Making Sense of Antisense Oligonucleotide Therapeutics Targeting Bcl-2
Abstract
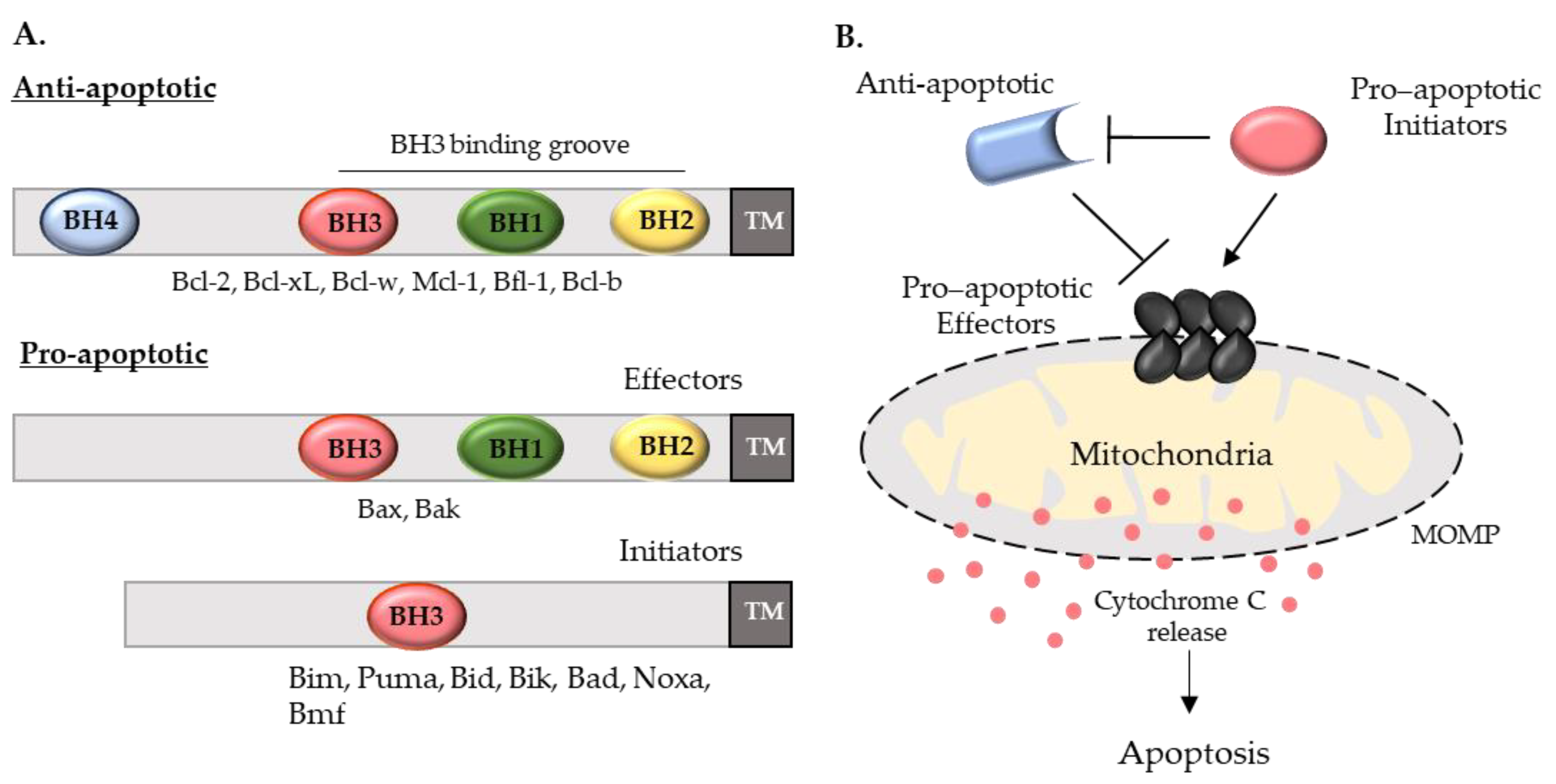
1. Introduction
2. The Beginning of Bcl-2 ASO Therapies
2.1. Initial Bcl-2 ASOs In Vitro Studies
2.2. Bcl-2/Bcl-xL-Bispecific ASO
2.3. Oblimersen
3. Optimizations of Bcl-2 ASOs
3.1. SPC2996
3.2. PNT2258
3.3. BP1002
4. Outlook on Bcl-2 ASO and Alternative Oligonucleotide Therapies
4.1. Overcoming Venetoclax Resistance
4.2. Alternative Oligonucleotide Therapies
4.2.1. MRX34
4.2.2. TargomiR
4.2.3. siBCL-2 NKExos
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arya, R.; White, K. Cell death in development: Signaling pathways and core mechanisms. Semin. Cell Dev. Biol. 2015, 39, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, R.S.; Ness, J.M.; Roth, K.A. Bcl-2 family regulation of neuronal development and neurodegeneration. Biochim. Biophys. Acta Mol. Cell Res. 2004, 1644, 189–203. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.L.; Abel, P.; Foster, C.S.; Lalani, E.N. Bcl-2: Role in epithelial differentiation and oncogenesis. Hum. Pathol. 1996, 27, 102–110. [Google Scholar] [CrossRef]
- Kelekar, A.; Thompson, C.B. Bcl-2-family proteins: The role of the BH3 domain in apoptosis. Trends Cell Biol. 1998, 8, 324–330. [Google Scholar] [CrossRef]
- Westphal, D.; Kluck, R.M.; Dewson, G. Building blocks of the apoptotic pore: How bax and bak are activated and oligomerize during apoptosis. Cell Death Differ. 2013, 21, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Pihán, P.; Carreras-Sureda, A.; Hetz, C. BCL-2 Family: Integrating stress responses at the ER to control cell demise. Cell Death Differ. 2017, 24, 1478–1487. [Google Scholar] [CrossRef]
- Kønig, S.M.; Rissler, V.; Terkelsen, T.; Lambrughi, M.; Papaleo, E. Alterations of the interactome of Bcl-2 proteins in breast cancer at the transcriptional, mutational and structural level. PLoS Comput. Biol. 2019, 15, e1007485. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Gorham, J.; Cossman, J.; Jaffe, E.; Croce, C.M. The t(14;18) chromosome translocations involved in B-cell neoplasms result from mistakes in VDJ joining. Science 1985, 229, 1390–1393. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Finger, L.R.; Yunis, J.; Nowell, P.C.; Croce, C.M. Cloning of the Chromosome Breakpoint of Neoplastic B Cells with the t(14;18) Chromosome Translocation. Science 1984, 226, 1097–1099. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Cossman, J.; Jaffe, E.; Croce, C.M. Involvement of the Bcl-2 gene in human follicular lymphoma. Science 1985, 228, 1440–1443. [Google Scholar] [CrossRef]
- Eberle, J.; Hossini, A. Expression and function of Bcl-2 proteins in melanoma. Curr. Genom. 2008, 9, 409–419. [Google Scholar] [CrossRef]
- Merino, D.; Lok, S.W.; Visvader, J.E.; Lindeman, G.J. Targeting BCL-2 to enhance vulnerability to therapy in estrogen receptor-positive breast cancer. Oncogene 2016, 35, 1877–1887. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhang, X.; Yao, M.; Lv, K.; Wang, J.; Chen, L.; Chen, Y.; Wang, S.; Fu, P. Bcl-2 promotes metastasis through the epithelial-to-mesenchymal transition in the BCap37 medullary breast cancer cell line. Oncol. Lett. 2018, 15, 8991–8998. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.-P.; Ding, X.-W.; Peng, J.-P.; Zheng, Y.-X.; Zhang, S.-Z. Prognostic significance of Bcl-2 and P53 expression in colorectal carcinoma. J. Zhejiang Univ. Sci. B 2005, 6, 1163–1169. [Google Scholar] [CrossRef]
- Anagnostou, V.K.; Lowery, F.J.; Zolota, V.; Tzelepi, V.; Gopinath, A.; Liceaga, C.; Panagopoulos, N.; Frangia, K.; Tanoue, L.; Boffa, D.; et al. High expression of BCL-2 predicts favorable outcome in non-small cell lung cancer patients with non squamous histology. BMC Cancer 2010, 10, 186. [Google Scholar] [CrossRef]
- Monni, O.; Joensuu, H.; Franssila, K.; Klefstrom, J.; Alitalo, K.; Knuutila, S. Bcl-2 overexpression associated with chromosomal amplification in diffuse large B-cell lymphoma. Blood 1997, 90, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Novak, U.; Piovan, E.; Basso, K.; Sumazin, P.; Schneider, C.; Crespo, M.; Shen, Q.; Bhagat, G.; Califano, A.; et al. Bcl-6 suppression of Bcl-2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc. Natl. Acad. Sci. USA 2009, 106, 11294–11299. [Google Scholar] [CrossRef]
- Catz, S.D.; Johnson, J.L. Transcriptional regulation of Bcl-2 by nuclear factor ΚB and its significance in prostate cancer. Oncogene 2001, 20, 7342–7351. [Google Scholar] [CrossRef]
- Cimmino, A.; Calin, G.A.; Babbri, M.; Iorio, M.V.; Ferracin, M.; Shimizu, M.; Wojcik, S.E.; Aqeilan, R.I.; Zupo, S.; Dono, M.; et al. MiR-15 and MiR-16 induce apoptosis by targeting BCL2. Proc. Natl. Acad. Sci. USA 2005, 39, 13944–13949. [Google Scholar] [CrossRef]
- Gabellini, C.; Trisciuoglio, D.; Del Bufalo, D. Non-canonical roles of Bcl-2 and Bcl-XL proteins: Relevance of BH4 domain. Carcinogenesis 2017, 38, 579–587. [Google Scholar] [CrossRef]
- Folkman, J. Role of angiogenesis in tumor growth and metastasis. Semin. Oncol. 2002, 29, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Karl, E.; Warner, K.; Zeitlin, B.; Kaneko, T.; Wurtzel, L.; Jin, T.; Chang, J.; Wang, S.; Wang, C.-Y.; Strieter, R.M.; et al. Bcl-2 acts in a proangiogenic signaling pathway through nuclear factor-ΚB and CXC chemokines. Cancer Res. 2005, 65, 5063–5069. [Google Scholar] [CrossRef]
- Iervolino, A.; Trisciuoglio, D.; Ribatti, D.; Candiloro, A.; Biroccio, A.; Zupi, G.; Del Bufalo, D. Bcl-2 overexpression in human melanoma cells increases angiogenesis through VEGF MRNA stabilization and HIF-1-mediated transcriptional activity. FASEB J. 2002, 16, 1453–1455. [Google Scholar] [CrossRef]
- Sun, T.; Sun, B.C.; Zhao, X.L.; Zhao, N.; Dong, X.Y.; Che, N.; Yao, Z.; Ma, Y.M.; Gu, Q.; Zong, W.K.; et al. Promotion of tumor cell metastasis and vasculogenic mimicry by way of transcription coactivation by Bcl-2 and Twist1: A study of hepatocellular carcinoma. Hepatology 2011, 54, 1690–1706. [Google Scholar] [CrossRef] [PubMed]
- Nör, J.E.; Christensen, J.; Mooney, D.J.; Polverini, P.J. Vascular Endothelial Growth Factor (VEGF)-mediated angiogenesis is associated with enhanced endothelial cell survival and induction of Bcl-2 expression. Am. J. Pathol. 1999, 154, 375–384. [Google Scholar] [CrossRef]
- Trisciuoglio, D.; Gabellini, C.; Desideri, M.; Ragazzoni, Y.; De Luca, T.; Ziparo, E.; Del Bufalo, D. Involvement of BH4 domain of Bcl-2 in the regulation of HIF-1-mediated VEGF expression in hypoxic tumor cells. Cell Death Differ. 2011, 18, 1024–1035. [Google Scholar] [CrossRef] [PubMed]
- Gabellini, C.; De Luca, T.; Trisciuoglio, D.; Desideri, M.; Di Martile, M.; Passeri, D.; Candiloro, A.; Biffoni, M.; Rizzo, M.G.; Orlandi, A.; et al. BH4 domain of Bcl-2 protein is required for its proangiogenic function under hypoxic condition. Carcinogenesis 2013, 34, 2558–2567. [Google Scholar] [CrossRef]
- Ricca, A.; Biroccio, A.; Del Bufalo, D.; Mackay, A.R.; Santoni, A.; Cippitelli, M. Bcl-2 over-expression enhances NF-ΚB activity and induces Mmp-9 transcription in human MCF7(ADR) breast-cancer cells. Int. J. Cancer 2000, 86, 188–196. [Google Scholar] [CrossRef]
- Regula, K.M.; Ens, K.; Kirshenbaum, L.A. IKKβ is required for Bcl-2-mediated NF-ΚB activation in ventricular myocytes. J. Biol. Chem. 2002, 277, 38676–38682. [Google Scholar] [CrossRef]
- Pires, B.R.B.; Mencalha, A.L.; Ferreira, G.M.; De Souza, W.F.; Morgado-Díaz, J.A.; Maia, A.M.; Corrêa, S.; Abdelhay, E.S.F.W. NF-KappaB is involved in the regulation of EMT genes in breast cancer cells. PLoS ONE 2017, 12, e0169622. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choi, K.; Benveniste, E.N.; Hong, Y.-S.; Lee, J.-H.; Kim, J.; Park, K. Bcl-2 promotes invasion and lung metastasis by inducing matrix metalloproteinase-2. Cancer Res. 2005, 65, 5554–5560. [Google Scholar] [CrossRef]
- Montero, J.; Letai, A. Why do BCL-2 inhibitorswork and where should we use them in the clinic? Cell Death Differ. 2018, 25, 56–64. [Google Scholar] [CrossRef]
- Certo, M.; Moore, V.D.G.; Nishino, M.; Wei, G.; Korsmeyer, S.; Armstrong, S.A.; Letai, A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell 2006, 9, 351–365. [Google Scholar] [CrossRef]
- Sarosiek, K.A.; Fraser, C.; Muthalagu, N.; Bhola, P.D.; Chang, W.; McBrayer, S.K.; Cantlon, A.; Fisch, S.; Golomb-Mello, G.; Ryan, J.A.; et al. Developmental regulation of mitochondrial apoptosis by C-myc governs age- and tissue-specific sensitivity to cancer therapeutics. Cancer Cell 2017, 31, 142–156. [Google Scholar] [CrossRef]
- Perini, G.F.; Ribeiro, G.N.; Pinto Neto, J.V.; Campos, L.T.; Hamerschlak, N. BCL-2 as therapeutic target for hematological malignancies. J. Hematol. Oncol. 2018, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Velankar, M.; Brahmandam, M.; Hideshima, T.; Podar, K.; Richardson, P.; Schlossman, R.; Ghobrial, I.; Raje, N.; Munshi, N.; et al. A novel Bcl-2/Bcl-XL/Bcl-w inhibitor ABT-737 as therapy in multiple myeloma. Oncogene 2007, 26, 2374–2380. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, M.; Ashizawa, A.T. The challenges and strategies of antisense oligonucleotide drug delivery. Biomedicines 2021, 9, 433. [Google Scholar] [CrossRef] [PubMed]
- Dias, N.; Stein, C.A. Antisense oligonucleotides: Basic concepts and mechanisms. Mol. Cancer Ther. 2002, 1, 347–355. [Google Scholar]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense oligonucleotides: An emerging area in drug discovery and development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef]
- Eckstein, F. Phosphorothioates, essential components of therapeutic oligonucleotides. Nucleic Acid Ther. 2014, 24, 374–387. [Google Scholar] [CrossRef]
- Baker, B.; Lot, S.; Condon, T.; Cheng-Flournoy, S.; Lesnik, E.; Sasmor, H.; Bennett, C. 2’-O-(2-Methoxy)Ethyl-modified anti-intercellular adhesion molecule 1 (ICAM-1) oligonucleotides selectively increase the ICAM-1 MRNA level and inhibit formation of the ICAM-1 translation initiation complex in human umbilical vein endothelial cells. J. Biol. Chem. 1997, 272, 11994–12000. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; De Hoyos, C.L.; Sun, H.; Vickers, T.A.; Liang, X.H.; Crooke, S.T. Acute hepatotoxicity of 2 fluoro-modified 5–10–5 gapmer phosphorothioate oligonucleotides in mice correlates with intracellular protein binding and the loss of DBHS proteins. Nucleic Acids Res. 2018, 46, 2204–2217. [Google Scholar] [CrossRef]
- Luan, X.; Sansanaphongpricha, K.; Myers, I.; Chen, H.; Yuan, H.; Sun, D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 2017, 38, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Vasquez, G.; Freestone, G.C.; Wan, W.B.; Low, A.; De Hoyos, C.L.; Yu, J.; Prakash, T.P.; Ostergaard, M.E.; Liang, X.H.; Crooke, S.T.; et al. Site-Specific Incorporation of 5′-methyl DNA enhances the therapeutic profile of gapmer ASOs. Nucleic Acids Res. 2021, 49, 1828–1839. [Google Scholar] [CrossRef]
- Khvorova, A.; Watts, J. The chemical evolution of oligonucleotide therapies of clinical utility. Physiol. Behav. 2017, 35, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Cuddy, M.; Slabiak, T.; Croce, C.M.; Nowell, P.C. Oncogenic potential of Bcl-2 demonstrated by gene transfer. Nature 1988, 336, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.C.; Kitada, S.; Takayama, S.; Miyashita, T. Regulation of chemoresistance by the Bcl-2 oncoprotein in non-Hodgkin’s lymphoma and lymphocytic leukemia cell lines. Ann. Oncol. 1994, 5, 61–65. [Google Scholar] [CrossRef]
- Maung, Z.T.; MacLean, F.R.; Reid, M.M.; Pearson, A.D.J.; Proctor, S.J.; Hamilton, P.J.; Hall, A.G. The relationship between Bcl-2 expression and response to chemotherapy in acute leukaemia. Br. J. Haematol. 1994, 88, 105–109. [Google Scholar] [CrossRef]
- Kitada, S.; Takayama, S.; de Riel, K.; Tanaka, S.; Reed, J.C. Reversal of chemoresistance of lymphoma cells by antisense-mediated reduction of Bcl-2 gene expression. Antisense Res. Dev. 1994, 4, 71–79. [Google Scholar] [CrossRef]
- Dang, C.V.; Reddy, E.P.; Shokat, K.M.; Soucek, L. Drugging the “undruggable” cancer targets. Nat. Rev. Cancer 2017, 17, 502–508. [Google Scholar] [CrossRef]
- Reed, J.C.; Yum, S.; Stein, C.; Subasinghe, C.; Cohen, J.; Haldar, S.; Croce, C.M. Antisense-mediated inhibition of BCL2 protooncogene expression and leukemic cell growth and survival: Comparisons of phosphodiester and phosphorothioate oligodeoxynucleotides. Cancer Res. 1990, 50, 6565–6570. [Google Scholar] [PubMed]
- Campos, L.; Sabido, O.; Rouault, J.; Guyotat, D. Effects of BCL-2 Antisense oligodeoxynucleotides on in vitro proliferation and survival of normal marrow progenitors and leukemic cells. Blood 1994, 84, 595–600. [Google Scholar] [CrossRef]
- Ziegler, A.; Luedke, G.H.; Fabbro, D.; Altmann, K.H.; Stahel, R.A.; Zangemeister-Wittke, U. Induction of Apoptosis in small-cell lung cancer cells by an antisense oligodeoxynucleotide targeting the Bcl-2 coding sequence. J. Natl. Cancer Inst. 1997, 89, 1027–1036. [Google Scholar] [CrossRef]
- Zangemeister-Wittke, U.; Schenker, T.; Luedke, G.H.; Stahel, R.A. Synergistic cytotoxicity of Bcl-2 antisense oligodeoxynucleotides and etoposide, doxorubicin and cisplatin on small-cell lung cancer cell lines. Br. J. Cancer 1998, 78, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Fanfone, D.; Idbaih, A.; Mammi, J.; Gabut, M.; Ichim, G. Profiling anti-apoptotic Bcl-Xl protein expression in glioblastoma tumorspheres. Cancers 2020, 12, 2853. [Google Scholar] [CrossRef]
- Bessou, M.; Lopez, J.; Gadet, R.; Deygas, M.; Popgeorgiev, N.; Poncet, D.; Nougarède, A.; Billard, P.; Mikaelian, I.; Gonzalo, P.; et al. The apoptosis inhibitor Bcl-XL controls breast cancer cell migration through mitochondria-dependent reactive oxygen species production. Oncogene 2020, 39, 3056–3074. [Google Scholar] [CrossRef] [PubMed]
- Trisciuoglio, D.; Tupone, M.G.; Desideri, M.; Di Martile, M.; Gabellini, C.; Buglioni, S.; Pallocca, M.; Alessandrini, G.; D’Aguanno, S.; Del Bufalo, D. BCL-XL overexpression promotes tumor progression-associated properties. Cell Death Dis. 2017, 8, 1–15. [Google Scholar] [CrossRef]
- Lebedeva, I.; Rando, R.; Ojwang, J.; Cossum, P.; Stein, C.A. Bcl-XL in prostate cancer cells: Effects of overexpression and down-regulation on chemosensitivity. Cancer Res. 2000, 60, 6052–6060. [Google Scholar] [PubMed]
- Simonian, P.L.; Grillot, D.A.M.; Nuñez, G. Bcl-2 and Bcl-XL can differentially block chemotherapy-induced cell death. Blood 1997, 90, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Chatterjee, D.; Early, J.; Pantazis, P.; Hendrickson, E.A.; Wyche, J.H. Isolation and characterization of an apoptosis-resistant variant of human leukemia HL-60 cells that has switched expression from Bcl-2 to Bcl-XL 1. Cancer Res. 1996, 56, 1621–1628. [Google Scholar] [PubMed]
- Fiebig, A.A.; Zhu, W.; Hollerbach, C.; Leber, B.; Andrews, D.W. Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer 2006, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zangemeister-Wittke, U.; Leech, S.H.; Olie, R.A.; Simões-Wüst, A.P.; Gautschi, O.; Luedke, G.H.; Natt, F.; Häner, R.; Martin, P.; Hall, J.; et al. A novel bispecific antisense oligonucleotide inhibiting both Bcl-2 and Bcl-XL expression efficiently induces apoptosis in tumor cells. Clin. Cancer Res. 2000, 6, 2547–2555. [Google Scholar]
- Gautschi, O.; Tschopp, S.; Olie, R.A.; Leech, S.H.; Simões-Wüst, A.P.; Ziegler, A.; Baumann, B.; Odermatt, B.; Hall, J.; Stahel, R.A.; et al. Activity of a novel Bcl-2/Bcl-XL-bispecific antisense oligonucleotide against tumors of diverse histologic origins. J. Natl. Cancer Inst. 2001, 93, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Del Bufalo, D.; Trisciuoglio, D.; Scarsella, M.; Zangemeister-Wittke, U.; Zupi, G. Treatment of melanoma cells with a Bcl-2/Bcl-XL antisense oligonucleotide induces antiangiogenic activity. Oncogene 2003, 22, 8441–8447. [Google Scholar] [CrossRef]
- Yamanaka, K.; Rocchi, P.; Miyake, H.; Fazli, L.; So, A.; Zangemeister-Wittke, U.; Gleave, M.E. Induction of apoptosis and enhancement of chemosensitivity in human prostate cancer LNCaP cells using bispecific antisense oligonucleotide targeting Bcl-2 and Bcl-XL genes. BJU Int. 2006, 97, 1300–1308. [Google Scholar] [CrossRef]
- Yamanaka, K.; Rocchi, P.; Miyake, H.; Fazli, L.; Vessella, B.; Zangemeister-Wittke, U.; Gleave, M.E. A novel antisense oligonucleotide inhibiting several antiapoptotic Bcl-2 Family members induces apoptosis and enhances chemosensitivity in androgen-independent human prostate cancer PC3 cells. Mol. Cancer Ther. 2005, 4, 1689–1698. [Google Scholar] [CrossRef]
- Simões-Wüst, A.P.; Hopkins-Donaldson, S.; Sigrist, B.; Belyanskaya, L.; Stahel, R.A.; Zangemeister-Wittke, U. A Functionally improved locked nucleic acid antisense oligonucleotide inhibits Bcl-2 and Bcl-XL expression and facilitates tumor cell apoptosis. Oligonucleotides 2004, 14, 199–209. [Google Scholar] [CrossRef]
- Schlagbauer-Wadl, H.; Klosner, G.; Heere-Ress, E.; Waltering, S.; Moll, I.; Wolff, K.; Pehamberger, H.; Jansen, B. Bcl-2 Antisense oligonucleotides (G3139) inhibit merkel cell carcinoma growth in SCID mice. J. Investig. Dermatol. 2000, 114, 725–730. [Google Scholar] [CrossRef]
- Webb, A.; Cunningham, D.; Cotter, F.; Clarke, P.A.; Di Stefano, F.; Ross, P.; Corbo, M.; Dziewanowska, Z. BCL-2 Antisense therapy in patients with non-hodgkin lymphoma. Lancet 1997, 349, 1137–1141. [Google Scholar] [CrossRef]
- Oblimersen: Augmerosen, BCL-2 Antisense Oligonucleotide—Genta, G 3139, GC 3139, oblimersen sodium. Drugs R D 2007, 8, 321–334. [CrossRef]
- Kitada, S.; Miyashita, T.; Tanaka, S.; Reed, J.C. Investigations of antisense oligonucleotides targeted against Bcl-2 RNAs. Antisense Res. Dev. 1993, 3, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Klasa, R.J.; Bally, M.B.; Ng, R.; Goldie, J.H.; Gascoyne, R.D.; Wong, F.M.P. Eradication of human non-hodgkin’s lymphoma in SCID mice by BCL-2 antisense oligonucleotides combined with low-dose cyclophosphamide. Clin. Cancer Res. 2000, 6, 2492–2500. [Google Scholar] [PubMed]
- Herbst, R.S.; Frankel, S.R. Oblimersen sodium (Genasense Bcl-2 antisense oligonucleotide): A rational therapeutic to enhance apoptosis in therapy of lung cancer. Clin. Cancer Res. 2004, 10, 4245s–4248s. [Google Scholar] [CrossRef]
- Leleu, X.; Gay, J.; Roccaro, A.M.; Moreau, A.S.; Paulain, S.; Dulery, R.; Champs, B.; Robu, D.; Ghobrial, I.M. Update on therapeutic options in waldenström macroglobulinemia. Eur. J. Haematol. 2011, 82, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Geyer, S.M.; Badros, A.; Kahl, B.S.; Erlichman, C. Early results of a phase I trial of oblimersen sodium for relapsed or refractory Waldenström’s macroglobulinemia. Clin. Lymphoma 2005, 5, 282–284. [Google Scholar] [CrossRef]
- Morris, M.J.; Tong, W.P.; Cordon-Cardo, C.; Drobnjak, M.; Kelly, W.K.; Slovin, S.F.; Terry, K.L.; Siedlecki, K.; Swanson, P.; Rafi, M.; et al. Phase I trial of BCL-2 antisense oligonucleotide (G3139) administered by continuous intravenous infusion in patients with advanced cancer. Clin. Cancer Res. 2002, 8, 679–683. [Google Scholar]
- O’Brien, S.M.; Cunningham, C.C.; Golenkov, A.K.; Turkina, A.G.; Novick, S.C.; Rai, K.R. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 Antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J. Clin. Oncol. 2005, 23, 7697–7702. [Google Scholar] [CrossRef]
- Bedikian, A.Y.; Millward, M.; Pehamberger, H.; Conry, R.; Gore, M.; Trefzer, U.; Pavlick, A.C.; DeConti, R.; Hersh, E.M.; Hersey, P.; et al. Bcl-2 antisense (Oblimersen Sodium) plus dacarbazine in patients with advanced melanoma: The oblimersen melanoma study group. J. Clin. Oncol. 2006, 24, 4738–4745. [Google Scholar] [CrossRef]
- Chanan-Khan, A.; Niesvizky, R.; Hohl, R.J.; Zimmerman, T.M.; Christiansen, N.P.; Schiller, G.J.; Callander, N.; Lister, J.; Oken, M.; Jagannath, S. Phase III randomised study of dexamethasone with or without oblimersen sodium for patients with advanced multiple myeloma. Leuk. Lymphoma 2009, 50, 559–565. [Google Scholar] [CrossRef]
- O’Brien, S.; Moore, J.O.; Boyd, T.E.; Larratt, L.M.; Skotnicki, A.; Koziner, B.; Chanan-Khan, A.A.; Seymour, J.F.; Bociek, R.G.; Pavletic, S.; et al. Randomized phase III trial of fludarabine plus cyclophosphamide with or without oblimersen sodium (Bcl-2 Antisense) in patients with relapsed or refractory chronic lymphocytic leukemia. J. Clin. Oncol. 2007, 25, 1114–1120. [Google Scholar] [CrossRef]
- Marcucci, G.; Stock, W.; Dai, G.; Klisovic, R.B.; Liu, S.; Klisovic, M.I.; Blum, W.; Kefauver, C.; Sher, D.A.; Green, M.; et al. Phase I study of oblimersen sodium, an antisense to Bcl-2, in untreated older patients with acute myeloid leukemia: Pharmacokinetics, pharmacodynamics, and clinical activity. J. Clin. Oncol. 2005, 23, 3404–3411. [Google Scholar] [CrossRef]
- Walker, A.R.; Marcucci, G.; Yin, J.; Blum, W.; Stock, W.; Kohlschmidt, J.; Mrózek, K.; Carroll, A.J.; Eisfeld, A.K.; Wang, E.S.; et al. Phase 3 randomized trial of chemotherapy with or without oblimersen in older AML patients: CALGB 10201 (Alliance). Blood Adv. 2021, 5, 2775–2787. [Google Scholar] [CrossRef] [PubMed]
- Bedikian, A.Y.; Garbe, C.; Conry, R.; Lebbe, C.; Grob, J.J. Dacarbazine with or without Oblimersen (a Bcl-2 Antisense Oligonucleotide) in chemotherapy-naive patients with advanced melanoma and low-normal serum lactate dehydrogenase: “The AGENDA trial”. Melanoma Res. 2014, 24, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Frantz, S. Lessons learnt from Genasense’s failure. Nat. Rev. Drug Discov. 2004, 3, 542–543. [Google Scholar] [CrossRef] [PubMed]
- Stein, C.A.; Hansen, J.B.; Lai, J.; Wu, S.; Voskresenskiy, A.; Høg, A.; Worm, J.; Hedtjärn, M.; Souleimanian, N.; Miller, P.; et al. Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res. 2010, 38, e3. [Google Scholar] [CrossRef]
- Dürig, J.; Dührsen, U.; Klein-Hitpass, L.; Worm, J.; Hansen, J.B.R.; Orum, H.; Wissenbach, M. The novel antisense Bcl-2 inhibitor SPC2996 causes rapid leukemic cell clearance and immune activation in chronic lymphocytic leukemia. Leukemia 2011, 25, 638–647. [Google Scholar] [CrossRef][Green Version]
- Lamphier, M.S.; Sirois, C.M.; Verma, A.; Golenbock, D.T.; Latz, E. TLR9 and the recognition of self and non-self nucleic acids. Ann. N. Y. Acad. Sci. 2006, 1082, 31–43. [Google Scholar] [CrossRef]
- Dampmann, M.; Görgens, A.; Möllmann, M.; Murke, F.; Dührsen, U.; Giebel, B.; Dürig, J. CpG Stimulation of chronic lymphocytic leukemia cells induces a polarized cell shape and promotes migration in vitro and in vivo. PLoS ONE 2020, 15, e0228674. [Google Scholar] [CrossRef]
- ProNAi Announces Dna Interference (DNAI(R)) Drug Development Strategy For 2007 | DNA RNA and Cells | News Channels. Available online: https://pipelinereview.com/index.php/200701099082/DNA-RNA-and-Cells/ProNAi-Announces-Dna-Interference-DNAIR-Drug-Development-Strategy-For-2007.html (accessed on 16 October 2021).
- Maston, G.A.; Evans, S.K.; Green, M.R. Transcriptional regulatory elements in the human genome. Annu. Rev. Genom. Hum. Genet. 2006, 7, 29–59. [Google Scholar] [CrossRef]
- Gaffney, D.J. Mapping and predicting gene–Enhancer interactions. Nat. Genet. 2019, 51, 1662–1663. [Google Scholar] [CrossRef]
- Duan, H.; Xiang, H.; Ma, L.; Boxer, L.M. Functional long-range interactions of the IgH 3′ enhancers with the Bcl-2 promoter region in t(14;18) lymphoma cells. Oncogene 2008, 27, 6720–6728. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, A.S.; Kandouz, M.; Liddane, A.; Sabbagh, H.; Hou, Y.; Li, C.; Al-Katib, A. PNT2258, a novel deoxyribonucleic acid inhibitor, induces cell cycle arrest and apoptosis via a distinct mechanism of action: A new class of drug for non-Hodgkin’s lymphoma. Oncotarget 2016, 7, 42374–42384. [Google Scholar] [CrossRef]
- Adami, R.C.; Seth, S.; Harvie, P.; Johns, R.; Fam, R.; Fosnaugh, K.; Zhu, T.; Farber, K.; McCutcheon, M.; Goodman, T.T.; et al. An Amino Acid-Based Amphoteric Liposomal Delivery System for Systemic Administration of SiRNA. Mol. Ther. 2011, 19, 1141–1151. [Google Scholar] [CrossRef] [PubMed]
- Rodrigueza, W.V.; Woolliscroft, M.J.; Ebrahim, A.S.; Forgey, R.; McGovren, P.J.; Endert, G.; Wagner, A.; Holewa, D.; Aboukameel, A.; Gill, R.D.; et al. Development and antitumor activity of a BCL-2 targeted single-stranded DNA oligonucleotide. Cancer Chemother. Pharmacol. 2014, 74, 151–166. [Google Scholar] [CrossRef]
- Siepi, E.; Lutz, S.; Meyer, S.; Panzner, S. An ion switch regulates fusion of charged membranes. Biophys. J. 2011, 100, 2412. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, A.S.; Kandouz, M.; Emara, N.; Sugalski, A.B.; Lipovich, L.; Al-Katib, A.M. Unintended target effect of anti-BCL-2 DNAi. Cancer Manag. Res. 2017, 9, 427. [Google Scholar] [CrossRef]
- Tolcher, A.W.; Rodrigueza, W.V.; Rasco, D.W.; Patnaik, A.; Papadopoulos, K.P.; Amaya, A.; Moore, T.D.; Gaylor, S.K.; Bisgaier, C.L.; Sooch, M.P.; et al. A phase 1 study of the BCL2-targeted deoxyribonucleic acid inhibitor (DNAi) PNT2258 in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2014, 73, 363–371. [Google Scholar] [CrossRef]
- Harb, W.; Lakhani, N.J.; Messmann, R.; Klencke, B.; Al-Katib, A.M. A phase 2 study of PNT2258 for treatment of relapsed or refractory B-cell malignancies. Clin. Lymphoma Myeloma Leuk. 2021, 21, 823–830. [Google Scholar] [CrossRef]
- ProNAi Reports Interim Data from Wolverine Phase 2 Trial of PNT2258 in DLBCL. Available online: https://www.prnewswire.com/news-releases/pronai-reports-interim-data-from-wolverine-phase-2-trial-of-pnt2258-in-dlbcl-581947101.html (accessed on 17 October 2021).
- Gutiérrez-Puente, Y.; Tari, A.M.; Stephens, C.; Rosenblum, M.; Guerra, R.T.; Lopez-Berestein, G. Safety, Pharmacokinetics, and tissue distribution of liposomal P-ethoxy antisense oligonucleotides targeted to Bcl-2. J. Pharmacol. Exp. Ther. 1999, 291, 865–869. [Google Scholar]
- Fresquet, V.; Rieger, M.; Carolis, C.; García-Barchino, M.J.; Martinez-Climent, J.A. Acquired mutations in BCL2 family proteins conferring resistance to the BH3 mimetic ABT-199 in lymphoma. Blood 2014, 123, 4111–4119. [Google Scholar] [CrossRef] [PubMed]
- Tari-Ashizawa, A.; Guiterrez-Puente, Y.; Ford, R.J.; Lopez-Berestein, G. Activity of Bcl-2 antisense therapeutic in aggressive non-Hodgkin’ s lymphoma. In Proceedings of the AACR Annual Meeting 2017, Washington, DC, USA, 1–5 April 2017. [Google Scholar]
- Konopleva, M.; Tari, A.M.; Estrov, Z.; Harris, D.; Xie, Z.; Zhao, S.; López-Berestein, G.; Andreeff, M. Liposomal Bcl-2 antisense oligonucleotides enhance proliferation, sensitize acute myeloid leukemia to cytosine-arabinoside, and induce apoptosis independent of other antiapoptotic proteins. Blood 2000, 95, 3929–3938. [Google Scholar] [CrossRef]
- Campone, M.; Vavasseur, F.; Le Cabellec, M.T.; Meflah, K.; Vallette, F.M.; Oliver, L. Induction of chemoresistance in HL-60 cells concomitantly causes a resistance to apoptosis and the synthesis of P-glycoprotein. Leukemia 2001, 15, 1377–1387. [Google Scholar] [CrossRef]
- Kornblau, S.M.; Thall, P.F.; Estrov, Z.; Walterscheid, M.; Patel, S.; Theriault, A.; Keating, M.J.; Kantarjian, H.; Estey, E.; Andreeff, M. The prognostic impact of BCL2 protein expression in acute myelogenous leukemia varies with cytogenetics. Clin. Cancer Res. 1999, 5, 1758–1766. [Google Scholar]
- Frazier, K.S. Antisense oligonucleotide therapies: The promise and the challenges from a toxicologic pathologist’s perspective. Toxicol. Pathol. 2015, 43, 78–89. [Google Scholar] [CrossRef]
- Waters, J.S.; Webb, A.; Cunningham, D.; Clarke, P.A.; Raynaud, F.; Di Stefano, F.; Cotter, F.E. Phase I clinical and pharmacokinetic study of Bcl-2 antisense oligonucleotide therapy in patients with non-Hodgkin’s lymphoma. J. Clin. Oncol. 2000, 18, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Sewing, S.; Roth, A.B.; Winter, M.; Dieckmann, A.; Bertinetti-Lapatki, C.; Tessier, Y.; McGinnis, C.; Huber, S.; Koller, E.; Ploix, C.; et al. Assessing single-stranded oligonucleotide drug-induced effects in vitro reveals key risk factors for thrombocytopenia. PLoS ONE 2017, 12, e0187574. [Google Scholar] [CrossRef]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J.; et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Konopleva, M.; Pollyea, D.A.; Potluri, J.; Chyla, B.; Hogdal, L.; Busman, T.; McKeegan, E.; Salem, A.H.; Zhu, M.; Ricker, J.L.; et al. Efficacy and biological correlates of response in a phase II study of venetoclax monotherapy in patients with acute myelogenous leukemia. Cancer Discov. 2016, 6, 1106–1117. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Al-Sawaf, O.; Hallek, M. Preventing and monitoring for tumor lysis syndrome and other toxicities of venetoclax during treatment of chronic lymphocytic leukemia. Hematol. Am. Soc. Hematol. Educ. Progr. 2020, 2020, 357. [Google Scholar] [CrossRef]
- Tausch, E.; Close, W.; Dolnik, A.; Bloehdorn, J.; Chyla, B.; Bullinger, L.; Döhner, H.; Mertens, D.; Stilgenbauer, S. Venetoclax Resistance and acquired BCL2 mutations in chronic lymphocytic leukemia. Haematologica 2019, 104, E434–E437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ren, Y.; Lawlor, M.; Shah, B.D.; Park, P.M.C.; Lwin, T.; Wang, X.; Liu, K.; Wang, M.; Gao, J.; et al. BCL2 amplicon loss and transcriptional remodeling drives ABT-199 resistance in B cell lymphoma models. Cancer Cell 2019, 35, 752–766.e9. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.Y.; Chen, Q.; He, J.S. Combination strategies to overcome resistance to the BCL2 inhibitor venetoclax in hematologic malignancies. Cancer Cell Int. 2020, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Salah, H.T.; Dinardo, C.D.; Konopleva, M.; Khoury, J.D. Potential biomarkers for treatment response to the Bcl-2 inhibitor venetoclax: State of the art and future directions. Cancers 2021, 13, 2974. [Google Scholar] [CrossRef]
- Choudhary, G.S.; Al-Harbi, S.; Mazumder, S.; Hill, B.T.; Smith, M.R.; Bodo, J.; Hsi, E.D.; Almasan, A. MCL-1 and BCL-XL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/MTOR activation in lymphoid malignancies. Cell Death Dis. 2015, 6, e1593. [Google Scholar] [CrossRef]
- Lin, K.H.; Winter, P.S.; Xie, A.; Roth, C.; Martz, C.A.; Stein, E.M.; Anderson, G.R.; Tingley, J.P.; Wood, K.C. Targeting MCL-1/BCL-XL forestalls the acquisition of resistance to ABT-199 in acute myeloid leukemia. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Li, Z.; He, S.; Look, A.T. The MCL1-specific inhibitor S63845 acts synergistically with venetoclax/ABT-199 to induce apoptosis in T-cell acute lymphoblastic leukemia cells. Leukemia 2019, 33, 262–266. [Google Scholar] [CrossRef]
- Chen, X.; Glytsou, C.; Zhou, H.; Narang, S.; Reyna, D.E.; Lopez, A.; Sakellaropoulos, T.; Gong, Y.; Kloetgen, A.; Yap, Y.S.; et al. Targeting mitochondrial structure sensitizes acute myeloid leukemia to venetoclax treatment. Cancer Discov. 2019, 9, 890–909. [Google Scholar] [CrossRef]
- Sharon, D.; Cathelin, S.; Mirali, S.; Di Trani, J.M.; Yanofsky, D.J.; Keon, K.A.; Rubinstein, J.L.; Schimmer, A.D.; Ketela, T.; Chan, S.M. Inhibition of mitochondrial translation overcomes venetoclax resistance in AML through activation of the integrated stress response. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Blombery, P.; Anderson, M.A.; Gong, J.N.; Thijssen, R.; Birkinshaw, R.W.; Thompson, E.R.; Teh, C.E.; Nguyen, T.; Xu, Z.; Flensburg, C.; et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2019, 9, 342–353. [Google Scholar] [CrossRef]
- Gagliardi, M.; Tari Ashizawa, A. The combination of liposomal Bcl-2 antisense oligonucleotide (BP1002) with decitabine is efficacious in venetoclax-resistant cells. Cancer Res. 2021, 81, 939. [Google Scholar]
- Ohanian, M.; Tari Ashizawa, A.; Garcia-Manero, G.; Pemmaraju, N.; Kadia, T.; Jabbour, E.; Ravandi, F.; Borthakur, G.; Andreeff, M.; Konopleva, M.; et al. Liposomal Grb2 antisense oligodeoxynucleotide (BP1001) in patients with refractory or relapsed haematological malignancies: A single-centre, open-label, dose-escalation, phase 1/1b trial. Lancet Haematol. 2018, 5, e136–e146. [Google Scholar] [CrossRef]
- Roca-Portoles, A.; Rodriguez-Blanco, G.; Sumpton, D.; Cloix, C.; Mullin, M.; Mackay, G.M.; O’Neill, K.; Lemgruber, L.; Luo, X.; Tait, S.W.G. Venetoclax causes metabolic reprogramming independent of BCL-2 inhibition. Cell Death Dis. 2020, 11, 616. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Cannell, I.G.; Kong, Y.W.; Bushell, M. How do MicroRNAs regulate gene expression? Biochem. Soc. Trans. 2008, 36, 1224–1231. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. Elegans heterochronic gene Lin-4 encodes small RNAs with antisense complementarity to Lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Friedman, R.C.; Farh, K.K.H.; Burge, C.B.; Bartel, D.P. Most mammalian MRNAs are conserved targets of MicroRNAs. Genome Res. 2009, 19, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Lionetti, M.; Musto, P.; Di Martino, M.T.; Fabris, S.; Agnelli, L.; Todoerti, K.; Tuana, G.; Mosca, L.; Cantafio, M.E.G.; Grieco, V.; et al. Biological and clinical relevance of MiRNA expression signatures in primary plasma cell leukemia. Clin. Cancer Res. 2013, 19, 3130–3142. [Google Scholar] [CrossRef]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of MicroRNAs in cancer drug resistance. Clin. Epigenet. 2019, 11, 1–24. [Google Scholar] [CrossRef]
- Rokavec, M.; Li, H.; Jiang, L.; Hermeking, H. The P53/MiR-34 axis in development and disease. J. Mol. Cell Biol. 2014, 6, 214–230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Ren, R.J.; Tang, J.H. MicroRNA-34a: A potential therapeutic target in human cancer. Cell Death Dis. 2014, 5, e1327. [Google Scholar] [CrossRef] [PubMed]
- Bader, A.G.; Brown, D.; Stoudemire, J.; Lammers, P. Developing therapeutic MicroRNAs for cancer. Gene Ther. 2011, 18, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.A.; Attiyeh, E.F.; Mosse, Y.P.; Laquaglia, M.J.; Diskin, S.J.; Brodeur, G.M.; Maris, J.M. A functional screen identifies MiR-34a as a candidate neuroblastoma tumor suppressor gene. Mol. Cancer Res. 2008, 6, 735–742. [Google Scholar] [CrossRef]
- Yang, F.; Li, Q.J.; Gong, Z.B.; Zhou, L.; You, N.; Wang, S.; Li, X.L.; Li, J.J.; An, J.Z.; Wang, D.S.; et al. MicroRNA-34a targets Bcl-2 and sensitizes human hepatocellular carcinoma cells to sorafenib treatment. Technol. Cancer Res. Treat. 2014, 13, 77–86. [Google Scholar] [CrossRef]
- Lin, X.; Guan, H.; Huang, Z.; Liu, J.; Li, H.; Wei, G.; Cao, X.; Li, Y. Downregulation of Bcl-2 expression by Mir-34a mediates palmitate-induced Min6 cells apoptosis. J. Diabetes Res. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, X. MiR-34a targets BCL-2 to suppress the migration and invasion of sinonasal squamous cell carcinoma. Oncol. Lett. 2018, 16, 6566–6572. [Google Scholar] [CrossRef]
- Daige, C.L.; Wiggins, J.F.; Priddy, L.; Nelligan-Davis, T.; Zhao, J.; Brown, D. Systemic delivery of a MiR34a mimic as a potential therapeutic for liver cancer. Mol. Cancer Ther. 2014, 13, 2352–2360. [Google Scholar] [CrossRef]
- Craig, V.J.; Tzankov, A.; Flori, M.; Schmid, C.A.; Bader, A.G.; Müller, A. Systemic MicroRNA-34a delivery induces apoptosis and abrogates growth of diffuse large B-cell lymphoma in vivo. Leukemia 2012, 26, 2421–2424. [Google Scholar] [CrossRef] [PubMed]
- Wiggins, J.F.; Ruffino, L.; Kelnar, K.; Omotola, M.; Patrawala, L.; Brown, D.; Bader, A.G. Development of a lung cancer therapeutic based on the tumor suppressor MicroRNA-34. Cancer Res. 2010, 70, 5923–5930. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.; Kim, T.; et al. Phase 1 study of MRX34, a liposomal MiR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Lovat, F.; Fassan, M.; Sacchi, D.; Ranganathan, P.; Palamarchuk, A.; Bill, M.; Karunasiri, M.; Gasparini, P.; Nigita, G.; Distefano, R.; et al. Knockout of both MiR-15/16 loci induces acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2018, 115, 13069–13074. [Google Scholar] [CrossRef]
- Calin, G.A.; Dumitru, C.D.; Shimizu, M.; Bichi, R.; Zupo, S.; Noch, E.; Aldler, H.; Rattan, S.; Keating, M.; Rai, K.; et al. Frequent deletions and down-regulation of Micro-RNA genes MiR15 and MiR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. USA 2002, 99, 15524–15529. [Google Scholar] [CrossRef]
- Reid, G.; Pel, M.E.; Kirschner, M.B.; Cheng, Y.Y.; Mugridge, N.; Weiss, J.; Williams, M.; Wright, C.; Edelman, J.J.B.; Vallely, M.P.; et al. Restoring expression of MiR-16: A novel approach to therapy for malignant pleural mesothelioma. Ann. Oncol. 2013, 24, 3128–3135. [Google Scholar] [CrossRef] [PubMed]
- Van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and activity of MicroRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: A first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Arrighetti, N.; Corbo, C.; Evangelopoulos, M.; Tasciotti, E. Exosome-like nanovectors for drug delivery in cancer. Curr. Med. Chem. 2020, 26, 6132–6148. [Google Scholar] [CrossRef]
- Wu, C.H.; Li, J.; Li, L.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C.; Jong, A.Y. Extracellular vesicles derived from natural killer cells use multiple cytotoxic proteins and killing mechanisms to target cancer cells. J. Extracell. Vesicles 2019, 8, 1588538. [Google Scholar] [CrossRef]
- Di Pace, A.L.; Tumino, N.; Besi, F.; Alicata, C.; Conti, L.A.; Munari, E.; Maggi, E.; Vacca, P.; Moretta, L. Characterization of human NK cell-derived exosomes: Role of DNAM1 receptor in exosome-mediated cytotoxicity against tumor. Cancers 2020, 12, 661. [Google Scholar] [CrossRef]
- Jong, A.Y.; Wu, C.H.; Li, J.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell. Vesicles 2017, 6, 1294368. [Google Scholar] [CrossRef]
- Kaban, K.; Hinterleitner, C.; Zhou, Y.; Salva, E.; Kantarci, A.G.; Salih, H.R.; Märklin, M. Therapeutic silencing of Bcl-2 using Nk cell-derived exosomes as a novel therapeutic approach in breast cancer. Cancers 2021, 13, 2397. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Liao, C.; Liang, S.; Ye, B.-C. A novel peptide-equipped exosomes platform for delivery of antisense oligonucleotides. ACS Appl. Mater. Interfaces 2021, 13, 10760–10767. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and challenges of liposome assisted drug delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Briolay, T.; Petithomme, T.; Fouet, M.; Nguyen-Pham, N.; Blanquart, C.; Boisgerault, N. Delivery of cancer therapies by synthetic and bio-inspired nanovectors. Mol. Cancer 2021, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, P.; Tan, H.; Chen, X.; Wang, Q.; Chen, T. Exosomes as smart nanoplatforms for diagnosis and therapy of cancer. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gagliardi, M.; Ashizawa, A.T. Making Sense of Antisense Oligonucleotide Therapeutics Targeting Bcl-2. Pharmaceutics 2022, 14, 97. https://doi.org/10.3390/pharmaceutics14010097
Gagliardi M, Ashizawa AT. Making Sense of Antisense Oligonucleotide Therapeutics Targeting Bcl-2. Pharmaceutics. 2022; 14(1):97. https://doi.org/10.3390/pharmaceutics14010097
Chicago/Turabian StyleGagliardi, Maria, and Ana Tari Ashizawa. 2022. "Making Sense of Antisense Oligonucleotide Therapeutics Targeting Bcl-2" Pharmaceutics 14, no. 1: 97. https://doi.org/10.3390/pharmaceutics14010097
APA StyleGagliardi, M., & Ashizawa, A. T. (2022). Making Sense of Antisense Oligonucleotide Therapeutics Targeting Bcl-2. Pharmaceutics, 14(1), 97. https://doi.org/10.3390/pharmaceutics14010097




