Evaluation of the Predictive Performance of Population Pharmacokinetic Models of Adalimumab in Patients with Inflammatory Bowel Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Design
2.3. Patients and Data Collection
2.4. Evaluation of Model Adequacy
2.5. Evaluation of Predictive Performance
2.6. Clinical Impact
2.7. Software
2.8. Ethical Considerations
2.8.1. Ethics Approval
2.8.2. Consent
3. Results
3.1. Literature Search
3.2. Patients
3.3. Evaluation of Model Adequacy
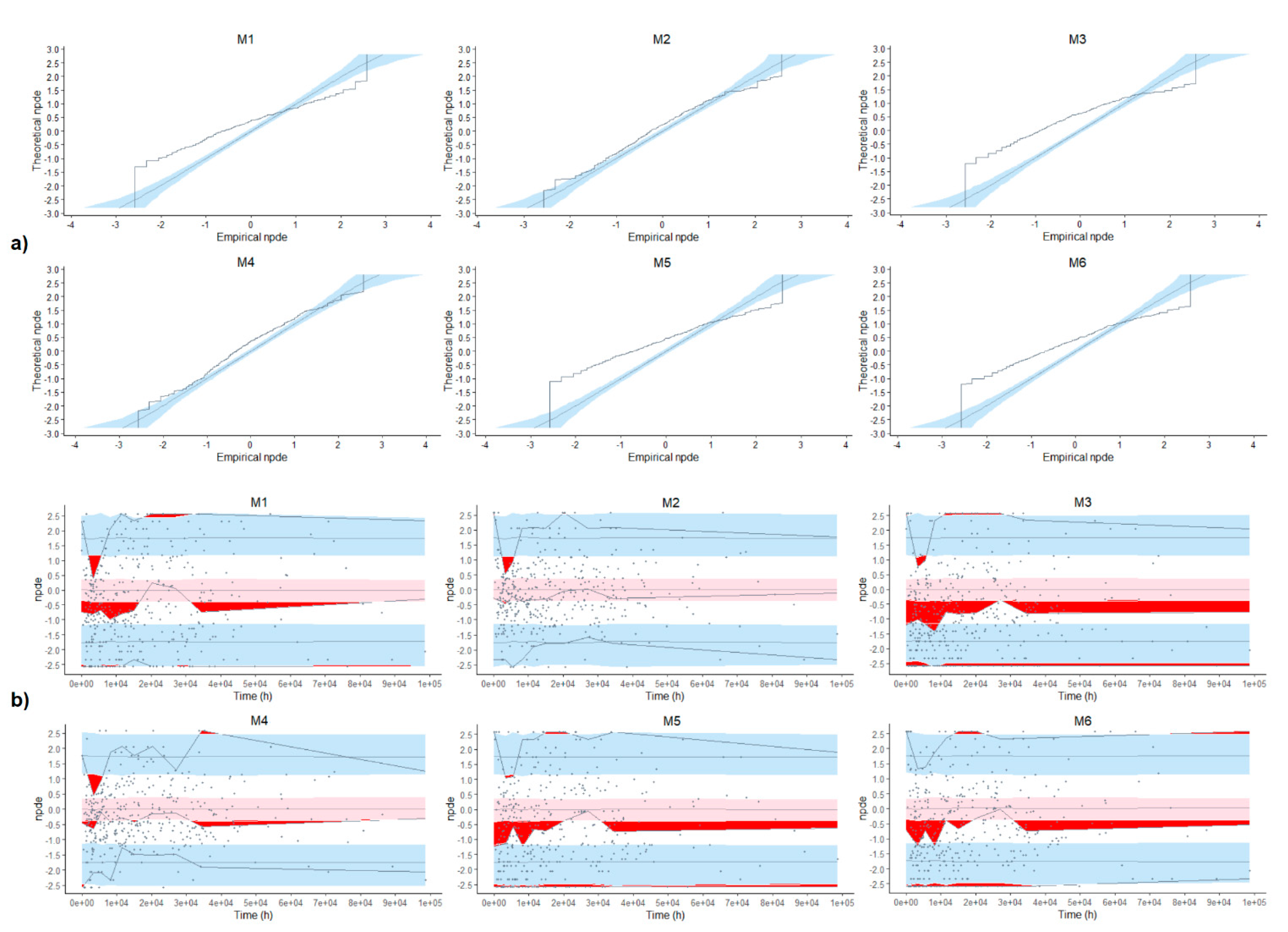
3.4. Evaluation of Predictive Performance
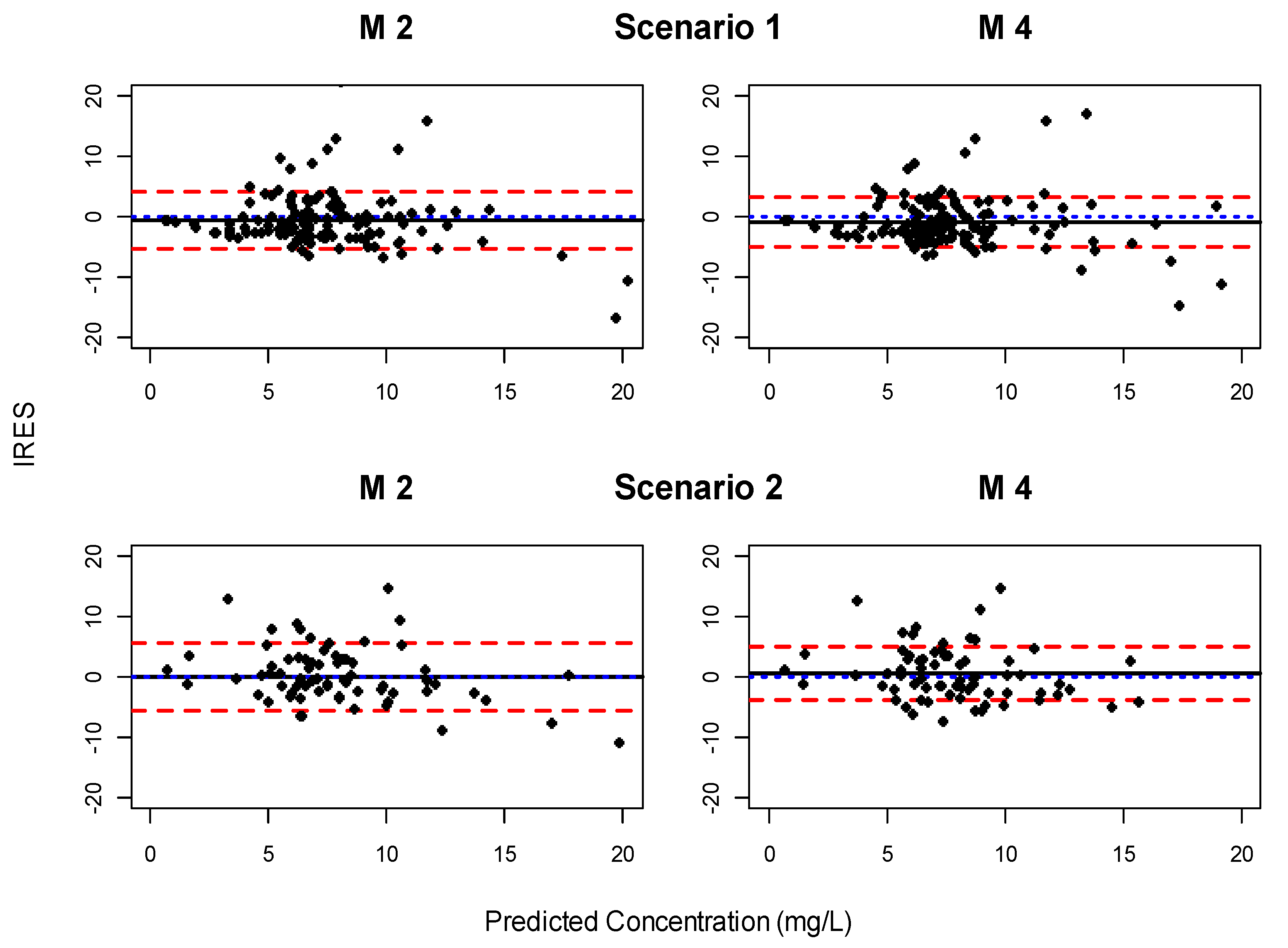
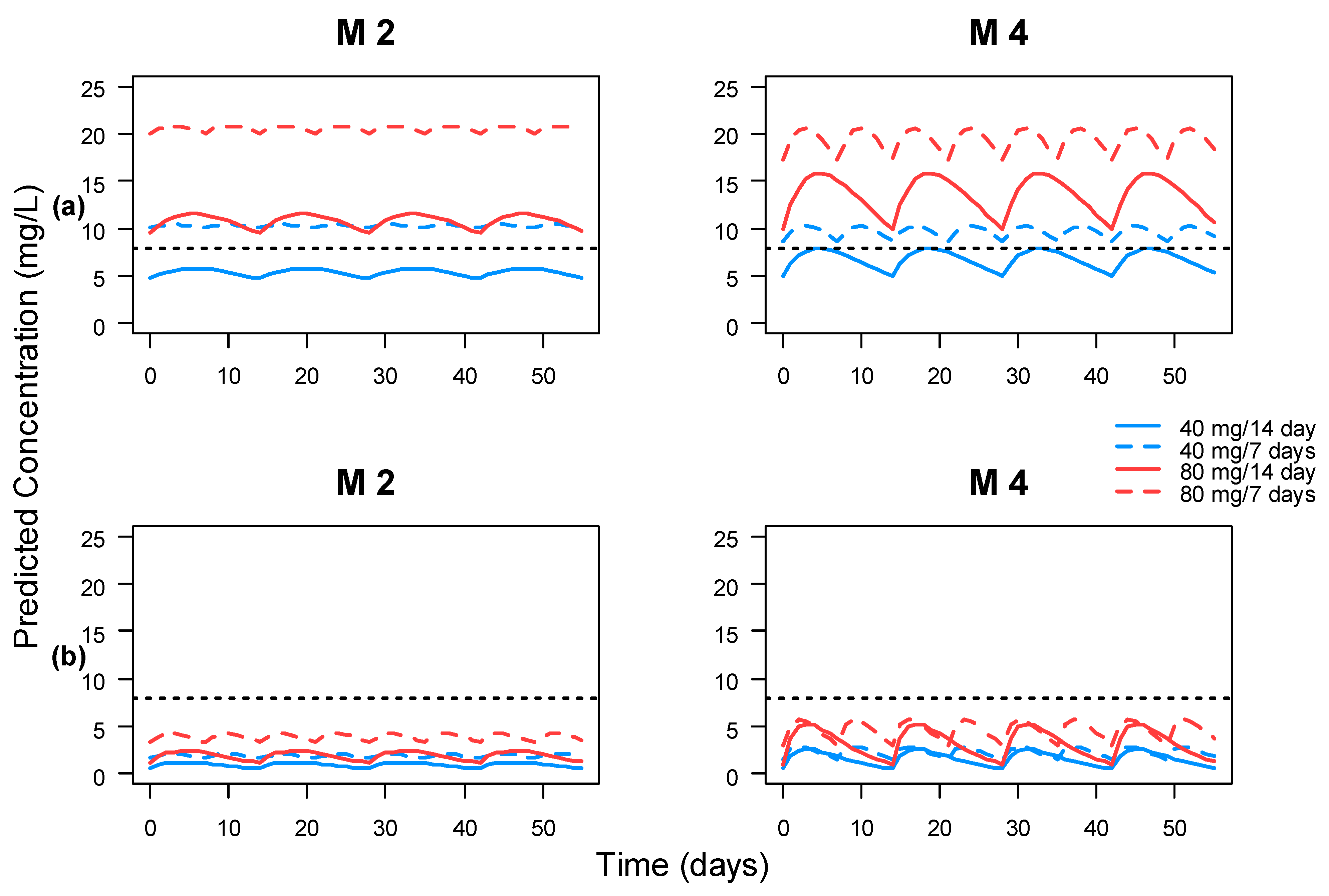
3.5. Clinical Impact
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomollón, F.; Dignass, A.; Annese, V.; Tilg, H.; Van Assche, G.; Lindsay, J.O.; Peyrin-Biroulet, L.; Cullen, G.J.; Daperno, M.; Kucharzik, T.; et al. 3rd European Evidence-based Consensus on the diagnosis and management of Crohn’s disease 2016: Part 1: Diagnosis and medical management. J. Crohn’s Colitis 2017, 11, 3–25. [Google Scholar] [CrossRef] [Green Version]
- Baumgart, D.C.; Sandborn, W.J. Inflammatory bowel disease: Clinical aspects and established and evolving therapies. Lancet 2007, 369, 1641–1657. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Eliakim, A.; Fedail, S.; Fried, M.; Gearry, R.; Goh, K.; Hamid, S.; Khan, A.G.; Khalif, I.; Ng, S.C.; et al. World Gastroenterology Organisation Global Guidelines inflammatory bowel disease. J. Clin. Gastroenterol. 2016, 50, 803–818. [Google Scholar] [CrossRef] [Green Version]
- Fakhoury, M.; Negrulj, R.; Mooranian, A.; Al-Salami, H. Inflammatory bowel disease: Clinical aspects and treatments. J. Inflamm. Res. 2014, 7, 113–120. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency (EMA). HUMIRA: Summary of Product Characteristics. EMA. 2021. Available online: https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf (accessed on 11 August 2021).
- Cohen, L.B.; Nanau, R.M.; Delzor, F.; Neuman, M.G. Biologic therapies in inflammatory bowel disease. Transl. Res. 2014, 163, 533–556. [Google Scholar] [CrossRef] [PubMed]
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.F. Loss of response to anti-TNFs: Definition, epidemiology, and management. Clin. Transl. Gastroenterol. 2016, 7, e135. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Huang, V.; Fedorak, D.K.; Kroeker, K.I.; Dieleman, L.A.; Halloran, B.P.; Fedorak, R.N. Adalimumab dose escalation is effective for managing secondary loss of response in Crohn’s disease. Aliment. Pharmacol. Ther. 2014, 40, 1044–1055. [Google Scholar] [CrossRef]
- Yanai, H.; Lichtenstein, L.; Assa, A.; Mazor, Y.; Weiss, B.; Levine, A.; Ron, Y.; Kopylov, U.; Bujanover, Y.; Rosenbach, Y.; et al. Levels of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin. Gastroenterol. Hepatol. 2015, 13, 522–530. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Gils, A. Pharmacokinetics of anti-TNF monoclonal antibodies in inflammatory bowel disease: Adding value to current practice. J. Clin. Pharmacol. 2015, 55, 39–50. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Gerardi, V.; Papa, V.; Scaldaferri, F.; Rapaccini, G.L.; Antonio Gasbarrini, A.; Papa, A. Can we predict the efficacy of anti-TNF-α agents? Int. J. Mol. Sci. 2017, 18, 1973. [Google Scholar] [CrossRef]
- Ding, N.S.; Hart, A.; De Cruz, P. Systematic review: Predicting and optimising response to anti-TNF therapy in Crohn’s disease—algorithm for practical management. Aliment. Pharmacol. Ther. 2016, 43, 30–51. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Gils, A.; Rutgeerts, P.; Levesque, B.G.; Vermeire, S.; Sandborn, W.J.; Vande Casteele, N. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD: Evolution in the definition and management of primary nonresponse. Inflamm. Bowel Dis. 2015, 21, 182–197. [Google Scholar] [CrossRef]
- Papamichael, K.; Vande Casteele, N.; Ferrante, M.; Gils, A.; Cheifetzl, A.S. Therapeutic drug monitoring during induction of antitumor necrosis factor therapy in inflammatory bowel disease: Defining a therapeutic drug window. Inflamm. Bowel Dis. 2017, 23, 1510–1515. [Google Scholar] [CrossRef] [Green Version]
- Papamichael, K.; Vogelzang, E.H.; Lambert, J.; Wolbink, G.; Cheifetz, A.S. Therapeutic drug monitoring with biologic agents in immune mediated inflammatory diseases. Expert Rev. Clin. Immunol. 2019, 15, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, K.; Cheifetz, A.S.; Melmed, G.Y.; Irving, P.M.; Vande Casteele, N.; Kozuch, P.L.; Raffals, L.E.; Baidoo, L.; Bressler, B.; Devlin, S.M.; et al. Appropriate Therapeutic Drug Monitoring of Biologic Agents for Patients With Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2019, 17, 1655–1668. [Google Scholar] [CrossRef] [Green Version]
- Darwich, A.S.; Ogungbenro, K.; Vinks, A.A.; Powell, J.R.; Reny, J.L.; Marsousi, N.; Daali, Y.; Fairman, D.; Cook, J.; Lesko, L.J.; et al. Why has model-informed precision dosing not yet become common clinical reality? Lessons from the past and a roadmap for the future. Clin. Pharmacol. Ther. 2017, 101, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Ungar, B.; Levy, I.; Yavne, Y.; Yavzori, M.; Picard, O.; Fudim, E.; Loebstein, R.; Chowers, Y.; Eliakim, R.; Kopylov, U.; et al. Optimizing anti-TNFα therapy: Serum levels of infliximab and adalimumab associate with mucosal healing in patients with inflammatory bowel diseases. Clin. Gastroenterol. Hepatol. 2015, 14, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Papamichael, K.; Juncadella, A.; Wong, D.; Rakowsky, S.; Sattler, L.A.; Campbell, J.P.; Vaughn, B.P.; Cheifetz, A.S. Proactive therapeutic drug monitoring of adalimumab is associated with better long-term outcomes compared to standard of care in patients with inflammatory bowel disease. J. Crohn’s Colitis 2019, 13, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Brendel, K.; Comets, E.; Laffont, C.; Laveille, C.; Mentre, F. Metrics for external model evaluation with an application to the population pharmacokinetics of gliclazide. Pharm. Res. 2006, 23, 2036–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brendel, K.; Comets, E.; Laffont, C.; Laveille, C.; Mentre, F. Evaluation of different tests based on observations for external model evaluation of population analyses. J. Pharmacokinet. Pharmacodyn. 2010, 37, 49–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verstockt, B.; Moors, G.; Bian, S.; Van Stappen, T.; Van Assche, G.; Vermeire, S.; Gils, A.; Ferrante, M. Influence of early adalimumab serum levels on immunogenicity and long-term outcome of anti-TNF naive Crohn’s disease patients: The usefulness of rapid testing. Aliment. Pharmacol. Ther. 2018, 48, 731–739. [Google Scholar] [CrossRef]
- Beal, S.L.; Sheiner, L.B.; Boeckmann, A.J.; Bauer, R.J. NONMEM 7.4.0 Users Guides. (1989–2013); ICON Development Solutions: Hanover, MD, USA, 2021; Available online: https://iconplc.com/innovation/nonmem/ (accessed on 10 May 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.R-project.org/ (accessed on 10 May 2021).
- RStudio. RStudio: Integrated Development Environment for R; RStudio: Boston, MA, USA, 2021; Available online: http://www.rstudio.org/ (accessed on 10 May 2021).
- Food and Drug Administration (FDA). HUMIRA: Clinical Pharmacology and Biopharmaceutics Review(s). FDA. 2008. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/bla/2007/125057_S0089.pdf (accessed on 11 August 2021).
- Ternant, D.; Karmiris, K.; Vermeire, S.; Desvignes, C.; Azzopardi, N.; Bejan-Angoulvant, T.; van Assche, G.; Paintaud, G. Pharmacokinetics of adalimumab in Crohn’s disease. Eur. J. Clin. Pharmacol. 2015, 71, 1155–1157. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Eckert, D.; Hyams, J.S.; Mensing, S.; Thakkar, R.B.; Robinson, A.M.; Rosh, J.R.; Ruemmele, F.M.; Awni, W.M. Pharmacokinetics and exposure-efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn’s disease: Results from a randomized, multicenter, phase-3 study Pharmacokinetics and exposure-efficacy relationship of adalimumab in pediatric patients with moderate to severe Crohn’s disease: Results from a randomized, multicenter, phase-3 study. Inflamm. Bowel Dis. 2015, 21, 783–792. [Google Scholar] [CrossRef]
- Berends, S.E.; Strik, A.S.; Van Selm, J.C.; Löwenberg, M.; Ponsioen, C.Y.; DʼHaens, G.R.; Mathôt, R.A. Explaining interpatient variability in adalimumab pharmacokinetics in patients with Crohn’s disease. Ther. Drug Monit. 2018, 40, 202–211. [Google Scholar] [CrossRef]
- Vande Casteele, N.; Baert, F.; Bian, S.; Dreesen, E.; Compernolle, G.; Van Assche, G.; Ferrante, M.; Vermeire, S.; Gils, A. Subcutaneous absorption contributes to observed interindividual variability in adalimumab serum concentrations in Crohn’s disease: A prospective multicentre study. J. Crohn’s Colitis 2019, 27, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, J.G.; Pérez-Blanco, J.S.; Rebollo, N.; Muñoz, F.; Prieto, V.; Calvo, M.V. Biomarkers of disease activity and other factors as predictors of adalimumab pharmacokinetics in inflammatory bowel disease. Eur. J. Pharm. Sci. 2020, 150, 105369. [Google Scholar] [CrossRef] [PubMed]
- Hemperly, A.; Vande Casteele, N. Clinical Pharmacokinetics and Pharmacodynamics of Infliximab in the Treatment of Inflammatory Bowel Disease. Clin. Pharmacokinet. 2018, 57, 929–942. [Google Scholar] [CrossRef]
- Brandse, J.F.; Mould, D.; Smeekes, O.; Ashruf, Y.; Kuin, S.; Strik, A.; van den Brink, G.R.; DʼHaens, G.R. A Real-life Population Pharmacokinetic Study Reveals Factors Associated with Clearance and Immunogenicity of Infliximab in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 650–660. [Google Scholar] [CrossRef] [Green Version]
- Juncadella, A.; Papamichael, K.; Vaughn, B.P.; Cheifetz, A.S. Maintenance adalimumab concentrations are associated with biochemical, endoscopic, and histologic remission in inflammatory bowel disease. Dig. Dis. Sci. 2018, 63, 3067–3073. [Google Scholar] [CrossRef]
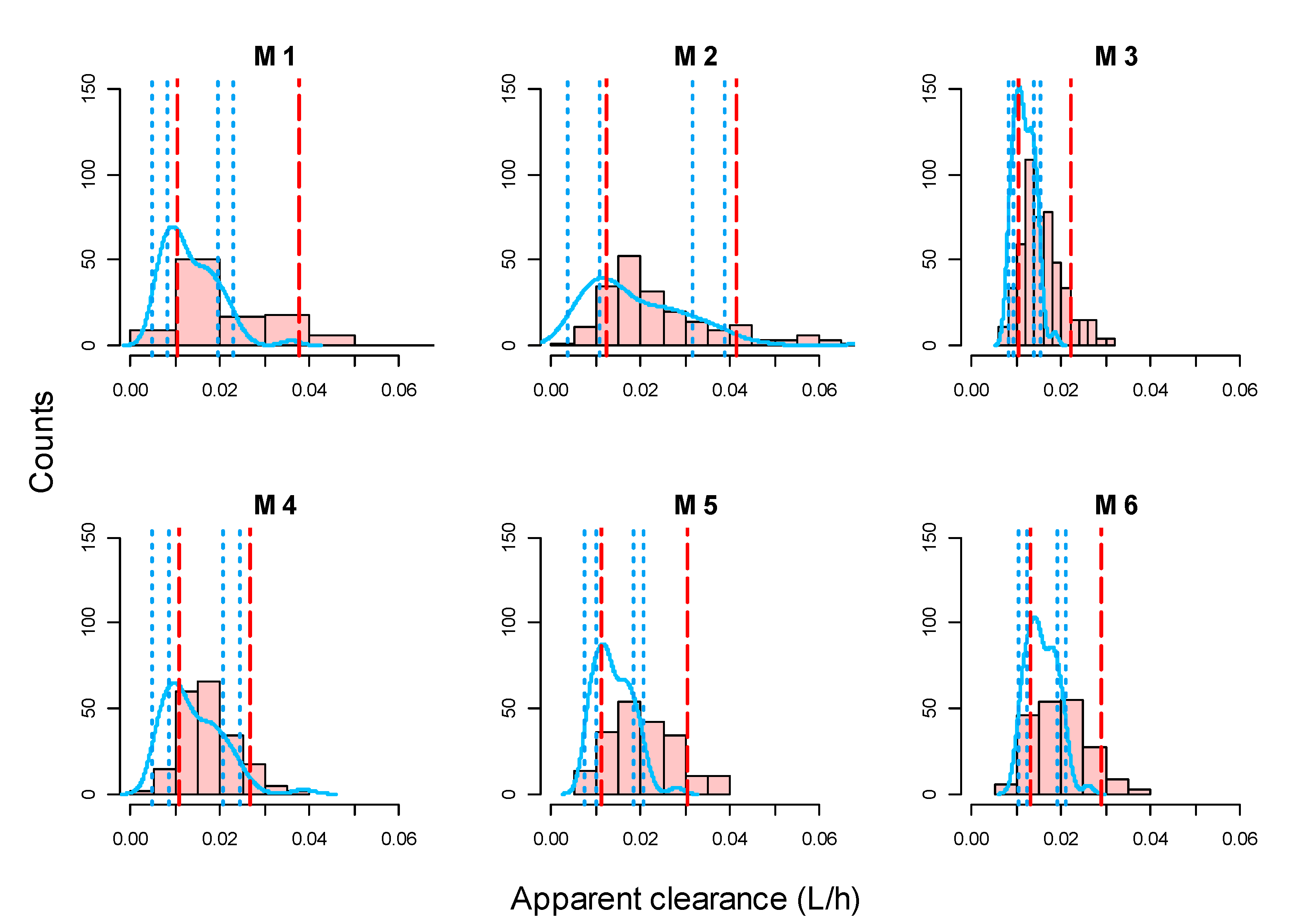
| Model No. | Study | No. of Patients (Total No. of Samples) | Parameter Values and Covariate Relationships Included | IIV (CV) | Residual Variability |
|---|---|---|---|---|---|
| M1 | FDA, 2008 | 646 adult patients (NA) | IIV-CL/F: 16.4% IIV-V/F: 35.1% | Prop = 31.6% | |
| M2 | Ternant D et al., 2015 | 65 adult CD patients (341) | IIV-CL/F: 65% IIV-V/F: 48% | Add = 1.8 mg/L Prop = 16% | |
| M3 | Sharma S et al., 2015 | 189 pediatric CD patients (852) | IIV-CL/F: 21.1% | Add = 1.9 mg/L Prop = 7.1% | |
| M4 | Berends SE et al., 2018 | 96 adult CD patients (181) | IIV-CL/F: 49.1% | Add = 1.02 mg/L Prop = 9% | |
| M5 | Vande Castelee et al., 2019 | 28 adult CD patients (185) | IIV-CL/F: 32.6% IIV-V/F: 35.6% IIV-ka: 103.9% | Prop = −16.6% | |
| M6 | Sánchez-Hernández et al., 2020 | 104 adult IBD patients (303) | IIV-CL/F: 23.2% | Prop = 21.7% |
| Model No. | Age (yr) | Weight (kg) | Disease (cd/uc) | Sex (m/f) | AAA Positive (%) | Albumin (g/dL) | Dosage Regimen | Measured Adalimumab Concentration | Measured AAA |
|---|---|---|---|---|---|---|---|---|---|
| M1 | NA | NA | NA | NA | NA | NA |
| ELISA | ELISA |
| M2 | 37 (17–61) | 68 (43–109) | 100/0 | 17/48 | 9 (13.8%) | NA |
| ELISA | Double-antigen ELISA |
| M3 | 13.6 (6–17) | 45.2 (18–119) | 100/0 | 105/84 | 83 (43.9%) | 4.0 (2.4–5.3) |
| Double-antigen ELISA | Bridging ELISA |
| M4 | 38 (32–44) | 65 (58–76) | 100/0 | 35/96 | 17 (18%) | 4.3 (4.05–4.5) |
| TNF ELISA | Antigen-binding test |
| M5 | 37 (30–49) | 66 (55–73) | 100/0 | 13/28 | 5 (17.9%) | 3.99 (3.6–4.4) |
| In-house developed TNF-coated ELISA | In-house developed drug resistant AAA assay |
| M6 | 43 (32–56) | 68 (56–80) | 84/20 | 58/46 | 0 | 4.5 (4.3–4.7) |
| ELISA | ELISA |
| Characteristics | Count (%)/Median (Percentile 25th–75th) | Missings, n (%) |
|---|---|---|
| Patients | 134 | 0 |
| Age (yr) | 45 (34–57) | 0 |
| Sex, male, n (%) | 70 (52.2%) | 0 |
| Weight (kg) | 66 (58–75) | 1 (0.75%) |
| Body mass index (kg/m2) | 23.85 (20.52–27.36) | 10 (7.46%) |
| Lean Body Weight (kg) | 46.84 (42.60–52.10) | 10 (7.46%) |
| Albumin (g/dL) | 3.84 (3.53–4.12) | 5 (3.73%) |
| CRP (mg/dL) | 0.64 (0.25–2.1) | 37 (27.61%) |
| FCP (mg/kg) | 487 (217.11–884.68) | 37 (27.61%) |
| IBD type, CD, n (%) | 115 (85.8%) | 0 |
| Concomitant immunomodulator, n (%) | ||
| Aminosalicylate | 7 (5.2%) | 0 |
| Methotrexate | 10 (7.5%) | 0 |
| Azathioprine | 53 (39.6%) | 0 |
| 6-Mercaptopurine | 6 (4.5%) | 0 |
| Corticosteroids | 16 (11.9%) | 0 |
| Combined | 14 (10.4%) | 0 |
| Adalimumab serum samples | 398 | 0 |
| Adalimumab serum concentrations (mg/L) | 6.75 (4.58–8.65) | 0 |
| AAA serum concentrations (mg/L) | 29 (4.53–76.30) | 0 |
| AAA positive, n (%) | 11 (8%) | 0 |
| Model | Scenario 1 | Scenario 2 | ||
|---|---|---|---|---|
| Bias | Precision | Bias | Precision | |
| M2 | −0.59 (−1.37:0.19) | 4.61 (3.55:5.67) | 0.012 (−1.27:1.29) | 5.43 (3.81:7.06) |
| M4 | −0.91 (−1.62:−0.19) | 4.30 (3.47:5.12) | 0.52 (−0.52:1.56) | 4.43 (3.49:5.37) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquez-Megias, S.; Ramon-Lopez, A.; Más-Serrano, P.; Diaz-Gonzalez, M.; Candela-Boix, M.R.; Nalda-Molina, R. Evaluation of the Predictive Performance of Population Pharmacokinetic Models of Adalimumab in Patients with Inflammatory Bowel Disease. Pharmaceutics 2021, 13, 1244. https://doi.org/10.3390/pharmaceutics13081244
Marquez-Megias S, Ramon-Lopez A, Más-Serrano P, Diaz-Gonzalez M, Candela-Boix MR, Nalda-Molina R. Evaluation of the Predictive Performance of Population Pharmacokinetic Models of Adalimumab in Patients with Inflammatory Bowel Disease. Pharmaceutics. 2021; 13(8):1244. https://doi.org/10.3390/pharmaceutics13081244
Chicago/Turabian StyleMarquez-Megias, Silvia, Amelia Ramon-Lopez, Patricio Más-Serrano, Marcos Diaz-Gonzalez, Maria Remedios Candela-Boix, and Ricardo Nalda-Molina. 2021. "Evaluation of the Predictive Performance of Population Pharmacokinetic Models of Adalimumab in Patients with Inflammatory Bowel Disease" Pharmaceutics 13, no. 8: 1244. https://doi.org/10.3390/pharmaceutics13081244
APA StyleMarquez-Megias, S., Ramon-Lopez, A., Más-Serrano, P., Diaz-Gonzalez, M., Candela-Boix, M. R., & Nalda-Molina, R. (2021). Evaluation of the Predictive Performance of Population Pharmacokinetic Models of Adalimumab in Patients with Inflammatory Bowel Disease. Pharmaceutics, 13(8), 1244. https://doi.org/10.3390/pharmaceutics13081244







