Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
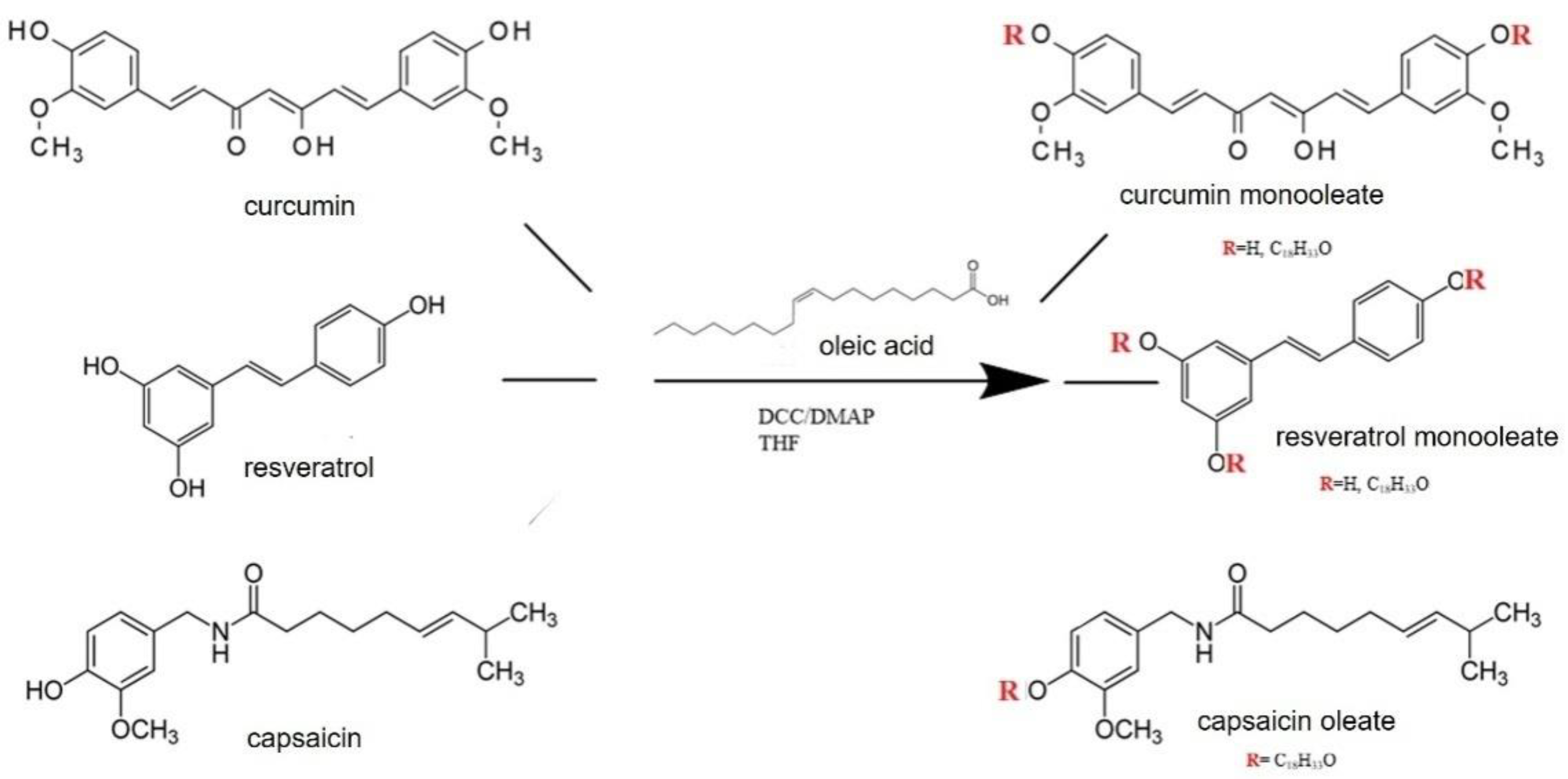
2.3. Synthesis of Capsaicin, CU and RVesters with Oleic Acid
2.4. SLN Preparation
2.5. Encapsulation Efficiency Determination
2.6. Transmission Electron Microscopy (TEM)
2.7. In Vitro Skin Penetration Studies
2.8. Evaluation of Antioxidant Activity
2.9. Cell Lines
2.10. MTT Assay
2.11. ELISA Analysis of IL-6 and MCP-1 Pro-Inflammatory Cytokines
2.12. Statistical Analysis
3. Results and Discussion
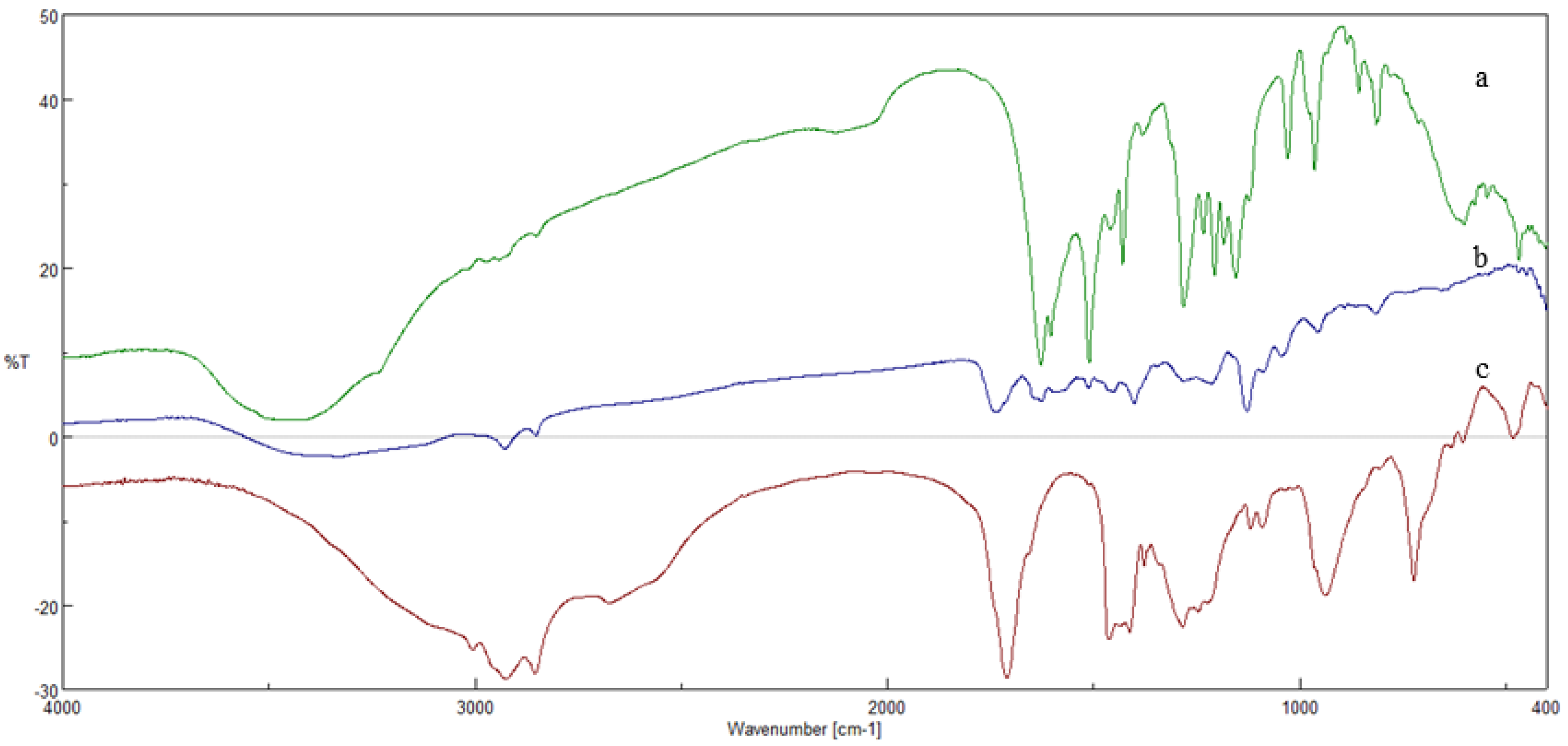
3.1. Esterification Reactions
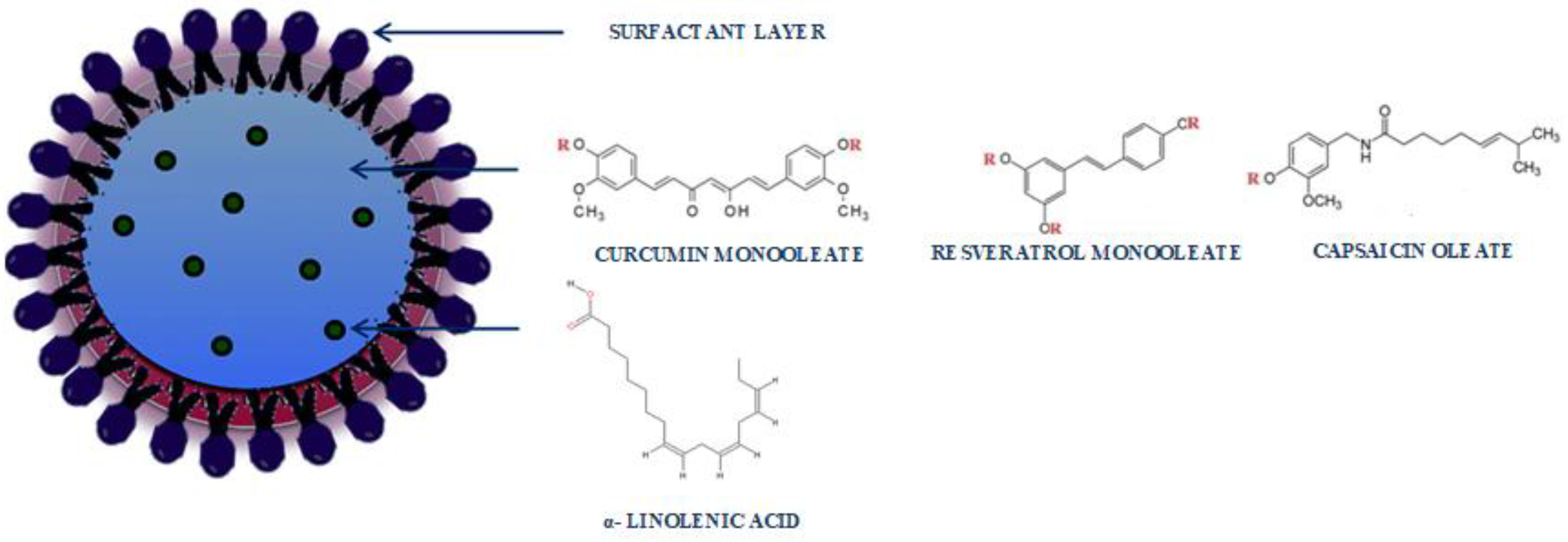
3.2. Preparation and Characterization of SLNs Based on CU-Monooleate, Capsaicin Oleate and RV-Monooleate
3.3. Characterization of SLNs
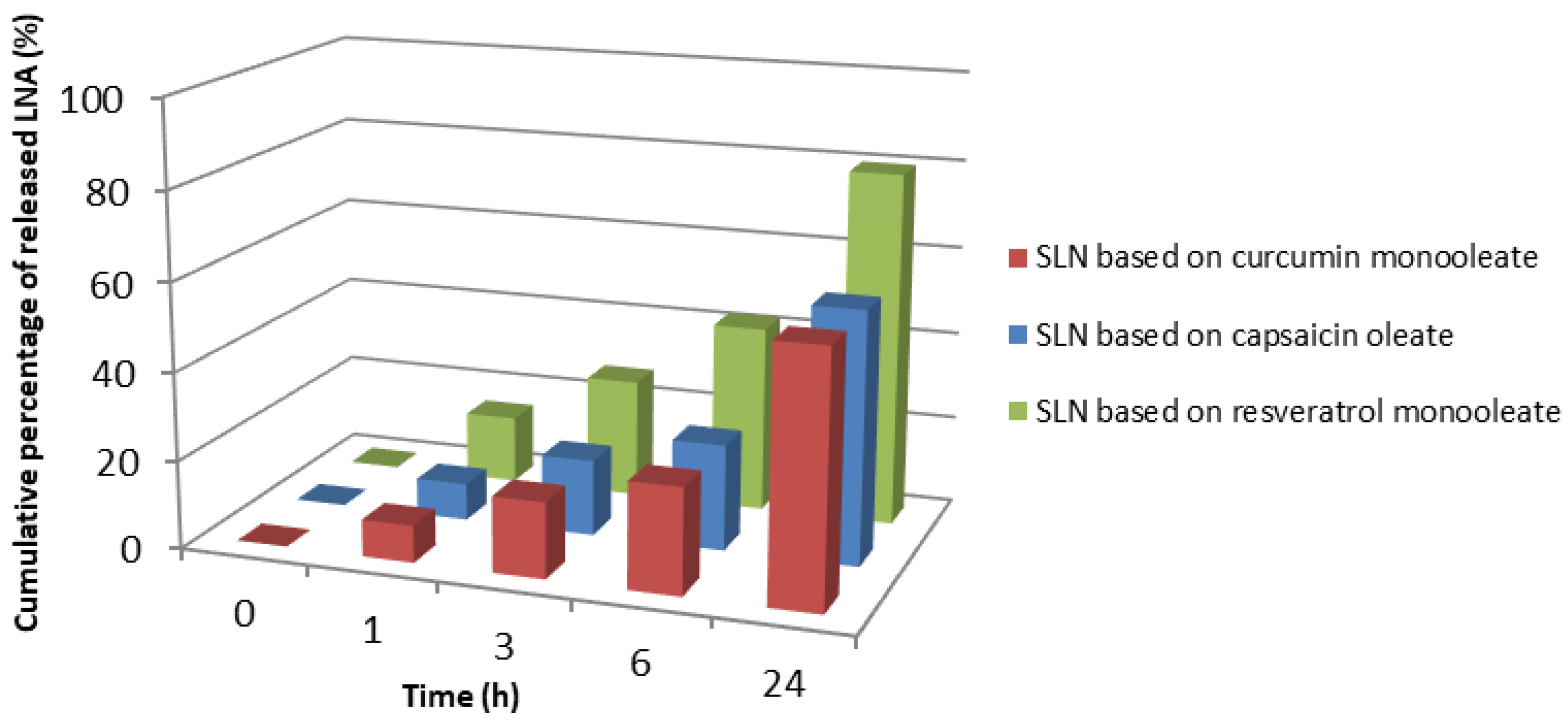
3.4. In Vitro Skin Permeation Studies
3.5. Evaluation of Antioxidant Activity
3.6. Stability Study of Nanoparticles
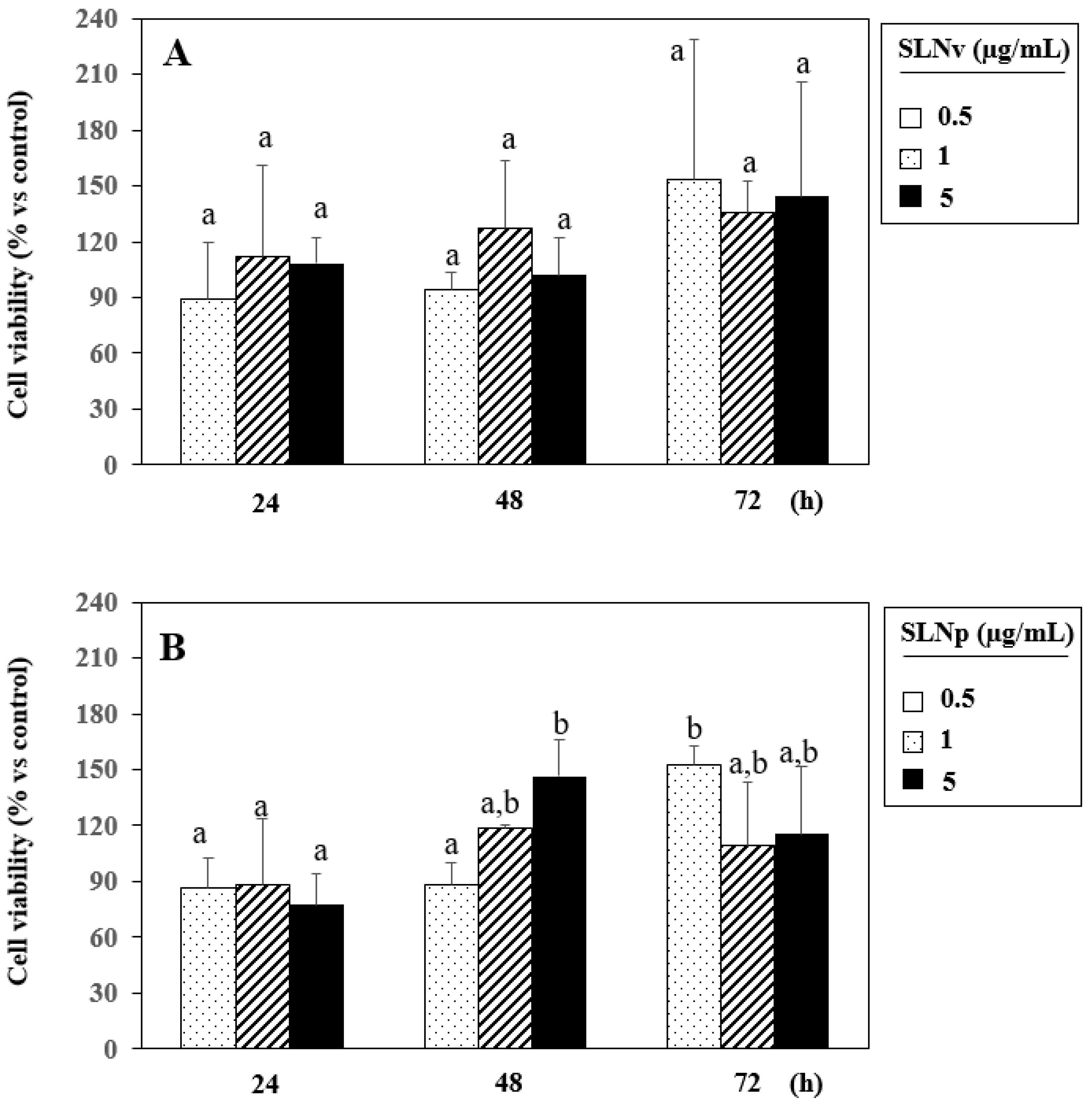
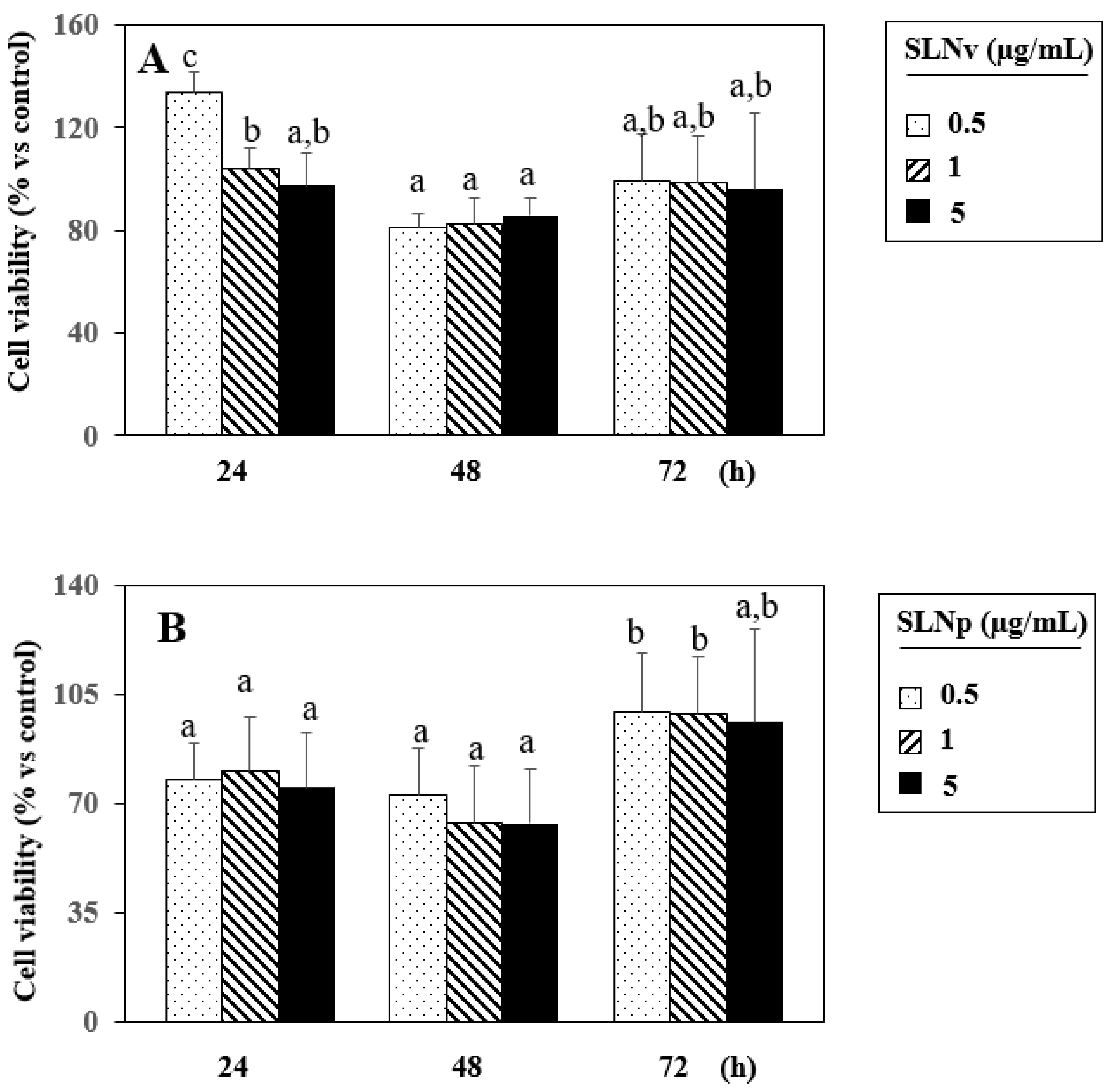
3.7. Evaluation of Cell Viability by MTT Assay
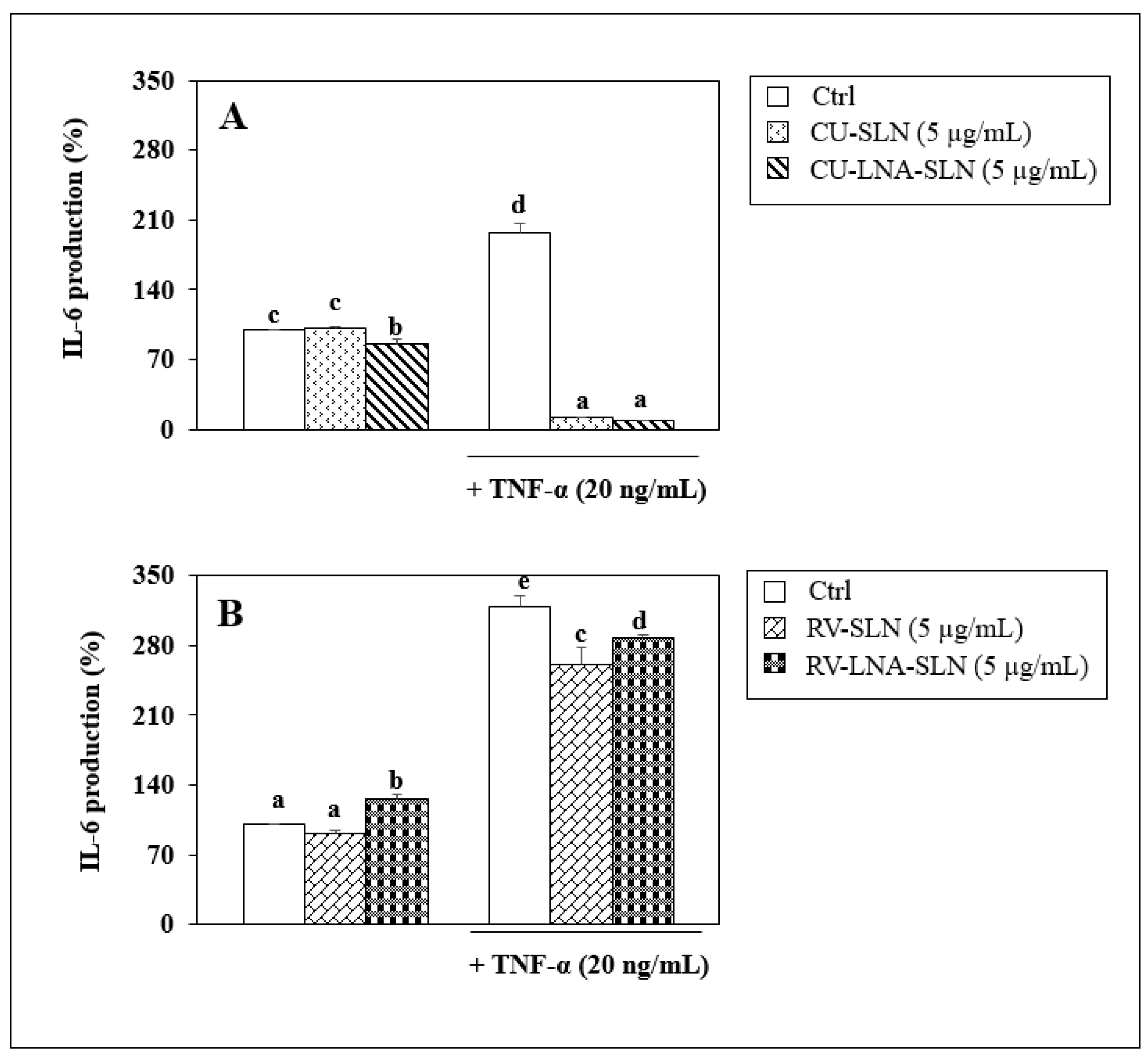
3.8. Evaluation of Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frazier, W.T.; Bhardwaj, N. Atopic dermatitis: Diagnosis and treatment. Am. Fam. Physician 2020, 101, 590–598. [Google Scholar] [PubMed]
- Ratchataswan, T.; Banzon, T.M.; Thyssen, J.P.; Weidinger, S.; Guttman-Yassky, E.; Phipatanakul, W. Biologics for Treatment of Atopic Dermatitis: Current Status and Future Prospect. J. Allergy Clin. Immunol. Pract. 2021, 9, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, D.S.; Mandlik, S.K. Atopic dermatitis: New insight into the etiology, pathogenesis, diagnosis and novel treatment strategies. Immunopharmacol. Immunotoxicol. 2021, 43, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Parekh, K.; Mehta, T.A.; Dhas, N.; Kumar, P.; Popat, A. Emerging Nanomedicines for the Treatment of Atopic Dermatitis. AAPS PharmSciTech 2021, 22, 55. [Google Scholar] [CrossRef]
- Reynolds, K.A.; Juhasz, M.L.W.; Mesinkovska, N.A. The role of oral vitamins and supplements in the management of atopic dermatitis: A systematic review. Int. J. Dermatol. 2019, 58, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Li, X.; Wan, L.; Wang, H.; Mai, Q.; Deng, Z.; Ding, H. Ameliorative effect of orally administered different linoleic acid/α-linolenic acid ratios in a mouse model of DNFB-induced atopic dermatitis. J. Funct. Foods 2020, 65, 103754. [Google Scholar] [CrossRef]
- Dyall, S.C.; Balas, L.; Bazan, N.G.; Brenna, J.T.; Chiang, N.; Souza, F.d.; Dalli, J.; Durand, T.; Galano, J.M.; Lein, P.J.; et al. Polyunsaturated fatty acids and fatty acid-derived lipid mediators: Recent advances in the understanding of their biosynthesis, structures, and functions. Prog. Lipid Res. 2022, 86, 101165. [Google Scholar] [CrossRef]
- Andreassi, M.; Forleo, P.; Dilohjo, A. Efficacy of γ-Linolenic Acid in the Treatment of Patients with Atopic Dermatitis. J. Int. Med. Res. 1997, 25, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Sroczyk, E.A.; Berniak, K.; Jaszczur, M.; Stachewicz, U. Topical electrospun patches loaded with oil for effective gamma linoleic acid transport and skin hydration towards atopic dermatitis skincare. Chem. Eng. J. 2022, 429, 132256. [Google Scholar] [CrossRef]
- Yuan, Q.; Xie, F.; Huang, W.; Hu, M.; Yan, Q.; Chen, Z.; Zheng, Y.; Liu, L. The review of alpha-linolenic acid: Sources, metabolism, and pharmacology. Phytother. Res. 2022, 36, 164–188. [Google Scholar] [CrossRef] [PubMed]
- Mashhadi, S.N.Y.; Askari, V.R.; Ghorani, V.; Jelodar, G.A.; Boskabady, M.H. The effect of Portulaca oleracea and α-linolenic acid on oxidant/antioxidant biomarkers of human peripheral blood mononuclear cells. Indian J. Pharmacol. 2018, 50, 177–184. [Google Scholar]
- Steinritz, D.; Schmidt, A.; Simons, A.T.; Ibrahim, M.; Morguet, C.; Balszuweit, F.; Thiermann, H.K.; Kehe, K.; Bloch, W.; Bölck, B. Chlorambucil (nitrogen mustard) induced impairment of early vascular endothelial cell migration-effects of α-linolenic acid and N-acetylcysteine. Chem. Biol. Interact. 2014, 219, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; Singh, M.; Rawat, J.K.; Gautam, S.; Saraf, S.A.; Kaithwas, G. Effect of enteral administration ofα-linolenic acid and linoleicacid against methotrexate induced intestinal toxicity in albino rats. RSC Adv. 2014, 4, 60397–60403. [Google Scholar] [CrossRef]
- Pal, M.; Ghosh, M. Prophylactic effect ofα-linolenic acid andα-eleostearic acid against MeHg induced oxidative stress, DNA dam-age and structural changes in RBC membrane. Food Chem. Toxicol. 2012, 50, 2811–2818. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Ghosh, M. Studies on comparative efficacy ofα-linolenic acid andα-eleostearic acid on prevention of organicmercury-induced oxidative stress in kidney and liver of rat. Food Chem. Toxicol. 2012, 50, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sethi, G.S.; Naura, A.S. Curcumin Ameliorates Ovalbumin-Induced Atopic Dermatitis and Blocks the Progression of Atopic March in Mice. Inflammation 2020, 43, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; de Amicis, F.; Servidio, C.; Curcio, F.; Trombino, S. Preparation, characterization and in vitro evaluation of resveratrol-loaded nanospheres potentially useful for human breast carcinoma. J. Drug Deliv. Sci. Technol. 2020, 57, 101748. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, J. Resveratrol Exerts Therapeutic Effects on Mice With Atopic Dermatitis. Compend. Clin. Res. Pract. 2019, 31, 279–284. [Google Scholar]
- Andersen, H.; Elberling, J.; Arendt-Nielsen, L. High-concentration topical capsaicin may abolish the clinical manifestations of allergic contact dermatitis by effects on induction and elicitation. Med. Hypotheses 2017, 99, 53–56. [Google Scholar] [CrossRef]
- Amaralb, G.M.H.; Lobob, J.M.S.; Silva, A.C. Formulations based on solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for cutaneous use. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar]
- Cassano, R.; Mellace, S.; Marrelli, M.; Conforti, F.; Trombino, S. α-Tocopheryllinolenate solid lipid nanoparticles for the encapsulation, protection, and release of the omega-3 polyunsaturated fatty acid: In vitro anti-melanoma activity evaluation. Colloids Surf. B Biointerfaces 2017, 151, 128–133. [Google Scholar] [CrossRef]
- Trombino, S.; Servidio, C.; Laganà, A.; Conforti, F.; Marrelli, M.; Cassano, R. Viscosified Solid Lipidic Nanoparticles Based onNaringenin and Linolenic Acid for the Release of Cyclosporine A on the Skin. Molecules 2020, 25, 3535. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Russo, R.; Mellace, S.; Verano, G.P.; Laganà, A.S.; Martucci, F.; Cassano, R. Solid lipid nanoparticles made of trehalose monooleate for cyclosporin-Atopic release. J. Drug Deliv. Sci. Technol. 2019, 49, 563–569. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Corsetto, P.A.; Rizzo, A.M.; Calviello, G.; Trombino, S. Omega-3 PUFA Loaded in Resveratrol-BasedSolid Lipid Nanoparticles: Physicochemical Properties and Antineoplastic Activities in Human Colorectal Cancer Cells In Vitro. Int. J. Mol. Sci. 2018, 19, 586. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Bruni, M.; Servidio, C.; Calviello, G.; Trombino, S. Characterization of a hyaluronic acid and folic acid-based hydrogel for cisplatin delivery: Antineoplastic effect in human ovarian cancer cells in vitro. Int. J. Pharm. 2021, 606, 120899. [Google Scholar] [CrossRef] [PubMed]







| Esters | Oleic Acid (Oa) | Tetrahydrofuran (THF) | Dicyclohexycarbodiimide (DCC) | 4-Dimethylaminopyridine (DMAP) |
|---|---|---|---|---|
| Capsaicin (1 × 10−3 mol) | 1 × 10 −3 mol | 20 mL | 1.3 × 10−3 mol | 5 × 10−4 mol |
| Curcumin (1 × 10−3 mol) | 1 × 10 −3 mol | 20 mL | 1.3 × 10−3 mol | 5 × 10−4 mol |
| Resveratrol (3.5 × 10−3 mol) | 3.5 × 10−3 mol | 20 mL | 4.5 × 10−3 mol | 2 × 10−3 mol |
| Esters | Tween 20 | Butanol | Sodium Taurocholate | Linolenic Acid |
|---|---|---|---|---|
| 0.05 g | 0.029 mL | 0.012 mL | 0.023 g | 0.0021 g |
| Formulation | Dimensional Analysis | Polydispersion Index (PI) |
|---|---|---|
| Empty SLN based on: | ||
| Curcumin monooleate | 258.9 ± 8.3 | 0.005 |
| Capsaicin oleate | 325.4 ± 13.5 | 0.275 ± 0.015 |
| Resveratrol monooleate | 395 ± 39.5 | 0.220 ± 0.150 |
| LNA-loaded SLN based on: | ||
| Curcumin monooleate | 493.6 ± 183.90 | 263 ± 0.043 |
| Capsaicin oleate | 277.4 ± 12.0 | 0.192 ± 0.095 |
| Resveratrol monooleate | 271.8 ± 4.0 | 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassano, R.; Serini, S.; Curcio, F.; Trombino, S.; Calviello, G. Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid. Pharmaceutics 2022, 14, 1593. https://doi.org/10.3390/pharmaceutics14081593
Cassano R, Serini S, Curcio F, Trombino S, Calviello G. Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid. Pharmaceutics. 2022; 14(8):1593. https://doi.org/10.3390/pharmaceutics14081593
Chicago/Turabian StyleCassano, Roberta, Simona Serini, Federica Curcio, Sonia Trombino, and Gabriella Calviello. 2022. "Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid" Pharmaceutics 14, no. 8: 1593. https://doi.org/10.3390/pharmaceutics14081593
APA StyleCassano, R., Serini, S., Curcio, F., Trombino, S., & Calviello, G. (2022). Preparation and Study of Solid Lipid Nanoparticles Based on Curcumin, Resveratrol and Capsaicin Containing Linolenic Acid. Pharmaceutics, 14(8), 1593. https://doi.org/10.3390/pharmaceutics14081593










