Pharmacokinetics of Levodopa and 3-O-Methyldopa in Parkinsonian Patients Treated with Levodopa and Ropinirole and in Patients with Motor Complications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Design
Determination of Plasma Concentrations of Levodopa, 3-OMD, and Ropinirole
2.3. Mathematical Modelling and Parameter Estimation of Levodopa and 3-OMD
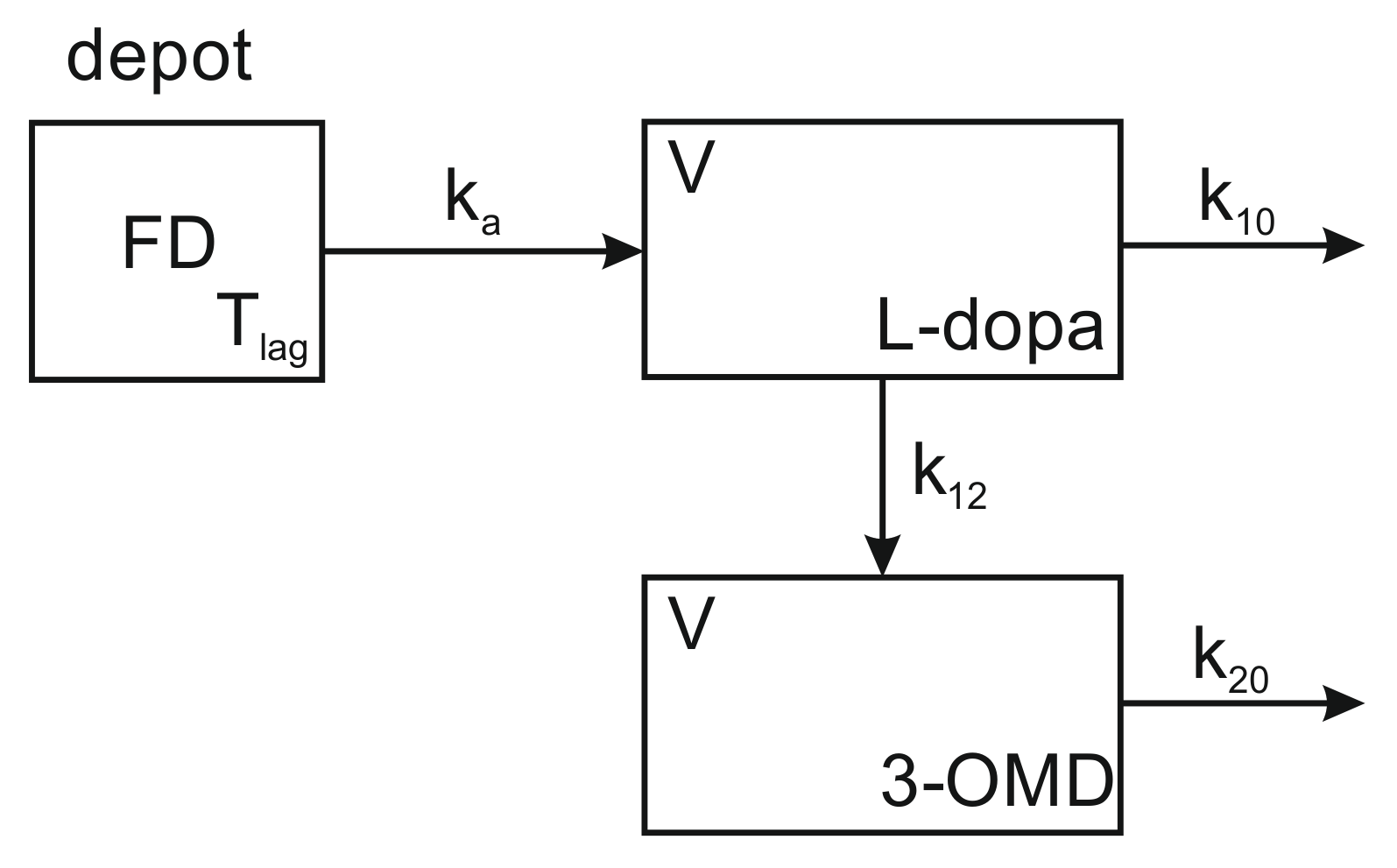
2.3.1. Pharmacokinetic Model for the Drug-Metabolite System
2.3.2. Population PK Modelling
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Plasma Concentration of Levodopa and 3-OMD
3.3. Pharmacokinetic Population Modelling
3.4. Pharmacokinetic Parameters of Levodopa and 3-OMD
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tambasco, N.; Romoli, M.; Calabresi, P. Levodopa in Parkinson’s disease: Current Status and Future Developments. Curr. Neuropharmacol. 2018, 16, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Marsot, A.; Guilhaumou, R.; Azulay, J.-P.; Blin, O. Levodopa in Parkinson’s disease: A Review of Population Pharmacokinetics/Pharmacodynamics Analysis. J. Pharm. Pharm. Sci. 2017, 20, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Horynkiewicz, O. Basic Research on Dopamine in Parkinson’s disease and the Discovery of the Nigrostratial Dopamine Pathway: The View of an Eyewitness. Neurodegener. Dis. 2008, 5, 114–117. [Google Scholar] [CrossRef]
- Simon, N.; Viallet, F.; Boulamery, A.; Eusebio, A.; Gayraund, D.; Azulay, J.-P. A combine pharmacokinetic/pharmacodynamic model of levodopa motor response and dyskinesias in Parkinson’s disease patients. Eur. J. Clin. Pharmacol. 2016, 72, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Nutt, J.G.; Fellman, J.H. Pharmacokinetics of levodopa. Clin. Neuropharmacol. 1984, 7, 35–49. [Google Scholar] [CrossRef]
- Camargo, S.M.R.; Vuille-dit-Bille, R.N.; Mariotta, L.; Ramadan, T.; Huggel, K.; Singer, D.; Gotze, O.; Verrey, F. The molecular mechanism of intestinal levodopa absorption and its possible implications for the treatment of Parkinson’s disease. J. Pharmacol. Exp. Ther. 2014, 351, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Khor, S.P.; Hsu, A. The Pharmacokinetics and Pharmacodynamics of Levodopa in the Treatment of Parkinson’s disease. Curr. Clin. Pharmacol. 2007, 2, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Nyholm, D. Pharmacokinetic Optimisationin the Treatment of Parkinson’s disease. Clin. Pharmacokinet. 2006, 45, 109–136. [Google Scholar] [CrossRef]
- Contin, M.; Martinelli, P. Pharmacokinetics of levodopa. J. Neurol. 2010, 257, 253–261. [Google Scholar] [CrossRef]
- Hauser, R.A. Levodopa: Past, present, and future. Eur. Neurol. 2009, 62, 1–8. [Google Scholar] [CrossRef]
- Nutt, J.G.; Woodward, W.R.; Anderson, J.L. The effect of carbidopa on the pharmacokinetics of intravenously administered levodopa: The mechanism of action in the treatment of parkinsonism. Ann. Neurol. 1985, 18, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Levodopa strengths and weaknesses. Neurology 2002, 58 (Suppl. 1), S19–S32. [Google Scholar] [CrossRef]
- Lee, E.S.; Chen, H.; King, J.; Charlton, C. The role of 3-O-methylda in the side effects of L-dopa. Neurochem. Res. 2008, 33, 401–411. [Google Scholar] [CrossRef]
- Wade, L.N.; Katzman, R. 3-O-Methyldopa inhibition of L-dopa at the blood-brain barrier. Life Sci. 1975, 17, 131–136. [Google Scholar] [CrossRef]
- Marsden, C.D. Problems with long-term levodopa therapy for Parkinson’s disease. Clin. Neuropharmacol. 1994, 17 (Suppl. 2), S32–S44. [Google Scholar] [CrossRef]
- Bjornestad, A.; Forsaa, E.B.; Pedersen, K.F.; Tysnes, O.B.; Larsen, J.P.; Alves, G. Risk and course of motor complications in a population-based incident Parkinson’s disease cohort. Parkinsonism Relat. Disord. 2016, 22, 48–53. [Google Scholar] [CrossRef]
- Kulisevsky, J.; Pagonabarraga, J. Tolerability and safety of ropinirole versus other dopamine agonists and levodopa in the treatment of Parkinson’s disease: Meta-analysis of randomized controlled trials. Drug Saf. 2010, 33, 147–161. [Google Scholar] [CrossRef]
- Whone, A.L.; Watts, R.L.; Stoessl, A.J.; Davis, M.; Reske, S.; Nahmias, C.; Lang, A.E.; Rascol, O.; Ribeiro, M.J.; Remy, P.; et al. REAL-PET StudyGroup. Slower progression of Parkinson’s disease with ropinirole versus levodopa: The REAL-PET study. Ann. Neurol. 2003, 54, 93–101. [Google Scholar] [CrossRef]
- Fox, S.H.; Lang, A.E. ‘Don’t delay, start today’: Delaying levodopa does not delay motor complications. Brain 2014, 137, 2628–2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewitt, P.A. Relief of parkinsonism and dyskinesia: One and the same dopaminergic mechanism? Neurology 2010, 74, 1169–1170. [Google Scholar] [CrossRef] [PubMed]
- Ursino, M.; Magosso, E.; Lopane, G.; Calandra-Buonaura, G.; Cortelli, P.; Contin, M. Mathematical modeling and parameter estimation of levodopa motor response in patients with parkinson disease. PLoS ONE 2020, 15, e0229729. [Google Scholar] [CrossRef] [Green Version]
- Aquino, C.C.; Fox, S.H. Clinical spectrum of levodopa-induced complications. Mov. Disord. 2015, 30, 80–89. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saxer, C.; Niina, M.; Nakashima, A.; Nagae, Y.; Masuda, N. Simultaneous Determination of Levodopa and 3-O-methyldopain Human Plasma by Liquid Chromatography with Electrochemical Detection. J. Chromatogr. B 2004, 802, 299–305. [Google Scholar] [CrossRef]
- Adamiak-Giera, U.; Gawrońska-Szklarz, B. Simultaneous determination of levodopa and 3-O-methyldopa in patients with Parkinson’s disease by high-performance liquid chromatography with electrochemical detection. J. Liq. Chromatogr. Relat. Technol. 2019, 41, 1047–1051. [Google Scholar] [CrossRef]
- Bhatt, J.; Jangid, A.; Shetty, R.; Shah, B.; Kambli, S.; Subbaiah, G.; Singh, S. Rapid and sensitive liquid chromatography-mass spectrometry method for determination of ropinirole in human plasma. J. Pharm. Biomed. Anal. 2006, 40, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Mlxtran User Guide. Lixoft 2019. Available online: http://mlxtran.lixoft.com/mlxtran-user-guide/ (accessed on 30 April 2019).
- Monolix Documentation Version 2019. Lixoft 2019. Available online: http://monolix.lixoft.com/single-page/ (accessed on 30 April 2019).
- Kuhn, E.; Lavielle, M. Maximum likelihood estimation in nonlinear mixed effects models. Comput. Stat. Data Anal. 2005, 49, 1020–1038. [Google Scholar] [CrossRef]
- Lavielle, M.; Mentré, F. Estimation of population pharmacokinetic parameters of saquinavir in HIV patients with the MONOLIX software. J. Pharmacokinet. Pharmacodyn. 2007, 34, 229–249. [Google Scholar] [CrossRef] [Green Version]
- Lixoft. Standard Error Using the Fisher Information Matrix. Available online: http://monolix.lixoft.com/tasks/standard-error-using-the-fisher-information-matrix/ (accessed on 16 July 2020).
- Bonate, P.L. Pharmacokinetic-Pharmacodynamic Modeling and Simulation; Springer: Boston, MA, USA, 2011. [Google Scholar] [CrossRef]
- Brendel, K.; Comets, E.; Laffont, C.; Laveille, C.; Mentré, F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm. Res. 2006, 23, 2036–2049. [Google Scholar] [CrossRef] [Green Version]
- Tompson, D.J.; Vearer, D. Steady-State Pharmacokinetic Properties of a 24-Hour Prolonged-Release Formulation of Ropinirole: Results of Two Randomized Studies in Patients with Parkinson’s disease. Clin. Ther. 2007, 29, 2654–2666. [Google Scholar] [CrossRef]
- Nomotoa, M.; Nagaia, M.; Nishikawaa, N.; Andoa, R.; Kagamiishib, Y.; Yanob, K.; Saitob, S.; Takedac, A. Pharmacokinetics and safety/efficacy of levodopa pro-drug ONO-2160/carbidopa for Parkinson’s disease. Eneurologicalsci 2018, 13, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.C.; Beerahee, A.; Citerone, D.R.; Cyronak, M.J.; Leigh, T.J.; Fitzpatrick, K.L.; Lopez-Gil, A.; Vakil, S.D.; Burns, E.; Lennox, G. Lack of pharmacokinetic interaction at steady state between ropinirole and L-dopa in patients with Parkinson’s disease. Pharmacotherapy 1999, 19, 150–156. [Google Scholar] [CrossRef]
- Kaye, C.M.; Nicholls, B. Clinical pharmacokinetics of ropinirole. Clin. Pharmacokinet. 2000, 39, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Adamiak, U.; Kaldonska, M.; Klodowska-Duda, G.; Wyska, E.; Safranow, K.; Bialecka, M.; Gawronska-Szklarz, B. Pharmacokinetic-Pharmacodynamic Modeling of Levodopa in Patients with Advanced Parkinson Disease. Clin. Neuropharmacol. 2010, 33, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Hardoff, R.; Sula, M.; Tamir, A.; Soil, A.; Front, A.; Badarna, S.; Honigman, S.; Giladi, N. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov. Disord. 2001, 16, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Djaldetti, R.; Baron, J.; Ziv, I.; Melamed, E. Gastric emptying in Parkinson’s disease: Patients with and without response fluctuations. Neurology 1996, 46, 1051–1054. [Google Scholar] [CrossRef] [PubMed]




| Demographic and Clinical Data (Mean, SD, Range) | Patients Administrating Both Levodopa and Ropinirole (n = 13) | Patients Administrating Levodopa But Not Ropinirole (n = 14) | p-Value |
|---|---|---|---|
| Sex (M/F) | 10 M/3 F | 10 M/4 F | Ns |
| Age (yr) | 68.4 ± 7.3 57–83 | 70.9 ± 10.8 45–86 | Ns |
| Weight (kg) | 79.3 ± 16.4 60–113 | 79.8 ± 21.6 50–110 | Ns |
| UPDRS score | 25.3 ± 9.9 10–44 | 21.8 ± 10.6 6–36 | Ns |
| Disease duration (yr) | 8.5 ± 5.9 1–21 | 5.3 ± 3.2 1–11 | Ns |
| Levodopa treatment duration (yr) | 7.2 ± 4.9 0.5–20 | 3.9 ± 3.5 0.5–11 | Ns |
| Current levodopa dosage (mg/d) | 692.9 ± 245.6 400–1100 | 592.9 ± 200.8 300–950 | Ns |
| Ropinirole treatment duration (yr) | 3.3 ± 2.5 1–8 | - | - |
| Current ropinirole dosage (mg/d) | 7.3 ± 2.2 4–10 | - | - |
| Motor complications (fluctuations and/or dyskinesias) | 8 Yes/5 No | 4 Yes/10 No | Ns |
| Demographic and Clinical Data (Mean, SD, Range) | Patients with Motor Complications (Fluctuations or Dyskinesias) (n = 12) | Patients without Motor Complications (Fluctuations and/or Dyskinesias) (n = 15) | p-Value |
|---|---|---|---|
| Sex (M/F) | 8 M/5 F | 13 M/2 F | Ns |
| Age (yr) | 72.6 ± 9.0 57–86 | 67.1 ± 8.8 45–79 | Ns |
| Weight (kg) | 68.2 ± 12.3 50–85 | 88.7 ± 18.6 51–113 | <0.002 |
| UPDRS score | 28.1 ± 9.2 15–44 | 20.2 ± 9.8 6–35 | Ns |
| Disease duration (yr) | 10.5 ± 4.7 6–21 | 3.8 ± 2.4 1–8 | <0.001 |
| Levodopa treatment duration (yr) | 9.1 ± 4.0 4–20 | 2.4 ± 1.9 0.5–6 | <0.001 |
| Current levodopa dosage (mg/d) | 811.5 ± 167.3 600–1100 | 496.7 ± 158.6 300–900 | <0.001 |
| Ropinirole treatment duration (yr) | 3.6 ± 2.6 1–8 (n = 8) | 2.0 ± 1.4 1–3 (n = 5) | Ns |
| Current ropinirole dosage (mg/d) | 8.0 ± 2.2 4–10 (n = 8) | 6.0 ± 1.4 4–8 (n = 5) | Ns |
| PK Parameter | Covariate | Significance (p) |
|---|---|---|
| Weight | 0.013 | |
| Movement disorder | <0.001 | |
| Age | 0.007 | |
| Ropinirole therapy | 0.019 | |
| Sex | <0.001 | |
| Ropinirole concentration at 2 h | <0.001 |
| Parameter | Patients Administrating Both Levodopa and Ropinirole (n = 13) | Patients Administrating Levodopa but Not Ropinirole (n = 14) | p-Value |
|---|---|---|---|
| levodopa | levodopa | ||
| lag time (min) | 9.97 ± 11.3 | 16.6 ± 10,6 | Ns |
| V/F (L) | 32.4 ± 10.3 | 36.1 ± 11.8 | Ns |
| CL/F (L/min) | 0.816 ± 0.323 | 0.835 ± 0.336 | Ns |
| AUC0–∞ () | 150 ± 83 | 147 ± 83 | Ns |
| tmax (min) | 35.5 ± 34.6 | 46.4 ± 26.8 | Ns |
| Cmax (mg/L) | 2.27 ± 1.56 | 1.79 ± 0.95 | Ns |
| MRT (min) | 41.6 ± 6.6 | 45.1 ± 6.9 | Ns |
| ka (min−1) | 0.18 ± 0.16 | 0.31 ± 0.42 | Ns |
| k10 (min−1) | 0.0213 ± 0.0038 | 0.0195 ± 0.0034 | Ns |
| k12 (min−1) | 0.00333 ± 0.0056 | 0.00318 ± 0.00040 | Ns |
| k20 (min−1) | 0.000262 ± 0.000043 | 0.000204 ± 0.000029 | 0.002 |
| 3-OMD | 3-OMD | ||
| tmax 3-OMD (min) | 305 ± 207 | 304 ± 152 | Ns |
| MET3-OMD (min) | 3997 ± 666 | 5040 ± 612 | 0.001 |
| Cmax 3-OMD (mg/L) | 0.476 ± 0.316 | 0.423 ± 0.188 | Ns |
| AUC3-OMD [0–∞] () | 1993 ± 1277 | 2232 ± 1032 | Ns |
| Parameter | Patients with Motor Complications (Fluctuations and/or Dyskinesias) (n = 12) | Patients without Motor Complications (Fluctuations and/or Dyskinesias) (n = 15) | p-Value |
|---|---|---|---|
| levodopa | levodopa | ||
| lag time (min) | 1.51 ± 0.19 | 22.9 ± 4.1 | <0.0001 |
| V/F (L) | 28.2 ± 10.9 | 39.2 ± 8.7 | 0.01 |
| CL/F (L/min) | 0.674 ± 0.330 | 0.948 ± 0.271 | 0.01 |
| AUC0–∞ () | 191 ± 103 | 114 ± 34 | 0.01 |
| tmax (min) | 30.5 ± 31.2 | 49.6 ± 28.5 | 0.02 |
| Cmax (mg/L) | 2.47 ± 1.56 | 1.66 ± 0.90 | Ns |
| MRT (min) | 44.8 ± 8.1 | 42.2 ± 5.7 | Ns |
| ka (min−1) | 0.19 ± 0.18 | 0.29 ± 0.40 | Ns |
| k10 (min−1) | 0.0197 ± 0.0042 | 0.0208 ± 0.0032 | Ns |
| k12 (min−1) | 0.00327 ± 0.00047 | 0.00324 ± 0.00050 | Ns |
| k20 (min−1) | 0.000247 ± 0.000046 | 0.000220 ± 0.000044 | Ns |
| 3-OMD | 3-OMD | ||
| tmax 3-OMD (min) | 290 ± 174 | 315 ± 261 | Ns |
| MET3-OMD (min) | 4260 ± 812 | 4759 ± 784 | Ns |
| Cmax 3-OMD (mg/L) | 0.580 ± 0.324 | 0.343 ± 0.101 | 0.01 |
| AUC3-OMD [0–∞] () | 2588 ± 1425 | 1740 ± 688 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamiak-Giera, U.; Jawień, W.; Pierzchlińska, A.; Białecka, M.; Kobierski, J.D.; Janus, T.; Gawrońska-Szklarz, B. Pharmacokinetics of Levodopa and 3-O-Methyldopa in Parkinsonian Patients Treated with Levodopa and Ropinirole and in Patients with Motor Complications. Pharmaceutics 2021, 13, 1395. https://doi.org/10.3390/pharmaceutics13091395
Adamiak-Giera U, Jawień W, Pierzchlińska A, Białecka M, Kobierski JD, Janus T, Gawrońska-Szklarz B. Pharmacokinetics of Levodopa and 3-O-Methyldopa in Parkinsonian Patients Treated with Levodopa and Ropinirole and in Patients with Motor Complications. Pharmaceutics. 2021; 13(9):1395. https://doi.org/10.3390/pharmaceutics13091395
Chicago/Turabian StyleAdamiak-Giera, Urszula, Wojciech Jawień, Anna Pierzchlińska, Monika Białecka, Jan Dariusz Kobierski, Tomasz Janus, and Barbara Gawrońska-Szklarz. 2021. "Pharmacokinetics of Levodopa and 3-O-Methyldopa in Parkinsonian Patients Treated with Levodopa and Ropinirole and in Patients with Motor Complications" Pharmaceutics 13, no. 9: 1395. https://doi.org/10.3390/pharmaceutics13091395
APA StyleAdamiak-Giera, U., Jawień, W., Pierzchlińska, A., Białecka, M., Kobierski, J. D., Janus, T., & Gawrońska-Szklarz, B. (2021). Pharmacokinetics of Levodopa and 3-O-Methyldopa in Parkinsonian Patients Treated with Levodopa and Ropinirole and in Patients with Motor Complications. Pharmaceutics, 13(9), 1395. https://doi.org/10.3390/pharmaceutics13091395






