.; Wu, F.; Shen, L.; Wang, Y.J.; Lin, X. Multifunctional role of silica in pharmaceutical pormulations. AAPS PharmSciTech 2022, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Hyde, E.; Seyfaee, A.; Neville, F.; Moreno-Atanasio, R. Colloidal silica particle synthesis and future industrial manufacturing pathways: A review. Ind. Eng. Chem. Res. 2016, 55, 8891–8913. [Google Scholar] [CrossRef]
- Cai, C.F.; Liu, M.H.; Li, Y.; Guo, B.; Chang, H.; Zhang, X.R.; Yang, X.X.; Zhang, T.H. A silica-supported solid dispersion of bifendate using supercritical carbon dioxide method with enhanced dissolution rate and oral bioavailability. Drug Dev. Ind. Pharm. 2016, 42, 412–417. [Google Scholar] [CrossRef]
- Van Speybroeck, M.; Barillaro, V.; Do Thi, T.; Mellaerts, R.; Martens, J.; Van Humbeeck, J.; Vermant, J.; Annaert, P.; Van Den Mooter, G.; Augustijns, P. Ordered mesoporous silica material SBA-15: A broad-spectrum formulation platform for poorly soluble drugs. J. Pharm. Sci. 2009, 98, 2648–2658. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, S.; Singh, S.K. Nanosilica: Recent progress in Synthesis, functionalization, biocompatibility, and biomedical applications. ACS Biomater. Sci. Eng. 2019, 5, 4882–4898. [Google Scholar] [CrossRef]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef]
- Jonat, S.; Hasenzahl, S.; Drechsler, M.; Albers, P.; Wagner, K.G.; Schmidt, P.C. Investigation of compacted hydrophilic and hydrophobic colloidal silicon dioxides as glidants for pharmaceutical excipients. Powder Technol. 2004, 141, 31–43. [Google Scholar] [CrossRef]
- Tirapelle, M.; Volpato, S.; Santomaso, A.C. Shear-induced particle segregation in binary mixtures: Verification of a percolation theory. Particuology 2021, 57, 214–222. [Google Scholar] [CrossRef]
- Qu, L.; Stewart, P.J.; Hapgood, K.P.; Lakio, S.; Morton, D.A.V.; Zhou, Q. Single-step coprocessing of cohesive powder via mechanical dry coating for direct tablet compression. J. Pharm. Sci. 2017, 106, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Y.P.; Wu, F.; Hong, Y.L.; Shen, L.; Lin, X.; Feng, Y. Texture and surface feature-mediated striking improvements on multiple direct compaction properties of Zingiberis rhizoma extracted powder by coprocessing with nano-silica. Int. J. Pharm. 2021, 603, 16. [Google Scholar] [CrossRef] [PubMed]
- Hentzschel, C.M.; Alnaief, M.; Smirnova, I.; Sakmann, A.; Leopold, C.S. Tableting properties of silica aerogel and other silicates. Drug Dev. Ind. Pharm. 2012, 38, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Kunnath, K.; Huang, Z.H.; Chen, L.; Zheng, K.; Dave, R. Improved properties of fine active pharmaceutical ingredient powder blends and tablets at high drug loading via dry particle coating. Int. J. Pharm. 2018, 543, 288–299. [Google Scholar] [CrossRef]
- Li, P.; Feng, B.; Jiang, H.; Han, X.; Wu, Z.F.; Wang, Y.Q.; Lin, J.Z.; Zhang, Y.; Yang, M.; Han, L.; et al. A novel forming method of traditional Chinese medicine dispersible tablets to achieve rapid disintegration based on the powder modification principle. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef]
- Bin Ruzaidi, A.F.; Mandal, U.K.; Chatterjee, B. Glidant effect of hydrophobic and hydrophilic nanosilica on a cohesive powder: Comparison of different flow characterization techniques. Particuology 2017, 31, 69–79. [Google Scholar] [CrossRef]
- Capece, M.; Ho, R.; Strong, J.; Gao, P. Prediction of powder flow performance using a multi-component granular Bond number. Powder Technol. 2015, 286, 561–571. [Google Scholar] [CrossRef]
- Qu, L.; Zhou, Q.; Denman, J.A.; Stewart, P.J.; Hapgood, K.P.; Morton, D.A.V. Influence of coating material on the flowability and dissolution of dry-coated fine ibuprofen powders. Eur. J. Pharm. Sci. 2015, 78, 264–272. [Google Scholar] [CrossRef]
- Huang, Z.H.; Scicolone, J.V.; Gurumuthy, L.; Dave, R.N. Flow and bulk density enhancements of pharmaceutical powders using a conical screen mill: A continuous dry coating device. Chem. Eng. Sci. 2015, 125, 209–224. [Google Scholar] [CrossRef]
- Huang, Z.H.; Kunnath, K.T.; Han, X.; Deng, X.L.; Chen, L.; Dave, R.N. Ultra-fine dispersible powders coated with L-Leucine via two-step co-milling. Adv. Powder Technol. 2018, 29, 2957–2965. [Google Scholar] [CrossRef]
- Chattoraj, S.; Shi, L.M.; Sun, C.C. Profoundly improving flow properties of a cohesive cellulose powder by surface coating with nano-silica through comilling. J. Pharm. Sci. 2011, 100, 4943–4952. [Google Scholar] [CrossRef] [PubMed]
- Tanimura, S.; Tahara, K.; Takeuchi, H. Spray-dried composite particles of erythritol and porous silica for orally disintegrating tablets prepared by direct tableting. Powder Technol. 2015, 286, 444–450. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, L.M.; Marinaro, W.; Lu, Q.; Sun, C.C. Improving manufacturability of an ibuprofen powder blend by surface coating with silica nanoparticles. Powder Technol. 2013, 249, 290–296. [Google Scholar] [CrossRef]
- Yang, J.; Sliva, A.; Banerjee, A.; Dave, R.N.; Pfeffer, R. Dry particle coating for improving the flowability of cohesive powders. Powder Technol. 2005, 158, 21–33. [Google Scholar] [CrossRef]
- Tran, D.T.; Majerová, D.; Veselý, M.; Kulaviak, L.; Ruzicka, M.C.; Zámostný, P. On the mechanism of colloidal silica action to improve flow properties of pharmaceutical excipients. Int. J. Pharm. 2019, 556, 383–394. [Google Scholar] [CrossRef]
- Trementozzi, A.N.; Leung, C.-Y.; Osei-Yeboah, F.; Irdam, E.A.; Lin, Y.; Macphee, J.M.; Boulas, P.L.; Karki, S.B.; Zawaneh, P.N. Engineered particles demonstrate improved flow properties at elevated drug loadings for direct compression manufacturing. Int. J. Pharm. 2017, 523, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.L.; Ma, Y.L.; Zhu, J. Sustained drug release from electrostatic powder coated tablets with ultrafine ethylcellulose powders. Adv. Powder Technol. 2016, 27, 2145–2152. [Google Scholar] [CrossRef]
- Les, K.; Kowalski, K.; Opalinski, I. Optimisation of process parameters in high energy mixing as a method of cohesive powder floeability improvement. Chem. Process Eng. 2015, 36, 449–460. [Google Scholar] [CrossRef][Green Version]
- Knieke, C.; Azad, M.A.; To, D.; Bilgili, E.; Dave, R.N. Sub-100 micron fast dissolving nanocomposite drug powders. Powder Technol. 2015, 271, 49–60. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, L.M.; Chattoraj, S.; Sun, C.C. Preparation and characterization of surface-engineered coarse microcrystalline cellulose through dry coating with silica nanoparticles. J. Pharm. Sci. 2012, 101, 4258–4266. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Zhao, L.J.; Lin, X.; Shen, L. An update on microcrystalline cellulose in direct compression: Functionality, critical material attributes, and co-processed excipients. Carbohydr. Polym. 2022, 278, 15. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder design environment-A review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef]
- Elsergany, R.N.; Chan, L.W.; Heng, P.W.S. Cushioning pellets based on microcrystalline cellulose—Crospovidone blends for MUPS tableting. Int. J. Pharm. 2020, 586, 14. [Google Scholar] [CrossRef] [PubMed]
- Gil-Chavez, J.; Padhi, S.S.P.; Leopold, C.S.; Smirnova, I. Application of aquasolv lignin in ibuprofen-loaded pharmaceutical formulations obtained via direct compression and wet granulation. Int. J. Biol. Macromol. 2021, 174, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nakagawa, M.; Tanaka, C.; Yuasa, H.; Sakamoto, T. Utility of microcrystalline cellulose to prevent drug segregation in direct powder compression. J. Drug Deliv. Sci. Technol. 2019, 52, 386–392. [Google Scholar] [CrossRef]
- Nakamura, S.; Tanaka, C.; Yuasa, H.; Sakamoto, T. Utility of microcrystalline cellulose for improving drug content uniformity in tablet manufacturing using direct powder compression. AAPS PharmSciTech 2019, 20, 12. [Google Scholar] [CrossRef]
- Jacob, S.; Shirwaikar, A.; Joseph, A.; Srinivasan, K. Novel co-processed excipients of mannitol and microcrystalline cellulose for preparing fast dissolving tablets of glipizide. Indian J. Pharm. Sci. 2007, 69, 633–639. [Google Scholar]
- Zhang, Y.; Li, J.Z.; Gao, Y.T.; Wu, F.; Hong, Y.L.; Shen, L.; Lin, X. Improvements on multiple direct compaction properties of three powders prepared from Puerariae Lobatae Radix using surface and texture modification: Comparison of microcrystalline cellulose and two nano-silicas. Int. J. Pharm. 2022, 622, 18. [Google Scholar] [CrossRef]
- Mužíková, J.; Srbová, A.; Svačinová, P. A study of a novel coprocessed dry binder composed of α-lactose monohydrate, microcrystalline cellulose and corn starch. Pharm. Dev. Technol. 2017, 22, 964–971. [Google Scholar] [CrossRef]
- Dai, Y.W.; Meng, Q.; Mu, W.M.; Zhang, T. Recent advances in the applications and biotechnological production of mannitol. J. Funct. Foods 2017, 36, 404–409. [Google Scholar] [CrossRef]
- Tomaszewska, L.; Rywinska, A.; Gladkowski, W. Production of erythritol and mannitol by Yarrowia lipolytica yeast in media containing glycerol. J. Ind. Microbiol. Biotechnol. 2012, 39, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Tomita, T.; Kuroda, J.; Kageyu, A.; Yonekura, C.; Hiramura, Y.; Tahara, K.; Takeuchi, H. Characterization of mannitol granules and powder: A comparative study using two flowability testers. Int. J. Pharm. 2018, 547, 106–113. [Google Scholar] [CrossRef]
- Holm, T.P.; Meng-Lund, H.; Rantanen, J.; Jorgensen, L.; Grohganz, H. Screening of novel excipients for freeze-dried protein formulations. Eur. J. Pharm. Biopharm. 2021, 160, 55–64. [Google Scholar] [CrossRef]
- Al-Khattawi, A.; Koner, J.; Rue, P.; Kirby, D.; Perrie, Y.; Rajabi-Siahboomi, A.; Mohammed, A.R. A pragmatic approach for engineering porous mannitol and mechanistic evaluation of particle performance. Eur. J. Pharm. Biopharm. 2015, 94, 1–10. [Google Scholar] [CrossRef]
- Saffari, M.; Ebrahimi, A.; Langrish, T. A novel formulation for solubility and content uniformity enhancement of poorly water-soluble drugs using highly-porous mannitol. Eur. J. Pharm. Sci. 2016, 83, 52–61. [Google Scholar] [CrossRef]
- Gervelas, C.; Serandour, A.L.; Geiger, S.; Grillon, G.; Fritsch, P.; Taulelle, C.; Le Gall, B.; Benech, H.; Deverre, J.R.; Fattal, E.; et al. Direct lung delivery of a dry powder formulation of DTPA with improved aerosolization properties: Effect on lung and systemic decorporation of plutonium. J. Control. Release 2007, 118, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.Y.; Zhu, G.S.; Lin, H.M.; Zhang, W.W.; Sun, J.Y.; Li, S.G.; Qiu, S.L. A controlled release of ibuprofen by systematically tailoring the morphology of mesoporous silica materials. J. Solid State Chem. 2006, 179, 2027–2035. [Google Scholar] [CrossRef]
- Saffari, M.; Ebrahimi, A.; Langrish, T. Highly-porous mannitol particle production using a new templating approach. Food Res. Int. 2015, 67, 44–51. [Google Scholar] [CrossRef]
- Xia, W.; Chang, J. Well-ordered mesoporous bioactive glasses (MBG): A promising bioactive drug delivery system. J. Control. Release 2006, 110, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Gao, Y.; Du, X.X.; Guan, R.; He, Z.G.; Liu, H.Z. Combining co-amorphous-based spray drying with inert carriers to achieve improved bioavailability and excellent downstream manufacturability. Pharmaceutics 2020, 12, 1063. [Google Scholar] [CrossRef]
- Matos, R.L.; Lu, T.J.; Leeke, G.; Prosapio, V.; McConville, C.; Ingram, A. Single-step coprecipitation and coating to prepare curcumin formulations by supercritical fluid technology. J. Supercrit. Fluids 2020, 159, 15. [Google Scholar] [CrossRef]
- Takekuma, Y.; Ishizaka, H.; Sumi, M.; Sato, Y.; Sugawara, M. Difference in the dissolution behaviors of tablets containing polyvinylpolypyrrolidone (PVPP) depending on pharmaceutical formulation after storage under high temperature and humid conditions. J. Pharm. Pharm. Sci. 2016, 19, 511–519. [Google Scholar] [CrossRef]
- Zilic, S.; Sukalovic, V.H.T.; Dodig, D.; Maksimovic, V.; Maksimovic, M.; Basic, Z. Antioxidant activity of small grain cereals caused by phenolics and lipid soluble antioxidants. J. Cereal Sci. 2011, 54, 417–424. [Google Scholar] [CrossRef]
- Chen, W.L.; Guo, D.W.; Shen, Y.Y.; Guo, S.R.; Ruan, K.P. Effects of highly hygroscopic excipients on the hydrolysis of simvastatin in tablet at high relative humidity. Indian J. Pharm. Sci. 2012, 74, 527–534. [Google Scholar]
- Gohel, M.; Patel, M.; Amin, A.; Agrawal, R.; Dave, R.; Bariya, N. Formulation design and optimization of mouth dissolve tablets of nimesulide using vacuum drying technique. AAPS PharmSciTech 2004, 5, e36. [Google Scholar] [CrossRef]
- Mangal, S.; Meiser, F.; Lakio, S.; Morton, D.; Larson, I. The role of physico-chemical and bulk characteristics of co-spray dried L-leucine and polyvinylpyrrolidone on glidant and binder properties in interactive mixtures. Int. J. Pharm. 2015, 479, 338–348. [Google Scholar] [CrossRef]
- Solomon, S.; Ziaee, A.; Giraudeau, L.; O’Reilly, E.; Walker, G.; Albadarin, A.B. Particle engineering of excipients: A mechanistic investigation into the compaction properties of lignin and co -spray dried lignin. Int. J. Pharm. 2019, 563, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wen, H.; Desai, D. Lubrication in tablet formulations. Eur. J. Pharm. Biopharm. 2010, 75, 1–15. [Google Scholar] [CrossRef]
- Pingali, K.; Mendez, R.; Lewis, D.; Michniak-Kohn, B.; Cuitino, A.; Muzzio, F. Mixing order of glidant and lubricant—Influence on powder and tablet properties. Int. J. Pharm. 2011, 409, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Ghoroi, C.; Gurumurthy, L.; McDaniel, D.J.; Jallo, L.J.; Dave, R.N. Multi-faceted characterization of pharmaceutical powders to discern the influence of surface modification. Powder Technol. 2013, 236, 63–74. [Google Scholar] [CrossRef]
- Pfeffer, R.A.; Davé, R.N.; Wei, D.; Ramlakhan, M. Synthesis of engineered particulates with tailored properties using dry particle coating. Powder Technol. 2001, 117, 40–67. [Google Scholar] [CrossRef]
- Zhou, Q.; Armstrong, B.; Larson, I.; Stewart, P.J.; Morton, D.A.V. Improving powder flow properties of a cohesive lactose monohydrate powder by intensive mechanical dry coating. J. Pharm. Sci. 2010, 99, 969–981. [Google Scholar] [CrossRef]
- Bhatt, N.; Gupta, P.M.; Naithani, S. Hydroxypropyl cellulose from alpha-cellulose isolated from Lantana camara with respect to DS and rheological behavior. Carbohydr. Polym. 2011, 86, 1519–1524. [Google Scholar] [CrossRef]
- Nau, M.; Trosien, S.; Seelinger, D.; Boehm, A.K.; Biesalski, M. Spatially Resolved crosslinking of hydroxypropyl cellulose esters for the generation of functional surface-attached organogels. Front. Chem. 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lu, A.; Zhang, L.N. Recent advances in regenerated cellulose materials. Prog. Polym. Sci. 2016, 53, 169–206. [Google Scholar] [CrossRef]
- Godinho, M.H.; Gray, D.G.; Pieranski, P. Revisiting (hydroxypropyl) cellulose (HPC)/water liquid crystalline system. Liq. Cryst. 2017, 44, 2108–2120. [Google Scholar] [CrossRef]
- Lin, X.; Chyi, C.W.; Ruan, K.F.; Feng, Y.; Heng, P.W. Development of potential novel cushioning agents for the compaction of coated multi-particulates by co-processing micronized lactose with polymers. Eur. J. Pharm. Biopharm. 2011, 79, 406–415. [Google Scholar] [CrossRef]
- Goczo, H.; Szabo-Revesz, P.; Farkas, B.; Hasznos-Nezdei, M.; Serwanis, S.F.; Pintye-Hodi, A.K.; Kasa, P., Jr.; Eros, I.; Antal, I.; Marto, S. Development of spherical crystals of acetylsalicylic acid for direct tablet-making. Chem. Pharm. Bull. 2000, 48, 1877–1881. [Google Scholar] [CrossRef]
- Nokhodchi, A.; Maghsoodi, M. Preparation of spherical crystal agglomerates of naproxen containing disintegrant for direct tablet making by spherical crystallization technique. AAPS PharmSciTech 2008, 9, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Patil, A.T. Preparation, Evaluation and need of spherical crystallization in case of high speed direct tabletting. Curr. Drug Deliv. 2014, 11, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Aburub, A.; Sun, C.C. Direct compression tablet containing 99% active ingredient—A tale of spherical crystallization. J. Pharm. Sci. 2019, 108, 1396–1400. [Google Scholar] [CrossRef]
- McDonagh, A.F.; Duff, B.; Brennan, L.; Tajber, L. The impact of the degree of intimate mixing on the compaction properties of materials produced by crystallo-co-spray drying. Eur. J. Pharm. Sci. 2020, 154, 12. [Google Scholar] [CrossRef] [PubMed]
- Moussa, E.; Siepmann, F.; Flament, M.P.; Benzine, Y.; Penis, F.; Siepmann, J.; Karrout, Y. Controlled release tablets based on HPMC: Lactose blends. J. Drug Deliv. Sci. Technol. 2019, 52, 607–617. [Google Scholar] [CrossRef]
- Medarevic, D.; Djuris, J.; Krkobabic, M.; Ibric, S. Improving tableting performance of lactose monohydrate by fluid-bed melt granulation co-processing. Pharmaceutics 2021, 13, 2165. [Google Scholar] [CrossRef]
- Lamesic, D.; Planinsek, O.; Lavric, Z.; Ilic, I. Spherical agglomerates of lactose with enhanced mechanical properties. Int. J. Pharm. 2017, 516, 247–257. [Google Scholar] [CrossRef]
- Raval, M.K.; Garala, K.C.; Patel, J.M.; Parikh, R.K.; Sheth, N.R. Functionality improvement of chlorzoxazone by crystallo-co-agglomeration using multivariate analysis approach. Part. Sci. Technol. 2021, 39, 689–711. [Google Scholar] [CrossRef]
- Singh, S.; Nwabor, O.F.; Ontong, J.C.; Voravuthikunchai, S.P. Characterization and assessment of compression and compactibility of novel spray-dried, co-processed bio-based polymer. J. Drug Deliv. Sci. Technol. 2020, 56, 16. [Google Scholar] [CrossRef]
- Oliveira, L.J.; Veiga, A.; Stofella, N.C.F.; Cunha, A.C.; Toledo, M.D.T.; Andreazza, I.F.; Murakami, F.S. Development and Evaluation of Orodispersible Tablets Containing Ketoprofen. Curr. Drug Deliv. 2020, 17, 348–360. [Google Scholar] [CrossRef]
- Garg, N.; Pandey, P.; Kaushik, D.; Dureja, H. Development of novel multifunction directly compressible co-processed excipient by melt granulation technique. Int. J. Pharm. Investig. 2015, 5, 266–274. [Google Scholar]
- Zhou, Q.; Denman, J.A.; Gengenbach, T.; Das, S.; Qu, L.; Zhang, H.L.; Larson, I.; Stewart, P.J.; Morton, D.A.V. Characterization of the Surface Properties of a Model Pharmaceutical Fine Powder Modified with a Pharmaceutical Lubricant to Improve Flow via a Mechanical Dry Coating Approach. J. Pharm. Sci. 2011, 100, 3421–3430. [Google Scholar] [CrossRef]
- Koskela, J.; Morton, D.A.V.; Stewart, P.J.; Juppo, A.M.; Lakio, S. The effect of mechanical dry coating with magnesium stearate on flowability and compactibility of plastically deforming microcrystalline cellulose powders. Int. J. Pharm. 2018, 537, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Jallo, L.J.; Ghoroi, C.; Gurumurthy, L.; Patel, U.; Dave, R.N. Improvement of flow and bulk density of pharmaceutical powders using surface modification. Int. J. Pharm. 2012, 423, 213–225. [Google Scholar] [CrossRef]
- Mansour, F.R.; Waheed, S.; Paull, B.; Maya, F. Porogens and porogen selection in the preparation of porous polymer monoliths. J. Sep. Sci. 2020, 43, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Huang, T.H.; Kao, C.T.; Wu, Y.H.; Chen, W.C.; Shie, M.Y. Mesoporous calcium silicate nanoparticles with drug delivery and odontogenesis properties. J. Endod. 2017, 43, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Saffari, M.; Langrish, T. Spray drying and post-processing production of highly-porous lactose particles using sugars as templating agents. Powder Technol. 2015, 283, 171–177. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Saffari, M.; Dehghani, F.; Langrish, T. Incorporation of acetaminophen as an active pharmaceutical ingredient into porous lactose. Int. J. Pharm. 2016, 499, 217–227. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Saffari, M.; Langrish, T. Developing a new production process for high-porosity lactose particles with high degrees of crystallinity. Powder Technol. 2015, 272, 45–53. [Google Scholar] [CrossRef]
- Swaminathan, V.; Kildsig, D.O. The effect of particle morphology on the physical stability of pharmaceutical powder mixtures: The effect of surface roughness of the carrier on the stability of ordered mixtures. Drug Dev. Ind. Pharm. 2000, 26, 365–373. [Google Scholar] [CrossRef]
- Salonen, J.; Laitinen, L.; Kaukonen, A.M.; Tuura, J.; Bjorkqvist, M.; Heikkila, T.; Vaha-Heikkila, K.; Hirvonen, J.; Lehto, V.P. Mesoporous silicon microparticles for oral drug delivery: Loading and release of five model drugs. J. Control. Release 2005, 108, 362–374. [Google Scholar] [CrossRef]
- Sharma, S.; Sher, P.; Badve, S.; Pawar, A.P. Adsorption of meloxicam on porous calcium silicate: Characterization and tablet formulation. AAPS PharmSciTech 2005, 6, E618–E625. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Kwok, P.C.L.; Fukushige, K.; Prud’homme, R.K.; Chan, H.K. Enhanced dissolution of inhalable cyclosporine nano-matrix particles with mannitol as matrix former. Int. J. Pharm. 2011, 420, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, M.; Barghi, L. Design of agglomerated crystals of ibuprofen during crystallization: Influence of surfactant. Iran. J. Basic Med. Sci. 2011, 14, 57–66. [Google Scholar]
- Raval, M.K.; Sorathiya, K.R.; Chauhan, N.P.; Patel, J.M.; Parikh, R.K.; Sheth, N.R. Influence of polymers/excipients on development of agglomerated crystals of secnidazole by crystallo-co-agglomeration technique to improve processability. Drug Dev. Ind. Pharm. 2013, 39, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Stone, E.H.; Henriques, J. A fundamental study on compression properties and strain rate sensitivity of spray-dried amorphous solid dispersions. AAPS PharmSciTech 2022, 23, 11. [Google Scholar] [CrossRef]
- Takahashi, H.; Chen, R.; Okamoto, H.; Danjo, K. Acetaminophen particle design using chitosan and a spray-drying technique. Chem. Pharm. Bull. 2005, 53, 37–41. [Google Scholar] [CrossRef][Green Version]
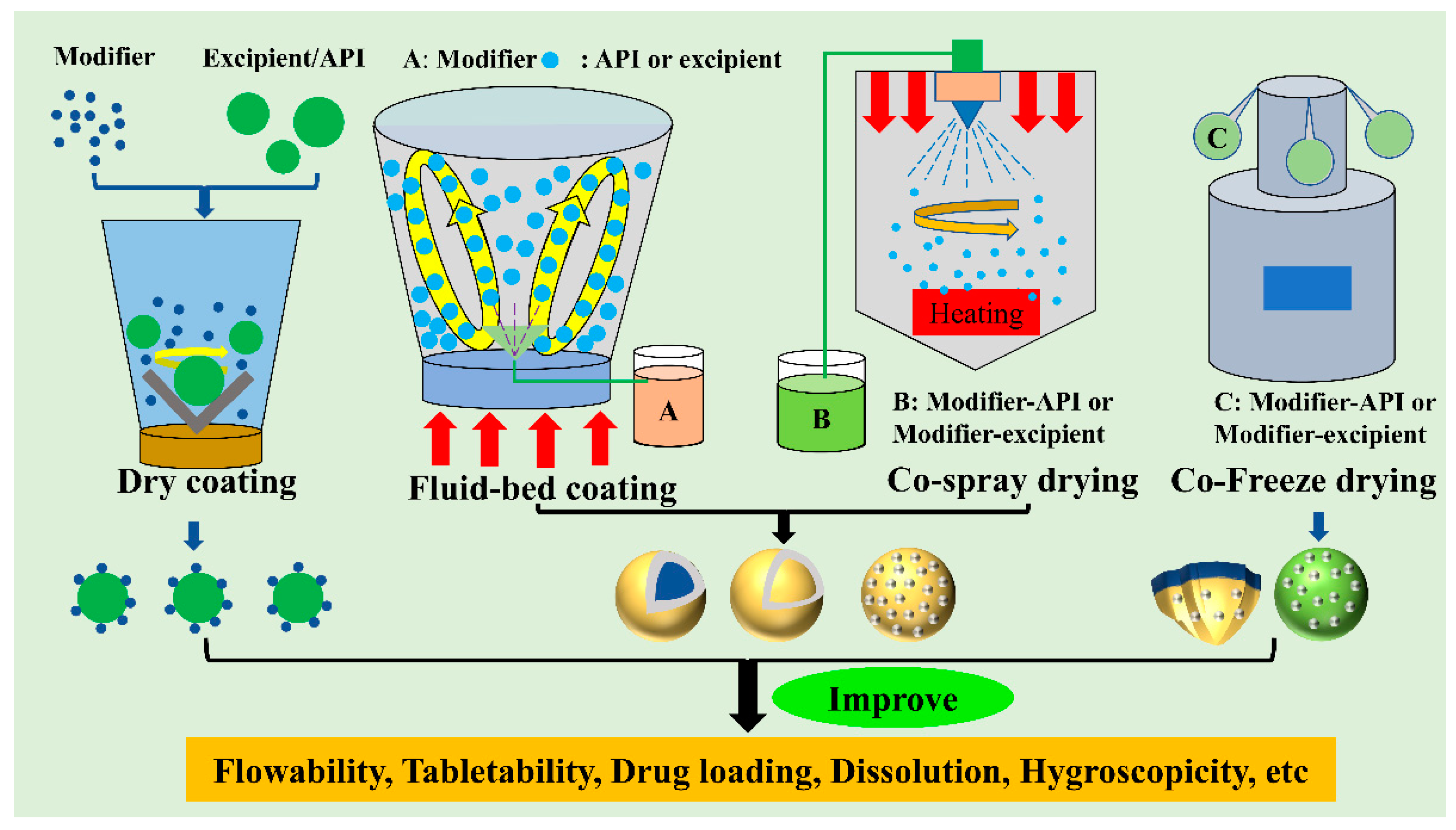
| Material | Modifier | Type | Processing | Improved Functional Properties | Ref. |
|---|---|---|---|---|---|
| Metformin | HPMC-mannitol | Binary modifier | Freeze-dried | Dissolution: disintegration time, ↓, 41%; Tabletability (1): TS, ↑, 2.5~5.2-fold | [64] |
| Andrographis paniculate extract, Gardenia extract | HPMC E3 (7%)-mannitol | Binary modifier | Fluid-bed coating | Flowability (1):AR, ↓, 26.54%; CI, ↓, 36.52%; Flowability (2): AR, ↓, 26.95%; CI, ↓, 37.95%; Tabletability (1): TS, ↑, 1.54~4.58-fold Drug loading (A and G): ↑, 75% and 50% | [30] |
| Andrographis paniculate extract, Gardenia extract | HPMC E3 (7%)-mannitol | Binary modifier | Spray-dried | Flowability (1):AR, ↓, 29.91%; CI, ↓, 37.77%; Flowability (2): AR, ↓, 30.29%; CI, ↓, 40.22%; Tabletability (1):TS, ↑, 2.28~3.07-fold; Drug loading (A and G): ↑, 75% and 50% | [30] |
| Andrographis paniculate extract, Gardenia extract | HPMC E3 (7%)-CC | Binary modifier | Fluid-bed coating | Flowability (1):AR, ↓, 20.30%; CI, ↓, 40.82%; Flowability (2): AR, ↓, 21.95%; CI, ↓, 39.22%; Tabletability (1): TS, ↑, 3.28~5.98-fold Drug loading (A and G): ↑, 75% and 25% | [30] |
| Andrographis paniculate extract, Gardenia extract | HPMC E3 (7%)-CC | Binary modifier | Spray-dried | Flowability (1):AR, ↓, 10.57%; CI, ↓, 21.65%; Flowability (2): AR, ↓, 12.42%; CI, ↓, 22.64%; Tabletability (1): TS, ↑, 2.61~5.11-fold Drug loading (A and G): ↑, 75% and 25% | [30] |
| Ibuprofen | HPMC | Unitary modifier | Fluid-bed coating | Flowability (2): flow rate: ↑, 1.08~2.5-fold | [96] |
| Three kinds of alcohol extracted medicinal powders | HPMC (7%) | Unitary modifier | Fluid-bed coating | Flowability (1): AR↓, 14.89%; 25.38%; 31.00%; Flowability (2): AR↓, 14.30%; 25.97%; 16.38%; Tabletability (1): AUTCC↑, 2.20-fold; 40.60- fold; 0→8.786 MPa.kN; Tabletability (2): AUTCC↑, 2.50-fold; 30.70- fold; 0→8.786 MPa.kN; Hygroscopicity (2): f(ZR)↓, 16.94% | [97] |
| The Andrographis herba extract | HPMC (6%) | Unitary modifier | Fluid-bed coating | Flowability (1): AR↓, 27.11%; Flowability (2): AR↓, 26.88% Tabletability (1): AUTCC↑, 1.96-fold; Tabletability (2): AUTCC↑, 1.95-fold | [18] |
| The Andrographis herba extract | HPMC (9%, 12%) | Unitary modifier | Fluid-bed coating | Flowability (2): AR↓, 28.92%; 30.48%; Tabletability (2): AUTCC↑, 2.07- fold; 2.26-fold | [18] |
| Zingiberis rhizoma extracted powder | HPMC E3 (7%) | Unitary modifier | Fluid-bed coating | Flowability (1): AR↓, 25.76%; Flowability (2): AR↓, 20.26% Tabletability (1): AUTCC↑, 0→8.786 MPa.kN Tabletability (2): AUTCC↑, 0→8.786 MPa.kN Hygroscopicity (1): f↓, 8.72%; Hygroscopicity (2): f↓, 16.94% | [6] |
| Zingiberis rhizoma extracted powder | HPMC E3 (7%)-SiO2 (1%) | Binary modifier | Fluid-bed coating | Flowability (1): AR, No significant improvement Flowability (2): AR↓, 23.17; Tabletability (1): AUTCC↑, 6.07-fold Tabletability (2): AUTCC↑, 0→14.401 MPa.kN Hygroscopicity (1): f↓, 15.71%; Hygroscopicity (2): f↓, 20.77% | [6] |
| Zingiberis rhizoma extracted powder | HPMC E3 (1.4%)-mannitol (5.6%) | Binary modifier | Fluid-bed coating | Flowability (1): AR↓, 20.52%; Flowability (2): AR↓, 22.18% Tabletability (1): AUTCC↑, 0→6.405 MPa.kN Tabletability (2): AUTCC↑, 0→6.405 MPa.kN Hygroscopicity (1): f↓, 0.53%; Hygroscopicity (2): f↓, 7.83% | [6] |
| The ethanol extract of pueraria lobatae radix | NH4HCO3 (6.67%, 10.00%, 13.33%) | Unitary modifier | Spray-dried | Flowability (2): AR, No significant improvement Tabletability (2): TS↑, 2.43-fold; 3.16~3.40-fold; 4.32-7.03-fold Disintegration: dissolution rate↑, ~2-fold | [98] |
| lactose, corn starch, mannitol, | HPMC | Unitary modifier | Spray-dried | Tabletability (1): TS↑, 2.43-fold; 4.20~6.28-fold; 1.63~4.12-fold; 1.81~2.69-fold; 1.34~1.63-fold; 1.05~1.24 -fold; Flowability: AR, 43 ± 0.15~48 ± 0.25 Tabletability (2): TS↑, 2.43-fold | [5] |
| Starch | HPMC-PVPP (3.5%) | Binary modifier | Spray-dried | Disintegration time (C): ↓, 4.77–7.58% | [5] |
| Calcium hydrogen phosphate dihydrate (19%~44%) | HPMC E3 (3.5%~10.5%) | Unitary modifier | Spray-dried | Flowability: AR, 29 ± 0.19~34 ± 0.15°; Tabletability: TS (a), 2.20 ± 0.04~3.55 ± 0.01 MPa; Disintegration time (b): 11.33 ± 0.08~59.67 ± 0.07 min | [5] |
| Calcium hydrogen phosphate dihydrate | HPMC E3 -PVPP (3.5%) | Binary modifier | Spray-dried | Disintegration time (C): ↓, 0.35–75.82% | [5] |
| Starch (19%~44%)- | HPMC E3 (3.5%~10.5%) | Binary modifier | Spray-dried | Flowability: AR, 29 ± 0.31~34 ± 0.13°; Tabletability: TS (a), 3.15 ± 0.04~5.27 ± 0.05 MPa; Disintegration time (b): 6.06 ± 0.03~13.29 ± 0.01 min | [5] |
| Metformin | HPMC vlV -lactose- | Binary modifier | Freeze-dried | Disintegration time (2): ↓, 24.68%; 50.00% | [44] |
| Lactose | HPMC | Unitary modifier | Dry coating | Drug loading (2): ↑, 46.15% | [99] |
| Metformin hydrochloride | HPMC E3 (2.5%, 5%) | Unitary modifier | Spray-dried | Tabletability (2): TS ↑, 0→1.89, 2.67 MPa; 0→2.65, 5.17 MPa; 0→3.15, 2.81 MPa; 0→1.62, 2.00 KPa; 0→2.07, 2.15 KPa; Tabletability (2): TS ↑, 192-fold | [28] |
| Carvedilol Matrix tablets | HPMC (K 4 M) | Unitary modifier | Dry coating | Tabletability (2): TS↑, 1.32-fold | [100] |
| Mannitol, lactose, anhydrous dibasic calcium phosphate , calcium carbonate Chitosan | HPMC E3 (7%) | Unitary modifier | Spray-dried | Flowability (1): AR↓, 36.04%; 28.31%; 28.57%; 29.87%; 30.26% Flowability (2): AR↓, 11.51%; 23.45%; 28.71%; 26.60%; 7.79% Tabletability (1): Tableting ratio↓, 0.94%; 15.68%; 11.90%; 26.42%; 2.21%; Tabletability (2): Tableting ratio↓, 10.62%; 5.74%; 23.67%; 33.05%; 15.70% | [62] |
| Material | Modifier | Type | Processing | Improved Functional Properties | Ref. |
|---|---|---|---|---|---|
| Acetaminophen | PVP K30 (1.25%, 1.5 %, 5%) | Unitary modifier | Spray-dried | Tabletability (2): The same compaction (kN), crushing strength↑, ~3.90-fold; ~5.00-fold; ~7.00-fold; Dissolution (2): Disintegration time↓, 36.61%; 52.29%; 74.31%; Dissolution (3): Disintegration time↓, 65.48%; 73.60%; 85.79% | [108] |
| Silicon dioxide | PVP | Unitary modifier | Dry coating | Tabletability (1): TS, ↑, 0→2.25 kN | [116] |
| Paracetamol | PVP (5%)- Lactose (20%) | Binary modifier | Spray-dried | Tabletability (2): TS, ↑, 1.67-fold; Tabletability (3): TS, ↑, 1.73-fold Flowability (3): FFC↑, 2.23-fold; Compared with adding PVP and lactose alone, adding PVP and lactose at the same time FFC↑, 1.12-fold; TS↑, 3.18-fold | [115] |
| Lactose | PVP K30(1 %, 2%, 3%) | Binary modifier | Spray-dried | Flowability (2): AR↓, 6.84%; 10.60%; 11.70%; CI↓, 22.68%; 30.00%; 21,22%; Flowability (3): AR↓, 25.57%; 28.57%; 29.54%; CI↓, 32.55%; 38.94%; 31.28%; | [113] |
| Curcumin | PVP K30(1 %, 2%, 3%) -lactose | Binary modifier | Spray-dried | Dissolution (2): Cumulative dissolution percentage (%) ↑, at 90 min, 3.31-fold, 3.77-fold, 3.58-fold; Dissolution (3): Cumulative dissolution percentage (%) ↑, at 90 min, 3.74-fold, 4.26-fold, 4.04-fold | [113] |
| Leflunomide | PVP | Unitary modifier | Freeze-drying | Dissolution: ↑, Dissolution efficiency (%), At 10 min, 60 min, 120 min, 0→10.73; 0→61.46; 1.05→78.44 | [114] |
| Paracetamol | PVP (5%)- Mannitol (20%) | Binary modifier | Spray-dried | Tabletability (1): TS, ↑, 8.00-fold; 16.00-fold; 10.17-fold | [117] |
| Three kinds of water extracted medicinal powders (A, G, GF) | PVP | Unitary modifier | Fluid-bed coating | Flowability (1): AR↓, 32.47%; 24.81%; 33.77% Flowability (2): AR↓, 32.51%; 24.30%; 32.72% Tabletability (1): AUTCC↑, 1.31-fold; 1.64-fold; 3.07-fold Tabletability (2): AUTCC↑, 1.36-fold; 1.99-fold; 3.12-fold | [97] |
| Ibuprofen | PVP (10%) MgSt (0.1–5%) | Binary modifier | Dry coating | Tabletability (2): TS↑, 1.23-fold; Flowability (2): CI↓, 29% | [36] |
| Metformin hydrochloride | PVP K30 (5.0%) | Unitary modifier | Spray-dried | Tabletability (2): TS ↑, 0→1.62 KPa; work of compaction, ↑, 72.8%; elastic recovery, ↓, 1.5%; tablet tensile strength at porosity 0.15, ↑, from 0 to 2.00 MPa. | [28] |
| Desloratadine | PVP | Unitary modifier | Spray-dried | Dissolution (3): Apparent solubility↑, 0→1.62, 2.00 KPa | [118] |
| Artemisinin | PVP (1:1) | Unitary modifier | Spray-dried | Dissolution: at 30 min, relative dissolution rate (1) ↑, 3.17-fold; relative dissolution rate (2) ↑, 1.78-fold, relative dissolution rate (3) ↑, 0 →4.78; at 30 min, percent dissolution efficiency (1) (%) ↑, 3.16-fold; percent dissolution efficiency (2) (%) ↑, 1.72-fold; percent dissolution efficiency (3) (%) ↑, 4.78-fold at 30 min, concentration of drug dissolved (1) (μg/mL) ↑, 3.15-fold, concentration of drug dissolved (2) (%) ↑,1.74-fold, concentration of drug dissolved (3) (%) ↑, 4.78-fold | [119] |
| Artemisinin | PVP (1:2, 1:4, 1:6) | Unitary modifier | Spray-dried | Dissolution: at 30 min, relative dissolution rate (2) ↑, 2.16-fold, 2.43-fold, 2.90-fold; relative dissolution rate (3) ↑, 0→5.78; 0→6.52; 0→7.76; at 30 min, percent dissolution efficiency (2) (%) ↑, 2.09-fold, 2.35-fold, 2.80-fold; percent dissolution efficiency (3) (%) ↑, 5.78-fold, 6.52-fold, 7.76-fold; at 30 min, concentration of drug dissolved (2) (%) ↑, 2.08-fold, 2.35-fold, 2.80-fold; concentration of drug dissolved (3) (%) ↑, 5.78-fold, 6.52-fold, 7.77-fold | [119] |
| Telmisartan | PVP K30 (1:5) | Unitary modifier | Spray-dried | Dissolution: at 5 min, percentage of drug dissolved (2) ↑, 4.05-fold; percentage of drug dissolved(3) ↑, 16.17-fold; at 15 min, percentage of drug dissolved (2) ↑, 3.63-fold; percentage of drug dissolved(3) ↑, 19.47-fold; at 5 min, percentage of dissolution efficiency (2) ↑, 4.15-fold; percentage of dissolution efficiency (3) ↑, 13.46-fold; at 15 min, percentage of dissolution efficiency (2) ↑, 3.85-fold; percentage of dissolution efficiency (3) ↑, 16.98-fold; at 5 min, percentage of drug released (2) ↑, 3.67-fold; percentage of drug released (3) ↑, 8.25-fold at 15 min, percentage of drug released (2) ↑,3.94-fold; percentage of drug released (3) ↑, 21.00-fold Saturation solubility (2) (mg = mL) ↑, 11.45-fold Saturation solubility (2) (mg = mL) ↑, 21.40-fold | [120] |
| Telmisartan | PVP- Aerosil200/Sylysia350 (1:5:2) | Binary modifier | Spray-dried | Dissolution: at 5 min, percentage of drug dissolved (2) ↑, 4.89-fold, 5.65-fold; percentage of drug dissolved (3) ↑, 19.58-fold, 22.57-fold; at 15 min, percentage of drug dissolved (2) ↑, 4.70-fold, 5.59-fold; percentage of drug dissolved (3) ↑, 25.26-fold, 30.03-fold; at 5 min, percentage of dissolution efficiency (2) ↑, 4.58-fold, 5.18-fold; percentage of dissolution efficiency (3) ↑, 14.86-fold, 16.81-fold; at 15 min, percentage of dissolution efficiency (2) ↑, 4.79-fold, 5.44-fold; percentage of dissolution efficiency (3) ↑, 21.12-fold, 23.89-fold; at 5 min, percentage of drug released (2) ↑, 4.00-fold, 4.57-fold; percentage of drug released (3) ↑, 14.00-fold, 16.00-fold; at 15 min, percentage of drug released (2) ↑,4.56-fold, 5.56-fold; percentage of drug released (3) ↑, 20.5-fold, 25.00-fold; Saturation solubility (2) (mg/mL) ↑, 17.65-fold, 25.56-fold Saturation solubility (2) (mg/mL) ↑, 33.00-fold, 47.79-fold | [120] |
| Loratadine | PVP K30 | Unitary modifier | Dry coating | Dissolution: Solubility (1) ↑, 1.17-fold; Solubility (3) ↑, 1.19-fold; at 60 min, percentage of drug dissolved (1) ↑, 1.22-fold, percentage of drug dissolved (3) ↑, 3.33-fold, | [121] |
| Irbesartan | PVP-sodium dodecyl sulfate | Binary modifier | Anti-solvent precipitation Spray-dried | Dissolution: at 30 min, percentage of drug dissolved (1) ↑, 8%→100% percentage of drug dissolved (3) ↑, 40%→100% | [122] |
| Zingiberis rhizoma extracted powder | PVP K30 (7%) | Unitary modifier | Fluid-bed coating | Flowability (1): AR↓, 26.88%; Flowability (2): AR↓, 23.49%; Tabletability (1): AUTCC↑,0→9.279 MPa.kN; Tabletability (2): AUTCC↑,0→9.279 MPa.kN; Hygroscopicity (1): f↓, 9.82%; Hygroscopicity (2): f↓, 14.63% | [6] |
| Effervescent tablets | PVP K30 (6%) | Unitary modifier | Fluid-bed coating | Flowability (1): AR↓, 7.42%; Flowability (3): AR↓, 33.43%; Tabletability (1): TS↑, 2.09-fold; Tabletability (3): TS↑, 2.30-fold; Sticking of citric acid (mg) (1) ↓, 70.83→0.24; Sticking of citric acid (mg) (3) ↓, 22.80→0.24 | [123] |
| Material | Modifier | Type | Processing | Improved Functional Properties | Ref. |
|---|---|---|---|---|---|
| Zingiberis rhizoma extracted powder | SiO2 (1%) | Unitary modifier | Freeze-dried | Flowability (3): ffc, ↑, mean↑, 2.60-fold; Mean ffc: 4.00→10.06 Tabletability (3): TS↓, 28.57% | [142] |
| Erythritol | Porous SiO2 (2:1) | Unitary modifier | Dry coating | Dissolution: Disintegration time↓, 40%; Tabletability (1): TS↑, 28.57% | [143] |
| Zingiberis rhizoma extracted powder | SiO2 (1:0.06, 1:0.5, 1:0.25) | Unitary modifier | Liquid dispersion | Flowability (1): AR↓, 5.30%, 18.54%; Flowability (3): AR↓, 7.94%, 20.82%, 18.24%; Tabletability (1): CR↑, 1.25%; Tabletability (3): CR↓, 7.69%, ↑, 1.92%, ↑, 3.85% | [133] |
| Ibuprofen 50 | SiO2 | Unitary modifier | Dry coating | Dissolution: time for 80% of drug to dissolve↓, 12min→3min; Flowability (3): AR↓, 19.15%, FFC↑, 2.96-fold; Drug loading (3): at 60%, AR:38°, TS: 3.40 MPa, FFC = 8 (at 60%, AR:47°, TS:2.30, FFC = 2.7) | [25] |
| Ibuprofen powder | SiO2-PVP 40 (4:1) | Binary modifier | Dry coating | Flowability (2): AR↓, 28.30%, FFC↑, 6.10-fold, Cohesion (KPa) ↓, 40% Flowability (3): AR↓, 32.07%, FFC↑, 5.90-fold, Cohesion (KPa) ↓, 42.50%; Dissolution (2): Dissolution rate↑, 2.00-fold; Dissolution (3): Dissolution rate↑, 3.00-fold | [53] |
| Ibuprofen (Ibu50, Ibu90) | SiO2 (1%-M5P, Aerosil R972P) | Unitary modifier | Dry coating | Flowability (3): FFC↑, hydrophilic M5P ↑, 1.38-fold, 2.67-fold hydrophobic Aerosil R972↑, 5.00-fold, 3.11-fold | [140] |
| Ibuprofen, mannitol, lactose | SiO2 (1% -Aerosil R972P, Aerosil A200) | Unitary modifier | Dry coating | Flowability (3): FFC↑, hydrophobic Aerosil R972 ↑, 3.33-fold, 1.74-fold, 1.71-fold; hydrophilic Aerosil A200↑, \, 2.28-fold, 1.84-fold | [144] |
| Cornstarch | 1%, 0.1%, silica EH-5; 20%, silica COSMO-55 | Unitary modifier | Dry coating | Flowability (3): AR↓, silica EH-5 ↓, 36.54%, 34.62% silica COSMO-55↓, 13.46% | [51] |
| Danshen, Notoginseng, bornel, formulated | 1%-silica nanoparticles | Unitary modifier | Dry coating | Tabletability (3): Compressibility↓, 56.64%, 24.04%, 7.60%, 63.59% TS↑, 1.67-fold, 1.75-fold, 1.53-fold, 1.74-fold; Flowability (3): AR↓, 53.45%, 27.27%, 16.67%, 44.33% | [145] |
| MCC (particle size 20, 25, 30, 35 μm) | Hydrophobic (Aerosil R972P), hydrophilic (Aerosil A200) silicas | Unitary modifier | Dry coating | Flowability (3): FFC↑, hydrophobic Aerosil R972P ↑, 1.70-fold, 2.00-fold, 2.78-fold, 3.00-fold | [33] |
| Acetaminophen (micronized and coarse) | SiO2 (M5P, R972P) | Binary modifier | Dry coating | Flowability (3): FFC↑, hydrophobic Aerosil R972P ↑, 2.20-fold, 1.88-fold hydrophilic M5P↑, 3.50-fold, 3.09-fold | [140] |
| Microcrystalline cellulose | Colloidal silica | Binary modifier | Dry coating | Flowability (3): Flowability energy↑, 1, 92-fold | [146] |
| API | Silica colloidal anhydrous-MCC-MgSt | Ternary modifier | Dry coating | Flowability: FFC↑, 2.00~2.50-fold; Drug loading: ↑, 50%→80% | [147] |
| EC | Colloidal silicon (1%)-lactose (5%) | Binary modifier | Dry coating | Sustained drug release: Dissolution rate↓, 4.00-fold; Flowability (3): AR↓, 11.70% | [148] |
| CaCO3 | Aerosil nanoparticle | Unitary modifier | Dry coating | Flowability (3): AR↓, 12.76%, CI↓, 12.24% | [149] |
| Fenofibrate | Hydrophilic nano-silica (M5P) (0.1%, 0.17%, 1%) | Unitary modifier | Dry coating | Flowability (3): AR↓, 45.22%, 45.40%, 39.40%; FFC↑, 3.57-fold, 3.57-fold, 2.16-fold | [150] |
| Potassium chloride | Silica | Unitary modifier | Dry coating | Flowability (3): Flow function coefficient↑, 1.26-fold; Cohesion↓, 25.53% | [141] |
| Avicel PH-102 | Nano-silica (1%) | Unitary modifier | Dry coating | Flowability (3): Flow function coefficient↑, 3.00-fold; Tabletability(3): TS↑, 1.26-fold | [14] |
| MCC | Silica nanoparticles (0.1%, 0.5%, 1%, 2%) | Unitary modifier | Dry coating | Flowability (3): Flow factor↑, 1.20-fold, 3.20-fold, 4.20-fold, 5.20-fold | [151] |
| Acetaminophen | Silica nanoparticles (0.1%)- MCC PH102, MCC PH105 | Binary modifier | Dry coating | Flowability (3): Flow function coefficient↑, At drug loading 20%, 40%, 60%, 80%, PH102↑, 3.02-fold, 4.30-fold, 5.00-fold, 5.00-fold; PH105↑, 4.20-fold, 3.65-fold, 3.70-fold, 3.25-fold; Tabletability (3): TS↑, At drug loading 20%, 40%, 60% PH102↑, 1.24-fold, 1.08-fold, 1.33-fold; PH105↑, 1.61-fold, 1.29-fold, 1.38-fold | [34] |
| Micronized acetaminophen, coarse acetaminophen, micronized ibuprofen | nano-silica (hydrophobic, hydrophilic) | Unitary modifier | Dry coating | Flowability (3): Flow function coefficient↑, hydrophobic Aerosil R972P↑, 3.80-fold, 4.41-fold, 3.90-fold; hydrophilic CAB-O-SIL M5P ↑, 2.44-fold, 2.96-fold, 3.15-fold; Tabletability (3): TS↑, hydrophobic Aerosil R972P↑, 1.50-fold, 1.07-fold, 1.09-fold; hydrophilic CAB-O-SIL M5P ↑, 3.00-fold, 1.43-fold, 1.08-fold | [135] |
| Micronized acetaminophen, Avicel PH105, Pharmatose 450 M | Silica | Unitary modifier | Dry coating | Flowability (3): Flow function coefficient↑, 2.50-fold, 2.37-fold, 6.33-fold; Tabletability (3): compressibility (%) ↓, 40.00%, 54.05%, 68.97% Drug loading at 60% had suitable; flowability and tabletability (FFC > 8, TS = 2) | [35] |
| Ibuprofen powder | Silica-R972 (1%) | Unitary modifier | Dry coating | Flowability: Cohesion (3) ↓, 81.95%; flow function (3) ↑, 5.14-fold | [139] |
| Material | Modifier | Type | Processing | Improved Functional Properties | Ref. |
|---|---|---|---|---|---|
| Crospovidone | MCC- sodium chloride | Unitary modifier | Fluid-bed Freeze dryer | Tabletability (1): Porosity↑, 2.14-fold, 2.57-fold; TS↑, 1.57-fold, 9.28-fold | [154] |
| Sacubitril valsartan | MCC | Unitary modifier | Spray-dried | Dissolution (1): solubility↑, 11.5-fold, 3.12-fold; Bioavailability (1): Relative bioavailability %↑, 14.49-fold, 11.21-fold, 5.64-fold, 1.98 -fold; Bioavailability (2): Relative bioavailability %↑, 1.54-fold, 1.11-fold | [171] |
| Zingiberis rhizoma extracted powder | MCC (1:0.06, 1:0.25) | Unitary modifier | Dry coating | Flowability (3): AR↓, 1.07%, 4.94%; Tabletability (3): CR↓, 17.41%, 18.15% | [133] |
| Curcumin | MCC | Unitary modifier | Fluid-bed | Flowability (3): Carr’s index↓, 84.00%; Hausner ratio↓, 46.12% | [172] |
| Indomethacin and Nifedipine | Mannitol (porous) | Unitary modifier | Spray-dried | Dissolution: The area under dissolution curve↑; Cumulative drug release (1) ↑, at 10 min, ↑, 2.79-fold, 2.00-fold; balance drug release (1) ↑, 2.04-fold, 1.69-fold; Drug loading (3): ↑, 2.84-fold, 3.07-fold | [166] |
| Metformin | HPMC-mannitol | Binary modifier | Freeze-dried | Dissolution: DT, ↓,41%; Tabletability (3): TS, ↑, 2.5~5.2-fold | [64] |
| Mannitol | NH4HCO3 (5% w/v) | Binary modifier | Spray-dried | Dissolution: Porosity (3) ↑, 2.5~5.2-fold; Disintegration time (3) ↑, 50%~70%; Tabletability (3): Tablet hardness, ↑, 1.46-fold | [165] |
| Material | Modifier | Type | Processing | Improved Functional Properties | Ref. |
|---|---|---|---|---|---|
| Paracetamol | Alpha-lactose-hydrate | Unitary modifier | Spray-dried | Tabletability (1): TS, ↑, 2.9-fold | [194] |
| Propranolol | lactose-HPMC (1:1) | Binary modifier | Spray-dried | Tabletability (1): Hardness ↑, 1.22-fold; Flowability (1): Flow time ↑, 1.33-fold | [195] |
| Lactose monohydrate | PEG 4000-popoxamer | Binary modifier | Fluid-bed granulation | Flowability (3): Flow rate ↑, 7.73-fold; compressibility index↑, 4.33-fold; Hausner ratio↑, 1.42-fold; Tabletability (1): TS ↑, 2.83-fold | [196] |
| Curcumin | MCC-PVP | Unitary modifier | Fluid-bed coating | Flowability (3): Carr’s index↓, 84.00%; Hausner ratio↓, 46.12% | [172] |
| Curcumin | Lactose-PVP | Unitary modifier | Fluid-bed coating | Flowability (3): Carr’s index↓, 6.67%; Hausner ratio↓, 9.13% | [172] |
| Lactose | Magnesium stearate | Unitary modifier | Spherical agglomerates | Tabletability (3): TS ↑, 3.50-fold; Flowability (3): Flow time↑, 2.00-fold | [197] |
| Mannitol | NH4HCO3 (5% w/v) | Unitary modifier | Spray-dried | Dissolution: Porosity (3) ↑, 2.5~5.2-fold; Disintegration time (3) ↑, 50%~70%; Tabletability (3): Tablet hardness, ↑, 1.46-fold | [165] |
| Pueraria lobatae radix | NH4HCO3 (6.67%, 10%, 13.33%) | Unitary modifier | Spray-dried | Flowability (3): AR↓, 9.33%, 10.34%, 12.37%; CI↑, 1.28-fold, 1.28-fold, 1.27-fold; HR↑, 1.21-fold, 1.20-fold, 1.19-fold; Flowability (2): AR↓, 1.11%, 2.21%, 4.42%; CI↑, 1.01-fold, 1.00-fold, 1.00-fold; HR↑, 1.01-fold, 1.01-fold, 1.00-fold; Tabletability (3): TS↑, 3.42-fold, 4.29-fold, 7.71-fold; Tabletability (2): TS↑, 1.50-fold, 1.88-fold, 3.38-fold | [98] |
| Pueraria lobatae radix | NH4HCO3 (10%) | Unitary modifier | Spray-dried | Dissolution (3): Dissolution rate↑, 2.00-fold | [98] |
| Lignin | Sodium lauryl sulphate (SLS) | Unitary modifier | Spray-dried | Tabletability (3): TS↑, 1.33-fold | [178] |
| Chlorzoxazone | HPC-Eudragit S100 | Binary modifier | Crystallo-co-agglomeration | Flowability (3): CI↑, 47.87%; Tabletability (3): TS↑, 3.33-fold Dissolution (3): Dissolution rate↑, 2.00-fold | [198] |
| Potassium chloride | Leucine (0.5%,1%, 2%, 5%, 10%) | Unitary modifier | Dry coating | Flowability (3): Flow function coefficient↑, 1.37-fold, 2.37-fold, 2.42-fold, 2.84-fold, 1.84-fold; Cohesion↓, 41.70%, 62.50%, 64.58%, 68.75%, 72.92% | [141] |
| Lactose | Silica | Ternary modifier | Spray-dried | Tabletability (3): TS↑, 1.89-fold | [199] |
| Ketoprofen | Croscarmellose, crospovidone, starch glycolate | Binary modifier | Spray-dried | Tabletability (3): TS↑, 3.33-fold; Dissolution (3): Ketoprofen effectively in 20 min | [200] |
| Dibasic calcium phosphate | Anhydrous polyethylene glycol-crospovidone | Binary modifier | Dry coating | Dissolution (3): Disintegration time↓, 48.13%; Cumulative drug release↑, 18.48% | [201] |
| Ibuprofen | MgSt (0.1%, 1%, 5%) | Unitary modifier | Dry coating | Flowability: Cohesion (1) ↓, 7.65%, 52.94%, 59.80%; Cohesion (3) ↓, 29.22%, 74.51%, 81.37%; flow function (1) ↑, 1.14-fold, 2.02-fold, 2.43-fold; flow function (3) ↑, 1.24-fold, 2.19-fold, 2.64-fold | [36] |
| Ibuprofen powder | MgSt, l-leucine | Unitary modifier | Dry coating | Flowability: Cohesion (3) ↓, 20.30%, 30.08%, 81.95%; flow function (3) ↑, 1.14-fold, 1.44-fold, 5.14-fold | [139] |
| Fine lactose powder | MgSt | Unitary modifier | Dry coating | Flowability (3): higher dispersive energy | [202] |
| MCC-E 50M | MgSt (1%) | Unitary modifier | Dry coating | Flowability (3): CI↓, 41.62% | [203] |
| Modifier | Pros | Cons |
|---|---|---|
| HPMC | (1) High glass transition temperature (170–180 °C) and viscosity; excellent bonding capability, tableting performance, and crystal growth inhibitor; (2) low hygroscopicity and surface tension for aqueous solutions; (3) non-toxic and improves stability. | (1) Long disintegration time. |
| PVP | (1) High glass transition temperature (160 °C); excellent solubility, plastic deformability, biocompatibility, and crystal growth inhibitor; (2) Almost no effect on the disintegration time of tablets; (3) non-toxic and increases stability. | (1) High hygroscopicity. |
| SiO2 | (1) High porosity, surface area, surface free energy, and drug loading; excellent flowability, dispersion, and biocompatibility; safety; (2) Low cohesion, sticking and static effect; and (3) can be classified into hydrophilic and hydrophobic groups. | (1) Low density. |
| MCC | (1) Good plastic deformation, compressibility, and compactibility; excellent dilution capacity and disintegration behavior. | /. |
| Mannitol | (1) Amorphous state is conducive to improve disintegration, crystallizing excipient; (2) low viscosity; and (3) edible. | (1) Crystal state is bad for disintegration. |
| PVPP | (1) excellent plastic deformability, biocompatibility, and crystal growth inhibitor, (2) super disintegrant for orally disintegrating tablets. | (1) Poor flowability. |
| Ammonium bicarbonate | (1) Porous agent; and (2) improves the disintegration of tablets. | (1) Unstable chemical substance. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.-C.; Liu, W.-J.; Zhu, W.-F.; Yang, L.-Y.; Zhang, J.-W.; Feng, Y.; Ming, L.-S.; Li, Z. Surface Modifiers on Composite Particles for Direct Compaction. Pharmaceutics 2022, 14, 2217. https://doi.org/10.3390/pharmaceutics14102217
Chen F-C, Liu W-J, Zhu W-F, Yang L-Y, Zhang J-W, Feng Y, Ming L-S, Li Z. Surface Modifiers on Composite Particles for Direct Compaction. Pharmaceutics. 2022; 14(10):2217. https://doi.org/10.3390/pharmaceutics14102217
Chicago/Turabian StyleChen, Fu-Cai, Wen-Jun Liu, Wei-Feng Zhu, Ling-Yu Yang, Ji-Wen Zhang, Yi Feng, Liang-Shan Ming, and Zhe Li. 2022. "Surface Modifiers on Composite Particles for Direct Compaction" Pharmaceutics 14, no. 10: 2217. https://doi.org/10.3390/pharmaceutics14102217
APA StyleChen, F.-C., Liu, W.-J., Zhu, W.-F., Yang, L.-Y., Zhang, J.-W., Feng, Y., Ming, L.-S., & Li, Z. (2022). Surface Modifiers on Composite Particles for Direct Compaction. Pharmaceutics, 14(10), 2217. https://doi.org/10.3390/pharmaceutics14102217






