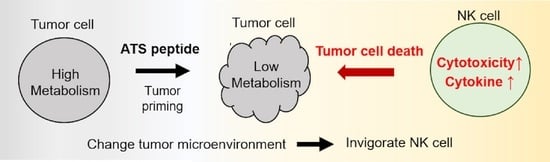
Peptide Adjuvant to Invigorate Cytolytic Activity of NK Cells in an Obese Mouse Cancer Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Culture Media, and Peptide Preparation
2.2. Assays of In Vitro Cytotoxic Effects of Peptide and NK Cells on Cancer Cells
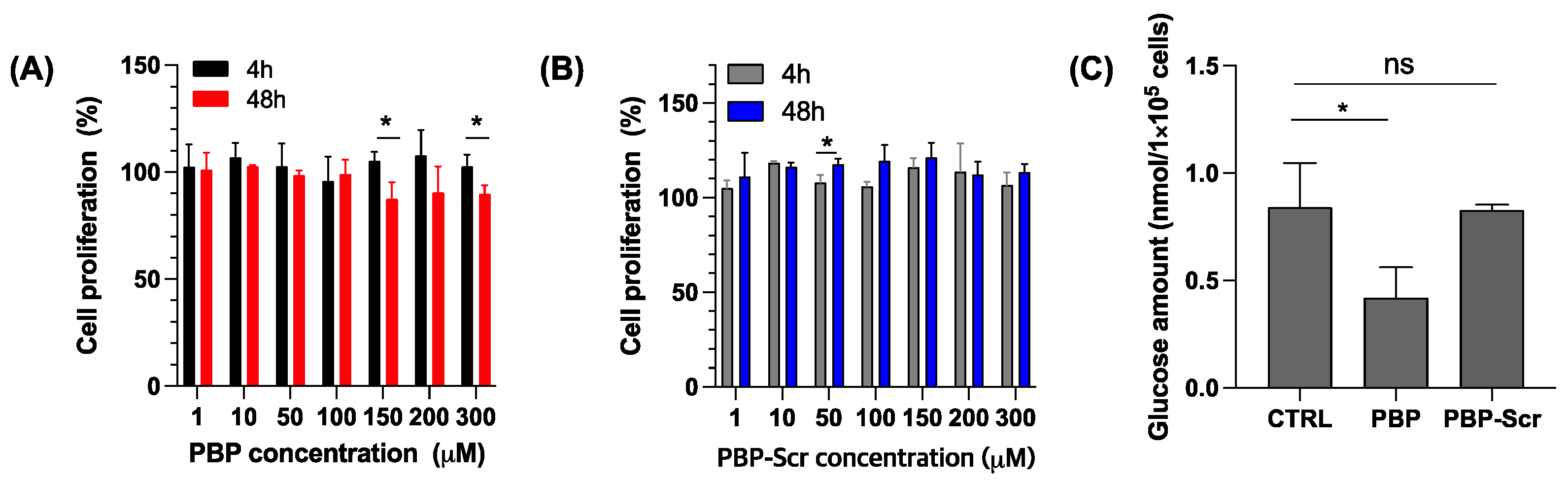
2.3. Confirmation of Glucose Metabolism Inhibition by PBP
2.4. Animal Model
2.5. In Vivo Cancer Model and Measures of Treatment Efficacy
2.6. Ex Vivo Tissue Analysis: Cytokine and Phenotype of NK Cell
2.7. Statistical Analysis
3. Results
3.1. PBP Priming Effect on Cancer Cells
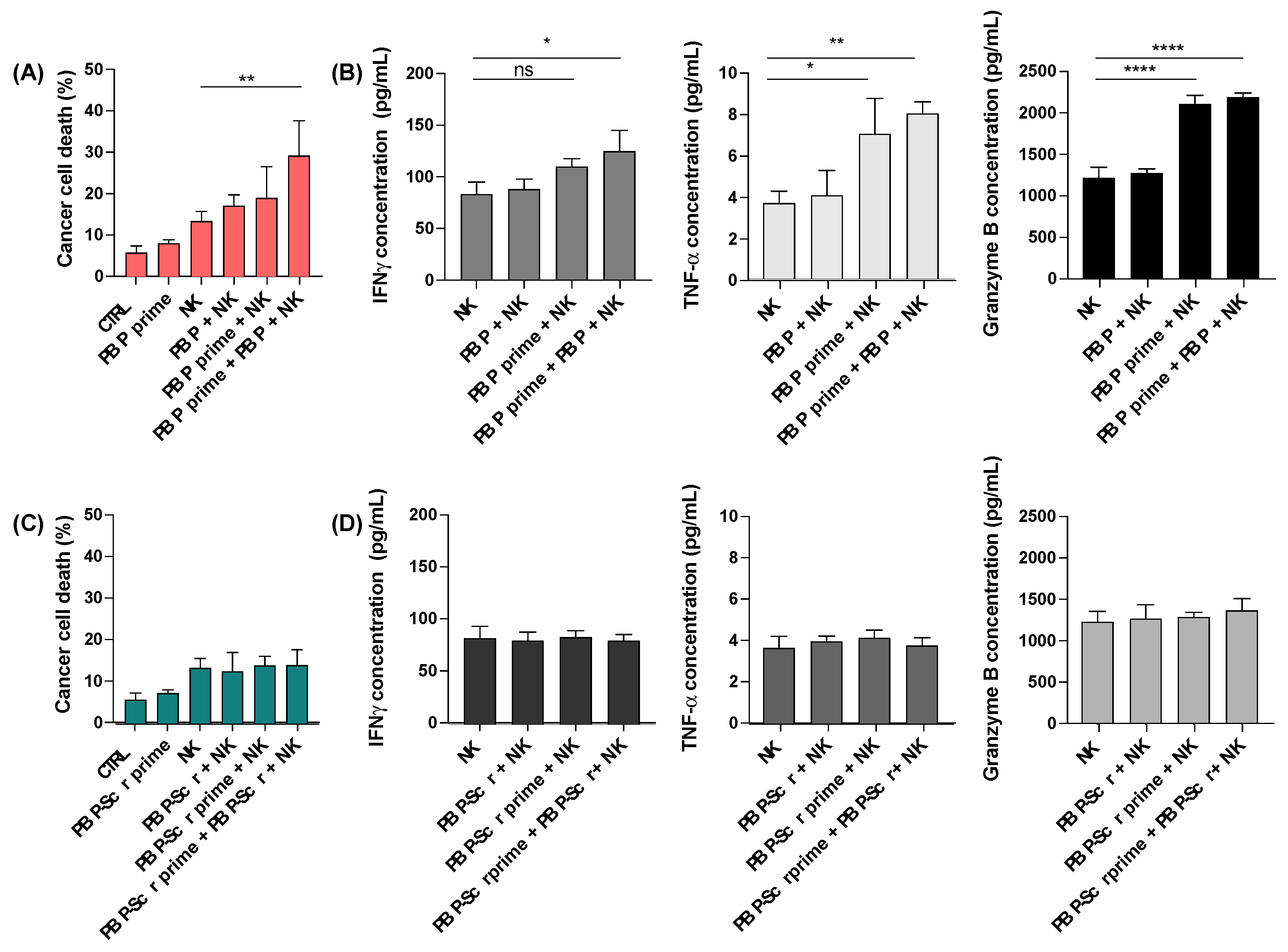
3.2. In Vitro Tumor Priming with PBP Enhances Anti-Cancer Activity of NK Cells
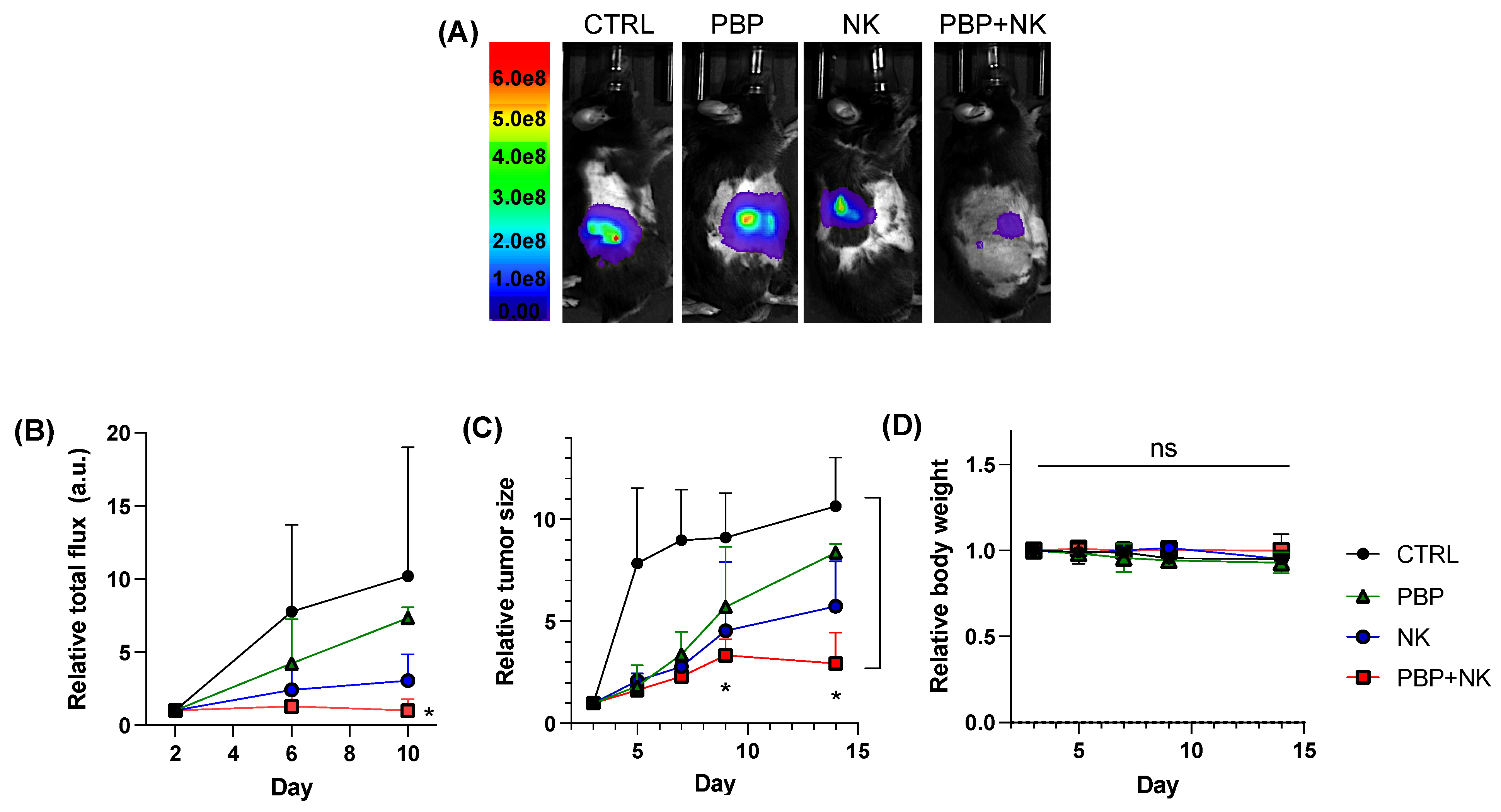
3.3. PBP-Priming Enhances NK Cell-Mediated Inhibition of Tumor Growth
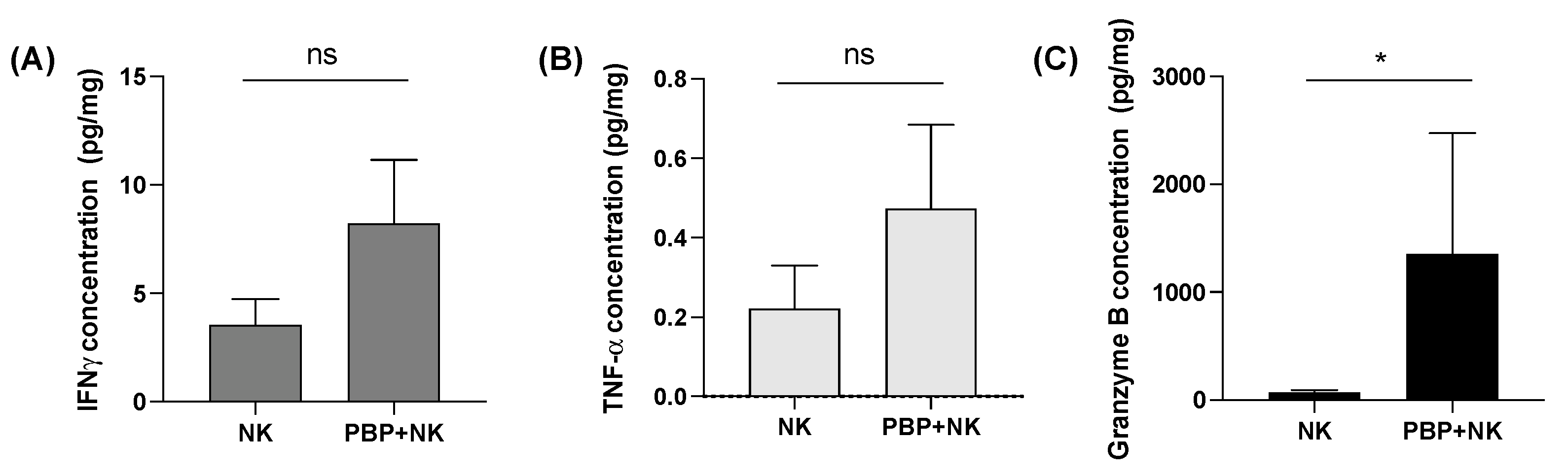
3.4. Tumor Priming with PBP Enhances In Vivo Cytokine Secretion by NK Cells
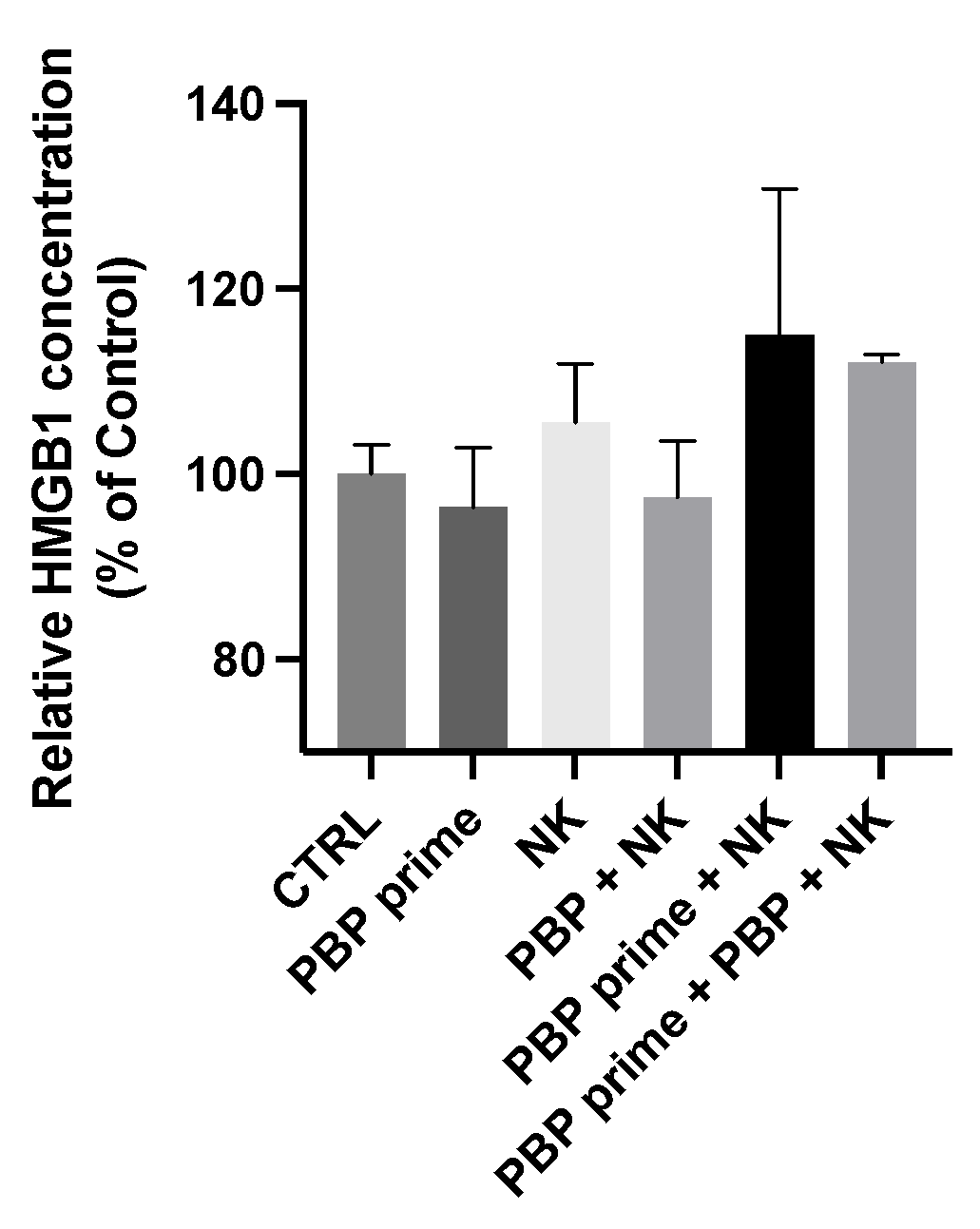
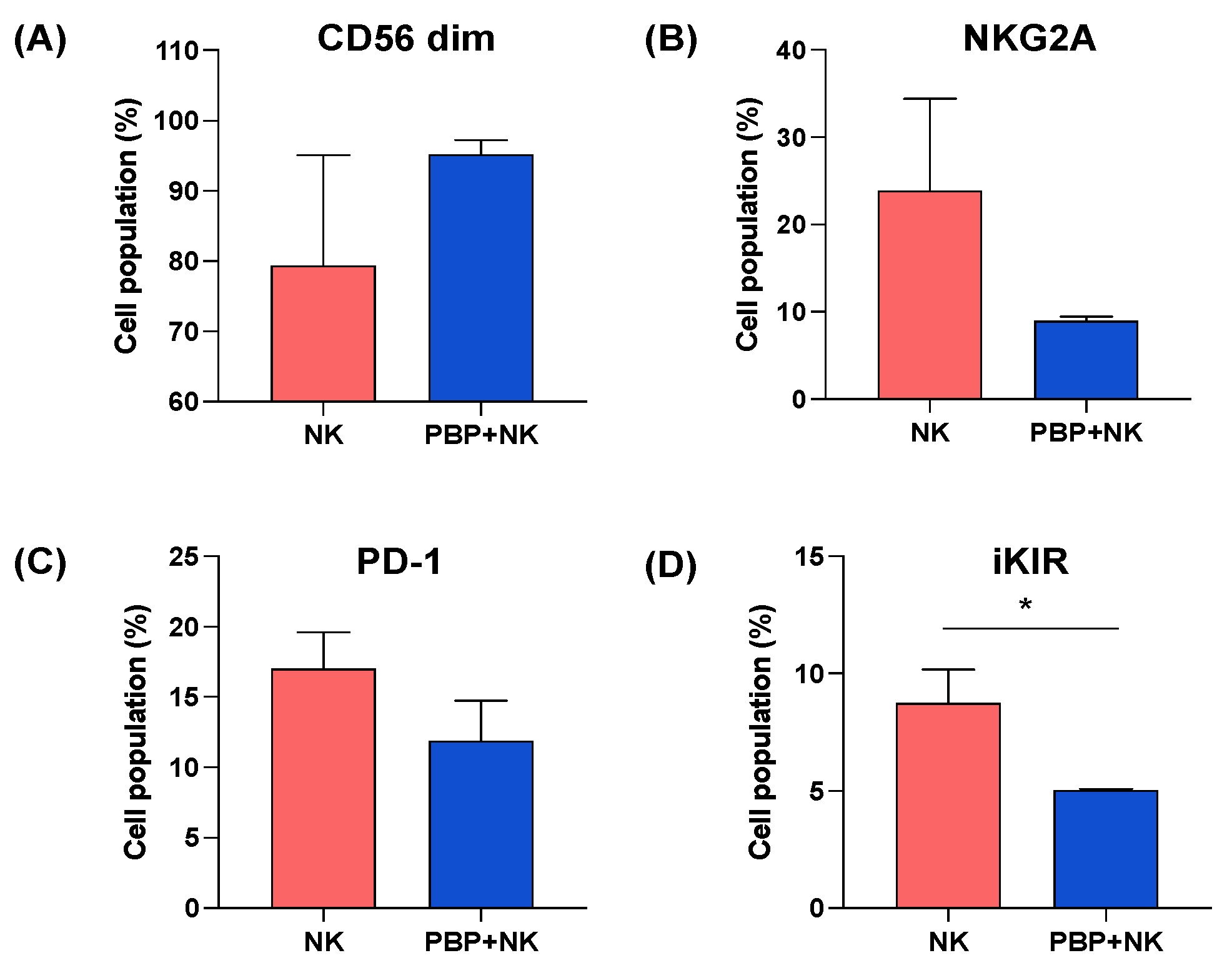
3.5. NK Cells Change Phenotype after Injection into In Vivo PBP-Primed Tumors
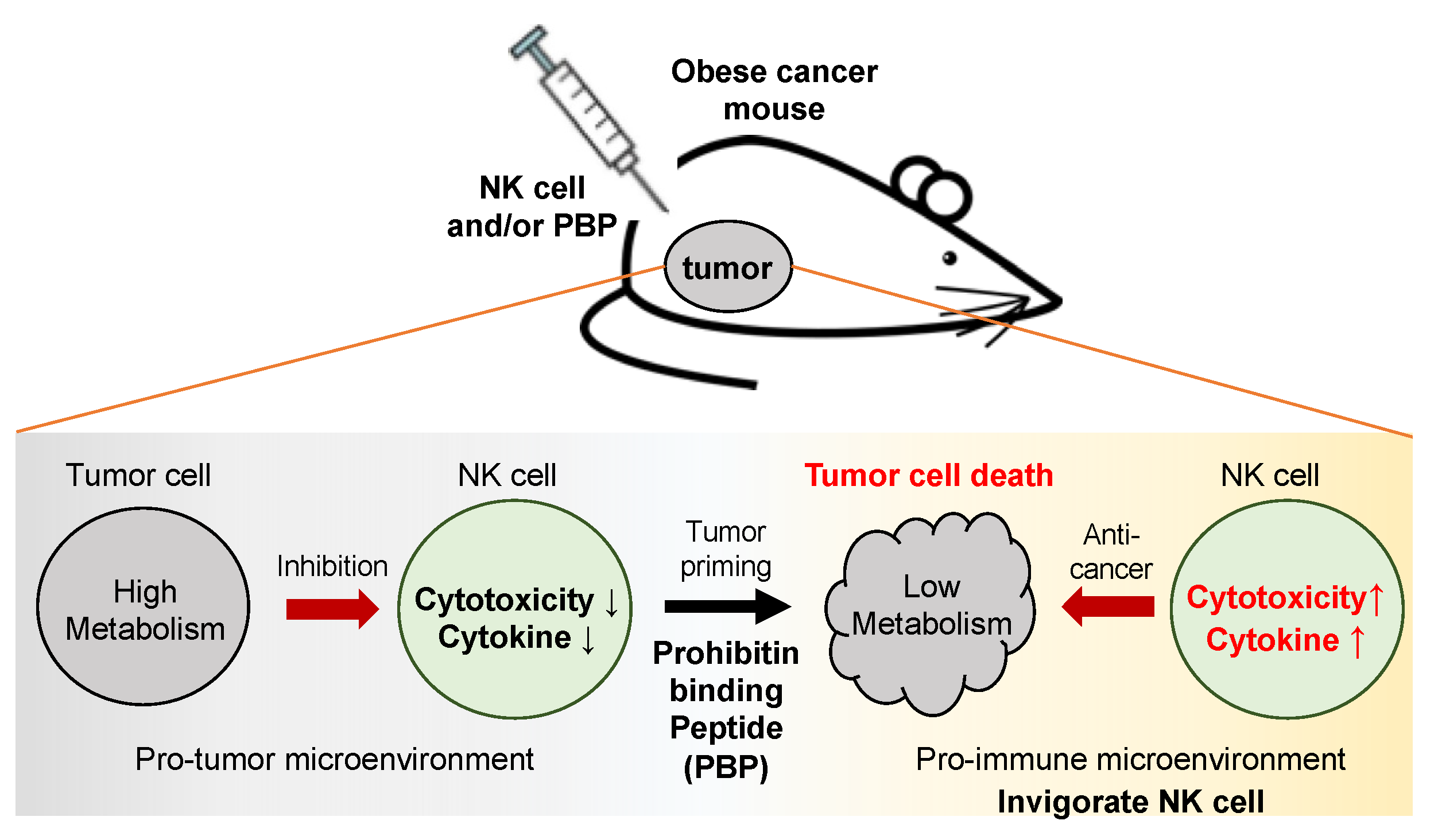
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Michelet, X.; Dyck, L.; Hogan, A.; Loftus, R.M.; Duquette, D.; Wei, K.; Beyaz, S.; Tavakkoli, A.; Foley, C.; Donnelly, R.; et al. Metabolic reprogramming of natural killer cells in obesity limits antitumor responses. Nat. Immunol. 2018, 19, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Ina Bähr, J.S. Dagmar Quandt, and Heike Kielstein. Obesity-Associated Alterations of Natural Killer Cells and Immunosurveillance of Cancer. Front. Immunol. 2020, 11, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tobin, L.M.; Mavinkurve, M.; Carolan, E.; Kinlen, D.; O’Brien, E.C.; Little, M.A.; Finlay, D.K.; Cody, D.; Hogan, A.E.; O’Shea, D. NK cells in childhood obesity are activated, metabolically stressed, and functionally deficient. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Bi, J.; Tian, Z. NK cell Exhaustion. Front. Immunol. 2017, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Doerstling, S.S.; O’Flanagan, C.H.; Hursting, S.D. Obesity and Cancer Metabolism: A Perspective on Interacting Tumor–Intrinsic and Extrinsic Factors. Front. Oncol. 2017, 7, 216. [Google Scholar] [CrossRef]
- Booth, A.; Magnuson, A.; Fouts, J.; Foster, M. Adipose tissue, obesity and adipokines: Role in cancer promotion. Horm. Mol. Biol. Clin. Investig. 2015, 21, 57–74. [Google Scholar] [CrossRef]
- Lee, C.; Woo, Y.; Wang, Y.; Yeung, C.; Xu, A.; Lam, K. Obesity, adipokines and cancer: An update. Clin. Endocrinol. 2015, 83, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Terrén, I.; Orrantia, A.; Vitallé, J.; Zenarruzabeitia, O.; Borrego, F. NK Cell Metabolism and Tumor Microenvironment. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Kouidhi, S.; Ben Ayed, F.; Benammar Elgaaied, A. Targeting Tumor Metabolism: A New Challenge to Improve Immunotherapy. Front. Immunol. 2018, 9, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naik, A.M.A.; Decock, J. The Obesity Paradox in Cancer, Tumor Immunology, and Immunotherapy: Potential Therapeutic Implications in Triple Negative Breast Cancer. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Pal, M.; Schwab, L.; Yermakova, A.; Mace, E.M.; Claus, R.; Krahl, A.C.; Woiterski, J.; Hartwig, U.F.; Orange, J.S.; Handgretinger, R.; et al. Tumor-priming converts NK cells to memory-like NK cells. Oncoimmunology 2017, 6, e1317411. [Google Scholar] [CrossRef] [Green Version]
- Moulin, C.M.; Marguti, I.; Peron, J.P.; Halpern, A.; Rizzo, L.V. Bariatric surgery reverses natural killer (NK) cell activity and NK-related cytokine synthesis impairment induced by morbid obesity. Obes. Surg. 2011, 21, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Jahn, J.; Spielau, M.; Brandsch, C.; Stangl, G.I.; Delank, K.-S.; Bähr, I.; Berreis, T.; Wrann, C.D.; Kielstein, H. Decreased NK cell functions in obesity can be reactivated by fat mass reduction. Obesity 2015, 23, 2233–2241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signorile, A.; Sgaramella, G.; Bellomo, F.; De Rasmo, D. Prohibitins: A Critical Role in Mitochondrial Functions and Implication in Diseases. Cells 2019, 8, 71. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Li, B.; He, Q.Y. Significance of prohibitin domain family in tumorigenesis and its implication in cancer diagnosis and treatment. Cell Death Dis. 2018, 9, 580. [Google Scholar] [CrossRef]
- Mishra, S.; Murphy, L.C.; Nyomba, B.L.; Murphy, L.J. Prohibitin: A potential target for new therapeutics. Trends Mol. Med. 2005, 11, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Chatterjee, S.K.; Vrbanac, V.; Chung, I.; Mu, C.J.; Olsen, R.R.; Waghorne, C.; Zetter, B.R. Rescue of paclitaxel sensitivity by repression of Prohibitin1 in drug-resistant cancer cells. Proc. Natl. Acad. Sci. USA 2010, 107, 2503–2508. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, Y.; Kajimoto, K.; Harashima, H. Anti-angiogenic nanotherapy via active targeting systems to tumors and adipose tissue vasculature. Biomater. Sci. 2015, 3, 1253–1265. [Google Scholar] [CrossRef]
- Ande, S.R.; Nguyen, K.H.; Nyomba, B.L.G.; Mishra, S. Prohibitin in Adipose and Immune Functions. Trends Endocrinol. Metab. 2016, 27, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Ande, S.R.; Nguyen, K.H.; Gregoire Nyomba, B.L.; Mishra, S. Prohibitin-induced, obesity-associated insulin resistance and accompanying low-grade inflammation causes NASH and HCC. Sci. Rep. 2016, 6, 23608. [Google Scholar] [CrossRef] [PubMed]
- Ande, S.R.; Nguyen, K.H.; Padilla-Meier, G.P.; Nyomba, B.L.; Mishra, S. Expression of a mutant prohibitin from the aP2 gene promoter leads to obesity-linked tumor development in insulin resistance-dependent manner. Oncogene 2016, 35, 4459–4470. [Google Scholar] [CrossRef]
- Thuaud, F.; Ribeiro, N.; Nebigil, C.G.; Desaubry, L. Prohibitin ligands in cell death and survival: Mode of action and therapeutic potential. Chem. Biol. 2013, 20, 316–331. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Tabti, R.; Elderwish, S.; Abou-Hamdan, H.; Djehal, A.; Yu, P.; Yurugi, H.; Rajalingam, K.; Nebigil, C.G.; Desaubry, L. Prohibitin ligands: A growing armamentarium to tackle cancers, osteoporosis, inflammatory, cardiac and neurological diseases. Cell Mol. Life Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kolonin, M.; Saha, P.; Chan, L. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004, 10, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Salameh, A.; Daquinag, A.C.; Staquicini, D.I.; An, Z.; Hajjar, K.A.; Pasqualini, R.; Arap, W.; Kolonin, M.G. Prohibitin/annexin 2 interaction regulates fatty acid transport in adipose tissue. JCI Insight 2016, 1, e86351. [Google Scholar] [CrossRef]
- Won, Y.W.; Adhikary, P.P.; Lim, K.S.; Kim, H.J.; Kim, J.K.; Kim, Y.H. Oligopeptide complex for targeted non-viral gene delivery to adipocytes. Nat. Mater. 2014, 13, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Xu, X.; Zhang, X.Q.; Farokhzad, O.C.; Langer, R. Preventing diet-induced obesity using nanoparticle. Proc. Natl. Acad. Sci. USA 2016, 113, 5552–5557. [Google Scholar] [CrossRef] [Green Version]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT Pathway in Insulin-Mediated Glucose Uptake. Blood Glucose Levels 2018, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Djehal, A.; Krayem, M.; Najem, A.; Hammoud, H.; Cresteil, T.; Nebigil, C.G.; Wang, D.; Yu, P.; Bentouhami, E.; Ghanem, G.E.; et al. Targeting prohibitin with small molecules to promote melanogenesis and apoptosis in melanoma cells. Eur. J. Med. Chem. 2018, 155, 880–888. [Google Scholar] [CrossRef]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef] [Green Version]
- Reefman, E.; Kay, J.G.; Wood, S.M.; Offenhäuser, C.; Brown, D.L.; Roy, S.; Stanley, A.C.; Low, P.C.; Manderson, A.P.; Stow, J.L. Cytokine Secretion Is Distinct from Secretion of Cytotoxic Granules in NK Cells. J. Immunol. 2010, 184, 4852–4862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jochems, C.; Hodge, J.W.; Fantini, M.; Fujii, R.; Maurice, Y.M. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget 2016, 7, 86359–86373. [Google Scholar] [CrossRef] [Green Version]
- Schmitz, K.H.; Neuhouser, M.L.; Agurs-Collins, T.; Zanetti, K.A.; Cadmus-Bertram, L.; Dean, L.T.; Drake, B.F. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J. Natl. Cancer Inst. 2013, 105, 1344–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [Green Version]
- Gismondi, A.; Stabile, H.; Nisti, P.; Santoni, A. Effector Functions of Natural Killer Cell Subsets in the Control of Hematological Malignancies. Front. Immunol. 2015, 6, 567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherif, S.; Farag, M.A.C. Human natural killer cell development and biology. Blood Rev. 2006, 20. [Google Scholar] [CrossRef]
- Lünemann, A.; Lünemann, J.D.; Münz, C. Regulatory NK-Cell Functions in Inflammation and Autoimmunity. Mol. Med. 2009, 15, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Penack, O.; Gentilini, C.; Fischer, L.; Asemissen, A.M.; Scheibenbogen, C.; Thiel, E.; Uharek, L. CD56dimCD16neg cells are responsible for natural cytotoxicity against tumor targets. Leukemia 2005, 19, 835–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.H.; Cho, H. Attenuated anti-tumor activity of NK-92 cells by invasive human breast carcinoma MDA-MB-231 cells. Mol. Cell. Toxicol. 2020, 16, 139–147. [Google Scholar] [CrossRef]
- Kamiya, T.; Seow, S.V.; Wong, D.; Robinson, M.; Campana, D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J. Clin. Investig. 2019, 129, 2094–2106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- André, P.; Denis, C.; Soulas, C.; Bourbon-Caillet, C.; Lopez, J.; Arnoux, T.; Bléry, M.; Bonnafous, C.; Gauthier, L.; Morel, A.; et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing both T and NK Cells. Cell 2018, 175, 1731–1743. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Wang, X.M.; Li, S.R.; Twelkmeyer, T.; Wang, W.H.; Zhang, S.Y.; Wang, S.F.; Chen, J.Z.; Jin, X.; Wu, Y.Z.; et al. NKG2A is a NK cell exhaustion checkpoint for HCV persistence. Nat. Commun. 2019, 10, 1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiossone, L.; Vienne, M.; Kerdiles, Y.M.; Vivier, E. Natural killer cell immunotherapies against cancer: Checkpoint inhibitors and more. Semin. Immunol. 2017, 31, 55–63. [Google Scholar] [CrossRef]
- Bi, J.; Tian, Z. NK Cell Dysfunction and Checkpoint Immunotherapy. Front. Immunol. 2019, 10, 1999. [Google Scholar] [CrossRef] [PubMed]
- Ande, S.; Xu, Z.; Gu, Y. Prohibitin has an important role in adipocyte differentiation. Int. J. Obes. 2012, 36, 1236–1244. [Google Scholar] [CrossRef] [Green Version]
- Quail, D.F.; Dannenberg, A.J. The obese adipose tissue microenvironment in cancer development and progression. Nat. Rev. Endocrinol. 2019, 15, 139–154. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Jung, M.; Kim, A.S.; Lee, D.Y.; Cha, B.-H.; Putnam, C.W.; Lim, K.S.; Bull, D.A.; Won, Y.-W. Peptide Adjuvant to Invigorate Cytolytic Activity of NK Cells in an Obese Mouse Cancer Model. Pharmaceutics 2021, 13, 1279. https://doi.org/10.3390/pharmaceutics13081279
Han S, Jung M, Kim AS, Lee DY, Cha B-H, Putnam CW, Lim KS, Bull DA, Won Y-W. Peptide Adjuvant to Invigorate Cytolytic Activity of NK Cells in an Obese Mouse Cancer Model. Pharmaceutics. 2021; 13(8):1279. https://doi.org/10.3390/pharmaceutics13081279
Chicago/Turabian StyleHan, Seungmin, Minjin Jung, Angela S. Kim, Daniel Y. Lee, Byung-Hyun Cha, Charles W. Putnam, Kwang Suk Lim, David A. Bull, and Young-Wook Won. 2021. "Peptide Adjuvant to Invigorate Cytolytic Activity of NK Cells in an Obese Mouse Cancer Model" Pharmaceutics 13, no. 8: 1279. https://doi.org/10.3390/pharmaceutics13081279
APA StyleHan, S., Jung, M., Kim, A. S., Lee, D. Y., Cha, B.-H., Putnam, C. W., Lim, K. S., Bull, D. A., & Won, Y.-W. (2021). Peptide Adjuvant to Invigorate Cytolytic Activity of NK Cells in an Obese Mouse Cancer Model. Pharmaceutics, 13(8), 1279. https://doi.org/10.3390/pharmaceutics13081279