Co-Delivery of Dihydroartemisinin and Indocyanine Green by Metal-Organic Framework-Based Vehicles for Combination Treatment of Hepatic Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
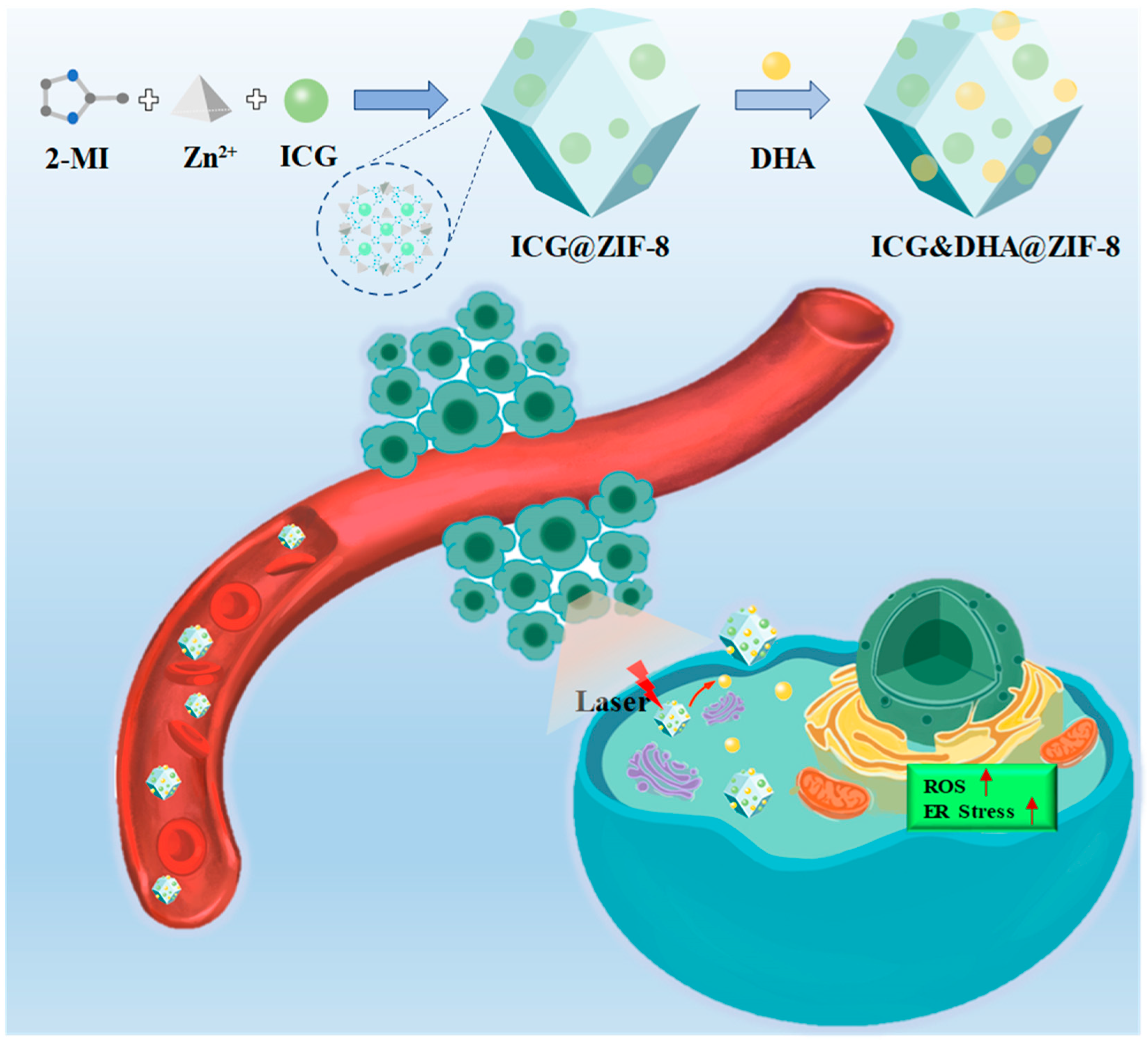
2.2. Preparation and Characterization of ICG&DHA@ZIF-8
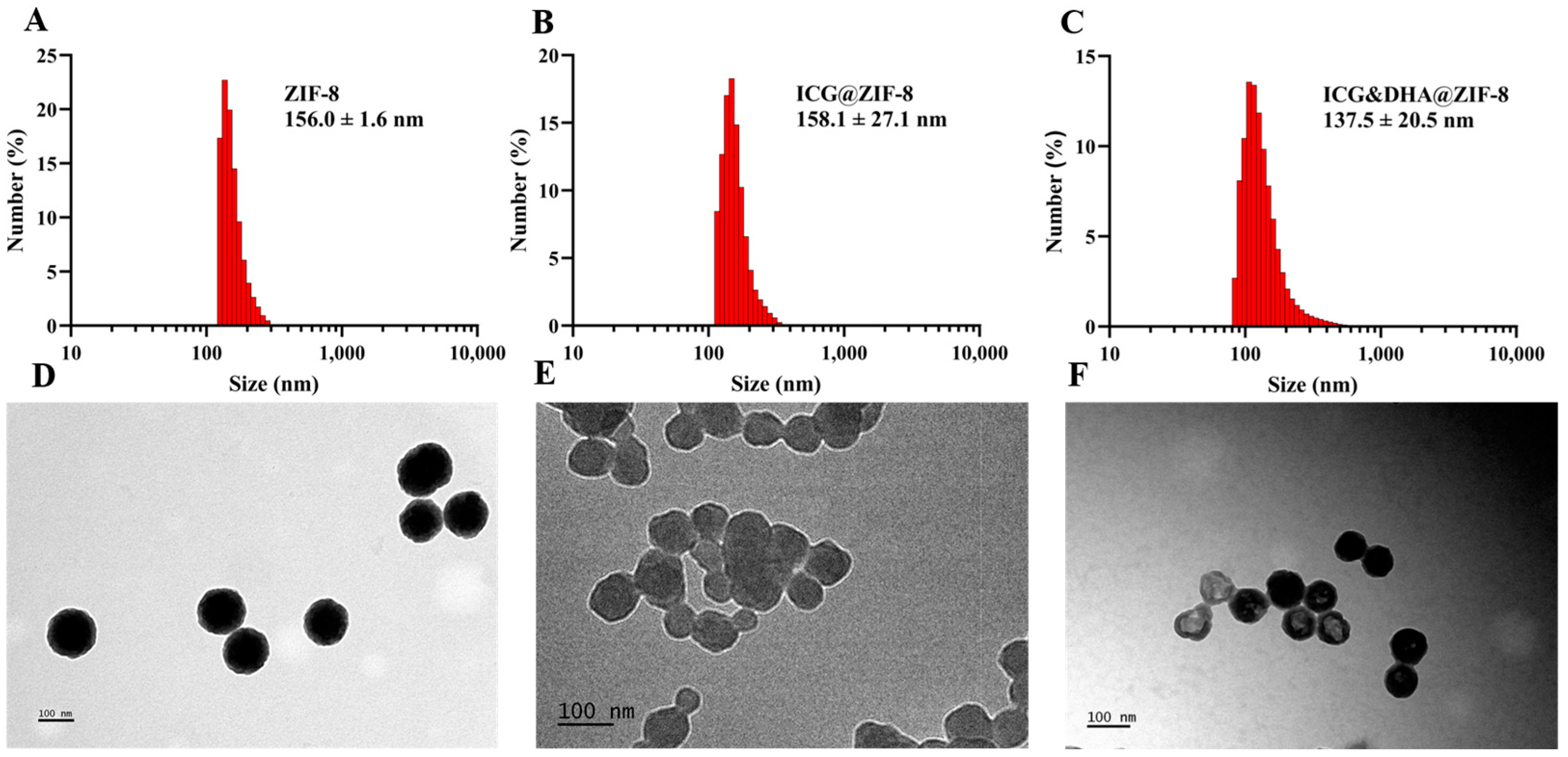
2.3. Size and Morphology
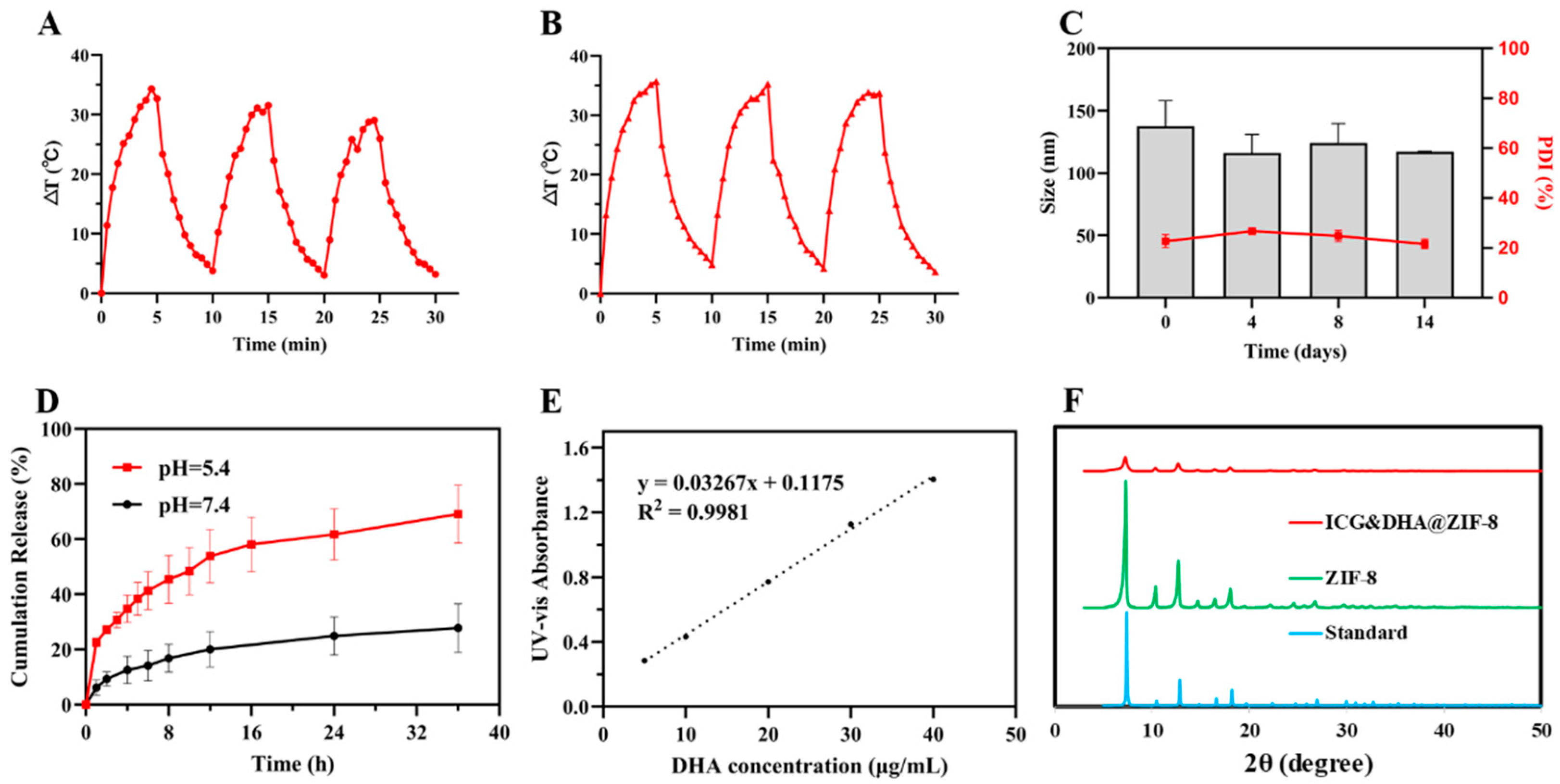
2.4. Photothermal Conversion Effect of ICG&DHA@ZIF-8
2.5. Drug Release Assays
2.6. In Vitro Antitumor Efficacy
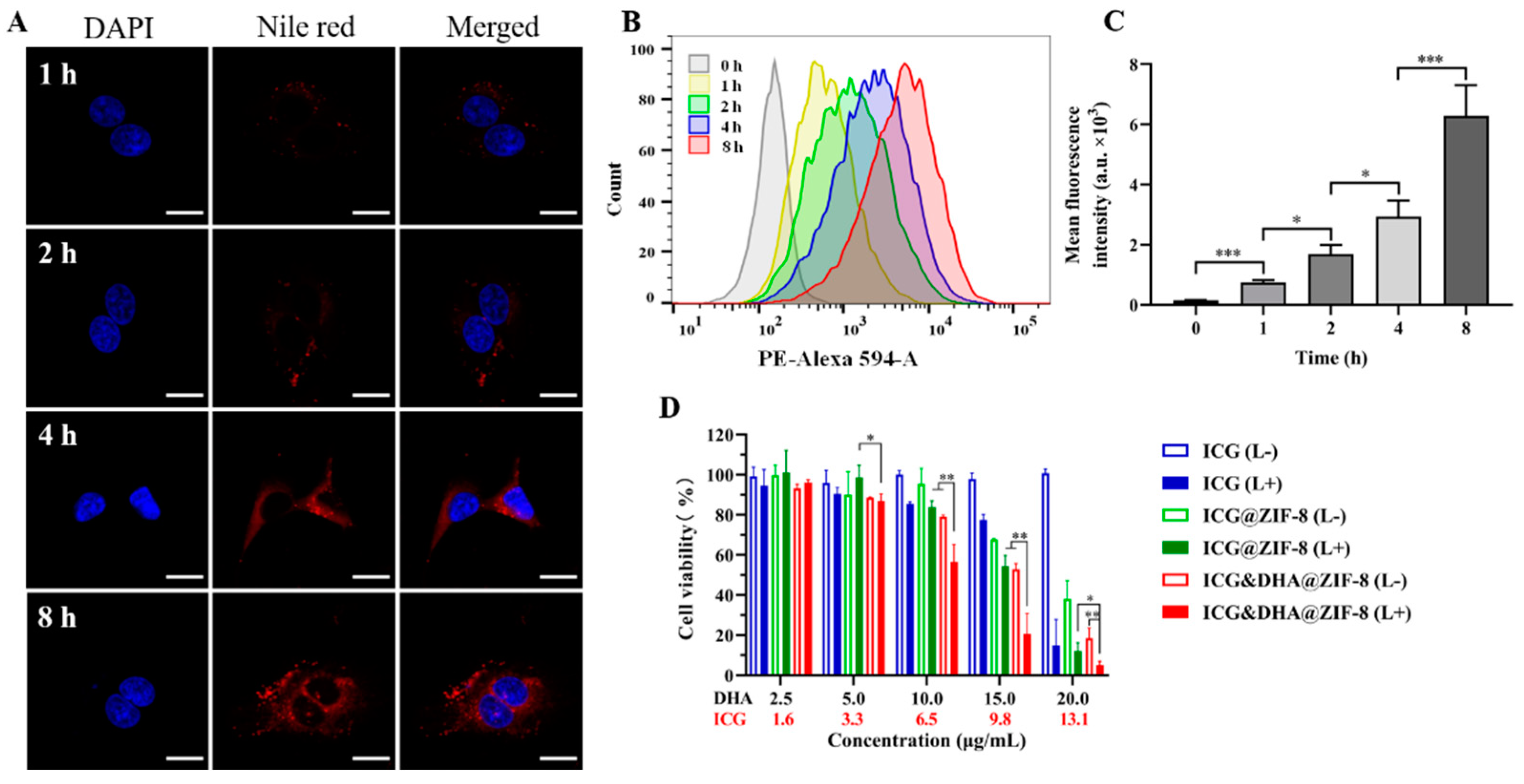
2.7. Cellular Uptake Assays
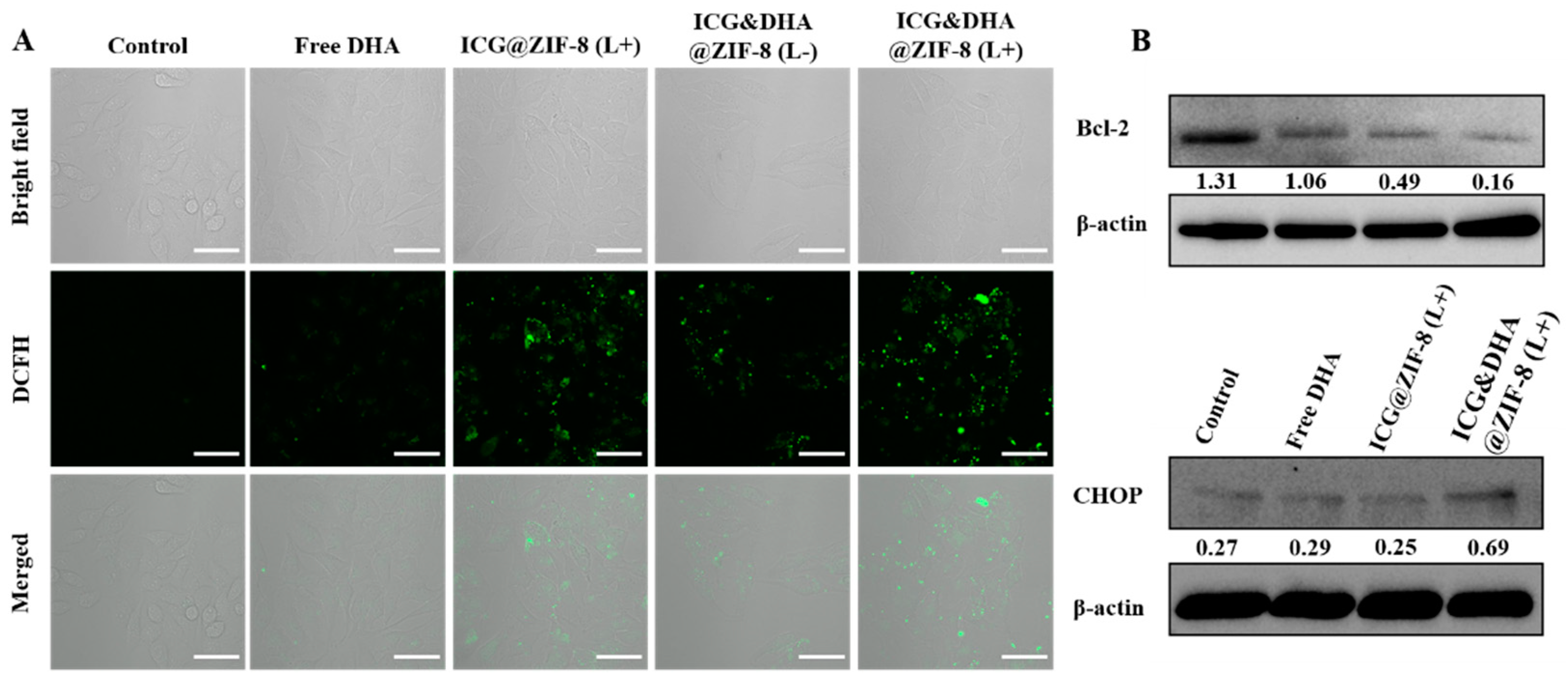
2.8. Cellular ROS Level Assays
2.9. Western Bolt Assays
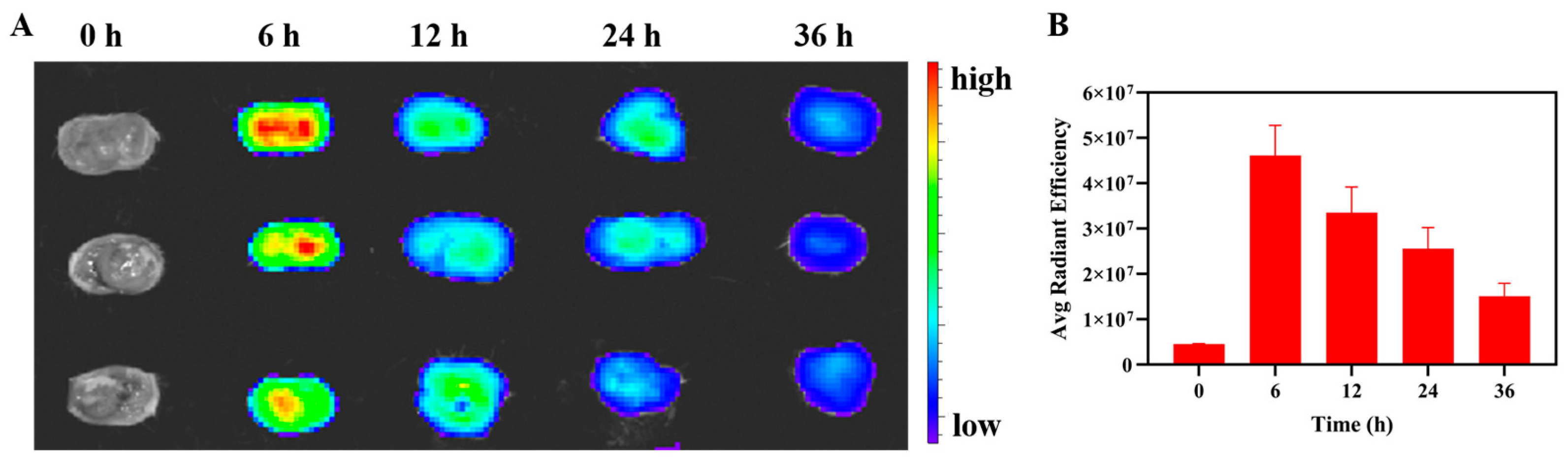
2.10. Ex-Vivo Imaging
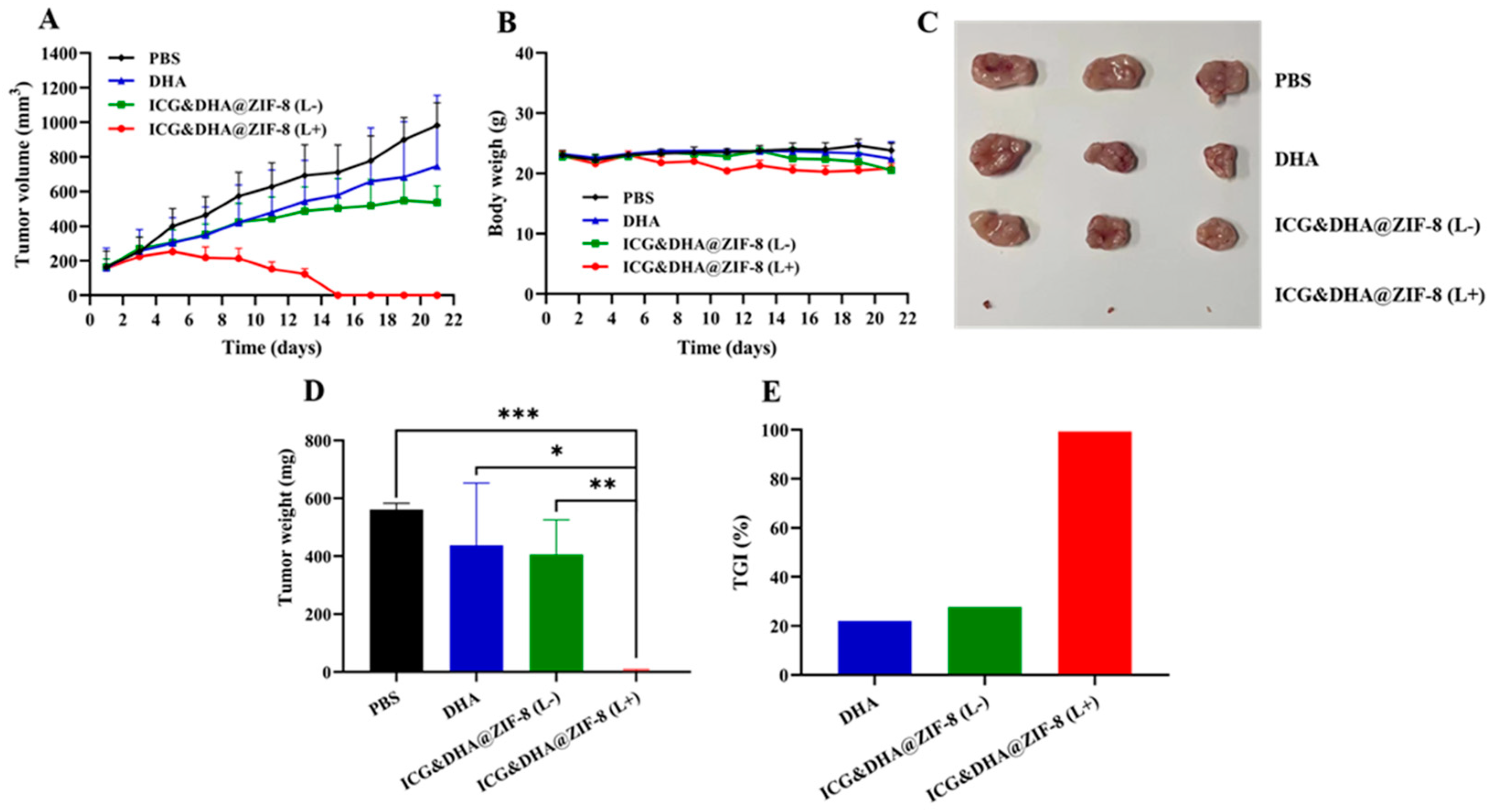
2.11. In Vivo Antitumor Efficiency
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ICG&DHA@ZIF-8
3.2. Photothermal Properties of ICG&DHA@ZIF-8
3.3. pH Responsive DHA Release of ICG&DHA@ZIF-8
3.4. Cellular Uptake and Cytotoxicity of ICG&DHA@ZIF-8
3.5. ROS Level Detection
3.6. Western Blot Assays
3.7. Ex Vivo Tumor Imaging Study
3.8. In Vivo Evaluation of the Antitumor Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Pluijm, R.W.; Tripura, R.; Hoglund, R.M.; Pyae Phyo, A.; Lek, D.; Ul Islam, A.; Anvikar, A.R.; Satpathi, P.; Satpathi, S.; Behera, P.K.; et al. Triple artemisinin-based combination therapies versus artemisinin-based combination therapies for uncomplicated Plasmodium falciparum malaria: A multicentre, open-label, randomised clinical trial. Lancet 2020, 395, 1345–1360. [Google Scholar] [CrossRef]
- Efferth, T. From ancient herb to modern drug: Artemisia annua and artemisinin for cancer therapy. Semin. Cancer Biol. 2017, 46, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Duan, X.; Ni, K.; Li, Y.; Chan, C.; Lin, W. Co-delivery of dihydroartemisinin and pyropheophorbide-iron elicits ferroptosis to potentiate cancer immunotherapy. Biomaterials 2022, 280, 121315. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Jia, X.; Zhen, W.; Cheng, W.; Jiang, X. A facile ion-doping strategy to regulate tumor microenvironments for enhanced multimodal tumor theranostics. J. Am. Chem. Soc. 2018, 140, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chan, C.; Han, W.; Guo, N.; Weichselbaum, R.R.; Lin, W. Immunostimulatory nanomedicines synergize with checkpoint blockade immunotherapy to eradicate colorectal tumors. Nat. Commun. 2019, 10, 1899. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, H.; Huang, Y.; Lian, B.; Ma, C.; Han, L.; Chen, Y.; Wu, S.; Li, N.; Zhang, W.; et al. A self-assembling amphiphilic peptide dendrimer-based drug delivery system for cancer therapy. Pharmaceutics 2021, 13, 1092. [Google Scholar] [CrossRef]
- Lv, X.; Xu, Y.; Ruan, X.; Yang, D.; Shao, J.; Hu, Y.; Wang, W.; Cai, Y.; Tu, Y.; Dong, X. An injectable and biodegradable hydrogel incorporated with photoregulated NO generators to heal MRSA-infected wounds. Acta Biomater. 2022, 146, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, Y.; Yuan, Z. Breast cancer cell membrane camouflaged lipid nanoparticles for tumor-targeted NIR-II phototheranostics. Pharmaceutics 2022, 14, 1367. [Google Scholar] [CrossRef]
- Yang, D.; Chen, F.; He, S.; Shen, H.; Hu, Y.; Feng, N.; Wang, S.; Weng, L.; Luo, Z.; Wang, L. One-pot growth of triangular SnS nanopyramids for photoacoustic imaging and photothermal ablation of tumors. New J. Chem. 2019, 43, 13256–13262. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2019, 48, 2053–2108. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, Z.; Zhao, H.; Qi, D.; Li, X.; Gao, D.; Li, M.; Fan, Q.; Shen, Q.; Huang, W. NIR-II fluorescence imaging guided tumor-specific NIR-II photothermal therapy enhanced by starvation mediated thermal sensitization strategy. Biomaterials 2021, 275, 120935. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.A.; Franke, D.; Caram, J.R.; Perkinson, C.F.; Saif, M.; Askoxylakis, V.; Datta, M.; Fukumura, D.; Jain, R.K.; Bawendi, M.G.; et al. Shortwave infrared fluorescence imaging with the clinically approved near-infrared dye indocyanine green. Proc. Natl. Acad. Sci. USA 2018, 115, 4465–4470. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, X.; Hu, D.; Wang, P.; Liu, Q.; Zhang, X.; Jiang, J.; Liu, X.; Sheng, Z.; Liu, B.; et al. Phototheranostics: Active targeting of orthotopic glioma using biomimetic proteolipid nanoparticles. ACS Nano 2019, 13, 386–398. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kuo, P.W.; Chen, C.J.; Sue, C.J.; Hsu, Y.F.; Pan, M.C. Indocyanine green-camptothecin co-loaded perfluorocarbon double-layer nanocomposite: A versatile nanotheranostics for photochemotherapy and FDOT diagnosis of breast cancer. Pharmaceutics 2021, 13, 1499. [Google Scholar] [CrossRef] [PubMed]
- Yue, D.; Cai, X.; Fan, M.; Zhu, J.; Tian, J.; Wu, L.; Jiang, Q.; Gu, Z. An alternating irradiation strategy-driven combination therapy of PDT and RNAi for highly efficient inhibition of tumor growth and metastasis. Adv. Healthc. Mater. 2021, 10, 2001850. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Cai, X.; Zhu, H.; Li, J.; Shi, D.; Su, D.; Yue, D.; Gu, Z. PDT-driven highly efficient intracellular delivery and controlled release of CO in combination with sufficient singlet oxygen production for synergistic anticancer therapy. Adv. Funct. Mater. 2018, 28, 1804324. [Google Scholar] [CrossRef]
- Yang, D.; Tu, Y.; Wang, X.; Cao, C.; Hu, Y.; Shao, J.; Weng, L.; Mou, X.; Dong, X. A photo-triggered antifungal nanoplatform with efflux pump and heat shock protein reversal activity for enhanced chemo-photothermal synergistic therapy. Biomater. Sci. 2021, 9, 3293–3299. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Huo, Q.; Wang, M.; Sun, Y.; Liu, H.; Chang, J.; He, B.; Liang, Y. Targeted polymeric nanoparticles based on mangiferin for enhanced protection of pancreatic beta-cells and type 1 diabetes mellitus efficacy. ACS Appl. Mater. Interfaces 2022, 14, 11092–11103. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Chen, Y.; Pan, W.; Li, N.; Liu, Z.; Tang, B. Antitumor agents based on metal-organic frameworks. Angew. Chem. Int. Ed. Engl. 2021, 60, 16763–16776. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ovais, M.; Zhou, H.; Rui, Y.; Chen, C. Tailoring metal-organic frameworks-based nanozymes for bacterial theranostics. Biomaterials 2021, 275, 120951. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.K.; Falcaro, P.; Doonan, C.J. Metal-organic framework-based enzyme biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Quan, G.; Niu, B.; Zhou, Y.; Zhao, Y.; Lu, C.; Pan, X.; Wu, C. Versatile nanoscale metal-organic frameworks (nMOFs): An emerging 3D nanoplatform for drug delivery and therapeutic applications. Small 2021, 17, 2005064. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhou, M.; Jia, F.; Ruan, L.; Lu, H.; Zhang, J.; Zhu, B.; Liu, X.; Chen, J.; Chai, Z.; et al. D-arginine-loaded metal-organic frameworks nanoparticles sensitize osteosarcoma to radiotherapy. Biomaterials 2021, 269, 120642. [Google Scholar] [CrossRef]
- Li, Y.; Song, Y.; Zhang, W.; Xu, J.; Hou, J.; Feng, X.; Zhu, W. MOF nanoparticles with encapsulated dihydroartemisinin as a controlled drug delivery system for enhanced cancer therapy and mechanism analysis. J. Mater. Chem. B 2020, 8, 7382–7389. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, S.; Zou, Z.; Hai, L.; Yang, X.; Jia, X.; Zhang, A.; He, D.; He, X.; Wang, K. A zeolitic imidazolate frame-work-8-based indocyanine green theranostic agent for infrared fluorescence imaging and photothermal therapy. J. Mater. Chem. B 2018, 6, 3914–3921. [Google Scholar] [CrossRef]
- Xiao, Y.; Huang, W.; Zhu, D.; Wang, Q.; Chen, B.; Liu, Z.; Wang, Y.; Liu, Q. Cancer cell membrane-camouflaged MOF na-noparticles for a potent dihydroartemisinin-based hepatocellular carcinoma therapy. RSC Adv. 2020, 10, 7194–7205. [Google Scholar] [CrossRef]
- Yu, W.; Sun, J.; Liu, F.; Yu, S.; Hu, J.; Zhao, Y.; Wang, X.; Liu, X. Treating immunologically cold tumors by precise cancer photoimmunotherapy with an extendable nanoplatform. ACS Appl. Mater. Interfaces 2020, 12, 40002–40012. [Google Scholar] [CrossRef]
- Li, F.; Chen, T.; Wang, F.; Chen, J.; Zhang, Y.; Song, D.; Li, N.; Lin, X.H.; Lin, L.; Zhuang, J. Enhanced cancer starvation therapy enabled by an autophagy inhibitors-encapsulated biomimetic ZIF-8 nanodrug: Disrupting and harnessing dual pro-survival autophagic responses. ACS Appl. Mater. Interfaces 2022, 14, 21860–21871. [Google Scholar] [CrossRef]
- Li, N.; Cai, H.; Jiang, L.; Hu, J.; Bains, A.; Hu, J.; Gong, Q.; Luo, K.; Gu, Z. Enzyme-sensitive and amphiphilic PEGylated dendrimer-paclitaxel prodrug-based nanoparticles for enhanced stability and anticancer efficacy. ACS Appl. Mater. Interfaces 2017, 9, 6865–6877. [Google Scholar] [CrossRef]
- Li, N.; Duan, Z.; Wang, L.; Guo, C.; Zhang, H.; Gu, Z.; Gong, Q.; Luo, K. An amphiphilic PEGylated peptide dendron-gemcitabine prodrug-based nanoagent for cancer therapy. Macromol. Rapid Commun. 2021, 42, 2100111. [Google Scholar] [CrossRef]
- Gao, L.; Chen, Q.; Gong, T.; Liu, J.; Li, C. Recent advancement of imidazolate framework (ZIF-8) based nanoformulations for synergistic tumor therapy. Nanoscale 2019, 11, 21030–21045. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; He, G.; Xiong, C.; Wang, C.; Lian, X.; Hu, L.; Li, Z.; Dalgarno, S.J.; Yang, Y.W.; Tian, J. One-pot fabrication of hollow porphyrinic MOF nanoparticles with ultrahigh drug loading toward controlled delivery and synergistic cancer therapy. ACS Appl. Mater. Interfaces 2021, 13, 3679–3693. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.S.; Chen, T.T.; Nie, C.P.; Yi, J.T.; Liu, C.; Hu, Y.L.; Chu, X. In situ synthesis of ultrathin ZIF-8 film-coated MSNs for codelivering Bcl 2 siRNA and doxorubicin to enhance chemotherapeutic efficacy in drug-resistant cancer cells. ACS Appl. Mater. Interfaces 2018, 10, 33070–33077. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative stress in cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Zhou, L.; Lei, Y.; Zhang, Y.; Huang, C. Redox signaling and unfolded protein response coordinate cell fate decisions under ER stress. Redox Biol. 2019, 25, 101047. [Google Scholar] [CrossRef] [PubMed]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Pihan, P.; Carreras-Sureda, A.; Hetz, C. BCL-2 family: Integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017, 24, 1478–1487. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Wang, B.; Chen, W.; Wang, T.; Li, M.; Shen, Z.; Wang, F.; Jia, J.; Li, F.; Huang, X.; et al. Co-Delivery of Dihydroartemisinin and Indocyanine Green by Metal-Organic Framework-Based Vehicles for Combination Treatment of Hepatic Carcinoma. Pharmaceutics 2022, 14, 2047. https://doi.org/10.3390/pharmaceutics14102047
Chen Y, Wang B, Chen W, Wang T, Li M, Shen Z, Wang F, Jia J, Li F, Huang X, et al. Co-Delivery of Dihydroartemisinin and Indocyanine Green by Metal-Organic Framework-Based Vehicles for Combination Treatment of Hepatic Carcinoma. Pharmaceutics. 2022; 14(10):2047. https://doi.org/10.3390/pharmaceutics14102047
Chicago/Turabian StyleChen, Yang, Bin Wang, Wenping Chen, Tao Wang, Min Li, Zucheng Shen, Fang Wang, Jing Jia, Fenglan Li, Xiangyu Huang, and et al. 2022. "Co-Delivery of Dihydroartemisinin and Indocyanine Green by Metal-Organic Framework-Based Vehicles for Combination Treatment of Hepatic Carcinoma" Pharmaceutics 14, no. 10: 2047. https://doi.org/10.3390/pharmaceutics14102047
APA StyleChen, Y., Wang, B., Chen, W., Wang, T., Li, M., Shen, Z., Wang, F., Jia, J., Li, F., Huang, X., Zhuang, J., & Li, N. (2022). Co-Delivery of Dihydroartemisinin and Indocyanine Green by Metal-Organic Framework-Based Vehicles for Combination Treatment of Hepatic Carcinoma. Pharmaceutics, 14(10), 2047. https://doi.org/10.3390/pharmaceutics14102047





