The Effect of Humidity on the Dissolution Kinetics and Tablet Properties of Immediate-Release Tablet Formulation Containing Lamotrigine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Preparation of Tablets
2.4. Setting Conditions for High (75%) and Low (30%) Humidity
2.5. Evaluation of Flow Properties of Powder Blend
2.6. Characteristics of Tablets Evaluation
2.7. UV/Vis Spectrophotometry
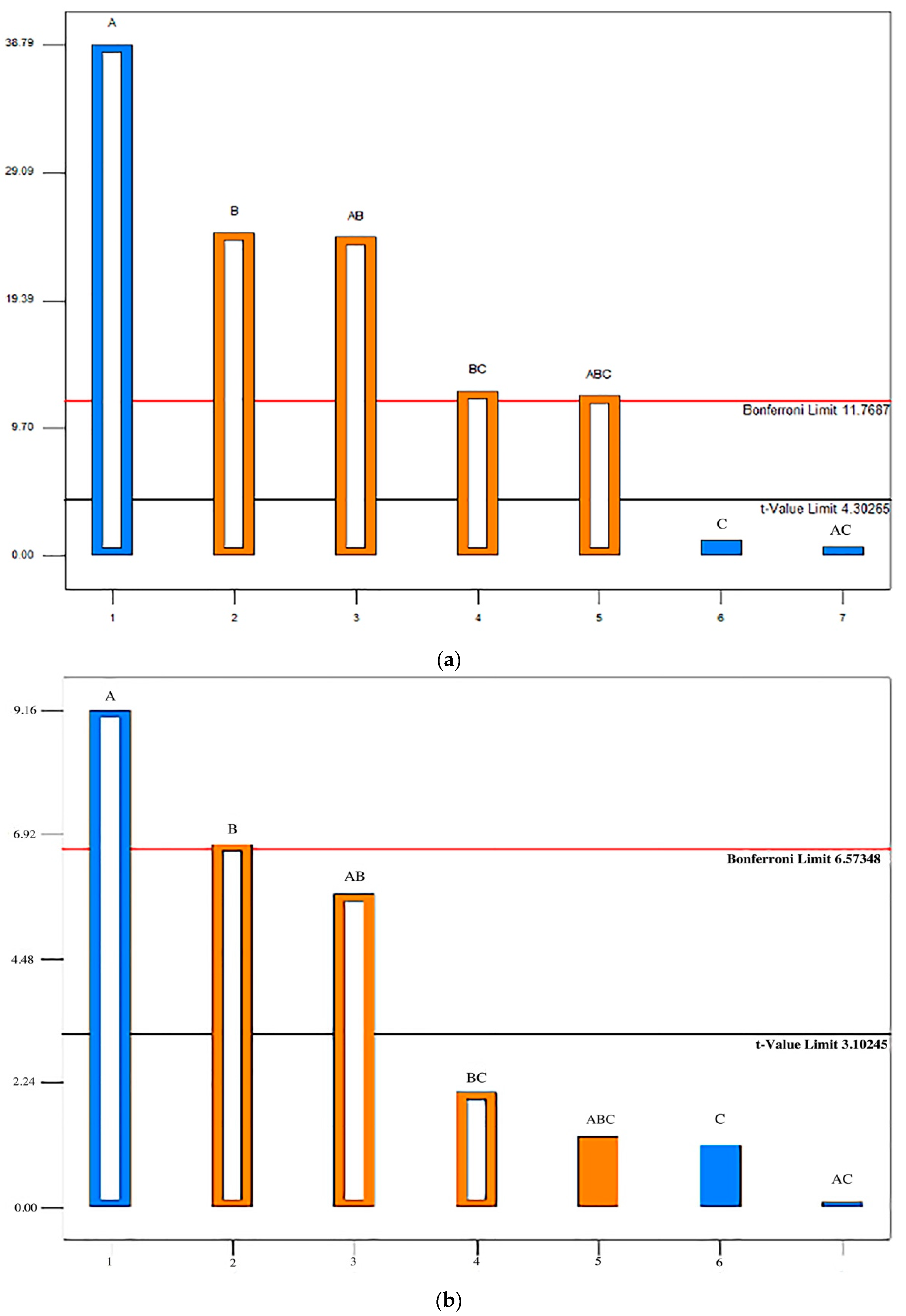
2.8. Statistical Analysis
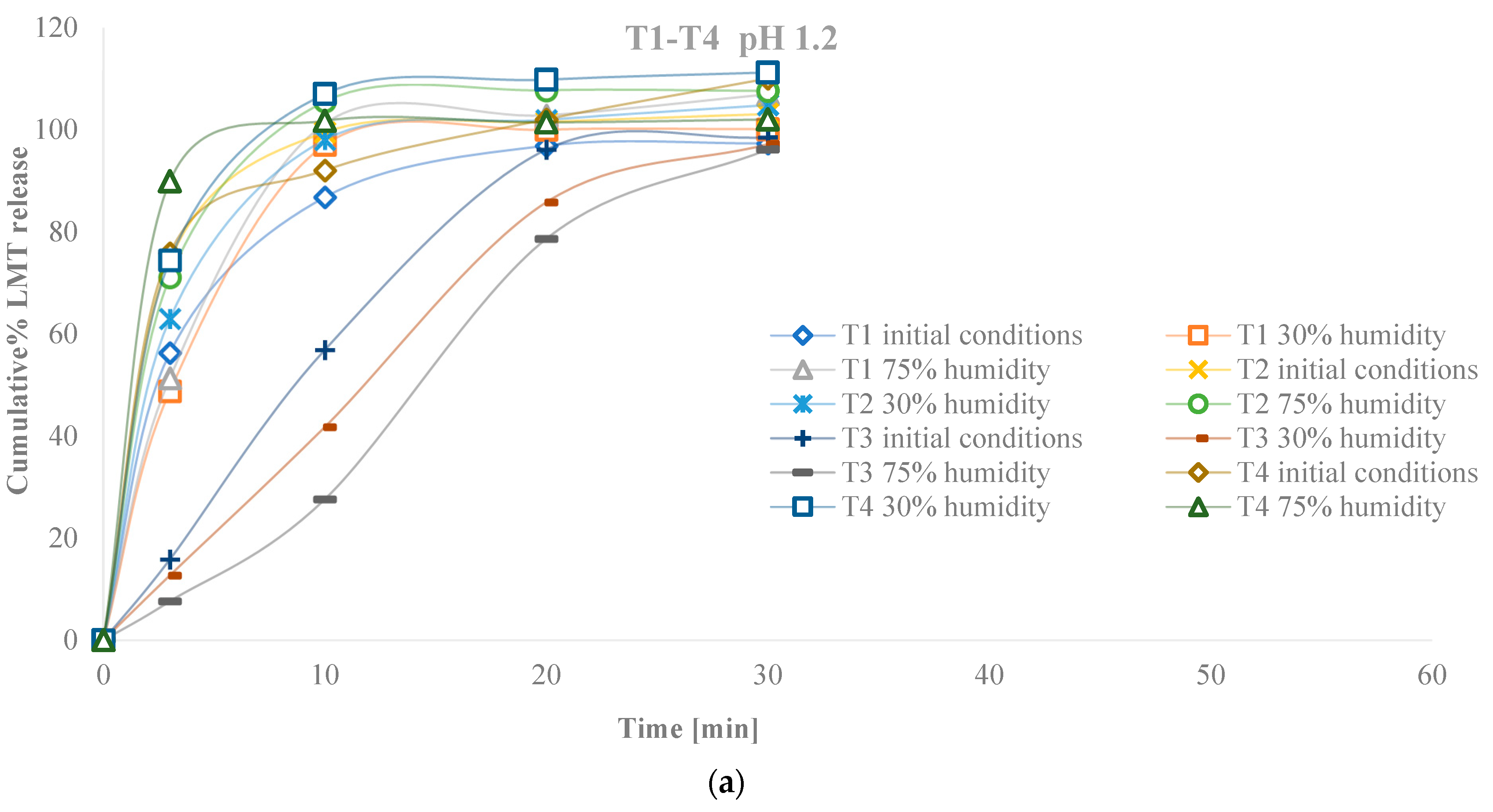
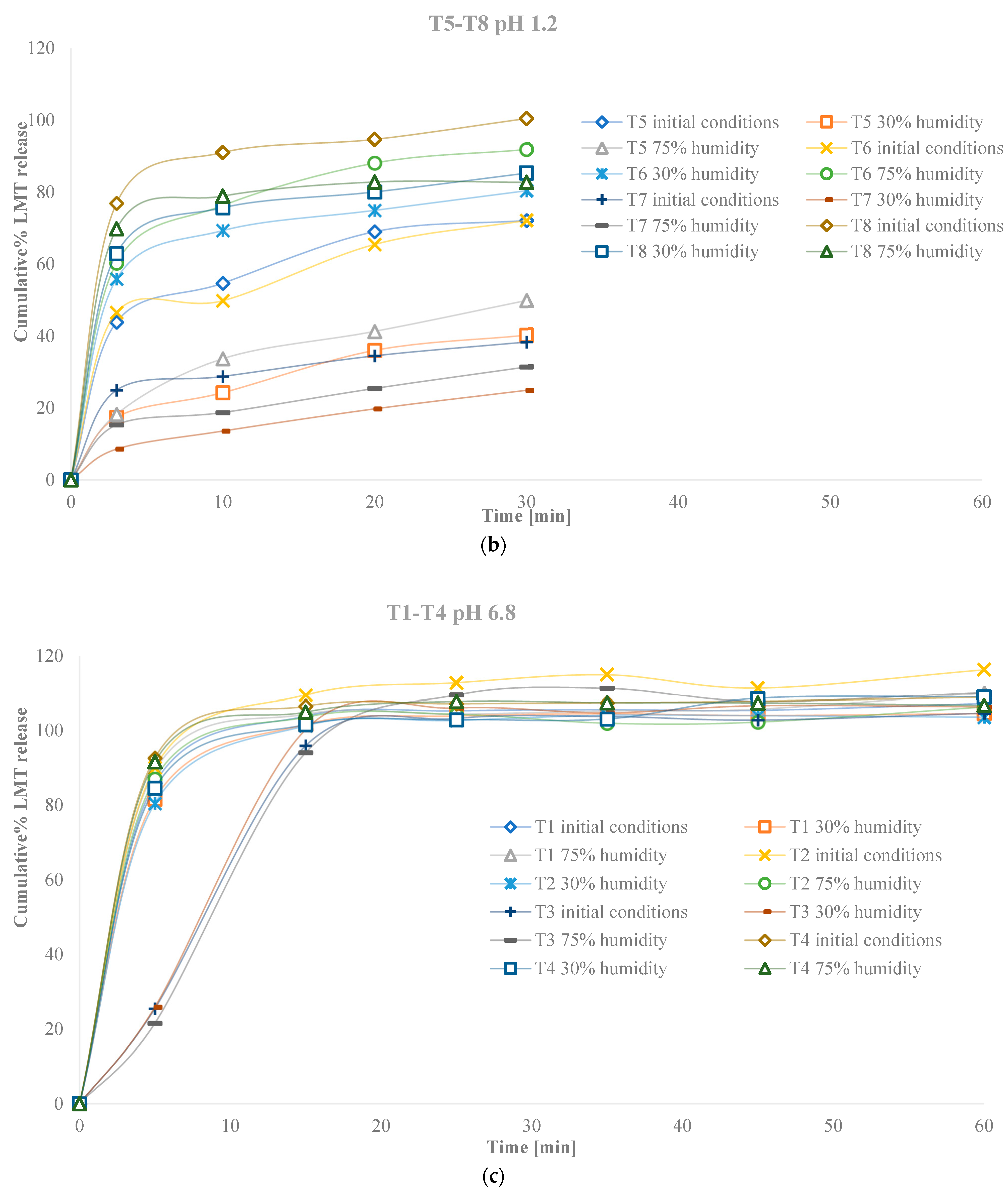
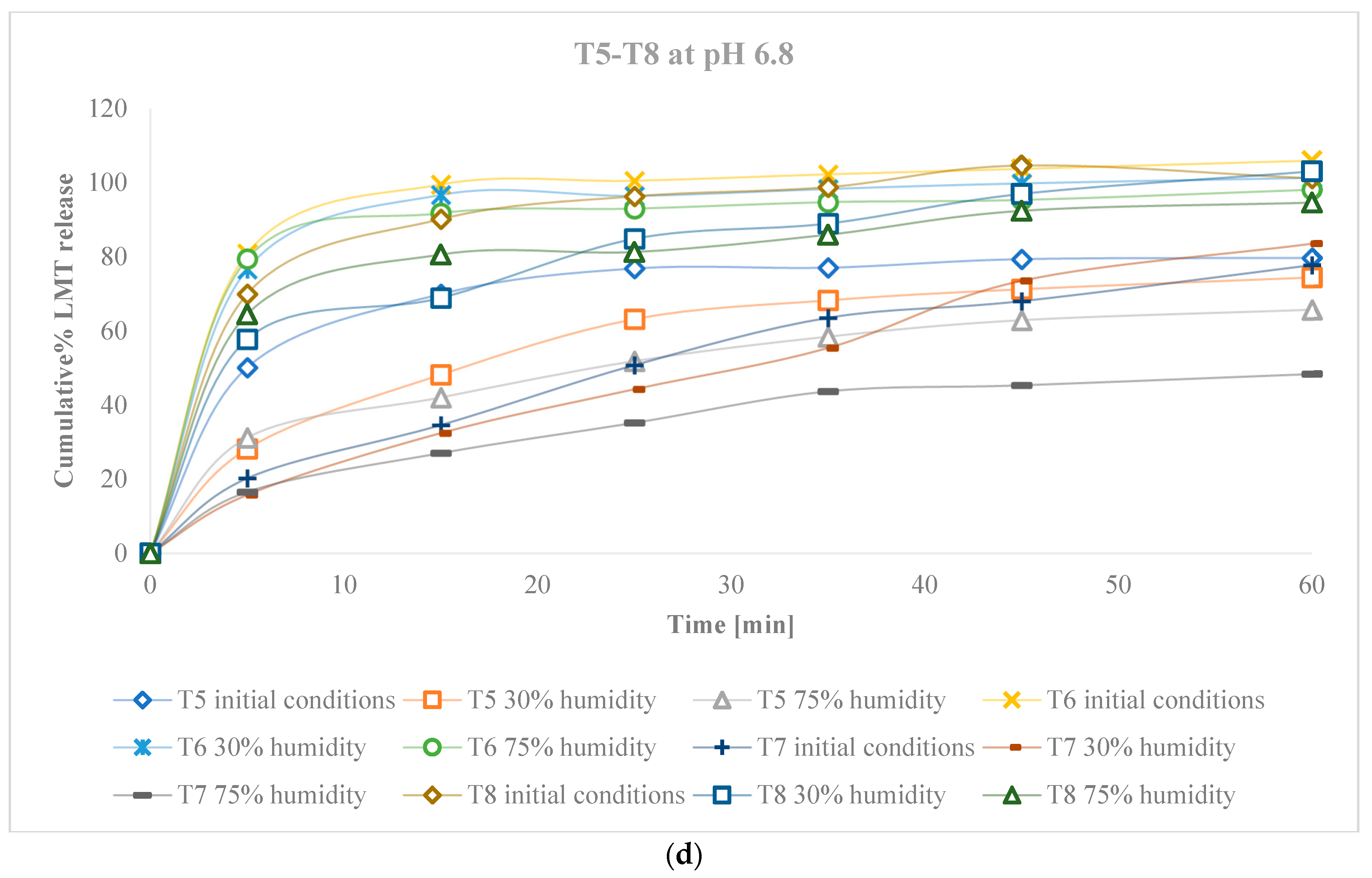
3. Results
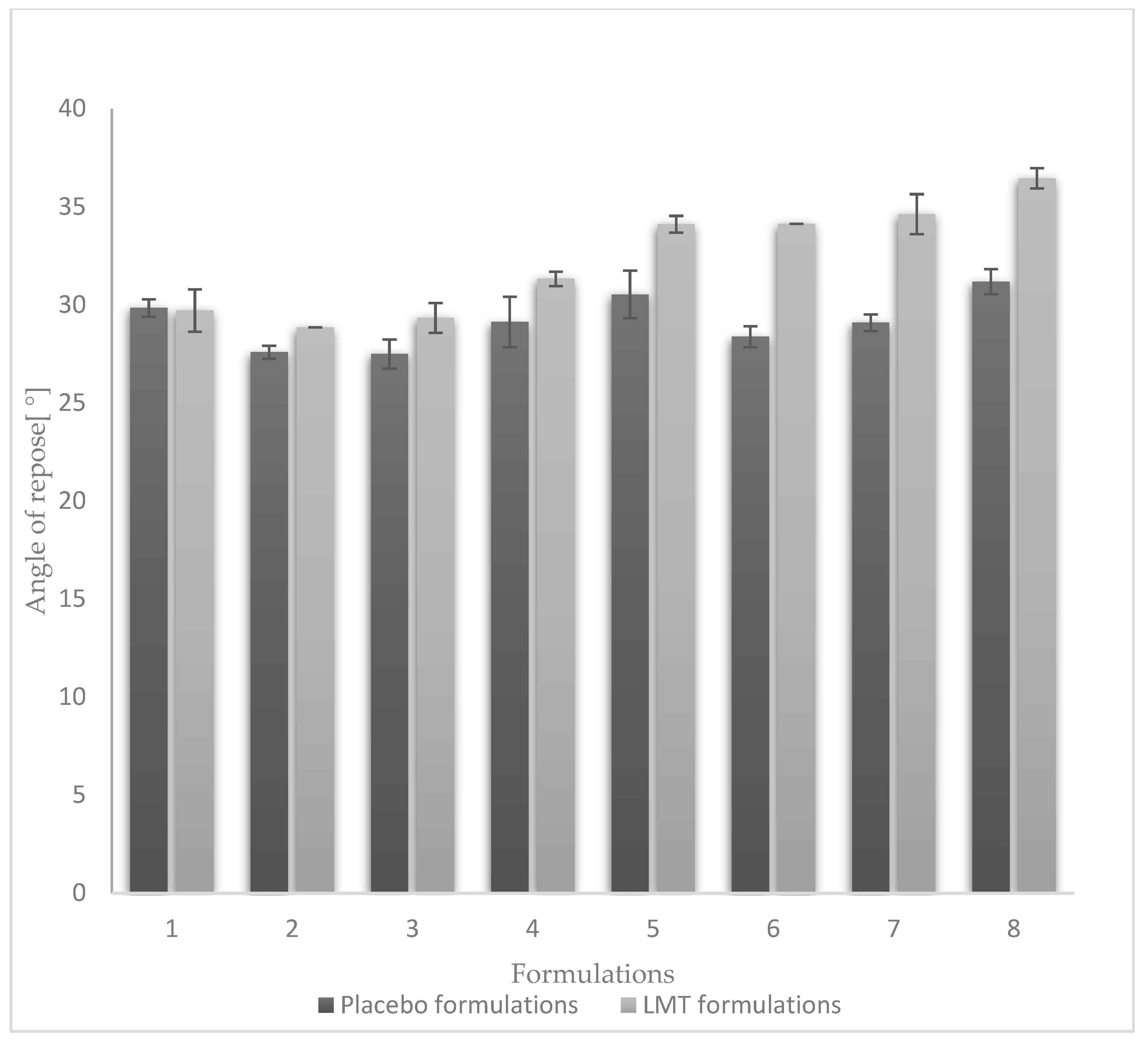
3.1. Powder Blend Flowability
3.2. Characteristics of Tablets
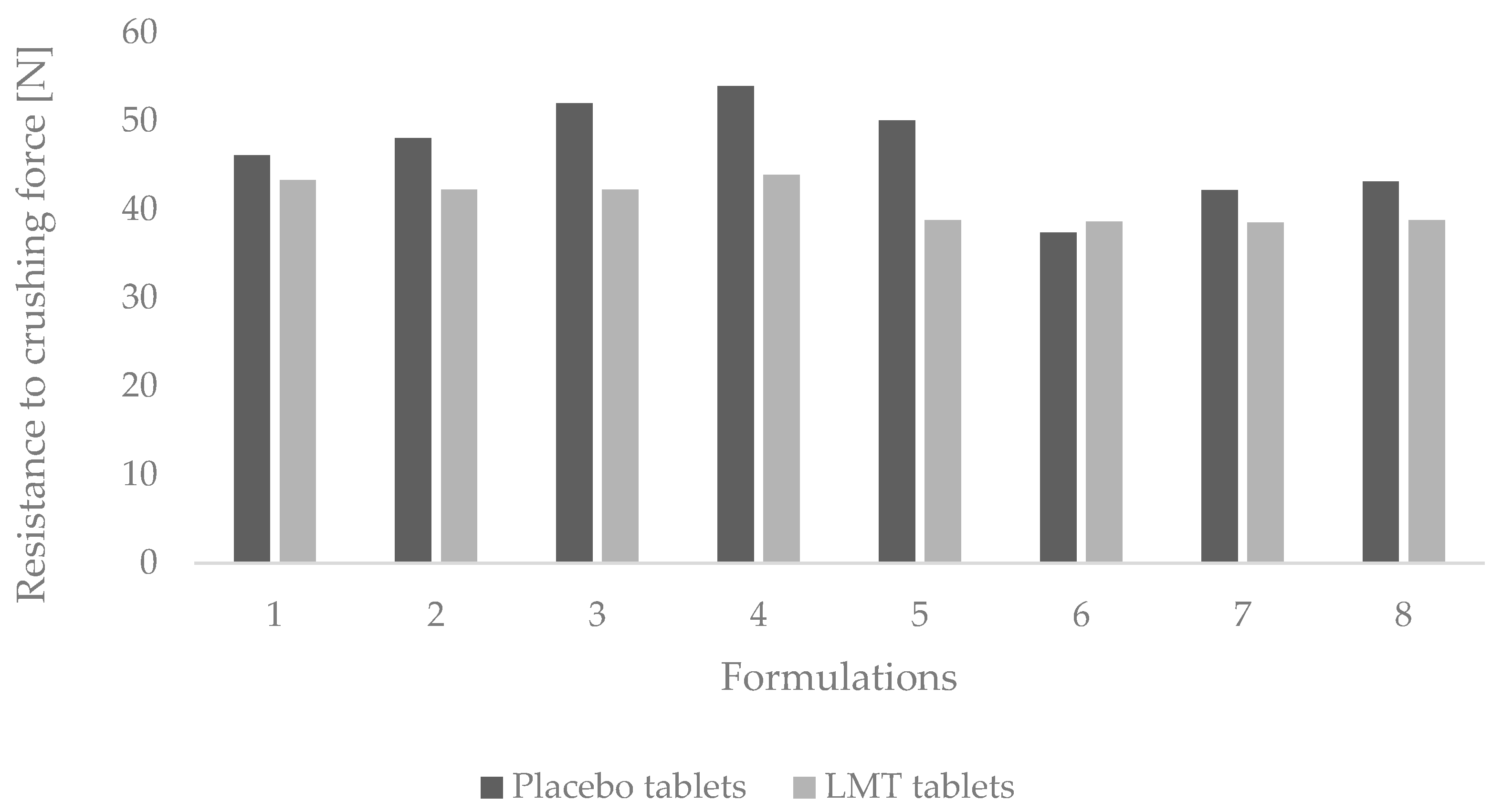
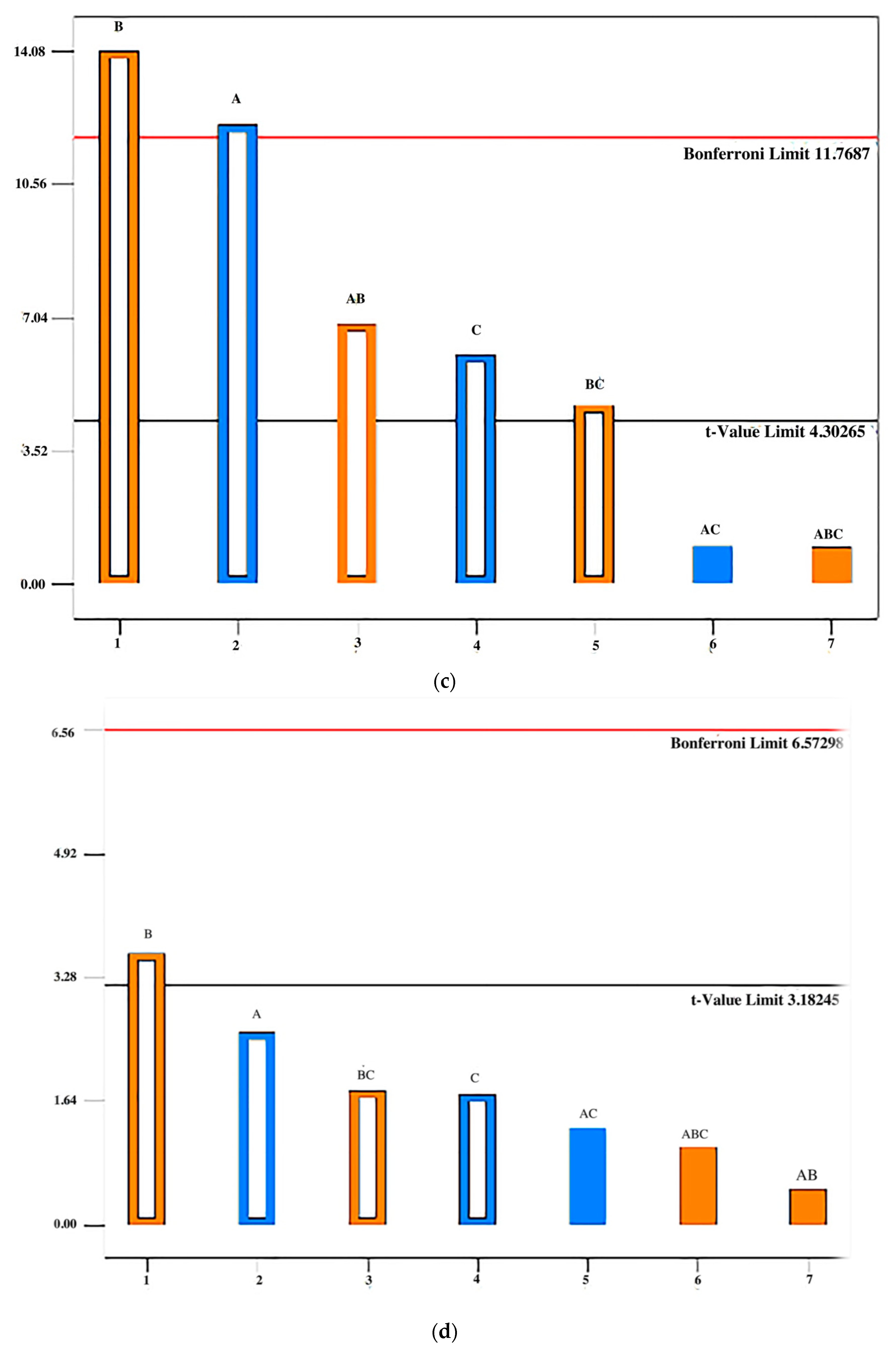
3.2.1. Resistance to Crushing of Tablets
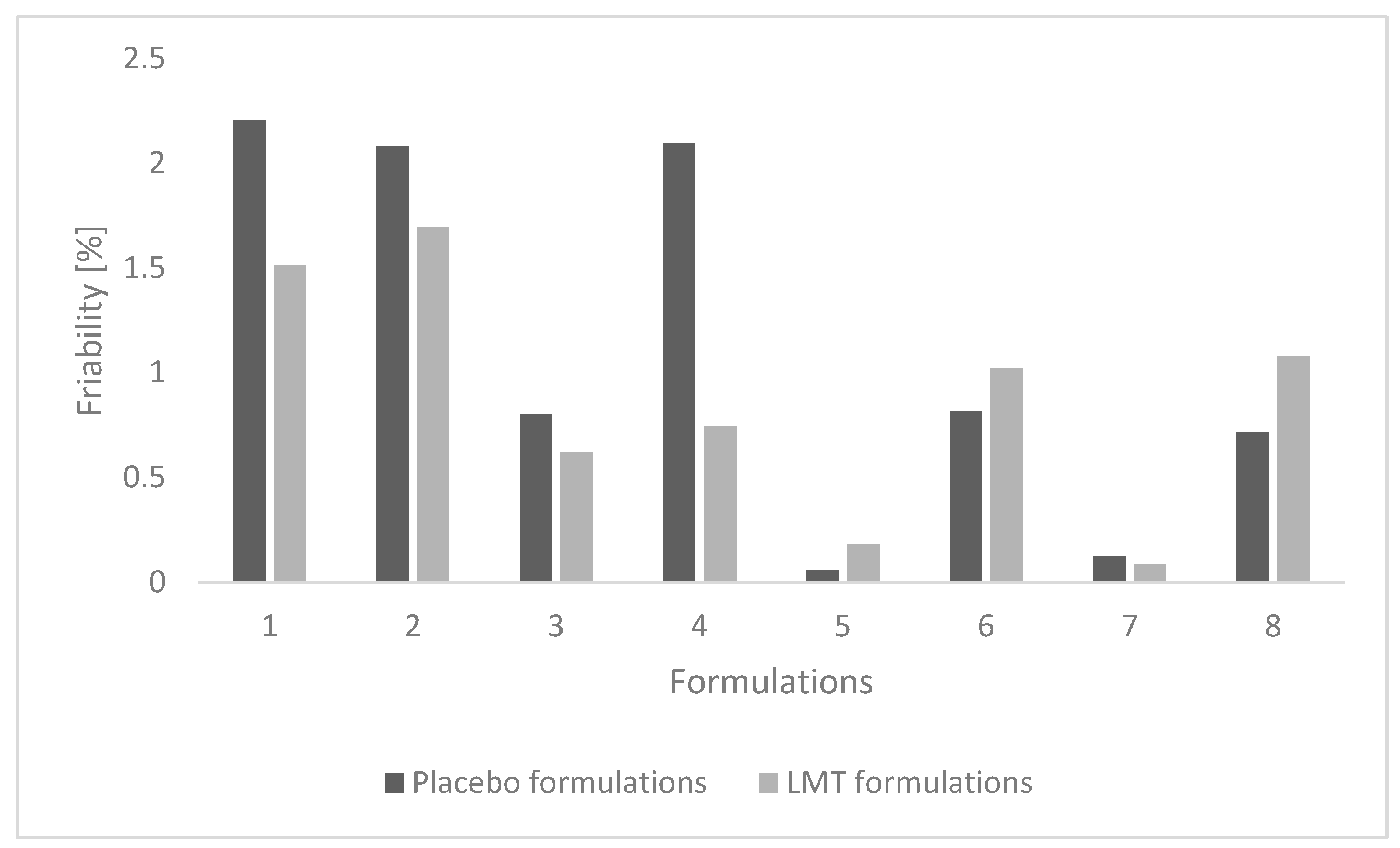
3.2.2. Friability
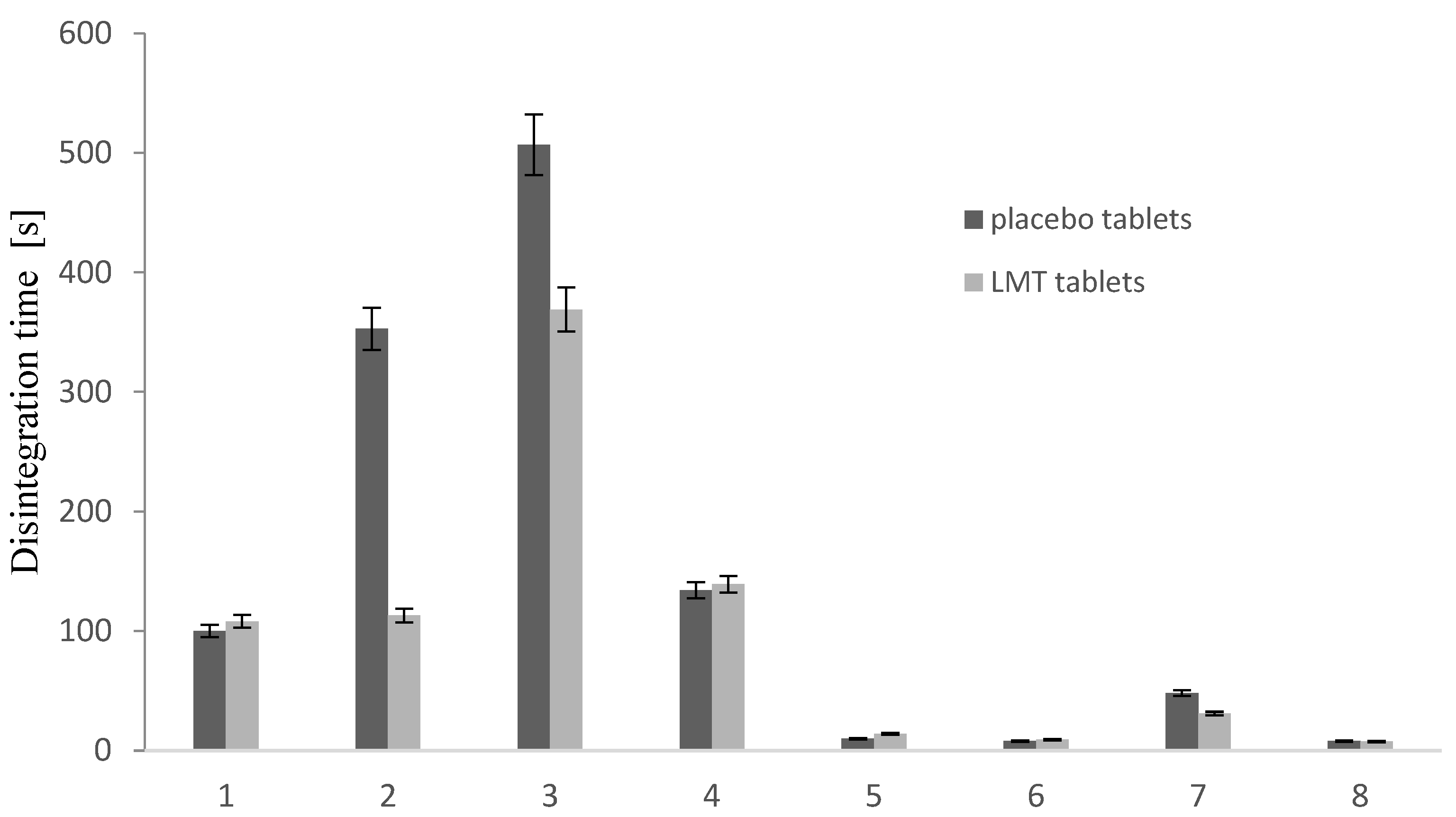
3.2.3. Disintegration Time
3.2.4. Characteristics of Tablets Stored in Conditions of Increased and Decreased Humidity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shang-Yang, L. Effect of excipients on tablet properties and dissolution behavior of theophylline tableted microcapsules under different compression forces. J. Pharm. Sci. 1988, 77, 229–232. [Google Scholar] [CrossRef]
- Shaw, L.R.; Irwin, W.J.; Grattan, T.J.; Conway, B.R. The effect of selected water-soluble excipients on the dissolution of paracetamol and ibuprofen. Drug Dev. Ind. Pharm. 2005, 31, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Mah, P.T.; Peltonen, L.; Novakovic, D.; Rades, T.; Strachan, C.J.; Laaksonen, T. The effect of surfactants on the dissolution behavior of amorphous formulations. Eur. J. Pharm. Biopharm. 2016, 103, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Vadlamudi, M.K.; Dhanaraj, S. Significance of excipients to enhance the bioavailability of poorly water-soluble drugs in oral solid dosage forms: A Review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 263, pp. 1–16. [Google Scholar] [CrossRef]
- Elliott, R.A. Appropriate use of dose administration aids. Aust. Prescr. 2014, 37, 46–50. [Google Scholar] [CrossRef]
- García, E.R.; Thalhauser, S.; Loscertales, H.R.; Modamio, P.; Lastra, C.F.; Mariño, E.L. Current evidence in the stability of 399 medicines in dose administration aids: Implications for patient safety. Expert Opin. Drug Deliv. 2018, 15, 577–587. [Google Scholar] [CrossRef]
- Haywood, A.; D Glass, B. Evidence of stability of medicines repackaged in compliance aids: A review. Curr. Drug Saf. 2016, 11, 69–77. [Google Scholar] [CrossRef]
- Vaithianathan, S.; Raman, S.; Jiang, W.; Ting, T.Y.; Kane, M.A.; Polli, J.E. Biopharmaceutic risk assessment of brand and generic lamotrigine tablets. Mol. Pharm. 2015, 12, 2436–2443. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.K.; Ginsburg, J.; Hickey, A.J.; Gheyas, F. Composite method to quantify powder flow as a screening method in early tablet or capsule formulation development. AAPS J. 2000, 1, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Hart, E.; Ji, Y. A novel approach for predicting the dissolution profiles of pharmaceutical tablets. Eur. J. Pharm. Biopharm. 2015, 96, 53–64. [Google Scholar] [CrossRef]
- Jivraj, M.; Martini, L.G.; Thomson, C.M. An overview of the different excipients useful for the direct compression of tablets. Pharm. Sci. Technol. 2000, 3, 58–63. [Google Scholar] [CrossRef]
- United States Pharmacopeial Convention, Inc. U.S. Pharmacopoeia-National Formulary (USP 29 NF 24); United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 2006. [Google Scholar]
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. J. Res. Natl. Bur. Stand. 1977, 81, 89–96. [Google Scholar] [CrossRef]
- Jagia, M.; Trivedi, M.; Dave, R.H. To evaluate the effect of solvents and different relative humidity conditions on thermal and rheological properties of microcrystalline cellulose 101 using METHOCEL™ E15LV as a binder. AAPS J. 2016, 17, 995–1006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- World Health Organization. Technical Report Series No. 953. Annex 2: Stability Testing of Active Pharmaceutical Ingredients and Finished Pharmaceutical Products. 2009. Available online: http://www.rsc.org/suppdata/c5/ra/c5ra07716h/c5ra07716h1.pdf (accessed on 21 July 2022).
- ICH Harmonsied Tripartite Guideline Stability Testing: Stability Testing of New Drug Substances and Products Q1A(R2). International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). Available online: https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf (accessed on 21 July 2022).
- Maclean, N.; Khadra, I.; Mann, J.; Williams, H.; Abbott, A.; Mead, H.; Markl, D. Investigating the role of excipients on the physical stability of directly compressed tablets. Int. J. Pharm. 2022, 4, 100106. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 10th ed.; European Directorate for the Quality of Medicines & Healthcare; Council of Europe: Strasbourg, France, 2019.
- Martins, M.T.; Paim, C.S.; Steppe, M. Development of a dissolution test for lamotrigine in tablet form using an ultraviolet method. Braz. J. Pharm. Sci. 2010, 46, 179–186. [Google Scholar] [CrossRef]
- Mohan, A.; Ghosh, S. New Method for Spectrophotometric Estimation of New Anti Epileptic Drugs in Solid Dosage Form. AJRC. 2009, 2, 322–324. [Google Scholar]
- Sharma, R.; Setia, G. Mechanical dry particle coating on cohesive pharmaceutical powders for improving flowability—A review. Powder Technol. 2019, 356, 458–479. [Google Scholar] [CrossRef]
- Faqih, A.M.N.; Mehrotra, A.; Hammond, S.V.; Muzzio, F.J. Effect of humidity and magnesium stearate concentration on flow properties of cohesive granular materials. Int. J. Pharm. 2007, 336, 338–345. [Google Scholar] [CrossRef]
- Guchardi, R.; Frei, M.; John, E.; Kaerger, J. Influence of fine lactose and magnesium stearate on low dose dry powder inhaler formulations. Int. J. Pharm. 2008, 348, 10–17. [Google Scholar] [CrossRef]
- Reier, G.E.; Shangraw, R.F. Microcrystalline cellulose in tableting. J. Pharm. Sci. 1966, 55, 510–514. [Google Scholar] [CrossRef]
- Carpin, M.; Bertelsen, H.; Bech, J.; Jeantet, R.; Risbo, J.; Schuck, P. Caking of lactose: A critical review. Trends Food Sci. Technol. 2016, 53, 1–12. [Google Scholar] [CrossRef]
- John, C.T.; Xu, W.; Lupton, L.K.; Harmon, P.A. Formulating weakly basic HCl salts: Relative ability of common excipients to induce disproportionation and the unique deleterious effects of magnesium stearate. Pharm. Res. 2013, 30, 1628–1641. [Google Scholar] [CrossRef] [PubMed]
- Guerrieri, P.; Taylor, L.S. Role of salt and excipient properties on disproportionation in the solid-state. Pharm. Res. 2009, 26, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Xu, W.; Ren, J.; Taylor, L.S.; Marsac, P.J.; John, C.T.; Byrn, S.R. Impact of metallic stearates on disproportionation of hydrochloride salts of weak bases in solid-state formulations. Mol. Pharm. 2016, 13, 3541–3552. [Google Scholar] [CrossRef]
- Merritt, J.M.; Viswanath, S.K.; Stephenson, G.A. Implementing quality by design in pharmaceutical salt selection: A modeling approach to understanding disproportionation. Pharm. Res. 2013, 30, 203–217. [Google Scholar] [CrossRef]
- Khuroo, T.; Verma, D.; Talegaonkar, S.; Padhi, S.; Panda, A.K.; Iqbal, Z. Topotecan–tamoxifen duple PLGA polymeric nanoparticles: Investigation of in vitro, in vivo and cellular uptake potential. Int. J. Pharm. 2014, 473, 384–394. [Google Scholar] [CrossRef]
- Verma, D.; Thakur, P.S.; Padhi, S.; Khuroo, T.; Talegaonkar, S.; Iqbal, Z. Design expert assisted nanoformulation design for co-delivery of topotecan and hymoquinone: Optimization, in vitro characterization and stability assessment. J. Mol. Liq. 2017, 242, 382–394. [Google Scholar] [CrossRef]
- Jha, R.; Singh, A.; Sharma, P.K.; Fuloria, N.K. Smart carbon nanotubes for drug delivery system: A comprehensive study. J. Drug. Deliv. Sci. Technol. 2020, 58, 101811. [Google Scholar] [CrossRef]
- Sathish, D.; Eman, M.M.; Khuroo, T.; Ali, H.I.; Reddy, I.K.; Rahman, Z.; Khan, M.A. In-use stability assessment of FDA approved metformin immediate release and extended release products for N-Nitrosodimethylamine and dissolution quality attributes. Int. J. Pharm. 2022, 625, 121923. [Google Scholar] [CrossRef]
- Khuroo, T.; Eman, M.M.; Dharani, S.; Immadi, S.; Nutan, M.T.H.; Lu, D.; Ali, H.I.; Khan, M.A.; Rahman, Z. In-Situ Implant Formulation of Laurate and Myristate Prodrugs of Dolutegravir for Ultra-Long Delivery. J. Pharm. Sci. 2022, 111, 2312–2321. [Google Scholar] [CrossRef]
- Khuroo, T.; Dharani, S.; Eman, M.M.; Immadi, S.; Wu, Z.; Khan, M.A.; Lu, D.; Nehete, P.; Rahman, Z. Ultra-long acting prodrug of dolutegravir and delivery system—Physicochemical, pharmacokinetic and formulation characterizations. Int. J. Pharm. 2021, 607, 120889. [Google Scholar] [CrossRef] [PubMed]


| Formulations *** | LAC | MCC | MgST [%] | NaSG [%] | LMT [%] Adjusted for Dose 25 mg per Tablet |
|---|---|---|---|---|---|
| T1 | q.s.* | / | 0.25 | 0.5 | q.s. ** |
| T2 | q.s. | / | 5 | 4 | q.s. ** |
| T3 | q.s. | / | 5 | 0.5 | q.s. ** |
| T4 | q.s. | / | 0.25 | 4 | q.s. ** |
| T5 | / | q.s. | 0.25 | 0.5 | q.s. ** |
| T6 | / | q.s. | 5 | 4 | q.s. ** |
| T7 | / | q.s. | 5 | 0.5 | q.s. ** |
| T8 | / | q.s. | 0.25 | 4 | q.s. ** |
| Conditions | Formulations | Moisture Content [%] | Crushing Force [N] | Weight Variation [g] | LMT Content [%] |
|---|---|---|---|---|---|
| Initials | T1 | 0.16 ± 0.04 | 53.48 ± 2.11 | 0.56 ± 0.01 | 97.41 ± 8.55 |
| T2 | 0.72 ± 0.06 | 56.35 ± 9.38 | 0.54 ± 0.02 | 94.44 ± 3.13 | |
| T3 | 0.27 ± 0.08 | 50.225 ± 4.69 | 0.54 ± 0.02 | 95.27 ± 5.20 | |
| T4 | 0.49 ± 0.09 | 53.90 ± 8.00 | 0.57 ± 0.02 | 97.63 ± 8.89 | |
| T5 | 3.63 ± 0.49 | 56.35 ± 2.68 | 0.34 ± 0.01 | 100.48 ± 1.97 | |
| T6 | 4.59 ± 0.31 | 62.88 ± 6.51 | 0.36 ± 0.02 | 99.12 ± 6.22 | |
| T7 | 3.88 ± 0.52 | 74.725 ± 2.45 | 0.34 ± 0.02 | 103.90 ± 11.89 | |
| T8 | 4.59 ± 0.31 | 58.80 ± 6.93 | 0.34 ± 0.02 | 99.89 ± 4.98 | |
| 30% humidity | T1 | 0.12 ± 0.03 | 69.83 ± 4.69 | 0.55 ± 0.01 | 93.55 ± 3.18 |
| T2 | 0.52 ± 0.01 | 52.68 ± 2.45 | 0.54 ± 0.01 | 96.17 ± 5.52 | |
| T3 | 0.19 ± 0.10 | 72.77 ± 4.26 | 0.54 ± 0.01 | 101.22 ± 3.87 | |
| T4 | 0.43 ± 0.07 | 44.10 ± 12.00 | 0.53 ± 0.01 | 99.99 ± 4.89 | |
| T5 | 3.06 ± 0.27 | 61.25 ± 4.90 | 0.34 ± 0.01 | 95.17 ± 3.69 | |
| T6 | 3.69 ± 0.55 | 61.99 ± 2.32 | 0.35 ± 0.01 | 103.22 ± 2.54 | |
| T7 | 3.11 ± 0.97 | 68.60 ± 4.00 | 0.35 ± 0.01 | 101.29 ± 9.89 | |
| T8 | 3.98 ± 0.28 | 49.82 ± 4.82 | 0.33 ± 0.01 | 97.93 ± 3.75 | |
| 75% humidity | T1 | 0.47 ± 0.22 | 99.63 ± 12.65 | 0.55 ± 0.01 | 98.18 ± 7.81 |
| T2 | 1.81 ± 0.23 | 64.19 ± 0.98 | 0.55 ± 0.01 | 92.25 ± 4.11 | |
| T3 | 1.14 ± 0.00 | 88.20 ± 5.66 | 0.54 ± 0.01 | 98.20 ± 4.80 | |
| T4 | 1.52 ± 0.07 | 45.33 ± 6.17 | 0.55 ± 0.01 | 95.23 ± 9.03 | |
| T5 | 7.01 ± 0.10 | 66.15 ± 2.83 | 0.35 ± 0.01 | 99.49 ± 0.55 | |
| T6 | 7.07 ± 0.02 | 27.83 ± 4.68 | 0.37 ± 0.01 | 100.98 ± 4.35 | |
| T7 | 6.50 ± 0.05 | 46.06 ± 2.68 | 0.35 ± 0.01 | 104.22 ± 6.27 | |
| T8 | 6.84 ± 0.10 | 27.44 ± 2.68 | 0.34 ± 0.01 | 98.12 ± 3.37 |
| Conditions | Formulations | Moisture Content [%] | Crushing Force [N] | Weight Variation [g] |
|---|---|---|---|---|
| Initials | F1 | 0.26 ± 0.07 | 57.21 ± 4.82 | 0.55 ± 0.01 |
| F2 | 0.75 ± 0.03 | 62.11 ± 5.62 | 0.55 ± 0.02 | |
| F3 | 0.37 ± 0.03 | 53.94 ± 4.77 | 0.56 ± 0.01 | |
| F4 | 0.96 ± 0.07 | 96.43 ± 8.35 | 0.56 ± 0.02 | |
| F5 | 4.33 ± 0.42 | 60.46 ± 4.82 | 0.35 ± 0.01 | |
| F6 | 4.69 ± 0.31 | 59.81 ± 7.33 | 0.36 ± 0.02 | |
| F7 | 3.98 ± 0.32 | 54.58 ± 3.52 | 0.34 ± 0.02 | |
| F8 | 4.29 ± 0.51 | 59.81 ± 4.77 | 0.35 ± 0.02 | |
| 30% humidity | F1 | 0.32 ± 0.01 | 48.71 ± 4.32 | 0.55 ± 0.01 |
| F2 | 0.71 ± 0.02 | 84.99 ± 7.52 | 0.55 ± 0.02 | |
| F3 | 0.30 ± 0.07 | 65.70 ± 8.85 | 0.53 ± 0.01 | |
| F4 | 0.51 ± 0.11 | 106.24 ± 9.29 | 0.53 ± 0.01 | |
| F5 | 3.16 ± 0.31 | 61.44 ± 6.12 | 0.35 ± 0.03 | |
| F6 | 3.88 ± 0.45 | 60.13 ± 5.21 | 0.34 ± 0.01 | |
| F7 | 3.54 ± 0.89 | 59.81 ± 5.11 | 0.35 ± 0.01 | |
| F8 | 3.92 ± 0.31 | 62.75 ± 4.33 | 0.34 ± 0.01 | |
| 75% humidity | F1 | 1.41 ± 0.22 | 67.01 ± 6.12 | 0.56 ± 0.01 |
| F2 | 2.04 ± 0.23 | 71.92 ± 5.28 | 0.52 ± 0.02 | |
| F3 | 2.63 ± 0.00 | 70.28 ± 6.34 | 0.54 ± 0.01 | |
| F4 | 1.82 ± 0.47 | 39.23 ± 2.88 | 0.55 ± 0.02 | |
| F5 | 7.96 ± 0.14 | 53.13 ± 3.88 | 0.37 ± 0.01 | |
| F6 | 7.96 ± 0.13 | 49.81 ± 3.23 | 0.36 ± 0.01 | |
| F7 | 6.71 ± 0.21 | 48.50 ± 3.22 | 0.36 ± 0.01 | |
| F8 | 6.74 ± 0.32 | 50.46 ± 2.87 | 0.35 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lalić-Popović, M.; Švonja Parezanović, G.; Todorović, N.; Zeković, Z.; Pavlić, B.; Milošević, N.; Čanji Panić, J.; Stjepanović, A.; Andrijević, L. The Effect of Humidity on the Dissolution Kinetics and Tablet Properties of Immediate-Release Tablet Formulation Containing Lamotrigine. Pharmaceutics 2022, 14, 2096. https://doi.org/10.3390/pharmaceutics14102096
Lalić-Popović M, Švonja Parezanović G, Todorović N, Zeković Z, Pavlić B, Milošević N, Čanji Panić J, Stjepanović A, Andrijević L. The Effect of Humidity on the Dissolution Kinetics and Tablet Properties of Immediate-Release Tablet Formulation Containing Lamotrigine. Pharmaceutics. 2022; 14(10):2096. https://doi.org/10.3390/pharmaceutics14102096
Chicago/Turabian StyleLalić-Popović, Mladena, Gordana Švonja Parezanović, Nemanja Todorović, Zoran Zeković, Branimir Pavlić, Nataša Milošević, Jelena Čanji Panić, Ana Stjepanović, and Ljiljana Andrijević. 2022. "The Effect of Humidity on the Dissolution Kinetics and Tablet Properties of Immediate-Release Tablet Formulation Containing Lamotrigine" Pharmaceutics 14, no. 10: 2096. https://doi.org/10.3390/pharmaceutics14102096
APA StyleLalić-Popović, M., Švonja Parezanović, G., Todorović, N., Zeković, Z., Pavlić, B., Milošević, N., Čanji Panić, J., Stjepanović, A., & Andrijević, L. (2022). The Effect of Humidity on the Dissolution Kinetics and Tablet Properties of Immediate-Release Tablet Formulation Containing Lamotrigine. Pharmaceutics, 14(10), 2096. https://doi.org/10.3390/pharmaceutics14102096








