Transfer Phenomena of Nanoliposomes by Live Imaging of Primary Cultures of Cortical Neurons
Abstract
1. Introduction
2. Materials and Methods
2.1. Lecithin Extraction
2.2. Fatty Acid Composition Analysis
2.3. Lipid Classes of Nanoliposomes
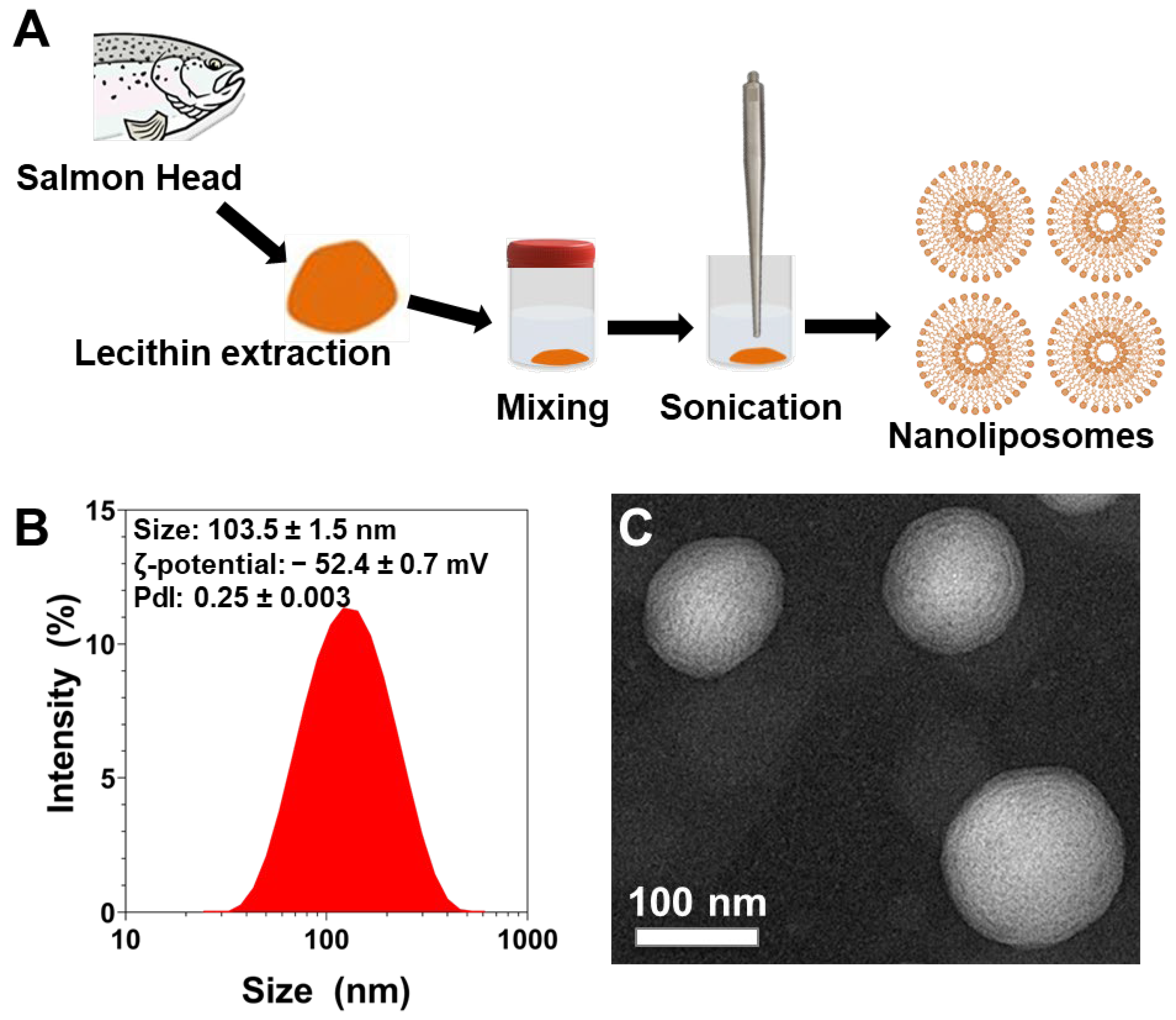
2.4. Preparation of Nanoliposomes
2.5. Nanoliposomes Characterization
2.6. Transmission Electron Microscopy
2.7. Primary Cultures of Cortical Neurons
2.8. Microscopy Imaging of Live Cortical Neurons
2.9. Image Analysis of Nanoliposomes in Cortical Neurons
2.10. Mean Square Displacement and Diffusion Coefficient
2.11. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition of Salmon Lecithin
3.2. Lipid Classes of Salmon Lecithin
3.3. Physicochemical Characterization of Nanoliposomes after Their Preparation
3.4. Diameter and Average Speed of Nanoliposomes over Time after Incubation with Cortical Neurons
3.5. Mean Square Displacement and Diffusion Coefficient
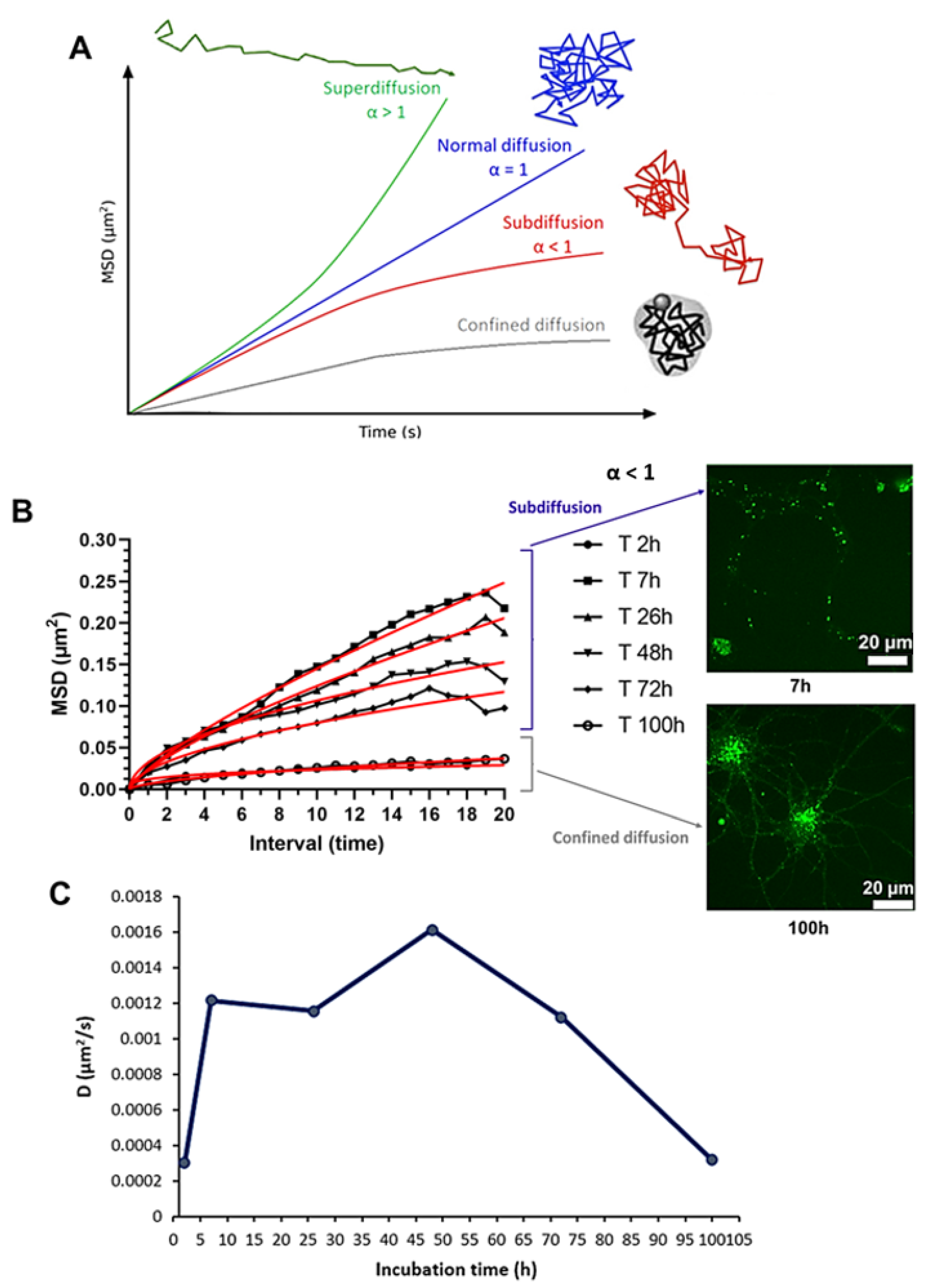
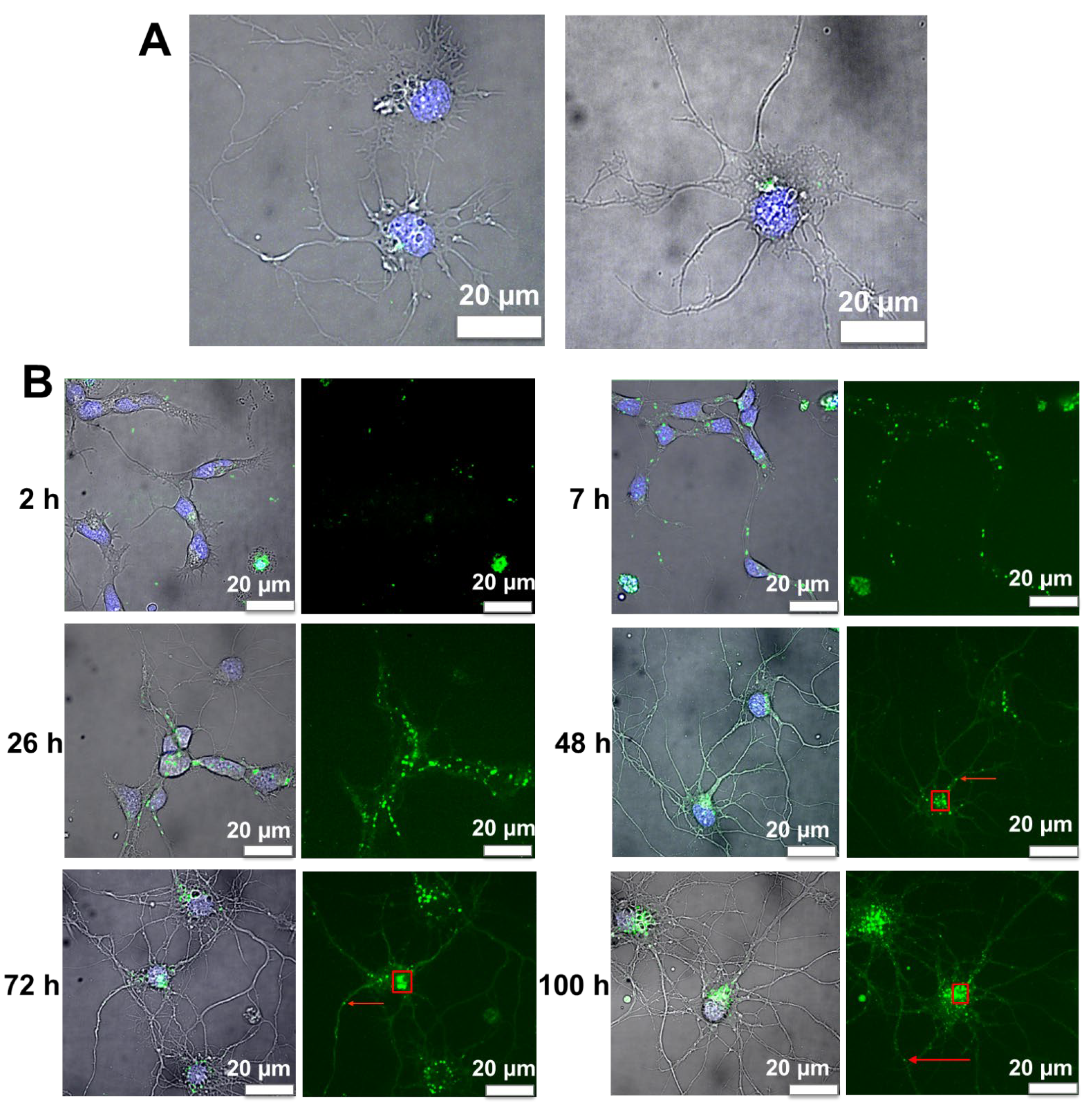
3.6. Internalization of Nanoliposomes Inside Cortical Neurons
3.7. Image Analysis of Cortical Neurons Morphology after Incubation over Time
3.8. Fluorescence Image Analysis of Nanoliposomes over Time
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Mozafari, M.R. Liposomes: An overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar] [PubMed]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A Review of Manufacturing Techniques and Targeting Strategies. Available online: http://www.eurekaselect.com/73978/article (accessed on 7 March 2019).
- Mozafari, M.R.; Johnson, C.; Hatziantoniou, S.; Demetzos, C. Nanoliposomes and Their Applications in Food Nanotechnology. J. Liposome Res. 2008, 18, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef]
- Barnabas, W. Drug targeting strategies into the brain for treating neurological diseases. J. Neurosci. Methods 2018, 311, 133–146. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Chen, I.-Y.; Rajesh, R. Use of functionalized liposomes loaded with antioxidants to permeate the blood–brain barrier and inhibit β-amyloid-induced neurodegeneration in the brain. J. Taiwan Inst. Chem. Eng. 2018, 87, 1–14. [Google Scholar] [CrossRef]
- Agrawal, M.; Ajazuddin; Tripathi, D.K.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Mourtas, S.; Hammarlund-Udenaes, M.; Alexander, A. Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. J. Control. Release 2017, 260, 61–77. [Google Scholar] [CrossRef]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef]
- Khorasani, S.; Danaei, M.; Mozafari, M. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Malaplate, C.; Poerio, A.; Huguet, M.; Soligot, C.; Passeri, E.; Kahn, C.J.F.; Linder, M.; Arab-Tehrany, E.; Yen, F.T. Neurotrophic Effect of Fish-Lecithin Based Nanoliposomes on Cortical Neurons. Mar. Drugs 2019, 17, 406. [Google Scholar] [CrossRef]
- Passeri, E.; Elkhoury, K.; Garavito, M.C.J.; Desor, F.; Huguet, M.; Soligot-Hognon, C.; Linder, M.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Use of Active Salmon-Lecithin Nanoliposomes to Increase Polyunsaturated Fatty Acid Bioavailability in Cortical Neurons and Mice. Int. J. Mol. Sci. 2021, 22, 11859. [Google Scholar] [CrossRef]
- Qian, H.; Sheetz, M.; Elson, E. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys. J. 1991, 60, 910–921. [Google Scholar] [CrossRef]
- Ruthardt, N.; Lamb, D.C.; Bräuchle, C. Single-particle Tracking as a Quantitative Microscopy-based Approach to Unravel Cell Entry Mechanisms of Viruses and Pharmaceutical Nanoparticles. Mol. Ther. 2011, 19, 1199–1211. [Google Scholar] [CrossRef]
- Saxton, M.J.; Jacobson, K. Single-Particle Trackng: Applications to Membrane Dynamics. Annu. Rev. Biophys. Biomol. Struct. 1997, 26, 373–399. [Google Scholar] [CrossRef]
- Weiss, M.; Nilsson, T. In a mirror dimly: Tracing the movements of molecules in living cells. Trends Cell Biol. 2004, 14, 267–273. [Google Scholar] [CrossRef]
- Tehrany, E.A.; Kahn, C.J.; Baravian, C.; Maherani, B.; Belhaj, N.; Wang, X.; Linder, M. Elaboration and characterization of nanoliposome made of soya; rapeseed and salmon lecithins: Application to cell culture. Colloids Surf. B Biointerfaces 2012, 95, 75–81. [Google Scholar] [CrossRef]
- Linder, M.; Fanni, J.; Parmentier, M.; Regnault, P. Procédé d’extraction d’huile Par Voie Enzymatique et Obtention d’hydrolysats Protéiques à Fonctionnalités Dirigées. Brev. FR 2002, 2, 703. [Google Scholar]
- Ackman, R.G. Remarks on official methods employing boron trifluoride in the preparation of methyl esters of the fatty acids of fish oils. J. Am. Oil Chem. Soc. 1998, 75, 541–545. [Google Scholar] [CrossRef]
- Hasan, M.; Belhaj, N.; Benachour, H.; Barberi-Heyob, M.; Kahn, C.J.F.; Jabbari, E.; Linder, M.; Arab-Tehrany, E. Liposome encapsulation of curcumin: Physico-chemical characterizations and effects on MCF7 cancer cell proliferation. Int. J. Pharm. 2014, 461, 519–528. [Google Scholar] [CrossRef]
- Hasan, M.; ElKhoury, K.; Kahn, C.J.F.; Arab-Tehrany, E.; Linder, M. Preparation, Characterization, and Release Kinetics of Chitosan-Coated Nanoliposomes Encapsulating Curcumin in Simulated Environments. Molecules 2019, 24, 2023. [Google Scholar] [CrossRef]
- Li, J.; ElKhoury, K.; Barbieux, C.; Linder, M.; Grandemange, S.; Tamayol, A.; Francius, G.; Arab-Tehrany, E. Effects of Bioactive Marine-Derived Liposomes on Two Human Breast Cancer Cell Lines. Mar. Drugs 2020, 18, 211. [Google Scholar] [CrossRef]
- ElKhoury, K.; Sanchez-Gonzalez, L.; Lavrador, P.; Almeida, R.; Gaspar, V.; Kahn, C.; Cleymand, F.; Arab-Tehrany, E.; Mano, J.F. Gelatin Methacryloyl (GelMA) Nanocomposite Hydrogels Embedding Bioactive Naringin Liposomes. Polymers 2020, 12, 2944. [Google Scholar] [CrossRef]
- Hasan, M.; ElKhoury, K.; Belhaj, N.; Kahn, C.; Tamayol, A.; Barberi-Heyob, M.; Arab-Tehrany, E.; Linder, M. Growth-Inhibitory Effect of Chitosan-Coated Liposomes Encapsulating Curcumin on MCF-7 Breast Cancer Cells. Mar. Drugs 2020, 18, 217. [Google Scholar] [CrossRef]
- Colas, J.-C.; Shi, W.; Rao, V.M.; Omri, A.; Mozafari, M.R.; Singh, H. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron 2007, 38, 841–847. [Google Scholar] [CrossRef]
- Colin, J.; Allouche, A.; Chauveau, F.; Corbier, C.; Pauron-Gregory, L.; Lanhers, M.-C.; Claudepierre, T.; Yen, F.T.; Oster, T.; Malaplate-Armand, C. Improved Neuroprotection Provided by Drug Combination in Neurons Exposed to Cell-Derived Soluble Amyloid-β Peptide. J. Alzheimer’s Dis. 2016, 52, 975–987. [Google Scholar] [CrossRef]
- Saxton, M. Single-particle tracking: The distribution of diffusion coefficients. Biophys. J. 1997, 72, 1744–1753. [Google Scholar] [CrossRef]
- Mady, M.M.; Darwish, M.M.; Khalil, S.; Khalil, W.M. Biophysical studies on chitosan-coated liposomes. Eur. Biophys. J. 2009, 38, 1127–1133. [Google Scholar] [CrossRef]
- Latifi, S.; Tamayol, A.; Habibey, R.; Sabzevari, R.; Kahn, C.; Geny, D.; Eftekharpour, E.; Annabi, N.; Blau, A.; Linder, M.; et al. Natural lecithin promotes neural network complexity and activity. Sci. Rep. 2016, 6, 25777. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Latifi, S.; Kahn, C.; Tamayol, A.; Habibey, R.; Passeri, E.; Linder, M.; Arab-Tehrany, E. The Positive Role of Curcumin-Loaded Salmon Nanoliposomes on the Culture of Primary Cortical Neurons. Mar. Drugs 2018, 16, 218. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896. [Google Scholar] [CrossRef]
- Bahari, L.A.S.; Hamishehkar, H. The Impact of Variables on Particle Size of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers; A Comparative Literature Review. Adv. Pharm. Bull. 2016, 6, 143–151. [Google Scholar] [CrossRef]
- Oliveira, F.A.; Ferreira, R.M.S.; Lapas, L.C.; Vainstein, M.H. Anomalous Diffusion: A Basic Mechanism for the Evolution of Inhomogeneous Systems. Front. Phys. 2019, 7. [Google Scholar] [CrossRef]
- Gajowczyk, M.; Szwabiński, J. Detection of Anomalous Diffusion with Deep Residual Networks. Entropy 2021, 23, 649. [Google Scholar] [CrossRef]
- Höfling, F.; Franosch, T. Anomalous transport in the crowded world of biological cells. Rep. Prog. Phys. 2013, 76, 046602. [Google Scholar] [CrossRef]
- Kapanidis, A.N.; Uphoff, S.; Stracy, M. Understanding Protein Mobility in Bacteria by Tracking Single Molecules. J. Mol. Biol. 2018, 430, 4443–4455. [Google Scholar] [CrossRef]
- Alves, S.B.; de Oliveira, G.F.; de Oliveira, L.C.; de Silans, T.P.; Chevrollier, M.; Oriá, M.; Cavalcante, H.L.D.S. Characterization of diffusion processes: Normal and anomalous regimes. Phys. A Stat. Mech. Its Appl. 2016, 447, 392–401. [Google Scholar] [CrossRef]
- Debets, V.E.; Janssen, L.M.C.; Saric, A. Characterising the diffusion of biological nanoparticles on fluid and cross-linked membranes. Soft Matter 2020, 16, 10628–10639. [Google Scholar] [CrossRef]
- Krapf, D. Mechanisms Underlying Anomalous Diffusion in the Plasma Membrane. Curr. Top. Membr. 2015, 75, 167–207. [Google Scholar] [CrossRef]
- Saxton, M. Anomalous diffusion due to binding: A Monte Carlo study. Biophys. J. 1996, 70, 1250–1262. [Google Scholar] [CrossRef]
- Saxton, M. Anomalous diffusion due to obstacles: A Monte Carlo study. Biophys. J. 1994, 66, 394–401. [Google Scholar] [CrossRef]
- Ghosh, R.; Webb, W. Automated detection and tracking of individual and clustered cell surface low density lipoprotein receptor molecules. Biophys. J. 1994, 66, 1301–1318. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Monne, H.M.-S.; Javanainen, M.; Metzler, R. Anomalous Diffusion of Phospholipids and Cholesterols in a Lipid Bilayer and its Origins. Phys. Rev. Lett. 2012, 109, 188103. [Google Scholar] [CrossRef]
- Sokolov, I.M. Models of anomalous diffusion in crowded environments. Soft Matter 2012, 8, 9043–9052. [Google Scholar] [CrossRef]
- Babayekhorasani, F.; Dunstan, D.E.; Krishnamoorti, R.; Conrad, J.C. Nanoparticle diffusion in crowded and confined media. Soft Matter 2016, 12, 8407–8416. [Google Scholar] [CrossRef]
- Guigas, G.; Weiss, M. Sampling the Cell with Anomalous Diffusion—The Discovery of Slowness. Biophys. J. 2008, 94, 90–94. [Google Scholar] [CrossRef]
- Korabel, N.; Han, D.; Taloni, A.; Pagnini, G.; Fedotov, S.; Allan, V.; Waigh, T. Local Analysis of Heterogeneous Intracellular Transport: Slow and Fast Moving Endosomes. Entropy 2021, 23, 958. [Google Scholar] [CrossRef]
- Oh, N.; Park, J.-H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014, 9 (Suppl. S1), 51–63. [Google Scholar] [CrossRef]
- Elkin, S.R.; Lakoduk, A.M.; Schmid, S.L. Endocytic pathways and endosomal trafficking: A primer. Wien. Med. Wochenschr. 2016, 166, 196–204. [Google Scholar] [CrossRef]
- Grant, B.D.; Donaldson, J.G. Pathways and mechanisms of endocytic recycling. Nat. Rev. Mol. Cell Biol. 2009, 10, 597–608. [Google Scholar] [CrossRef]
- Manzanares, D.; Ceña, V. Endocytosis: The Nanoparticle and Submicron Nanocompounds Gateway into the Cell. Pharmaceutics 2020, 12, 371. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; McMahon, H.T. Mechanisms of Endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Foroozandeh, P.; Aziz, A.A. Insight into Cellular Uptake and Intracellular Trafficking of Nanoparticles. Nanoscale Res. Lett. 2018, 13, 339. [Google Scholar] [CrossRef]
- De Almeida, M.S.; Susnik, E.; Drasler, B.; Taladriz-Blanco, P.; Petri-Fink, A.; Rothen-Rutishauser, B. Understanding nanoparticle endocytosis to improve targeting strategies in nanomedicine. Chem. Soc. Rev. 2021, 50, 5397–5434. [Google Scholar] [CrossRef]
- Bareford, L.M.; Swaan, P.W. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev. 2007, 59, 748–758. [Google Scholar] [CrossRef]
| Fatty Acids (% Total) | Salmon Lecithin |
|---|---|
| Myristic acid (C14:0) | 1.67 ± 0.02 |
| Pentadecanoic acid (C15:0) | 0.42 ± 0.01 |
| Palmitic acid (C16:0) | 20.08 ± 0.11 |
| Heptadecanoic acid (C17:0) | 0.41 ± 0.03 |
| Stearic acid (C18:0) | 4.98 ± 0.04 |
| Saturated Fatty Acids | 27.56 |
| Palmitoleic acid (C16:1n-7) | 1.74 ± 0.01 |
| Oleic acid (C18:1n-9) | 26.16 ± 0.21 |
| Vaccenic acid (C18:1n-7) | 2.56 ± 0.04 |
| Eicosenoic acid (C20:1n-9) | 0.63 ± 0.02 |
| Monounsaturated Fatty Acids | 31.09 |
| Linoleic acid (C18:2n-6) | 6.98 ± 0.11 |
| Gamma-linolenic acid (C18:3n-6) | 3.13 ± 0.05 |
| Arachidonic acid (C20:4n-6) | 2.11 ± 0.03 |
| n-6 total | 12.22 |
| Alpha-linolenic acid (C18:3n-3) | 1.63 ± 0.07 |
| Eicosapentaenoic acid (C20:5n-3) | 7.55 ± 0.05 |
| Docosapentaenoic acid (C22:5n-3) | 1.91 ± 0.08 |
| Docosahexaenoic acid (C22:6n-3) | 18.04 ± 0.09 |
| n-3 total | 29.13 |
| Polyunsaturated Fatty Acids | 41.35 |
| DHA/EPA | 2.39 |
| n-6/n-3 | 0.42 |
| Incubation Time | Diameter (μm) | Avg. Speed (μm/s) |
|---|---|---|
| 2 h | 0.312 ± 0.044 | 0.007 ± 0.004 c,d,e,f,g,h,i,j,k,l,m,n,o |
| 3 h | 0.317 ± 0.046 | 0.012 ± 0.002 d,e,f,g,h,i,j,k,l,m |
| 5 h | 0.317 ± 0.058 | 0.017 ± 0.007 a,h,i |
| 6 h | 0.339 ± 0.039 | 0.022 ± 0.006 a,b,o,p,q |
| 7 h | 0.315 ± 0.042 | 0.023 ± 0.005 a,b,i,o,p,q |
| 8 h | 0.290 ± 0.054 | 0.024 ± 0.003 a,b,i,o,p,q |
| 26 h | 0.325 ± 0.042 | 0.024 ± 0.005 a,b,n,o,p,q |
| 34 h | 0.335 ± 0.055 | 0.027 ± 0.004 a,b,c,m,n,o,p,q |
| 48 h | 0.329 ± 0.037 | 0.029 ± 0.006 a,b,c,e,k,l,m,n,o,p,q |
| 50 h | 0.314 ± 0.050 | 0.024 ± 0.007 a,b,n,o,p,q |
| 55 h | 0.303 ± 0.051 | 0.022 ± 0.005 a,b,i,o,p,q |
| 72 h | 0.333 ± 0.041 | 0.020 ± 0.005 a,b,i,o,p,q |
| 76 h | 0.330 ± 0.042 | 0.019 ± 0.006 a,b,h,i,o,p,q |
| 80 h | 0.295 ± 0.048 | 0.018 ± 0.006 a,g,h,i,jo,p,q |
| 96 h | 0.294 ± 0.051 | 0.013 ± 0.005 a,d,e,f,g,h,i,jk,l,m,n |
| 100 h | 0.324 ± 0.046 | 0.012 ± 0.004 d,e,f,g,h,i,j,kl,m,n |
| 104 h | 0.300 ± 0.059 | 0.012 ± 0.004 d,e,f,g,h,i,j,k,l,m |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Passeri, E.; Bun, P.; Elkhoury, K.; Linder, M.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Transfer Phenomena of Nanoliposomes by Live Imaging of Primary Cultures of Cortical Neurons. Pharmaceutics 2022, 14, 2172. https://doi.org/10.3390/pharmaceutics14102172
Passeri E, Bun P, Elkhoury K, Linder M, Malaplate C, Yen FT, Arab-Tehrany E. Transfer Phenomena of Nanoliposomes by Live Imaging of Primary Cultures of Cortical Neurons. Pharmaceutics. 2022; 14(10):2172. https://doi.org/10.3390/pharmaceutics14102172
Chicago/Turabian StylePasseri, Elodie, Philippe Bun, Kamil Elkhoury, Michel Linder, Catherine Malaplate, Frances T. Yen, and Elmira Arab-Tehrany. 2022. "Transfer Phenomena of Nanoliposomes by Live Imaging of Primary Cultures of Cortical Neurons" Pharmaceutics 14, no. 10: 2172. https://doi.org/10.3390/pharmaceutics14102172
APA StylePasseri, E., Bun, P., Elkhoury, K., Linder, M., Malaplate, C., Yen, F. T., & Arab-Tehrany, E. (2022). Transfer Phenomena of Nanoliposomes by Live Imaging of Primary Cultures of Cortical Neurons. Pharmaceutics, 14(10), 2172. https://doi.org/10.3390/pharmaceutics14102172









