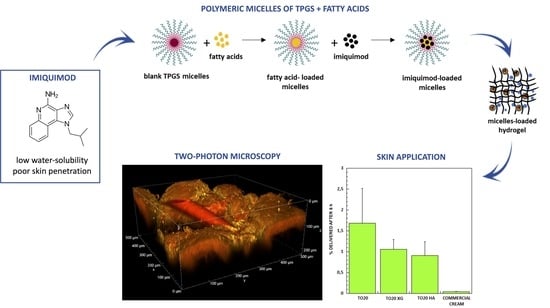
Improvement of Imiquimod Solubilization and Skin Retention via TPGS Micelles: Exploiting the Co-Solubilizing Effect of Oleic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Imiquimod Quantification Method
2.3. Fatty Acids Quantification Method
2.4. Preparation of Blank Polymeric Micelles
2.4.1. Method 1
2.4.2. Method 2
2.4.3. Method 3
2.5. Imiquimod Loading in the Polymeric Micelles
2.6. Micelles Characterization and Stability
2.7. Formulation of Water-Based and Micelles-Based Hydrogels
Hydrogels Preparation
2.8. Evaluation of Micelles Diffusion in Water-Based Hydrogels
2.9. Skin Accumulation and Permeation Experiments
2.10. Statistical Analysis
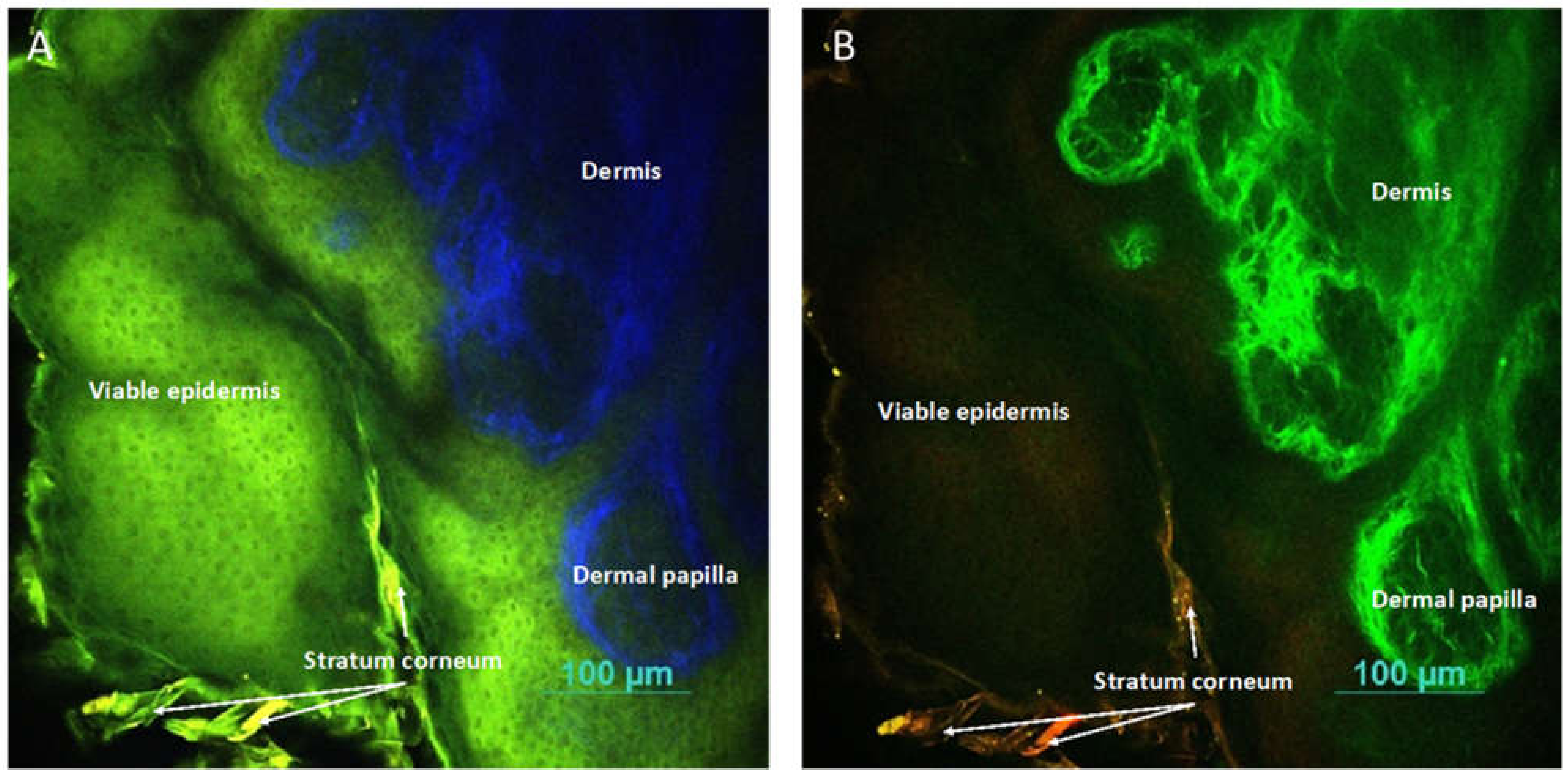
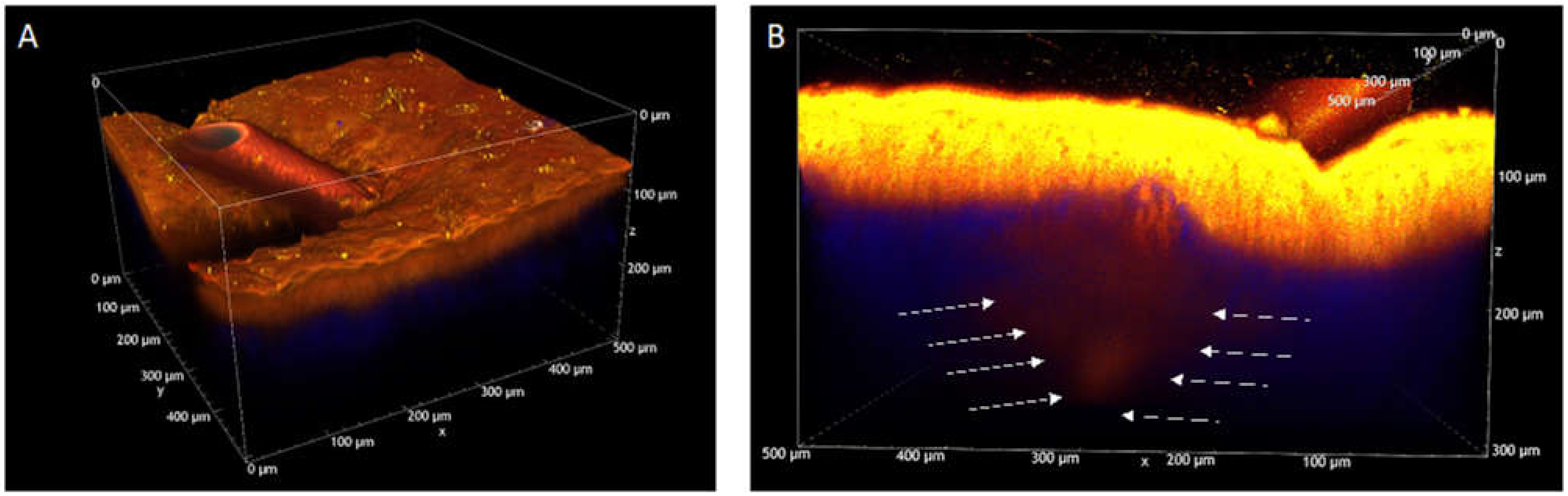
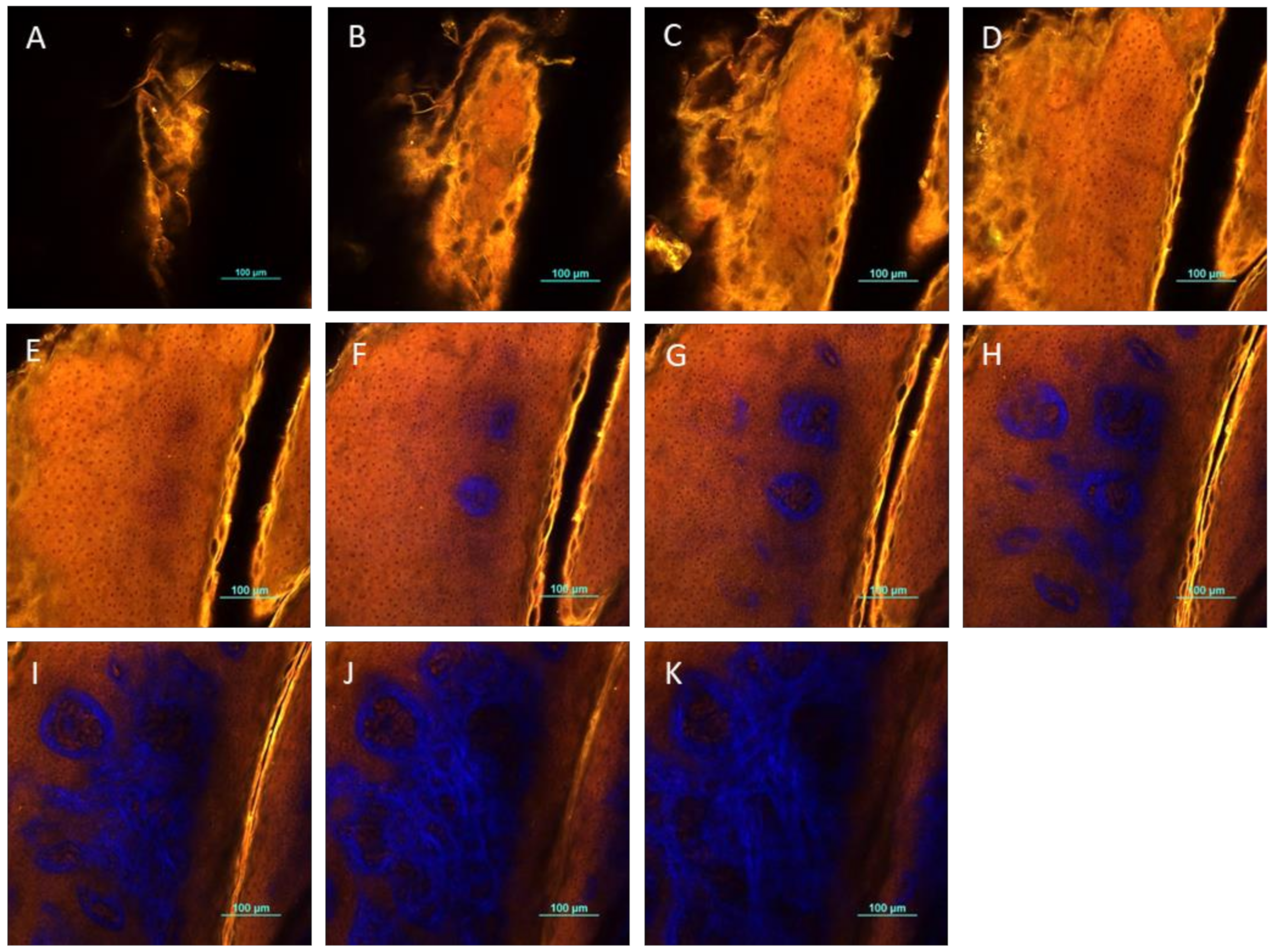
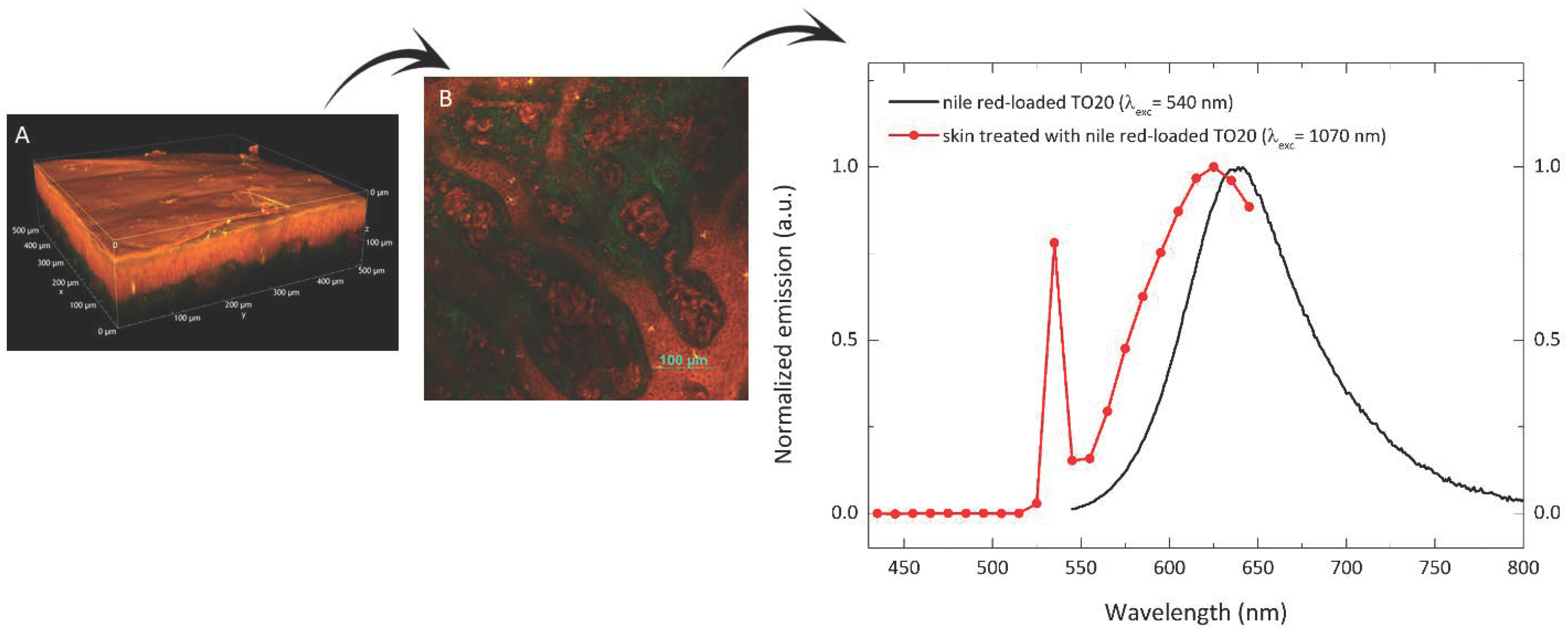
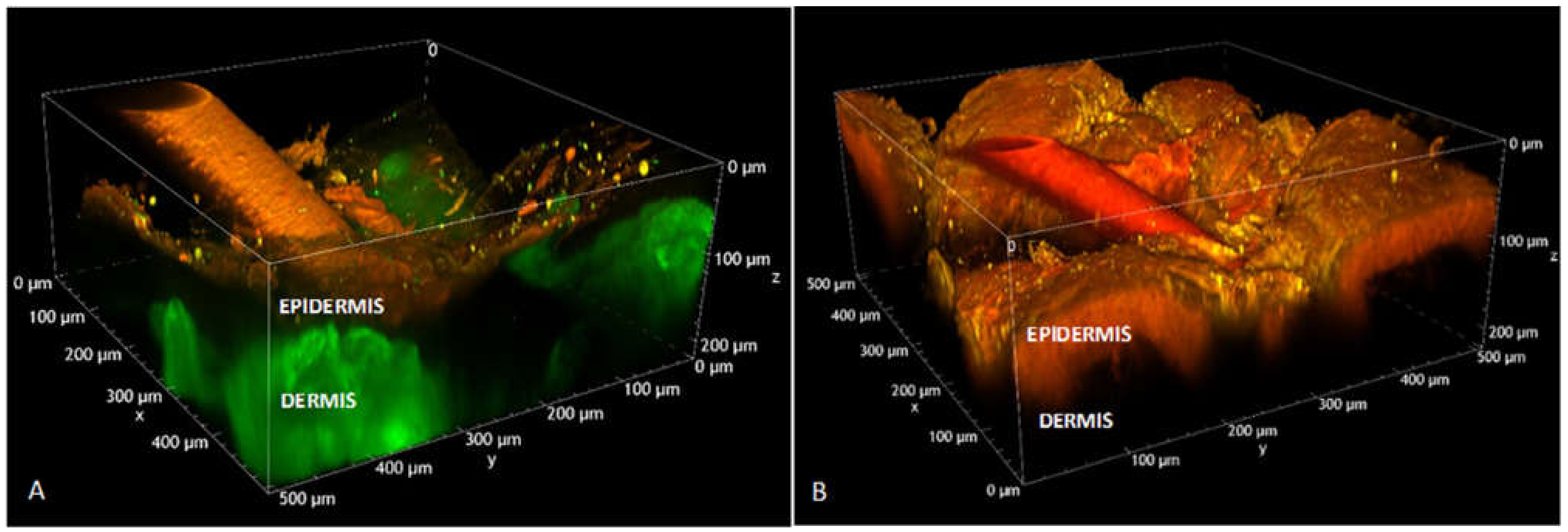
2.11. Two-Photon Microscopy Method
3. Results
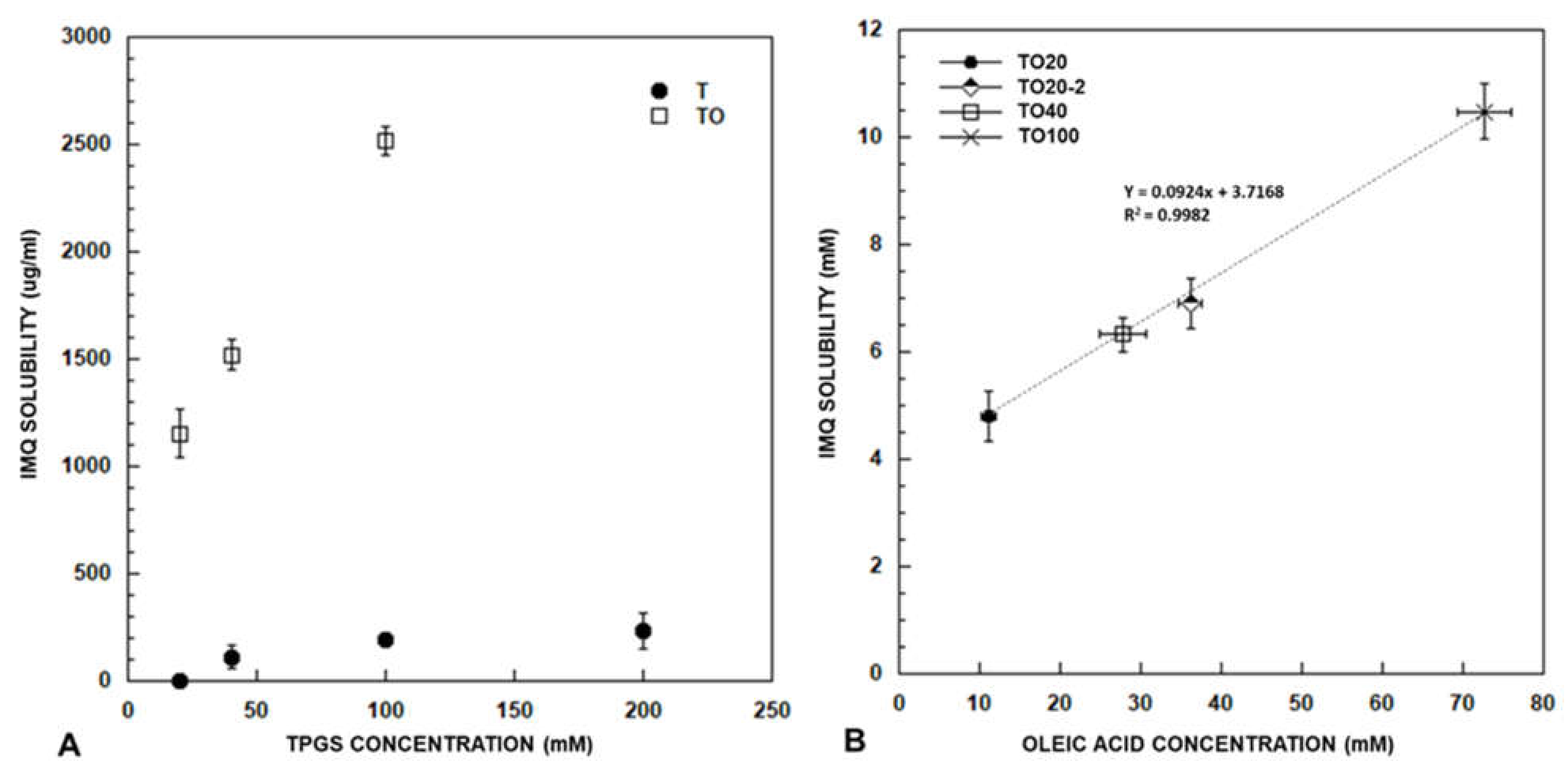
3.1. Imiquimod Solubilisation
3.1.1. Effect of Co-Encapsulation of Fatty Acids and Oleic Acid Esters
3.1.2. Effect of TPGS Concentration
3.1.3. Effect of Preparation Method
3.2. Size Determination
3.3. Loading of Polymeric Micelles in Hydrogels
3.4. Imiquimod Skin Accumulation and Permeation
3.4.1. Imiquimod Skin Delivery from Micelles
3.4.2. Imiquimod Skin Delivery from Semisolid Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclosures
Appendix A

References
- De Oliveira, E.C.V.; da Motta, V.R.V.; Pantoja, P.C.; Ilha, C.S.O.; Magalhães, R.F.; Galadari, H.A.-O.; Leonardi, G.R. Actinic keratosis-review for clinical practice. Int. J. Dermatol. 2019, 58, 400–407. [Google Scholar] [CrossRef]
- Stanley, M.A. Imiquimod and the imidazoquinolones: Mechanism of action and therapeutic potential. Clin. Exp. Dermatol. 2002, 27, 571–577. [Google Scholar] [CrossRef]
- David, V. Topical Imiquimod: Mechanism of Action and Clinical Applications. Mini-Rev. Med. Chem. 2006, 6, 499–503. [Google Scholar]
- Bubna, A.K. Imiquimod-Its role in the treatment of cutaneous malignancies. Indian J. Pharmacol. 2015, 47, 354–359. [Google Scholar] [CrossRef]
- Miller, R.L.; Gerster Jf Fau-Owens, M.L.; Owens Ml Fau-Slade, H.B.; Slade Hb Fau-Tomai, M.A.; Tomai, M.A. Imiquimod applied topically: A novel immune response modifier and new class of drug. Int. J. Immunopharmacol. 1999, 21, 1–14. [Google Scholar] [CrossRef]
- Hanna, E.; Abadi, R.; Abbas, O. Imiquimod in dermatology: An overview. Int. J. Dermatol. 2016, 55, 831–844. [Google Scholar] [CrossRef]
- Peris, K.; Micantonio, T.; Fargnoli, M.C.; Lozzi, G.P.; Chimenti, S. Imiquimod 5% cream in the treatment of Bowen’s disease and invasive squamous cell carcinoma. J. Am. Acad. Dermatol. 2006, 55, 324–327. [Google Scholar] [CrossRef]
- Karabulut, G.O.; Kaynak, P.; Ozturker, C.; Fazil, K.; Ocak, O.B.; Taskapılı, M. Imiquimod 5% cream for the treatment of large nodular basal cell carcinoma at the medial canthal area. Indian J. Ophthalmol. 2017, 65, 48–51. [Google Scholar] [CrossRef]
- Bath-Hextall, F.; Ozolins, M.; Armstrong, S.J.; Colver, G.B.; Perkins, W.; Miller, P.S.; Williams, H.C. Surgical excision versus imiquimod 5% cream for nodular and superficial basal-cell carcinoma (SINS): A multicentre, non-inferiority, randomised controlled trial. Lancet Oncol. 2014, 15, 96–105. [Google Scholar] [CrossRef]
- Tio, D.; van der Woude, J.; Prinsen, C.A.C.; Jansma, E.P.; Hoekzema, R.; van Montfrans, C. A systematic review on the role of imiquimod in lentigo maligna and lentigo maligna melanoma: Need for standardization of treatment schedule and outcome measures. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 616–624. [Google Scholar] [CrossRef]
- Fan, Q.; Cohen, S.; John, B.; Riker, A.I. Melanoma in Situ Treated with Topical Imiquimod for Management of Persistently Positive Margins: A Review of Treatment Methods. Ochsner J. 2015, 15, 443–447. [Google Scholar]
- Troiani, T.; Napolitano, S.; Brancaccio, G.; Belli, V.; Nappi, A.; Miro, C.; Salvatore, D.; Dentice, M.; Caraglia, M.; Franco, R.; et al. Treatment of Cutaneous Melanoma Harboring SMO p.Gln216Arg Mutation with Imiquimod: An Old Drug with New Results. J. Pers. Med. 2021, 11, 206. [Google Scholar] [CrossRef]
- Luchner, M.; Reinke, S.; Milicic, A. TLR Agonists as Vaccine Adjuvants Targeting Cancer and Infectious Diseases. Pharmaceutics 2021, 13, 142. [Google Scholar] [CrossRef]
- Meneveau, M.O.; Petroni, G.R.; Salerno, E.P.; Lynch, K.T.; Smolkin, M.; Woodson, E.; Chianese-Bullock, K.A.; Olson, W.C.; Deacon, D.; Patterson, J.W.; et al. Immunogenicity in humans of a transdermal multipeptide melanoma vaccine administered with or without a TLR7 agonist. J. Immunother. Cancer 2021, 9, e002214. [Google Scholar] [CrossRef]
- Telò, I.; Pescina, S.; Padula, C.; Santi, P.; Nicoli, S. Mechanisms of imiquimod skin penetration. Int. J. Pharm. 2016, 511, 516–523. [Google Scholar] [CrossRef]
- Šmejkalová, D.; Muthný, T.; Nešporová, K.; Hermannová, M.; Achbergerová, E.; Huerta-Angeles, G.; Svoboda, M.; Čepa, M.; Machalová, V.; Luptáková, D.; et al. Hyaluronan polymeric micelles for topical drug delivery. Carbohydr. Polym. 2017, 156, 86–96. [Google Scholar] [CrossRef]
- Makhmalzade, B.S.; Chavoshy, F. Polymeric micelles as cutaneous drug delivery system in normal skin and dermatological disorders. J. Adv. Pharm. Technol. Res. 2018, 9, 2–8. [Google Scholar] [CrossRef]
- Xu, H.; Wen, Y.; Chen, S.; Zhu, L.; Feng, R.; Song, Z. Paclitaxel skin delivery by micelles-embedded Carbopol 940 hydrogel for local therapy of melanoma. Int. J. Pharm. 2020, 587, 119626. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. European J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Ghosh, I.; Michniak-Kohn, B. Design and characterization of submicron formulation for a poorly soluble drug: The effect of Vitamin E TPGS and other solubilizers on skin permeability enhancement. Int. J. Pharm. 2012, 434, 90–98. [Google Scholar] [CrossRef]
- Ahmed, T.A.; El-Say, K.M.; Ahmed, O.A.; Aljaeid, B.M. Superiority of TPGS-loaded micelles in the brain delivery of vinpocetine via administration of thermosensitive intranasal gel. Int. J. Nanomed. 2019, 14, 5555–5567. [Google Scholar] [CrossRef]
- Patil, P.H.; Mahajan, H.S. Mixed micelles for bioavailability enhancement of nelfinavir mesylate: In vitro characterisation and In vivo pharmacokinetic study. Mater. Technol. 2018, 33, 793–802. [Google Scholar] [CrossRef]
- Grimaudo, M.A.; Pescina, S.; Padula, C.; Santi, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Nicoli, S. Poloxamer 407/TPGS Mixed Micelles as Promising Carriers for Cyclosporine Ocular Delivery. Mol. Pharm 2018, 15, 571–584. [Google Scholar] [CrossRef]
- Tavares Luiz, M.; Delello Di Filippo, L.; Carolina Alves, R.; Sousa Araújo, V.H.; Lobato Duarte, J.; Maldonado Marchetti, J.; Chorilli, M. The use of TPGS in drug delivery systems to overcome biological barriers. Eur. Polym. J. 2021, 142, 110129. [Google Scholar] [CrossRef]
- Tavano, L.; Muzzalupo, R.; Picci, N.; de Cindio, B. Co-encapsulation of lipophilic antioxidants into niosomal carriers: Percutaneous permeation studies for cosmeceutical applications. Colloids Surf. B Biointerfaces 2014, 114, 144–149. [Google Scholar] [CrossRef]
- Kalhapure, R.S.; Mocktar, C.; Sikwal, D.R.; Sonawane, S.J.; Kathiravan, M.K.; Skelton, A.; Govender, T. Ion pairing with linoleic acid simultaneously enhances encapsulation efficiency and antibacterial activity of vancomycin in solid lipid nanoparticles. Colloids Surf. B Biointerfaces 2014, 117, 303–311. [Google Scholar] [CrossRef]
- Seedat, N.; Kalhapure, R.S.; Mocktar, C.; Vepuri, S.; Jadhav, M.; Soliman, M.; Govender, T. Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid–polymer hybrid nanoparticles: In vitro and in silico studies. Mater. Sci. Eng. C 2016, 61, 616–630. [Google Scholar] [CrossRef]
- Pescina, S.; Garrastazu, G.; del Favero, E.; Rondelli, V.; Cantù, L.; Padula, C.; Santi, P.; Nicoli, S. Microemulsions based on TPGS and isostearic acid for imiquimod formulation and skin delivery. Eur. J. Pharm. Sci. 2018, 125, 223–231. [Google Scholar] [CrossRef]
- ACD/Percepta, v12.1.0.50374, Advanced Chemistry Development Inc., Toronto, On, Canada. Available online: https://www.acdlabs.com/ (accessed on 30 July 2021).
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 30 July 2021).
- Ke, W.-T.; Lin, S.-Y.; Ho, H.-O.; Sheu, M.-T. Physical characterizations of microemulsion systems using tocopheryl polyethylene glycol 1000 succinate (TPGS) as a surfactant for the oral delivery of protein drugs. J. Control. Release 2005, 102, 489–507. [Google Scholar] [CrossRef]
- Kanicky, J.R.; Shah, D.O. Effect of degree, type, and position of unsaturation on the pKa of long-chain fatty acids. J. Colloid Interface Sci. 2002, 256, 201–207. [Google Scholar] [CrossRef]
- Silva, A.E.; Barratt, G.; Chéron, M.; Egito, E.S.T. Development of oil-in-water microemulsions for the oral delivery of amphotericin B. Int. J. Pharm. 2013, 454, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Chemical Book. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB2757258.htm (accessed on 30 July 2021).
- Gattefossé. Available online: https://www.gattefosse.com/pharmaceuticals-products/plurol-oleique-cc-497 (accessed on 30 July 2021).
- Kassem, M.G.A.; Ahmed, A.-M.M.; Abdel-Rahman, H.H.; Moustafa, A.H.E. Use of Span 80 and Tween 80 for blending gasoline and alcohol in spark ignition engines. Energy Rep. 2019, 5, 221–230. [Google Scholar] [CrossRef]
- Telò, I.; Favero, E.D.; Cantù, L.; Frattini, N.; Pescina, S.; Padula, C.; Santi, P.; Sonvico, F.; Nicoli, S. Gel-like TPGS-Based Microemulsions for Imiquimod Dermal Delivery: Role of Mesostructure on the Uptake and Distribution into the Skin. Mol. Pharm. 2017, 14, 3281–3289. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wu, T.; Qi, Y.; Zhang, Z. Recent Advances in the Application of Vitamin E TPGS for Drug Delivery. Theranostics 2018, 8, 464–485. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, J.; Tan, S.; Otieno, B.O.; Zhang, Z. The applications of Vitamin E TPGS in drug delivery. Eur. J. Pharm. Sci. 2013, 49, 175–186. [Google Scholar] [CrossRef]
- Sheu, M.-T.; Wu, A.-B.; Lin, K.-P.; Shen, C.-H.; Ho, H.-O. Effect of Tocopheryl Polyethylene Glycol Succinate on the Percutaneous Penetration of Minoxidil from Water/Ethanol/Polyethylene Glycol 400 Solutions. Drug Dev. Ind. Pharm. 2006, 32, 595–607. [Google Scholar] [CrossRef]
- Lapteva, M.; Möller, M.; Gurny, R.; Kalia, Y.N. Self-assembled polymeric nanocarriers for the targeted delivery of retinoic acid to the hair follicle. Nanoscale 2015, 7, 18651–18662. [Google Scholar] [CrossRef]
- Yuan, L.; Pan, M.; Lei, M.; Zhou, X.; Hu, D.; Liu, Q.; Chen, Y.; Li, W.; Qian, Z. A novel composite of micelles and hydrogel for improving skin delivery of hydrocortisone and application in atopic dermatitis therapy. Appl. Mater. Today 2020, 19, 100593. [Google Scholar] [CrossRef]
- Bachhav, Y.G.; Mondon, K.; Kalia, Y.N.; Gurny, R.; Möller, M. Novel micelle formulations to increase cutaneous bioavailability of azole antifungals. J. Control. Release 2011, 153, 126–132. [Google Scholar] [CrossRef]
- Chollet, J.L.; Jozwiakowski, M.J.; Phares, K.R.; Reiter, M.J.; Roddy, P.J.; Schultz, H.J.; Ta, Q.V.; Tomai, M.A. Development of a Topically Active Imiquimod Formulation. Pharm. Dev. Technol. 1999, 4, 35–43. [Google Scholar] [CrossRef]
- Argenziano, M.; Haimhoffer, A.; Bastiancich, C.; Jicsinszky, L.; Caldera, F.; Trotta, F.; Scutera, S.; Alotto, D.; Fumagalli, M.; Musso, T.; et al. In Vitro Enhanced Skin Permeation and Retention of Imiquimod Loaded in β-Cyclodextrin Nanosponge Hydrogel. Pharmaceutics 2019, 11, 138. [Google Scholar] [CrossRef]
- Cholkar, K.; Hariharan, S.; Gunda, S.; Mitra, A.K. Optimization of Dexamethasone Mixed Nanomicellar Formulation. AAPS Pharm. Sci. Tech. 2014, 15, 1454–1467. [Google Scholar] [CrossRef]
- Pescina, S.; Lucca, L.G.; Govoni, P.; Padula, C.; Favero, E.D.; Cantù, L.; Santi, P.; Nicoli, S. Ex Vivo Conjunctival Retention and Transconjunctival Transport of Poorly Soluble Drugs Using Polymeric Micelles. Pharmaceutics 2019, 11, 476. [Google Scholar] [CrossRef]
- Pooja, D.; Kulhari, H.; Singh, M.K.; Mukherjee, S.; Rachamalla, S.S.; Sistla, R. Dendrimer–TPGS mixed micelles for enhanced solubility and cellular toxicity of taxanes. Colloids Surf. B Biointerfaces 2014, 121, 461–468. [Google Scholar] [CrossRef]
- Pescina, S.; Sonvico, F.; Clementino, A.; Padula, C.; Santi, P.; Nicoli, S. Preliminary Investigation on Simvastatin-Loaded Polymeric Micelles in View of the Treatment of the Back of the Eye. Pharmaceutics 2021, 13, 855. [Google Scholar] [CrossRef] [PubMed]
- Tampucci, S.; Guazzelli, L.; Burgalassi, S.; Carpi, S.; Chetoni, P.; Mezzetta, A.; Nieri, P.; Polini, B.; Pomelli, C.S.; Terreni, E.; et al. pH-Responsive Nanostructures Based on Surface Active Fatty Acid-Protic Ionic Liquids for Imiquimod Delivery in Skin Cancer Topical Therapy. Pharmaceutics 2020, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, F.; Mahato, R.I. Poly(ethylene glycol)-Block-Poly(2-methyl-2-benzoxycarbonyl-propylene Carbonate) Micelles for Rapamycin Delivery: In Vitro Characterization and Biodistribution. J. Pharm. Sci. 2011, 100, 2418–2429. [Google Scholar] [CrossRef]
- Guo, X.D.; Qian, Y.; Zhang, C.Y.; Nie, S.Y.; Zhang, L.J. Can drug molecules diffuse into the core of micelles? Soft Matter 2012, 8, 9989–9995. [Google Scholar] [CrossRef]
- Owen, S.C.; Chan, D.P.Y.; Shoichet, M.S. Polymeric micelle stability. Nano Today 2012, 7, 53–65. [Google Scholar] [CrossRef]
- Khuwijitjaru, P.; Kimura, Y.; Matsuno, R.; Adachi, S. Solubility of Oleic and Linoleic Acids in Subcritical Water. Food Sci. Technol. Res. 2004, 10, 261–263. [Google Scholar] [CrossRef]
- Panja, S.; Khatua, D.K.; Halder, M. Investigations on the Effect of Fatty Acid Additives on Casein Micelles: Role of Ethylenic Unsaturation on the Interaction and Structural Diversity. ACS Omega 2018, 3, 821–830. [Google Scholar] [CrossRef]
- Smith, A.; Lough, A.K. Micellar solubilization of fatty acids in aqueous media containing bile salts and phospholipids. Br. J. Nutr. 1976, 35, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Leekumjorn, S.; Cho, H.J.; Wu, Y.; Wright, N.T.; Sum, A.K.; Chan, C. The role of fatty acid unsaturation in minimizing biophysical changes on the structure and local effects of bilayer membranes. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1508–1516. [Google Scholar] [CrossRef]
- Ristroph, K.D.; Prud’homme, R.K. Hydrophobic ion pairing: Encapsulating small molecules, peptides, and proteins into nanocarriers. Nanoscale Adv. 2019, 1, 4207–4237. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Shin, E.; Wang, H.; Nolan, J.; Low, S.; Parsons, D.; Zale, S.; Ashton, S.; Ashford, M.; Ali, M.; et al. A novel in situ hydrophobic ion paring (HIP) formulation strategy for clinical product selection of a nanoparticle drug delivery system. J. Control. Release 2016, 229, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Quartier, J.; Lapteva, M.; Boulaguiem, Y.; Guerrier, S.; Kalia, Y.N. Polymeric micelle formulations for the cutaneous delivery of sirolimus: A new approach for the treatment of facial angiofibromas in tuberous sclerosis complex. Int. J. Pharm. 2021, 604, 120736. [Google Scholar] [CrossRef]
- Gilbert, L.; Savary, G.; Grisel, M.; Picard, C. Predicting sensory texture properties of cosmetic emulsions by physical measurements. Chemom. Intell. Lab. Syst. 2013, 124, 21–31. [Google Scholar] [CrossRef]
- Kong, D.D.; Kosar, T.F.; Dungan, S.R.; Phillips, R.J. Diffusion of proteins and nonionic micelles in agarose gels by holographic interferometry. AIChE J. 1997, 43, 25–32. [Google Scholar] [CrossRef]
- Rusanov, A.I. Surfactant micellization and diffusion at moderate concentrations. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 162–169. [Google Scholar] [CrossRef]
- Turq, P.; Drifford, M.; Hayoun, M.; Perera, A.; Tabony, J. Influence of monomer-micelle exchange on micelle diffusion. J. Phys. Lett. 1983, 44, 471–479. [Google Scholar] [CrossRef][Green Version]
- Lapteva, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Polymeric Micelle Nanocarriers for the Cutaneous Delivery of Tacrolimus: A Targeted Approach for the Treatment of Psoriasis. Mol. Pharm. 2014, 11, 2989–3001. [Google Scholar] [CrossRef]
- Starigazdová, J.; Nešporová, K.; Čepa, M.; Šínová, R.; Šmejkalová, D.; Huerta-Angeles, G.; Velebný, V. In vitro investigation of hyaluronan-based polymeric micelles for drug delivery into the skin: The internalization pathway. Eur. J. Pharm. Sci. 2020, 143, 105168. [Google Scholar] [CrossRef]
- Yotsumoto, K.; Ishii, K.; Kokubo, M.; Yasuoka, S. Improvement of the skin penetration of hydrophobic drugs by polymeric micelles. Int. J. Pharm. 2018, 553, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, B.; Cavalli, E.; Painelli, A.; Terenziani, F. Polar Dyes in Solution: A Joint Experimental and Theoretical Study of Absorption and Emission Band Shapes. J. Phys. Chem. A 2002, 106, 6286–6294. [Google Scholar] [CrossRef]
- Lapteva, M.; Mignot, M.; Mondon, K.; Möller, M.; Gurny, R.; Kalia, Y.N. Self-assembled mPEG-hexPLA polymeric nanocarriers for the targeted cutaneous delivery of imiquimod. Eur. J. Pharm. Biopharm. 2019, 142, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Hammerl, V.; Parlar, B.; Navarini, A.; Gantenbein, L.; Väth, H.; Mueller, S.M. Mucosal side effects in patients treated with topical imiquimod—A scoping review of the literature. Dermatol. Ther. 2021, 34, e14355. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Pan, J.; Long, Y.; Yu, K.; Wang, Y.; Pan, W.; Ruan, W.; Qin, M.; Wu, C.; Xu, Y. Dual roles of TPGS based microemulsion for tacrolimus: Enhancing the percutaneous delivery and anti-psoriatic efficacy. Int. J. Pharm. 2017, 528, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.; Kable, E.; Jones, A.; Fraser, I.; Manconi, F.; Gorrell, M.D. 3-Dimensional imaging of collagen using second harmonic generation. J. Struct. Biol. 2003, 141, 53–62. [Google Scholar] [CrossRef]


 ) represent IMQ concentration in the formulations applied. Data on Transcutol® saturated solution is from [15]. (B) Delivery efficiency (% delivered after 5 h) of TO20 and semisolid formulations. (p * < 0.05); p-values obtained from t-test analysis for epidermis, dermis and total amount of the drug in the skin for all formulations tested are reported in Table S4.
) represent IMQ concentration in the formulations applied. Data on Transcutol® saturated solution is from [15]. (B) Delivery efficiency (% delivered after 5 h) of TO20 and semisolid formulations. (p * < 0.05); p-values obtained from t-test analysis for epidermis, dermis and total amount of the drug in the skin for all formulations tested are reported in Table S4.
 ) represent IMQ concentration in the formulations applied. Data on Transcutol® saturated solution is from [15]. (B) Delivery efficiency (% delivered after 5 h) of TO20 and semisolid formulations. (p * < 0.05); p-values obtained from t-test analysis for epidermis, dermis and total amount of the drug in the skin for all formulations tested are reported in Table S4.
) represent IMQ concentration in the formulations applied. Data on Transcutol® saturated solution is from [15]. (B) Delivery efficiency (% delivered after 5 h) of TO20 and semisolid formulations. (p * < 0.05); p-values obtained from t-test analysis for epidermis, dermis and total amount of the drug in the skin for all formulations tested are reported in Table S4.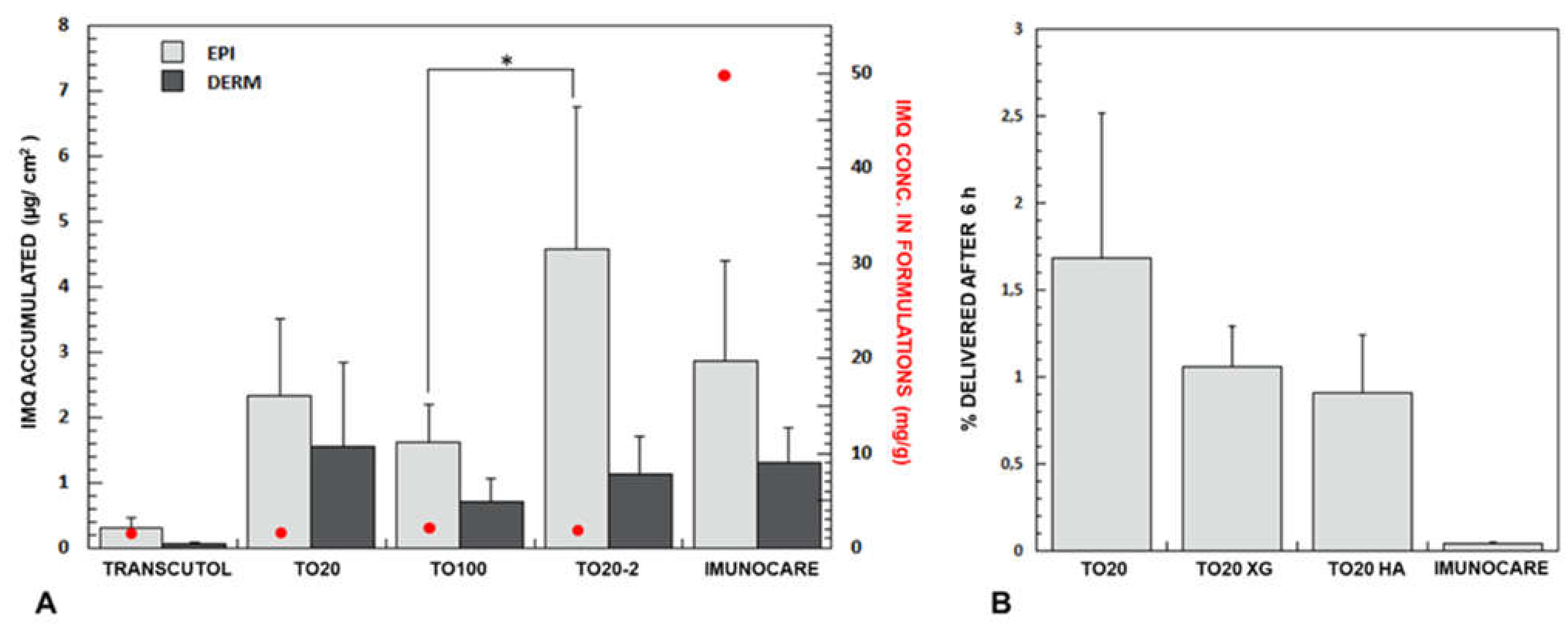
| # | Compound | Log P [29] | HLB | pKa | H-Bond Capacity [30] (Donor-Acceptor) | Molecular Weight (g/mol) |
|---|---|---|---|---|---|---|
| 1 | Imiquimod | 2.9 | - | 7.3 [29] | 2–4 | 240.30 |
| 2 | TPGS | - | 13.2 [31] | - | 1–6 | 1513.00 |
| 3 | Oleic acid (18:1) | 7.19 | - | 4.90 [29]–9.85 [32] | 1–2 | 282.50 |
| 4 | Linolenic acid (18:3) | 5.95 | - | 4.90 [29]–8.28 [32] | 1–2 | 278.43 |
| 5 | Linoleic acid (18:2) | 6.65 | - | 4.78 [29]–9.24 [32] | 1–2 | 280.45 |
| 6 | Isostearic acid (18:0) | 7.2 | - | 4.78 [29] | 1–2 | 284.50 |
| 7 | Peceol™ | - | 3 [33] | 13.16 [34] | 2–4 | 356.54 |
| 8 | Plurol® Oleique CC497 | - | 3 [35] | - | 20–27 | 1023.20 |
| 9 | Span® 80 | - | 4.3 [36] | - | 3–6 | 428.60 |
 | ||||||
| Code | Composition | Method | Imiquimod Solubility (µg/mL) | Fatty Acid Content (µg/mL) | pH |
|---|---|---|---|---|---|
| T20 | TPGS 20 mM | 1 | 1.70 ± 0.63 | - | 6.14 ± 0.14 |
| T40 | TPGS 40 mM | 1 | 109.81 ± 53.77 | - | 5.06 ± 0.45 |
| T100 | TPGS 100 mM | 1 | 188.76 ± 6.76 | - | 5.37 ± 0.43 |
| T200 | TPGS 200 mM | 1 | 231.51 ± 84.21 | - | - |
| TO20 | TPGS 20 mM + oleic acid | 1 | 1154.08 ± 112.78 | 3156.69 ± 882.31 | 4.79 ± 0.03 |
| TO40 | TPGS 40 mM + oleic acid | 1 | 1520.61 ± 72.98 | 7871.15 ± 808.39 | 4.69 ± 0.02 |
| TO100 | TPGS 100 mM + oleic acid | 1 | 2517.56 ± 70.26 | 20519 ± 956.79 | 4.92 ± 0.03 |
| TLN20 | TPGS 20 mM + linolenic acid | 1 | 912.59 ± 27.50 | 7898.09 ± 180.30 | 4.76 ± 0.06 |
| TL20 | TPGS 20 mM + linoleic acid | 1 | 731.77 ± 52.25 | 3998.00 ± 140.60 | 5.09 ± 0.04 |
| TI20 | TPGS 20 mM + isostearic acid | 1 | 393.24 ± 13.34 | n.d. a | 5.34 ± 0.01 |
| TP20 | TPGS 20 mM + Peceol™ | 1 | 180.21 ± 35.99 | - | 5.30 ± 0.10 |
| TPO20 | TPGS 20 mM + Plurol® Oleique | 1 | 126.55 ± 5.52 | - | 5.99 ± 1.00 |
| TSP20 | TPGS 20 mM + Span® 80 | 1 | 115.34 ± 76.82 | - | 6.20 ± 0.13 |
| TO20-2 | TPGS 20 mM + oleic acid | 2 | 1659.34 ± 109.87 | 8683.54 ± 344.36 | 4.63 ± 0.03 |
| TO20-3 | TPGS 20 mM + oleic acid | 3 | 304.76 ± 4.06 | n.d. | 3.44 ± 0.01 |
| Formulation | Blank Size (nm) | Intensity (%) | PDI | Loaded Size (nm) | Intensity (%) | PDI |
|---|---|---|---|---|---|---|
| T20 | 14.43 ± 0.39 647.80 ±37.75 | 86.93 26.13 | 0.28 | 14.01 ± 0.68 845.56 ± 289.37 | 86.33 12.77 | 0.29 |
| T40 | 13.71 ± 0.26 | 95.03 | 0.20 | 12.66 ± 0.11 | 100 | 0.08 |
| T100 | 12.26 ± 0.22 | 100 | 0.07 | 11.95 ± 0.06 | 100 | 0.06 |
| TO20 | 16.02 ± 0.61 | 96.07 | 0.17 | 15.31 ± 0.69 230.66 ± 66.18 | 61.67 42.96 | 0.57 |
| TO20-2 | 40.48 ± 0.52 | 100 | 0.12 | 16.74 ± 0.62 222.77 ± 2.08 | 13.33 86.67 | 0.63 |
| TO40 | 30.40 ± 1.19 | 99.43 | 0.22 | 20.13 ± 0.48 | 100 | 0.25 |
| TO100 | 13.42 ± 0.11 | 93.93 | 0.21 | 12.63 ± 0.06 | 100 | 0.04 |
| TI20 | 14.69 ± 0.18 511.77 ± 64.94 | 85.85 23.23 | 0.238 | 13.57 ± 0.76 612.44 ± 383.32 | 79.55 17.64 | 0.36 |
| Formulation | Epidermis (µg/cm2) | Dermis (µg/cm2) | Total (µg/cm2) |
|---|---|---|---|
| TRANSCUTOL® SS [15] | 0.32 ± 0.14 | 0.06 ± 0.04 | 0.38 ± 0.17 |
| TO20 | 2.32 ± 1.18 | 1.56 ± 1.28 | 3.89 ± 1.93 |
| TO100 | 1.63 ± 0.57 | 0.71 ± 0.34 | 2.34 ± 0.53 |
| TO20-2 | 4.57 ± 2.19 | 1.14 ± 0.56 | 5.46 ± 2.56 |
| Imunocare® | 2.87 ± 1.51 | 1.32 ± 0.53 | 4.19 ± 0.99 |
| TO20 XG | 1.64 ± 0.65 | 0.80 ± 0.24 | 2.44 ± 0.54 |
| TO20 HA | 1.59 ± 0.65 | 0.50 ± 0.22 | 2.10 ± 0.77 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghezzi, M.; Pescina, S.; Delledonne, A.; Ferraboschi, I.; Sissa, C.; Terenziani, F.; Remiro, P.D.F.R.; Santi, P.; Nicoli, S. Improvement of Imiquimod Solubilization and Skin Retention via TPGS Micelles: Exploiting the Co-Solubilizing Effect of Oleic Acid. Pharmaceutics 2021, 13, 1476. https://doi.org/10.3390/pharmaceutics13091476
Ghezzi M, Pescina S, Delledonne A, Ferraboschi I, Sissa C, Terenziani F, Remiro PDFR, Santi P, Nicoli S. Improvement of Imiquimod Solubilization and Skin Retention via TPGS Micelles: Exploiting the Co-Solubilizing Effect of Oleic Acid. Pharmaceutics. 2021; 13(9):1476. https://doi.org/10.3390/pharmaceutics13091476
Chicago/Turabian StyleGhezzi, Martina, Silvia Pescina, Andrea Delledonne, Ilaria Ferraboschi, Cristina Sissa, Francesca Terenziani, Paula De Freitas Rosa Remiro, Patrizia Santi, and Sara Nicoli. 2021. "Improvement of Imiquimod Solubilization and Skin Retention via TPGS Micelles: Exploiting the Co-Solubilizing Effect of Oleic Acid" Pharmaceutics 13, no. 9: 1476. https://doi.org/10.3390/pharmaceutics13091476
APA StyleGhezzi, M., Pescina, S., Delledonne, A., Ferraboschi, I., Sissa, C., Terenziani, F., Remiro, P. D. F. R., Santi, P., & Nicoli, S. (2021). Improvement of Imiquimod Solubilization and Skin Retention via TPGS Micelles: Exploiting the Co-Solubilizing Effect of Oleic Acid. Pharmaceutics, 13(9), 1476. https://doi.org/10.3390/pharmaceutics13091476