A Monocentric Retrospective Study of AUC/MIC Ratio of Vancomycin Associated with Clinical Outcomes and Nephrotoxicity in Patients with Enterococcal Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
2.2. Data Collection
2.3. Pharmacokinetic Data for Vancomycin
2.4. Statistical Analyses
2.5. Outcome Measurement
2.6. Antimicrobial Susceptibility Test
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schröder, U.C.; Beleites, C.; Assmann, C.; Glaser, U.; Hübner, U.; Pfister, W.; Fritzsche, W.; Popp, J.; Neugebauer, U. Detection of vancomycin resistances in enterococci within 3½ hours. Sci. Rep. 2015, 5, 8217. [Google Scholar] [CrossRef]
- NARST. Percent Susceptibility of Enterococci. Available online: http://narst.dmsc.moph.go.th/data/AMR%202000-2020-06M.pdf (accessed on 10 January 2021).
- Deryke, C.A.; Alexander, D.P. Optimizing vancomycin dosing through pharmacodynamic assessment targeting area under the concentration-time curve/minimum inhibitory concentration. Hosp. Pharm. 2009, 44, 751–765. [Google Scholar] [CrossRef]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R.; Craig, W., Jr.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American society of health-system pharmacists, the infectious diseases society of America, and the society of infectious diseases pharmacists. Am. J. Health-Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef]
- Moise-Broder, P.A.; Forrest, A.; Birmingham, M.C.; Schentag, J.J. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin. Pharm. 2004, 43, 925–942. [Google Scholar] [CrossRef] [PubMed]
- Kullar, R.; Davis, S.L.; Levine, D.P.; Rybak, M.J. Impact of vancomycin exposure on outcomes in patients with methicillin-resistant Staphylococcus aureus bacteremia: Support for consensus guidelines suggested targets. Clin. Infect. Dis. 2011, 52, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Takesue, Y.; Ohmagari, N.; Mochizuki, T.; Mikamo, H.; Seki, M.; Takakura, S.; Tokimatsu, I.; Takahashi, Y.; Kasahara, K.; et al. Practice guidelines for therapeutic drug monitoring of vancomycin: A consensus review of the Japanese society of chemotherapy and the Japanese society of therapeutic drug monitoring. J. Infect. Chemother. 2013, 19, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Rybak, M.J.; Le, J.; Lodise, T.P.; Levine, D.P.; Bradley, J.S.; Liu, C.; Mueller, B.A.; Pai, M.P.; Wong-Beringer, A.; Rotschafer, J.C.; et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American society of health-system pharmacists, the infectious diseases society of america, the pediatric infectious diseases society, and the society of infectious diseases pharmacists. Am. J. Health-Syst. Pharm. 2020, 77, 835–864. [Google Scholar]
- Jumah, M.; Vasoo, S.; Menon, S.R.; De, P.P.; Neely, M.; Teng, C.B. Pharmacokinetic/pharmacodynamic determinants of vancomycin efficacy in enterococcal bacteremia. Antimicrob. Agents Chemother. 2018, 62, e01602-17. [Google Scholar] [CrossRef]
- Nakakura, I.; Sakakura, K.; Imanishi, K.; Sako, R.; Yamazaki, K. Association between vancomycin pharmacokinetic/pharmacodynamic parameters, patient characteristics, and mortality in patients with bacteremia caused by vancomycin-susceptible Enterococcus faecium: A single-center retrospective study. J. Pharm. Health Care Sci. 2019, 5, 8. [Google Scholar] [CrossRef]
- Gawronski, K.M.; Goff, D.A.; Brown, J.; Khadem, T.M.; Bauer, K.A. A stewardship program’s retrospective evaluation of vancomycin AUC24/MIC and time to microbiological clearance in patients with methicillin-resistant Staphylococcus aureus bacteremia and osteomyelitis. Clin. Ther. 2013, 35, 772–779. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kawasaki, K.; Sato, Y.; Tokimatsu, I.; Itoh, H.; Hiramatsu, K.; Takeya-ma, M.; Kadota, J. Is peak concentration needed in therapeutic drug monitoring of vancomycin? A pharmacokinetic-pharmacodynamic analysis in patients with methicillin-resistant staphylococcus aureus pneumonia. Chemotherapy 2012, 58, 308–312. [Google Scholar] [CrossRef]
- Horan, T.C.; Andrus, M.; Dudeck, M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control. 2008, 36, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Roustit, M.; François, P.; Sellier, E.; Roch, N.; Vittoz, J.P.; Foroni, L.; Stahl, J.P.; Pavese, P. Evaluation of glycopeptide prescription and therapeutic drug monitoring at a university hospital. Scand. J. Infect. Dis. 2010, 42, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Jelliffe, R.W.; Bayard, D.; Schumitzky, A.; Milman, M.; Van Guilder, M. Pharmaco-Informatics: More precise drug therapy from ‘multiple model’ (MM) adaptive control regimens: Evaluation with simulated vancomycin therapy. Medinfo 1995, 8, 1106–1110. [Google Scholar]
- Nunn, M.O.; Corallo, C.E.; Aubron, C.; Poole, S.; Dooley, M.J.; Cheng, A.C. Vancomycin dosing: Assessment of time to thera-peutic concentration and predictive accuracy of pharmacokinetic modeling software. Ann. Pharmacother. 2011, 45, 757–763. [Google Scholar] [CrossRef]
- Felton, T.W.; Roberts, J.A.; Lodise, T.P.; Van Guilder, M.; Boselli, E.; Neely, M.N.; Hope, W.W. Individualization of piperacillin dosing for critically ill patients: Dosing software to optimize antimicrobial therapy. Antimicrob. Agents Chemother. 2014, 58, 4094–4102. [Google Scholar] [CrossRef] [PubMed]
- Marsot, A.; Boulamery, A.; Bruguerolle, B.; Simon, N. Vancomycin: A review of population pharmacokinetic analyses. Clin. Pharm. 2012, 51, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lodise, T.P.; Drusano, G.L.; Zasowski, E.; Dihmess, A.; Lazariu, V.; Cosler, L.; McNutt, L.A. Vancomycin exposure in patients with methicillin-resistant Staphylococcus aureus bloodstream infections: How much is enough? Clin. Infect. Dis. 2014, 59, 666–675. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; CLSI Document M100-S25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef] [PubMed]
- Hanberger, H.; Nilsson, L.E.; Maller, R.; Isaksson, B. Pharmacodynamics of daptomycin and vancomycin on Enterococcus faecalis and Staphylococcus aureus demonstrated by studies of initial killing and postantibiotic effect and influence of Ca2+ and albumin on these drugs. Antimicrob. Agents Chemother. 1991, 35, 1710–1716. [Google Scholar] [CrossRef]
- Lodise, T.P.; Lomaestro, B.; Graves, J.; Drusano, G.L. Larger vancomycin doses (at least four grams per day) are associated with an increased incidence of nephrotoxicity. Antimicrob. Agents Chemother. 2008, 52, 1330–1336. [Google Scholar] [CrossRef]
- Neely, M.N.; Kato, L.; Youn, G.; Kraler, L.; Bayard, D.; van Guilder, M.; Schumitzky, A.; Yamada, W.; Jones, B.; Minejima, E. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob. Agents Chemother. 2018, 62, e02042-17. [Google Scholar] [CrossRef]
- Muklewicz, J.D.; Steuber, T.D.; Edwards, J.D. Evaluation of area under the concentration-time curve-guided vancomycin dosing with or without piperacillin-tazobactam on the incidence of acute kidney injury. Int. J. Antimicrob. Agents. 2021, 57, 106234. [Google Scholar] [CrossRef]
- Zasowski, E.J.; Murray, K.P.; Trinh, T.D.; Finch, N.A.; Pogue, J.M.; Mynatt, R.P.; Rybak, M.J. Identification of vancomycin exposure-toxicity thresholds in hospitalized patients receiving intravenous vancomycin. Antimicrob. Agents Chemother. 2017, 62, e01684-17. [Google Scholar] [CrossRef]
- Cristallini, S.; Hites, M.; Kabtouri, H.; Roberts, J.A.; Beumier, M.; Cotton, F.; Lipman, J.; Jacobs, F.; Vincent, J.L.; Creteur, J.; et al. New regimen for continuous infusion of vancomycin in critically Ill patients. Antimicrob. Agents Chemother. 2016, 60, 4750–4756. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, T.P.; Harlow, G.; Hutchinson, J.; Dulhunty, J.M.; Lipman, J.; Whitehouse, T.; Roberts, J.A. Vancomycin-Associated nephrotoxicity in the critically ill: A retrospective multivariate regression analysis. Crit. Care Med. 2014, 42, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, T.; Whitehouse, T.; Lipman, J.; Roberts, J.A. Vancomycin-Associated nephrotoxicity: A meta-analysis of administration by continuous versus intermittent infusion. Int. J. Antimicrob. Agents. 2015, 46, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Katip, W.; Jaruratanasirikul, S.; Pattharachayakul, S.; Wongpoowarak, W.; Jitsurong, A.; Lucksiri, A. The pharmacokinetics of vancomycin during the initial loading dose in patients with septic shock. Infect. Drug Resist. 2016, 9, 253–260. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Potretzke, A.; Schreiber, H.L.; Pinkner, J.S.; Bauman, T.M.; Park, A.M.; Desai, A.; Hultgren, S.J.; Caparon, M.G. Antibody-Based therapy for enterococcal catheter-associated urinary tract infections. mBio 2016, 7, e01653-16. [Google Scholar] [CrossRef]
- Marschall, J.; Piccirillo, M.L.; Fraser, V.J.; Doherty, J.A.; Warren, D.K. Catheter removal versus retention in the management of catheter-associated enterococcal bloodstream infections. Can. J. Infect. Dis. Med. Microbiol. 2013, 24, e83–e87. [Google Scholar] [CrossRef]
- Assadi, F. Strategies for preventing catheter-associated urinary tract infections. Int. J. Prev. Med. 2018, 9, 50. [Google Scholar] [CrossRef]
- Sandoe, J.; Witherden, I.R.; Cove, J.H.; Heritage, J.; Wilcox, M.H. Correlation between enterococcal biofilm formation In Vitro and medical-device-related infection potential In Vivo. J. Med. Microbiol. 2003, 52, 547–550. [Google Scholar] [CrossRef]
- Raad, I.I.; Hanna, H.A.; Boktour, M.; Chaiban, G.; Hachem, R.Y.; Dvorak, T.; Lewis, R.; Murray, B.E. Vancomycin-Resistant Enterococcus faecium: Catheter colonization, esp gene, and decreased susceptibility to antibiotics in biofilm. Antimicrob. Agents Chemother. 2005, 49, 5046–5050. [Google Scholar] [CrossRef] [PubMed]
- Sandoe, J.A.; Wysome, J.; West, A.P.; Heritage, J.; Wilcox, M.H. Measurement of ampicillin, vancomycin, linezolid and gentamicin activity against enterococcal biofilms. J. Antimicrob. Chemother. 2006, 57, 767–770. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Coyle, E.A.; Raad, I.I.; Prince, R.A.; Lewis, R.E. Antibacterial activity of linezolid and vancomycin in an in vitro pharmacodynamic model of Gram-positive catheter-related bacteraemia. J. Antimicrob. Chemother. 2005, 55, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the infectious diseases society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef] [PubMed]
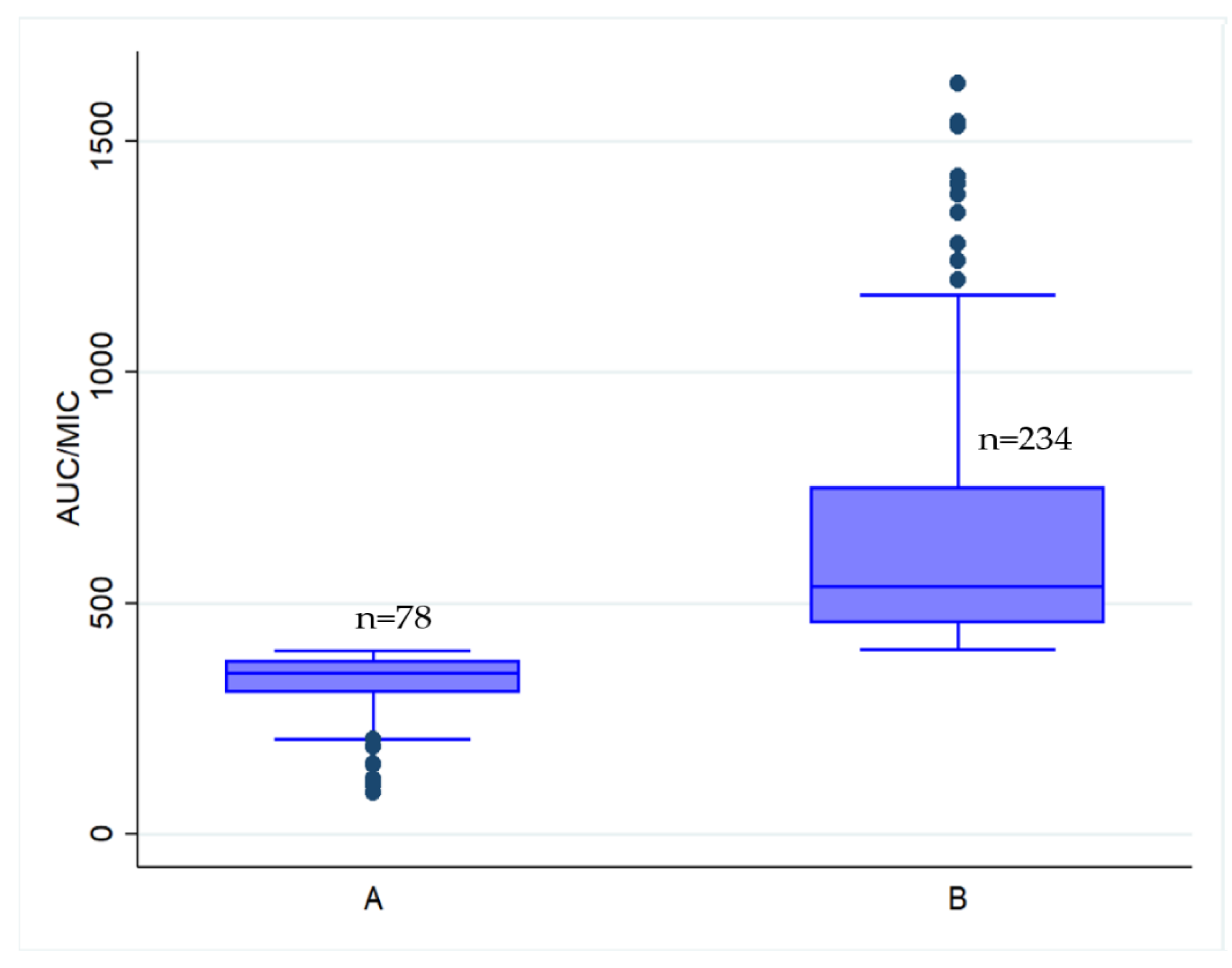
| Characteristic | AUC/MIC of <400 mg·h/L n = 78 | AUC/MIC of ≥400 mg·h/L n = 234 | p-Value |
|---|---|---|---|
| Male, n (%) | 35 (44.87) | 101 (43.16) | 0.794 |
| Female, n (%) | 43 (55.13) | 133 (56.84) | |
| Age, mean ± SD, years | 60.32 ± 18.93 | 61.60 ± 17.91 | 0.637 |
| Body weight, mean ± SD (kg) | 56.06 ± 11.65 | 56.01 ± 12.55 | 0.975 |
| Height, mean ± SD (cm) | 165.8 ± 7.3 | 165.6 ± 7.1 | 0.830 |
| Vancomycin dose, mean ± SD (mg/kg) | 22.4 ± 5.2 | 28.4 ± 5.2 | 0.001 |
| Diagnosis, n (%) | |||
| Urinary tract infection | 59 (75.64) | 160 (68.38) | 0.254 |
| Bacteremia | 8 (10.26) | 28 (11.97) | 0.838 |
| Wound infection | 6 (7.69) | 33 (14.10) | 0.168 |
| Intra-abdominal infection | 4 (5.13) | 13 (5.56) | 1.000 |
| Enterococcal species, n (%) | |||
| E. faecium | 56 (71.79) | 187 (79.91) | 0.156 |
| E. feacalis | 22 (28.21) | 47 (20.09) | |
| Comorbid disease states, n (%) | |||
| Cardiovascular disease | 28 (35.90) | 79 (33.76) | 0.783 |
| Chronic kidney disease | 22 (28.21) | 90 (38.46) | 0.133 |
| Solid tumor | 19 (24.36) | 48 (20.51) | 0.525 |
| Diabetes mellitus | 13 (16.67) | 35 (14.96) | 0.719 |
| Cerebrovascular disease | 7 (8.97) | 15 (6.41) | 0.448 |
| Hematologic malignancy | 1 (1.30) | 7 (2.99) | 0.684 |
| Chronic liver disease | 6 (7.69) | 23 (9.87) | 0.658 |
| Respiratory disease | 5 (6.41) | 14 (5.98) | 1.000 |
| Others * | 14 (17.95) | 59 (25.21) | 0.218 |
| Co-contaminant nephrotoxic drugs, n (%) | |||
| Furosemide | 26 (33.33) | 83 (35.47) | 0.785 |
| Piperacillin/tazobactam | 10 (12.82) | 34 (14.53) | 0.851 |
| Colistin | 4 (5.13) | 36 (15.38) | 0.019 |
| Aminoglycoside | 2 (2.56) | 2 (0.85) | 0.261 |
| Amphotericin B | 5 (6.41) | 5 (2.14) | 0.128 |
| Rifampin | 1 (1.28) | 5 (2.14) | 1.000 |
| Baseline Scr, mean ± SD, mg/dL | 1.62 ± 1.91 | 2.10 ± 2.12 | 0.078 |
| Baseline GFR, mean ± SD, ml/min | 45.32 ± 37.48 | 36.59 ± 33.23 | 0.053 |
| Duration of vancomycin therapy, mean ± SD, d | 9.08 ± 4.98 | 10.50 ± 7.11 | 0.105 |
| Duration of hospitalization, mean ± SD, d | 34.43 ± 20.67 | 33.93 ± 21.85 | 0.858 |
| APACHE II score, mean ± SD | 12.42 ± 5.22 | 13.50 ± 5.52 | 0.130 |
| Albumin | 2.96 ± 0.65 | 2.91 ± 0.78 | 0.607 |
| MIC of vancomycin for Enterococcus spp. mean ± SD, μg/mL | 1.27 ± 0.71 | 0.90 ± 0.23 | 0.001 |
| Vancomycin trough level, mean ± SD, mg/L | 10.90 ± 4.61 | 23.42 ± 9.72 | 0.001 |
| Parameter * | Mean | S.D. | Median | Min | Max |
|---|---|---|---|---|---|
| VS1 (L) | 0.295907 | 0.195098 | 0.221463 | 0.044752 | 0.584645 |
| KS1 (h−1) | 0.004154 | 0.002980 | 0.003265 | 0.000843 | 0.009051 |
| KPC (h−1) | 0.556593 | 0.699659 | 0.392091 | 0.080472 | 3.047390 |
| KCP (h−1) | 1.366962 | 1.897641 | 0.898388 | 0.107569 | 7.998810 |
| KI (h−1) | 0.002043 | 0 | 0.002043 | 0.002043 | 0.002043 |
| Variable | AUC/MIC of <400 mg·h/L, n (%) | AUC/MIC of ≥400 mg·h/L, n (%) | Crude HR (95% CI) | p-Value | Adjusted HR * (95% CI) | p-Value |
|---|---|---|---|---|---|---|
| Efficacy | ||||||
| Clinical failure | 17 (22.97) | 37 (15.55) | 0.59 (0.32–1.04) | 0.072 | 0.50 (0.26–0.97) | 0.042 |
| Microbiological failure | 9 (12.16) | 16 (6.72) | 0.44 (0.20–0.99) | 0.050 | 0.37 (0.14–0.94) | 0.036 |
| Safety | ||||||
| Nephrotoxicity | 3 (3.85) | 35 (14.96) | 3.34 (1.02–10.91) | 0.046 | 3.96 (1.09–14.47) | 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katip, W.; Oberdorfer, P. A Monocentric Retrospective Study of AUC/MIC Ratio of Vancomycin Associated with Clinical Outcomes and Nephrotoxicity in Patients with Enterococcal Infections. Pharmaceutics 2021, 13, 1378. https://doi.org/10.3390/pharmaceutics13091378
Katip W, Oberdorfer P. A Monocentric Retrospective Study of AUC/MIC Ratio of Vancomycin Associated with Clinical Outcomes and Nephrotoxicity in Patients with Enterococcal Infections. Pharmaceutics. 2021; 13(9):1378. https://doi.org/10.3390/pharmaceutics13091378
Chicago/Turabian StyleKatip, Wasan, and Peninnah Oberdorfer. 2021. "A Monocentric Retrospective Study of AUC/MIC Ratio of Vancomycin Associated with Clinical Outcomes and Nephrotoxicity in Patients with Enterococcal Infections" Pharmaceutics 13, no. 9: 1378. https://doi.org/10.3390/pharmaceutics13091378
APA StyleKatip, W., & Oberdorfer, P. (2021). A Monocentric Retrospective Study of AUC/MIC Ratio of Vancomycin Associated with Clinical Outcomes and Nephrotoxicity in Patients with Enterococcal Infections. Pharmaceutics, 13(9), 1378. https://doi.org/10.3390/pharmaceutics13091378






