Evaluation of the Pharmacokinetics of Intranasal Drug Delivery for Targeting Cervical Lymph Nodes in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Intravenous and Intranasal Drug Administration in Rats
2.3. Assay
2.4. Calculation of the Area under the Concentration–Time Curve (AUC) and Mean Resistance Time (MRT)
2.5. Calculation of Drug Targeting Index (DTI) and Direct Transport Percentage (DTP)
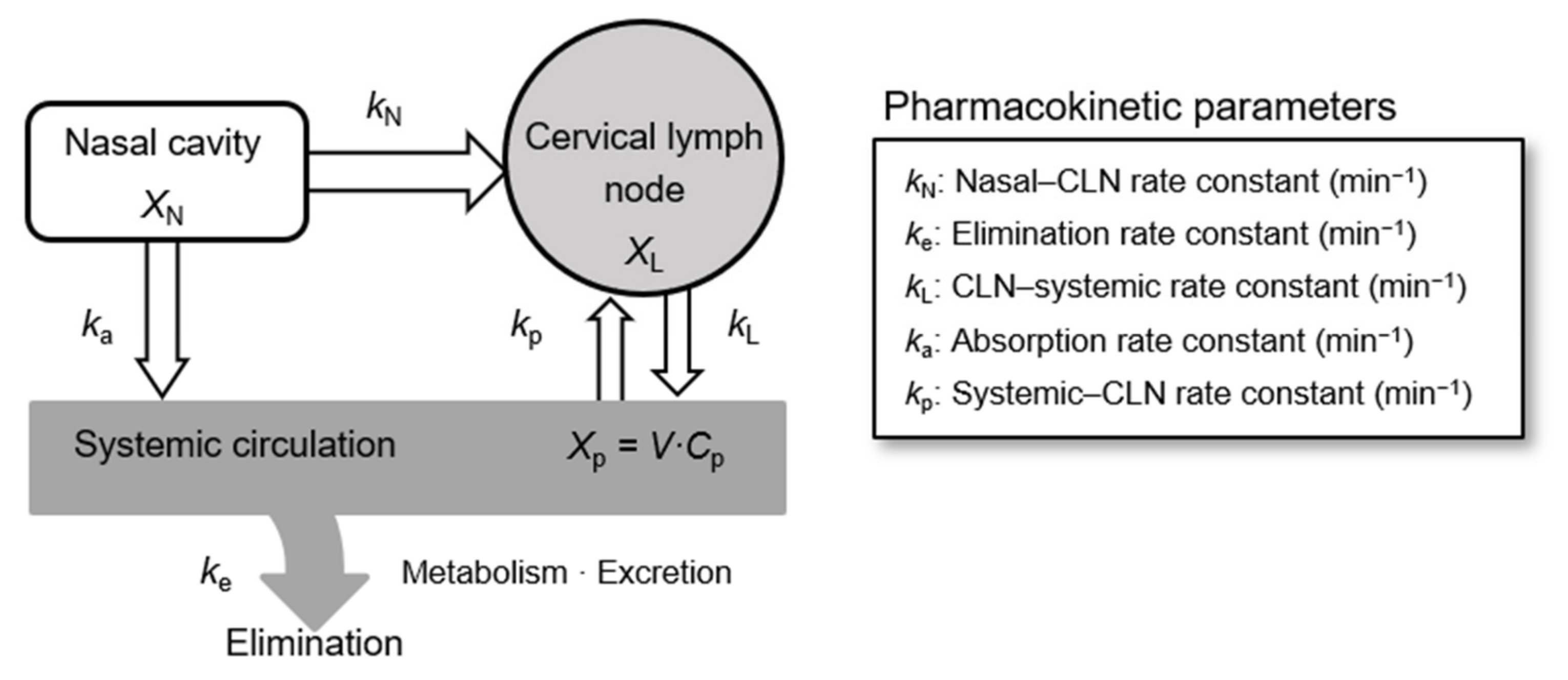
2.6. Analysis of Pharmacokinetic Parameters
2.7. Statistical Analysis
3. Results and Discussion
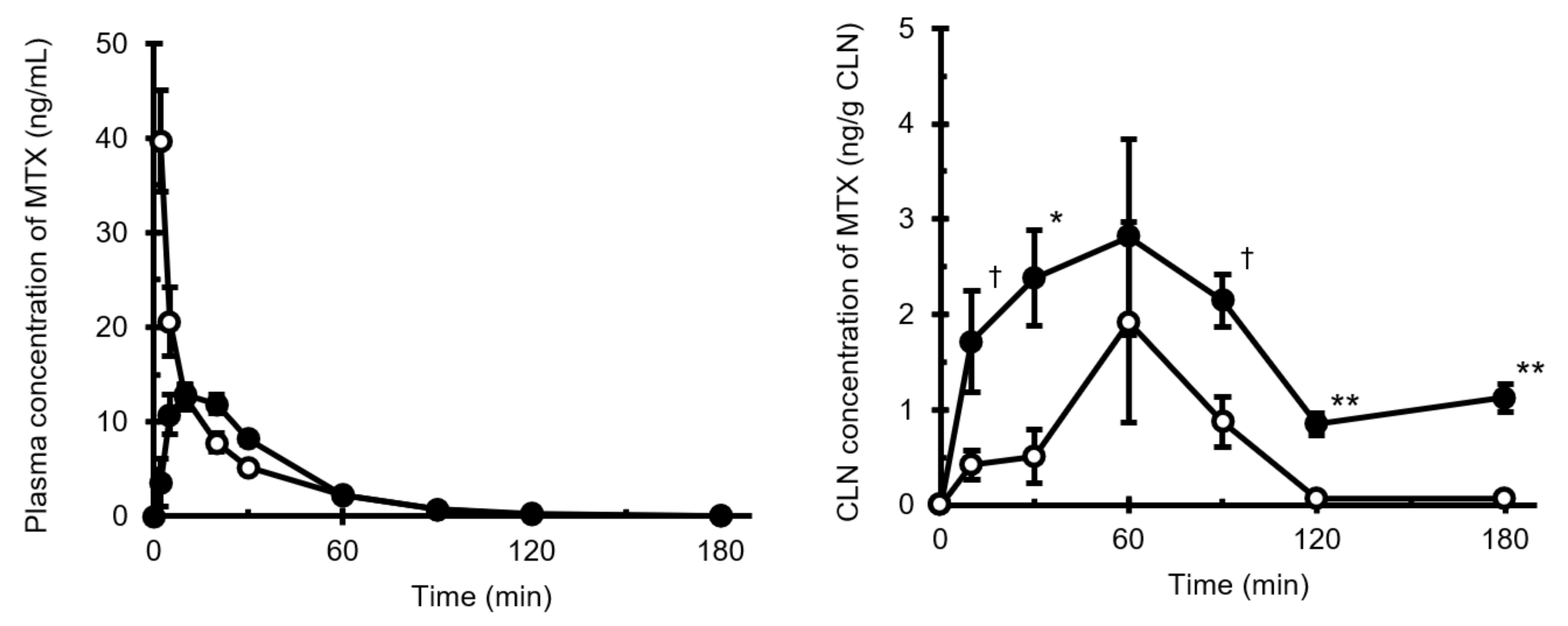
3.1. Intravenous and Intranasal Administration of MTX in Rats
3.2. Analysis of Pharmacokinetic Parameters
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tirucherai, G.S.; Yang, C.; Mitra, A.K. Prodrugs in nasal drug delivery. Expert Opin. Biol. Ther. 2001, 1, 49–66. [Google Scholar] [CrossRef]
- Illum, L. Nasal drug delivery-Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Pearson, R.G.; Masud, T.; Blackshaw, E.; Naylor, A.; Hinchcliffe, M.; Jeffery, K.; Jordan, F.; Shabir-Ahmed, A.; King, G.; Lewis, A.L.; et al. Nasal administration and plasma pharmacokinetics of parathyroid hormone peptide PTH 1-34 for the treatment of osteoporosis. Pharmaceutics 2019, 11, 265. [Google Scholar] [CrossRef]
- McMartin, C.; Hutchinson, L.E.F.; Hyde, R.; Peters, G.E. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J. Pharm. Sci. 1987, 76, 535–540. [Google Scholar] [CrossRef]
- Hosoya, K.; Kubo, H.; Natsume, H.; Sugibayashi, K.; Motrimoto, K.; Yamashita, S. The structural barrier of absorptive mucosae: Site difference of the permeability of fluorescein isothiocyanate-labelled dextran in rabbits. Biopharm. Drug Disposit. 1993, 14, 685–696. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.; Thakur, R.; Meidan, V.M.; Michniak, B. Mucosal drug delivery: Membranes, methodologies, and applications. Crit. Rev. Ther. Drug Carrier Syst. 2004, 21, 195–256. [Google Scholar] [CrossRef]
- Wu, H.Y.; Nguyen, H.H.; Russell, M.W. Nasal lymphoid tissue (NALT) as a mucosal immune inductive site. Scand. J. Immunol. 1997, 46, 506–513. [Google Scholar] [CrossRef]
- Ainai, A.; van Riet, E.; Ito, R.; Ikeda, K.; Senchi, K.; Suzuki, T.; Tamura, S.I.; Asanuma, H.; Odagiri, T.; Tashiro, M.; et al. Human immune responses elicited by an intranasal inactivated H5 influenza vaccine. Microbiol. Immunol. 2020, 64, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Hanai, N.; Eba, J.; Mizusawa, J.; Asakage, T.; Homma, A.; Kiyota, N.; Fukuda, H.; Hayashi, R. Randomized phase III study to evaluate the value of omission of prophylactic neck dissection for stage I/ II tongue cancer: Japan Clinical Oncology Group study (JCOG1601, RESPOND). Jpn. J. Clin. Oncol. 2018, 48, 1105–1108. [Google Scholar] [CrossRef]
- Rossa, T.M.; Martineza, P.M.; Rennera, J.C.; Thorneab, R.G.; Hansona, L.R.; Frey, W.H., II. Intranasal administration of interferon beta bypasses the blood–brain barrier to target the central nervous system and cervical lymph nodes: A non-invasive treatment strategy for multiple sclerosis. J. Neuroimmunol. 2004, 151, 66–77. [Google Scholar] [CrossRef]
- Abolmaali, S.S.; Tamaddon, A.M.; Dinarvand, R. A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother. Pharmacol. 2013, 71, 1115–1130. [Google Scholar] [CrossRef]
- Purcell, W.T.; Ettinger, D.S. Novel antifolate drugs. Curr. Oncol. Rep. 2003, 5, 114–125. [Google Scholar] [CrossRef]
- Furubayashi, T.; Kamaguchi, A.; Kawaharada, K.; Masaoka, Y.; Kataoka, M.; Yamashita, S.; Higashi, Y.; Sakane, T. Evaluation of the contribution of the nasal cavity and gastrointestinal tract to drug absorption following nasal application to rats. Biol. Pharm. Bull. 2007, 30, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Jiang, X.; Lu, W. Profiles of methotrexate in blood and CSF following intranasal and intravenous administration to rats. Int. J. Pharm. 2003, 263, 1–7. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, X.; Jiang, W.; Lu, W.; Su, L.; Shi, Z. Preparation of nimodipine-loaded microemulsion for intranasal delivery and evaluation on the targeting efficiency to the brain. Int. J. Pharm. 2004, 275, 85–96. [Google Scholar] [CrossRef]
- Sudo, J.; Iwase, H.; Terui, J.; Hayashi, T.; Soyama, M. Higher dopamine level in lymph from the cervical lymph trunk than in plasma following intravenous bolus injection of L-dopa in rats. Biol. Pharm. Bull. 1995, 18, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Serralheiro, A.; Alves, G.; Fortuna, A.; Falcão, A. Direct nose-to brain delivery of lamotrigine following intranasal administration to mice. Int. J. Pharm. 2015, 490, 39–46. [Google Scholar] [CrossRef]
- Ballard, B.E. Biopharmaceutical considerations in subcutaneous and intramuscular drug administration. J. Pharm. Sci. 1968, 57, 357–378. [Google Scholar] [CrossRef]
- Kihara, T. Das extravaskulare sSaftbahnsystem. Okajimas Folia Anat. Jpn. 1956, 28, 601–621. [Google Scholar] [CrossRef][Green Version]
- Kim, T.H.; Lee, S.H.; Moon, J.H.; Lee, H.M.; Lee, S.H.; Jung, H.H. Distributional characteristics of lymphatic vessels in normal human nasal mucosa and sinus mucosa. Cell Tissue Res. 2007, 327, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.; Zakharov, A.; Papaiconomou, C.; Salmasi, G.; Armstrong, D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cereb. Fluid Res. 2004, 1, 2. [Google Scholar] [CrossRef]
- Furubayashi, T.; Kamaguchi, A.; Kawaharada, K.; Masaoka, Y.; Kataoka, M.; Yamashita, S.; Higashi, Y.; Sakane, T. Kinetic model to predict the absorption of nasally applied drugs from in vitro transcellular permeability of drugs. Biol. Pharm. Bull. 2007, 30, 1007–1010. [Google Scholar] [CrossRef] [PubMed]


| i.v. | i.n. | |
|---|---|---|
| AUCplsm (ng·min/mL) | 554.9 | 536.8 |
| AUCCLN (ng·min/g CLN) | 129.9 | 491.0 |
| F (%) | 96.7 | |
| MRTplsm (min) | 24.7 | 31.1 |
| MRTCLN (min) | 56.6 | 146.1 |
| DTI | 3.78 | |
| DTP (%) | 74.3 |
| Rate Constants | min−1 |
|---|---|
| ke | 0.0740 ± 0.0208 |
| ka | 0.1014 ± 0.0211 |
| kN | 0.0047 ± 0.0013 |
| kL | 0.0095 ± 0.0039 |
| kp | 0.0021 ± 0.0009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furubayashi, T.; Inoue, D.; Kimura, S.; Tanaka, A.; Sakane, T. Evaluation of the Pharmacokinetics of Intranasal Drug Delivery for Targeting Cervical Lymph Nodes in Rats. Pharmaceutics 2021, 13, 1363. https://doi.org/10.3390/pharmaceutics13091363
Furubayashi T, Inoue D, Kimura S, Tanaka A, Sakane T. Evaluation of the Pharmacokinetics of Intranasal Drug Delivery for Targeting Cervical Lymph Nodes in Rats. Pharmaceutics. 2021; 13(9):1363. https://doi.org/10.3390/pharmaceutics13091363
Chicago/Turabian StyleFurubayashi, Tomoyuki, Daisuke Inoue, Shunsuke Kimura, Akiko Tanaka, and Toshiyasu Sakane. 2021. "Evaluation of the Pharmacokinetics of Intranasal Drug Delivery for Targeting Cervical Lymph Nodes in Rats" Pharmaceutics 13, no. 9: 1363. https://doi.org/10.3390/pharmaceutics13091363
APA StyleFurubayashi, T., Inoue, D., Kimura, S., Tanaka, A., & Sakane, T. (2021). Evaluation of the Pharmacokinetics of Intranasal Drug Delivery for Targeting Cervical Lymph Nodes in Rats. Pharmaceutics, 13(9), 1363. https://doi.org/10.3390/pharmaceutics13091363








