Mesoporous Silica Particles Functionalized with Newly Extracted Fish Oil (Omeg@Silica) Reducing IL-8 Counteract Cell Migration in NSCLC Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Extraction of AnchoisOil
2.2. Preparation of Omeg@Silica
2.3. Cell Culture and Treatment
2.4. Wound-Healing Assay
2.5. RNA Extraction
2.6. TaqMan RT-qPCR for IL-8 and miRNA-21-5p
2.7. IL-8 Protein Release
2.8. NF-κB Expression by Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of Fish Oil, Silica, and FOS on Cell Migration in A549 and NCI-H292 Cells
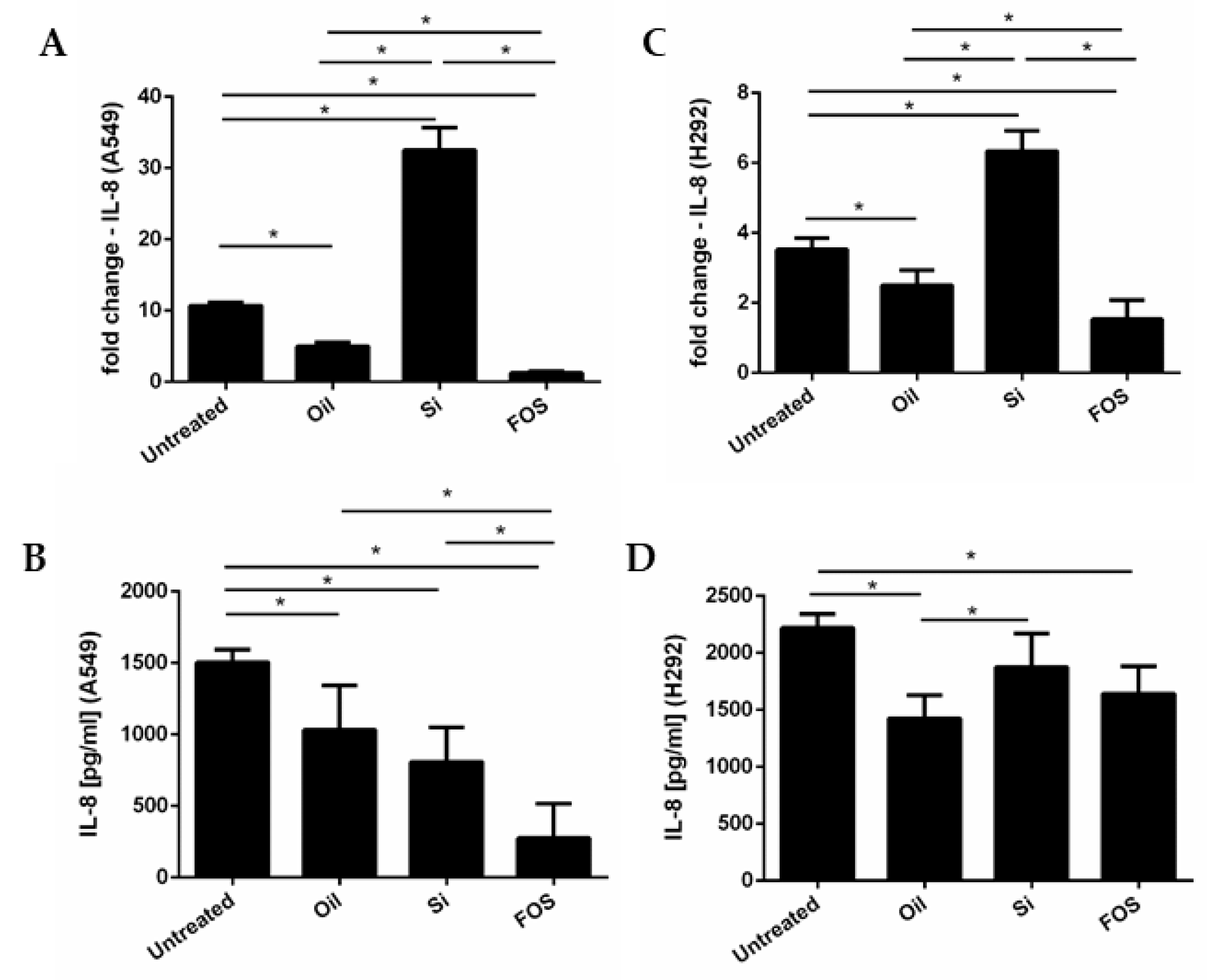
3.2. Effects of Fish Oil, Silica, and FOS on IL-8 Gene Expression and Release, in A549 and NCI-H292 Cells
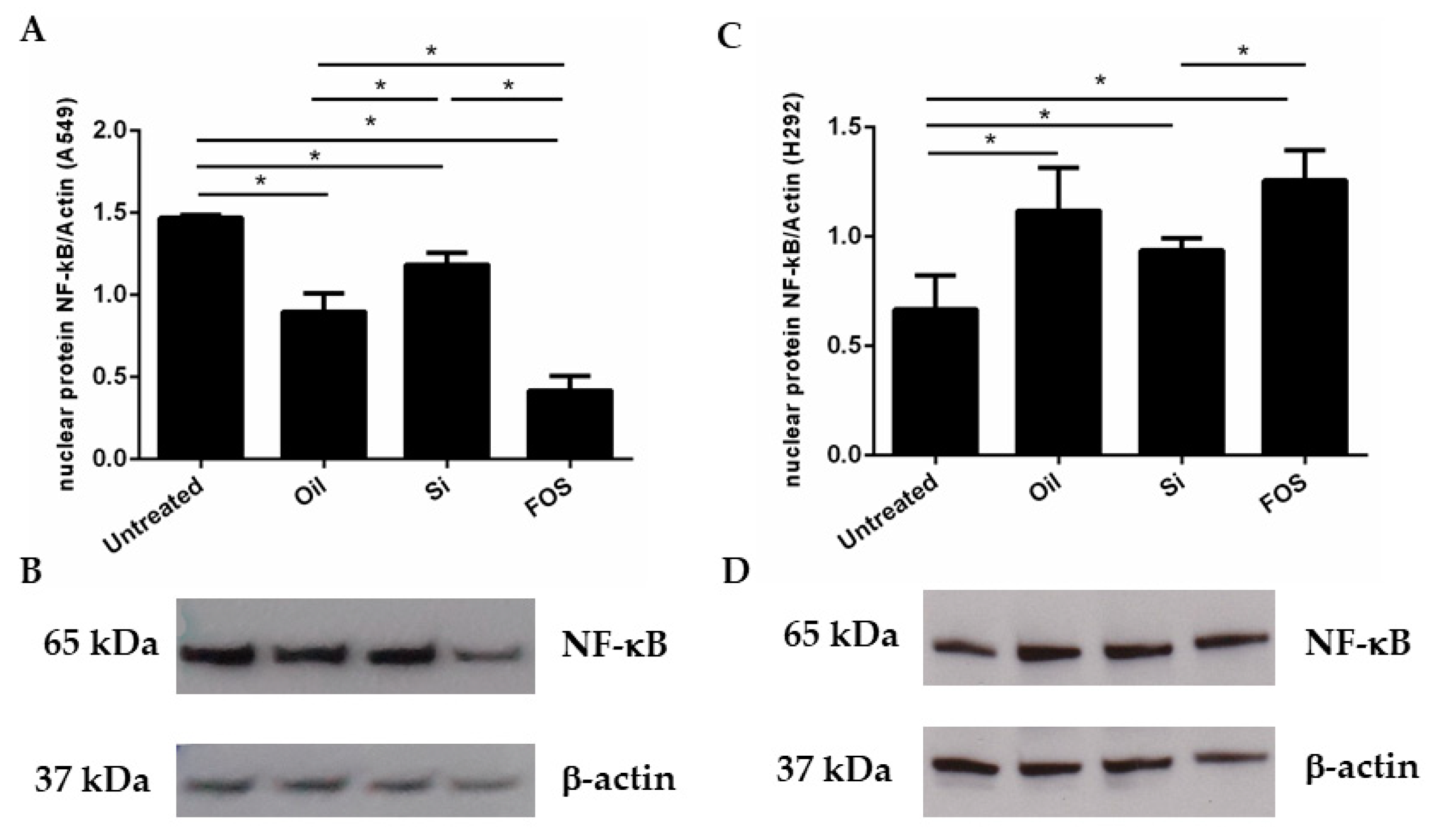
3.3. Effects of Fish Oil, Silica, and FOS on NF-κB Expression in A549 and NCI-H292 Cells
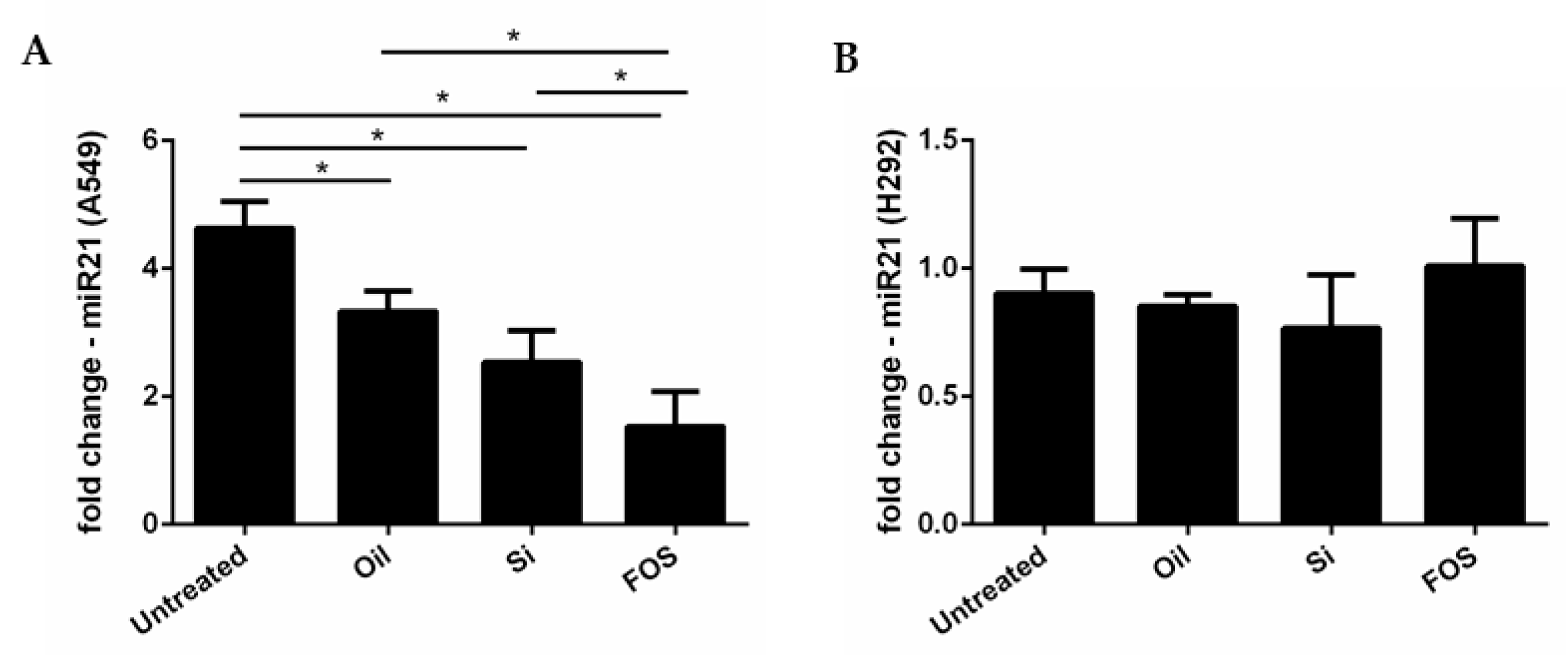
3.4. Effects of Fish Oil, Silica, and FOS on miRNA-21 Expression in A549 and NCI-H292 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nie, Y.; Feng, F.; Luo, W.; Sanders, A.J.; Zhang, Y.; Liang, J.; Chen, C.; Feng, W.; Gu, W.; Liao, W.; et al. Overexpressed transient receptor potential vanilloid 1 (TRPV1) in lung adenocarcinoma harbours a new opportunity for therapeutic targeting. Cancer Gene Ther. 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Park, Y.; Jo, J.R.; Jung, N.K.; Song, D.K.; Bae, J.; Keum, D.Y.; Kim, J.B.; Park, G.Y.; Jang, B.C.; et al. Overexpression of cyclooxygenase-2 in NCI-H292 human alveolar epithelial carcinoma cells: Roles of p38 MAPK, ERK-1/2, and PI3K/PKB signaling proteins. J. Cell Biochem. 2011, 112, 3015–3024. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Fillmore, C.M.; Hammerman, P.S.; Kim, C.F.; Wong, K.K. Non-small-cell lung cancers: A heterogeneous set of diseases. Nat. Rev. Cancer 2014, 14, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Di Sano, C.; D’Anna, C.; Scurria, A.; Lino, C.; Pagliaro, M.; Ciriminna, R.; Pace, E. Mesoporous silica particles functionalized with newly extracted fish oil (Omeg@Silica) inhibit lung cancer cell growth. Nanomedicine 2021, 16, 2061–2074. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Scurria, A.; Avellone, G.; Pagliaro, M. A Circular Economy Approach to Fish Oil Extraction. ChemistrySelect 2019, 4, 5106–5109. [Google Scholar] [CrossRef]
- Ciriminna, R.; Lino, C.; Pagliaro, M. Omeg@Silica: Entrapment and Stabilization of Sustainably Sourced Fish Oil. ChemistryOpen 2021, 10, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Lomeli-Rodriguez, M.; Cara, P.D.; Lopez-Sanchez, J.A.; Pagliaro, M. Limonene: A versatile chemical of the bioeconomy. Chem. Commun. 2014, 50, 15288–15296. [Google Scholar] [CrossRef]
- Vega, O.M.; Abkenari, S.; Tong, Z.; Tedman, A.; Huerta-Yepez, S. Omega-3 Polyunsaturated Fatty Acids and Lung Cancer: Nutrition or Pharmacology? Nutr. Cancer 2021, 73, 541–561. [Google Scholar] [CrossRef]
- Yin, Y.; Sui, C.; Meng, F.; Ma, P.; Jiang, Y. The omega-3 polyunsaturated fatty acid docosahexaenoic acid inhibits proliferation and progression of non-small cell lung cancer cells through the reactive oxygen species-mediated inactivation of the PI3K/Akt pathway. Lipids Health Dis. 2017, 16, 87. [Google Scholar] [CrossRef]
- Maucher, D.; Schmidt, B.; Kuhlmann, K.; Schumann, J. Polyunsaturated Fatty Acids of Both the Omega-3 and the Omega-6 Family Abrogate the Cytokine-Induced Upregulation of miR-29a-3p by Endothelial Cells. Molecules 2020, 25, 4466. [Google Scholar] [CrossRef]
- Lagiou, P.; Trichopoulos, D. Inflammatory biomarkers and risk of lung cancer. J. Natl. Cancer Inst. 2011, 103, 1073–1075. [Google Scholar] [CrossRef] [PubMed]
- Orditura, M.; De Vita, F.; Catalano, G.; Infusino, S.; Lieto, E.; Martinelli, E.; Morgillo, F.; Castellano, P.; Pignatelli, C.; Galizia, G. Elevated serum levels of interleukin-8 in advanced non-small cell lung cancer patients: Relationship with prognosis. J. Interferon Cytokine Res. 2002, 22, 1129–1135. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, J.; Wang, L.; Chen, C.; Yang, D.; Jin, M.; Bai, C.; Song, Y. Urban particulate matter triggers lung inflammation via the ROS-MAPK-NF-kappaB signaling pathway. J. Thorac. Dis. 2017, 9, 4398–4412. [Google Scholar] [CrossRef] [PubMed]
- Rom, O.; Avezov, K.; Aizenbud, D.; Reznick, A.Z. Cigarette smoking and inflammation revisited. Respir. Physiol. Neurobiol. 2013, 187, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Pace, E.; Di Vincenzo, S.; Di Salvo, E.; Genovese, S.; Dino, P.; Sangiorgi, C.; Ferraro, M.; Gangemi, S. MiR-21 upregulation increases IL-8 expression and tumorigenesis program in airway epithelial cells exposed to cigarette smoke. J. Cell Physiol. 2019, 234, 22183–22194. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, S.; Sangiorgi, C.; Ferraro, M.; Buscetta, M.; Cipollina, C.; Pace, E. Cigarette smoke extract reduces FOXO3a promoting tumor progression and cell migration in lung cancer. Toxicology 2021, 454, 152751. [Google Scholar] [CrossRef]
- Ferraro, M.; Di Vincenzo, S.; Dino, P.; Bucchieri, S.; Cipollina, C.; Gjomarkaj, M.; Pace, E. Budesonide, Aclidinium and Formoterol in combination limit inflammaging processes in bronchial epithelial cells exposed to cigarette smoke. Exp. Gerontol 2019, 118, 78–87. [Google Scholar] [CrossRef]
- Dickhoff, C.; Hartemink, K.J.; Kooij, J.; van de Ven, P.M.; Paul, M.A.; Smit, E.F.; Dahele, M. Is the routine use of trimodality therapy for selected patients with non-small cell lung cancer supported by long-term clinical outcomes? Ann. Oncol. 2017, 28, 185. [Google Scholar] [CrossRef]
- Chen, J.W.; Dhahbi, J. Lung adenocarcinoma and lung squamous cell carcinoma cancer classification, biomarker identification, and gene expression analysis using overlapping feature selection methods. Sci. Rep. 2021, 11, 13323. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef]
- Yanagitani, N.; Uchibori, K.; Koike, S.; Tsukahara, M.; Kitazono, S.; Yoshizawa, T.; Horiike, A.; Ohyanagi, F.; Tambo, Y.; Nishikawa, S.; et al. Drug resistance mechanisms in Japanese anaplastic lymphoma kinase-positive non-small cell lung cancer and the clinical responses based on the resistant mechanisms. Cancer Sci. 2020, 111, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, A.; Maggiora, M.; Martinasso, G.; Cotogni, P.; Canuto, R.A.; Muzio, G. Arachidonic and docosahexaenoic acids reduce the growth of A549 human lung-tumor cells increasing lipid peroxidation and PPARs. Chem. Biol. Interact. 2007, 165, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Zajdel, A.; Wilczok, A.; Tarkowski, M. Toxic effects of n-3 polyunsaturated fatty acids in human lung A549 cells. Toxicol. Vitr. 2015, 30, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Siena, L.; Cipollina, C.; Di Vincenzo, S.; Ferraro, M.; Bruno, A.; Gjomarkaj, M.; Pace, E. Electrophilic derivatives of omega-3 fatty acids counteract lung cancer cell growth. Cancer Chemother. Pharm. 2018, 81, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Monkkonen, T.; Debnath, J. Inflammatory signaling cascades and autophagy in cancer. Autophagy 2018, 14, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Alaarg, A.; Jordan, N.Y.; Verhoef, J.J.; Metselaar, J.M.; Storm, G.; Kok, R.J. Docosahexaenoic acid liposomes for targeting chronic inflammatory diseases and cancer: An in vitro assessment. Int. J. Nanomed. 2016, 11, 5027–5040. [Google Scholar] [CrossRef]
- Tantipaiboonwong, P.; Chaiwangyen, W.; Suttajit, M.; Kangwan, N.; Kaowinn, S.; Khanaree, C.; Punfa, W.; Pintha, K. Molecular Mechanism of Antioxidant and Anti-Inflammatory Effects of Omega-3 Fatty Acids in Perilla Seed Oil and Rosmarinic Acid Rich Fraction Extracted from Perilla Seed Meal on TNF-alpha Induced A549 Lung Adenocarcinoma Cells. Molecules 2021, 26, 6757. [Google Scholar] [CrossRef]
- Das, R.P.; Gandhi, V.V.; Singh, B.G.; Kunwar, A. Balancing loading, cellular uptake, and toxicity of gelatin-pluronic nanocomposite for drug delivery: Influence of HLB of pluronic. J. Biomed. Mater. Res. A 2022, 110, 304–315. [Google Scholar] [CrossRef]
- Scurria, A.; Lino, C.; Pitonzo, R.; Pagliaro, M.; Avellone, G.; Ciriminna, R. Vitamin D3 in fish oil extracted with limonene from anchovy leftovers. Chem. Data Collect. 2020, 25, 100311. [Google Scholar] [CrossRef]
- Gan, Q.; Dai, D.; Yuan, Y.; Qian, J.; Sha, S.; Shi, J.; Liu, C. Effect of size on the cellular endocytosis and controlled release of mesoporous silica nanoparticles for intracellular delivery. Biomed. Microdevices 2012, 14, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Gill, I.; Ballesteros, A. Bioencapsulation within synthetic polymers (Part 1): Sol–gel encapsulated biologicals. Tr. Biotechnol. 2000, 18, 282–296. [Google Scholar] [CrossRef]
- Fujita, Y.; Okamoto, M.; Goda, H.; Tano, T.; Nakashiro, K.; Sugita, A.; Fujita, T.; Koido, S.; Homma, S.; Kawakami, Y.; et al. Prognostic significance of interleukin-8 and CD163-positive cell-infiltration in tumor tissues in patients with oral squamous cell carcinoma. PLoS ONE 2014, 9, e110378. [Google Scholar] [CrossRef] [PubMed]
- Punyani, S.R.; Sathawane, R.S. Salivary level of interleukin-8 in oral precancer and oral squamous cell carcinoma. Clin. Oral Investig. 2013, 17, 517–524. [Google Scholar] [CrossRef]
- Herrero, A.B.; Garcia-Gomez, A.; Garayoa, M.; Corchete, L.A.; Hernandez, J.M.; San Miguel, J.; Gutierrez, N.C. Effects of IL-8 Up-Regulation on Cell Survival and Osteoclastogenesis in Multiple Myeloma. Am. J. Pathol. 2016, 186, 2171–2182. [Google Scholar] [CrossRef]
- Perbellini, O.; Cioffi, F.; Malpeli, G.; Zanolin, E.; Lovato, O.; Scarpa, A.; Pizzolo, G.; Scupoli, M.T. Up-regulation of CXCL8/interleukin-8 production in response to CXCL12 in chronic lymphocytic leukemia. Leuk. Lymphoma 2015, 56, 1897–1900. [Google Scholar] [CrossRef]
- Brew, R.; Erikson, J.S.; West, D.C.; Kinsella, A.R.; Slavin, J.; Christmas, S.E. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine 2000, 12, 78–85. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.P.; Sharma, B.; Owen, L.B.; Singh, R.K. CXCL8 and its cognate receptors in melanoma progression and metastasis. Future Oncol. 2010, 6, 111–116. [Google Scholar] [CrossRef]
- Yuan, A.; Yang, P.C.; Yu, C.J.; Chen, W.J.; Lin, F.Y.; Kuo, S.H.; Luh, K.T. Interleukin-8 messenger ribonucleic acid expression correlates with tumor progression, tumor angiogenesis, patient survival, and timing of relapse in non-small-cell lung cancer. Am. J. Respir. Crit. Care Med. 2000, 162, 1957–1963. [Google Scholar] [CrossRef]
- Masuya, D.; Huang, C.; Liu, D.; Kameyama, K.; Hayashi, E.; Yamauchi, A.; Kobayashi, S.; Haba, R.; Yokomise, H. The intratumoral expression of vascular endothelial growth factor and interleukin-8 associated with angiogenesis in nonsmall cell lung carcinoma patients. Cancer 2001, 92, 2628–2638. [Google Scholar] [CrossRef]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zang, Y.; Lv, L.; Cai, F.; Qian, T.; Zhang, G.; Feng, Q. IL8 promotes proliferation and inhibition of apoptosis via STAT3/AKT/NFkappaB pathway in prostate cancer. Mol. Med. Rep. 2017, 16, 9035–9042. [Google Scholar] [CrossRef] [PubMed]
- Refsnes, M.; Skuland, T.; Lag, M.; Schwarze, P.E.; Ovrevik, J. Differential NF-kappaB and MAPK activation underlies fluoride- and TPA-mediated CXCL8 (IL-8) induction in lung epithelial cells. J. Inflamm. Res. 2014, 7, 169–185. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.; Zhao, C.; Bourgoin, S.G. Differential Effects of Inhibitor Combinations on Lysophosphatidic Acid-Mediated Chemokine Secretion in Unprimed and Tumor Necrosis Factor-alpha-Primed Synovial Fibroblasts. Front. Pharm. 2017, 8, 848. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.W.; Lai, H.T.; Tsai, T.C.; Wu, Y.C.; Yang, Y.T.; Chen, K.Y.; Chen, C.M.; Li, Y.S.; Chen, C.N. Difference in the regulation of IL-8 expression induced by uropathogenic E. coli between two kinds of urinary tract epithelial cells. J. Biomed. Sci. 2009, 16, 91. [Google Scholar] [CrossRef]
- Medin, C.L.; Rothman, A.L. Cell type-specific mechanisms of interleukin-8 induction by dengue virus and differential response to drug treatment. J. Infect. Dis. 2006, 193, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Jatczak-Pawlik, I.; Gorzkiewicz, M.; Studzian, M.; Zinke, R.; Appelhans, D.; Klajnert-Maculewicz, B.; Pulaski, L. Nanoparticles for Directed Immunomodulation: Mannose-Functionalized Glycodendrimers Induce Interleukin-8 in Myeloid Cell Lines. Biomacromolecules 2021, 22, 3396–3407. [Google Scholar] [CrossRef]
- Relli, V.; Trerotola, M.; Guerra, E.; Alberti, S. Abandoning the Notion of Non-Small Cell Lung Cancer. Trends Mol. Med. 2019, 25, 585–594. [Google Scholar] [CrossRef]
- Sorrenti, V.; D’Amico, A.G.; Barbagallo, I.; Consoli, V.; Grosso, S.; Vanella, L. Tin Mesoporphyrin Selectively Reduces Non-Small-Cell Lung Cancer Cell Line A549 Proliferation by Interfering with Heme Oxygenase and Glutathione Systems. Biomolecules 2021, 11, 917. [Google Scholar] [CrossRef]
- Cullen, B.R. MicroRNAs as mediators of viral evasion of the immune system. Nat. Immunol. 2013, 14, 205–210. [Google Scholar] [CrossRef]
- Yang, Z.; Fang, S.; Di, Y.; Ying, W.; Tan, Y.; Gu, W. Modulation of NF-kappaB/miR-21/PTEN pathway sensitizes non-small cell lung cancer to cisplatin. PLoS ONE 2015, 10, e0121547. [Google Scholar] [CrossRef]
- Bica-Pop, C.; Cojocneanu-Petric, R.; Magdo, L.; Raduly, L.; Gulei, D.; Berindan-Neagoe, I. Overview upon miR-21 in lung cancer: Focus on NSCLC. Cell Mol. Life Sci. 2018, 75, 3539–3551. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Anna, C.; Di Sano, C.; Di Vincenzo, S.; Taverna, S.; Cammarata, G.; Scurria, A.; Pagliaro, M.; Ciriminna, R.; Pace, E. Mesoporous Silica Particles Functionalized with Newly Extracted Fish Oil (Omeg@Silica) Reducing IL-8 Counteract Cell Migration in NSCLC Cell Lines. Pharmaceutics 2022, 14, 2079. https://doi.org/10.3390/pharmaceutics14102079
D’Anna C, Di Sano C, Di Vincenzo S, Taverna S, Cammarata G, Scurria A, Pagliaro M, Ciriminna R, Pace E. Mesoporous Silica Particles Functionalized with Newly Extracted Fish Oil (Omeg@Silica) Reducing IL-8 Counteract Cell Migration in NSCLC Cell Lines. Pharmaceutics. 2022; 14(10):2079. https://doi.org/10.3390/pharmaceutics14102079
Chicago/Turabian StyleD’Anna, Claudia, Caterina Di Sano, Serena Di Vincenzo, Simona Taverna, Giuseppe Cammarata, Antonino Scurria, Mario Pagliaro, Rosaria Ciriminna, and Elisabetta Pace. 2022. "Mesoporous Silica Particles Functionalized with Newly Extracted Fish Oil (Omeg@Silica) Reducing IL-8 Counteract Cell Migration in NSCLC Cell Lines" Pharmaceutics 14, no. 10: 2079. https://doi.org/10.3390/pharmaceutics14102079
APA StyleD’Anna, C., Di Sano, C., Di Vincenzo, S., Taverna, S., Cammarata, G., Scurria, A., Pagliaro, M., Ciriminna, R., & Pace, E. (2022). Mesoporous Silica Particles Functionalized with Newly Extracted Fish Oil (Omeg@Silica) Reducing IL-8 Counteract Cell Migration in NSCLC Cell Lines. Pharmaceutics, 14(10), 2079. https://doi.org/10.3390/pharmaceutics14102079








