Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy
Abstract
1. Introduction
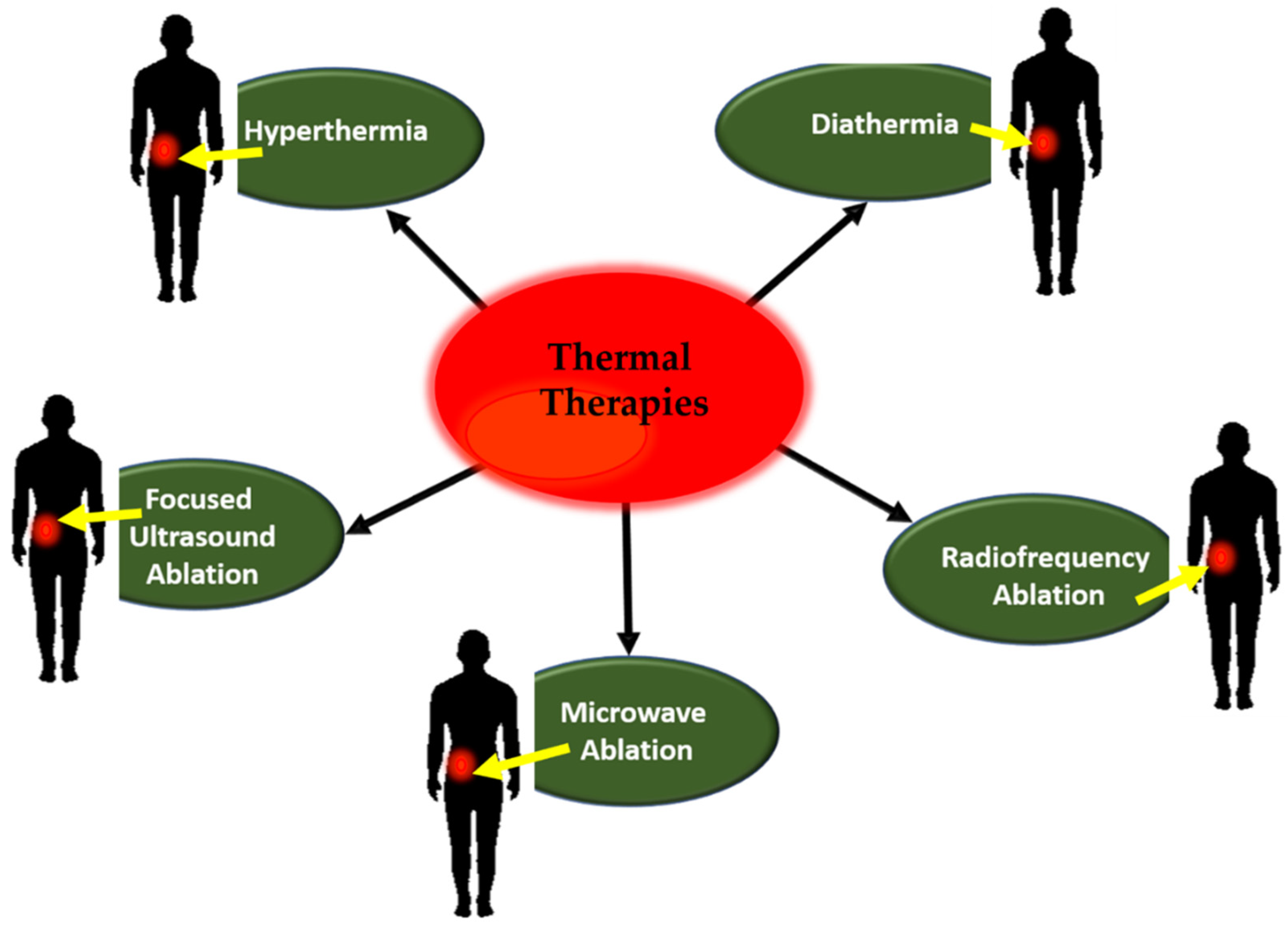
2. Thermal Therapies
2.1. Principle and Mechanism of Thermal Therapy
2.2. Hyperthermia
2.2.1. Local Hyperthermia
2.2.2. Regional Hyperthermia
- Regional perfusion
- Hyperthermic intraperitoneal chemotherapy
2.2.3. Whole Body Hyperthermia
2.3. Diathermia
2.4. Other Conventional Thermal Ablation Techniques
2.4.1. Radiofrequency Ablation
2.4.2. Microwave Ablation
2.4.3. Focused Ultrasound Ablation
2.5. Role of Nanotechnology in Thermal Therapies
2.5.1. Magnetic Nanoparticles Dependent Thermal Therapy: Mechanism of Action
Iron Oxide Nanoparticles
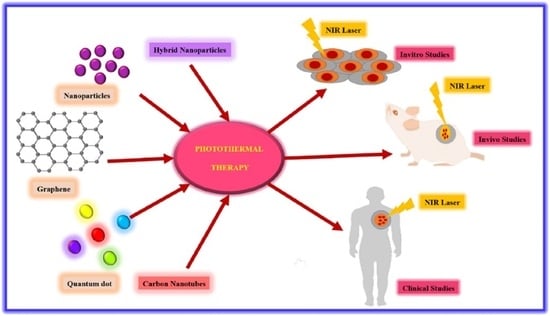
2.6. Photothermal Therapy by Nanoparticles: Mechanism of Action
2.7. Photothermal Therapy by Laser Applicator: Mechanism of Action
3. Laser Technology for Photothermal Therapy
Type of Lasers
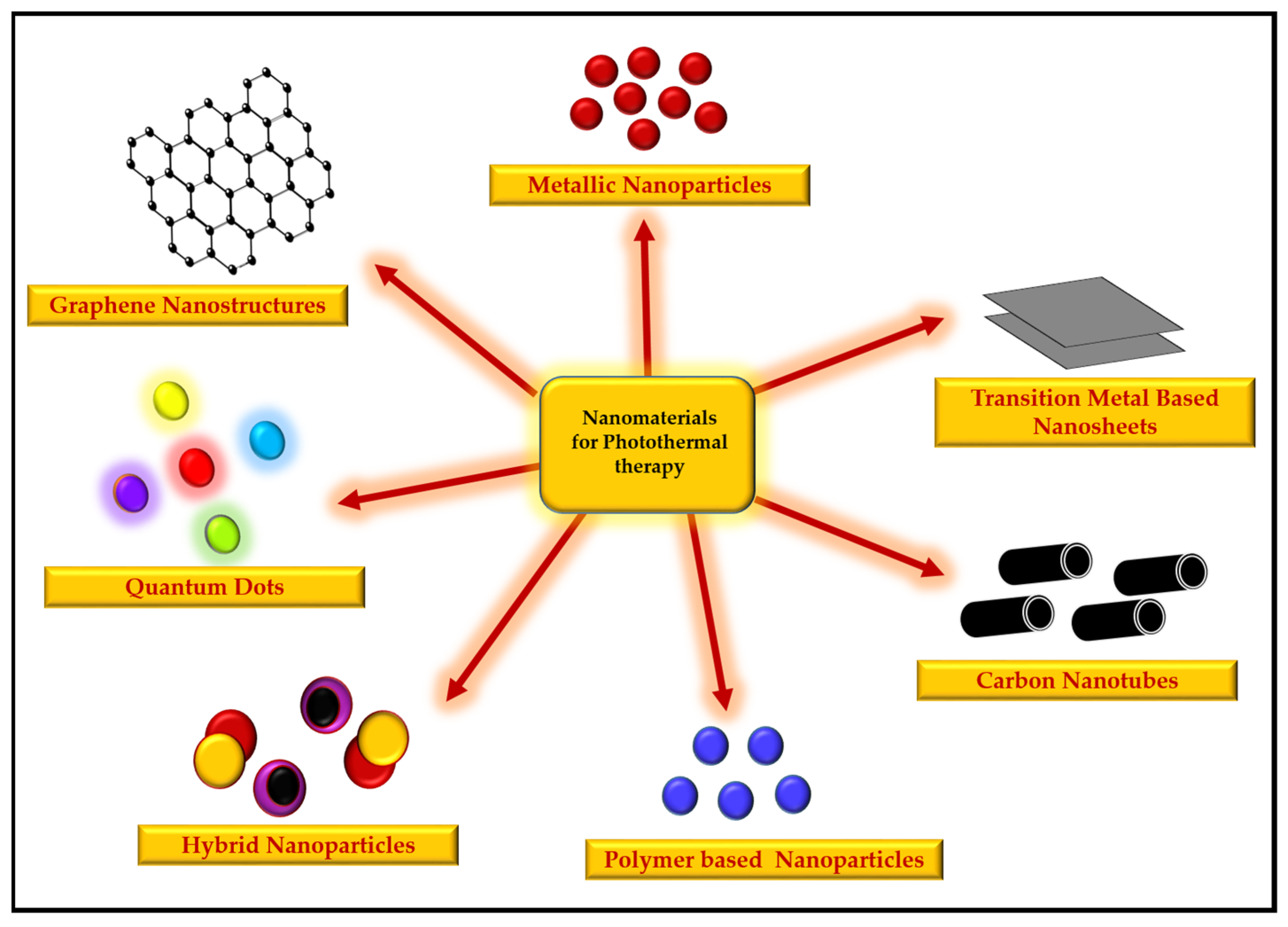
4. Potential of Nanomaterials for Photothermal Therapy
4.1. Metallic Nanoparticles
4.2. Graphene Nanostructures
4.3. Carbon Nanotubes
4.4. Quantum Dots
4.5. Hybrid Nanoparticles
4.6. Transition-Metal-Based Nanosheets
4.7. Polymer-Based Nanoparticles
5. Effect of Nanoparticle Shapes and Sizes on Photothermal Therapy
6. Application of Nanomaterials in Cancer Therapy Studies
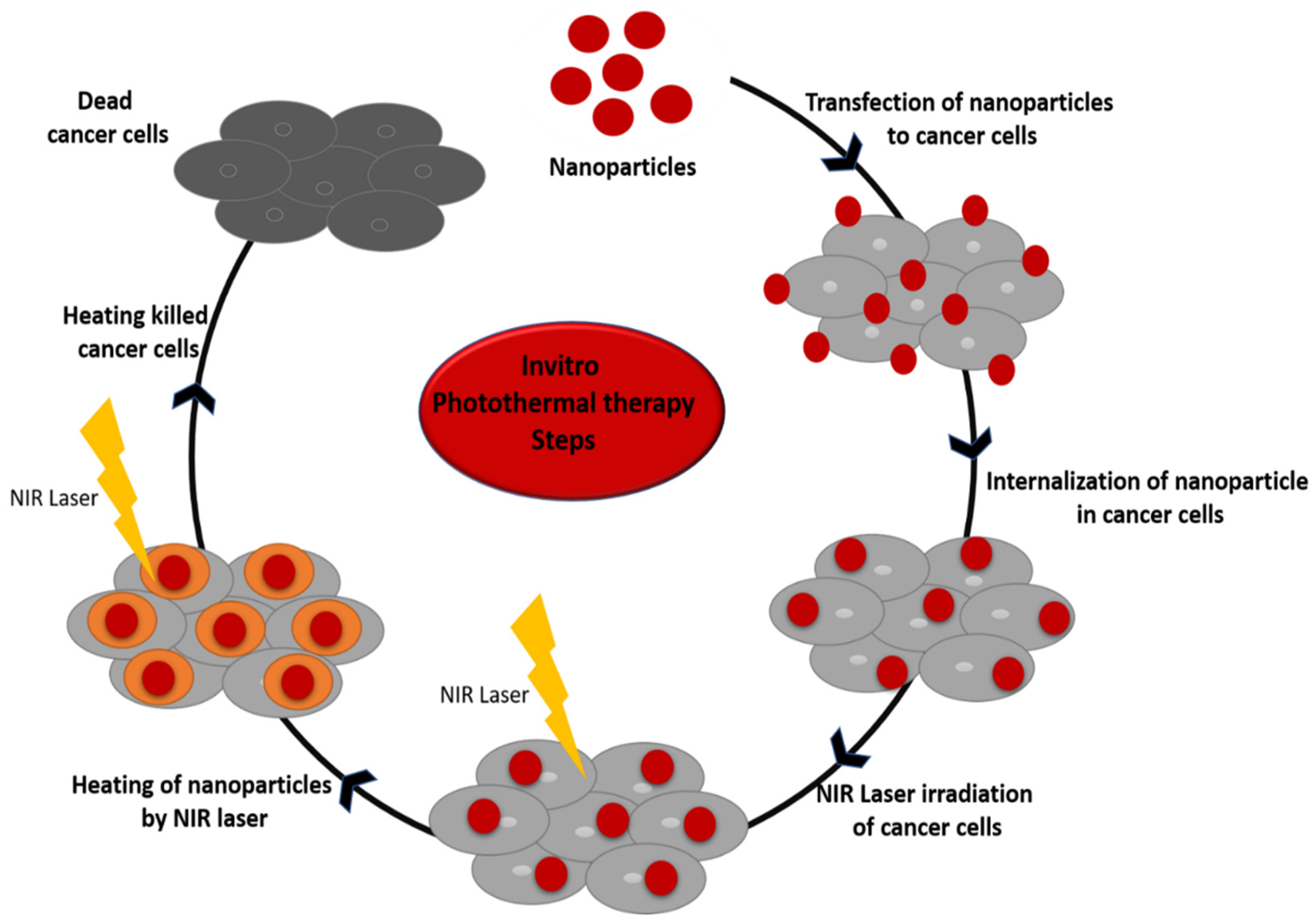
6.1. In Vitro Photothermal Therapy
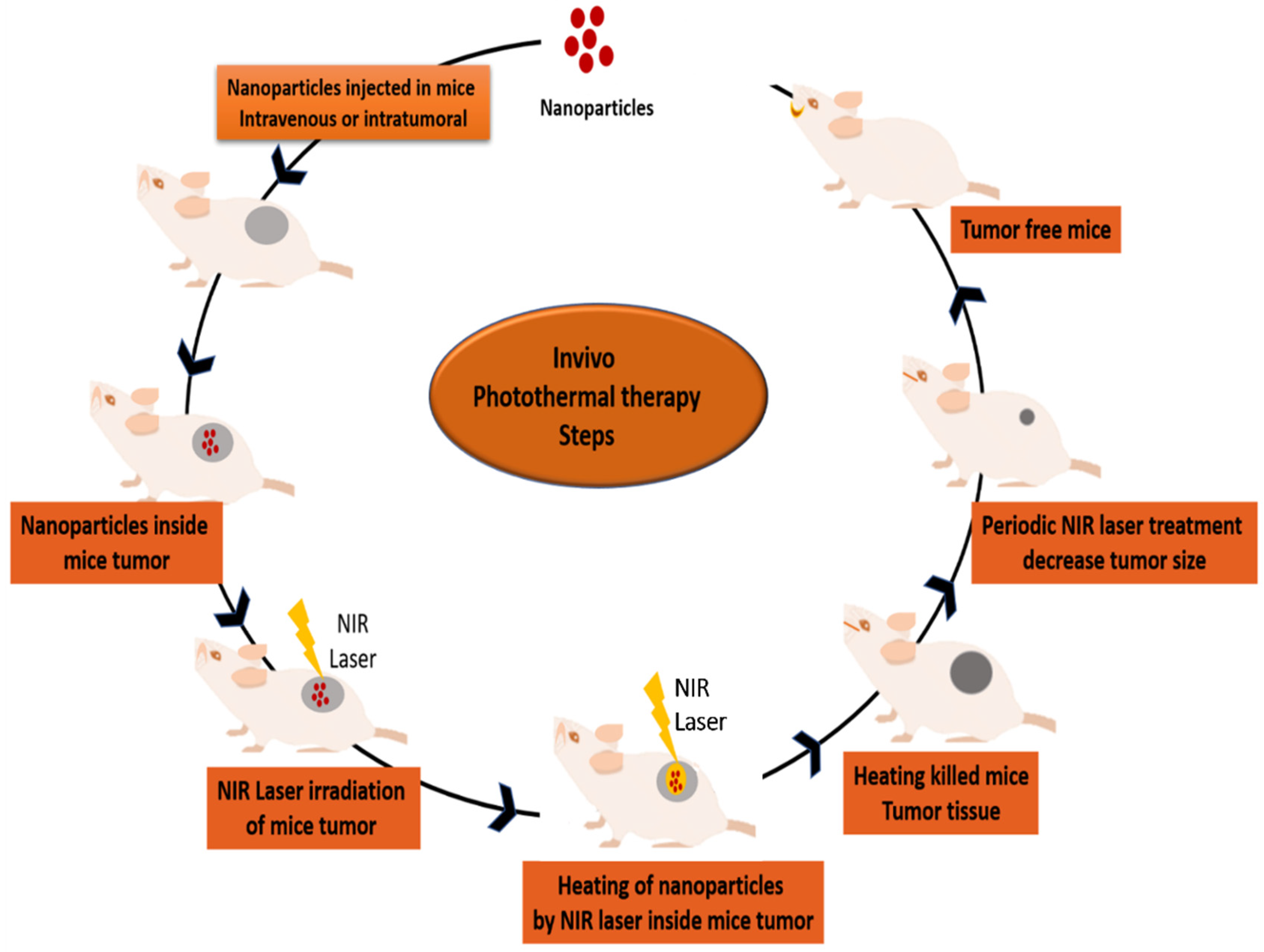
6.2. In Vivo Photothermal Therapy
6.3. Photothermal Therapy in Human Clinical Trials
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussein, E.A.; Zagho, M.M.; Nasrallah, G.K.; Elzatahry, A.A. Recent advances in functional nanostructures as cancer photothermal therapy. Int. J. Nanomed. 2018, 13, 2897. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA A Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current challenges in cancer treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tran, S.; DeGiovanni, P.J.; Piel, B.; Rai, P. Cancer nanomedicine: A review of recent success in drug delivery. Clin. Trans. Med. 2017, 6, 1–21. [Google Scholar] [CrossRef]
- Hildebrandt, B.; Wust, P.; Ahlers, O.; Dieing, A.; Sreenivasa, G.; Kerner, T.; Felix, R.; Riess, H. The cellular and molecular basis of hyperthermia. Crit. Rev. Oncol. Hematol. 2002, 43, 33–56. [Google Scholar] [CrossRef]
- Roti Roti, J.L. Cellular responses to hyperthermia (40–46 C): Cell killing and molecular events. Int. J. Hyperth. 2008, 24, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Chicheł, A.; Skowronek, J.; Kubaszewska, M.; Kanikowski, M. Hyperthermia–description of a method and a review of clinical applications. Rep. Pract. Oncol. Radiother. 2007, 12, 267–275. [Google Scholar] [CrossRef]
- Burlaka, A.; Lukin, S.; Prylutska, S.; Remeniak, O.; Prylutskyy, Y.; Shuba, M.; Maksimenko, S.; Ritter, U.; Scharff, P. Hyperthermic effect of multi-walled carbon nanotubes stimulated with near infrared irradiation for anticancer therapy: In vitro studies. Exp. Oncol. 2010, 32, 48–50. [Google Scholar] [PubMed]
- Mackowiak, P.A. Temperature regulation and the pathogenesis of fever. In Principles and Practice of Infectious Diseases; Mandell, G.L., Bennett, J.E., Dolin, R., Eds.; Elsevier Churchill Livingstone: Philadelphia, PA, USA, 2000. [Google Scholar]
- Rangel, L. (Ed.) Cancer Treatment: Conventional and Innovative Approaches; IntechOpen: London, UK, 2013. [Google Scholar]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Review Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, e497. [Google Scholar] [CrossRef]
- Van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 1: An introduction to thermal therapy. Crit. Rev. Biomed. Eng. 2006, 34, 459–489. [Google Scholar] [CrossRef]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, M.; Gneveckow, U.; Eckelt, L.; Feussner, A.; Waldöfner, N.; Scholz, R.; Deger, S.; Wust, P.; Loening, S.A.; Jordan, A. Clinical hyperthermia of prostate cancer using magnetic nanoparticles: Presentation of a new interstitial technique. Int. J. Hyperth. 2005, 21, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.P.; Brace, C.L. Interstitial microwave treatment for cancer: Historical basis and current techniques in antenna design and performance. Int. J. Hyperth. 2017, 33, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, G.; Szigeti, G.P.; Szász, A. Hyperthermia versus oncothermia: Cellular effects in complementary cancer therapy. Evid. Based Complementary Altern. Med. 2013, 2013, 672873. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Sharma, P.K.; Malviya, R. Hyperthermia: Role and risk factor for cancer treatment. Achiev. Life Sci. 2016, 10, 161–167. [Google Scholar] [CrossRef]
- Mellal, I.; Oukaira, A.; Kengene, E.; Lakhssassi, A. Thermal therapy modalities for cancer treatment: A review and future perspectives. Appl. Sci. Res. Rev. 2017, 4, 14. [Google Scholar] [CrossRef]
- Fisher, J.W.; Sarkar, S.; Buchanan, C.F.; Szot, C.S.; Whitney, J.; Hatcher, H.C.; Torti, S.V.; Rylander, C.G.; Rylander, M.N. Photothermal response of human and murine cancer cells to multiwalled carbon nanotubes after laser irradiation. Cancer Res. 2010, 70, 9855–9864. [Google Scholar] [CrossRef]
- Takahashi, H.; Niidome, T.; Nariai, A.; Niidome, Y.; Yamada, S. Photothermal reshaping of gold nanorods prevents further cell death. Nanotechnology 2006, 17, 4431. [Google Scholar] [CrossRef]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, part 2: Hyperthermia techniques. Crit. Rev. Biomed. Eng. 2006, 34, 491–542. [Google Scholar] [CrossRef]
- Glazer, E.S.; Curley, S.A. The ongoing history of thermal therapy for cancer. Surg. Oncol Clin. 2011, 20, 229–235. [Google Scholar] [CrossRef]
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Busetti, A.; Soncin, M.; Reddi, E.; Rodgers, M.A.; Kenney, M.E.; Jori, G. Photothermal sensitization of amelanotic melanoma cells by Ni (II)-octabutoxy-naphthalocyanine. J. Photochem. Photobiol. B Biol. 1999, 53, 103–109. [Google Scholar] [CrossRef]
- Seifert, G.; Budach, V.; Keilholz, U.; Wust, P.; Eggert, A.; Ghadjar, P. Regional hyperthermia combined with chemotherapy in paediatric, adolescent and young adult patients: Current and future perspectives. Radiat. Oncol. 2016, 11, 65. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.R.; Ordóñez, S.G.; Gaipl, U.S.; Paulides, M.M.; Crezee, H.; Gellermann, J.; Marder, D.; Puric, E.; Bodis, S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015, 41, 742–753. [Google Scholar] [CrossRef]
- Trefná, H.D.; Martinsson, B.; Petersson, T.; Renström, N.; Torstensson, M.; Ravanis, J.; Kok, P.; Persson, M. Multifrequency approach in hyperthermia treatment planning: Impact of frequency on SAR distribution in head and neck. In Proceedings of the 2017 11th European Conference on Antennas and Propagation (EUCAP), Paris, France, 19–24 March 2017; pp. 3710–3712. [Google Scholar]
- Behrouzkia, Z.; Joveini, Z.; Keshavarzi, B.; Eyvazzadeh, N.; Aghdam, R.Z. Hyperthermia: How can it be used? Oman Med. J. 2016, 31, 89. [Google Scholar] [CrossRef]
- Van der Zee, J.; Gonzalez, D.; van Rhoon, G.C. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000, 355, 1119–1125. [Google Scholar] [CrossRef]
- Lepock, J.R.; Cheng, K.H.; Al-qysi, H.; Sim, I.; Koch, C.J.; Kruuv, J. Hyperthermia-induced inhibition of respiration and mitochondrial protein denaturation in CHL cells. Int. J. Hyperth. 1987, 3, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Lepock, J.R.; Frey, H.E.; Rodahl, A.M.; Kruuv, J. Thermal analysis of CHL V79 cells using differential scanning calorimetry: Implications for hyperthermic cell killing and the heat shock response. J. Cell Physiol. 1988, 137, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Fingar, V.H. Relationship of tumor hypoxia and response to photodynamic treatment in an experimental mouse tumor. Cancer Res. 1987, 47, 3110–3114. [Google Scholar]
- Aziz, M.N.; Salim, M.I.; Wahab, A.A.; Abd Manaf, N. A feasibility study of ultrasound as a monitoring method for hyperthermia therapy. In Proceedings of the 2015 IEEE Student Conference on Research and Development (SCOReD), Kuala Lumpur, Malaysia, 13–14 December 2015; pp. 407–411. [Google Scholar]
- Gao, S.; Zheng, M.; Ren, X.; Tang, Y.; Liang, X. Local hyperthermia in head and neck cancer: Mechanism, application and advance. Onco. Targets 2016, 7, 57367. [Google Scholar] [CrossRef]
- Paulides, M.M.; Bakker, J.F.; Linthorst, M.; Van der Zee, J.; Rijnen, Z.; Neufeld, E.; Pattynaa, P.M.; Jansen, P.P.; Levendag, P.C.; Van Rhoon, G.C. The clinical feasibility of deep hyperthermia treatment in the head and neck: New challenges for positioning and temperature measurement. Phys. Med. Biol. 2010, 55, 2465. [Google Scholar] [CrossRef] [PubMed]
- Law, T. Brain Cooling. In The Future of Thermal Comfort in an Energy-Constrained World; Springer: Heidelberg, Germany, 2013; pp. 53–82. [Google Scholar]
- Garrett, C.L.; Draper, D.O.; Knight, K.L. Heat distribution in the lower leg from pulsed short-wave diathermy and ultrasound treatments. J. Athl. Train. 2000, 35, 50. [Google Scholar] [PubMed]
- Ni, Y.; Mulier, S.; Miao, Y.; Michel, L.; Marchal, G. A review of the general aspects of radiofrequency ablation. Abdom. Imaging 2005, 30, 381–400. [Google Scholar] [CrossRef]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal therapy, Part III: Ablation techniques. Crit. Rev. Biomed. Eng. 2007, 35, 37–121. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Bhowmik, A.; Repaka, R. Thermal analysis of induced damage to the healthy cell during RFA of breast tumor. J. Ther. Biol. 2016, 58, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.N.; Fornage, B.D.; Sneige, N.; Kuerer, H.M.; Newman, L.A.; Ames, F.C.; Singletary, S.E. Radiofrequency ablation of solid tumors. Cancer J. 2001, 7, 95–102. [Google Scholar]
- Zhao, W.; Chen, J.Z.; Hu, J.H.; Huang, J.Q.; Jiang, Y.N.; Luo, G.; Yi, G.F.; Peng, Z.H.; Wang, H.; Shen, J.; et al. In vivo effects of radiofrequency ablation on long bones and the repair process in swine models. Jpn. J. Radiol. 2017, 35, 31–39. [Google Scholar] [CrossRef]
- Knavel, E.M.; Brace, C.L. Tumor ablation: Common modalities and general practices. Tech. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef]
- Abadeer, N.S.; Murphy, C.J. Recent progress in cancer thermal therapy using gold nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, Y.Y.; Jon, S. A drug-loaded aptamer− gold nanoparticle bioconjugate for combined CT imaging and therapy of prostate cancer. ACS Nano 2010, 4, 3689–3696. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Sefah, K.; Bamrungsap, S.; Chang, H.T.; Tan, W. Selective photothermal therapy for mixed cancer cells using aptamer-conjugated nanorods. Langmuir 2008, 24, 11860–11865. [Google Scholar] [CrossRef]
- Sanhai, W.R.; Sakamoto, J.H.; Canady, R.; Ferrari, M. Seven challenges for nanomedicine. Nat. Nanotechnol. 2008, 3, 242–244. [Google Scholar] [CrossRef]
- Friedman, M.; Mikityansky, I.; Kam, A.; Libutti, S.K.; Walther, M.M.; Neeman, Z.; Locklin, J.K.; Wood, B.J. Radiofrequency ablation of cancer. Cardiovas. Interv. Radiol. 2004, 27, 427–434. [Google Scholar] [CrossRef]
- Glazer, E.S.; Curley, S.A. Non-invasive radiofrequency ablation of malignancies mediated by quantum dots, gold nanoparticles and carbon nanotubes. Ther. Deliv. 2011, 2, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.H.; Wainerdi, S.M.; Cherukuri, T.K.; Kittrell, C.; Wiley, B.J.; Nicholas, N.W.; Curley, S.A.; Kanzius, J.S.; Cherukuri, P. Size-dependent joule heating of gold nanoparticles using capacitively coupled radiofrequency fields. Nano Res. 2009, 2, 400–405. [Google Scholar] [CrossRef]
- Lubner, M.G.; Brace, C.L.; Hinshaw, J.L.; Lee Jr, F.T. Microwave tumor ablation: Mechanism of action, clinical results, and devices. J. Vasc. Interv. Radiol. 2010, 21, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Curto, S.; Taj-Eldin, M.; Fairchild, D.; Prakash, P. Microwave ablation at 915 MHz vs 2.45 GHz: A theoretical and experimental investigation. Med. Phys. 2015, 42, 6152–6161. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.O.; Mougenot, C.; Quesson, B.; Enholm, J.; Le Bail, B.; Laurent, C.; Moonen, C.T.; Ehnholm, G.J. Volumetric HIFU ablation under 3D guidance of rapid MRI thermometry. Med. Phys. 2009, 36, 3521–3535. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.B. High-intensity focused ultrasound tumor ablation: Review of ten years of clinical experience. Front. Med. China 2010, 4, 294–302. [Google Scholar] [CrossRef]
- Dubinsky, T.J.; Cuevas, C.; Dighe, M.K.; Kolokythas, O.; Hwang, J.H. High-intensity focused ultrasound: Current potential and oncologic applications. Am. J. Roentgenol. 2008, 190, 191–199. [Google Scholar] [CrossRef]
- Illing, R.O.; Kennedy, J.E.; Wu, F.; Ter Haar, G.R.; Protheroe, A.S.; Friend, P.J.; Gleeson, F.V.; Cranston, D.W.; Phillips, R.R.; Middleton, M.R. The safety and feasibility of extracorporeal high-intensity focused ultrasound (HIFU) for the treatment of liver and kidney tumours in a Western population. Br. J. Cancer 2005, 93, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Crouzet, S.; Rouviere, O.; Martin, X.; Gelet, A. High-intensity focused ultrasound as focal therapy of prostate cancer. Curr. Opin. Urol. 2014, 24, 225–230. [Google Scholar] [CrossRef]
- Poissonnier, L.; Chapelon, J.Y.; Rouviere, O.; Curiel, L.; Bouvier, R.; Martin, X.; Dubernard, J.M.; Gelet, A. Control of prostate cancer by transrectal HIFU in 227 patients. Eur. Urol. 2007, 51, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, G.A.; Mohazzabi, P.; McCormack, P.; Jabbari, S. Nanotechnology in cancer prevention, detection and treatment: Bright future lies ahead. World Rev. Sci. Tech. Sustain. Dev. 2007, 4, 226–257. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400. [Google Scholar] [CrossRef]
- Lu, B.Q.; Zhu, Y.J.; Ao, H.Y.; Qi, C.; Chen, F. Synthesis and characterization of magnetic iron oxide/calcium silicate mesoporous nanocomposites as a promising vehicle for drug delivery. ACS Appl. Mater. Interfaces 2012, 4, 6969–6974. [Google Scholar] [CrossRef]
- Fekrazad, R.; Naghdi, N.; Nokhbatolfoghahaei, H.; Bagheri, H. The combination of laser therapy and metal nanoparticles in cancer treatment originated from epithelial tissues: A literature review. J. Lasers Med. Sci. 2016, 7, 62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manthe, R.L.; Foy, S.P.; Krishnamurthy, N.; Sharma, B.; Labhasetwar, V. Tumor ablation and nanotechnology. Mol. Pharm. 2010, 7, 1880–1898. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Wust, P.; Fählin, H.; John, W.; Hinz, A.; Felix, R. Inductive heating of ferrimagnetic particles and magnetic fluids: Physical evaluation of their potential for hyperthermia. Int. J. Hyperth 1993, 9, 51–68. [Google Scholar] [CrossRef]
- Johannsen, M.; Thiesen, B.; Wust, P.; Jordan, A. Magnetic nanoparticle hyperthermia for prostate cancer. Int. J. Hyperth 2010, 26, 790–795. [Google Scholar] [CrossRef]
- Salloum, M.; Ma, R.H.; Weeks, D.; Zhu, L. Controlling nanoparticle delivery in magnetic nanoparticle hyperthermia for cancer treatment: Experimental study in agarose gel. Int. J. Hyperth 2008, 24, 337–345. [Google Scholar] [CrossRef]
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia enables tumor-specific nanoparticle delivery: Effect of particle size. Cancer Res. 2000, 60, 4440–4445. [Google Scholar] [PubMed]
- Vegerhof, A.; Motei, M.; Rudinzky, A.; Malka, D.; Popovtzer, R.; Zalevsky, Z. Thermal therapy with magnetic nanoparticles for cell destruction. Biomed. Opt. Exp. 2016, 7, 4581–4594. [Google Scholar] [CrossRef] [PubMed]
- Ashikbayeva, Z.; Tosi, D.; Balmassov, D.; Schena, E.; Saccomandi, P.; Inglezakis, V. Application of nanoparticles and nanomaterials in thermal ablation therapy of cancer. Nanomaterials 2019, 9, 1195. [Google Scholar] [CrossRef]
- Mornet, S.; Vasseur, S.; Grasset, F.; Duguet, E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 2004, 14, 2161–2175. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic particle hyperthermia: Nanoparticle magnetism and materials development for cancer therapy. J. Phys. Condens. Matter 2006, 18, S2919. [Google Scholar] [CrossRef]
- Thiesen, B.; Jordan, A. Clinical applications of magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2008, 24, 467–474. [Google Scholar] [CrossRef]
- Ito, A.; Shinkai, M.; Honda, H.; Kobayashi, T. Medical application of functionalized magnetic nanoparticles. J. Biosci. Bioeng. 2005, 100, 1–11. [Google Scholar] [CrossRef]
- Soustelle, L.; Aigouy, B.; Asensio, M.L.; Giangrande, A. UV laser mediated cell selective destruction by confocal microscopy. Neural Dev. 2008, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Campos, I.; Asín, L.; Torres, T.E.; Marquina, C.; Tres, A.; Ibarra, M.R.; Goya, G.F. Cell death induced by the application of alternating magnetic fields to nanoparticle-loaded dendritic cells. Nanotechnology 2011, 22, 205101. [Google Scholar] [CrossRef]
- Kobayashi, T. Intracellular Hyperthermia Using Magnetic Nanoparticles: A Novel Method for Hyperthermia Clinical Applications. Ther. Med. 2008, 2, 113–129. [Google Scholar]
- Laurent, S.; Mahmoudi, M. Superparamagnetic iron oxide nanoparticles: Promises for diagnosis and treatment of cancer. Int J. Mol. Epidemiol. Genet. 2011, 2, 367. [Google Scholar] [PubMed]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef]
- Lartigue, L.; Alloyeau, D.; Kolosnjaj-Tabi, J.; Javed, Y.; Guardia, P.; Riedinger, A.; Péchoux, C.; Pellegrino, T.; Wilhelm, C.; Gazeau, F. Biodegradation of iron oxide nanocubes: High-resolution in situ monitoring. ACS Nano 2013, 7, 3939–3952. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, J.T.; Choi, J.S.; Moon, S.H.; Noh, S.H.; Kim, J.W.; Kim, J.G.; Kim, I.S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol 2011, 6, 418–422. [Google Scholar] [CrossRef]
- Yang, H.W.; Hua, M.Y.; Liu, H.L.; Huang, C.Y.; Tsai, R.Y.; Lu, Y.J.; Chen, J.Y.; Tang, H.J.; Hsien, H.; Chang, Y.S.; et al. Self-protecting core-shell magnetic nanoparticles for targeted, traceable, long half-life delivery of BCNU to gliomas. Biomaterials 2011, 32, 6523–6532. [Google Scholar] [CrossRef]
- Sohail, A.; Ahmad, Z.; Bég, O.A.; Arshad, S.; Sherin, L. A review on hyperthermia via nanoparticle-mediated therapy. Bull. Cancer 2017, 104, 452–461. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Chu, M.; Shao, Y.; Peng, J.; Dai, X.; Li, H.; Wu, Q.; Shi, D. Near-infrared laser light mediated cancer therapy by photothermal effect of Fe3O4 magnetic nanoparticles. Biomaterials 2013, 34, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Xiu-Li, Y.; Fang, M.; Zhi-Fei, D. Multifunctional magnetic nanoparticles for magnetic resonance image-guided photothermal therapy for cancer. Chin. Phys. B 2014, 23, 044301. [Google Scholar]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer cells assemble and align gold nanorods conjugated to antibodies to produce highly enhanced, sharp, and polarized surface Raman spectra: A potential cancer diagnostic marker. Nano Lett. 2007, 7, 1591–1597. [Google Scholar] [CrossRef]
- Camerin, M.; Rello, S.; Villanueva, A.; Ping, X.; Kenney, M.E.; Rodgers, M.A.; Jori, G. Photothermal sensitisation as a novel therapeutic approach for tumours: Studies at the cellular and animal level. Eur. J. Cancer 2005, 41, 1203–1212. [Google Scholar] [CrossRef]
- Camerin, M.; Rodgers, M.A.; Kenney, M.E.; Jori, G. Photothermal sensitisation: Evidence for the lack of oxygen effect on the photosensitising activity. Photochem. Photobiol. Sci. 2005, 4, 251–253. [Google Scholar] [CrossRef]
- He, X.; Bischof, J.C. Quantification of temperature and injury response in thermal therapy and cryosurgery. Crit. Rev. Biomed. Eng. 2003, 31, 67. [Google Scholar] [CrossRef]
- Sheng, W.; He, S.; Seare, W.J.; Almutairi, A. Review of the progress toward achieving heat confinement—The holy grail of photothermal therapy. J. Biomed. Opt. 2017, 22, 080901. [Google Scholar] [CrossRef]
- Kalambur, V.S.; Han, B.; Hammer, B.E.; Shield, T.W.; Bischof, J.C. In vitro characterization of movement, heating and visualization of magnetic nanoparticles for biomedical applications. Nanotechnology 2005, 16, 1221. [Google Scholar] [CrossRef]
- Kawashita, M.; Domi, S.; Saito, Y.; Aoki, M.; Ebisawa, Y.; Kokubo, T.; Saito, T.; Takano, M.; Araki, N.; Hiraoka, M. In vitro heat generation by ferrimagnetic maghemite microspheres for hyperthermic treatment of cancer under an alternating magnetic field. J. Mater. Sci. Mater. Med. 2008, 19, 1897–1903. [Google Scholar] [CrossRef]
- Roggan, A.; Müller, G. Dosimetry and computer based irradiation planning for laser-induced interstitial thermotherapy (LITT). In Laser-Induced Interstitial Thermotherapy; SPIE Optical Engineering Press: Washington, DC, USA, 1995; pp. 114–156. [Google Scholar]
- Izzo, F. Other thermal ablation techniques: Microwave and interstitial laser ablation of liver tumors. Ann. Surg. Oncol. 2003, 10, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Stafford, R.J.; Fuentes, D.; Elliott, A.A.; Weinberg, J.S.; Ahrar, K. Laser-induced thermal therapy for tumor ablation. Crit. Rev. Biomed. Eng. 2010, 38, 79–100. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Schena, E.; Saccomandi, P.; Fong, Y. Laser ablation for cancer: Past, present and future. J. Funct. Biomater. 2017, 8, 19. [Google Scholar] [CrossRef]
- Bown, S.G. Phototherapy of tumors. World J. Surg 1983, 7, 700–709. [Google Scholar] [CrossRef]
- Muschter, R.; Hofstetter, A. Interstitial laser therapy outcomes in benign prostatic hyperplasia. J. Endourol. 1995, 9, 129–135. [Google Scholar] [CrossRef]
- Saccomandi, P.; Schena, E.; Caponero, M.A.; Di Matteo, F.M.; Martino, M.; Pandolfi, M.; Silvestri, S. Theoretical analysis and experimental evaluation of laser-induced interstitial thermotherapy in ex vivo porcine pancreas. IEEE Trans. Biomed. Eng. 2012, 59, 2958–2964. [Google Scholar] [CrossRef]
- Nikfarjam, M.; Christophi, C. Interstitial laser thermotherapy for liver tumours. Br. J. Surg. 2003, 90, 1033–1047. [Google Scholar] [CrossRef]
- Beuthan, J.; Dressler, C.; Minet, O.; Müller, G. Dosimetric investigations of laser-induced phase transition of MX1-cell membranes by use of quantum dots. Laser Phys. 2006, 16, 808–815. [Google Scholar] [CrossRef]
- Lee, J.; Chatterjee, D.K.; Lee, M.H.; Krishnan, S. Gold nanoparticles in breast cancer treatment: Promise and potential pitfalls. Cancer Lett. 2014, 347, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Khoury, C.G.; Wilson, C.M.; Grant, G.A.; Bennett, A.J.; Vo-Dinh, T. In vivo particle tracking and photothermal ablation using plasmon-resonant gold nanostars. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1355–1363. [Google Scholar] [CrossRef]
- Ye, X.; Gao, Y.; Chen, J.; Reifsnyder, D.C.; Zheng, C.; Murray, C.B. Seeded growth of monodisperse gold nanorods using bromide-free surfactant mixtures. Nano Lett. 2013, 13, 2163–2171. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, M.; Hasan, N.; Wu, H.F. Platinum nanoparticles for the photothermal treatment of Neuro 2A cancer cells. Biomaterials 2013, 34, 5833–5842. [Google Scholar] [CrossRef] [PubMed]
- Li, K.C.; Chu, H.C.; Lin, Y.; Tuan, H.Y.; Hu, Y.C. PEGylated copper nanowires as a novel photothermal therapy agent. ACS Appl. Mater. Interfaces 2016, 8, 12082–12090. [Google Scholar] [CrossRef]
- Jin, Y.; Wang, J.; Ke, H.; Wang, S.; Dai, Z. Graphene oxide modified PLA microcapsules containing gold nanoparticles for ultrasonic/CT bimodal imaging guided photothermal tumor therapy. Biomaterials 2013, 34, 4794–4802. [Google Scholar] [CrossRef]
- Chen, Y.W.; Chen, P.J.; Hu, S.H.; Chen, I.W.; Chen, S.Y. NIR-triggered synergic photo chemothermal therapy delivered by reduced graphene oxide/carbon/mesoporous silica nanocookies. Adv. Funct. Mater. 2014, 24, 451–459. [Google Scholar] [CrossRef]
- Gonçalves, G.; Marques, P.; Vila, M. Graphene-Based Materials in Health and Environment; Springer International Publishing: Berlin, Germany, 2017. [Google Scholar]
- Gong, F.; Liu, X.; Yang, Y.; Xia, D.; Wang, W.; Duong, H.M.; Papavassiliou, D.V.; Xu, Z.; Liao, J.; Wu, M. A facile approach to tune the electrical and thermal properties of graphene aerogels by including bulk MoS2. Nanomaterials 2017, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, J.Q.; Qian, W.Z.; Zhang, Y.Y.; Wei, F. The road for nanomaterials industry: A review of carbon nanotube production, post-treatment, and bulk applications for composites and energy storage. Small 2013, 9, 1237–1265. [Google Scholar] [CrossRef]
- Fang, Y.; Lv, Y.; Gong, F.; Wu, Z.; Li, X.; Zhu, H.; Zhou, L.; Yao, C.; Zhang, F.; Zheng, G.; et al. Interface tension-induced synthesis of monodispersed mesoporous carbon hemispheres. J. Am. Chem. Soc. 2015, 137, 2808–2811. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Gong, F.; Ding, Z.; Fang, Y.; Tong, C.J.; Xia, D.; Lv, Y.; Wang, B.; Papavassiliou, D.V.; Liao, J.; Wu, M. Enhanced electrochemical and thermal transport properties of graphene/MoS2 heterostructures for energy storage: Insights from multiscale modeling. ACS Appl. Mater. Interfaces 2018, 10, 14614–14621. [Google Scholar] [CrossRef]
- Gong, F.; Wang, W.; Li, H.; Xia, D.; Papavassiliou, D.V. Predictions of the thermal conductivity of multiphase nanocomposites with complex structures. J. Mater. Sci. 2018, 53, 12157–12166. [Google Scholar] [CrossRef]
- Gong, F.; Li, H.; Wang, W.; Xia, D.; Liu, Q.; Papavassiliou, D.V.; Xu, Z. Recent advances in graphene-based free-standing films for thermal management: Synthesis, properties, and applications. Coatings 2018, 8, 63. [Google Scholar] [CrossRef]
- Whitney, J.R.; Rodgers, A.; Harvie, E.; Carswell, W.F.; Torti, S.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; Rylander, C.G.; Rylander, M.N. Spatial and temporal measurements of temperature and cell viability in response to nanoparticle-mediated photothermal therapy. Nanomedicine 2012, 7, 1729–1742. [Google Scholar] [CrossRef] [PubMed]
- Hoseini-Ghahfarokhi, M.; Mirkiani, S.; Mozaffari, N.; Sadatlu, M.A.; Ghasemi, A.; Abbaspour, S.; Akbarian, M.; Farjadain, F.; Karimi, M. Applications of Graphene and Graphene Oxide in Smart Drug/Gene Delivery: Is the World Still Flat? Int. J. Nanomed. 2020, 15, 9469. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.L.; Carswell, W.F.; Rodgers, A.; Kosoglu, M.A.; Rylander, M.N.; Grant, D.; Robertson, J.L.; Rylander, C.G. Spatially controlled photothermal heating of bladder tissue through single-walled carbon nanohorns delivered with a fiberoptic microneedle device. Lasers Med. Sci. 2013, 28, 1143–1150. [Google Scholar] [CrossRef] [PubMed]
- Gong, F.; Duong, H.M.; Papavassiliou, D.V. Review of recent developments on using an off-lattice monte carlo approach to predict the effective thermal conductivity of composite systems with complex structures. Nanomaterials 2016, 6, 142. [Google Scholar] [CrossRef]
- Gong, F.F.; Li, H.; Wang, W.; Huang, J.; Xia, D.D.; Liao, J.; Wu, M.; Papavassiliou, D.V. Scalable, eco-friendly and ultrafast solar steam generators based on one-step melamine-derived carbon sponges toward water purification. Nano Energy 2019, 58, 322–330. [Google Scholar] [CrossRef]
- Xia, D.D.; Gong, F.; Pei, X.; Wang, W.; Li, H.; Zeng, W.; Wu, M.; Papavassiliou, D.V. Molybdenum and tungsten disulfides-based nanocomposite films for energy storage and conversion: A review. Chem. Eng. J. 2018, 348, 908–928. [Google Scholar] [CrossRef]
- Bussy, C.; Ali-Boucetta, H.; Kostarelos, K. Safety considerations for graphene: Lessons learnt from carbon nanotubes. Acc. Chem Res. 2013, 46, 692–701. [Google Scholar] [CrossRef]
- Kostarelos, K.; Novoselov, K.S. Exploring the interface of graphene and biology. Science 2014, 344, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Savchuk, O.A.; Carvajal, J.J.; Massons, J.; Aguiló, M.; Díaz, F. Determination of photothermal conversion efficiency of graphene and graphene oxide through an integrating sphere method. Carbon 2016, 103, 134–141. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Zhang, G.; Sun, X.; Lee, S.T.; Liu, Z. Graphene in mice: Ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010, 10, 3318–3323. [Google Scholar] [CrossRef]
- Yang, L.; Tseng, Y.T.; Suo, G.; Chen, L.; Yu, J.; Chiu, W.J.; Huang, C.C.; Lin, C.H. Photothermal therapeutic response of cancer cells to aptamer–gold nanoparticle-hybridized graphene oxide under NIR illumination. ACS Appl. Mater. Interfaces 2015, 7, 5097–5106. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Bangsaruntip, S.; Drouvalakis, K.A.; Kam, N.W.; Shim, M.; Li, Y.; Kim, W.; Utz, P.J.; Dai, H. Noncovalent functionalization of carbon nanotubes for highly specific electronic biosensors. Proc. Natl. Acad. Sci. USA 2003, 100, 4984–4989. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J.J.; Wibowo, R.; Liu, J.; Yang, W.; Losic, D.; Orbons, S.; Mearns, F.J.; Shapter, J.G.; Hibbert, D.B. Protein electrochemistry using aligned carbon nanotube arrays. J. Am. Chem. Soc. 2003, 125, 9006–9007. [Google Scholar] [CrossRef]
- Baughman, R.H.; Cui, C.; Zakhidov, A.A.; Iqbal, Z.; Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Mazzoldi, A.; De Rossi, D.; Rinzler, A.G.; et al. Carbon nanotube actuators. Science 1999, 284, 1340–1344. [Google Scholar] [CrossRef]
- Pantarotto, D.; Briand, J.P.; Prato, M.; Bianco, A. Translocation of bioactive peptides across cell membranes by carbon nanotubes. Chem Commun. 2004, 1, 16–17. [Google Scholar] [CrossRef]
- Shi Kam, N.W.; Jessop, T.C.; Wender, P.A.; Dai, H. Nanotube molecular transporters: Internalization of carbon nanotube− protein conjugates into mammalian cells. J. Am. Chem. Soc. 2004, 126, 6850–6851. [Google Scholar] [CrossRef]
- Sobhani, Z.; Behnam, M.A.; Emami, F.; Dehghanian, A.; Jamhiri, I. Photothermal therapy of melanoma tumor using multiwalled carbon nanotubes. Int. J. Nanomed. 2017, 12, 4509. [Google Scholar] [CrossRef]
- Kam, N.W.; O’Connell, M.; Wisdom, J.A.; Dai, H. Carbon nanotubes as multifunctional biological transporters and near-infrared agents for selective cancer cell destruction. Proc. Natl. Acad. Sci. USA 2005, 102, 11600–11605. [Google Scholar] [CrossRef] [PubMed]
- O’connell, M.J.; Bachilo, S.M.; Huffman, C.B.; Moore, V.C.; Strano, M.S.; Haroz, E.H.; Rialon, K.L.; Boul, P.J.; Noon, W.H.; Kittrell, C.; et al. Band gap fluorescence from individual single-walled carbon nanotubes. Science 2002, 297, 593–596. [Google Scholar] [CrossRef]
- Shao, W.; Arghya, P.; Yiyong, M.; Rodes, L.; Prakash, S. Carbon nanotubes for use in medicine: Potentials and limitations. Synth. Appl. Carbon Nanotub. Compos. 2013, 13, 285–311. [Google Scholar]
- Murphy, F.A.; Poland, C.A.; Duffin, R.; Al-Jamal, K.T.; Ali-Boucetta, H.; Nunes, A.; Byrne, F.; Prina-Mello, A.; Volkov, Y.; Li, S.; et al. Length-dependent retention of carbon nanotubes in the pleural space of mice initiates sustained inflammation and progressive fibrosis on the parietal pleura. Am. J. Pathol. 2011, 178, 2587–2600. [Google Scholar] [CrossRef]
- Cheng, X.; Zhong, J.; Meng, J.; Yang, M.; Jia, F.; Xu, Z.; Kong, H.; Xu, H. Characterization of multiwalled carbon nanotubes dispersing in water and association with biological effects. J. Nanomater. 2011, 2011. [Google Scholar] [CrossRef]
- Ling, X.; Wei, Y.; Zou, L.; Xu, S. The effect of different order of purification treatments on the purity of multiwalled carbon nanotubes. Appl. Surf. Sci. 2013, 276, 159–166. [Google Scholar] [CrossRef]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Torti, S.V. Carbon nanotubes in hyperthermia therapy. Adv. Drug Deliv. Rev. 2013, 65, 2045–2060. [Google Scholar] [CrossRef]
- Zhou, F.; Da, X.; Ou, Z.; Wu, B.; Resasco, D.E.; Chen, W.R. Cancer photothermal therapy in the near-infrared region by using single-walled carbon nanotubes. J. Biomed. Opt. 2009, 14, 021009. [Google Scholar] [CrossRef] [PubMed]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.J.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Pan, X.; Zhang, D.; Wu, Q.; Peng, J.; Hai, W. The therapeutic efficacy of CdTe and CdSe quantum dots for photothermal cancer therapy. Biomaterials 2012, 33, 7071–7083. [Google Scholar] [CrossRef]
- Das, R.K.; Panda, S.; Bhol, C.S.; Bhutia, S.K.; Mohapatra, S. N-Doped Carbon Quantum Dot (NCQD)-Deposited Carbon Capsules for Synergistic Fluorescence Imaging and Photothermal Therapy of Oral Cancer. Langmuir 2019, 35, 15320–15329. [Google Scholar] [CrossRef]
- Yu, H.; Chen, M.; Rice, P.M.; Wang, S.X.; White, R.L.; Sun, S. Dumbbell-like bifunctional Au−Fe3O4 nanoparticles. Nano Lett. 2005, 5, 379–382. [Google Scholar] [CrossRef]
- Mezni, A.; Balti, I.; Mlayah, A.; Jouini, N.; Smiri, L.S. Hybrid Au–Fe3O4 nanoparticles: Plasmonic, surface enhanced Raman scattering, and phase transition properties. J. Phys. Chem. C 2013, 117, 16166–16174. [Google Scholar] [CrossRef]
- Khafaji, M.; Vossoughi, M.; Hormozi-Nezhad, M.R.; Dinarvand, R.; Börrnert, F.; Irajizad, A. A new bifunctional hybrid nanostructure as an active platform for photothermal therapy and MR imaging. Sci. Rep. 2016, 6, 27847. [Google Scholar] [CrossRef]
- Cai, X.; Ding, S.; Shi, Q.; Lyu, Z.; Liu, D.; Dong, W.J.; Du, M.; Dutta, P.; Song, Y.; Du, D.; et al. Eyeball-Like Yolk–Shell Bimetallic Nanoparticles for Synergistic Photodynamic–Photothermal Therapy. ACS Appl. Bio Mater. 2020, 3, 5922–5929. [Google Scholar] [CrossRef]
- Zhu, X.; Ji, X.; Kong, N.; Chen, Y.; Mahmoudi, M.; Xu, X.; Ding, L.; Tao, W.; Cai, T.; Li, Y.; et al. Intracellular mechanistic understanding of 2D MoS2 nanosheets for anti-exocytosis-enhanced synergistic cancer therapy. ACS Nano 2018, 12, 2922–2938. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Williams, G.R.; Fu, L.; Wu, J.; Wang, H.; Liang, R.; Weng, X.; Wei, M. Cancer Theranostics: Multicomponent Transition Metal Dichalcogenide Nanosheets for Imaging-Guided Photothermal and Chemodynamic Therapy (Adv. Sci. 23/2020). Adv. Sci. 2020, 7, 2070128. [Google Scholar] [CrossRef]
- Liu, T.; Wang, C.; Gu, X.; Gong, H.; Cheng, L.; Shi, X.; Feng, L.; Sun, B.; Liu, Z. Drug delivery with PEGylated MoS2 nano-sheets for combined photothermal and chemotherapy of cancer. Adv. Mat. 2014, 26, 3433–3440. [Google Scholar] [CrossRef]
- Feng, W.; Chen, L.; Qin, M.; Zhou, X.; Zhang, Q.; Miao, Y.; Qiu, K.; Zhang, Y.; He, C. Flower-like PEGylated MoS2 nanoflakes for near-infrared photothermal cancer therapy. Sci. Rep. 2015, 5, 1–3. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, C.; He, Q.; Khalil, A.; Xiang, T.; Liu, D.; Zhou, Y.; Wang, J.; Song, L. Stable metallic 1T-WS2 ultrathin nanosheets as a promising agent for near-infrared photothermal ablation cancer therapy. Nano Res. 2015, 8, 3982–3991. [Google Scholar] [CrossRef]
- Xiao, L.; Chen, X.; Yang, X.; Sun, J.; Geng, J. Recent Advances in Polymer-Based Photothermal Materials for Biological Applications. ACS Appl. Poly. Mat. 2020, 2, 4273–4288. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xu, H.; Cheng, L.; Sun, C.; Wang, J.; Liu, Z. In vitro and in vivo near-infrared photothermal therapy of cancer using polypyrrole organic nanoparticles. Adv. Mater. 2012, 24, 5586–5592. [Google Scholar] [CrossRef]
- Chen, J.; Ning, C.; Zhou, Z.; Yu, P.; Zhu, Y.; Tan, G.; Mao, C. Nanomaterials as photothermal therapeutic agents. Prog. Mat. Sci. 2019, 99, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Lim, D.J.; Park, H. Contemporary polymer-based nanoparticle systems for photothermal therapy. Polymers 2018, 10, 1357. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Z.; Zhu, X.; Wang, X.; Liao, Y.; Ma, Z.; Li, F. NIR photothermal therapy using polyaniline nanoparticles. Biomaterials 2013, 34, 9584–9592. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yan, R.; Guo, F.; Yu, M.; Tan, F.; Li, N. Targeted lipid-polyaniline hybrid nanoparticles for photoacoustic imaging guided photothermal therapy of cancer. Nanotechnology 2016, 27, 285102. [Google Scholar] [CrossRef]
- Manivasagan, P.; Quang Bui, N.; Bharathiraja, S.; Santha Moorthy, M.; Oh, Y.-O.; Song, K.; Seo, H.; Yoon, M.; Oh, J. Multifunctional biocompatible chitosan-polypyrrole nanocomposites as novel agents for photoacoustic imaging-guided photothermal ablation of cancer. Sci. Rep. 2017, 7, 43593. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, Y.; Tang, R. Spindle-like polypyrrole hollow nanocapsules as multifunctional platforms for highly effective chemo-photothermal combination therapy of cancer cells in vivo. Chemistry 2014, 20, 11826–11834. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, D.; Zeng, Y.; Wu, L.; Liu, X.; Liu, J. Nanocluster of superparamagnetic iron oxide nanoparticles coated with poly (dopamine) for magnetic field-targeting, highly sensitive MRI and photothermal cancer therapy. Nanotechnology 2015, 26, 115102. [Google Scholar] [CrossRef]
- Gong, H.; Cheng, L.; Xiang, J.; Xu, H.; Feng, L.; Shi, X.; Liu, Z. Near-Infrared Absorbing Polymeric Nanoparticles as a Versatile Drug Carrier for Cancer Combination Therapy. Adv. Funct. Mater. 2013, 23, 6059–6067. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, P.; Li, Z.; Li, X.; Djaker, N.; Spadavecchia, J. HIV-1 Tat Peptide-Gemcitabine Gold (III)-PEGylated Complex—Nanoflowers: A Sleek Thermosensitive Hybrid Nanocarrier as Prospective Anticancer. Part. Part. Syst Char 2018, 35, 1800082. [Google Scholar] [CrossRef]
- Singh, R.; Nalwa, H.S. Medical applications of nanoparticles in biological imaging, cell labeling, antimicrobial agents, and anticancer nanodrugs. J. Biomed. Nanotechnol. 2011, 7, 489–503. [Google Scholar] [CrossRef]
- Issaad, D.; Moustaoui, H.; Medjahed, A.; Lalaoui, L.; Spadavecchia, J.; Bouafia, M.; de la Chapelle, M.L.; Djaker, N. Scattering correlation spectroscopy and raman spectroscopy of thiophenol on gold nanoparticles: Comparative study between nanospheres and nanourchins. J. Phys. Chem. C 2017, 121, 18254–18262. [Google Scholar] [CrossRef]
- Juluri, B.K.; Zheng, Y.B.; Ahmed, D.; Jensen, L.; Huang, T.J. Effects of geometry and composition on charge-induced plasmonic shifts in gold nanoparticles. J. Phys. Chem. C 2008, 112, 7309–7317. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 668–677. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Theoretical considerations. In Optical Properties of Metal Clusters; Springer: Berlin/Heidelberg, Germany, 1995; pp. 13–201. [Google Scholar]
- Grand, J.; Adam, P.M.; Grimault, A.S.; Vial, A.; De La Chapelle, M.L.; Bijeon, J.L.; Kostcheev, S.; Royer, P. Optical extinction spectroscopy of oblate, prolate and ellipsoid shaped gold nanoparticles: Experiments and theory. Plasmonics 2006, 1, 135–140. [Google Scholar] [CrossRef]
- Van de Broek, B.; Devoogdt, N.; D’Hollander, A.; Gijs, H.L.; Jans, K.; Lagae, L.; Muyldermans, S.; Maes, G.; Borghs, G. Specific cell targeting with nanobody conjugated branched gold nanoparticles for photothermal therapy. ACS Nano 2011, 5, 4319–4328. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, H.; Rahman, D.S.; Sengupta, M.; Ghosh, S.K. Gold nanostars in plasmonic photothermal therapy: The role of tip heads in the thermoplasmonic landscape. J. Phys. Chem. C 2018, 122, 13082–13094. [Google Scholar] [CrossRef]
- Pu, Y.; Zhao, Y.; Zheng, P.; Li, M. Elucidating the growth mechanism of plasmonic gold nanostars with tunable optical and photothermal properties. Inorg. Chem. 2018, 57, 8599–8607. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and properties of nanocrystals of different shapes. Chem. Rev. 2005, 105, 1025–1102. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, C.; He, Q.; Liu, D.; Khalil, A.; Xiang, T.; Wu, Z.; Wang, J.; Song, L. Ultrathin carbon layer coated MoO2 nanoparticles for high-performance near-infrared photothermal cancer therapy. Chem. Commun. 2015, 51, 10054–10057. [Google Scholar] [CrossRef]
- Chen, C.H.; Wu, Y.J.; Chen, J.J. Gold nanotheranostics: Photothermal therapy and imaging of Mucin 7 conjugated antibody nanoparticles for urothelial cancer. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, W.; Huang, Q.; Li, C.; Chen, W. Copper sulfide nanoparticles for photothermal ablation of tumor cells. Nanomedicine 2010, 5, 1161–1171. [Google Scholar] [CrossRef]
- Chen, M.; Fang, X.; Tang, S.; Zheng, N. Polypyrrole nanoparticles for high-performance in vivo near-infrared photothermal cancer therapy. Chem. Commun. 2012, 48, 8934–8936. [Google Scholar] [CrossRef]
- Cheng, L.; Yang, K.; Chen, Q.; Liu, Z. Organic stealth nanoparticles for highly effective in vivo near-infrared photothermal therapy of cancer. ACS Nano 2012, 6, 5605–5613. [Google Scholar] [CrossRef]
- Green, H.N.; Crockett, S.D.; Martyshkin, D.V.; Singh, K.P.; Grizzle, W.E.; Rosenthal, E.L.; Mirov, S.B. A histological evaluation and in vivo assessment of intratumoral near infrared photothermal nanotherapy-induced tumor regression. Int. J. Nanomed. 2014, 9, 5093. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Feng, L.; Zhang, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging. Biomaterials 2018, 155, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Han, J.; Liu, S.; Wang, X.; Wang, Z.Y.; Xie, Z. Tailor-made semiconducting polymers for second near-infrared photothermal therapy of orthotopic liver cancer. ACS Nano 2019, 13, 7345–7354. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Dou, J.H.; Liu, S.; Wang, X.; Zheng, X.; Wang, Y.; Pei, J.; Xie, Z. Second near-infrared conjugated polymer nanoparticles for photoacoustic imaging and photothermal therapy. ACS Appl. Mater. Interfaces 2018, 10, 7919–7926. [Google Scholar] [CrossRef]
- Wu, X.; Suo, Y.; Shi, H.; Liu, R.; Wu, F.; Wang, T.; Ma, L.; Liu, H.; Cheng, Z. Deep-tissue photothermal therapy using laser illumination at NIR-iia window. Nano-Micro Lett. 2020, 12, 1–3. [Google Scholar] [CrossRef]
- Han, H.S.; Choi, K.Y. Advances in Nanomaterial-Mediated Photothermal Cancer Therapies: Toward Clinical Applications. Biomedicines 2021, 9, 305. [Google Scholar] [CrossRef]
- Rastinehad, A.R.; Anastos, H.; Wajswol, E.; Winoker, J.S.; Sfakianos, J.P.; Doppalapudi, S.K.; Carrick, M.R.; Knauer, C.J.; Taouli, B.; Lewis, S.C.; et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA 2019, 116, 18590–18596. [Google Scholar] [CrossRef] [PubMed]

| Nanomaterials | Configuration | Wavelength | Power and Duration | Reference |
|---|---|---|---|---|
| Metallic Nanostructures | ||||
| Gold | Gold nanostar | 980 nm | 15 W cm−2, 5 min | [108] |
| Gold | PEG-gold nanostar | 785 nm | 1.1 W cm−2, 5 min | [108] |
| Copper | PEG-coated copper nanowires | 808 nm | - | [111] |
| Graphene nanostructures | ||||
| Graphene oxide (GO) | Glycol functionalized graphene oxide (GO-PEG) nanosheet | 808 nm | 2 W cm−2 | [132] |
| Graphene oxide (GO) | Gold-nanoparticle-specific aptamer–graphene oxide | 808 nm | 3 W cm−2, 5 min | [133] |
| Carbon nanotubes | ||||
| Single-walled carbon nanotubes (SWNT) | folate (FA)-functionalized SWNT | 980 nm | 0.5 to 1 W cm−2, 2 min | [148] |
| carbon nanotubes (CNT) | Oxidized CNT (O-CNT), and further functionalized it with PEG | 808 nm | 2 W cm−2, 10 min | [139] |
| Quantum dots | ||||
| CdSe quantum dot and CdTe quantum dots | CdSe, CdTe quantum dots | 671 nm | 0.16 W cm−2, 0–20 min | [154] |
| CdSe quantum dot and CdTe quantum dots | Mesoporous hollow NCQD captured carbon sphere | 980 nm | 1 W cm−2, 5 min | [155] |
| Hybrid Nanoparticles | ||||
| Au–Fe3O4 hybrid nanoparticles | Au-Fe3O4 hybrid nanoparticles | 808 nm | 0.5 W cm−2 | [158] |
| Au–Pd hybrid nanoparticles | Au-Pd hybrid nanoparticles, functionalized with folic acid and chlorin e6 | 808 nm | 1.5 W cm−2, 5 min | [159] |
| Transition metal based nanosheets | ||||
| CoFeMn dichalcogenides (CFMS) nanosheets | CoFeMn dichalcogenides (CFMS) nanosheets modified with polyvinyl pyrrolidone | 808 nm | 1 W cm−2, 5 on/off cycle, 10 min each cycle | [161] |
| MoS2 nano-sheets | PEGylated MoS2 nano-sheets | 808 nm | Varying power, 5 min | [162] |
| MoS2 nanoflakes | PEG modified MoS2 nanoflakes (MoS2-PEG) | 808 nm | 2 W cm−2, 10 min | [163] |
| WS2 nanosheets | Ammonium ion intercalated 1T-WS2 ultrathin nanosheets (N-WS2) | 808 nm | 0.6 W cm−2, 10 min | [164] |
| Polymer nanoparticles | ||||
| polyaniline nanoparticles | F-127 functionalized polyaniline nanoparticles (F-PANP) | 808 nm | 0.5 W cm−2, 3 min | [170] |
| polyaniline nanoparticles | Folic-acid-functionalized lipid-coated polyaniline nanoparticles | 808 nm | 2 W cm−2, 5 min | [171] |
| polypyrrole | chitosan-polypyrrole-based nanocomposites | 808 nm | 2.0 W cm−2, 5 min | [172] |
| polypyrrole | DOX-loaded polypyrrole hollow nanocapsules (PPy HNCs) | 980 nm | 1.0 W cm−2 | [173] |
| polydopamine | Polydopamine-coated cluster of iron oxide nanoparticles | 808 nm | 2 W cm−2, 10 min | [174] |
| poly-(3,4-ethylenedioxythiophene):poly(4-styrenesulfonate) (PEDOT:PSS) | polyethylene glycol (PEG)-coated PEDOT:PSS nanoparticles-based drug carrier | 808 nm | 0.15 W cm−2, 20 min | [175] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Sharma, N.; Sahi, S.V. Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics 2021, 13, 1174. https://doi.org/10.3390/pharmaceutics13081174
Kumari S, Sharma N, Sahi SV. Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics. 2021; 13(8):1174. https://doi.org/10.3390/pharmaceutics13081174
Chicago/Turabian StyleKumari, Sangeeta, Nilesh Sharma, and Shivendra V. Sahi. 2021. "Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy" Pharmaceutics 13, no. 8: 1174. https://doi.org/10.3390/pharmaceutics13081174
APA StyleKumari, S., Sharma, N., & Sahi, S. V. (2021). Advances in Cancer Therapeutics: Conventional Thermal Therapy to Nanotechnology-Based Photothermal Therapy. Pharmaceutics, 13(8), 1174. https://doi.org/10.3390/pharmaceutics13081174