Abstract
Cancer cells normally develop the ability to rewire or reprogram themselves to become resistant to treatments that were previously effective. Despite progress in understanding drug resistance, knowledge gaps remain regarding the underlying biological causes of drug resistance and the design of cancer treatments to overcome it. So, resistance acquisition remains a major problem in cancer treatment. Targeted therapeutics are considered the next generation of cancer therapy because they overcome many limitations of traditional treatments. Numerous tumor cells overexpress several receptors that have a high binding affinity for hyaluronic acid (HA), while they are poorly expressed in normal body cells. HA and its derivatives have the advantage of being biocompatible and biodegradable and may be conjugated with a variety of drugs and drug carriers for developing various formulations as anticancer therapies such as micelles, nanogels, and inorganic nanoparticles. Due to their stability in blood circulation and predictable delivery patterns, enhanced tumor-selective drug accumulation, and decreased toxicity to normal tissues, tumor-targeting nanomaterial-based drug delivery systems have been shown to represent an efficacious approach for the treatment of cancer. In this review, we aim to provide an overview of some in vitro and in vivo studies related to the potential of HA as a ligand to develop targeted nanovehicles for future biomedical applications in cancer treatment.
1. Introduction
Cancer is considered the highest clinical, social, and economic burden in terms of cause-specific disability-adjusted life years (DALYs) among all human diseases. In 2020, there were approximately 19.3 million new cancer cases and almost 10 million cancer deaths, and by 2018, in Europe alone, the total cost of cancer was EUR 199 billion [1]. For a long time, several options for cancer therapy have been developed, but successful cancer treatment remains one of the most important goals of present medical science. Current treatment approaches include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy. Even though they present a good cytotoxicity capacity, chemotherapy and radiotherapy lead to acute side effects (such as neuropathies, suppression of bone marrow, gastrointestinal and skin disorders, hair loss, and fatigue) and high risk of recurrences. In the case of targeted therapy, multi-drug resistance commonly occurs, limiting therapeutic efficacy, and in immunotherapy, in addition to the increased risk of autoimmune disease, a reduced efficiency against solid tumors has also been observed [2].
With the aim to enhance patients’ response to the considered anticancer treatments and to improve their general healthcare status, new advances in nanotechnology have made it possible to develop new and promising therapies based on the fundamental biology of cancer [2]. In the last several years, nanoparticles have shown great potential in numerous biomedical applications. Among them, silver nanoparticles have been studied owing to their specific physicochemical properties and their great potential in killing cancer cells [3]. Recently, it has been shown that starch-capped silver nanoparticles, synthesized through a green method, successfully induced damage in cytoplasmic membranes and mitochondria, leading to cell cycle arrest and consequent blockage of cell proliferation and death in prostate cancer cells, showing the potential of silver nanoparticles as anticancer agents [4]. Nanomedicine has been shown to overcome some of the limitations of current drugs used in cancer treatment, such as poor water solubility, lack of specificity to the tumor site, and systemic side effects [5,6,7]. In fact, several nanocarrier-based drug delivery systems have already been proposed, using materials such as liposomes, micelles, protein conjugates, and polymers, and are being tested in clinical trials [8].
Hyaluronic acid (HA) is a polymer with a much wider range of applications than the facial treatments with which it is typically associated. Recent findings and progression in research aim to demonstrate the various formulations of HA to design drug carriers and advances in HA-based drug delivery systems for promising improved cancer therapies [9]. Considering the great interest in HA from different fields and the fast-growing number of studies, a comprehensive review is needed regarding this polysaccharide and its potentialities.
2. Hyaluronic Acid
Hyaluronic acid is a natural anionic polysaccharide with a simple chemical structure (Figure 1) composed of two alternating repeats: disaccharide units of β-1,3-N-acetyl-D-glucosamine and β-1,4-D-glucuronic acid. It can be obtained by extraction from animal tissues, microbial production, or enzymatic synthesis. This polysaccharide is physiologically synthetized at the plasma membrane by three different hyaluronan synthases (HAS 1–3) and its molecular weight (MW) may range from 5 to 20,000 kDa in vivo [9].
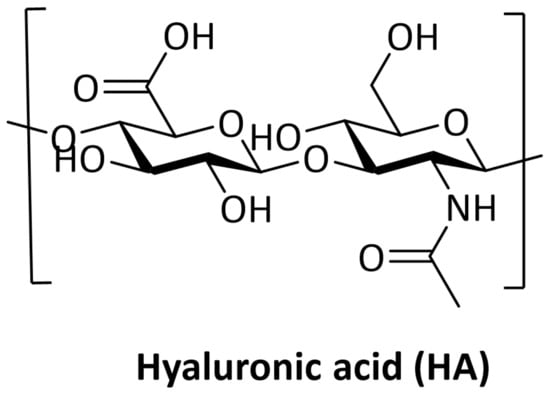
Figure 1.
Chemical structure of Hyaluronic Acid. Created with ChemDraw Software version 12.
It is quite difficult for the body to absorb a polysaccharide. In 2008, Nozomi Hisada and co-workers performed a study in which, using Caco-2 cells (intestinal epithelial model), they revealed that HA with a MW greater than 100 kDa is rarely absorbed. In fact, the amount of HA absorbed by Caco-2 cells increases as the MW of HA decreases to 70, 20, or 5 kDa [10]. Thus, HA is not absorbed into the body as a high-MW polymer after ingestion. The half-life of HA is very short (approximately 1–2 days in the skin and 24 h in the bloodstream). Its degradation in the human body is carried out by two distinct mechanisms: one is specific, mediated by enzymes (hyaluronidases, HYALs), while the other is non-specific, determined by oxidative damage due to reactive oxygen species (ROS). By catalyzing the hydrolysis of HA, HYALs decrease the viscosity of HA, thereby increasing tissue permeability [11].
The balance between the synthesis and degradation processes of HA plays an essential regulatory role in the human body, as it determines not only the amount of HA, but also its MW, and the MW determines the various biological actions/functions of the HA [9]. HA synthesis and degradation depends on the tissue microenvironment and is regulated by intra- and intercellular signaling factors. In cancer, the degradation of HA by HYALs is highly affected by malignancy, angiogenesis, and metastasis. The hypoxic status of a tumor and its microenvironment has a positive effect in HYALs’ activity, resulting in the production of small-sized HA fragments that promote angiogenesis and help the cancer to spread in the body. In fact, high levels of HYALs have been observed in various tumor types such as brain, bladder, and metastatic breast cancer [12]. There is no rigorous definition of high-MW and low-MW HA, but generally speaking, high-MW HA is responsible for the maintenance of the homeostatic condition, with anti-angiogenic, immunosuppressive, and anti-inflammatory properties; low-MW HA plays an opposite effect, having a key role in pathological conditions [13].
Since HA is produced by almost all cell types, in normal biological conditions, HA has multiple essential biological functions. HA can be involved in several cellular interactions (differentiation, proliferation, development, and antigen recognition) and biological functions (lubrication, hydration, matrix structure, and steric interactions). Its natural negative charge (due to the carboxylate groups) allows it to bind to a large amount of water, forming a highly viscous gel. This gel lubricates joints and acts as a buffer for the surrounding tissues, as well as contributing to tissue regeneration and remodeling processes, for example, during the healing process [14].
Owing to its high hydrophilicity, biodegradability, good biocompatibility, low toxicity, and modification flexibility, HA possesses great potential in biomedical and pharmaceutical applications, such as drug delivery systems, ophthalmic surgery, osteoarthritis treatment, and tissue engineering. It is also used in cosmetics applications, notably as dermal fillers and moisturizers [15,16]. Moreover, due to the differential expression of HA receptors in different tissues, HA also presents selectivity to target-specific sites, which increases its potential in these applications.
3. Hyaluronic Acid Receptors
HA is an important constituent of the extracellular matrix (ECM) that binds to ECM molecules and cell surface receptors (Figure 2), thereby regulating cellular behavior via control of the tissue’s macro- and microenvironments [11]. The three main classes of cell surface receptors for HA binding are: (1) cluster of differentiation 44 (CD44), a membrane glycoprotein, (2) receptor for hyaluronate-mediated motility (RHAMM), and (3) intercellular adhesion molecule 1 (ICAM-1).
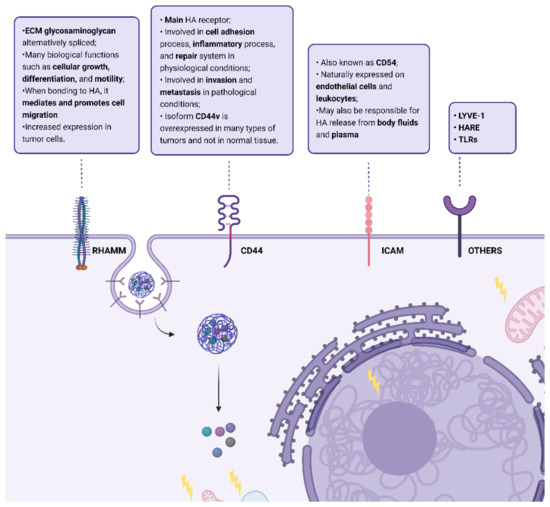
Figure 2.
Summary of HA cell surface receptors: cluster of differentiation 44 (CD44), receptor for hyaluronic acid-mediated motility (RHAMM), Intercellular Adhesion Molecule 1 (ICAM); lymphatic vessel endothelial hyaluronan receptor (LYVE-1), hyaluronic acid receptor for endocytosis (HARE), and Toll-like receptors (TLRs) and some of their actions when bonded to HA. Created with “https://biorender.com/, accessed on 28 September 2022”.
The receptor CD44 is considered the main HA receptor and their interaction activates many pathways involved in biological processes such as inflammation, wound healing, morphogenesis, and cancer. It is endogenously expressed in different cells in normal tissues, but in low levels, and requires activation [17]. CD44 is subject to extensive alternative splicing and, thus, is a transmembrane glycoprotein family with several isoforms. In normal physiology, this receptor is involved in the cell adhesion process (aggregation and migration), inflammatory process, and repair system [18]. However, in the case of pathological physiology, as cancer, it is involved in invasion and metastasis [19]. This is due to the activation of HER2 tyrosine kinase and Src, RhoA, and Rac1, as well as to the promotion of association of CD44 isoforms to cytoskeleton proteins caused by its interaction with HA [20]. Nevertheless, in cancer cells, the structure of CD44 is modified. These cells stimulate alternative splicing and post-translational modifications, producing different isoforms of CD44 protein with enhanced binding to HA [19]. Thus, the CD44 gene can encode more than 100 isoforms, from 80 to 200 kDa. The standard isoform, CD44s, is the smaller form (85–95 kDa) without variable exons, encoded by conserved exons and is ubiquitously expressed, being composed of a single-chain molecule with various domains: N-terminal, a membrane-proximal region, comprising ligand-binding sites, a cytoplasmic domain, and transmembrane domain [16]. The isoform CD44v is the major form upregulated in cancer cells and CD44v6, a specific CD44v isoform, has been identified as the major isoform of this receptor which is overexpressed in many types of tumors, and not in normal tissue [16,21]. Additionally, CD44 has already been identified in cancer stem cells (CSCs), improving their motility, and in macrophages, making these tumors immunosuppressive [22,23].
RHAMM (also designated CD168) is an ECM glycosaminoglycan which is alternatively spliced, and its truncated forms can be found not only in the cell membrane, but also in cell cytoplasm, the nucleus, and the cytoskeleton [20,24,25]. It has a role in many biological functions such as cellular growth, differentiation, and motility. When bonded to HA, the cell surface receptor RHAMM mediates and promotes cell migration, and the intracellular RHAMM mediates the cell cycle, namely the formation and integration of the mitotic spindle [11]. This interaction is important in inflammation and tissue repair because it triggers many signaling pathways and controls cells such as fibroblasts and macrophages [26]. In the case of human cancer, it is present in solid tumors in the following organs: stomach, prostate, breast, colon, and lungs [27,28]. RHAMM is poorly expressed in the majority of common normal tissues, but shows increased expression in tumor cells, which has already been correlated with tumoral progression, invasion, metastasis development, and poor survival rate [25]. The RHAMM receptor co-exists with the CD44 receptor, which is the major cell surface HA-binding protein, but in 23% of cases, RHAMM is overexpressed in the absence of CD44 [29].
ICAM-1 (also known as CD54) is a cell surface metabolic receptor for HA and is naturally expressed on endothelial cells and leukocytes. Its structure is characterized by heavy glycosylation and the protein extracellular domain is composed of multiple loops created by disulfide bonds within the protein. The binding of HA to this receptor triggers a regulated cascade of events that feed the endocytic vesicles. This molecule may also be responsible for the release of HA from body fluid and plasma, which is responsible for most of its turnover throughout the body [30].
In addition to these HA receptors, others have been identified: the lymphatic vessel endothelial hyaluronan receptor (LYVE-1), the hyaluronic acid receptor for endocytosis (HARE), and Toll-like receptors (TLRs) [31].
CD44 and RHAM’s overexpression in most tumors and their correlation with poor prognosis lead to the development of therapeutic approaches through signaling targeting and drug delivery mediation by HA [16]. HA oligosaccharides (oHA) were able to abrogate signaling pathways such as the PI3K/Akt pathway and the association between CD44 and receptor tyrosine kinases. Additionally, they were able to inhibit CD44 clustering on the plasma membrane as well as block its interaction with emmprin and with different drug transporters [32]. Additionally, because of HA’s strong binding affinity for these receptors, HA has been used through targeted delivery of chemotherapeutic drugs or other novel treatments with different studies showing successful results both in vitro and in vivo [16].
4. Therapeutic Applications of HA in Cancer
HA represents a key molecule in a variety of medical, pharmaceutical, nutritional, and cosmetic applications since it has many useful advantages, including biocompatibility, chemical versatility, non-toxicity, biodegradability, and high hydrophilicity [31]. For many years, it has been used in the treatment of osteoarthritis, cosmetics, and in ophthalmology, but there has been a growing interest in HA’s application in other fields of medicine such as skin wound healing, tissue engineering, dentistry, and targeted drug delivery systems [13]. In recent years, HA has been studied as an anticancer delivering system, not only for drugs, but also for imaging agents, gene plasmids, and photosensitizers [16]. In fact, in the field of cancer therapy, the progress of nanotechnology facilitated the development of nanodrug delivery systems that are highly tumor-selective and allow for the slow release of active anticancer drugs, which pose as great advantages since unfunctionalized nanomaterials are potentially cytotoxic and lack cell-specific function. The concept of the “3S” transition has been recently proposed in nanotechnology referring to stability, surface, and size transition and states that if these three concepts are satisfied in drug delivery systems, all barriers in delivery processes can be overcome and the drug will be effective. HA-based nanomaterials are said to be one of the few biopolymers that can satisfy the “3S” transition approach for anticancer drugs [33]. Size is, indeed, an important factor affecting half-life in vivo and accumulation in tumor tissue. Large particles tend to stay in the tumor tissue, but their penetration ability is low, whereas small particles have the opposite characteristics, and are easily removed from blood circulation. Thus, to reach a good enhanced permeability and retention effect, drugs need to be kept in a large amount in the blood circulation and less near the tumor tissue. In the case of nanomaterials containing HA—CD44, LYVE-1, and RHAMM function as selective tumor targets. After being taken up by cancer cells through receptor-mediated endocytosis, HA is degraded to low-molecular-weight components by hyaluronidase [11]. Additionally, HA’s several functional groups (carboxylic acid, hydroxyl, and N-acetyl groups) allow several chemical conjugations and modifications and the consequent delivery of synergistic cancer therapies.
Because of these properties, HA-based nanomaterials have been studied as drug delivery systems through passive and active targeting [16]. Drug delivery systems have raised attention in overcoming drug resistance as well as increasing the therapeutic index and decreasing side effects of treatments [34]. An example of such drug delivery systems are polymeric conjugates of chemotherapy drugs. These are endocytosed, accumulating in lysosomes which leads to a release of the drug from the polymer closest to its target and makes it less prone to membrane-linked drug efflux mechanisms. Their size also constrains the extravasation of the drug to normal tissues, which diminishes toxicity. Additionally, they retain the ability to cross the irregular neo-vasculature characteristics of solid tumors and are capable of accumulating in tumor interstitium [35]. The use of nanomaterials to improve immunotherapy results has also been raising attention, since their combination can potentiate the cancer-immunity cycle through enhancement of antigen release, antigen processing, antigen presentation, and immune cell-mediated tumor killing [36]. In the same way, research regarding gene therapy has been increasing. Gene therapy delivers genetic material (such as RNA or DNA), through a vector, into the target or is used to reshape cells removed from the host which are then re-administered [37]. The use of nonviral delivery vectors, such as nanomedicine, led to lower immunogenicity and toxicity, was easier to prepare, and was able to load a higher capacity [38]. Therefore, the use of HA-based nanomaterials to deliver non-coding RNAs such as siRNAs, miRNAs, and lnc-RNAs has been studied in the last several years. Thus, the present review intends to summarize the current evidence regarding these nanomaterials and their potential application in cancer.
5. Evidence Acquisition
A literature search in PubMed was conducted using the search term “Hyaluronic acid-based nanomaterials in cancer”. Papers between January 2017–March 2022 were included. A total of 366 papers were selected and after analysis and 207 papers were excluded due to the following exclusion criteria: they were review papers; we did not have access to the full text; they did not fit into the main classes of HA nanomaterials; or they were not related to cancer. The papers were then divided into major categories, corresponding to the four main classes of HA nanomaterials (Figure 3): HA–drug conjugates, HA-based hydrogels, HA micelles, and HA-based nanoparticles and their evidence was summarized in the following tables.
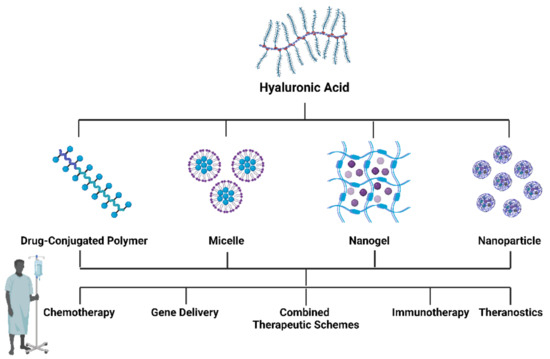
Figure 3.
Different formulations of HA-based nanomaterials and their possible applications in cancer therapy. Created with “https://biorender.com/ accessed on 28 September 2022”.
6. HA–Drug Conjugates
Taking into account the specific binding of HA to receptors on the surface of cancer cells, it can be used as a carrier of other drugs through the formation of conjugates, generating new compounds with promising antitumor effects [9]. This direct conjugation made by covalent bonds is not easily broken in the blood, but can be disrupted through hydrolysis by intracellular enzymes after reaching the target and releasing the drug [39]. Besides this targeting ability, HA–drug conjugates can improve drug solubility, stability, circulation time, and change its distribution in vivo, increasing its accumulation in tumor tissue by enhancing the osmotic retention effect. In fact, hyaluronan has already been conjugated to different antineoplastic drugs, generating new compounds with promising antitumor effects (Table 1).

Table 1.
Recent application of HA-based drug conjugates in cancer models.
Lai and collaborators have proposed the conjugation of curcumin and hyaluronic acid to form amphiphilic HA–ADH–CUR conjugates. These conjugates were efficiently internalized through CD44 receptor-mediated endocytosis by breast cancer cells and in an in vivo context. Moreover, curcumin was successfully released in an acidic lysosome environment, which is characteristic of the tumoral microenvironment, and was able to achieve significative therapeutic effects for tumor growth suppression, showing potential as a promising nanocarrier for curcumin to enhance cancer therapy with good biosafety [40]. On the other hand, DaEun Kim and collaborators conjugated S-nitrosoglutathione with HA to improve doxorubicin anticancer activity and observed that it was capable to generate NO within cells that made breast cancer cells vulnerable to doxorubicin, reinforcing its apoptotic activity. At the same time, the drug conjugate alone exhibited negligible cytotoxic effects. These results were reinforced in vivo where there was effective accumulation in the solid tumor and effective tumor growth suppression [41]. Additionally, Xiaoyu Xu and collaborators have conjugated cinnamaldehyde with hyaluronic acid and encapsulated the photosensitized protoporphyrin combining a ROS-based dual chemo/photodynamic treatment modality. The generated ROS was used as a mechanism to avoid undesired elimination of protoporphyrin and, in fact, this drug conjugate was able to induce antitumor effects both in vitro and in vivo [42].
These studies show the potential of drugs conjugated with HA as a new class of bioconjugated and tumor-targeted chemotherapeutic drugs for cancer treatment due to their innovative carrier-mediated drug delivery systems characterized by CD44-mediated endocytosis of HA and intracellular drug release with great potential.
7. HA-Based Hydrogel
Hydrogels are three-dimensional hydrated polymeric networks (with high water content), formed from crosslinked polymer chains with highly porous structures that enable drug release in a controlled manner [46]. In recent years, on account of their advantages such as low cytotoxicity, viscoelasticity, and bioconjugation, as well as prevention of enzymatic degradation, hydrophilic hydrogels have been widely investigated for biomedical applications such as cell therapy, tissue engineering, drug delivery, and diagnostics [47,48]. HA does not natively form physical gels alone and is susceptible to endogenous degradation; thus, the hydroxyl- and carboxyl-reactive groups in HA are often subjected to chemical modifications, crosslinking, and gelling agents to develop HA-based hydrogels with structural, mechanical, and degradation properties while maintaining native biological functions [49]. Thus, HA-based hydrogels are macroscopic networks of randomly interconnected HA chains at crosslinking points established by covalent bonds, such as hydrogen bonds, hydrophobic/hydrophilic interactions, and ionic/electrostatic interactions [50]. In the last several years, they have been studied for the controlled release of loaded anticancer drugs (Table 2).

Table 2.
Recent application of HA-based hydrogels in cancer models.
Barbarisi M and collaborators synthesized a nanohydrogel that was able to carry quercetin combined with temozolomide and was administrated to glioblastoma cells in vitro. This nanocarrier increased the internalization of quercetin, which, when co-delivered with temozolomide, contributed to an improved anticancer effect as well as a reduction inIL-8, IL-6, and vascular endothelial growth factor (VEGF) production. The increased internalization was due to the ability of the nanohydrogel to recognize the CD44 receptor through an energy- and caveolae-dependent internalization mechanism, demonstrating the ability of hyaluronic acid nanocarriers in targeting glioblastoma cells [51]. Interestingly, Zhiwen Cao and collaborators synthesized a nanogel using hyaluronic acid and β-cyclodextrin derivative to carry auraptene and cisplatin. This nanogel showed excellent physiological stability and its delivery was affected by pH value, favoring a selective release to the tumor microenvironment. Additionally, it demonstrated a selective cytotoxicity to breast cancer cells compared to normal ones, which is a great indicator of biosafety. This is enhanced by the in vivo results, since the nanogel was able to reduce tumor volume while showing reduced systemic toxicity [52]. Nanogel application in theranostics has been demonstrated by Pan et al. [53]. They reported a one-step assembly of an HA-based multifunctional theranostic nanoplatform. Histidine was conjugated with HA and Mn2+ was used as a magnetic resonance imaging (MRI) contrast agent. Doxorubicin and chlorin e6 were then loaded as chemotherapeutic agents. This nanogel showed high biosafety and tumor microenvironment responsiveness in a melanoma cell line. The targeted responsive release of doxorubicin, chlorin e6, and Mn2+ was able to induce cell death in vitro and suppress tumor growth in vivo, showing potential both in combined chemo-photodynamic therapy and T1-weighted MR imaging [53].
Thus, the optimal formulations of hydrogels can increase the therapeutic efficacy of the local treatment of cancer, resulting in promising injectable formulations for the treatment of local and metastatic tumors.
8. HA Micelles
The functional groups presented in HA can be modified with hydrophobic substances such as hydrophobic drugs or polymers via esterification or amidation, allowing the binding of hydrophobic macromolecules with positive charges via electrostatic interactions to form micelles or micellar NPs for loading drugs [32]. Thus, HA can form self-assembling micelles generating amphiphilic nanocarriers. Micelles have an amphiphilic nature, displaying a spherical structure with a hydrophilic shell and hydrophobic core [62]. Therefore, they have the ability to carry hydrophobic drugs and increase their bio-availability and half-life. There are several characteristics that have made micelles the target of study in the last several years (Table 3), of which high dissolution capacity, high stability along with prolonged release, long-term circulation and the capacity to stay in the tumor for a greater amount of time are examples [63].

Table 3.
Recent application of HA-based micelles in cancer models.
Tao Yu and collaborators synthesized an HA-based nanocarrier, incorporating doxorubicin and cisplatin as a CD44-targeting anticancer drug delivery system. These micelles with dual cargo were tested in breast cancer and normal cells, showing an increased drug release under acidic conditions, which is characteristic of the tumoral microenvironment. Additionally, the studies indicated a good cellular uptake and a higher cellular growth inhibition than doxorubicin and cisplatin alone. It is important to note that this was not observed in the normal breast cells, meaning there was a great polarity of the micelles to CD44+. These micelles were also tested in vivo, using a mammary cancer-bearing mouse model and, when compared to the free drugs, there was a higher inhibitory effect of the micelles, a lower toxicity, and higher tumor accumulation. These results showed the importance of HA in the formulation of nanocarriers of existing cancer drugs. [64]. On the other hand, an interesting work performed by Ying Yu and collaborators was the incorporation of a chemical radiosensitizer, doxorubicin, into the micelle’s core. These DOX-loaded ROS-sensitive nanomicelles were tested in breast cancer cells and, upon radiation stimulus, they were oxidized, generating ROS and leading to the micelles’ destruction and doxorubicin release. Additionally, when combined with radiotherapy, the DOX released by the micelles showed enhanced cytotoxicity and a sensitization of the cells to radiotherapy. This was further shown in in vivo studies in which these micelles showed longer circulation time, better tumor accumulation, and a greater tumor inhibition rate. In fact, when the tumor sites were irradiated, the release of doxorubicin was combined with the cytotoxic effect of radiotherapy with a tumor inhibition rate of about 70%. The study is an indication of the possibilities opened up by nanomedicine using HA in encapsulating anticancer drugs, maximizing their effect in combination with radiotherapy [65]. Recently, Bingjie Wang and collaborators synthesized a novel nanocarrier material for synchronous delivery of curcumin and baicalin, targeting both lung cells and tumor-associated macrophages, to effectively overcome tumor resistance. They demonstrated through in vitro cellular studies that these micelles have good cellular penetration and tumor cytotoxicity. In vivo antitumor experiments confirmed effective antitumor activity and reduced side effects in A549 tumor-bearing nude mice [66]. Even though these and other studies point to micelles as promising carriers for the delivery of anticancer drugs, there is little clinical research that proves their safety and clinical antitumor effect [78,79].
Another frequently used method is the coating of HA onto other nanocarriers, such as liposomes or inorganic nanoparticles, made by electrostatic attraction or covalent bonds, especially unstable bonds. A carrier system must be biocompatible, inert, and able to efficiently carry a high concentration of drug [80]. The slow release of drug from the carrier allows the drug to remain in the tumor tissue at a higher concentration and lower plasma drug concentration [81]. In Table 4, we summarize the studies with HA-based nanoparticles and their possible applications in cancer.

Table 4.
Recent application of HA-based nanoparticles in cancer models.
Carla Serri and collaborators synthesized biodegradable NPs coated with HA and loaded with gemcitabine and quercetin [82]. These HA–NPs enhanced the cellular uptake and cytotoxicity in two cell lines of pancreatic ductal adenocarcinoma, highlighting the effect of HA on targeting CD44 overexpressed in cancer cells. Furthermore, a result demonstrated the capacity of the NPs to slow the release of the incorporated drug and allow it to remain at higher concentration due to the enhancement in the anti-inflammatory properties of quercetin, showing a decrease in the interleukin cellular levels, in both cell lines pre-stimulated with lipopolysaccharides. This is an interesting result taking into account the role of interleukins in progression, metastatic processes, and drug resistance of human pancreas cancer cells, and is a study that demonstrates the benefits of using HA–NPs to improve cancer therapy [82]. Another study performed by Shaoxuan Yu and collaborators combined the advantages of curcumin, zeolitic imidazolate framework-8 nanoparticles, and hyaluronic acid for breast cancer therapy. They concluded that during storage in different media, these NPs had good stability and that under acidic conditions, a characteristic of the tumoral microenvironment, the NPs showed a slower drug release. The in vitro results obtained with these nanoparticles indicated that they have good cellular uptake which leads to several anticancer effects such as higher cytotoxicity and higher release of lactate dehydrogenase, cell cycle arrest, induction of apoptosis and production of reactive oxygen species. In this study, in vivo experiments were also performed, using mammary cancer-bearing mice models, showing that these NPs are able to strongly inhibit the tumor growth and pulmonary metastasis, remarking the properties obtained with the introduction of HA in the nanoparticles [83].
Taking into account that combinational cancer therapy has been considered a promising strategy to achieve synergetic therapeutic effects and suppression of multidrug resistance, in 2018, Yang Li and collaborators developed a dual-targeting delivery system [163]. These NPs were based on a ligand of CD44 receptors (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-hyaluronic acid) and a selective ligand of folate receptors (MTX) with a focus on combining methotrexate (MTX), which act on cytoplasm, and 10-hydroxycamptothecin (HCPT), an alkaloid acting on nuclei, to treat breast cancer. The efficiency of selective internalization of these NPs via CD44/folate receptors was confirmed by cellular uptake results. Additionally, in vivo studies indicated NP accumulation at the tumor sites through passive-plus-active targeting, leading to synergetic tumor cell death and inhibition of tumor growth, showing that these NPs can be an efficient delivery system for tumor-targeting therapy [163]. Another example of a dual-targeting delivery system was performed in 2019 by Safia Naz and collaborators based on mesoporous silica NPs, which performed as a drug delivery system, demonstrating enzyme-responsive and multistage-targeted anticancer effects with doxorubicin (DOX) as a cargo. To obtain this delivery system, the authors grafted the mesoporous silica NPs with triphenylphosphine (TPP) and capped them with HA. The resulting NPs had dual-targeting, CD44 (HA), and mitochondrial-targeting properties (TPP), which was confirmed by the results showing that cancer cells favorably uptake these NPs via CD44 receptor-mediated endocytosis and are largely accumulated in mitochondria. In cancer cells, overexpressed HAase enabled HA degradation leading to the enzyme-responsive release of DOX, killing cells while exhibiting much lower cytotoxicity than normal ones [84].
Additionally, Mengjiao Zhou and collaborators synthesized carrier-free drug NPs that carry paclitaxel and DOX modified with cis-aconitic anhydride, coated with a crosslinker based on HA. Based on the unique pH and redox environment of tumor tissues, the objective of the authors was to obtain NPs with pH- and redox-responsive release capable of CD44 targeting. The results showed that these NPs at a neutral pH level, such as that of the blood stream, are stable and have a very low drug release. However, at acidic pH levels, such as that in the tumor environment, they observed a significant increase in drug release. The authors also tested the tumor selectivity, using normal and cancer cells, concluding that these NPs preferentially target cancer cells, expected, due to the presence of HA. Further, In vivo studies showed high accumulation in tumors and excellent inhibition of tumor growth. The authors observed a greater anticancer effect than the individual paclitaxel and DOX; together, these results presented the ability of HA–NPs in targeting cancer cells and increasing the drug availability [85]. In 2018, Dalia Kabary and collaborators, focusing on lung cancer therapy, developed inhalable nanocomposites with the ability to deliver the hydrophobic mTOR inhibitor rapamycin (RAP) and the hydrophilic herbal drug berberine (BER) [148]. In order to decrease the delivery gap between the two drugs, the authors created two types of multi-compartmental nanocarriers by enveloping a BER hydrophobic ion pair-lipid nanocore within a shell of RAP-phospholipid complex bilayer. Then, they were coated with cationic lactoferrin and anionic hyaluronate to improve their tumor targeting. The authors performed in vivo studies using lung cancer-bearing mice, in order to compare the anticancer efficiency of inhaled free drugs to the inhalable nanocomposites, and it was possible to see a remarkable decrease in lung weight and in the number and diameters of lung adenomatous foci and angiogenic markers. This study showed a potential application of NPs for localized delivery to tumor cells via inhalable multi-compartmental nanocomposites, which is promising in the management of lung cancer [148]. In 2020, Jinying Liang and collaborators created and characterized lipid/hyaluronic acid (HA)-coated DOX–Fe3O4 and determined its safety and effectiveness on breast cancer [98]. As it was described, DOX was conjugated onto the Fe3O4 NP surface and then coated with a tumor cell-targeting HA ligand, phosphatidylcholine (PC) lipid, in order to obtain a dual-targeting NP. The objective of the authors was to obtain a drug delivery system capable of transporting DOX into cancer cells, decreasing its cardiotoxicity and addressing any MDR problems. The results showed the synergistic interaction of this coated PC/HA surface with DOX–Fe3O4, resulting in good antitumor efficacy for MDR cancer therapy and diminutive DOX cardiotoxicity, showing the potential of PC/HA@DOX–Fe3O4 NPs as efficient nanocarriers to overcome MDR tumors and the cardiotoxicity of DOX [98]. In the same year, Jing Yang and collaborators used HA as an alternative to plasmid DNA to construct a novel type of cationic liposome carrier that can carry siRNA [192]. The objective of the investigators was to create a carrier that targets melanoma survivin and evaluate the efficacy of this carrier and the potential of this target. They concluded that these NPs inhibit melanoma proliferation in time and dose matters, in vitro, and target survivin. In addition, NPs inhibited the metastatic ability of melanoma cells. In the in vivo experiments, the cationic liposome NPs were injected into a mouse tumor model, and an inhibition of tumor growth and a significant reduction of the expression of survivin mRNA and protein were observed. This is a good study that shows that siRNA cationic liposome NPs are highly stable and have notable properties of low immunogenicity and toxicity, and can effectively inhibit melanoma cells by inhibiting survivin expression [192].
Together, the studies discussed here and the ones presented in the Table 4 show that HA–NPs are a highly promising nanocarrier and are efficient as a delivery system to perform enhanced cancer therapy with good biosafety.
9. Conclusions and Future Perspectives
Considering the numerous publications in recent years, it is clear that nanomaterials with hyaluronic acid as a biomaterial designed to target different tumors are perceived as a promising and attractive strategy to improve cancer therapy.
The application of nanomaterials in the field of biomedicines has had a great impact on the delivery of anti-neoplastics. In the last several years, the development of nanodrug delivery systems for targeted tumor treatment has been the focus of many researchers, namely regarding glycosaminoglycans that target CD44, since this receptor is overexpressed in different tumor cells. HA-based nanomaterials have potential applications in chemotherapy, gene therapy, immunotherapy, and combination therapy for cancer treatment due to the negatively charged surfaces that make them beneficial for prolonged blood circulation, protecting drugs from absorption by endothelial cells. Additionally, all biological properties of HA—such as the cell surface receptors with which HA can interact, including the CD44 receptor, which is widely expressed in cancer cells when compared to normal ones—are a relevant aspect. It is the interaction with these receptors that enables targeted delivery to target locations, resulting in greater cellular uptake and, therefore, beneficial results.
Nevertheless, there are some major points of potential improvement in order to overcome some of the possible obstacles that make it difficult to translate HA-based nanomaterials to clinical applications. Firstly, more extensive and in-depth investigation needs to be performed in order to improve the uptake of the different biomaterials, namely regarding CD44 binding. Secondly, some chemical modifications in the HA structure may affect CD44 targeting and also affect HA degradation, causing undesirable cellular uptake and drug release. Thus, the study of drug release and nanomaterial degradation kinetics is highly needed to improve the ability to apply these biomaterials in the clinical practice. One of the main concerns regarding hyaluronic acid’s ability to target the nanomaterial to tumor cells is that CD44 is expressed in normal cells, even if in a lower concentration. Therefore, the possibility to improve these nanomaterials would involve modifying it in order to target CD44v, an isoform of CD44 expressed in tumor cells. The path to clinical implementation of a drug is long, and both 3D spheroids and in vivo studies are needed to strengthen the potential of these nanomaterials and their biosafety in order to apply them both as therapeutic agents and also as theranostic agents. More specifically, there is a lack of studies regarding biodistribution, toxicity, and availability in physiological conditions. The study of these parameters is strongly needed in order to clinically implement hyaluronic acid-based nanomaterials.
Author Contributions
V.M. wrote the main manuscript text; M.M. re-wrote some sections of the manuscript and prepared figures; R.M. supervision and project administration. All authors reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
V.M. is funded by RNCCC/P.CCC/CI-IPOP/Lab2/InvJun under the project NORTE-01-0145-FEDER-072678 and M.M. is funded by FCT through the grant 2020.08193.BD.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This article is part of the project “P.CCC: Centro Compreensivo de Cancro do Porto”—NORTE-01-0145-FEDER-072678, supported by the Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hofmarcher, T.; Lindgren, P.; Wilking, N.; Jönsson, B. The cost of cancer in Europe 2018. Eur. J. Cancer 2020, 129, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Gavas, S.; Quazi, S.; Karpiński, T.M. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.; Teixeira, A.L.; Dias, F.; Machado, V.; Medeiros, R.; Prior, J.A.V. Cytotoxic Effect of Silver Nanoparticles Synthesized by Green Methods in Cancer. J. Med. Chem. 2020, 63, 14308–14335. [Google Scholar] [CrossRef]
- Morais, M.; Machado, V.; Dias, F.; Palmeira, C.; Martins, G.; Fonseca, M.; Martins, C.; Teixeira, A.; Prior, J.; Medeiros, R. Starch-Capped AgNPs’ as Potential Cytotoxic Agents against Prostate Cancer Cells. Nanomaterials 2021, 11, 256. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Bauleth-Ramos, T.; Hirvonen, J.; Sarmento, B.; Santos, H.A. Chapter 1—The emerging role of multifunctional theranostic materials in cancer nanomedicine. In Handbook of Nanomaterials for Cancer Theranostics; Conde, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–31. [Google Scholar]
- Zhang, M.; Qin, X.; Zhao, Z.; Du, Q.; Li, Q.; Jiang, Y.; Luan, Y. A self-amplifying nanodrug to manipulate the Janus-faced nature of ferroptosis for tumor therapy. Nanoscale Horiz. 2022, 7, 198–210. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, N.; Li, Q.; Zhou, Y.; Luan, Y. A two-pronged photodynamic nanodrug to prevent metastasis of basal-like breast cancer. Chem. Commun. 2021, 57, 2305–2308. [Google Scholar] [CrossRef]
- Chiang, C.-L.; Cheng, M.-H.; Lin, C.-H. From Nanoparticles to Cancer Nanomedicine: Old Problems with New Solutions. Nanomaterials 2021, 11, 1727. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, M.S.; Jeong, W.Y.; Han, D.-W.; Kim, K.S. Hyaluronic Acid-Based Theranostic Nanomedicines for Targeted Cancer Therapy. Cancers 2020, 12, 940. [Google Scholar] [CrossRef]
- Hisada, N.; Satsu, H.; Mori, A.; Totsuka, M.; Kamei, J.-I.; Nozawa, T.; Shimizu, M. Low-Molecular-Weight Hyaluronan Permeates through Human Intestinal Caco-2 Cell Monolayers via the Paracellular Pathway. Biosci. Biotechnol. Biochem. 2008, 72, 1111–1114. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- McAtee, C.O.; Barycki, J.J.; Simpson, M.A. Emerging Roles for Hyaluronidase in Cancer Metastasis and Therapy. Adv. Cancer Res. 2014, 123, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Šoltés, L. Hyaluronan as a Prominent Biomolecule with Numerous Applications in Medicine. Int. J. Mol. Sci. 2021, 22, 7077. [Google Scholar] [CrossRef]
- Marcotti, S.; Maki, K.; Reilly, G.C.; Lacroix, D.; Adachi, T. Hyaluronic acid selective anchoring to the cytoskeleton: An atomic force microscopy study. PLoS ONE 2018, 13, e0206056. [Google Scholar] [CrossRef]
- Lapcik, L., Jr.; Lapcik, L.; De Smedt, S.; Demeester, J.; Chabreček, P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Dai, Y.; Gao, H. Development and application of hyaluronic acid in tumor targeting drug delivery. Acta Pharm. Sin. B 2019, 9, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Cichy, J.; Puré, E. The liberation of CD44. J. Cell Biol. 2003, 161, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef]
- Turley, E.A.; Noble, P.W.; Bourguignon, L.Y. Signaling Properties of Hyaluronan Receptors. J. Biol. Chem. 2002, 277, 4589–4592. [Google Scholar] [CrossRef]
- Günthert, U.; Hofmann, M.; Rudy, W.; Reber, S.; Zöller, M.; Hauβmann, I.; Matzku, S.; Wenzel, A.; Ponta, H.; Herrlich, P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 1991, 65, 13–24. [Google Scholar] [CrossRef]
- Zöller, M. CD44: Can a cancer-initiating cell profit from an abundantly expressed molecule? Nat. Rev. Cancer 2011, 11, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Jaggupilli, A.; Elkord, E. Significance of CD44 and CD24 as Cancer Stem Cell Markers: An Enduring Ambiguity. Clin. Dev. Immunol. 2012, 2012, 708036. [Google Scholar] [CrossRef] [PubMed]
- Crainie, M.; Belch, A.R.; Mant, M.J.; Pilarski, L.M. Overexpression of the receptor for hyaluronan-mediated motility (RHAMM) characterizes the malignant clone in multiple myeloma: Identification of three distinct RHAMM variants. Blood 1999, 93, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Gust, K.M.; Hofer, M.D.; Perner, S.R.; Kim, R.; Chinnaiyan, A.M.; Varambally, S.; Moller, P.; Rinnab, L.; Rubin, M.; Greiner, J.; et al. RHAMM (CD168) Is Overexpressed at the Protein Level and May Constitute an Immunogenic Antigen in Advanced Prostate Cancer Disease. Neoplasia 2009, 11, 956–963. [Google Scholar] [CrossRef]
- Vigetti, D.; Karousou, E.; Viola, M.; Deleonibus, S.; De Luca, G.; Passi, A. Hyaluronan: Biosynthesis and signaling. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, P.; Brown, T.J.; Ng, R.; Jennens, R.; Cinc, E.; Pho, M.; Michael, M.; Fox, R.M. A Pilot Human Evaluation of a Formulation of Irinotecan and Hyaluronic Acid in 5-Fluorouracil-Refractory Metastatic Colorectal Cancer Patients. Chemotherapy 2009, 55, 49–59. [Google Scholar] [CrossRef]
- Augustin, F.; Fiegl, M.; Schmid, T.; Pomme, G.; Sterlacci, W.; Tzankov, A. Receptor for hyaluronic acid-mediated motility (RHAMM, CD168) expression is prognostically important in both nodal negative and nodal positive large cell lung cancer. J. Clin. Pathol. 2015, 68, 368–373. [Google Scholar] [CrossRef]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of Inflammation and Cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Mirza, S.; Jordan, A. Targeting Hyaluronic Acid Family for Cancer Chemoprevention and Therapy. Adv. Cancer Res. 2014, 123, 35–65. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Pang, L.; Feng, H.; Dong, H.; Wang, S.; Cong, H.; Shen, Y.; Bing, Y. Recent advantage of hyaluronic acid for anti-cancer application: A review of “3S” transition approach. Carbohydr. Polym. 2020, 238, 116204. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.-J.; De Geest, B.G. Nanomedicine and cancer immunotherapy. Acta Pharmacol. Sin. 2020, 41, 879–880. [Google Scholar] [CrossRef]
- Belete, T.M. The Current Status of Gene Therapy for the Treatment of Cancer. Biol. Targets Ther. 2021, 15, 67–77. [Google Scholar] [CrossRef]
- Keles, E.; Song, Y.; Du, D.; Dong, W.-J.; Lin, Y. Recent progress in nanomaterials for gene delivery applications. Biomater. Sci. 2016, 4, 1291–1309. [Google Scholar] [CrossRef]
- Chen, B.; Miller, R.J.; Dhal, P.K. Hyaluronic Acid-Based Drug Conjugates: State-of-the-Art and Perspectives. J. Biomed. Nanotechnol. 2014, 10, 4–16. [Google Scholar] [CrossRef]
- Lai, H.; Ding, X.; Ye, J.; Deng, J.; Cui, S. pH-responsive hyaluronic acid-based nanoparticles for targeted curcumin delivery and enhanced cancer therapy. Colloids Surf. B Biointerfaces 2021, 198, 111455. [Google Scholar] [CrossRef]
- Kim, D.E.; Kim, C.W.; Lee, H.J.; Min, K.H.; Kwack, K.H.; Lee, H.-W.; Bang, J.; Chang, K.; Lee, S.C. Intracellular NO-Releasing Hyaluronic Acid-Based Nanocarriers: A Potential Chemosensitizing Agent for Cancer Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 26870–26881. [Google Scholar] [CrossRef]
- Xu, X.; Huang, B.; Zeng, Z.; Chen, J.; Huang, Z.; Guan, Z.; Chen, M.; Huang, Y.; Zhao, C. Broaden sources and reduce expenditure: Tumor-specific transformable oxidative stress nanoamplifier enabling economized photodynamic therapy for reinforced oxidation therapy. Theranostics 2020, 10, 10513–10530. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, Y.; Xu, L.; Shao, Y.; Zhang, H.; Shi, F.; Chen, J.; Cui, S.; Chen, X.; Zhu, D.; et al. Hyaluronic acid-shelled, peptide drug conjugate-cored nanomedicine for the treatment of hepatocellular carcinoma. Mater. Sci. Eng. C 2020, 117, 111261. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.T.; Du, J.-K.; Yang, Y.-Q.; Li, L.; Zhang, D.-W.; Liang, C.-L.; Wang, J.; Mei, J.; Wang, G.-H. pH and redox dual stimulate-responsive nanocarriers based on hyaluronic acid coated mesoporous silica for targeted drug delivery. Mater. Sci. Eng. C 2017, 81, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Yang, Z.; Chen, S.; Wu, H.; Liu, Y.; Wright, A.; Lu, J.-W.; Xia, X.; Lee, A.; Zhang, J.; et al. Bioreducible Zinc(II)–Dipicolylamine Functionalized Hyaluronic Acid Mediates Safe siRNA Delivery and Effective Glioblastoma RNAi Therapy. ACS Appl. Bio Mater. 2019, 2, 362–369. [Google Scholar] [CrossRef]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Mendes, B.B.; Daly, A.C.; Reis, R.L.; Domingues, R.M.; Gomes, M.E.; Burdick, J.A. Injectable hyaluronic acid and platelet lysate-derived granular hydrogels for biomedical applications. Acta Biomater. 2021, 119, 101–113. [Google Scholar] [CrossRef]
- Liu, C.; Bae, K.H.; Yamashita, A.; Chung, J.E.; Kurisawa, M. Thiol-Mediated Synthesis of Hyaluronic Acid–Epigallocatechin-3-O-Gallate Conjugates for the Formation of Injectable Hydrogels with Free Radical Scavenging Property and Degradation Resistance. Biomacromolecules 2017, 18, 3143–3155. [Google Scholar] [CrossRef]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Barbarisi, M.; Iaffaioli, R.V.; Armenia, E.; Schiavo, L.; De Sena, G.; Tafuto, S.; Barbarisi, A.; Quagliariello, V. Novel nanohydrogel of hyaluronic acid loaded with quercetin alone and in combination with temozolomide as new therapeutic tool, CD44 targeted based, of glioblastoma multiforme. J. Cell. Physiol. 2018, 233, 6550–6564. [Google Scholar] [CrossRef]
- Cao, Z.; Li, W.; Liu, R.; Li, C.; Song, Y.; Liu, G.; Chen, Y.; Lu, C.; Lu, A.; Liu, Y. pH-Responsive Fluorescence Enhanced Nanogel for Targeted Delivery of AUR and CDDP Against Breast Cancer. Int. J. Nanomed. 2020, 15, 8369–8382. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.T.; Ding, Y.-F.; Han, Z.-H.; Yuwen, L.; Ye, Z.; Mok, G.S.; Li, S.; Wang, L.-H. Hyaluronic acid-based nanogels derived from multicomponent self-assembly for imaging-guided chemo-photodynamic cancer therapy. Carbohydr. Polym. 2021, 268, 118257. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Iscaro, A.; Zambito, G.; Mijiti, Y.; Minicucci, M.; Essand, M.; Lowik, C.; Muthana, M.; Censi, R.; Mezzanotte, L.; et al. Development of a New Hyaluronic Acid Based Redox-Responsive Nanohydrogel for the Encapsulation of Oncolytic Viruses for Cancer Immunotherapy. Nanomaterials 2021, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, T.F.; Couturaud, B.; Szarpak-Jankowska, A.; Fournier, D.; Louage, B.; Garcia, F.P.; Nakamura, C.V.; De Geest, B.G.; Woisel, P.; van der Sanden, B.; et al. Coumarin-containing thermoresponsive hyaluronic acid-based nanogels as delivery systems for anticancer chemotherapy. Nanoscale 2017, 9, 12150–12162. [Google Scholar] [CrossRef]
- Onder, F.C.; Suner, S.S.; Sahiner, N.; Ay, M.; Ozpolat, B. Delivery of Small Molecule EF2 Kinase Inhibitor for Breast and Pancreatic Cancer Cells Using Hyaluronic Acid Based Nanogels. Pharm. Res. 2020, 37, 63. [Google Scholar] [CrossRef]
- Quagliariello, V.; Iaffaioli, R.V.; Armenia, E.; Clemente, O.; Barbarisi, M.; Nasti, G.; Berretta, M.; Ottaiano, A.; Barbarisi, A. Hyaluronic Acid Nanohydrogel Loaded with Quercetin Alone or in Combination to a Macrolide Derivative of Rapamycin RAD001 (Everolimus) as a New Treatment for Hormone-Responsive Human Breast Cancer. J. Cell. Physiol. 2017, 232, 2063–2074. [Google Scholar] [CrossRef]
- Xiao, T.; Hu, W.; Fan, Y.; Shen, M.; Shi, X. Macrophage-mediated tumor homing of hyaluronic acid nanogels loaded with polypyrrole and anticancer drug for targeted combinational photothermo-chemotherapy. Theranostics 2021, 11, 7057–7071. [Google Scholar] [CrossRef]
- Faraji, N.; Esrafili, A.; Esfandiari, B.; Abednezhad, A.; Naghizadeh, M.; Arasteh, J. Synthesis of pH-sensitive hyaluronic acid nanogels loaded with paclitaxel and interferon gamma: Characterization and effect on the A549 lung carcinoma cell line. Colloids Surf. B Biointerfaces 2021, 205, 111845. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, H.; Hu, F.; Pei, Z.; Xu, Y.; Shuai, Q. Multifunctional nanogel engineering with redox-responsive and AIEgen features for the targeted delivery of doxorubicin hydrochloride with enhanced antitumor efficiency and real-time intracellular imaging. Artif. Cells Nanomed. Biotechnol. 2018, 46, S900–S910. [Google Scholar] [CrossRef]
- Štaka, I.; Cadete, A.; Surikutchi, B.T.; Abuzaid, H.; Bradshaw, T.D.; Alonso, M.J.; Marlow, M. A novel low molecular weight nanocomposite hydrogel formulation for intra-tumoural delivery of anti-cancer drugs. Int. J. Pharm. 2019, 565, 151–161. [Google Scholar] [CrossRef]
- Biswas, S.; Vaze, O.S.; Movassaghian, S.; Torchilin, V.P. Polymeric micelles for the delivery of poorly soluble drugs. In Drug Delivery Strategies for Poorly Water-Soluble Drugs; Douroumis, D., Fahr, A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 411–476. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, Y.; Gu, X.; Li, Q. Development of a Hyaluronic Acid-Based Nanocarrier Incorporating Doxorubicin and Cisplatin as a pH-Sensitive and CD44-Targeted Anti-Breast Cancer Drug Delivery System. Front. Pharmacol. 2020, 11, 532457. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, Z.; Liu, J.; Hou, X.; Zhou, X.; Gao, J.; Wang, W.; Zhang, Y.; Li, G.; Liu, J. γ-Ray-Triggered Drug Release of Reactive Oxygen Species-Sensitive Nanomedicine for Enhanced Concurrent Chemoradiation Therapy. ACS Omega 2021, 6, 19445–19457. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, W.; Zhou, X.; Liu, M.; Hou, X.; Cheng, Z.; Chen, D. Development of dual-targeted nano-dandelion based on an oligomeric hyaluronic acid polymer targeting tumor-associated macrophages for combination therapy of non-small cell lung cancer. Drug Deliv. 2019, 26, 1265–1279. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, S.; Zhang, T.; He, D.; Tu, J.; Shen, Y. Enhanced cytotoxicity of a redox-sensitive hyaluronic acid-based nanomedicine toward different oncocytes via various internalization mechanisms. Drug Deliv. 2020, 27, 128–136. [Google Scholar] [CrossRef]
- Bae, K.H.; Tan, S.; Yamashita, A.; Ang, W.X.; Gao, S.J.; Wang, S.; Chung, J.E.; Kurisawa, M. Hyaluronic acid-green tea catechin micellar nanocomplexes: Fail-safe cisplatin nanomedicine for the treatment of ovarian cancer without off-target toxicity. Biomaterials 2017, 148, 41–53. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Dong, L.; Zhang, P.; Mu, Y.; Cui, X.; Zhou, J.; Huo, M.; Yin, T. Hyaluronic acid-decorated redox-sensitive chitosan micelles for tumor-specific intracellular delivery of gambogic acid. Int. J. Nanomed. 2019, 14, 4649–4666. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Chen, T.; Yang, Q.; Huang, T.; Fu, Y.; Gong, T.; Zhang, Z. Hyaluronic Acid-Modified Micelles Encapsulating Gem-C12 and HNK for Glioblastoma Multiforme Chemotherapy. Mol. Pharm. 2018, 15, 1203–1214. [Google Scholar] [CrossRef]
- Sun, F.; Zhang, P.; Liu, Y.; Lu, C.; Qiu, Y.; Mu, H.; Duan, J. A photo-controlled hyaluronan-based drug delivery nanosystem for cancer therapy. Carbohydr. Polym. 2018, 206, 309–318. [Google Scholar] [CrossRef]
- Xia, J.; Du, Y.; Huang, L.; Chaurasiya, B.; Tu, J.; Webster, T.J.; Sun, C. Redox-responsive micelles from disulfide bond-bridged hyaluronic acid-tocopherol succinate for the treatment of melanoma. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 713–723. [Google Scholar] [CrossRef]
- Su, Y.; Liu, Y.; Xu, X.; Zhou, J.; Xu, L.; Xu, X.; Wang, D.; Li, M.; Chen, K.; Wang, W. On-Demand Versatile Prodrug Nanomicelle for Tumor-Specific Bioimaging and Photothermal-Chemo Synergistic Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 38700–38714. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sutrisno, L.; Hou, Y.; Fei, Y.; Xue, C.; Hu, Y.; Li, M.; Luo, Z. A redox-activatable biopolymer-based micelle for sequentially enhanced mitochondria-targeted photodynamic therapy and hypoxia-dependent chemotherapy. Chem. Commun. 2020, 56, 9978–9981. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Arshad, R.; Razzaq, S.; Nousheen, K.; Kiani, M.H.; Shahnaz, G. Formulation and evaluation of hyaluronic acid-based mucoadhesive self nanoemulsifying drug delivery system (SNEDDS) of tamoxifen for targeting breast cancer. Int. J. Biol. Macromol. 2020, 152, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, Y.; Tian, J.; Liu, Z.; Zhuang, A.; Gu, P.; Zhou, H.; Zhang, W.; Fan, X. Fluorinated-functionalized hyaluronic acid nanoparticles for enhanced photodynamic therapy of ocular choroidal melanoma by ameliorating hypoxia. Carbohydr. Polym. 2020, 237, 116119. [Google Scholar] [CrossRef]
- Sun, C.-Y.; Zhang, B.-B.; Zhou, J.-Y. Light-activated drug release from a hyaluronic acid targeted nanoconjugate for cancer therapy. J. Mater. Chem. B 2019, 7, 4843–4853. [Google Scholar] [CrossRef]
- Gao, Q.-Q.; Zhang, C.-M.; Zhang, E.-X.; Chen, H.-Y.; Zhen, Y.-H.; Zhang, S.-B. Zwitterionic pH-responsive hyaluronic acid polymer micelles for delivery of doxorubicin. Colloids Surf. B Biointerfaces 2019, 178, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Chai, Z.; Teng, C.; Yang, L.; Ren, L.; Yuan, Z.; Xu, S.; Cheng, M.; Wang, Y.; Yan, Z.; Qin, C.; et al. Doxorubicin delivered by redox-responsive Hyaluronic Acid–Ibuprofen prodrug micelles for treatment of metastatic breast cancer. Carbohydr. Polym. 2020, 245, 116527. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Zhang, W.; Zhao, Y.; Zhang, S.; Zhang, S. Drug delivery systems based on CD44-targeted glycosaminoglycans for cancer therapy. Carbohydr. Polym. 2021, 251, 117103. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Serri, C.; Quagliariello, V.; Iaffaioli, R.V.; Fusco, S.; Botti, G.; Mayol, L.; Biondi, M. Combination therapy for the treatment of pancreatic cancer through hyaluronic acid-decorated nanoparticles loaded with quercetin and gemcitabine: A preliminary in vitro study. J. Cell. Physiol. 2019, 234, 4959–4969. [Google Scholar] [CrossRef]
- Yu, S.; Wang, S.; Xie, Z.; Yu, S.; Li, L.; Xiao, H.; Song, Y. Hyaluronic acid coating on the surface of curcumin-loaded ZIF-8 nanoparticles for improved breast cancer therapy: An in vitro and in vivo study. Colloids Surf. B Biointerfaces 2021, 203, 111759. [Google Scholar] [CrossRef] [PubMed]
- Naz, S.; Wang, M.; Han, Y.; Hu, B.; Teng, L.; Zhou, J.; Zhang, H.; Chen, J. Enzyme-responsive mesoporous silica nanoparticles for tumor cells and mitochondria multistage-targeted drug delivery. Int. J. Nanomed. 2019, 14, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wei, W.; Chen, X.; Xu, X.; Zhang, X.; Zhang, X. pH and redox dual responsive carrier-free anticancer drug nanoparticles for targeted delivery and synergistic therapy. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102008. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, H.; Khan, A.R.; Yang, X.; Xu, J.; Ji, J.; Zhai, G. Redox-responsive hyaluronic acid-based nanoparticles for targeted photodynamic therapy/chemotherapy against breast cancer. J. Colloid Interface Sci. 2021, 598, 213–228. [Google Scholar] [CrossRef]
- Carton, F.; Chevalier, Y.; Nicoletti, L.; Tarnowska, M.; Stella, B.; Arpicco, S.; Malatesta, M.; Jordheim, L.P.; Briançon, S.; Lollo, G. Rationally designed hyaluronic acid-based nano-complexes for pentamidine delivery. Int. J. Pharm. 2019, 568, 118526. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Hong, E.-H.; Lee, S.Y.; Lee, J.-Y.; Song, J.-H.; Ko, S.-H.; Shim, J.-S.; Choe, S.; Kim, D.-D.; Ko, H.-J.; et al. Boronic acid-tethered amphiphilic hyaluronic acid derivative-based nanoassemblies for tumor targeting and penetration. Acta Biomater. 2017, 53, 414–426. [Google Scholar] [CrossRef]
- Alves, C.G.; de Melo-Diogo, D.; Sousa, A.R.L.; Costa, E.C.; Correia, I.J. Hyaluronic acid functionalized nanoparticles loaded with IR780 and DOX for cancer chemo-photothermal therapy. Eur. J. Pharm. Biopharm. 2019, 137, 86–94. [Google Scholar] [CrossRef]
- Phua, S.Z.F.; Yang, G.; Lim, W.Q.; Verma, A.; Chen, H.; Thanabalu, T.; Zhao, Y. Catalase-Integrated Hyaluronic Acid as Nanocarriers for Enhanced Photodynamic Therapy in Solid Tumor. ACS Nano 2019, 13, 4742–4751. [Google Scholar] [CrossRef]
- Lu, G.; Cao, L.; Zhu, C.; Xie, H.; Hao, K.; Xia, N.; Wang, B.; Zhang, Y.; Liu, F. Improving lung cancer treatment: Hyaluronic acid-modified and glutathione-responsive amphiphilic TPGS-doxorubicin prodrug-entrapped nanoparticles. Oncol. Rep. 2019, 42, 361–369. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, S.; Sarkar, A.; Kundu, M.; Sil, P.C. Targeted delivery of curcumin in breast cancer cells via hyaluronic acid modified mesoporous silica nanoparticle to enhance anticancer efficiency. Colloids Surf. B Biointerfaces 2021, 197, 111404. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, J.; Yang, M.; Xu, W.; Wang, J.; Hou, G.; Ji, L.; Suo, A. Doxorubicin/cisplatin co-loaded hyaluronic acid/chitosan-based nanoparticles for in vitro synergistic combination chemotherapy of breast cancer. Carbohydr. Polym. 2019, 225, 115206. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Luo, B.; Chen, Z.; Yuan, Y.; Kuang, Y.; Wan, L.; Yao, L.; Chen, X.; Jiang, B.; Liu, J.; et al. Host-guest fabrication of dual-responsive hyaluronic acid/mesoporous silica nanoparticle based drug delivery system for targeted cancer therapy. Int. J. Biol. Macromol. 2020, 146, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.; Koul, M.; Mintoo, M.J.; Khare, V.; Gupta, R.; Rawat, N.; Sharma, P.R.; Singh, S.K.; Mondhe, D.M.; Gupta, P.N. Development and characterization of hyaluronic acid modified PLGA based nanoparticles for improved efficacy of cisplatin in solid tumor. Biomed. Pharmacother. 2017, 95, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Cho, H.-J. Mitochondria Targeting and Destabilizing Hyaluronic Acid Derivative-Based Nanoparticles for the Delivery of Lapatinib to Triple-Negative Breast Cancer. Biomacromolecules 2019, 20, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, M.; Xie, Q.; Li, L.; Zhu, L.; Ma, Q.; Gao, S. Construction and evaluation of hyaluronic acid-based copolymers as a targeted chemotherapy drug carrier for cancer therapy. Nanotechnology 2020, 31, 305702. [Google Scholar] [CrossRef]
- Liang, J.; Yang, X.; Liu, D.; Cong, M.; Song, Y.; Bai, S. Lipid/Hyaluronic Acid–Coated Doxorubicin-Fe3O4 as a Dual-Targeting Nanoparticle for Enhanced Cancer Therapy. AAPS PharmSciTech 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Sun, W.; Du, Y.; Liang, X.; Yua, C.; Fanga, J.; Lua, W.; Guoa, X.; Tianbcef, J.; Jina, Y.; Zhenga, J. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials 2019, 217, 119264. [Google Scholar] [CrossRef]
- Gao, D.; Wong, R.C.; Wang, Y.; Guo, X.; Yang, Z.; Lo, P.C. Shifting the absorption to the near-infrared region and inducing a strong photothermal effect by encapsulating zinc(II) phthalocyanine in poly(lactic-co-glycolic acid)-hyaluronic acid nanoparticles. Acta Biomater. 2020, 116, 329–343. [Google Scholar] [CrossRef]
- Saneja, A.; Nayak, D.; Srinivas, M.; Kumar, A.; Khare, V.; Katoch, A.; Goswami, A.; Vishwakarma, R.A.; Sawant, S.D.; Gupta, P.N. Development and mechanistic insight into enhanced cytotoxic potential of hyaluronic acid conjugated nanoparticles in CD44 overexpressing cancer cells. Eur. J. Pharm. Sci. 2017, 97, 79–91. [Google Scholar] [CrossRef]
- Chen, C.; Sun, W.; Wang, X.; Wang, Y.; Wang, P. pH-responsive nanoreservoirs based on hyaluronic acid end-capped mesoporous silica nanoparticles for targeted drug delivery. Int. J. Biol. Macromol. 2018, 111, 1106–1115. [Google Scholar] [CrossRef]
- Pereira, F.M.; Melo, M.N.; Santos, K.M.; Oliveira, K.V.; Diz, F.M.; Ligabue, R.A.; Morrone, F.B.; Severino, P.; Fricks, A.T. Hyaluronic acid-coated chitosan nanoparticles as carrier for the enzyme/prodrug complex based on horseradish peroxidase/indole-3-acetic acid: Characterization and potential therapeutic for bladder cancer cells. Enzym. Microb. Technol. 2021, 150, 109889. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, V.; Gauche, C.; Mazzaferro, S.; Couvet, M.; Vanwonterghem, L.; Henry, M.; Didier, C.; Vollaire, J.; Josserand, V.; Coll, J.-L.; et al. Anti-tumor efficacy of hyaluronan-based nanoparticles for the co-delivery of drugs in lung cancer. J. Control. Release 2018, 275, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Perumalsamy, H.; Castro-Aceituno, V.; Kim, D.; Markus, J.; Lee, S.; Kim, S.; Liu, Y.; Yang, D.C. Photoluminescent And Self-Assembled Hyaluronic Acid-Zinc Oxide-Ginsenoside Rh2 Nanoparticles and Their Potential Caspase-9 Apoptotic Mechanism Towards Cancer Cell Lines. Int. J. Nanomed. 2019, 14, 8195–8208. [Google Scholar] [CrossRef]
- Xu, Y.; Asghar, S.; Yang, L.; Li, H.; Wang, Z.; Ping, Q.; Xiao, Y. Lactoferrin-coated polysaccharide nanoparticles based on chitosan hydrochloride/hyaluronic acid/PEG for treating brain glioma. Carbohydr. Polym. 2017, 157, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, H.; Tang, W.; Zhang, Q.; Li, M.; Jin, H.; Huang, Z.; Cui, Z.; Xu, J.; Wang, K.; et al. A multifunctional magnetic nanosystem based on “two strikes” effect for synergistic anticancer therapy in triple-negative breast cancer. J. Control. Release 2020, 322, 401–415. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, J.; Liu, D.; Zhu, W.; Guan, S.; Fan, L.; Cai, D. Targeted delivery of honokiol by zein/hyaluronic acid core-shell nanoparticles to suppress breast cancer growth and metastasis. Carbohydr. Polym. 2020, 240, 116325. [Google Scholar] [CrossRef]
- Wang, S.; Shao, M.; Zhong, Z.; Wang, A.; Cao, J.; Lu, Y.; Wang, Y.; Zhang, J. Co-delivery of gambogic acid and TRAIL plasmid by hyaluronic acid grafted PEI-PLGA nanoparticles for the treatment of triple negative breast cancer. Drug Deliv. 2017, 24, 1791–1800. [Google Scholar] [CrossRef]
- Gupta, B.; Poudel, B.K.; Ruttala, H.B.; Regmi, S.; Pathak, S.; Gautam, M.; Jin, S.G.; Jeong, J.-H.; Choi, H.-G.; Ku, S.K.; et al. Hyaluronic acid-capped compact silica-supported mesoporous titania nanoparticles for ligand-directed delivery of doxorubicin. Acta Biomater. 2018, 80, 364–377. [Google Scholar] [CrossRef]
- Fang, Z.; Li, X.; Xu, Z.; Du, F.; Wang, W.; Shi, R.; Gao, D. Hyaluronic acid-modified mesoporous silica-coated superparamagnetic Fe3O4 nanoparticles for targeted drug delivery. Int. J. Nanomed. 2019, 14, 5785–5797. [Google Scholar] [CrossRef]
- Matha, K.; Lollo, G.; Taurino, G.; Respaud, R.; Marigo, I.; Shariati, M.; Bussolati, O.; Vermeulen, A.; Remaut, K.; Benoit, J.-P. Bioinspired hyaluronic acid and polyarginine nanoparticles for DACHPt delivery. Eur. J. Pharm. Biopharm. 2020, 150, 1–13. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, R.; Zhong, Z. Facile fabrication of robust, hyaluronic acid-surfaced and disulfide-crosslinked PLGA nanoparticles for tumor-targeted and reduction-triggered release of docetaxel. Acta Biomater. 2021, 125, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, T.; Li, Z.; Luo, C.; Wu, Y.; Zhang, J.; Zhang, X.; Fan, W. Hyaluronate-Based Self-Stabilized Nanoparticles for Immunosuppression Reversion and Immunochemotherapy in Osteosarcoma Treatment. ACS Biomater. Sci. Eng. 2021, 7, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Yuan, X.; Peng, H.; Shan, G.; Gao, M.; Yi, X.; He, X. Targeted delivery and stimulus-responsive release of anticancer drugs for efficient chemotherapy. Drug Deliv. 2021, 28, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, M.; Cao, N.; Qin, W.; Zhao, M.; Wu, J.; Lin, D. Construction of a tumor microenvironment pH-responsive cleavable PEGylated hyaluronic acid nano-drug delivery system for colorectal cancer treatment. Biomater. Sci. 2020, 8, 1885–1896. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-Y.; Lee, C.-C.; Lin, H.-M. Hyaluronidase-Responsive Mesoporous Silica Nanoparticles with Dual-Imaging and Dual-Target Function. Cancers 2019, 11, 697. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Chen, H.; Miao, T.; Ni, J.; Han, L. Prodrug Delivery Using Dual-Targeting Nanoparticles To Treat Breast Cancer Brain Metastases. Mol. Pharm. 2021, 18, 2694–2702. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Cui, M.; Bai, Y.; Liu, Y.; Liu, D.; Song, T. A supramolecular nanoparticle system based on β-cyclodextrin-conjugated poly-l-lysine and hyaluronic acid for co-delivery of gene and chemotherapy agent targeting hepatocellular carcinoma. Colloids Surf. B Biointerfaces 2017, 155, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.S.; Shan, M.H.; Chu, C.C. Inclusion complex from cyclodextrin-grafted hyaluronic acid and pseudo protein as biodegradable nano-delivery vehicle for gambogic acid. Acta Biomater. 2017, 62, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jia, Y.; Zheng, C.W.; Shao, D.; Zhao, Y.; Wang, Z.; Dawulietia, J.; Liu, W.; Sun, M.; Sun, W.; et al. Janus nanocarrier-based co-delivery of doxorubicin and berberine weakens chemotherapy-exacerbated hepatocellular carcinoma recurrence. Acta Biomater. 2019, 100, 352–364. [Google Scholar] [CrossRef]
- Cerqueira, B.B.S.; Lasham, A.; Shelling, A.N.; Al-Kassas, R. Development of biodegradable PLGA nanoparticles surface engineered with hyaluronic acid for targeted delivery of paclitaxel to triple negative breast cancer cells. Mater. Sci. Eng. C 2017, 76, 593–600. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Guo, Q.; Wang, N.; Wu, B.; Zhu, C.; Zhao, W.; Qiang, W.; Zheng, M. A Novel Nanoprobe for Targeted Imaging and Photothermal/Photodynamic Therapy of Lung Cancer. Langmuir 2022, 38, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Park, J.-H.; Ko, S.-H.; Shim, J.-S.; Kim, D.-D.; Cho, H.-J. Mussel-Inspired Hyaluronic Acid Derivative Nanostructures for Improved Tumor Targeting and Penetration. ACS Appl. Mater. Interfaces 2017, 9, 22308–22320. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Zhang, Y.; Ren, X.-H.; He, X.-W.; Li, W.-Y.; Zhang, Y.-K. HA targeted-biodegradable nanocomposites responsive to endogenous and exogenous stimulation for multimodal imaging and chemo-/photothermal therapy. Nanoscale 2021, 13, 886–900. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gu, X.; Wang, H.; Yang, J.; Mao, S. Enhanced delivery of doxorubicin to the liver through self-assembled nanoparticles formed via conjugation of glycyrrhetinic acid to the hydroxyl group of hyaluronic acid. Carbohydr. Polym. 2018, 195, 170–179. [Google Scholar] [CrossRef]
- Sargazi, A.; Kamali, N.; Shiri, F.; Majd, M.H. Hyaluronic acid/polyethylene glycol nanoparticles for controlled delivery of mitoxantrone. Artif. Cells Nanomed. Biotechnol. 2018, 46, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zheng, H.; Fei, Z.; Lu, B.; Zheng, H.; Li, D.; Xiong, X.; Yi, Y. Tumor-targeting and pH-responsive nanoparticles from hyaluronic acid for the enhanced delivery of doxorubicin. Int. J. Biol. Macromol. 2018, 113, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Niu, S.; Williams, G.R.; Wu, J.; Chen, X.; Zheng, H.; Zhu, L.-M. Dual-responsive nanoparticles based on chitosan for enhanced breast cancer therapy. Carbohydr. Polym. 2019, 221, 84–93. [Google Scholar] [CrossRef]
- Gaio, E.; Conte, C.; Esposito, D.; Miotto, G.; Quaglia, F.; Moret, F.; Reddi, E. Co-delivery of Docetaxel and Disulfonate Tetraphenyl Chlorin in One Nanoparticle Produces Strong Synergism between Chemo- and Photodynamic Therapy in Drug-Sensitive and -Resistant Cancer Cells. Mol. Pharm. 2018, 15, 4599–4611. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, D.; Gou, S.; Canup, B.S.B.; Zhang, G.; Dai, F.; Li, C.; Xiao, B. Quadruple-responsive nanoparticle-mediated targeted combination chemotherapy for metastatic breast cancer. Nanoscale 2021, 13, 5765–5779. [Google Scholar] [CrossRef]
- Bhatnagar, P.; Kumari, M.; Pahuja, R.; Pant, A.B.; Shukla, Y.; Kumar, P.; Gupta, K.C. Hyaluronic acid-grafted PLGA nanoparticles for the sustained delivery of berberine chloride for an efficient suppression of Ehrlich ascites tumors. Drug Deliv. Transl. Res. 2018, 8, 565–579. [Google Scholar] [CrossRef]
- Shi, J.; Ren, Y.; Ma, J.; Luo, X.; Li, J.; Wu, Y.; Gu, H.; Fu, C.; Cao, Z.; Zhang, J. Novel CD44-targeting and pH/redox-dual-stimuli-responsive core–shell nanoparticles loading triptolide combats breast cancer growth and lung metastasis. J. Nanobiotechnol. 2021, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Zhu, R.; Sun, W.; Cai, K.; Chen, Y.; Yin, L. Selective cancer treatment via photodynamic sensitization of hypoxia-responsive drug delivery. Nanoscale 2018, 10, 2856–2865. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; He, H.; Liu, Y.; Cao, D.; Yan, J.; Duan, S.; Chen, Y.; Yin, L. Cancer-Selective Bioreductive Chemotherapy Mediated by Dual Hypoxia-Responsive Nanomedicine upon Photodynamic Therapy-Induced Hypoxia Aggravation. Biomacromolecules 2019, 20, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, Y.; Yang, D.; Gao, X.; Wen, T.; Fu, J.; Wen, X.; Quan, G.; Pan, X.; Wu, C. Intelligent and spatiotemporal drug release based on multifunctional nanoparticle-integrated dissolving microneedle system for synergetic chemo-photothermal therapy to eradicate melanoma. Acta Biomater. 2021, 135, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, T.; Cui, M.; Li, N.; Li, Q.; Zhu, L.; Duan, S.; Wang, Y.; Guo, Y. A novel targeted co-delivery nanosystem for enhanced ovarian cancer treatment via multidrug resistance reversion and mTOR-mediated signaling pathway. J. Nanobiotechnol. 2021, 19, 444. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, B.; Zhou, W.; Liu, Y. High-Efficiency Synergistic Effect of Supramolecular Nanoparticles Based on Cyclodextrin Prodrug on Cancer Therapy. Biomacromolecules 2020, 21, 4998–5007. [Google Scholar] [CrossRef]
- Yan, J.; Su, T.; Cheng, F.; Cao, J.; Zhang, H.; He, B. Multifunctional nanoparticles self-assembled from polyethylenimine-based graft polymers as efficient anticancer drug delivery. Colloids Surf. B Biointerfaces 2017, 155, 118–127. [Google Scholar] [CrossRef]
- Qiao, H.; Fang, D.; Zhang, L.; Gu, X.; Lu, Y.; Sun, M.; Sun, C.; Ping, Q.; Li, J.; Chen, Z.; et al. Nanostructured Peptidotoxins as Natural Pro-Oxidants Induced Cancer Cell Death via Amplification of Oxidative Stress. ACS Appl. Mater. Interfaces 2018, 10, 4569–4581. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Hao, Y.; Li, L.; Zheng, C.; Zhao, H.; Niu, M.; Yin, Y.; Zhang, Z.; Zhang, Y. Tumor-targeting core-shell structured nanoparticles for drug procedural controlled release and cancer sonodynamic combined therapy. J. Control. Release 2018, 286, 74–84. [Google Scholar] [CrossRef]
- Li, Y.; Shi, S.; Ming, Y.; Wang, L.; Li, C.; Luo, M.; Li, Z.; Li, B.; Chen, J. Specific cancer stem cell-therapy by albumin nanoparticles functionalized with CD44-mediated targeting. J. Nanobiotechnol. 2018, 16, 99. [Google Scholar] [CrossRef]
- Li, T.; Geng, Y.; Zhang, H.; Wang, J.; Feng, Y.; Chen, Z.; Xie, X.; Qin, X.; Li, S.; Wu, C.; et al. A versatile nanoplatform for synergistic chemo-photothermal therapy and multimodal imaging against breast cancer. Expert Opin. Drug Deliv. 2020, 17, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, Y.; Huang, X.; Liu, B.; Hu, L.; Ma, C.; Liu, J.; Xue, J.; Qu, W.; Liu, F.; et al. A stage-specific cancer chemotherapy strategy through flexible combination of reduction-activated charge-conversional core-shell nanoparticles. Theranostics 2019, 9, 6532–6549. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qiao, L.; Zhang, S.; Wan, G.; Chen, B.; Zhou, P.; Zhang, N.; Wang, Y. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef]
- Hou, G.; Qian, J.; Guo, M.; Xu, W.; Wang, J.; Wang, Y.; Suo, A. Hydrazided hyaluronan/cisplatin/indocyanine green coordination nanoprodrug for photodynamic chemotherapy in liver cancer. Carbohydr. Polym. 2022, 276, 118810. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Chen, R.; Chen, X.; Zhang, H.; Song, L.; Cui, W.; Zhang, J.; Ye, D.; Zhang, Y.; Wang, Z. Oridonin-loaded and GPC1-targeted gold nanoparticles for multimodal imaging and therapy in pancreatic cancer. Int. J. Nanomed. 2018, 13, 6809–6827. [Google Scholar] [CrossRef]
- Kabary, D.M.; Helmy, M.W.; Abdelfattah, E.-Z.A.; Fang, J.-Y.; Elkhodairy, K.A.; Elzoghby, A.O. Inhalable multi-compartmental phospholipid enveloped lipid core nanocomposites for localized mTOR inhibitor/herbal combined therapy of lung carcinoma. Eur. J. Pharm. Biopharm. 2018, 130, 152–164. [Google Scholar] [CrossRef]
- Jin, R.; Xie, J.; Yang, X.; Tian, Y.; Yuan, P.; Bai, Y.; Liu, S.; Cai, B.; Chen, X. A tumor-targeted nanoplatform with stimuli-responsive cascaded activities for multiple model tumor therapy. Biomater. Sci. 2020, 8, 1865–1874. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, X.; Nie, W.; Yang, Y.; Wu, C.; Liu, W.; Zhang, K.; Zhang, Z.; Shi, J. Tumor cell-activated “Sustainable ROS Generator” with homogeneous intratumoral distribution property for improved anti-tumor therapy. Theranostics 2021, 11, 379–396. [Google Scholar] [CrossRef]
- Seo, J.-H.; Lee, S.Y.; Hwang, C.; Yang, M.; Lee, J.; Lee, S.-H.; Cho, H.-J. Multi-layered cellulose nanocrystal system for CD44 receptor-positive tumor-targeted anticancer drug delivery. Int. J. Biol. Macromol. 2020, 162, 798–809. [Google Scholar] [CrossRef]
- Liu, T.; Huang, X.; Zhao, L.; Xiao, Z.; Li, Z.; Xin, Y.; Yang, S.; Guo, D.; Zhao, W.; Mi, Y.; et al. Distinguishable Targeting of Non-Small Cell Lung Cancer Using Hyaluronan Functionalized Platinum Nanoclusters and Their Inhibition Behaviors of Proliferation, Invasion, Migration. ChemistryOpen 2021, 10, 882–888. [Google Scholar] [CrossRef]
- Fan, R.; Chuan, D.; Hou, H.; Chen, H.; Han, B.; Zhang, X.; Zhou, L.; Tong, A.; Xu, J.; Guo, G. Development of a hybrid nanocarrier-recognizing tumor vasculature and penetrating the BBB for glioblastoma multi-targeting therapy. Nanoscale 2019, 11, 11285–11304. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Lai, C.-J.; Lin, Y.-N.; Huang, C.-M.; Lin, Y.-H. Multifunctional nanoparticles for targeting the tumor microenvironment to improve synergistic drug combinations and cancer treatment effects. J. Mater. Chem. B 2020, 8, 10416–10427. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, L.; Lu, Q.; Yang, S.; Yang, L.; Cheng, Y.; Wang, Y.; Wang, S.; Song, Y.; Tan, F.; et al. Ultrasmall MoS2 Nanodots-Doped Biodegradable SiO2 Nanoparticles for Clearable FL/CT/MSOT Imaging-Guided PTT/PDT Combination Tumor Therapy. ACS Appl. Mater. Interfaces 2019, 11, 5771–5781. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lu, T.; Wang, Y.; Song, Y.; Wang, S.; Lu, Q.; Yang, L.; Tan, F.; Li, J.; Li, N. Glutathione-Mediated Clearable Nanoparticles Based on Ultrasmall Gd2O3 for MSOT/CT/MR Imaging Guided Photothermal/Radio Combination Cancer Therapy. Mol. Pharm. 2019, 16, 3489–3501. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tan, X.; Wang, J.; Wang, Y.; Song, Y.; You, Q.; Sun, Q.; Liu, L.; Wang, S.; Tan, F.; et al. Polymer-based gadolinium oxide nanocomposites for FL/MR/PA imaging guided and photothermal/photodynamic combined anti-tumor therapy. J. Control. Release 2018, 277, 77–88. [Google Scholar] [CrossRef]
- Lin, X.; Cao, Y.; Xue, Y.; Wu, F.; Yu, F.; Wu, M.; Zhu, X. Multifunctional theranostic agents based on prussian blue nanoparticles for tumor targeted and MRI—Guided photodynamic/photothermal combined treatment. Nanotechnology 2020, 31, 135101. [Google Scholar] [CrossRef]
- Elsey, J.; Bubley, J.A.; Zhu, L.; Rao, S.; Sasaki, M.; Pollack, B.P.; Yang, L.; Arbiser, J.L. Palladium based nanoparticles for the treatment of advanced melanoma. Sci. Rep. 2019, 9, 3255. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, P.; Meng, Y.; Hu, S.; Ding, J.; Zhou, W. Nanoscale Copper(II)–Diethyldithiocarbamate Coordination Polymer as a Drug Self-Delivery System for Highly Robust and Specific Cancer Therapy. Mol. Pharm. 2020, 17, 2864–2873. [Google Scholar] [CrossRef]
- Liao, J.; Zheng, H.; Hu, R.; Cao, J.; Wei, X.; Li, D.; Zheng, H.; Yin, Y. Hyaluronan Based Tumor-Targeting and pH-Responsive Shell Cross-Linkable Nanoparticles for the Controlled Release of Doxorubicin. J. Biomed. Nanotechnol. 2018, 14, 496–509. [Google Scholar] [CrossRef]
- Sheng, S.; Liu, F.; Lin, L.; Yan, N.; Wang, Y.; Xu, C.; Tian, H.; Chen, X. Nanozyme-mediated cascade reaction based on metal-organic framework for synergetic chemo-photodynamic tumor therapy. J. Control. Release 2020, 328, 631–639. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Y.; Ma, J.; Lin, J.; Zhang, Y.; Fan, Z.; Su, G.; Xie, L.; Zhu, X.; et al. Integration of phospholipid-hyaluronic acid-methotrexate nanocarrier assembly and amphiphilic drug-drug conjugate for synergistic targeted delivery and combinational tumor therapy. Biomater. Sci. 2018, 6, 1818–1833. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, J.; Yan, X.; Li, C.; Elsabahy, M.; Chen, L.; Yang, Y.-W.; Gao, H. A dendritic polyamidoamine supramolecular system composed of pillar[5]arene and azobenzene for targeting drug-resistant colon cancer. J. Mater. Chem. B 2021, 9, 9594–9605. [Google Scholar] [CrossRef]
- Zeng, S.; Liu, S.; Lan, Y.; Qiu, T.; Zhou, M.; Gao, W.; Huang, W.; Ge, L.; Zhang, J. Combined Photothermotherapy and Chemotherapy of Oral Squamous Cell Carcinoma Guided by Multifunctional Nanomaterials Enhanced Photoacoustic Tomography. Int. J. Nanomed. 2021, 16, 7373–7390. [Google Scholar] [CrossRef]
- Xu, Y.; Asghar, S.; Gao, S.; Chen, Z.; Huang, L.; Yin, L.; Ping, Q.; Xiao, Y. Polysaccharide-based nanoparticles for co-loading mitoxantrone and verapamil to overcome multidrug resistance in breast tumor. Int. J. Nanomed. 2017, 12, 7337–7350. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.-Y.; Zhang, J.-J.; Zhang, Y.-X.; Wang, W.; Li, C.; Zhou, S.-Y.; Zhang, B.-L. Construction of redox-responsive tumor targeted cisplatin nano-delivery system for effective cancer chemotherapy. Int. J. Pharm. 2020, 580, 119190. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Shi, Z.; Qi, H.; Zhao, M.; Huang, K.; Han, D.; Zhou, J.; Liu, C.; Liu, Y.; Lu, Y.; et al. A novel Granzyme B nanoparticle delivery system simulates immune cell functions for suppression of solid tumors. Theranostics 2019, 9, 7616–7627. [Google Scholar] [CrossRef]
- Cui, X.; Deng, X.; Liang, Z.; Lu, J.; Shao, L.; Wang, X.; Jia, F.; Pan, Z.; Hu, Q.; Xiao, X.; et al. Multicomponent-assembled nanodiamond hybrids for targeted and imaging guided triple-negative breast cancer therapy via a ternary collaborative strategy. Biomater. Sci. 2021, 9, 3838–3850. [Google Scholar] [CrossRef]
- Singh, B.; Maharjan, S.; Pan, D.C.; Zhao, Z.; Gao, Y.; Zhang, Y.S.; Mitragotri, S. Imiquimod-gemcitabine nanoparticles harness immune cells to suppress breast cancer. Biomaterials 2022, 280, 121302. [Google Scholar] [CrossRef]
- Gou, S.; Yang, J.; Ma, Y.; Zhang, X.; Zu, M.; Kang, T.; Liu, S.; Ke, B.; Xiao, B. Multi-responsive nanococktails with programmable targeting capacity for imaging-guided mitochondrial phototherapy combined with chemotherapy. J. Control. Release 2020, 327, 371–383. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Li, K.; Wang, Y.; Wu, J.; Zeng, Y.; Wu, D. Versatile hyaluronic acid modified AQ4N-Cu(II)-gossypol infinite coordination polymer nanoparticles: Multiple tumor targeting, highly efficient synergistic chemotherapy, and real-time self-monitoring. Biomaterials 2018, 154, 197–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, Q.; Wu, T.; He, Z.; Li, Y.; Li, Z.; Hou, X.; He, Y.; Ruan, S.; Wang, Z.; et al. A novel multi-functionalized multicellular nanodelivery system for non-small cell lung cancer photochemotherapy. J. Nanobiotechnol. 2021, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, L.; Bai, X.; Cao, X.; Jiao, X.; Huang, Y.; Li, Y.; Qin, Y.; Wen, Y. Stimuli-Responsive Nanocarrier for Co-delivery of MiR-31 and Doxorubicin to Suppress High MtEF4 Cancer. ACS Appl. Mater. Interfaces 2018, 10, 22767–22775. [Google Scholar] [CrossRef] [PubMed]
- Cong, Z.; Zhang, L.; Ma, S.-Q.; Lam, K.S.; Yang, F.-F.; Liao, Y.-H. Size-Transformable Hyaluronan Stacked Self-Assembling Peptide Nanoparticles for Improved Transcellular Tumor Penetration and Photo–Chemo Combination Therapy. ACS Nano 2020, 14, 1958–1970. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Tao, Y.; Li, X.; Pang, Y.; Yang, C.; Jiang, G.; Liu, Y. CD44-Targeting Oxygen Self-Sufficient Nanoparticles for Enhanced Photodynamic Therapy Against Malignant Melanoma. Int. J. Nanomed. 2020, 15, 10401–10416. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; You, Q.; Wang, J.; Song, Y.; Cheng, Y.; Wang, Y.; Yang, S.; Yang, L.; Li, P.; Lu, Q.; et al. MSOT/CT/MR imaging-guided and hypoxia-maneuvered oxygen self-supply radiotherapy based on one-pot MnO2-mSiO2@Au nanoparticles. Nanoscale 2019, 11, 6270–6284. [Google Scholar] [CrossRef]
- Shen, J.; Ma, M.; Zhang, H.; Yu, H.; Xue, F.; Hao, N.; Chen, H. Microfluidics-Assisted Surface Trifunctionalization of a Zeolitic Imidazolate Framework Nanocarrier for Targeted and Controllable Multitherapies of Tumors. ACS Appl. Mater. Interfaces 2020, 12, 45838–45849. [Google Scholar] [CrossRef]
- Torino, E.; Auletta, L.; Vecchione, D.; Orlandella, F.M.; Salvatore, G.; Iaccino, E.; Fiorenza, D.; Grimaldi, A.M.; Sandomenico, A.; Albanese, S.; et al. Multimodal imaging for a theranostic approach in a murine model of B-cell lymphoma with engineered nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 483–491. [Google Scholar] [CrossRef]
- Yalcin, E.; Kara, G.; Celik, E.; Pinarli, F.A.; Saylam, G.; Sucularli, C.; Ozturk, S.; Yilmaz, E.; Bayır, O.; Korkmaz, M.H.; et al. Preparation and characterization of novel albumin-sericin nanoparticles as siRNA delivery vehicle for laryngeal cancer treatment. Prep. Biochem. Biotechnol. 2019, 49, 659–670. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, W.; Li, H.; Li, X.; Ma, H. H2O2 -Responsive Organosilica-Doxorubicin Nanoparticles for Targeted Imaging and Killing of Cancer Cells Based on a Synthesized Silane-Borate Precursor. ChemMedChem 2019, 14, 1079–1085. [Google Scholar] [CrossRef]
- Huang, S.; Li, C.; Wang, W.; Li, H.; Sun, Z.; Song, C.; Li, B.; Duan, S.; Hu, Y. A54 peptide-mediated functionalized gold nanocages for targeted delivery of DOX as a combinational photothermal-chemotherapy for liver cancer. Int. J. Nanomed. 2017, 12, 5163–5176. [Google Scholar] [CrossRef]
- Xie, R.; Lian, S.; Peng, H.; Ouyang, C.; Li, S.; Lu, Y.; Cao, X.; Zhang, C.; Xu, J.; Jia, L. Mitochondria and Nuclei Dual-Targeted Hollow Carbon Nanospheres for Cancer Chemophotodynamic Synergistic Therapy. Mol. Pharm. 2019, 16, 2235–2248. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, J.; Liu, L.; Sun, Q.; You, Q.; Cheng, Y.; Wang, Y.; Wang, S.; Tan, F.; Li, N. One-Pot Synthesis of a Bismuth Selenide Hexagon Nanodish Complex for Multimodal Imaging-Guided Combined Antitumor Phototherapy. Mol. Pharm. 2018, 15, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sun, D.; Liao, H.; Wang, Y.; Zhao, S.; Zhang, Y.; Lv, G.; Ma, X.; Liu, Y.; Sun, G. Synthesis and characterization of a bimodal nanoparticle based on the host-guest self-assembly for targeted cellular imaging. Talanta 2017, 171, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Guo, Z.; Zheng, K.; Ma, K.; Cui, C.; Wang, L.; Yuan, Y.; Tang, Y. Dual targeting mesoporous silica nanoparticles for inhibiting tumour cell invasion and metastasis. Int. J. Pharm. 2017, 534, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Dong, Y.; Yue, S.; Qiu, X.; Sun, H.; Zhong, Z. Dually Active Targeting Nanomedicines Based on a Direct Conjugate of Two Purely Natural Ligands for Potent Chemotherapy of Ovarian Tumors. ACS Appl. Mater. Interfaces 2019, 11, 46548–46557. [Google Scholar] [CrossRef]
- Su, X.; Zhao, F.; Wang, Y.; Yan, X.; Jia, S.; Du, B. CuS as a gatekeeper of mesoporous upconversion nanoparticles-based drug controlled release system for tumor-targeted multimodal imaging and synergetic chemo-thermotherapy. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1761–1772. [Google Scholar] [CrossRef]
- Feng, X.; Xiong, X.; Ma, S. Docetaxel-Loaded Novel Nano-Platform for Synergistic Therapy of Non-Small Cell Lung Cancer. Front. Pharmacol. 2022, 13, 832725. [Google Scholar] [CrossRef]
- Trivedi, M.; Singh, A.; Talekar, M.; Pawar, G.; Shah, P.; Amiji, M. MicroRNA-34a Encapsulated in Hyaluronic Acid Nanoparticles Induces Epigenetic Changes with Altered Mitochondrial Bioenergetics and Apoptosis in Non-Small-Cell Lung Cancer Cells. Sci. Rep. 2017, 7, 3636. [Google Scholar] [CrossRef]
- Li, H.; Zhuang, S.; Yang, Y.; Zhou, F.; Rong, J.; Zhao, J. ATP/Hyals dually responsive core-shell hyaluronan/chitosan-based drug nanocarrier for potential application in breast cancer therapy. Int. J. Biol. Macromol. 2021, 183, 839–851. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, R.; Feng, Q.; Zhuo, X.; Wang, R. Development of a carrier system containing hyaluronic acid and protamine for siRNA delivery in the treatment of melanoma. Investig. New Drugs 2021, 39, 66–76. [Google Scholar] [CrossRef]
- Parayath, N.; Parikh, A.; Amiji, M.M. Repolarization of Tumor-Associated Macrophages in a Genetically Engineered Nonsmall Cell Lung Cancer Model by Intraperitoneal Administration of Hyaluronic Acid-Based Nanoparticles Encapsulating MicroRNA-125b. Nano Lett. 2018, 18, 3571–3579. [Google Scholar] [CrossRef] [PubMed]
- Parayath, N.N.; Gandham, S.K.; Leslie, F.; Amiji, M.M. Improved anti-tumor efficacy of paclitaxel in combination with MicroRNA-125b-based tumor-associated macrophage repolarization in epithelial ovarian cancer. Cancer Lett. 2019, 461, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liang, G.-F.; Li, Y.; Li, J.-H.; Jing, A.-H.; Feng, W.-P.; Li, G.-D.; Du, J.-X.; Feng, S.-Y. Preparation and Evaluation of Upconversion Nanoparticles Based miRNA Delivery Carrier in Colon Cancer Mice Model. J. Biomed. Nanotechnol. 2019, 15, 2240–2250. [Google Scholar] [CrossRef] [PubMed]
- Aldawsari, H.M.; Dhaliwal, H.K.; Aljaeid, B.M.; Alhakamy, N.A.; Banjar, Z.M.; Amiji, M.M. Optimization of the Conditions for Plasmid DNA Delivery and Transfection with Self-Assembled Hyaluronic Acid-Based Nanoparticles. Mol. Pharm. 2019, 16, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.-L.; Li, Y.; Zhao, L.-M.; Su, L.-W.; Ding, G. Delivery of MTH1 inhibitor (TH287) and MDR1 siRNA via hyaluronic acid-based mesoporous silica nanoparticles for oral cancers treatment. Colloids Surf. B Biointerfaces 2019, 173, 599–606. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Lan, Y.; He, X.; Liu, K.; Liang, Y. Antitumor effect of hyaluronic-acid-modified chitosan nanoparticles loaded with siRNA for targeted therapy for non-small cell lung cancer. Int. J. Nanomed. 2019, 14, 5287–5301. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Qiu, Y.; Liu, Y.; Ding, M.; Zhang, Y. Anti-miRNA21 and resveratrol-loaded polysaccharide-based mesoporous silica nanoparticle for synergistic activity in gastric carcinoma. J. Drug Target. 2019, 27, 1135–1143. [Google Scholar] [CrossRef]
- Luo, K.; Yin, S.; Zhang, R.; Yu, H.; Wang, G.; Li, J. Multifunctional composite nanoparticles based on hyaluronic acid-paclitaxel conjugates for enhanced cancer therapy. Int. J. Pharm. 2020, 589, 119870. [Google Scholar] [CrossRef]
- Edelman, R.; Assaraf, Y.G.; Levitzky, I.; Shahar, T.; Livney, Y.D. Hyaluronic acid-serum albumin conjugate-based nanoparticles for targeted cancer therapy. Oncotarget 2017, 8, 24337–24353. [Google Scholar] [CrossRef]
- Zhang, X.; Pan, J.; Yao, M.; Mendes, L.P.; Sarisozen, C.; Mao, S.; Torchilin, V.P. Charge reversible hyaluronic acid-modified dendrimer-based nanoparticles for siMDR-1 and doxorubicin co-delivery. Eur. J. Pharm. Biopharm. 2020, 154, 43–49. [Google Scholar] [CrossRef]
- Wu, J.; Hu, X.; Liu, R.; Zhang, J.; Song, A.; Luan, Y. pH-responsive and self-targeting assembly from hyaluronic acid-based conjugate toward all-in-one chemo-photodynamic therapy. J. Colloid Interface Sci. 2019, 547, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Rangasami, V.K.; Samanta, S.; Parihar, V.S.; Asawa, K.; Zhu, K.; Varghese, O.P.; Teramura, Y.; Nilsson, B.; Hilborn, J.; Harris, R.A.; et al. Harnessing hyaluronic acid-based nanoparticles for combination therapy: A novel approach for suppressing systemic inflammation and to promote antitumor macrophage polarization. Carbohydr. Polym. 2021, 254, 117291. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Xiao, F.; Wang, Z.; Wang, B.; Pan, Z.; Zhao, W.; Zhu, Z.; Zhang, J. Redox-Sensitive Hyaluronic Acid Polymer Prodrug Nanoparticles for Enhancing Intracellular Drug Self-Delivery and Targeted Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 6, 4106–4115. [Google Scholar] [CrossRef] [PubMed]
- Janik-Hazuka, M.; Szafraniec-Szczęsny, J.; Kamiński, K.; Odrobińska, J.; Zapotoczny, S. Uptake and in vitro anticancer activity of oleic acid delivered in nanocapsules stabilized by amphiphilic derivatives of hyaluronic acid and chitosan. Int. J. Biol. Macromol. 2020, 164, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cai, H.; Yin, T.; Huo, M.; Ma, P.; Zhou, J.; Lai, W. Paclitaxel-loaded redox-sensitive nanoparticles based on hyaluronic acid-vitamin E succinate conjugates for improved lung cancer treatment. Int. J. Nanomed. 2018, 13, 1585–1600. [Google Scholar] [CrossRef] [PubMed]
- Molina-Crespo, A.; Cadete, A.; Sarrio, D.; Gámez-Chiachio, M.; Martinez, L.; Chao, K.; Olivera, A.; Gonella, A.; Díaz, E.; Palacios, J.; et al. Intracellular Delivery of an Antibody Targeting Gasdermin-B Reduces HER2 Breast Cancer Aggressiveness. Clin. Cancer Res. 2019, 25, 4846–4858. [Google Scholar] [CrossRef]
- Rata, D.M.; Cadinoiu, A.N.; Atanase, L.I.; Popa, M.; Mihai, C.-T.; Solcan, C.; Ochiuz, L.; Vochita, G. Topical formulations containing aptamer-functionalized nanocapsules loaded with 5-fluorouracil—An innovative concept for the skin cancer therapy. Mater. Sci. Eng. C 2021, 119, 111591. [Google Scholar] [CrossRef]
- Chen, Q.; Li, X.; Xie, Y.; Hu, W.; Cheng, Z.; Zhong, H.; Zhu, H. Azo modified hyaluronic acid based nanocapsules: CD44 targeted, UV-responsive decomposition and drug release in liver cancer cells. Carbohydr. Polym. 2021, 267, 118152. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).