Lipid-Polymeric Films: Composition, Production and Applications in Wound Healing and Skin Repair
Abstract
:1. Introduction
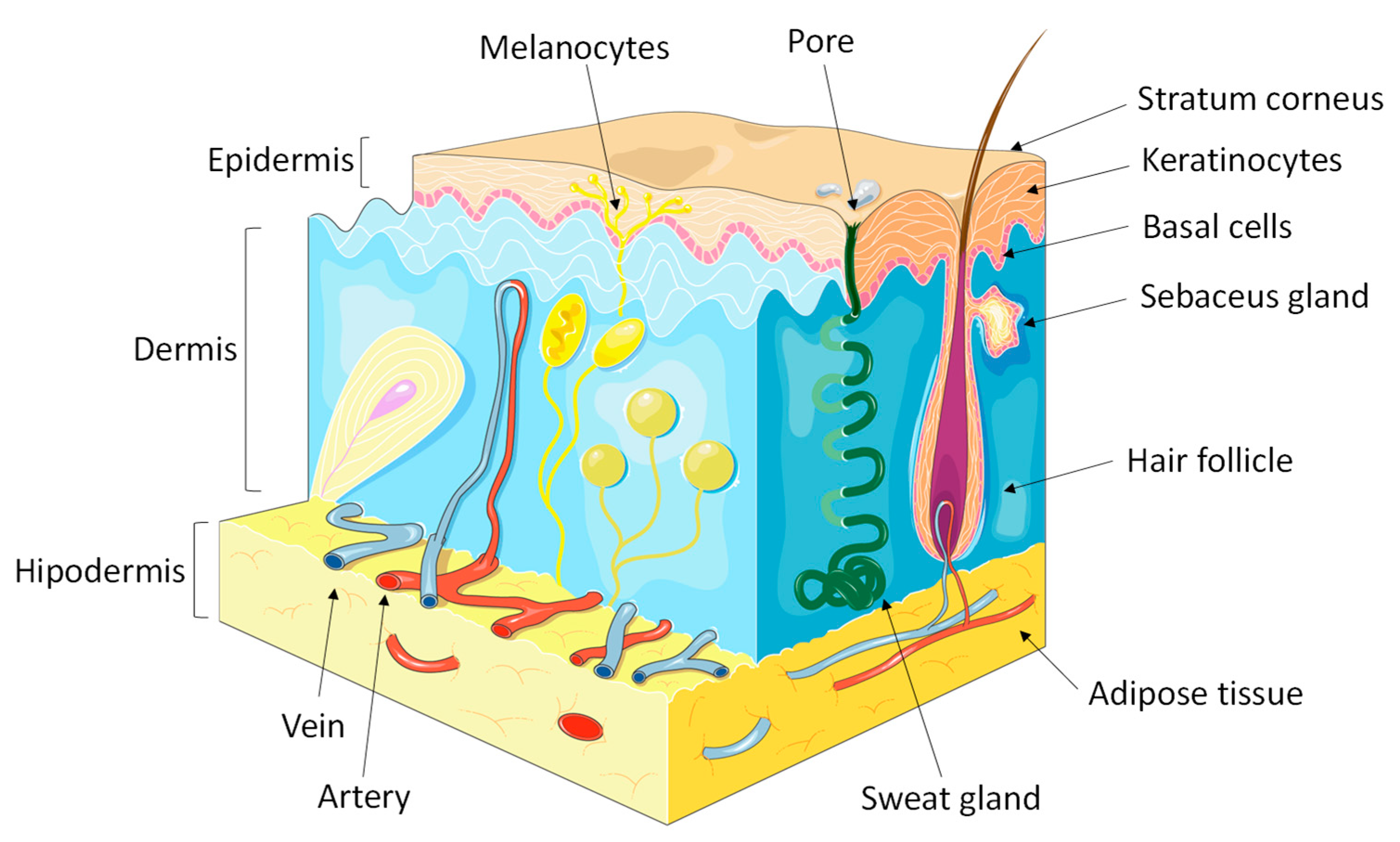
2. Lipid Barriers of the Epidermis
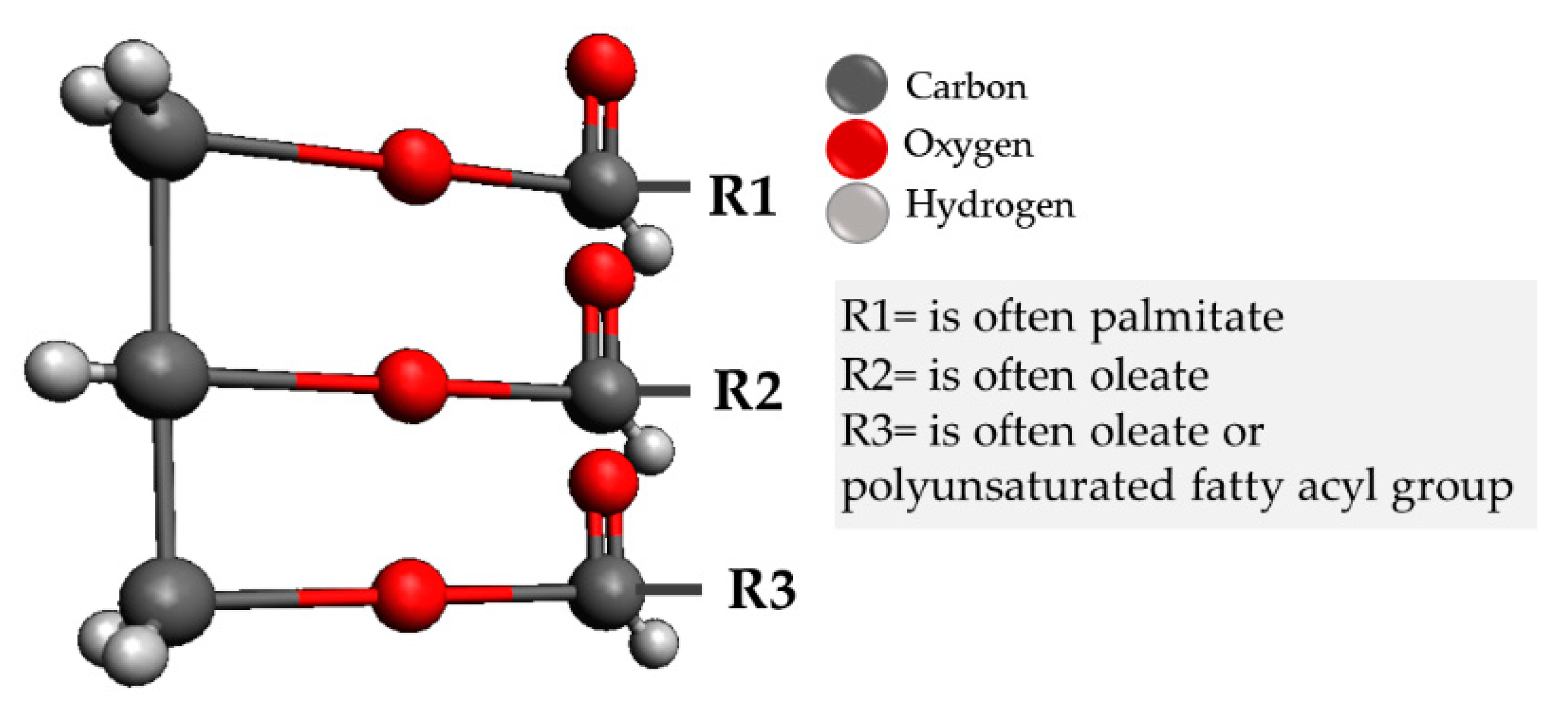
2.1. Sebaceous Lipids
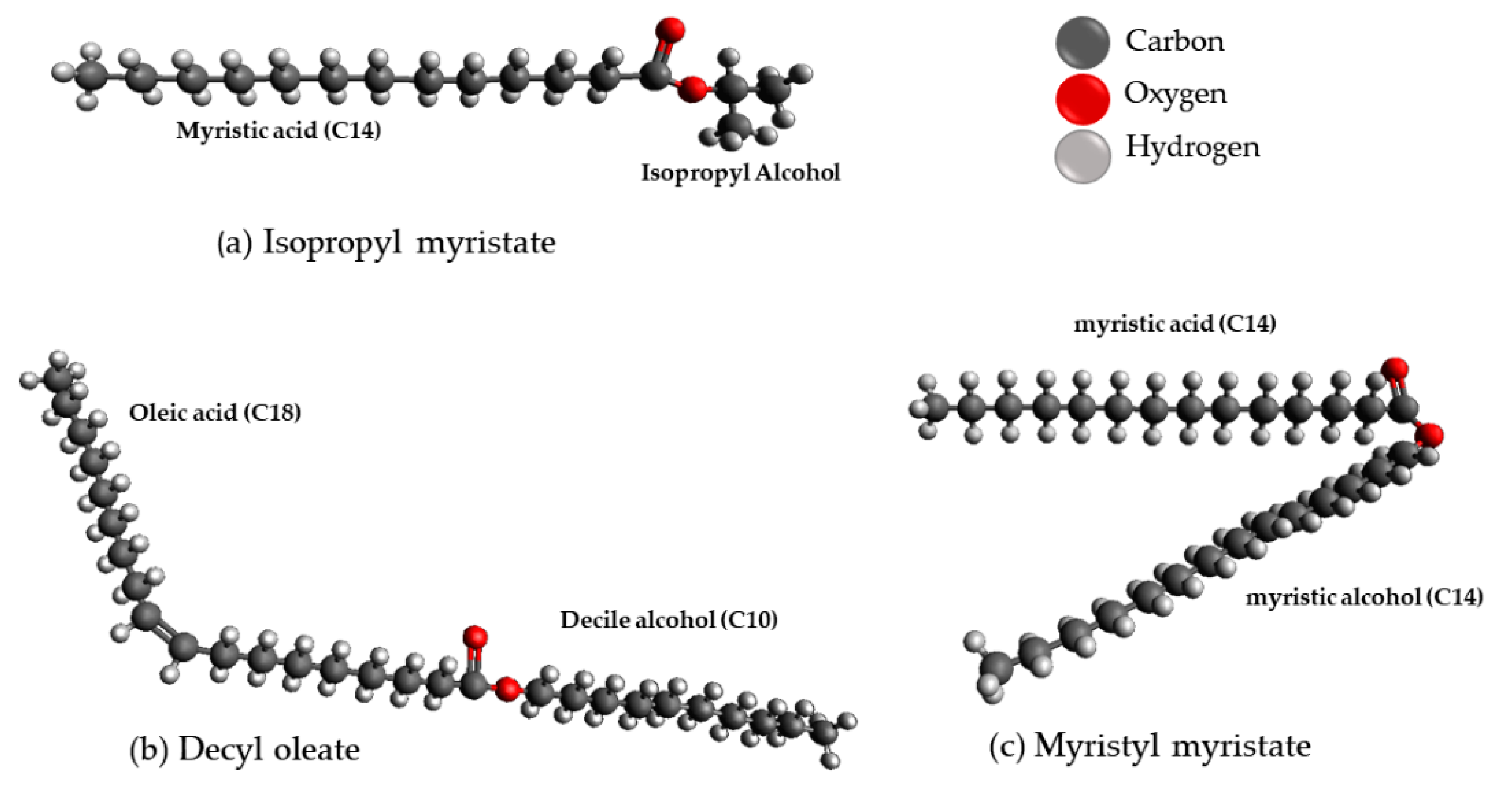
2.2. Lipids Used in Cosmetic and Dermatological Formulations
2.2.1. Beeswax
2.2.2. Squalene
2.2.3. Sterols
3. Films Containing Lipids
3.1. Emulsification of Lipids in Polymers
3.2. Main Emulsification Techniques
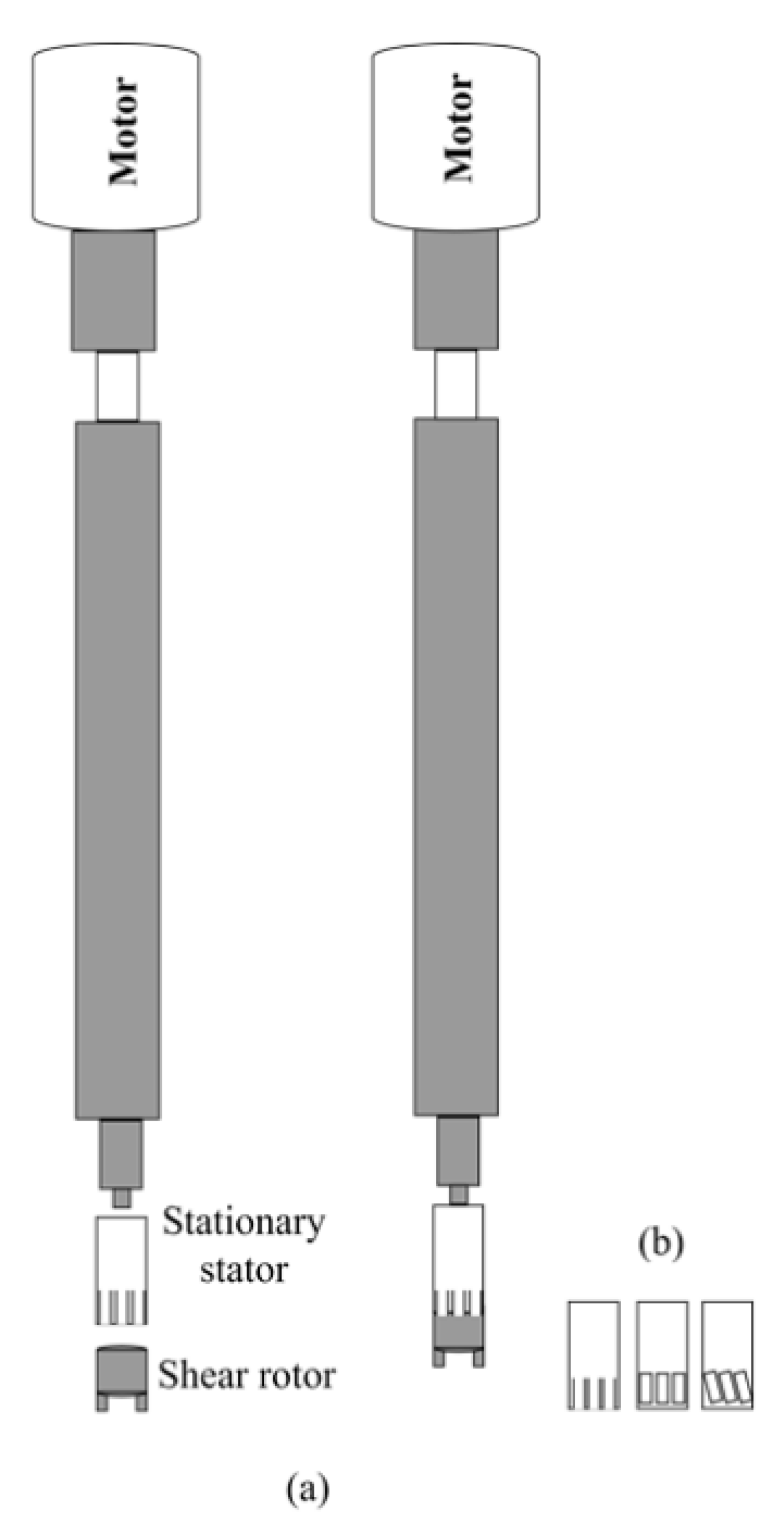
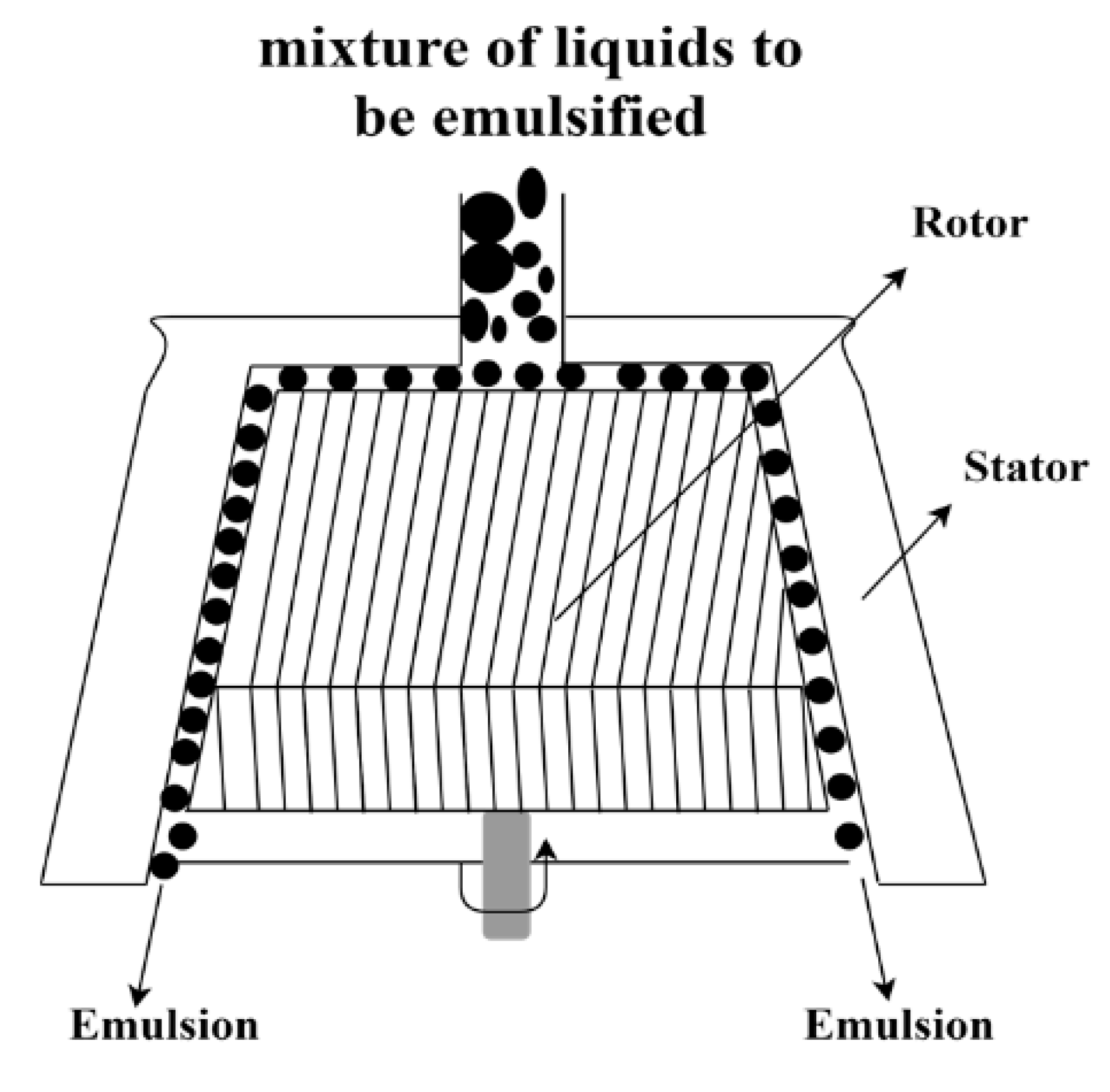
3.3. Rotor-Stator Systems
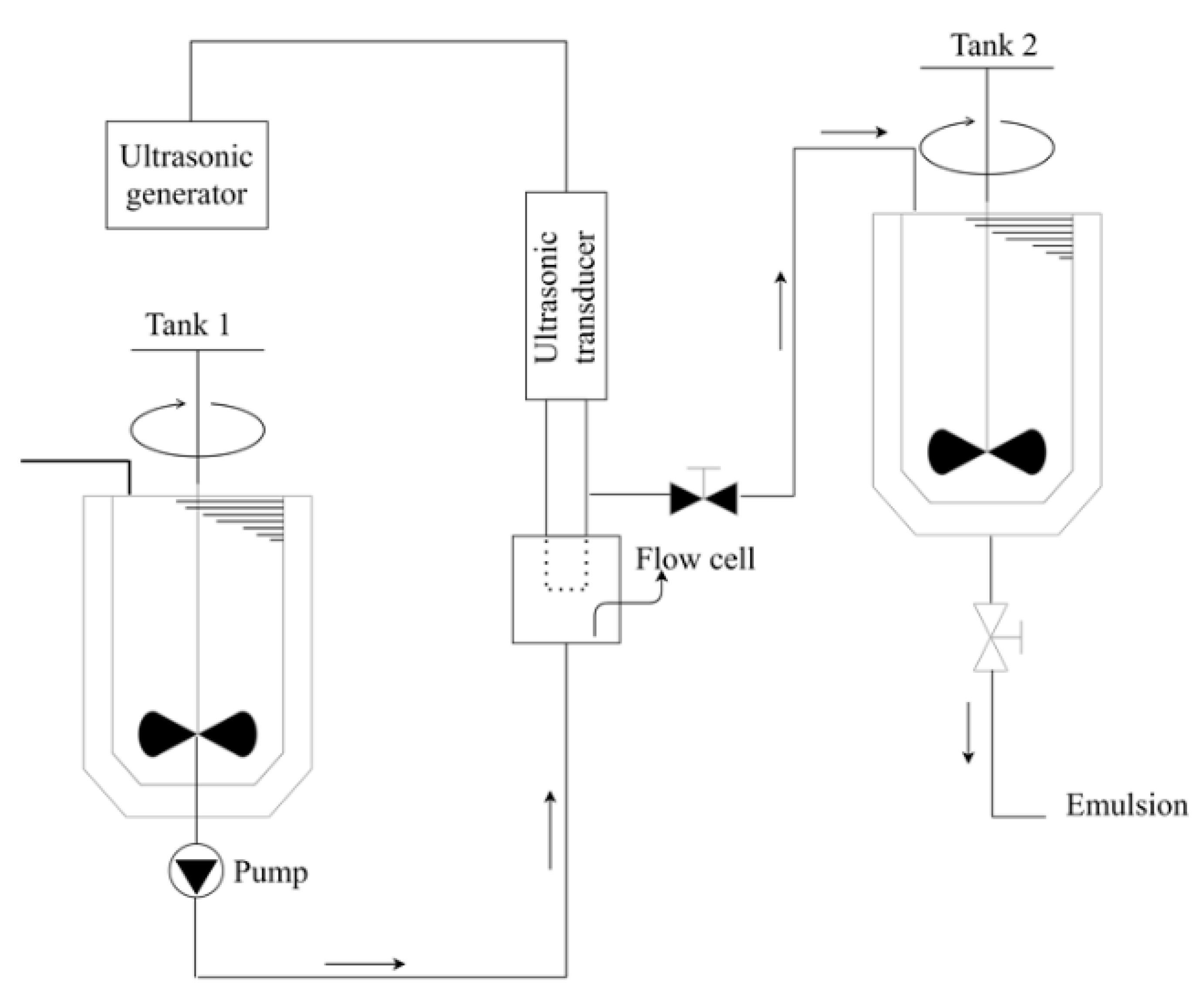
3.3.1. Sonicators (Ultrasound Processes)
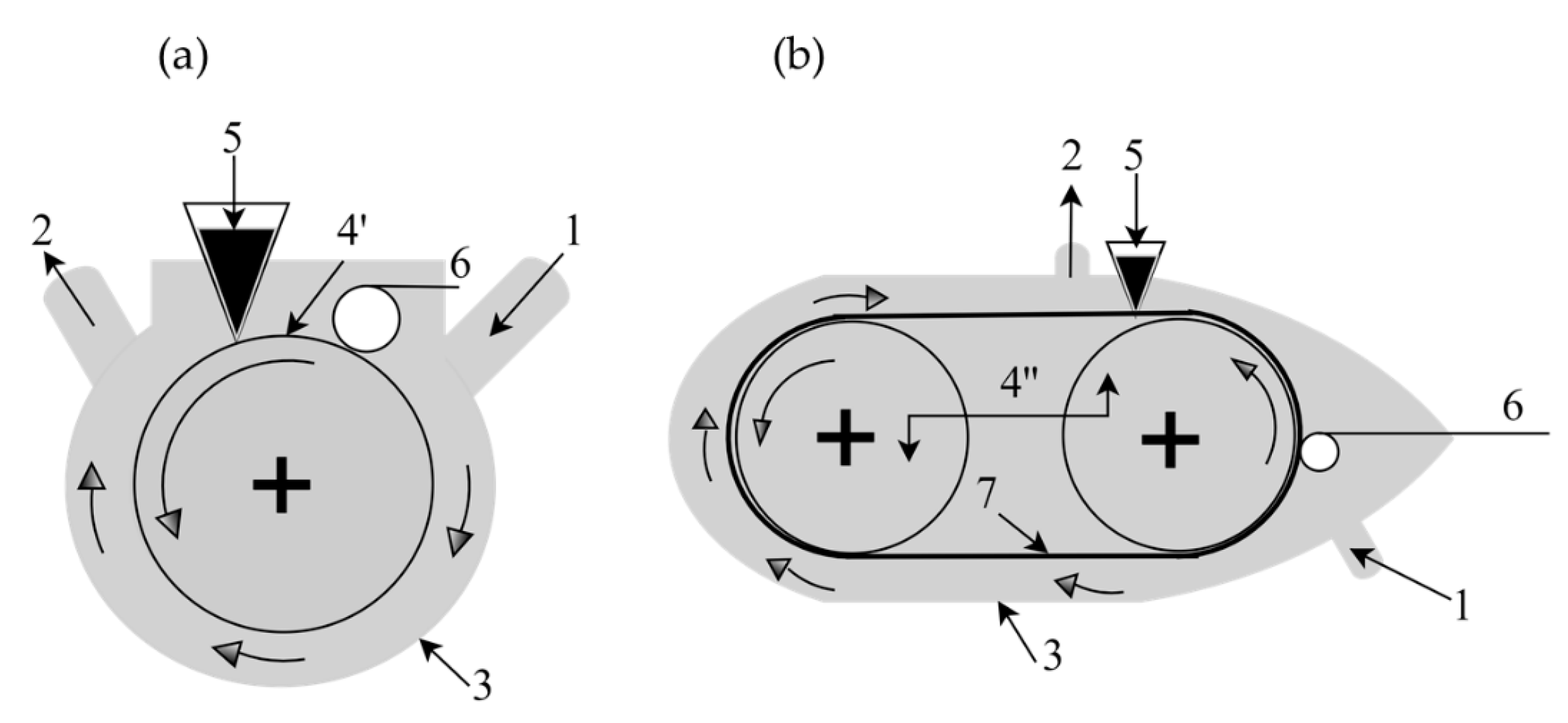
3.3.2. Membrane Emulsification
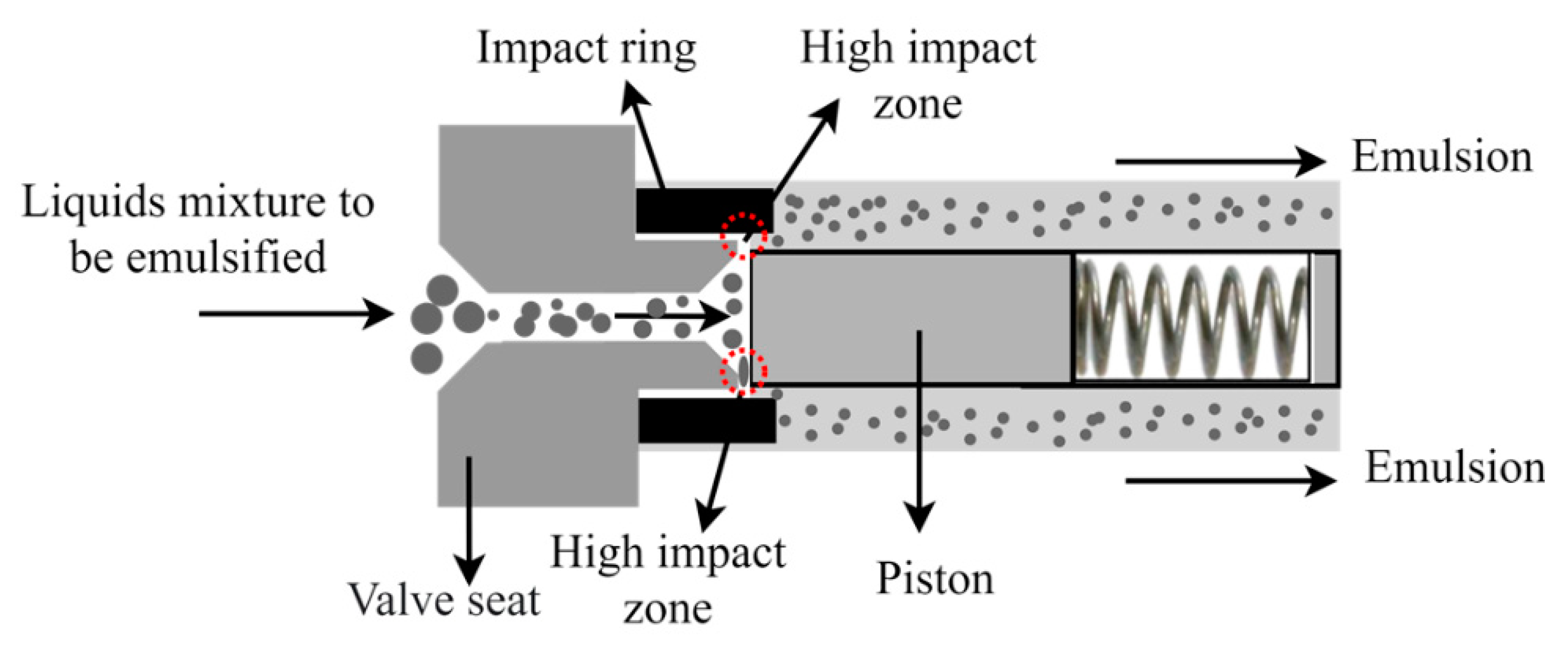
3.3.3. High Pressure Homogenizers
3.4. Formation of the Emulsified Film
3.5. Electrospinning Scaffolds and Nanofibers Fabrication
4. Film Properties
4.1. Mechanical Properties
4.2. Gas Barrier Property
5. Application of Films in Cosmetics and Dermatology
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gallo, R.L. Human Skin Is the Largest Epithelial Surface for Interaction with Microbes. J. Investig. Dermatol. 2017, 137, 1213–1214. [Google Scholar] [CrossRef] [Green Version]
- Madiedo-Podvrsan, S.; Belaïdi, J.-P.; Desbouis, S.; Simonetti, L.; Ben-Khalifa, Y.; Soeur, J.; Rielland, M. Utilization of patterned bioprinting for heterogeneous and physiologically representative reconstructed epidermal skin models. Sci. Rep. 2021, 11, 6217. [Google Scholar] [CrossRef] [PubMed]
- Kantor, J. May 2021: Heterogeneity in reported skin manifestations of COVID-19 and vaccines. J. Am. Acad. Dermatol. 2021, 84, 1251. [Google Scholar] [CrossRef] [PubMed]
- Osseiran, S.; Cruz, J.D.; Jeong, S.; Wang, H.; Fthenakis, C.; Evans, C.L. Characterizing stratum corneum structure, barrier function, and chemical content of human skin with coherent Raman scattering imaging. Biomed. Opt. Express 2018, 9, 6425–6443. [Google Scholar] [CrossRef]
- Mahant, S.; Rao, R.; Souto, E.B.; Nanda, S. Analytical tools and evaluation strategies for nanostructured lipid carrier-based topical delivery systems. Expert Opin. Drug Deliv. 2020, 17, 963–992. [Google Scholar] [CrossRef]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Batistela, M.A.; Chorilli, M.; Leonardi, G.R. Approach to the process knowledge of skin aging among different ethnics. Rev. Bras. Farm. 2007, 88, 59–62. [Google Scholar]
- Freeman, S.C.; Sonthalia, S. Histology, Keratohyalin Granules. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Sundberg, J.P.; Nanney, L.B.; Fleckman, P.; King, L.E. 23—Skin and Adnexa. In Comparative Anatomy and Histology; Treuting, P.M., Dintzis, S.M., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 433–455. [Google Scholar] [CrossRef]
- Wertz, P.W. Roles of Lipids in the Permeability Barriers of Skin and Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 5229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Duan, E. Fighting against Skin Aging: The Way from Bench to Bedside. Cell Transplant. 2018, 27, 729–738. [Google Scholar] [CrossRef]
- De Luca, M.; Pappalardo, I.; Limongi, A.R.; Viviano, E.; Radice, R.P.; Todisco, S.; Martelli, G.; Infantino, V.; Vassallo, A. Lipids from Microalgae for Cosmetic Applications. Cosmetics 2021, 8, 52. [Google Scholar] [CrossRef]
- Imokawa, G.; Kuno, H.; Kawai, M. Stratum corneum lipids serve as a bound-water modulator. J. Investig. Dermatol. 1991, 96, 845–851. [Google Scholar] [CrossRef]
- Naik, A.; Kalia, Y.N.; Guy, R.H. Transdermal drug delivery: Overcoming the skin’s barrier function. Pharm. Sci. Technol. Today. 2000, 3, 318–326. [Google Scholar] [CrossRef]
- Purnamawati, S.; Indrastuti, N.; Danarti, R.; Saefudin, T. The Role of Moisturizers in Addressing Various Kinds of Dermatitis: A Review. Clin. Med. Res. 2017, 15, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Rosso, J.D.; Zeichner, J.; Alexis, A.; Cohen, D.; Berson, D. Understanding the Epidermal Barrier in Healthy and Compromised Skin: Clinically Relevant Information for the Dermatology Practitioner: Proceedings of an Expert Panel Roundtable Meeting. J. Clin. Aesthet. Dermatol. 2016, 9, S2–S8. [Google Scholar]
- Chandan, N.; Rajkumar, J.R.; Shi, V.Y.; Lio, P.A. A new era of moisturizers. J. Cosmet. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rieger, M. Skin constituents as cosmetic ingredients. Cosmet. Toilet. 1992, 107, 85–94. [Google Scholar]
- Rieger, M. Skin lipids and their importance to cosmetic science. Cosmet. Toilet. 1987, 102, 36–50. [Google Scholar]
- Pappas, A. Epidermal surface lipids. Dermatoendocrinol 2009, 1, 72–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonardi, G.R.; Gaspar, L.R.; Maia Campos, P.M.B.G. Study of pH variation on the skin using cosmetic formulation s with and without vitamins A, E or ceramide: By a non-invasive method. Bras. Dermatol. 2002, 77, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Leonardi, G.R. Cosmetologia Aplicada; MedFarma: São Paulo, Brazil, 2008. [Google Scholar]
- Özcan, M.M.; Al Juhaimi, F.; Ghafoor, K.; Babiker, E.E.; Özcan, M.M. Characterization of physico-chemical and bioactive properties of oils of some important almond cultivars by cold press and soxhlet extraction. J. Food Sci. Technol. 2020, 57, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Callegarin, F.; Quezada Gallo, J.-A.; Debeaufort, F.d.r.; Voilley, A.e. Lipids and biopackaging. J. Am. Oil. Chem. Soc. 1997, 74, 1183–1192. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Fairus, S.; Zulfarina, M.S.; Naina Mohamed, I. The Efficacy of Squalene in Cardiovascular Disease Risk-A Systematic Review. Nutrients 2020, 12, 414. [Google Scholar] [CrossRef] [Green Version]
- Pal, K.; Paulson, A.T.; Rousseau, D. 14—Biopolymers in Controlled-Release Delivery Systems. In Handbook of Biopolymers and Biodegradable Plastics; Ebnesajjad, S., Ed.; William Andrew Publishing: Boston, MA, USA, 2013; pp. 329–363. [Google Scholar] [CrossRef]
- Kallis, P.J.; Friedman, A.J. Collagen Powder in Wound Healing. J. Drugs Dermatol. JDD 2018, 17, 403–408. [Google Scholar] [PubMed]
- Nyman, E.; Henricson, J.; Ghafouri, B.; Anderson, C.D.; Kratz, G. Hyaluronic Acid Accelerates Re-epithelialization and Alters Protein Expression in a Human Wound Model. Plast. Reconstr. Surg. Glob Open 2019, 7, e2221. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, C.M.P.; Pacheco, M.S.; de Moraes, M.A.; Lopes, P.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Effect of Chitosan and Aloe Vera Extract Concentrations on the Physicochemical Properties of Chitosan Biofilms. Polymers 2021, 13, 1187. [Google Scholar] [CrossRef]
- Negut, I.; Dorcioman, G.; Grumezescu, V. Scaffolds for Wound Healing Applications. Polymers 2020, 12, 2010. [Google Scholar] [CrossRef]
- Gerhardt, L.C.; Schiller, A.; Müller, B.; Spencer, N.D.; Derler, S. Fabrication, Characterisation and Tribological Investigation of Artificial Skin Surface Lipid Films. Tribol. Lett. 2009, 34, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Hou, Z.; Li, T. Novel sample preparation method of polymer emulsion for SEM observation. Microsc. Res. Tech. 2007, 70, 847–850. [Google Scholar] [CrossRef]
- Tadros, T. Principles of emulsion stabilization with special reference to polymeric surfactants. J. Cosmet. Sci. 2006, 57, 153–169. [Google Scholar]
- Gall, V.; Runde, M.; Schuchmann, H.P. Extending Applications of High-Pressure Homogenization by Using Simultaneous Emulsification and Mixing (SEM)—An Overview. Processes 2016, 4, 46. [Google Scholar] [CrossRef] [Green Version]
- Tripodi, E.; Lazidis, A.; Norton, I.T.; Spyropoulos, F. Production of Oil-in-Water Emulsions with Varying Dispersed-Phase Content using Confined Impinging Jet Mixers. Ind. Eng. Chem. Res. 2019, 58, 14859–14872. [Google Scholar] [CrossRef]
- Souto, E.B.; Doktorovova, S.; Zielinska, A.; Silva, A.M. Key production parameters for the development of solid lipid nanoparticles by high shear homogenization. Pharm. Dev. Technol. 2019, 24, 1181–1185. [Google Scholar] [CrossRef]
- Maa, Y.F.; Hsu, C. Liquid-liquid emulsification by rotor/stator homogenization. J. Control Release 1996, 38, 219–228. [Google Scholar] [CrossRef]
- Urban, K.; Wagner, G.; Schaffner, D.; Röglin, D.; Ulrich, J. Rotor-Stator and Disc Systems for Emulsification Processes. Chem. Eng. Technol. 2006, 29, 24–31. [Google Scholar] [CrossRef]
- Suslick, K.S.; Price, G.J. Applications of Ultrasound to Materials Chemistry. Ann. Rev. Mater. Sci. 1999, 29, 295–326. [Google Scholar] [CrossRef] [Green Version]
- Oh, D.H.; Balakrishnan, P.; Oh, Y.-K.; Kim, D.-D.; Yong, C.S.; Choi, H.-G. Effect of process parameters on nanoemulsion droplet size and distribution in SPG membrane emulsification. Int. J. Pharm. 2011, 404, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Schultz, S.; Wagner, G.; Urban, K.; Ulrich, J. High-Pressure Homogenization as a Process for Emulsion Formation. Chem. Eng. Technol. 2004, 27, 361–368. [Google Scholar] [CrossRef]
- Siemann, U. Solvent cast technology—A versatile tool for thin film production. Progr. Colloid. Polym. Sci. 2005, 130, 1–14. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Boateng, J.S.; Snowden, M.J.; Douroumis, D. A Review of Hot-Melt Extrusion: Process Technology to Pharmaceutical Products. ISRN Pharm. 2012, 2012, 9. [Google Scholar] [CrossRef] [Green Version]
- Chokshi, R.; Zia, H. Hot-Melt Extrusion Technique: A Review. Iran. J. Pharm. Res. 2004, 3, 3–16. [Google Scholar]
- Juncos Bombin, A.D.; Dunne, N.J.; McCarthy, H.O. Electrospinning of natural polymers for the production of nanofibres for wound healing applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 110994. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Aghamohamadi, N.; Sanjani, N.S.; Majidi, R.F.; Nasrollahi, S.A. Preparation and characterization of Aloe vera acetate and electrospinning fibers as promising antibacterial properties materials. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Pan, H.; Ji, D.; Li, Y.; Duan, H.; Pan, W. Curcumin-loaded sandwich-like nanofibrous membrane prepared by electrospinning technology as wound dressing for accelerate wound healing. Mater. Sci. Eng. 2021, 127, 112245. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yuan, F.; Xu, L.; Yan, Q.; Yang, Y.; Wu, D.; Guo, F.; Yang, G. An Update of Moisture Barrier Coating for Drug Delivery. Pharmaceutics 2019, 11, 436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.; Mellinas, C.; Solaberrieta, I.; Garrigós, M.C.; Jiménez, A. Emulsions Incorporated in Polysaccharide-Based Active Coatings for Fresh and Minimally Processed Vegetables. Foods 2021, 10, 665. [Google Scholar] [CrossRef]
- Debeaufort, F.; Quezada-Gallo, J.-A.; Voilley, A. Edible Films and Coatings: Tomorrow’s Packagings: A Review. Crit. Rev. Food Sci. Nutr. 1998, 38, 299–313. [Google Scholar] [CrossRef]
- Debeaufort, F.; Voilley, A. Edible Films and Coatings for Food Applications. In Lipid-Based Edible Films and Coatings; Embuscado, M.E., Huber, K.C., Eds.; Springer Nature: Heidelberg, Germany, 2009; pp. 135–168. [Google Scholar]
- Ditzinger, F.; Price, D.J.; Ilie, A.-R.; Köhl, N.J.; Jankovic, S.; Tsakiridou, G.; Aleandri, S.; Kalantzi, L.; Holm, R.; Nair, A.; et al. Lipophilicity and hydrophobicity considerations in bio-enabling oral formulations approaches—A PEARRL review. J. Pharm. Pharmacol. 2019, 71, 464–482. [Google Scholar] [CrossRef] [Green Version]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Kamper, S.L.; Fennema, O. Use of an Edible Film to Maintain Water Vapor Gradients in Foods. J. Food. Sci. 1985, 50, 382–384. [Google Scholar] [CrossRef]
- Wong, D.W.S.; Gastineau, F.A.; Gregorski, K.S.; Tillin, S.J.; Pavlath, A.E. Chitosan-lipid films: Microstructure and surface energy. J. Agric. Food Chem. 1992, 40, 540–544. [Google Scholar] [CrossRef]
- Teixeira, M.D.C.; Santini, A.; Souto, E.B. Chapter 8—Delivery of Antimicrobials by Chitosan-Composed Therapeutic Nanostructures. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 8; pp. 203–222. [Google Scholar] [CrossRef]
- Ataide, J.A.; Gerios, E.F.; Cefali, L.C.; Fernandes, A.R.; Teixeira, M.D.C.; Ferreira, N.R.; Tambourgi, E.B.; Jozala, A.F.; Chaud, M.V.; Oliveira-Nascimento, L.; et al. Effect of Polysaccharide Sources on the Physicochemical Properties of Bromelain-Chitosan Nanoparticles. Polymers 2019, 11, 1681. [Google Scholar] [CrossRef] [Green Version]
- Cuq, B.; Aymard, C.; Cuq, J.-L.; Guilbert, S. Edible Packaging Films Based on Fish Myofibrillar Proteins: Formulation and Functional Properties. J. Food Sci. 1995, 60, 1369–1374. [Google Scholar] [CrossRef]
- Mendes, J.F.; Norcino, L.B.; Manrich, A.; Pinheiro, A.C.M.; Oliveira, J.E.; Mattoso, L.H.C. Characterization of Pectin Films Integrated with Cocoa Butter by Continuous Casting: Physical, Thermal and Barrier Properties. J. Polym. Environ. 2020, 28, 2905–2917. [Google Scholar] [CrossRef]
- Kunst, S.R.; Cardoso, H.R.P.; Ortega, V.M.R.; Beltrami, L.V.R.; Menezes, T.L.; Malfatti, C.F. The effects of curing temperature on bilayer and monolayer hybrid films: Mechanical and electrochemical properties. J. Appl. Electrochem. 2014, 44, 759–771. [Google Scholar] [CrossRef]
- Sherwin, C.P.; Smith, D.E.; Fulcher, R.G. Effect of Fatty Acid Type on Dispersed Phase Particle Size Distributions in Emulsion Edible Films. J Agric. Food Chem. 1998, 46, 4534–4538. [Google Scholar] [CrossRef]
- Yousuf, B.; Sun, Y.; Wu, S. Lipid and Lipid-containing Composite Edible Coatings and Films. Food Rev. Int. 2021. [Google Scholar] [CrossRef]
- Fairley, P.; Krochta, J.M.; German, J.B. Interfacial interactions in edible emulsion films from whey protein isolate. Food Hydrocoll. 1997, 11, 245–252. [Google Scholar] [CrossRef]
- Gontard, N.; Duchez, C.; Cuq, J.-L.; Guilbert, S. Edible composite films of wheat gluten and lipids: Water vapour permeability and other physical properties. Int. J. Food Sci. Technol. 1994, 29, 39–50. [Google Scholar] [CrossRef]
- McHugh, T.H.; Krochta, J.M. Water vapor permeability properties of edible whey protein-lipid emulsion films. J. Am. Oil Chem. Soc. 1994, 71, 307–312. [Google Scholar] [CrossRef]
- Koelsch, C.M.; Labuza, T.P. Functional, physical and morphological properties of methyl cellulose and fatty acid-based edible barriers. Lebensm. Wiss. Und Technol. 1992, 25, 404–411. [Google Scholar]
- Yoshida, C.M.P.; Oliveira-Junior, E.N.; Franco, T.T. Chitosan Films: Additives effects on barrier and mechanical properties. Packag. Sci. Technol. 2009, 22, 161–170. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.J.; Krochta, J.M. Water Vapor Permeability of Caseinate-Based Edible Films as Affected by pH, Calcium Crosslinking and Lipid Content. J. Food Sci. 1993, 58, 904–907. [Google Scholar] [CrossRef]
- Sapru, V.; Labruza, T.P. Dispersed phase concentration effect on water vapor permeability in composite methyl cellulose-stearic acid edible films. J. Food Process. Preserv. 1994, 18, 359–368. [Google Scholar] [CrossRef]
- Guilbert, S.; Gontard, N.; Cuq, B. Technology and applications of edible protective films. Packag. Technol. Sci. 1995, 8, 339–346. [Google Scholar] [CrossRef]
- Martins, M.P.; Dagostin, J.L.A.; Franco, T.S.; de Muñiz, G.I.B.; Masson, M.L. Application of Cellulose Nanofibrils Isolated from an Agroindustrial Residue of Peach Palm in Cassava Starch Films. Food Biophys. 2020, 15, 323–334. [Google Scholar] [CrossRef]
- Robertson, G.L. Food Packaging: Principles and Practice; Marcell Dekker Inc.: New York, NY, USA, 1993. [Google Scholar]
- Krywko-Cendrowska, A.; di Leone, S.; Bina, M.; Yorulmaz-Avsar, S.; Palivan, C.G.; Meier, W. Recent Advances in Hybrid Biomimetic Polymer-Based Films: From Assembly to Applications. Polymers 2020, 12, 1003. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Antunes, A.C.B.; Antunes, L.J.; Antunes, A.J. Water vapour permeability of whey protein emulsion films. Milchwissenchaft 2004, 59, 48–51. [Google Scholar]
- Yoshida, C.M.P.; Antunes, A.J. Characterization of whey protein emulsion films. Braz. J. Chem. Eng. 2004, 21, 247–252. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Bastos, C.E.N.; Franco, T.T. Modeling of potassium sorbate diffusion through chitosan films. Lebensm. Wiss. Technol. 2010, 43, 584–589. [Google Scholar] [CrossRef]
- Oliveira, D.M.L.; Rezende, P.S.; Barbosa, T.C.; Andrade, L.N.; Bani, C.; Tavares, D.S.; da Silva, C.F.; Chaud, M.V.; Padilha, F.; Cano, A.; et al. Double membrane based on lidocaine-coated polymyxin-alginate nanoparticles for wound healing: In vitro characterization and in vivo tissue repair. Int. J. Pharm. 2020, 591, 120001. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Olewnik-Kruszkowska, E.; Reczyńska, K.; Pamuła, E. Is Dialdehyde Chitosan a Good Substance to Modify Physicochemical Properties of Biopolymeric Materials? Int. J. Mol. Sci. 2021, 22, 3391. [Google Scholar] [CrossRef]
- Wardhono, E.Y.; Pinem, M.P.; Kustiningsih, I.; Agustina, S.; Oudet, F.; Lefebvre, C.; Clausse, D.; Saleh, K.; Guénin, E. Cellulose Nanocrystals to Improve Stability and Functional Properties of Emulsified Film Based on Chitosan Nanoparticles and Beeswax. Nanomaterials 2019, 9, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiva, J.M.; Geffroy, E. Evolution of the Size Distribution of an Emulsion under a Simple Shear Flow. Fluids 2018, 3, 46. [Google Scholar] [CrossRef] [Green Version]
- Ochoa, T.A.; Almendárez, B.E.G.; Reyes, A.A.; Pastrana, D.M.R.; López, G.F.G.; Belloso, O.M.; González, C.R. Design and Characterization of Corn Starch Edible Films Including Beeswax and Natural Antimicrobials. Food Bioprocess Technol. 2017, 10, 103–114. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Trends in Chitosan as a Primary Biopolymer for Functional Films and Coatings Manufacture for Food and Natural Products. Polymers 2021, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.B.; Oliveira, W.d.S.; Araújo, R.L.; Honório França, A.C.; Pertuzatti, P.B. Buriti (Mauritia Flexuosa L.) pulp oil as an immunomodulator against enteropathogenic Escherichia coli. Ind. Crop. Prod. 2020, 149, 112330. [Google Scholar] [CrossRef]
- Batista, J.S.; Olinda, R.G.; Medeiros, V.B.; Rodrigues, C.M.F.; Oliveira, A.F.; Paiva, E.S.; Freitas, C.I.A.; Medeiros, A.d.C. Atividade antibacteriana e cicatrizante do óleo de buriti Mauritia flexuosa L. Ciência Rural. 2012, 42, 136–141. [Google Scholar] [CrossRef]
- Maia Campos, P.M.B.G. Estudos da Estabilidade Química e da Absorção In Vivo da Vitamina A em Preparações Cosméticas Para a Pele; São Paulo University: São Paulo, Brazil, 1993. [Google Scholar]
- Dias-Ferreira, J.; Fernandes, A.R.; Soriano, J.L.; Naveros, B.C.; Severino, P.; da Silva, C.F.; Souto, E.B. Chapter —Skin rejuvenation: Biopolymers applied to UV sunscreens and sheet masks. In Biopolymer Membranes and Films; de Moraes, M.A., da Silva, C.F., Vieira, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 309–330. [Google Scholar] [CrossRef]
- Alves, T.F.R.; Morsink, M.; Batain, F.; Chaud, M.V.; Almeida, T.; Fernandes, D.A.; da Silva, C.F.; Souto, E.B.; Severino, P. Applications of Natural, Semi-Synthetic, and Synthetic Polymers in Cosmetic Formulations. Cosmetics 2020, 7, 75. [Google Scholar] [CrossRef]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
- Gubitosa, J.; Rizzi, V.; Fini, P.; Cosma, P. Hair Care Cosmetics: From Traditional Shampoo to Solid Clay and Herbal Shampoo, A Review. Cosmetics 2019, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Zielinska, A.; Souto, S.B.; Durazzo, A.; Lucarini, M.; Santini, A.; Silva, A.M.; Atanasov, A.G.; Marques, C.; Andrade, L.N.; et al. (+)-Limonene 1,2-Epoxide-Loaded SLNs: Evaluation of Drug Release, Antioxidant Activity, and Cytotoxicity in an HaCaT Cell Line. Int. J. Mol. Sci. 2020, 21, 1449. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.S.; Loureiro, K.C.; Rezende, P.S.; Andrade, L.N.; de Melo Barbosa, R.; Santini, A.; Santos, A.C.; Ferreira da Silva, C.; Souto, E.B.; de Sousa, D.P.; et al. Innovative nanocompounds for cutaneous administration of classical antifungal drugs: A systematic review. J. Dermatolog. Treat. 2019, 30, 617–626. [Google Scholar] [CrossRef]
- Carbone, C.; Teixeira, M.D.C.; Sousa, M.D.C.; Martins-Gomes, C.; Silva, A.M.; Souto, E.M.B.; Musumeci, T. Clotrimazole-Loaded Mediterranean Essential Oils NLC: A Synergic Treatment of Candida Skin Infections. Pharmaceutics 2019, 11, 231. [Google Scholar] [CrossRef] [Green Version]
- Rigon, R.B.; Goncalez, M.L.; Severino, P.; Alves, D.A.; Santana, M.H.A.; Souto, E.B.; Chorilli, M. Solid lipid nanoparticles optimized by 2(2) factorial design for skin administration: Cytotoxicity in NIH3T3 fibroblasts. Colloids Surf. B Biointerfaces 2018, 171, 501–505. [Google Scholar] [CrossRef] [Green Version]
- Severino, P.; Silveira, E.F.; Loureiro, K.; Chaud, M.V.; Antonini, D.; Lancellotti, M.; Sarmento, V.H.; da Silva, C.F.; Santana, M.H.A.; Souto, E.B. Antimicrobial activity of polymyxin-loaded solid lipid nanoparticles (PLX-SLN): Characterization of physicochemical properties and in vitro efficacy. Eur. J. Pharm. Sci. 2017, 106, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Doktorovova, S.; Kovacevic, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Lulla, A.; Malhotra, G. (Inventor); Cipla Pvt. Ltd., Assignee. Transdermal Pharmaceutical Spray Formulations Comprising a vp/va Copolymer and a Non-Aqueous Vehicle. U.S. Patent 2005041943A1, 12 May 2005. [Google Scholar]
- Ahmad, A.; Ahsan, H. Lipid-based formulations in cosmeceuticals and biopharmaceuticals. Biomed. Dermatol. 2020, 4, 12. [Google Scholar] [CrossRef]
- Cárdenas, G.; Anaya, P.; von Plessing, C.; Rojas, C.; Sepúlveda, J. Chitosan composite films. Biomedical applications. J. Mater. Sci. Mater. Med. 2008, 19, 2397–2405. [Google Scholar] [CrossRef] [PubMed]
- Altiok, D.; Altiok, E.; Tihminlioglu, F. Physical, antibacterial and antioxidant properties of chitosan films incorporated with thyme oil for potential wound healing applications. J. Mater. Sci. 2010, 21, 2227–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, G.P.; Debone, H.S.; Severino, P.; Souto, E.B.; da Silva, C.F. Design and characterization of chitosan/zeolite composite films—Effect of zeolite type and zeolite dose on the film properties. Mater. Sci. Eng. C 2016, 60, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Hissae Yassue-Cordeiro, P.; Zandonai, C.H.; Pereira Genesi, B.; Santos Lopes, P.; Sanchez-Lopez, E.; Garcia, M.L.; Camargo Fernandes-Machado, N.R.; Severino, P.; Souto, E.B.; da Silva, C.F. Development of Chitosan/Silver Sulfadiazine/Zeolite Composite Films for Wound Dressing. Pharmaceutics 2019, 11, 535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khater, D.; Nsairat, H.; Odeh, F.; Saleh, M.; Jaber, A.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Design, Preparation, and Characterization of Effective Dermal and Transdermal Lipid Nanoparticles: A Review. Cosmetics 2021, 8, 39. [Google Scholar] [CrossRef]
- Nosari, A.B.F.L. Development of Microparticles Containing Green Coffee Oil by Spray Congealing; Universidade de São Paulo: Ribeirão Preto, Brazil, 2012. [Google Scholar]
- Swathi, G.; Prasanthi, N.L.; Manikiran, S.S.; Ramarao, N. Solid Lipid nanoparticles: Colloidal carrier systems for drug delivery. Int. J. Pharm. Sci. Res. 2010, 1, 1–16. [Google Scholar]
- Favaro-Trindade, C.S.; de Matos Junior, F.E.; Okuro, P.K.; Dias-Ferreira, J.; Cano, A.; Severino, P.; Zielinska, A.; Souto, E.B. Encapsulation of active pharmaceutical ingredients in lipid nanoparticles by spray-cooling. Pharmaceutics 2021, 13, 1186. [Google Scholar] [CrossRef]
- Passerini, N.; Gavini, E.; Albertini, B.; Rassu, G.; Di Sabatino, M.; Sanna, V.; Giunchedi, P.; Rodriguez, L. Evaluation of solid lipid microparticles produced by spray congealing for topical application of econazole nitrate. J. Pharm. Pharmacol. 2009, 61, 559–567. [Google Scholar] [CrossRef]
- Albertini, B.; Di Sabatino, M.; Calonghi, N.; Rodriguez, L.; Passerini, N. Novel multifunctional platforms for potential treatment of cutaneous wounds: Development and in vitro characterization. Int. J. Pharm. 2013, 440, 238–249. [Google Scholar] [CrossRef]
- Albertini, B.; Mezzena, M.; Passerini, N.; Rodriguez, L.; Scalia, S. Evaluation of spray congealing as technique for the preparation of highly loaded solid lipid microparticles containing the sunscreen agent, avobenzona. J. Pharm. Sci. 2009, 98, 2759–2769. [Google Scholar] [CrossRef]
- Albertini, B.; Passerini, N.; Di Sabatino, M.; Vitali, B.; Brigidi, B.; Rodriguez, L. Polymer-lipid based mucoadhesive microspheres prepared by spray-congealing for the vaginal delivery of econazole nitrate. Eur. J. Pharm. Sci. 2009, 36, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Al-Kassas, R.; Donnelly, R.F.; McCarron, P.A. Aminolevulinic acid-loaded Witepsol microparticles manufactured using a spray congealing procedure: Implications for topical photodynamic therapy. J. Pharm. Pharmacol. 2009, 61, 1125–1135. [Google Scholar] [CrossRef] [PubMed]



| Biopolymers | Therapeutic Properties |
|---|---|
| Fucoidan | Skin protection, antioxidant, antiaging, antiviral, anti-inflammatory, anticoagulant, and antitumor |
| Agar | Emollient |
| Collagen | Moisturizing and anti-inflammatory effect |
| Gelatin | Moisturizing effect and antioxidant activity |
| Chitosan | Healing, microbicide, mucoadhesiveness |
| Hyaluronic acid | Healing, swelling in soft tissue |
| Chondroitin and Chondroitin Sulfate | Healing and anti-inflammatory |
| Lipophilic Compounds | Sources |
|---|---|
| Emulsifiers and surface-active compounds | Lecithin, mono- and di-glyceride ester, fatty acids, sucrose fatty ester |
| Essential oils | Essential oils of camphor, mint, and citrus |
| Natural resins | Chile tree (M. zapota, M. chicle, M. staminodella, and M. bidentata), olibanum (extracted from trees of the genus Boswellia), castor seed, and others |
| Oils, fats, margarine | Animal or vegetable oils and fats (peanuts, coconut, milk, sebum and others) Fractioned, concentrated and/or reconstituted oils and fats (fatty acids, mono-, di-, triacylglycerols, and others) Hydrogenated and/or trans-esterified oils (margarines, and others) |
| Waxes | Natural vegetable and animal waxes: carnauba, bee, whale, and others) Artificial waxes: paraffins, minerals, microcrystalline, oxidized, and non-oxidized polyethylene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souto, E.B.; Yoshida, C.M.P.; Leonardi, G.R.; Cano, A.; Sanchez-Lopez, E.; Zielinska, A.; Viseras, C.; Severino, P.; Silva, C.F.d.; Barbosa, R.d.M. Lipid-Polymeric Films: Composition, Production and Applications in Wound Healing and Skin Repair. Pharmaceutics 2021, 13, 1199. https://doi.org/10.3390/pharmaceutics13081199
Souto EB, Yoshida CMP, Leonardi GR, Cano A, Sanchez-Lopez E, Zielinska A, Viseras C, Severino P, Silva CFd, Barbosa RdM. Lipid-Polymeric Films: Composition, Production and Applications in Wound Healing and Skin Repair. Pharmaceutics. 2021; 13(8):1199. https://doi.org/10.3390/pharmaceutics13081199
Chicago/Turabian StyleSouto, Eliana B., Cristiana M. P. Yoshida, Gislaine R. Leonardi, Amanda Cano, Elena Sanchez-Lopez, Aleksandra Zielinska, César Viseras, Patricia Severino, Classius F. da Silva, and Raquel de M. Barbosa. 2021. "Lipid-Polymeric Films: Composition, Production and Applications in Wound Healing and Skin Repair" Pharmaceutics 13, no. 8: 1199. https://doi.org/10.3390/pharmaceutics13081199
APA StyleSouto, E. B., Yoshida, C. M. P., Leonardi, G. R., Cano, A., Sanchez-Lopez, E., Zielinska, A., Viseras, C., Severino, P., Silva, C. F. d., & Barbosa, R. d. M. (2021). Lipid-Polymeric Films: Composition, Production and Applications in Wound Healing and Skin Repair. Pharmaceutics, 13(8), 1199. https://doi.org/10.3390/pharmaceutics13081199












