Formulation of a 3D Printed Biopharmaceutical: The Development of an Alkaline Phosphatase Containing Tablet with Ileo-Colonic Release Profile to Treat Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
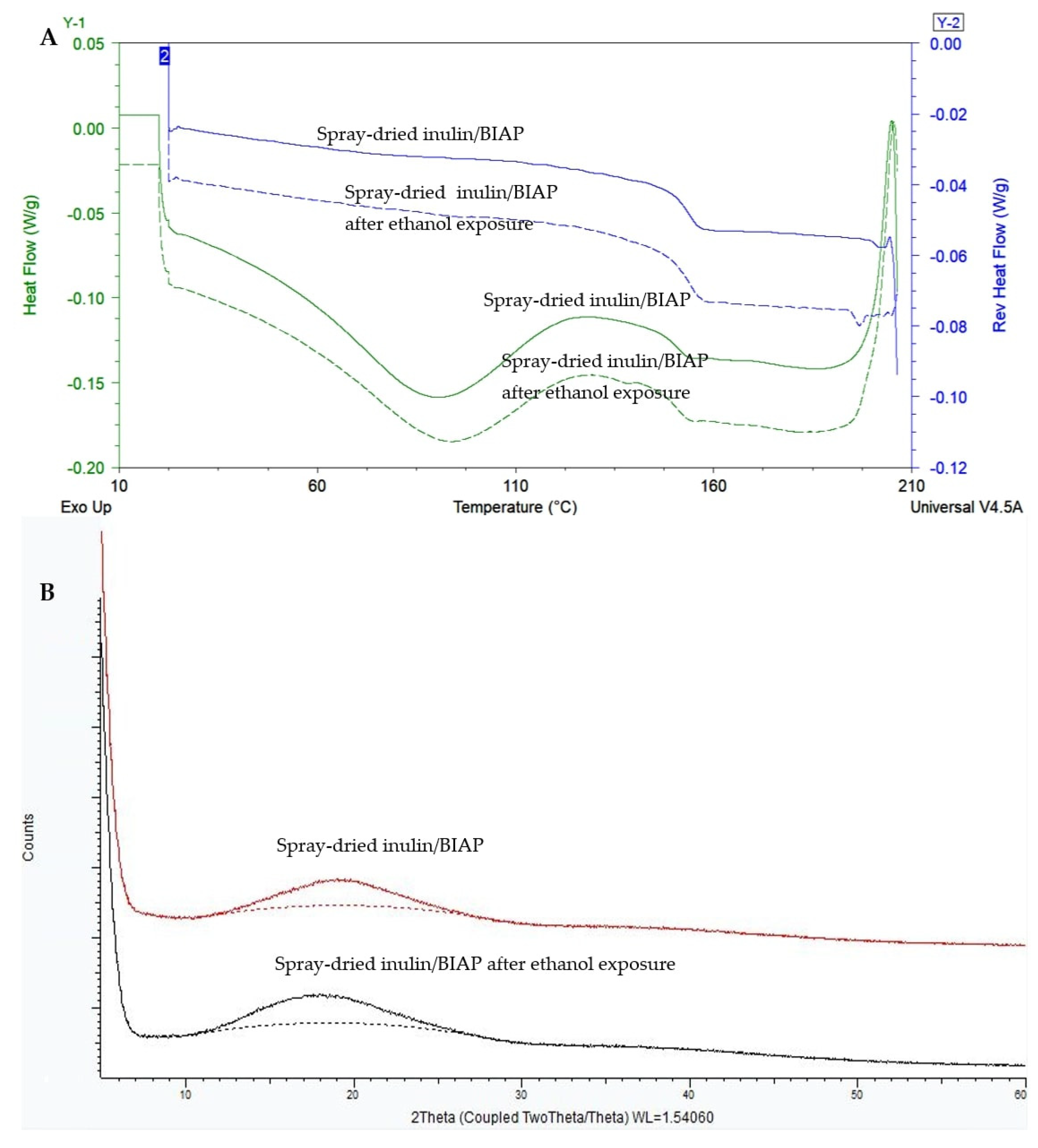
2.2. Spray Drying of Inulin Stabilized BIAP
2.3. Powder Mixture Preparation
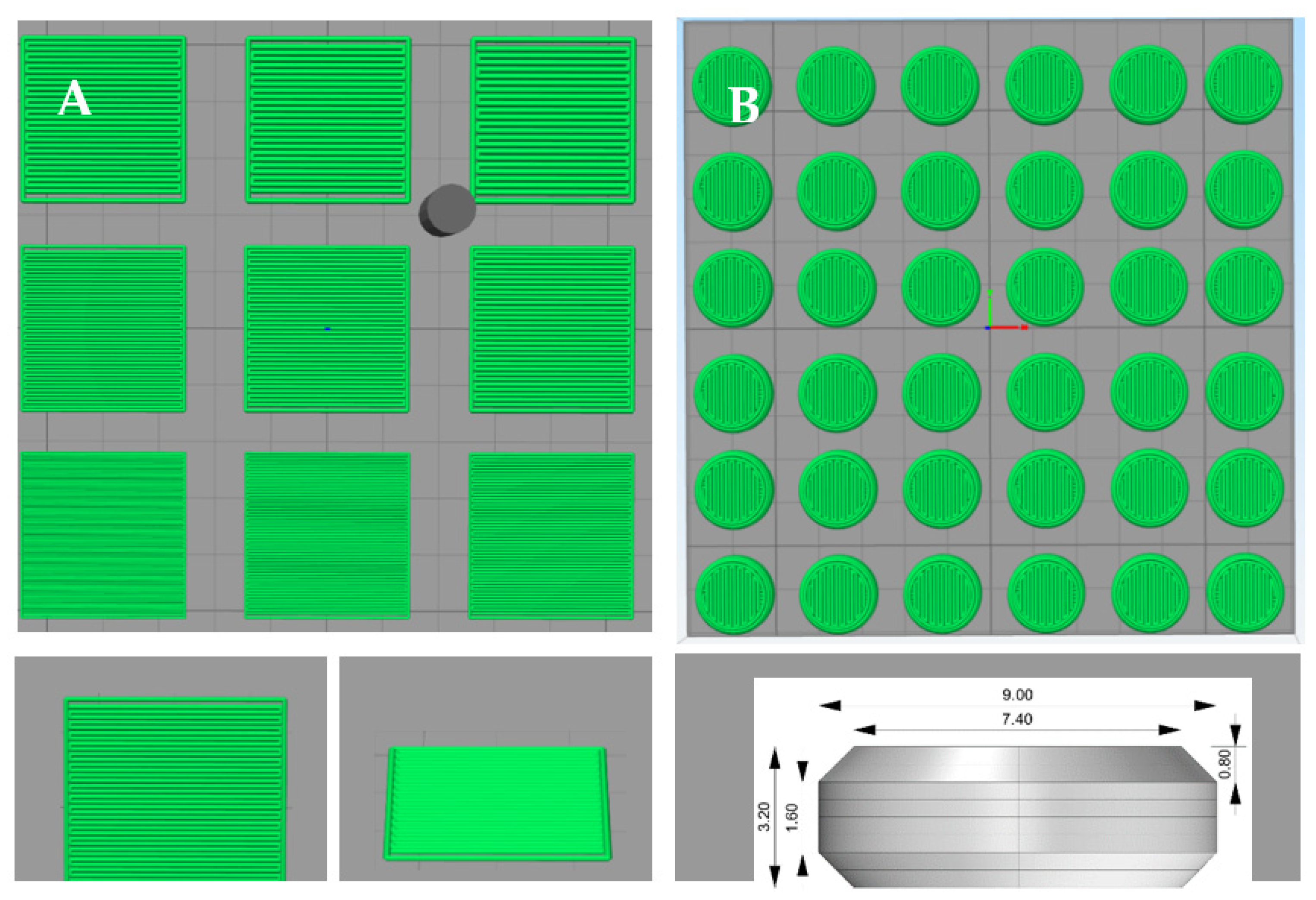
2.4. Tablet Design, Powder Bed Printing and Formulation Selection
2.5. Tablet Dimension and Weight
2.6. Crushing Strength
2.7. Friability
2.8. Disintegration Time
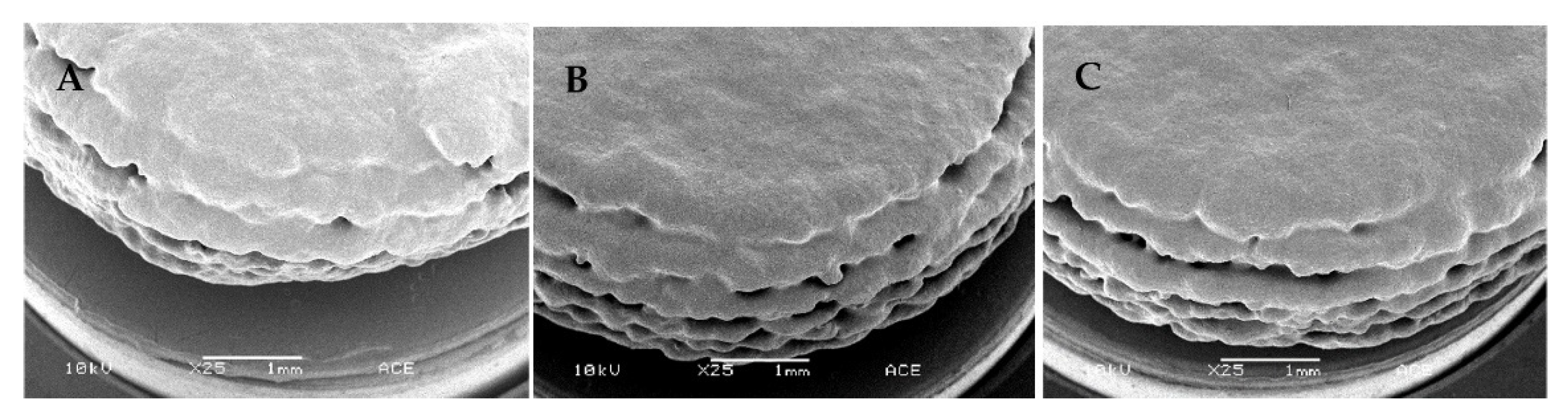
2.9. Scanning Electron Microscopy
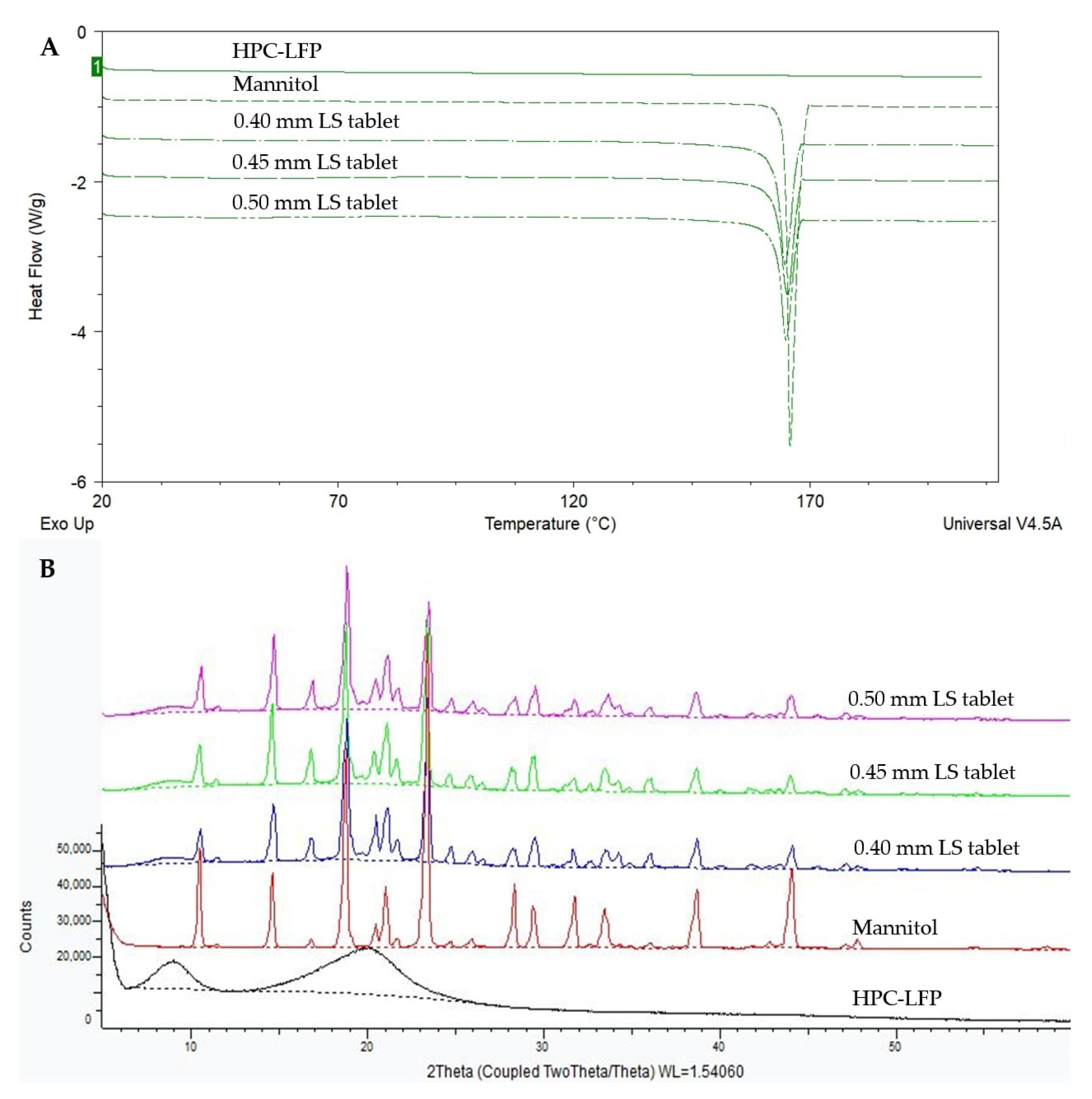
2.10. Solid-State Characterization
2.11. Enzymatic Assay
2.12. Stability of Alkaline Phosphatase in the Tablet
2.13. Sub-Coating and ColoPulse Coating
2.14. Drug Release Profile Testing in the Gastrointestinal Simulated System
2.15. Statistical Analysis
3. Results
3.1. Screening of LSs and Binders for the Printing Process
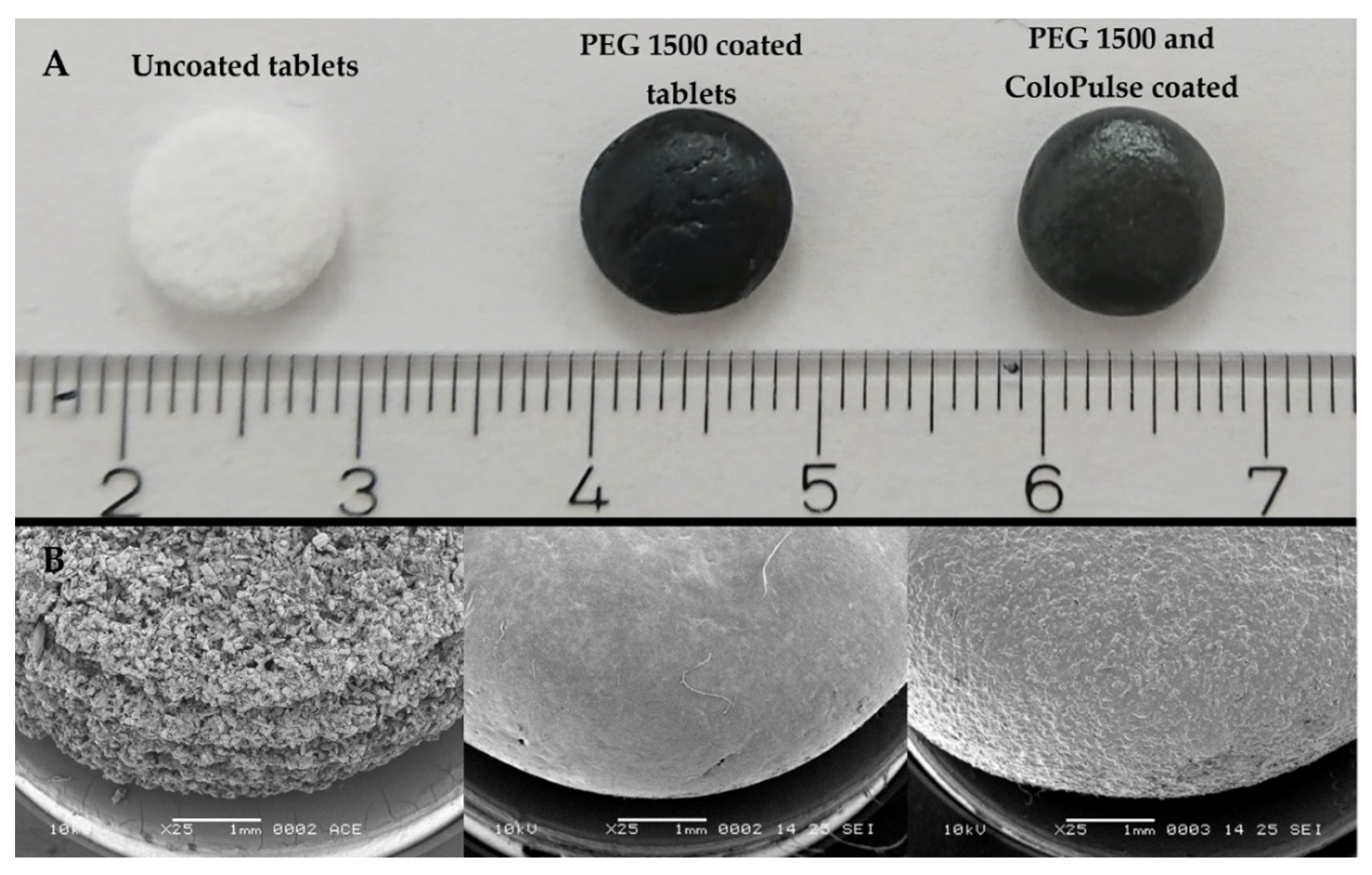
3.2. Three-Dimensional Printed Tablet Properties
3.3. Solid-State Characteristics of the Tablets
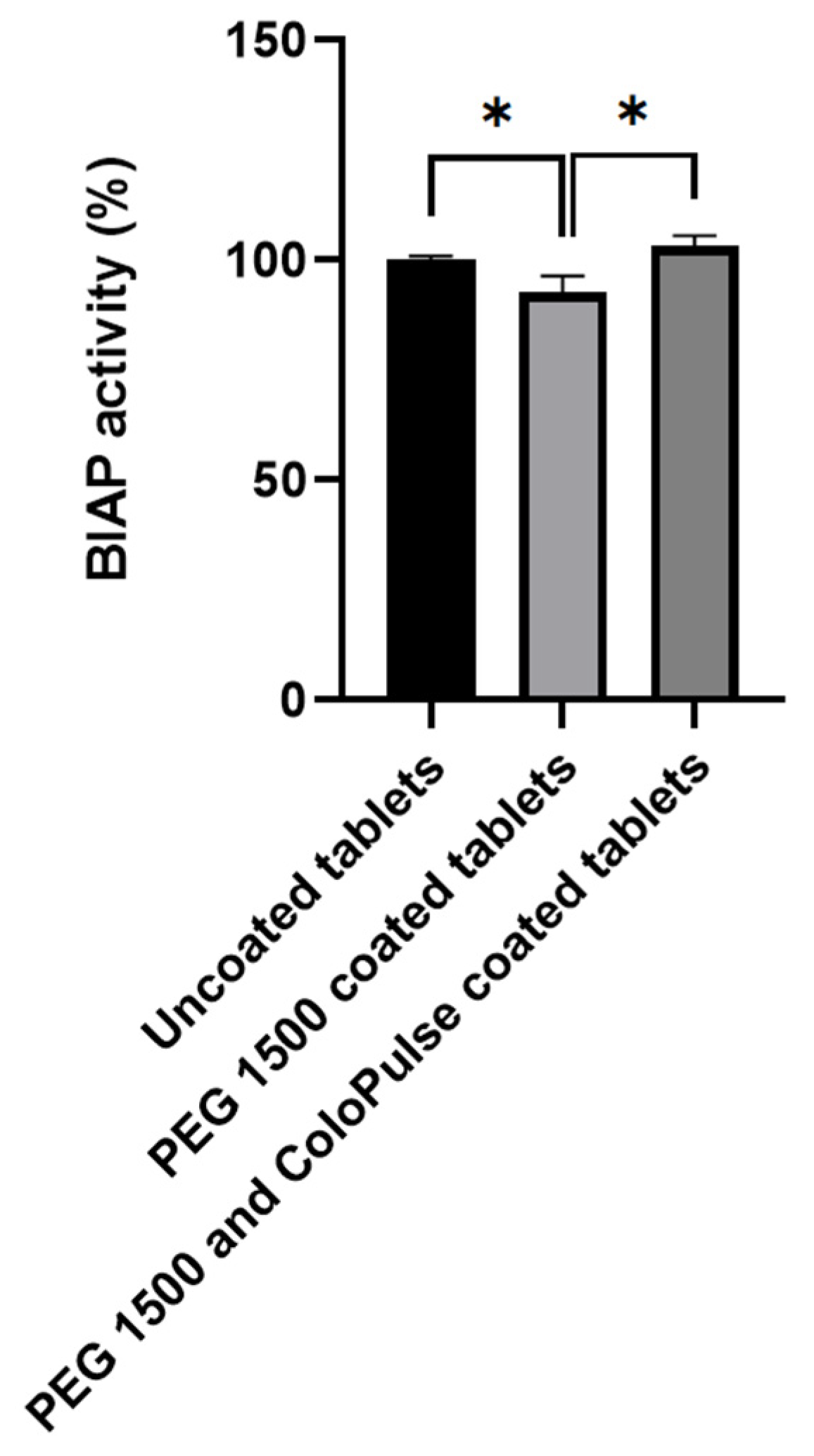
3.4. Enzymatic Activity of Alkaline Phosphatase
3.5. ColoPulse Coating and In Vitro Release Profile of the 3D Printed Tablets
3.5.1. Coating Process Optimization
3.5.2. In Vitro Release of Methylene Blue from ColoPulse-Coated 3D Printed Tablets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seoane-Viaño, I.; Trenfield, S.J.; Basit, A.W.; Goyanes, A. Translating 3D printed pharmaceuticals: From hype to real-world clinical applications. Adv. Drug Deliv. Rev. 2021, 174, 553–575. [Google Scholar] [CrossRef]
- Goole, J.; Amighi, K. 3D printing in pharmaceutics: A new tool for designing customized drug delivery systems. Int. J. Pharm. 2016, 499, 376–394. [Google Scholar] [CrossRef]
- Ali, S.F.B.; Mohamed, E.M.; Ozkan, T.; Kuttolamadom, M.A.; Khan, M.A.; Asadi, A.; Rahman, Z. Understanding the effects of formulation and process variables on the printlets quality manufactured by selective laser sintering 3D printing. Int. J. Pharm. 2019, 570, 118651. [Google Scholar]
- Zhou, Z.; Buchanan, F.; Mitchell, C.; Dunne, N. Printability of calcium phosphate: Calcium sulfate powders for the application of tissue engineered bone scaffolds using the 3D printing technique. Mater. Sci. Eng. C 2014, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Hiser, M.; Wu, W. Effect of particle size on three dimensional printed mesh structures. J. Powder Technol. 2009, 192, 178–183. [Google Scholar] [CrossRef]
- Turker, M.; Godlinski, D.; Petzoldt, F. Effect of production parameters on the properties of IN 718 superalloy by three-dimensional printing. Mater. Charact. 2008, 59, 1728–1735. [Google Scholar] [CrossRef]
- Buanz, A.; Saunders, M.H.; Basit, A.W.; Gaisford, S. Preparation of personalized-dose salbutamol sulphate oral films with thermal ink-jet printing. Pharm. Res. 2011, 28, 2386–2392. [Google Scholar] [CrossRef]
- Kolakovic, R.; Viitala, T.; Ihalainen, P.; Genina, N.; Peltonen, J.; Sandler, N. Printing technologies in fabrication of drug delivery systems. Expert Opin. Drug Deliv. 2013, 10, 1711–1723. [Google Scholar] [CrossRef]
- Infanger, S.; Haemmerli, A.; Iliev, S.; Baier, A.; Stoyanov, E.; Quodbach, J. Powder bed 3D-printing of highly loaded drug delivery devices with hydroxypropyl cellulose as solid binder. Int. J. Pharm. 2019, 555, 198–206. [Google Scholar] [CrossRef]
- Evans, S.E.; Harrington, T.; Rivero, M.C.R.; Rognin, E.; Tuladhar, T.; Daly, R. 2D and 3D inkjet printing of biopharmaceuticals–A review of trends and future perspectives in research and manufacturing. Int. J. Pharm. 2021, 599, 120443. [Google Scholar] [CrossRef] [PubMed]
- Tuin, A.; Poelstra, K.; de Jager-Krikken, A.; Bok, L.; Raaben, W.; Velders, M.P.; Dijkstra, G. Role of alkaline phosphatase in colitis in man and rats. Gut 2009, 58, 379–387. [Google Scholar] [CrossRef]
- Gareb, B.; Beugeling, M.; Posthumus, S.; Otten, A.T.; Dijkstra, G.; Kosterink, J.G.; Frijlink, H.W. Infliximab formulation strategy for a stable ileo-colonic targeted oral dosage form intended for the topical treatment of inflammatory bowel disease. J. Drug Deliv. Sci. Technol. 2021, 64, 102552. [Google Scholar] [CrossRef]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Molnár, K.; Vannay, Á.; Szebeni, B.; Bánki, N.F.; Sziksz, E.; Cseh, Á.; Győrffy, H.; Lakatos, P.L.; Papp, M.; Arató, A. Intestinal alkaline phosphatase in the colonic mucosa of children with inflammatory bowel disease. World J. Gastroenterol. 2012, 18, 3254. [Google Scholar] [PubMed]
- Lukas, M.; Drastich, P.; Konecny, M.; Gionchetti, P.; Urban, O.; Cantoni, F.; Bortlik, M.; Duricova, D.; Bulitta, M. Exogenous alkaline phosphatase for the treatment of patients with moderate to severe ulcerative colitis. Inflamm. Bowel Dis. 2010, 16, 1180–1186. [Google Scholar] [CrossRef]
- Schellekens, R.; Stellaard, F.; Mitrovic, D.; Stuurman, F.; Kosterink, J.; Frijlink, H. Pulsatile drug delivery to ileo-colonic segments by structured incorporation of disintegrants in pH-responsive polymer coatings. JCR 2008, 132, 91–98. [Google Scholar] [CrossRef]
- Gareb, B.; Posthumus, S.; Beugeling, M.; Koopmans, P.; Touw, D.J.; Dijkstra, G.; Kosterink, J.G.; Frijlink, H.W. Towards the oral treatment of ileo-colonic inflammatory bowel disease with Infliximab tablets: Development and validation of the production process. Pharmaceutics 2019, 11, 428. [Google Scholar] [CrossRef]
- Schellekens, R.C.; Baltink, J.H.; Woesthuis, E.M.; Stellaard, F.; Kosterink, J.G.; Woerdenbag, H.J.; Frijlink, H.W. Film coated tablets (ColoPulse technology) for targeted delivery in the lower intestinal tract: Influence of the core composition on release characteristics. Pharm. Dev. Technol. 2012, 17, 40–47. [Google Scholar] [CrossRef]
- Maurer, M.J.; Schellekens, R.C.; Wutzke, K.D.; Dijkstra, G.; Woerdenbag, H.J.; Frijlink, H.W.; Kosterink, J.G.; Stellaard, F. A non-invasive, low-cost study design to determine the release profile of colon drug delivery systems: A feasibility study. Pharm. Res. 2012, 29, 2070–2078. [Google Scholar] [CrossRef]
- Schellekens, R.; Stellaard, F.; Olsder, G.; Woerdenbag, H.; Frijlink, H.; Kosterink, J. Oral ileocolonic drug delivery by the colopulse-system: A bioavailability study in healthy volunteers. JCR 2010, 146, 334–340. [Google Scholar] [CrossRef]
- Schellekens, R.; Olsder, G.; Langenberg, S.; Boer, T.; Woerdenbag, H.; Frijlink, H.; Kosterink, J.; Stellaard, F. Proof-of-concept study on the suitability of 13C-urea as a marker substance for assessment of in vivo behaviour of oral colon-targeted dosage forms. Br. J. Pharmacol. 2009, 158, 532–540. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Broesder, A.; Woerdenbag, H.J.; Prins, G.H.; Nguyen, D.N.; Frijlink, H.W.; Hinrichs, W.L. pH-dependent ileocolonic drug delivery, part I: In vitro and clinical evaluation of novel systems. Drug Discov. Today 2020, 25, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Schneider, F.; Grimm, M.; Koziolek, M.; Modeß, C.; Dokter, A.; Roustom, T.; Siegmund, W.; Weitschies, W. Resolving the physiological conditions in bioavailability and bioequivalence studies: Comparison of fasted and fed state. Eur. J. Pharm. Biopharm. 2016, 108, 214–219. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Becker, D.; Iordanov, V.; Zou, H.; Shimizu, J.; Wanke, C.; Garbacz, G.; Weitschies, W. Investigation of pH and temperature profiles in the GI tract of fasted human subjects using the Intellicap® system. J. Pharm. Sci. 2015, 104, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Fallingborg, J.; Christensen, L.; Ingeman-Nielsen, M.; Jacobsen, B.; Abildgaard, K.; Rasmussen, H. pH-profile and regional transit times of the normal gut measured by a radiotelemetry device. Aliment. Pharmacol. Ther. 1989, 3, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Mikolajczyk, A.E.; Watson, S.; Surma, B.L.; Rubin, D.T. Assessment of tandem measurements of pH and total gut transit time in healthy volunteers. J. Clin. Gastroenterol. 2015, 6, e100. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, W.; Prinsen, M.; Frijlink, H. Inulin glasses for the stabilization of therapeutic proteins. Int. J. Pharm. 2001, 215, 163–174. [Google Scholar] [CrossRef]
- van den Heuvel, K.A.; de Wit, M.T.; Dickhoff, B.H. Evaluation of lactose based 3D powder bed printed pharmaceutical drug product tablets. Powder Technol. 2021, 390, 97–102. [Google Scholar] [CrossRef]
- Beugeling, M.; Grasmeijer, N.; Born, P.A.; van der Meulen, M.; van der Kooij, R.S.; Schwengle, K.; Baert, L.; Amssoms, K.; Frijlink, H.W.; Hinrichs, W.L. The mechanism behind the biphasic pulsatile drug release from physically mixed poly (dl-lactic (-co-glycolic) acid)-based compacts. Int. J. Pharm. 2018, 551, 195–202. [Google Scholar] [CrossRef]
- Dedroog, S.; Boel, E.; Kindts, C.; Appeltans, B.; Van den Mooter, G. The underestimated contribution of the solvent to the phase behavior of highly drug loaded amorphous solid dispersions. Int. J. Pharm. 2021, 609, 121201. [Google Scholar] [CrossRef] [PubMed]
- Grasmeijer, N.; Stankovic, M.; de Waard, H.; Frijlink, H.W.; Hinrichs, W.L. Unraveling protein stabilization mechanisms: Vitrification and water replacement in a glass transition temperature controlled system. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2013, 1834, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Schellekens, R.; Stuurman, F.; Van der Weert, F.; Kosterink, J.; Frijlink, H. A novel dissolution method relevant to intestinal release behaviour and its application in the evaluation of modified release mesalazine products. Eur. J. Pharm. Sci. 2007, 30, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide I: Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Mensink, M.A.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohydr. Polym. 2015, 134, 418–428. [Google Scholar] [CrossRef]
- Amorij, J.-P.; Saluja, V.; Petersen, A.H.; Hinrichs, W.L.; Huckriede, A.; Frijlink, H.W. Pulmonary delivery of an inulin-stabilized influenza subunit vaccine prepared by spray-freeze drying induces systemic, mucosal humoral as well as cell-mediated immune responses in BALB/c mice. Vaccine 2007, 25, 8707–8717. [Google Scholar] [CrossRef]
- Wilts, E.M.; Ma, D.; Bai, Y.; Williams, C.B.; Long, T.E. Comparison of linear and 4-arm star poly (vinyl pyrrolidone) for aqueous binder jetting additive manufacturing of personalized dosage tablets. ACS Appl. Mater. Interfaces 2019, 11, 23938–23947. [Google Scholar] [CrossRef]
- Ziaee, M.; Crane, N.B. Binder jetting: A review of process, materials, and methods. Addit. Manuf. 2019, 28, 781–801. [Google Scholar] [CrossRef]
- Sen, K.; Manchanda, A.; Mehta, T.; Ma, A.W.; Chaudhuri, B. Formulation design for inkjet-based 3D printed tablets. Int. J. Pharm. 2020, 584, 119430. [Google Scholar] [CrossRef]
- Yu, D.-G.; Branford-White, C.; Ma, Z.-H.; Zhu, L.-M.; Li, X.-Y.; Yang, X.-L. Novel drug delivery devices for providing linear release profiles fabricated by 3DP. Int. J. Pharm. 2009, 370, 160–166. [Google Scholar] [CrossRef]
- LEWIS, W.E.P.; Rowe, C.W.; Cima, M.J.; Materna, P.A. System and Method for Uniaxial Compression of an Article, Such as a Three-Dimensionally Printed Dosage Form. U.S. Patent 7,931,914, 26 April 2011. [Google Scholar]
- Wang, C.-C.; Tejwani, M.R.; Roach, W.J.; Kay, J.L.; Yoo, J.; Surprenant, H.L.; Monkhouse, D.C.; Pryor, T. Development of near zero-order release dosage forms using three-dimensional printing (3-DP™) technology. Drug Dev. Ind. Pharm 2006, 32, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Katstra, W.; Palazzolo, R.; Rowe, C.; Giritlioglu, B.; Teung, P.; Cima, M. Oral dosage forms fabricated by Three Dimensional PrintingTM. JCR 2000, 66, 1–9. [Google Scholar] [CrossRef]
- Rowe, C.W.; Wang, C.-C.; Monkhouse, D.C. TheriForm technology. In Modified-Release Drug Delivery Technology; CRC Press: Boca Raton, FL, USA, 2002; pp. 101–112. [Google Scholar]
- Goyanes, A.; Chang, H.; Sedough, D.; Hatton, G.B.; Wang, J.; Buanz, A.; Gaisford, S.; Basit, A.W. Fabrication of controlled-release budesonide tablets via desktop (FDM) 3D printing. Int. J. Pharm. 2015, 496, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Press, A.; Hauptmann, I.; Hauptmann, L.; Fuchs, B.; Fuchs, M.; Ewe, K.; Ramadori, G. Gastrointestinal pH profiles in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 1998, 12, 673–678. [Google Scholar] [CrossRef]
- Ewe, K.; Schwartz, S.; Petersen, S.; Press, A.G. Inflammation does not decrease intraluminal pH in chronic inflammatory bowel disease. Dig. Dis. Sci. 1999, 44, 1434–1439. [Google Scholar] [CrossRef]
- Gareb, B.; Dijkstra, G.; Kosterink, J.G.; Frijlink, H.W. Development of novel zero-order release budesonide tablets for the treatment of ileo-colonic inflammatory bowel disease and comparison with formulations currently used in clinical practice. Int. J. Pharm. 2019, 554, 366–375. [Google Scholar]
- Broesder, A.; Berends, J.M.; Scheepers, S.M.; Nguyen, D.N.; Frijlink, H.W.; Hinrichs, W.L. Ileo-Colon Targeting of the Poorly Water-Soluble Drug Celecoxib Using a pH-Dependent Coating in Combination with Self-Emulsifying Drug Delivery or Solid Dispersion Systems. Pharmaceutics 2021, 13, 731. [Google Scholar] [CrossRef]
- Mensink, M.A.; Van Bockstal, P.-J.; Pieters, S.; De Meyer, L.; Frijlink, H.W.; van der Voort Maarschalk, K.; Hinrichs, W.L.; De Beer, T. In-line near infrared spectroscopy during freeze-drying as a tool to measure efficiency of hydrogen bond formation between protein and sugar, predictive of protein storage stability. Int. J. Pharm. 2015, 496, 792–800. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Brown, M.B.; Quader, A.; Martin, G.P. Protective mechanism of stabilizing excipients against dehydration in the freeze-drying of proteins. Pharm. Res. 2002, 19, 1854–1861. [Google Scholar] [CrossRef]
- Chang, L.L.; Shepherd, D.; Sun, J.; Ouellette, D.; Grant, K.L.; Tang, X.C.; Pikal, M.J. Mechanism of protein stabilization by sugars during freeze-drying and storage: Native structure preservation, specific interaction, and/or immobilization in a glassy matrix? J. Pharm. Sci. 2005, 94, 1427–1444. [Google Scholar] [CrossRef]
- Massant, J.; Fleurime, S.; Batens, M.; Vanhaerents, H.; Van den Mooter, G. Formulating monoclonal antibodies as powders for reconstitution at high concentration using spray-drying: Trehalose/amino acid combinations as reconstitution time reducing and stability improving formulations. Eur. J. Pharm. Biopharm. 2020, 156, 131–142. [Google Scholar] [CrossRef] [PubMed]




| Phase | Segment Gastrointestinal Tract | pH | Volume (mL) | Time (h) |
|---|---|---|---|---|
| I | Stomach | 1.20 ± 0.20 | 500 | 2.0 |
| II | Jejunum | 6.80 ± 0.20 | 629 | 2.0 |
| III | Terminal ileum | 7.63 ± 0.12 | 940 | 0.5 |
| IV | Colon | 6.00 ± 0.25 | 1000 | 1.5 |
| Composition | Time Added to the Dissolution Vessel (h) | |
|---|---|---|
| Phase I | 1.00 g sodium chloride, 3.5 mL concentrated hydrochloric acid, add demineralized water to 500 ml | 0 |
| Phase I to phase II | 4.08 potassium dihydrogen phosphate, 30 mL sodium hydroxide 2.0 M (80 g/L), add demineralized water to 129 mL | 2.0 |
| Phase II to phase III | 2.04 g potassium dihydrogen phosphate, 12.0 mL sodium hydroxide 2.0 M (80 g/L), add demineralized water to 311 mL | 4.0 |
| Phase III to phase IV | 9 mL hydrochloric acid 3.0 M, add demineralized water of 60 mL | 4.5 |
| Binder | LS (mm) | Binder:Mannitol Ratio (w:w) | ||
|---|---|---|---|---|
| PVP K30 | 0.40 | 2:8 | 5:5 | 8:2 |
| 0.45 | 2:8 | 5:5 | 8:2 | |
| 0.50 | 2:8 | 5:5 | 8:2 | |
| HPC-SLFP | 0.45 | 2:8 | 5:5 | 8:2 |
| 0.50 | 2:8 | 5:5 | 8:2 | |
| 0.55 | 2:8 | 5:5 | 8:2 | |
| Kollidon VA64 | 0.40 | 2:8 | 8:2 | |
| 0.45 | 2:8 | 8:2 | ||
| 0.50 | 2:8 | 8:2 | ||
| Eudragit L100 | 0.35 | 2:8 | 5:5 | 8:2 |
| 0.40 | 2:8 | 5:5 | 8:2 | |
| 0.45 | 2:8 | 8:2 | ||
| HPC-LFP | 0.50 | 2:8 | 5:5 | 8:2 |
| 0.55 | 2:8 | 5:5 | 8:2 | |
| 0.60 | 2:8 | 5:5 | 8:2 | |
| 0.40 mm LS | 0.45 mm LS | 0.50 mm LS | |
|---|---|---|---|
| Average weight (mg) | 132.1 ± 7.4 | 115.9 ± 5.7 | 106.7 ± 5.5 |
| Diameter (mm) | 8.71 ± 0.09 | 8.66 ± 0.07 | 8.62 ± 0.02 |
| Thickness (mm) | 3.76 ± 0.21 | 3.46 ± 0.17 | 3.35 ± 0.22 |
| Crushing strength (N) | 67.0 ± 14.0 | 64.3 ± 4.6 | 39.0 ± 7.0 ∇ |
| Friability (percentage weight loss) | 2.58 ± 0.51 † | 1.19 ± 0.26 | 1.41 ± 0.39 ∇ |
| Disintegration time (minutes) | 12.26 ± 2.07 † | 5.95 ± 0.52 | 3.36 ± 0.95 ∇ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, K.T.T.; Heijningen, F.F.M.; Zillen, D.; van Bommel, K.J.C.; van Ee, R.J.; Frijlink, H.W.; Hinrichs, W.L.J. Formulation of a 3D Printed Biopharmaceutical: The Development of an Alkaline Phosphatase Containing Tablet with Ileo-Colonic Release Profile to Treat Ulcerative Colitis. Pharmaceutics 2022, 14, 2179. https://doi.org/10.3390/pharmaceutics14102179
Nguyen KTT, Heijningen FFM, Zillen D, van Bommel KJC, van Ee RJ, Frijlink HW, Hinrichs WLJ. Formulation of a 3D Printed Biopharmaceutical: The Development of an Alkaline Phosphatase Containing Tablet with Ileo-Colonic Release Profile to Treat Ulcerative Colitis. Pharmaceutics. 2022; 14(10):2179. https://doi.org/10.3390/pharmaceutics14102179
Chicago/Turabian StyleNguyen, Khanh T. T., Franca F. M. Heijningen, Daan Zillen, Kjeld J. C. van Bommel, Renz J. van Ee, Henderik W. Frijlink, and Wouter L. J. Hinrichs. 2022. "Formulation of a 3D Printed Biopharmaceutical: The Development of an Alkaline Phosphatase Containing Tablet with Ileo-Colonic Release Profile to Treat Ulcerative Colitis" Pharmaceutics 14, no. 10: 2179. https://doi.org/10.3390/pharmaceutics14102179
APA StyleNguyen, K. T. T., Heijningen, F. F. M., Zillen, D., van Bommel, K. J. C., van Ee, R. J., Frijlink, H. W., & Hinrichs, W. L. J. (2022). Formulation of a 3D Printed Biopharmaceutical: The Development of an Alkaline Phosphatase Containing Tablet with Ileo-Colonic Release Profile to Treat Ulcerative Colitis. Pharmaceutics, 14(10), 2179. https://doi.org/10.3390/pharmaceutics14102179








