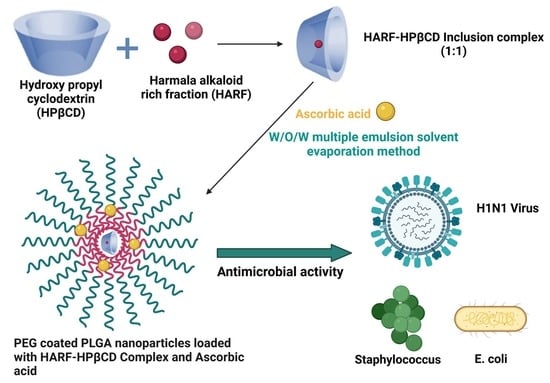
PLGA/PEG Nanoparticles Loaded with Cyclodextrin-Peganum harmala Alkaloid Complex and Ascorbic Acid with Promising Antimicrobial Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction and Isolation of Major P. harmala Alkaloids
2.3. Preparation of Harmala Alkaloid-Rich Fraction/2-Hydroxy Propyl ß Cyclodextrin Complex (HARF–HPßCD)
2.3.1. 1H NMR Spectroscopy of HARF–HPßCD Complex
2.3.2. Phase Solubility Study
2.4. Preparation of PEG-Coated PLGA Nanoparticles Dual-Loaded with Ascorbic Acid (AA) and HARF–HPßCD Inclusion Complex (HARF–HPßCD/AA@PLGA-PEG NPs)
2.5. Characterization of the Designed HARF–HPßCD/AA@PLGA-PEG NPs
2.6. Entrapment Efficiency
2.7. In Vitro Release Study of HARF–HPßCD Complex and Ascorbic Acid from HARF–HPßCD/AA@PLGA-PEG NPs
2.8. Antibacterial Assay for the Designed HARF–HPßCD/AA@PLGA-PEG NPs
2.8.1. Preparation of the Inoculum Using Colony Suspension Approach
2.8.2. Broth Macrodilution Method
2.9. Cytotoxicity and Antiviral Assays
3. Results and Discussion
3.1. Characterization of the Prepared HARF–HPßCD Complex
3.1.1. 1H NMR Spectroscopy of the HARF–HPßCD Complex
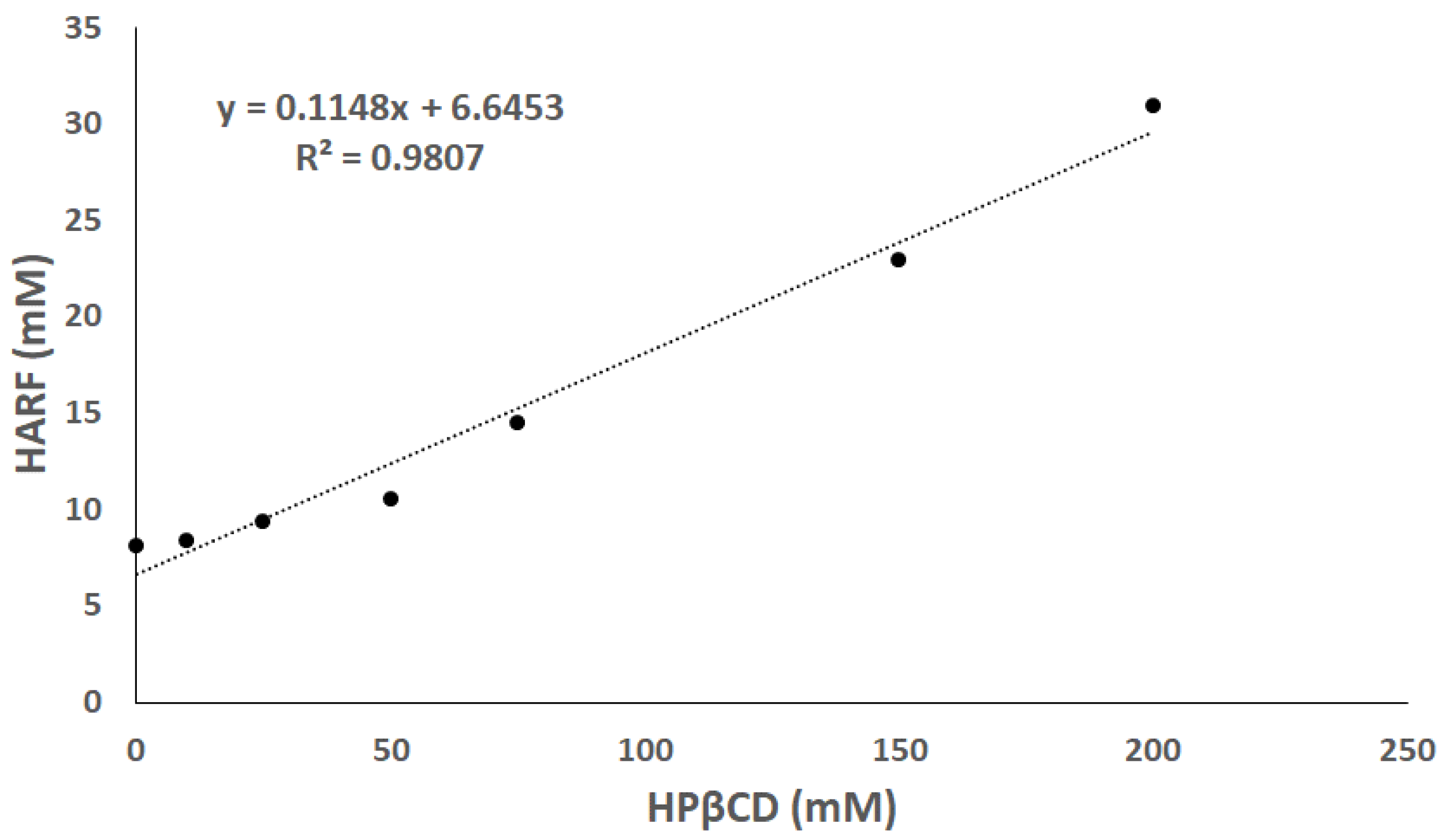
3.1.2. Phase Solubility Study
3.2. Characterization of the HARF–HPßCD/AA@PLGA-PEG NPs
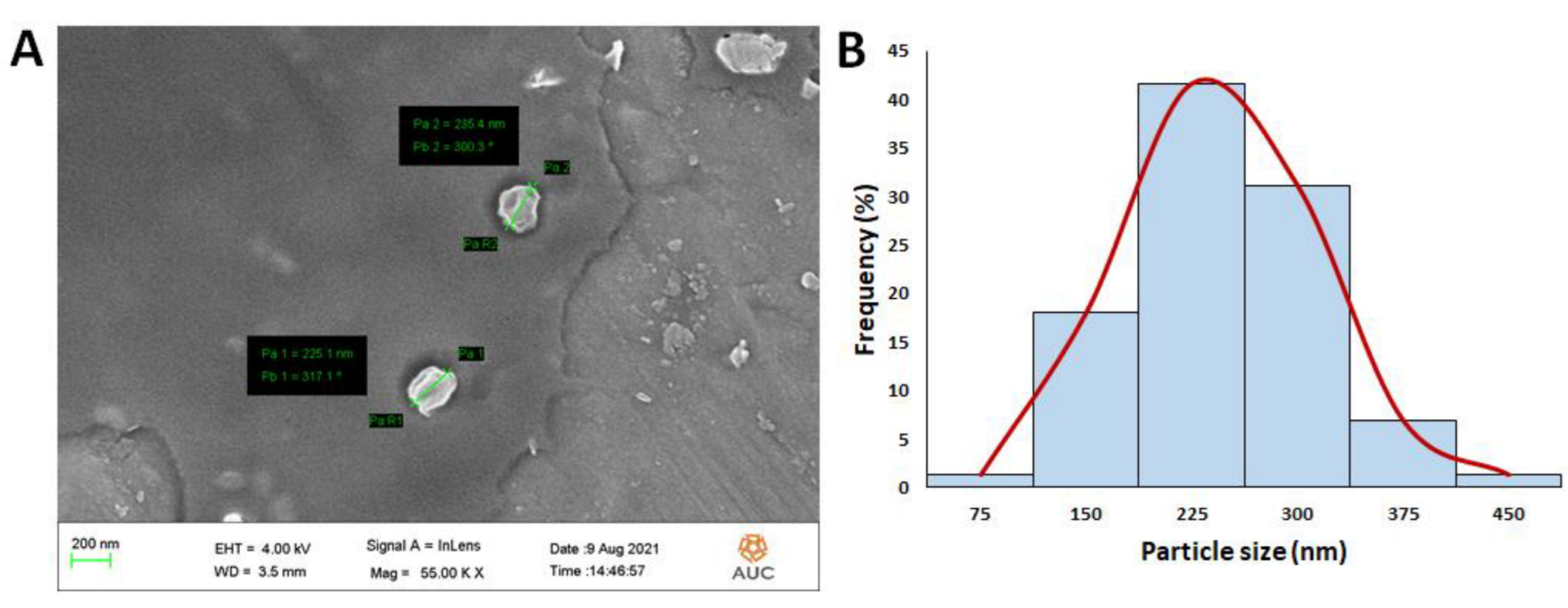
3.2.1. Particle Size, Polydispersity Index (PDI), ζ-Potential, Entrapment Efficiency (EE), and Morphological Features
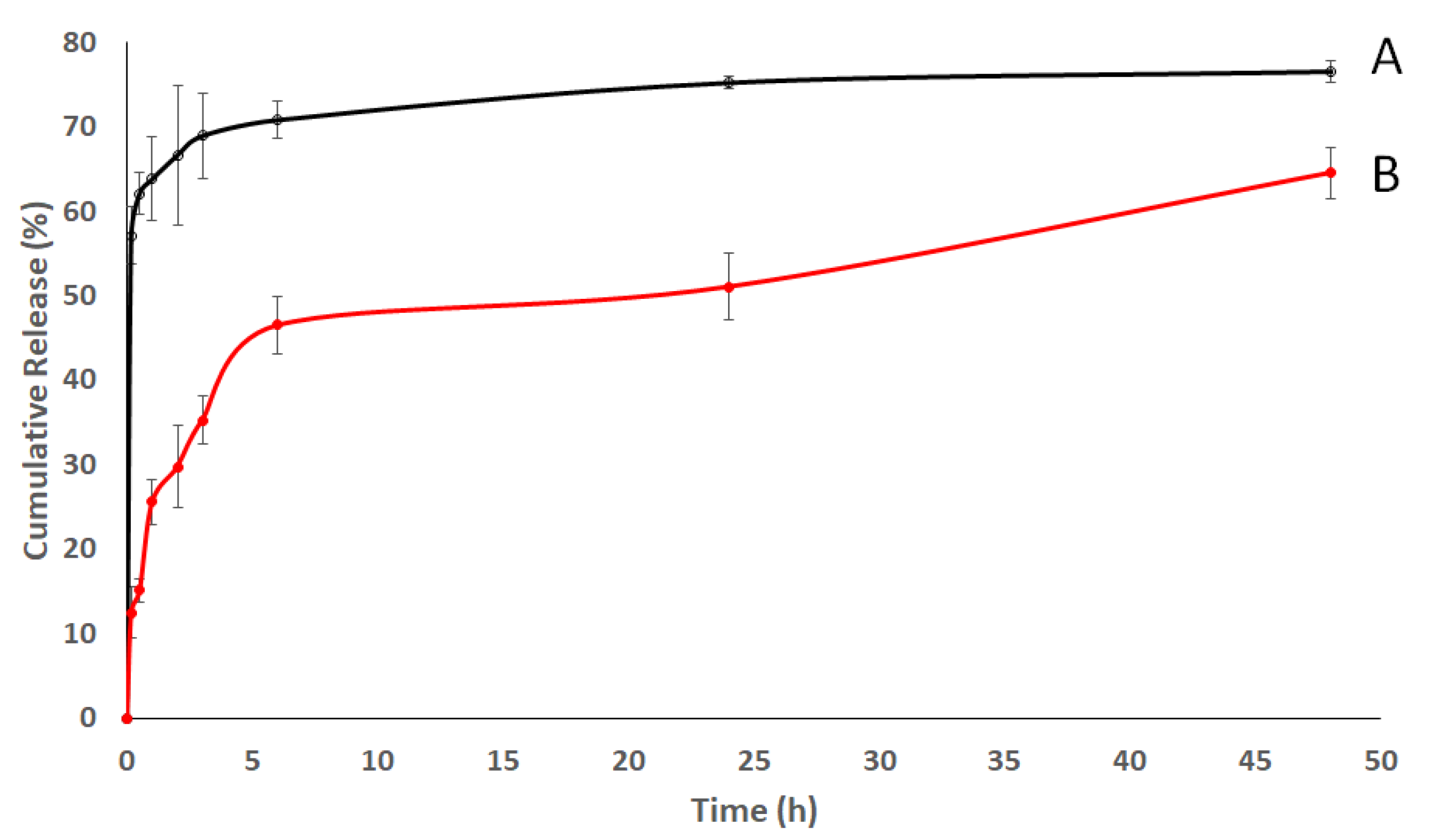
3.2.2. In Vitro Release Study
3.2.3. Antibacterial Assay for the Designed HARF–HPßCD/AA@PLGA-PEG NPs
3.2.4. Cytotoxicity and Antiviral Activity Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Influenza (Seasonal). Available online: https://www.who.int/en/news-room/fact-sheets/detail/influenza-(seasonal) (accessed on 22 November 2021).
- Mogana, R.; Adhikari, A.; Tzar, M.N.; Ramliza, R.; Wiart, C. Antibacterial activities of the extracts, fractions and isolated compounds from canarium patentinervium miq. Against bacterial clinical isolates. BMC Complement. Med. Ther. 2020, 20, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, S.A.; Mamdouh, W. Garlic oil–loaded PLGA nanoparticles with controllable size and shape and enhanced antibacterial activities. J. Appl. Polym. Sci. 2018, 135, 46133. [Google Scholar] [CrossRef]
- Omrani, M.; Keshavarz, M.; Nejad Ebrahimi, S.; Mehrabi, M.; McGaw, L.J.; Ali Abdalla, M.; Mehrbod, P. Potential Natural Products Against Respiratory Viruses: A Perspective to Develop Anti-COVID-19 Medicines. Front. Pharmacol. 2021, 11, 2115. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Fawzy, I.M.; Saleh, B.M.; Issa, M.Y.; Bakowsky, U.; Azzazy, H.M.E.-S. Green Synthesis of Platinum and Palladium Nanoparticles Using Peganum harmala L. Seed Alkaloids: Biological and Computational Studies. Nanomaterials 2021, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Issa, M.Y.; Saleh, B.M.; Meselhy, M.R.; Azzazy, H.M.E.A. Peganum harmala alkaloids self-assembled supramolecular nanocapsules with enhanced antioxidant and cytotoxic activities. ACS Omega 2021, 6, 11954–11963. [Google Scholar] [CrossRef]
- Azzazy, H.M.E.-S.; Fahmy, S.A.; Mahdy, N.K.; Meselhy, M.R.; Bakowsky, U. Chitosan-Coated PLGA Nanoparticles Loaded with Peganum harmala Alkaloids with Promising Antibacterial and Wound Healing Activities. Nanomaterials 2021, 11, 2438. [Google Scholar] [CrossRef] [PubMed]
- Pathan Aslam, R.; Vadnere Gautam, P.; Singhai Abhay, K.; Kulkarni Bharti, U. Peganum harmala: A Phyto-pharmacological Review. Inventi Rapid: Planta Activa 2012, 2012, 2–4. [Google Scholar]
- Jinous, A.; Ramezanloo, F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. Afr. J. Pharm. Pharmacol. 2012, 6, 1573–1580. [Google Scholar] [CrossRef]
- Grosso, G.; Bei, R.; Mistretta, A.; Marventano, S.; Calabrese, G.; Masuelli, L.; Giganti, M.G.; Modesti, A.; Galvano, F.; Gazzolo, D. Effects of vitamin C on health: A review of evidence. Front. Biosci. 2013, 18, 1017–1029. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin C and infections. Nutrients 2017, 9, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, S.A.; Ponte, F.; Fawzy, I.M.; Sicilia, E.; Bakowsky, U.; Azzazy, H.M.E.-S. Betaine host–guest complexation with a calixarene receptor: Enhanced in vitro anticancer effect. RSC Adv. 2021, 11, 24673–24680. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Alawak, M.; Brüßler, J.; Bakowsky, U.; El Sayed, M.M.H. Nanoenabled Bioseparations: Current Developments and Future Prospects. BioMed Res. Int. J. 2019, 2019, 4983291. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Ponte, F.; Abd El-Rahman, M.K.; Russo, N.; Sicilia, E.; Shoeib, T. Investigation of the host-guest complexation between 4-sulfocalix[4]arene and nedaplatin for potential use in drug delivery. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2018, 193, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, S.A.; Ponte, F.; Sicilia, E.; El-Said Azzazy, H.M. Experimental and Computational Investigations of Carboplatin Supramolecular Complexes. ACS Omega 2020, 5, 31456–31466. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Ponte, F.; Fawzy, I.M.; Sicilia, E.; Bakowsky, U.; Azzazy, H.M.E.A. Host-Guest Complexation of Oxaliplatin and Para-Sulfonatocalix[n]Arenes for Potential Use in Cancer Therapy. Molecules 2020, 25, 5926. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Brüßler, J.; Ponte, F.; Abd El-Rahman, M.K.; Russo, N.; Sicilia, E.; Bakowsky, U.; Shoeib, T. A study on the physicochemical properties and cytotoxic activity of p-sulfocalix[4]arene-nedaplatin complex. J. Phys. Conf. Ser. 2019, 1310, 012011. [Google Scholar] [CrossRef]
- Fahmy, S.A.; Brüßler, J.; Alawak, M.; El-Sayed, M.M.H.; Bakowsky, U.; Shoeib, T. Chemotherapy based on supramolecular chemistry: A promising strategy in cancer therapy. Pharmaceutics 2019, 11, 292. [Google Scholar] [CrossRef] [Green Version]
- Fahmy, S.A.; Ramzy, A.; Saleh, B.M.; Azzazy, H.M.E.-S. Stimuli-Responsive Amphiphilic Pillar[n]arene Nanovesicles for Targeted Delivery of Cancer Drugs. ACS Omega 2021, 6, 25876–25883. [Google Scholar] [CrossRef]
- Chen, J.; Qin, X.; Zhong, S.; Chen, S.; Su, W.; Liu, Y. Characterization of curcumin/cyclodextrin polymer inclusion complex and investigation on its antioxidant and antiproliferative activities. Molecules 2018, 23, 1179. [Google Scholar] [CrossRef] [Green Version]
- Mohan, L.J.; McDonald, L.; Daly, J.S.; Ramtoola, Z. Optimising PLGA-PEG nanoparticle size and distribution for enhanced drug targeting to the inflamed intestinal barrier. Pharmaceutics 2020, 12, 1114. [Google Scholar] [CrossRef]
- Tammam, S.N.; Azzazy, H.M.E.A.; Lamprecht, A. Biodegradable Particulate Carrier Formulation and Tuning for Targeted Drug Delivery. J. Biomed Nanotechnol. 2015, 11, 555–577. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Bonacucina, G.; Palmieri, G.F. PEGylated polylactide (PLA) and poly (lactic-co-glycolic acid) (PLGA) copolymers for the design of drug delivery systems. J. Pharm. Investig. 2019, 49, 443–458. [Google Scholar] [CrossRef]
- Devasvaran, K.; Jairaman, S.; Yahaya, N.A.; Jaganath, I.B.S.; Khung, Y.L.; Lim, V.; Ngalim, S.H. PEG-b-PLGA nanoparticles loaded with Geraniin from Phyllanthus watsonii extract as a phytochemical delivery model. Appl. Sci. 2020, 10, 4891. [Google Scholar] [CrossRef]
- Alhakamy, N.A. Development and Evaluation of Icariin-Loaded PLGA-PEG Nanoparticles for Potentiation the Proapoptotic Activity in Pancreatic Cancer Cells. AAPS PharmSciTech 2021, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, L.; Cano, A.; Ettcheto, M.; Souto, E.B.; Espina, M.; Camins, A.; García, M.L.; Sánchez-López, E. Surface functionalization of plga nanoparticles to increase transport across the bbb for alzheimer’s disease. Appl. Sci. 2021, 11, 4305. [Google Scholar] [CrossRef]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017, 12, 935–947. [Google Scholar] [CrossRef] [Green Version]
- Plenagl, N.; Duse, L.; Seitz, B.S.; Goergen, N.; Pinnapireddy, S.R.; Jedelska, J.; Brüßler, J.; Bakowsky, U. Photodynamic therapy–hypericin tetraether liposome conjugates and their antitumor and antiangiogenic activity. Drug Deliv. 2019, 26, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Higuchi, T.; Connors, K.A. Phase Solubility Techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Liu, L.; Zhao, T.; Cheng, X.; Wang, C.; Wang, Z. Characterization and determination of trace alkaloids in seeds extracts from Peganum harmala linn. Using LC-ESI-MS and HPLC. Acta Chromatogr. 2013, 25, 221–240. [Google Scholar] [CrossRef] [Green Version]
- Kurkov, S.V.; Ukhatskaya, E.V.; Loftsson, T. Drug/cyclodextrin: Beyond inclusion complexation. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 297–301. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. The complexation efficiency. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 545–552. [Google Scholar] [CrossRef]
- Tewes, F.; Munnier, E.; Antoon, B.; Okassa, L.N.; Cohen-Jonathan, S.; Marchais, H.; Douziech-Eyrolles, L.; Soucé, M.; Dubois, P.; Chourpa, I. Comparative study of doxorubicin-loaded poly(lactide-co-glycolide) nanoparticles prepared by single and double emulsion methods. Eur. J. Pharm. Biopharm. 2007, 66, 488–492. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Kong, L. Chitosan-modified PLGA nanoparticles with versatile surface for improved drug delivery. AAPS PharmSciTech 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinnapireddy, S.R.; Duse, L.; Strehlow, B.; Schäfer, J.; Bakowsky, U. Composite liposome-PEI/nucleic acid lipopolyplexes for safe and efficient gene delivery and gene knockdown. Colloids Surf. B Biointerfaces 2017, 158, 93–101. [Google Scholar] [CrossRef]
- Preis, E.; Baghdan, E.; Agel, M.R.; Anders, T.; Pourasghar, M.; Schneider, M.; Bakowsky, U. Spray dried curcumin loaded nanoparticles for antimicrobial photodynamic therapy. Eur. J. Pharm. Biopharm. 2019, 142, 531–539. [Google Scholar] [CrossRef]
- Duse, L.; Baghdan, E.; Pinnapireddy, S.R.; Engelhardt, K.H.; Jedelská, J.; Schaefer, J.; Quendt, P.; Bakowsky, U. Preparation and Characterization of Curcumin Loaded Chitosan Nanoparticles for Photodynamic Therapy. Phys. Status Solidi Appl. Mater. Sci. 2018, 215, 1–5. [Google Scholar] [CrossRef]
- Chen, M.X.; Li, T.; Peng, S.; Tao, D. Supramolecular nanocapsules from the self-assembly of amphiphilic calixarene as a carrier for paclitaxel. N. J. Chem. 2016, 40, 9923–9929. [Google Scholar] [CrossRef]
- El-Shafie, S.; Fahmy, S.A.; Ziko, L.; Elzahed, N.; Shoeib, T.; Kakarougkas, A. Encapsulation of nedaplatin in novel pegylated liposomes increases its cytotoxicity and genotoxicity against a549 and u2os human cancer cells. Pharmaceutics 2020, 12, 863. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Shan, M.; Di, X.; Gong, C.; Zhang, L.; Wang, Y.; Wu, G. A dual pH- and reduction-responsive anticancer drug delivery system based on PEG-SS-poly(amino acid) block copolymer. RSC Adv. 2017, 7, 30242–30249. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, M.P.; Patel, J.B.; Burnhman, C.-A.; ZImmer, B.L. M07-Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; Approved Standard—2018.
- Khiralla, A.; Spina, R.; Varbanov, M.; Philippot, S.; Lemiere, P.; Slezack-Deschaumes, S.; André, P.; Mohamed, I.; Yagi, S.M.; Laurain-Mattar, D. Evaluation of antiviral, antibacterial and antiproliferative activities of the endophytic fungus curvularia papendorfii, and isolation of a new polyhydroxyacid. Microorganisms 2020, 8, 1353. [Google Scholar] [CrossRef]
- Jang, Y.; Shin, J.S.; Lee, J.Y.; Shin, H.; Kim, S.J.; Kim, M. In vitro and in vivo antiviral activity of nylidrin by targeting the hemagglutinin 2-mediated membrane fusion of influenza A virus. Viruses 2020, 12, 581. [Google Scholar] [CrossRef]
- Pauwels, R.; Balzarini, J.; Baba, M.; Snoeck, R.; Schols, D.; Herdewijn, P.; Desmyter, J.; De Clercq, E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods 1988, 20, 309–321. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Jiang, J.; Li, X.; Zhao, L.; Fu, Y.; Ye, F. Encapsulation of thiabendazole in hydroxypropyl-β-cyclodextrin nanofibers via polymer-free electrospinning and its characterization. Pest Manag. Sci. 2020, 76, 3264–3272. [Google Scholar] [CrossRef] [PubMed]
- Veiga, F.J.B.; Fernandes, C.M.; Carvalho, R.A.; Geraldes, C.F.G.C. Molecular modelling and 1H-NMR: Ultimate tools for the investigation of tolbutamide: β-cyclodextrin and tolbutamide: Hydroxypropyl-β-cyclodextrin complexes. Chem. Pharm. Bull. 2001, 49, 1251–1256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, C.; Jin, Z.; Xu, X. Inclusion complex of astaxanthin with hydroxypropyl-β-cyclodextrin: UV, FTIR, 1H NMR and molecular modeling studies. Carbohydr. Polym. 2012, 89, 492–496. [Google Scholar] [CrossRef]
- Ge, X.; He, J.; Qi, F.; Yang, Y.; Huang, Z.; Lu, R.; Huang, L. Inclusion complexation of chloropropham with β-cyclodextrin: Preparation, characterization and molecular modeling. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2011, 81, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Meinguet, C.; Masereel, B.; Wouters, J. Preparation and characterization of a new harmine-based antiproliferative compound in complex with cyclodextrin: Increasing solubility while maintaining biological activity. Eur. J. Pharm. Sci. 2015, 77, 135–140. [Google Scholar] [CrossRef]
- Imran, M.; Shah, M.R.; Ullah, F.; Ullah, S.; Sadiq, A.; Ali, I.; Ahmed, F.; Nawaz, W. Double-tailed acyl glycoside niosomal nanocarrier for enhanced oral bioavailability of Cefixime. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.P.; Pei, Y.Y.; Zhang, X.Y.; Gu, Z.H.; Zhou, Z.H.; Yuan, W.F.; Zhou, J.J.; Zhu, J.H.; Gao, X.J. PEGylated PLGA nanoparticles as protein carriers: Synthesis, preparation and biodistribution in rats. J. Control. Release 2001, 71, 203–211. [Google Scholar] [CrossRef]
- Ullah, S.; Shah, M.R.; Shoaib, M.; Imran, M.; Elhissi, A.M.A.; Ahmad, F.; Ali, I.; Shah, S.W.A. Development of a biocompatible creatinine-based niosomal delivery system for enhanced oral bioavailability of clarithromycin. Drug Deliv. 2016, 23, 3480–3491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Locatelli, E.; Franchini, M.C. Biodegradable PLGA-b-PEG polymeric nanoparticles: Synthesis, properties, and nanomedical applications as drug delivery system. J. Nanoparticle Res. 2012, 14, 1–17. [Google Scholar] [CrossRef]
- Zhang, L.; Pornpattananangkul, D.; Hu, C.-M.; Huang, C.-M. Development of Nanoparticles for Antimicrobial Drug Delivery. Curr. Med. Chem. 2010, 17, 585–594. [Google Scholar] [CrossRef] [Green Version]
- Esmaeili, F.; Hosseini-Nasr, M.; Rad-Malekshahi, M.; Samadi, N.; Atyabi, F.; Dinarvand, R. Preparation and antibacterial activity evaluation of rifampicin-loaded poly lactide-co-glycolide nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Koopaei, M.N.; Maghazei, M.S.; Mostafavi, S.H.; Jamalifar, H.; Samadi, N.; Amini, M.; Malek, S.J.; Darvishi, B.; Atyabi, F.; Dinarvand, R. Enhanced antibacterial activity of roxithromycin loaded pegylated poly lactide-co-glycolide nanoparticles. DARU J. Pharm. Sci. 2012, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Moradi, M.T.; Karimi, A.; Rafieian-Kopaei, M.; Fotouhi, F. In vitro antiviral effects of Peganum harmala seed extract and its total alkaloids against Influenza virus. Microb. Pathog. 2017, 110, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, H.; Bae, S.; Choi, J.; Lim, S.Y.; Lee, N.; Kong, J.M.; Hwang, Y.; Kang, J.S.; Lee, W.J. Vitamin C is an Essential Factor on the Anti-viral Immune Responses through the Production of Interferon-α/β at the Initial Stage of Influenza A Virus (H3N2) Infection. Immune Netw. 2013, 13, 70. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, D.B.; Ismail, W.M.; Moatasim, Y.; Kutkat, O.; ElMeshad, A.N.; Ezzat, S.M.; El Deeb, K.S.; El-Fishawy, A.M.; Gomaa, M.R.; Kandeil, A.; et al. Delineating a potent antiviral activity of Cuphea ignea extract loaded nano-formulation against SARS-CoV-2: In silico and in vitro studies. J. Drug Deliv. Sci. Technol. 2021, 66, 102845. [Google Scholar] [CrossRef] [PubMed]
| Chemical Shifts (δ, ppm) | ||||||
|---|---|---|---|---|---|---|
| H-1 | H-2 | H-3 | H-4 | H-5 | H-6 | |
| HPßCD | 4.899 | 3.310 | 3.768 | 3.329 | 3.552 | 3.696 |
| HARF-HPßCD complex | 4.877 | 3.307 | 3.741 | 3.307 | 3.526 | 3.675 |
| Δδ | −0.022 | −0.003 | −0.027 | −0.022 | −0.026 | −0.021 |
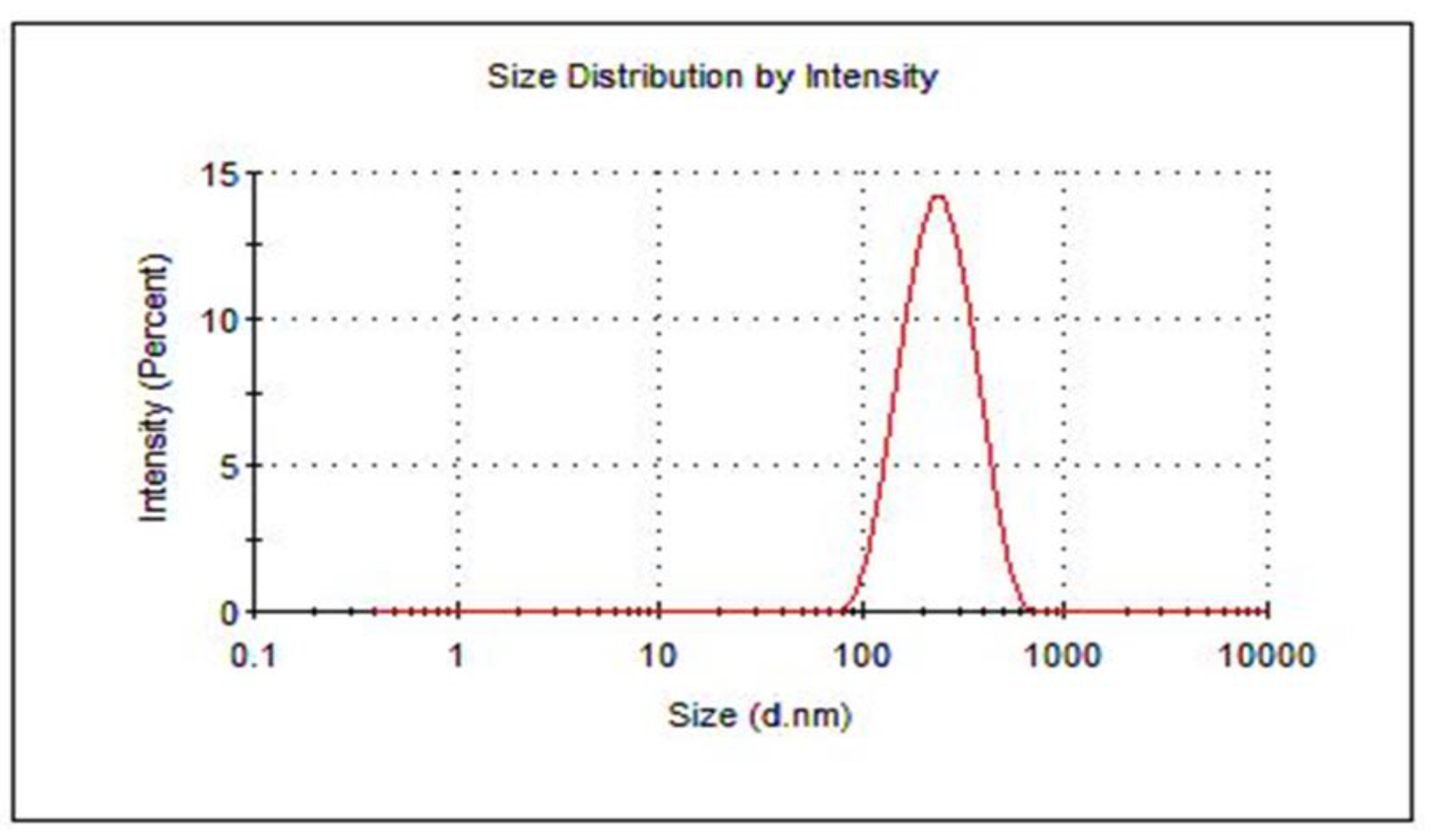
| Formula | Average Size (nm) | PDI | ζ-Potential (mV) ± SD | Encapsulation Efficiency (%) | |
|---|---|---|---|---|---|
| HARF | AA | ||||
| HARF–HPßCD/AA@ PLGA-PEG NPs | 207 ± 2.60 | 0.17 ± 0.01 | −31.60 ± 0.20 | 81.60 ± 1.20 | 87 ± 2.20 |
| Bacterial Strain | Minimum Inhibitory Concentration (MIC in mg/mL) | ||
|---|---|---|---|
| PLGA-PEG NPs | HARF | HARF–HPßCD/AA@PLGA-PEG NPs | |
| Staphylococcus aureus | 0 | 0.5 | 0.025 |
| Escherichia coli | 0 | 0.5 | 0.025 |
| Sample | CC50 (μg /mL) | IC50 (μg /mL) | SI |
|---|---|---|---|
| Plain PLGA-PEG | 0 | 0 | 0 |
| HARF | 238.8 | 30.2 | 7.9 |
| HARF–HPßCD/AA@PLGA-PEG NPs | 110.4 | 2.7 | 41.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fahmy, S.A.; Mahdy, N.K.; Al Mulla, H.; ElMeshad, A.N.; Issa, M.Y.; Azzazy, H.M.E.-S. PLGA/PEG Nanoparticles Loaded with Cyclodextrin-Peganum harmala Alkaloid Complex and Ascorbic Acid with Promising Antimicrobial Activities. Pharmaceutics 2022, 14, 142. https://doi.org/10.3390/pharmaceutics14010142
Fahmy SA, Mahdy NK, Al Mulla H, ElMeshad AN, Issa MY, Azzazy HME-S. PLGA/PEG Nanoparticles Loaded with Cyclodextrin-Peganum harmala Alkaloid Complex and Ascorbic Acid with Promising Antimicrobial Activities. Pharmaceutics. 2022; 14(1):142. https://doi.org/10.3390/pharmaceutics14010142
Chicago/Turabian StyleFahmy, Sherif Ashraf, Noha Khalil Mahdy, Hadeer Al Mulla, Aliaa Nabil ElMeshad, Marwa Y. Issa, and Hassan Mohamed El-Said Azzazy. 2022. "PLGA/PEG Nanoparticles Loaded with Cyclodextrin-Peganum harmala Alkaloid Complex and Ascorbic Acid with Promising Antimicrobial Activities" Pharmaceutics 14, no. 1: 142. https://doi.org/10.3390/pharmaceutics14010142
APA StyleFahmy, S. A., Mahdy, N. K., Al Mulla, H., ElMeshad, A. N., Issa, M. Y., & Azzazy, H. M. E.-S. (2022). PLGA/PEG Nanoparticles Loaded with Cyclodextrin-Peganum harmala Alkaloid Complex and Ascorbic Acid with Promising Antimicrobial Activities. Pharmaceutics, 14(1), 142. https://doi.org/10.3390/pharmaceutics14010142