Ethyl Acetate Fraction and Isolated Phenolics Derivatives from Mandevilla moricandiana Identified by UHPLC-DAD-ESI-MSn with Pharmacological Potential for the Improvement of Obesity-Induced Endothelial Dysfunction
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plant Material and Extraction
2.3. Purification of MMEtAc Fraction by Glass Column Chromatography
2.4. Ultra-High Performance Liquid Chromatography Coupled to Diode Array Detector and Electrospray Ionization Mass Spectrometry (UHPLC-DAD-ESI-MSn)
2.5. Purification by Semi-Preparative High-Performance Liquid Chromatography Coupled to Diode Array Detector (HPLC-DAD)
2.6. Nuclear Magnetic Resonance (NMR)
2.7. Animals
2.8. Preparation of Aortic Rings for Isometric Tension Recording
2.9. Vasodilatador Effect
2.10. Effects of MMEAF on Vascular Reactivity of Aorta from Obese Rats
2.11. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Assay
2.12. Oxygen Radical Absorbance Capacity (ORAC) Assay
2.13. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analysis of MMEAF and Its Subfractions
3.2. NMR Data
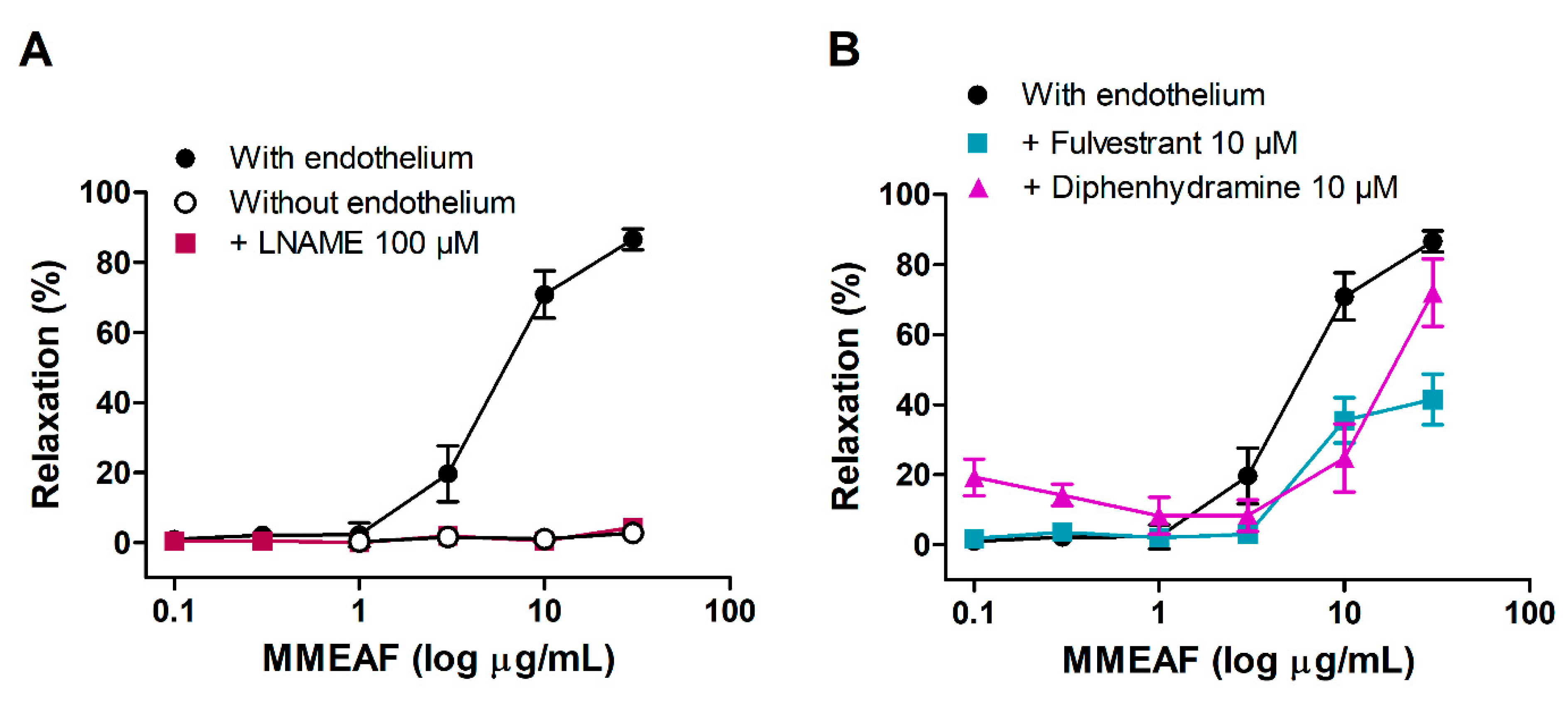
3.3. Vasodilator Effect of MMEAF
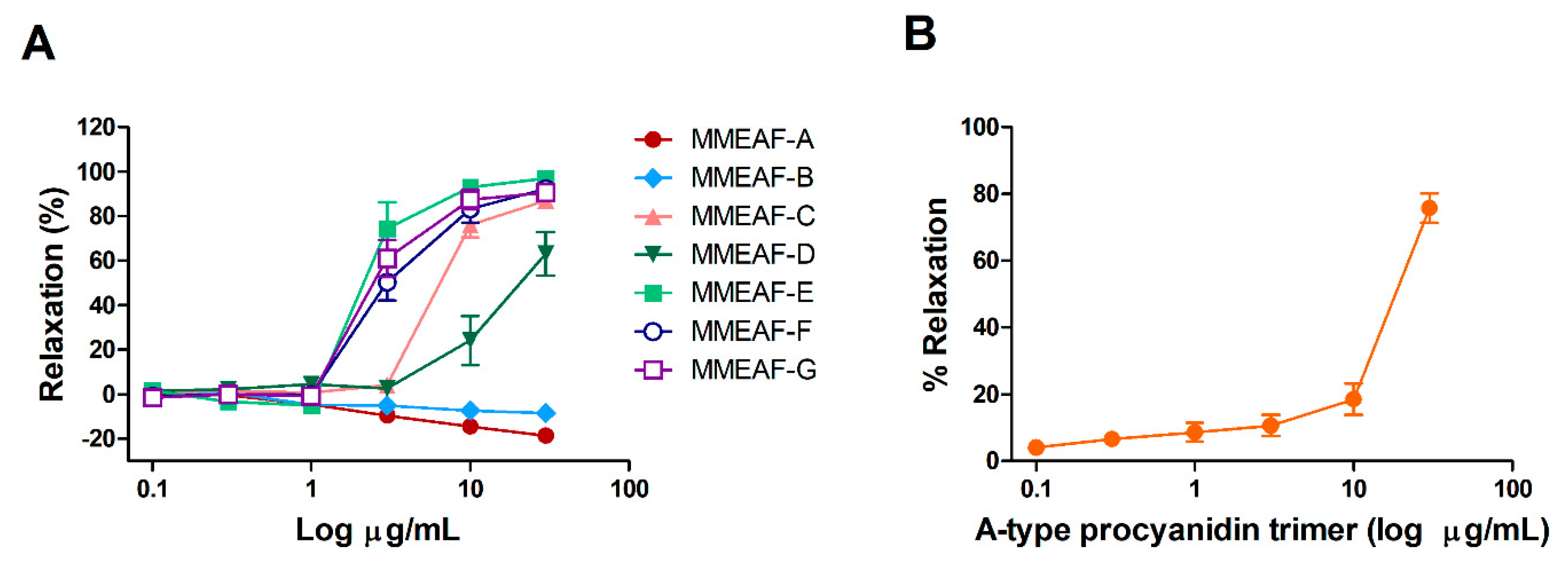
3.4. Vasodilatory Activity of MMEAF Subfractions and Isolated Compound
3.5. Antioxidant Acitivity of MMEAF and Its Subfractions
3.6. Effects of MMEAF on Vascular Reactivity of Aorta from Obese Rats
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Cardiovascular Diseases (CVDs) Fact Sheet. Available online: https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 11 June 2021).
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of nitric oxide synthases by oxidized LDLs: Role in vascular inflammation and atherosclerosis development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef]
- Heitzer, T.; Schlinzig, T.; Krohn, K.; Meinertz, T.; Munzel, T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation 2001, 104, 2673–2678. [Google Scholar] [CrossRef]
- Panza, J.A.; Quyyumi, A.A.; Brush, J.E.; Epstein, S.E. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N. Engl. J. Med. 1990, 323, 22–27. [Google Scholar] [CrossRef]
- Brown, I.A.M.; Diederich, L.; Good, M.E.; DeLalio, L.J.; Murphy, S.A.; Cortese-Krott, M.M.; Hall, J.L.; Le, T.H.; Isakson, B.E. Vascular smooth muscle remodeling in conductive and resistance arteries in hypertension. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1969–1985. [Google Scholar] [CrossRef]
- Engin, A. Endothelial dysfunction in obesity. In Advances in Experimental Medicine and Biology; Engin, A.B., Engin, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; Volume 960, pp. 345–379. ISBN 978-3-319-48380-1. [Google Scholar]
- Coutinho, T.; Turner, S.T.; Kullo, I.J. Adverse effects of long-term weight gain on microvascular endothelial function. Obes. Res. Clin. Pract. 2018, 12, 452–458. [Google Scholar] [CrossRef]
- Cercato, C.; Fonseca, F.A. Cardiovascular risk and obesity. Diabetol. Metab. Syndr. 2019, 11, 1–15. [Google Scholar] [CrossRef]
- Félétou, M.; Vanhoutte, P.M. Endothelial dysfunction: A multifaceted disorder (The Wiggers Award Lecture). Am. J. Physiol. Circ. Physiol. 2006, 291, H985–H1002. [Google Scholar] [CrossRef]
- Higashi, Y.; Noma, K.; Yoshizumi, M.; Kihara, Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ. J. 2009, 73, 411–418. [Google Scholar] [CrossRef]
- Griendling, K.K.; FitzGerald, G.A. Oxidative stress and cardiovascular injury: Part II: Animal and human studies. Circulation 2003, 108, 2034–2040. [Google Scholar] [CrossRef] [PubMed]
- Siti, H.N.; Kamisah, Y.; Kamsiah, J. The role of oxidative stress, antioxidants and vascular inflammation in cardiovascular disease (a review). Vascul. Pharmacol. 2015, 71, 40–56. [Google Scholar] [CrossRef]
- Zhang, H.; Park, Y.; Wu, J.; Chen, X.P.; Lee, S.; Yang, J.; Dellsperger, K.C.; Zhang, C. Role of TNF-α in vascular dysfunction. Clin. Sci. 2009, 116, 219–230. [Google Scholar] [CrossRef]
- Virdis, A.; Santini, F.; Colucci, R.; Duranti, E.; Salvetti, G.; Rugani, I.; Segnani, C.; Anselmino, M.; Bernardini, N.; Blandizzi, C.; et al. Vascular generation of tumor necrosis factor-α reduces nitric oxide availability in small arteries from visceral fat of obese patients. J. Am. Coll. Cardiol. 2011, 58, 238–247. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Y.; Wang, N. New paradigms in inflammatory signaling in vascular endothelial cells. Am. J. Physiol. Circ. Physiol. 2014, 306, H317–H325. [Google Scholar] [CrossRef]
- Haybar, H.; Shokuhian, M.; Bagheri, M.; Davari, N.; Saki, N. Involvement of circulating inflammatory factors in prognosis and risk of cardiovascular disease. J. Mol. Cell. Cardiol. 2019, 132, 110–119. [Google Scholar] [CrossRef]
- Sun, H.-J.; Wu, Z.-Y.; Nie, X.-W.; Bian, J.-S. Role of endothelial dysfunction in cardiovascular diseases: The link between inflammation and hydrogen sulfide. Front. Pharmacol. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future research. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Serino, A.; Salazar, G. Protective role of polyphenols against vascular inflammation, aging and cardiovascular disease. Nutrients 2019, 11, 53. [Google Scholar] [CrossRef]
- Battino, M.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Reboredo-Rodríguez, P.; Varela Lopez, A.; Quiles, J.L.; et al. Relevance of functional foods in the Mediterranean diet: The role of olive oil, berries and honey in the prevention of cancer and cardiovascular diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 893–920. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Wu, J.H.Y. Flavonoids, dairy foods, and cardiovascular and metabolic health: A review of emerging biologic pathways. Circ. Res. 2018, 122, 369–384. [Google Scholar] [CrossRef] [PubMed]
- Dagher, O.; Mury, P.; Thorin-Trescases, N.; Noly, P.E.; Thorin, E.; Carrier, M. Therapeutic potential of quercetin to alleviate endothelial dysfunction in age-related cardiovascular diseases. Front. Cardiovasc. Med. 2021, 8, 1–24. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Luna-Vázquez, F.J.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Rojas-Molina, I.; Zavala-Sánchez, M.Á. Vasodilator compounds derived from plants and their mechanisms of action. Molecules 2013, 18, 5814–5857. [Google Scholar] [CrossRef]
- Schini-Kerth, V.B.; Étienne-Selloum, N.; Chataigneau, T.; Auger, C. Vascular protection by natural product-derived polyphenols: In vitro and in vivo evidence. Planta Med. 2011, 77, 1161–1167. [Google Scholar] [CrossRef]
- Jardim Botâncio do Rio de Janeiro Mandevilla in Flora do Brasil 2020. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB4652 (accessed on 16 July 2021).
- Biondo, R.; Soares, A.M.; Bertoni, B.W.; França, S.C.; Pereira, A.M.S. Direct organogenesis of Mandevilla illustris (Vell) Woodson and effects of its aqueous extract on the enzymatic and toxic activities of Crotalus durissus terrificus snake venom. Plant Cell Rep. 2004, 22, 549–552. [Google Scholar] [CrossRef]
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal plants in Brazil: Pharmacological studies, drug discovery, challenges and perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef]
- Calixto, J.B.; Yunes, R.A. Effect of a crude extract of Mandevilla velutina on contractions induced by bradykinin and [des-Arg9]-bradykinin in isolated vessels of the rabbit. Br. J. Pharmacol. 1986, 88, 937–941. [Google Scholar] [CrossRef]
- Ferreira, L.; Gomes, M.; Paes, B.; do Carmo, P.; Konno, T.; Esteves, F.; Lopes, N.; Tomaz, J.; Leal, I.; Guimarães, D.; et al. The hydroalcoholic extract of leaves of Mandevilla moricandiana induces NO-mediated vascular relaxation. Planta Med. 2017, 83, 63–69. [Google Scholar] [CrossRef]
- Cordeiro, S.Z.; Simas, N.K.; Henriques, A.B.; Sato, A. In vitro conservation of Mandevilla moricandiana (Apocynaceae): Short-term storage and encapsulation-dehydration of nodal segments. Vitr. Cell. Dev. Biol. Plant 2014, 50, 326–336. [Google Scholar] [CrossRef]
- Cordeiro, S.Z.; Simas, N.K.; Henriques, A.B.; Lage, C.L.S.; Sato, A. Micropropagation of Mandevilla moricandiana (A.DC.) Woodson. Vitr. Cell. Dev. Biol. Plant 2012, 48, 620–626. [Google Scholar] [CrossRef]
- de Fátima Leão, V.; Raimundo, J.M.; Ferreira, L.L.D.M.; Santos-Silva, J.C.; Vettorazzi, J.F.; Bonfleur, M.L.; Carneiro, E.M.; Ribeiro, R.A. Effects of paternal hypothalamic obesity and taurine supplementation on adiposity and vascular reactivity in rat offspring. In Taurine 9. Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2015; Volume 803, pp. 749–763. ISBN 978-3-319-15126-7. [Google Scholar]
- Leão, V.F.; Ferreira, L.L.D.M.; Melo, C.M.; Bonfleur, M.L.; da Silva, L.L.; Carneiro, E.M.; Raimundo, J.M.; Ribeiro, R.A. Taurine supplementation prevents endothelial dysfunction and attenuates structural changes in aortas from hypothalamic obese rats. Eur. J. Nutr. 2019, 58, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, J.C.; Lage, L.F.O.; Camargos, C.R.D.; Amaral, J.C.; Costa, L.M.; De Souza, A.N.; Oliveira, F.Q. Antioxidant determination activity by DPPH method and assay for total flavonoids in leaves extracts of Bauhinia variegata L. Braz. J. Pharm. 2011, 92, 327–333. [Google Scholar]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacumtissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chem. Cent. J. 2014, 8, 1–10. [Google Scholar] [CrossRef]
- González-Abuín, N.; Martínez-Micaelo, N.; Blay, M.; Ardévol, A.; Pinent, M. Grape-seed procyanidins prevent the cafeteria-diet-induced decrease of glucagon-like peptide-1 production. J. Agric. Food Chem. 2014, 62, 1066–1072. [Google Scholar] [CrossRef]
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Baruca Arbeiter, A.; Peeters, K.; Miklavčič Višnjevec, A.; Jenko Pražnikar, Z. HPLC-DAD-ESI-QTOF-MS Determination of bioactive compounds and antioxidant activity comparison of the hydroalcoholic and water extracts from two Helichrysum italicum species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Souilem, F.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Harzallah-Skhiri, F.; Ferreira, I.C.F.R. Phenolic profile and bioactive properties of Carissa macrocarpa (Eckl.) A.DC.: An in vitro comparative study between leaves, stems, and flowers. Molecules 2019, 24, 1696. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Sun, X.; Guo, X.; Bai, H.; Liu, R.; Jin, Q.; Wang, X. Composition and antioxidant study of procyanidins from peanut skins. J. Food Meas. Charact. 2020, 14, 2781–2789. [Google Scholar] [CrossRef]
- Dudzinski, D.M.; Igarashi, J.; Greif, D.; Michel, T. The regulation and pharmacology of endothelial nitric oxide synthase. Annu. Rev. Pharmacol. Toxicol. 2006, 46, 235–276. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, M.; Tesse, A.; Martínez, M.C.; Rognan, D.; Arnal, J.-F.; Andriantsitohaina, R. Estrogen receptor alpha as a key target of red wine polyphenols action on the endothelium. PLoS ONE 2010, 5, e8554. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M.; Wickramasinghe, N.S.; Ivanova, M.M.; Dougherty, S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor αa-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008, 22, 2185–2197. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Nanashima, N.; Maeda, H. Phytoestrogenic effects of blackcurrant anthocyanins increased endothelial nitric oxide synthase (eNOS) expression in human endothelial cells and ovariectomized rats. Molecules 2019, 24, 1259. [Google Scholar] [CrossRef]
- Raimundo, J.M.; Trindade, A.P.F.; Velozo, L.S.M.; Kaplan, M.A.C.; Sudo, R.T.; Zapata-Sudo, G. The lignan eudesmin extracted from Piper truncatum induced vascular relaxation via activation of endothelial histamine H1 receptors. Eur. J. Pharmacol. 2009, 606, 150–154. [Google Scholar] [CrossRef]
- Kim, E.-Y.; Jho, H.-K.; Kim, D.-I.; Rhyu, M.R. Cirsium japonicum elicits endothelium-dependent relaxation via histamine H1-receptor in rat thoracic aorta. J. Ethnopharmacol. 2008, 116, 223–227. [Google Scholar] [CrossRef]
- Nakamura, Y.; Matsumoto, H.; Todoki, K. Endothelium-dependent vasorelaxation induced by black currant concentrate in rat thoracic aorta. Jpn. J. Pharmacol. 2002, 89, 29–35. [Google Scholar] [CrossRef]
- Rue, E.A.; Rush, M.D.; van Breemen, R.B. Procyanidins: A comprehensive review encompassing structure elucidation via mass spectrometry. Phytochem. Rev. 2018, 17, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, D.F.; Bing, B.; Maggi, D.A.; Fleming, R.C.; O’Malley, R.M. Vasodilating procyanidins derived from grape seeds. Ann. N. Y. Acad. Sci. 2002, 957, 78–89. [Google Scholar] [CrossRef]
- Matsui, T.; Korematsu, S.; Byun, E.B.; Nishizuka, T.; Ohshima, S.; Kanda, T. Apple procyanidins induced vascular relaxation in isolated rat aorta through NO/cGMP pathway in combination with hyperpolarization by multiple K+ channel activations. Biosci. Biotechnol. Biochem. 2009, 73, 2246–2251. [Google Scholar] [CrossRef]
- Byun, E.B.; Ishikawa, T.; Suyama, A.; Kono, M.; Nakashima, S.; Kanda, T.; Miyamoto, T.; Matsui, T. A procyanidin trimer, C1, promotes NO production in rat aortic endothelial cells via both hyperpolarization and PI3K/Akt pathways. Eur. J. Pharmacol. 2012, 692, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Tom, E.N.L.; Girard-Thernier, C.; Demougeot, C. The Janus face of chlorogenic acid on vascular reactivity: A study on rat isolated vessels. Phytomedicine 2016, 23, 1037–1042. [Google Scholar] [CrossRef]
- Taskova, R.; Mitova, M.; Mikhova, B.; Duddeck, H. Bioactive phenolics from Carthamus lanatus L. Z. Nat. C 2003, 58, 704–707. [Google Scholar] [CrossRef] [PubMed]
- Penso, J.; Cordeiro, K.C.F.A.; Da Cunha, C.R.M.; Da Silva Castro, P.F.; Martins, D.R.; Lião, L.M.; Rocha, M.L.; De Oliveira, V. Vasorelaxant activity of 7-β-O-glycosides biosynthesized from flavonoids. Eur. J. Pharmacol. 2014, 733, 75–80. [Google Scholar] [CrossRef]
- Gasparotto Junior, A.; dos Reis Piornedo, R.; Assreuy, J.; Da Silva-Santos, J.E. Nitric oxide and Kir 6.1 potassium channel mediate isoquercitrin-induced endothelium-dependent and independent vasodilation in the mesenteric arterial bed of rats. Eur. J. Pharmacol. 2016, 788, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Li, P.G.; Sun, L.; Han, X.; Ling, S.; Gan, W.T.; Xu, J.W. Quercetin induces rapid eNOS phosphorylation and vasodilation by an Akt-independent and PKA-dependent mechanism. Pharmacology 2012, 89, 220–228. [Google Scholar] [CrossRef]
- Pérez-Vizcaíno, F.; Ibarra, M.; Cogolludo, A.L.; Duarte, J.; Zaragozá-Arnáez, F.; Moreno, L.; López-López, G.; Tamargo, J. Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J. Pharmacol. Exp. Ther. 2002, 302, 66–72. [Google Scholar] [CrossRef]
- Taubert, D.; Berkels, R.; Klaus, W.; Roesen, R. Nitric oxide formation and corresponding relaxation of porcine coronary arteries induced by plant phenols: Essential structural features. J. Cardiovasc. Pharmacol. 2002, 40, 701–713. [Google Scholar] [CrossRef]
- Leeya, Y.; Mulvany, M.J.; Queiroz, E.F.; Marston, A.; Hostettmann, K.; Jansakul, C. Hypotensive activity of an n-butanol extract and their purified compounds from leaves of Phyllanthus acidus (L.) Skeels in rats. Eur. J. Pharmacol. 2010, 649, 301–313. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Stankevicius, E.; Herrera, M.D.; Østergaard, L.; Andersen, M.R.; Ruiz-Gutierrez, V.; Simonsen, U. Oleanolic acid induces relaxation and calcium-independent release of endothelium-derived nitric oxide. Br. J. Pharmacol. 2008, 155, 535–546. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Herrera, M.D.; Perona, J.S.; Ruiz-Gutiérrez, V. Potential vasorelaxant effects of oleanolic acid and erythrodiol, two triterpenoids contained in ‘orujo’ olive oil, on rat aorta. Br. J. Nutr. 2004, 92, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, R.; Perona, J.S.; Herrera, M.D.; Ruiz-Gutierrez, V. Triterpenic compounds from ‘Orujo’ olive oil elicit vasorelaxation in aorta from spontaneously hypertensive rats. J. Agric. Food Chem. 2006, 54, 2096–2102. [Google Scholar] [CrossRef]
- Aguirre-Crespo, F.; Vergara-Galicia, J.; Villalobos-Molina, R.; Javier López-Guerrero, J.; Navarrete-Vázquez, G.; Estrada-Soto, S. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci. 2006, 79, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Chen, C.P.; Dong, E.; Tanaka, T.; Nonaka, G.I.; Nishioka, I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem. Pharmacol. 1998, 56, 213–222. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, B.J.; Lee, E.H.; Osborne, N.N. Isoquercitrin is the most effective antioxidant in the plant Thuja orientalis and able to counteract oxidative-induced damage to a transformed cell line (RGC-5 cells). Neurochem. Int. 2010, 57, 713–721. [Google Scholar] [CrossRef]
- Riaz, A.; Rasul, A.; Hussain, G.; Zahoor, M.K.; Jabeen, F.; Subhani, Z.; Younis, T.; Ali, M.; Sarfraz, I.; Selamoglu, Z. Astragalin: A bioactive phytochemical with potential therapeutic activities. Adv. Pharmacol. Sci. 2018, 2018, 9794625. [Google Scholar] [CrossRef]
- Ahmad, R.; Ahmad, N.; Naqvi, A.; Exarchou, V.; Upadhyay, A.; Tuenter, E.; Foubert, K.; Apers, S.; Hermans, N.; Pieters, L. Antioxidant and antiglycating constituents from leaves of Ziziphus oxyphylla and Cedrela serrata. Antioxidants 2016, 5, 9. [Google Scholar] [CrossRef]
- Zang, Y.; Zhang, L.; Igarashi, K.; Yu, C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015, 6, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Dornas, W.C.; Oliveira, T.T.; Rodrigues-das-Dores, R.G.; Santos, A.F.; Nagem, T. Flavonóides: Potencial terapêutico no estresse oxidativo. Rev. Ciências Farm. Básica Apl. 2016, 28, 241–249. [Google Scholar]
- Balbo, S.L.; Grassiolli, S.; Ribeiro, R.A.; Bonfleur, M.L.; Gravena, C.; Brito, M.d.N.; Andreazzi, A.E.; Mathias, P.C.d.F.; Torrezan, R. Fat storage is partially dependent on vagal activity and insulin secretion of hypothalamic obese rat. Endocrine 2007, 31, 142–148. [Google Scholar] [CrossRef]
- da Cunha, N.V.; Pinge-Filho, P.; Panis, C.; Silva, B.R.; Pernomian, L.; Grando, M.D.; Cecchini, R.; Bendhack, L.M.; Martins-Pinge, M.C. Decreased endothelial nitric oxide, systemic oxidative stress, and increased sympathetic modulation contribute to hypertension in obese rats. Am. J. Physiol. Circ. Physiol. 2014, 306, H1472–H1480. [Google Scholar] [CrossRef]
- Lobato, N.S.; Filgueira, F.P.; Akamine, E.H.; Davel, A.P.C.; Rossoni, L.V.; Tostes, R.C.; Carvalho, M.H.C.; Fortes, Z.B. Obesity induced by neonatal treatment with monosodium glutamate impairs microvascular reactivity in adult rats: Role of NO and prostanoids. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 808–816. [Google Scholar] [CrossRef]
- Menendez, C.; Jimenez, R.; Moreno, L.; Galindo, P.; Cogolludo, A.; Duarte, J.; Perez-Vizcaino, F. Lack of synergistic interaction between quercetin and catechin in systemic and pulmonary vascular smooth muscle. Br. J. Nutr. 2011, 105, 1287–1293. [Google Scholar] [CrossRef]
- Redondo, A.; Estrella, N.; Lorenzo, A.G.; Cruzado, M.; Castro, C. Quercetin and catechin synergistically inhibit angiotensin II-induced redox-dependent signaling pathways in vascular smooth muscle cells from hypertensive rats. Free Radic. Res. 2012, 46, 619–627. [Google Scholar] [CrossRef]
- Kurin, E.; Atanasov, A.G.; Donath, O.; Heiss, E.H.; Dirsch, V.M.; Nagy, M. Synergy study of the inhibitory potential of red wine polyphenols on vascular smooth muscle cell proliferation. Planta Med. 2012, 78, 772–778. [Google Scholar] [CrossRef]
- Khandelwal, A.R.; Hebert, V.Y.; Kleinedler, J.J.; Rogers, L.K.; Ullevig, S.L.; Asmis, R.; Shi, R.; Dugas, T.R. Resveratrol and quercetin interact to inhibit neointimal hyperplasia in mice with a carotid injury. J. Nutr. 2012, 142, 1487–1494. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Yao, H.; Sun, J.; Wei, J.; Zhang, X.; Chen, B.; Lin, Y. Kaempferol protects blood vessels from damage induced by oxidative stress and inflammation in association with the Nrf2/HO-1 signaling pathway. Front. Pharmacol. 2020, 11, 1–11. [Google Scholar] [CrossRef]
- Xiao, H.-B.; Lu, X.-Y.; Sun, Z.-L.; Zhang, H.-B. Kaempferol regulates OPN–CD44 pathway to inhibit the atherogenesis of apolipoprotein E deficient mice. Toxicol. Appl. Pharmacol. 2011, 257, 405–411. [Google Scholar] [CrossRef]
- Crespo, I.; García-Mediavilla, M.V.; Gutiérrez, B.; Sánchez-Campos, S.; Tuñón, M.J.; González-Galjego, J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br. J. Nutr. 2008, 100, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Jones, H.S.; Gordon, A.; Magwenzi, S.G.; Naseem, K.; Atkin, S.L.; Courts, F.L. The dietary flavonol quercetin ameliorates angiotensin II-induced redox signaling imbalance in a human umbilical vein endothelial cell model of endothelial dysfunction via ablation of p47phox expression. Mol. Nutr. Food Res. 2016, 60, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.; Lodi, F.; Vera, R.; Villar, I.C.; Cogolludo, A.; Jimenez, R.; Moreno, L.; Romero, M.; Tamargo, J.; Perez-Vizcaino, F.; et al. Quercetin and isorhamnetin prevent endothelial dysfunction, superoxide production, and overexpression of p47phox induced by angiotensin II in rat aorta. J. Nutr. 2007, 137, 910–915. [Google Scholar] [CrossRef]
- Sánchez, M.; Galisteo, M.; Vera, R.; Villar, I.C.; Zarzuelo, A.; Tamargo, J.; Pérez-Vizcaíno, F.; Duarte, J. Quercetin downregulates NADPH oxidase, increases eNOS activity and prevents endothelial dysfunction in spontaneously hypertensive rats. J. Hypertens. 2006, 24, 75–84. [Google Scholar] [CrossRef]
- Galisteo, M.; García-Saura, M.F.; Jiménez, R.; Villar, I.C.; Wangensteen, R.; Zarzuelo, A.; Vargas, F.; Duarte, J. Effects of quercetin treatment on vascular function in deoxycorticosterone acetate-salt hypertensive rats. Comparative study with verapamil. Planta Med. 2004, 70, 334–341. [Google Scholar] [PubMed]
- Ajay, M.; Achike, F.I.; Mustafa, A.M.; Mustafa, M.R. Effect of quercetin on altered vascular reactivity in aortas isolated from streptozotocin-induced diabetic rats. Diabetes Res. Clin. Pract. 2006, 73, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Huang, S.; Xu, M.; Gong, Y.; Li, D.; Wan, C.; Wu, H.; Tang, Q. Isoquercitrin protects HUVECs against high glucose-induced apoptosis through regulating p53 proteasomal degradation. Int. J. Mol. Med. 2021, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jiang, M.; Xie, X.; Yang, H.; Wang, X.; Xiao, L.; Wang, N. Oleanolic acid ameliorates high glucose-induced endothelial dysfunction via PPARδ activation. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Li, D.; Ren, D.; Luo, Y.; Yang, X. Protective effects of ursolic acid against hepatotoxicity and endothelial dysfunction in mice with chronic high choline diet consumption. Chem. Biol. Interact. 2016, 258, 102–107. [Google Scholar] [CrossRef]
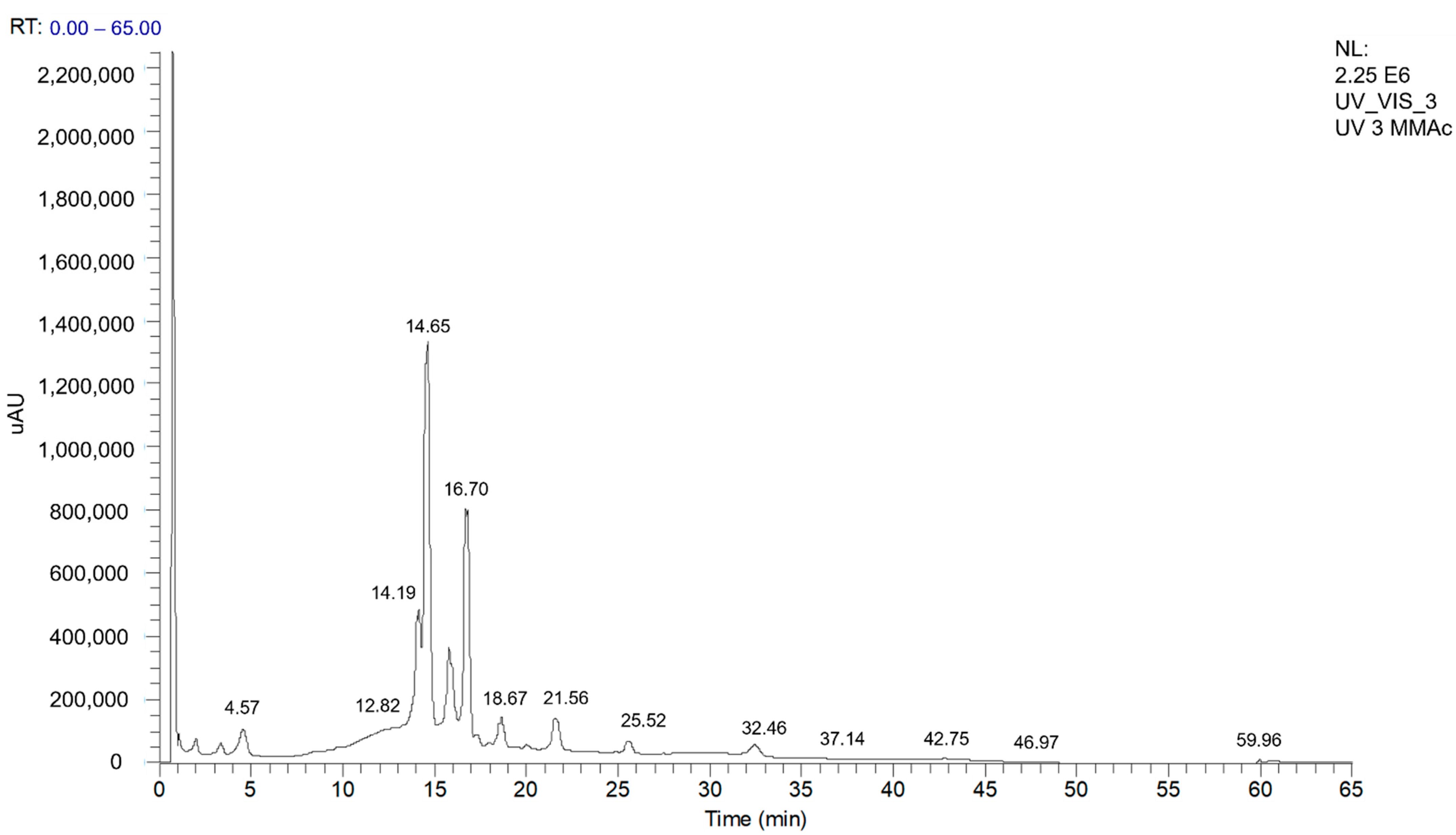
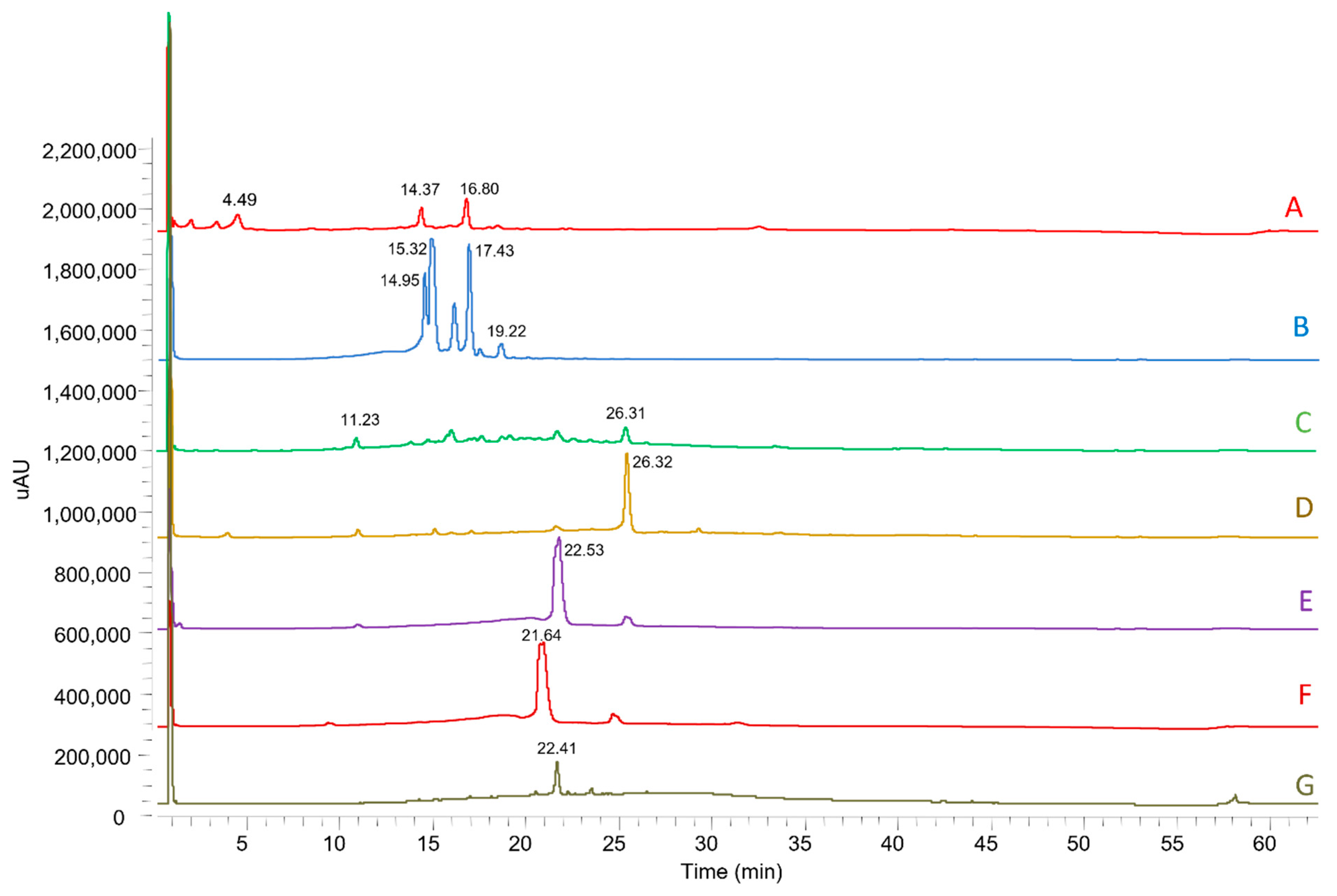
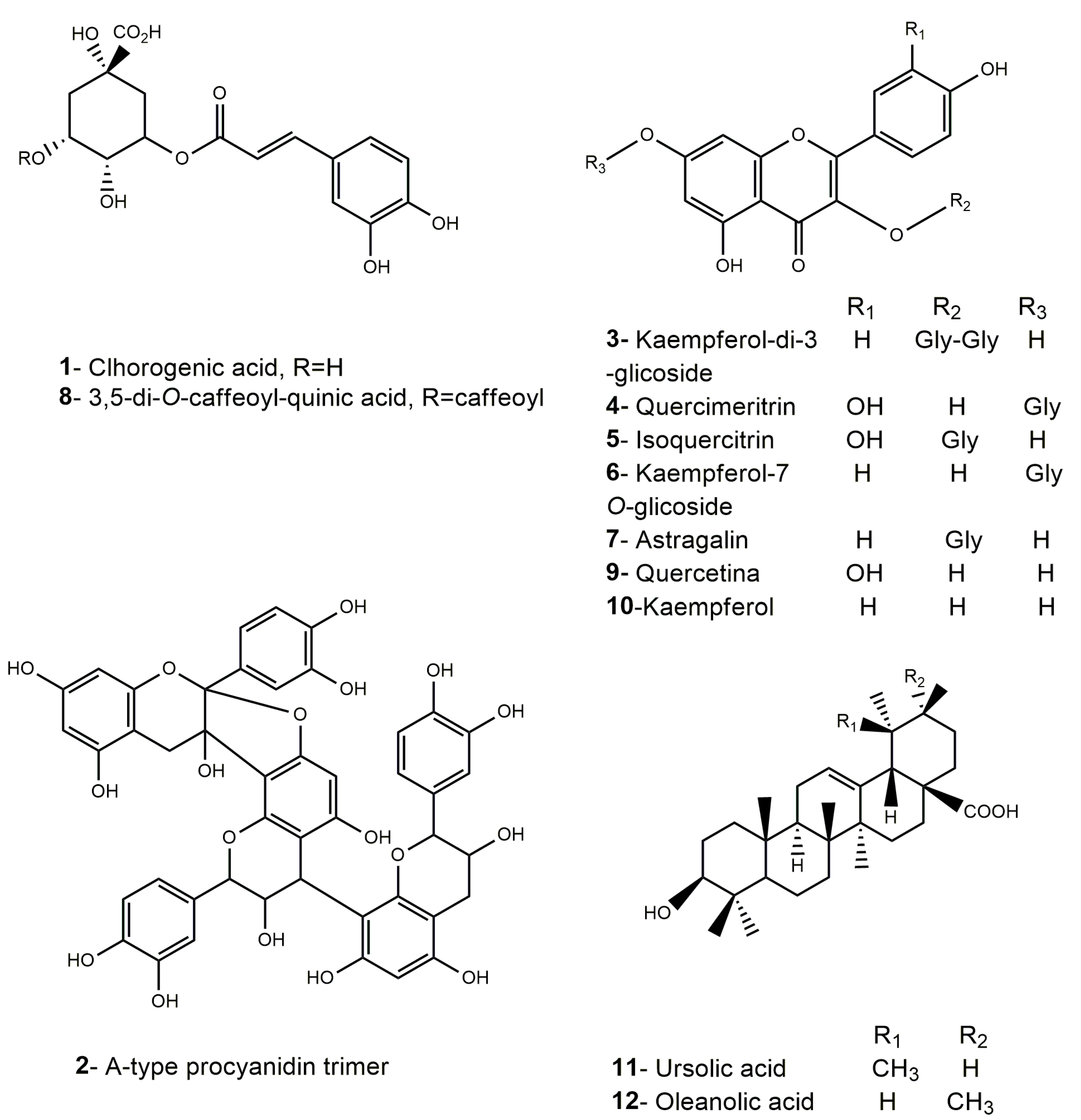

| Nº | Identification | Rt (min) | λmax (nm) | [M-H]− | MS2 Fragments (% Relative Area) | MMEAF-A | MMEAF-B | MMEAF-C | MMEAF-D | MMEAF-E | MMEAF-F | MMEAF-G |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chlorogenic acid | 4.49 | 239,325 | 353 | 191 (100) | + | ||||||
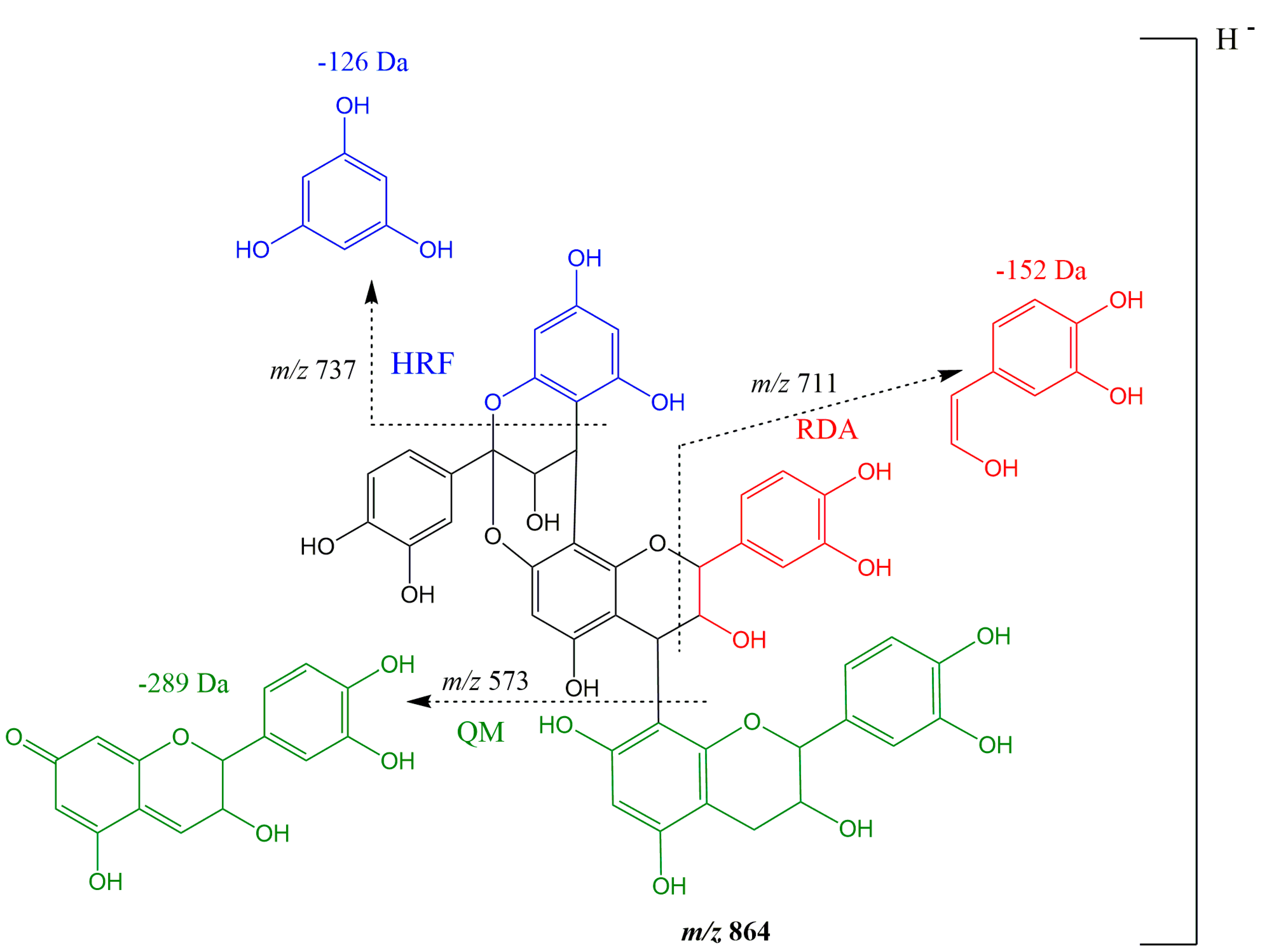
| 2 | Procyanidin A-type trimer | 11.21–11.25 | 195, 280 | 863 | 711 (100), 411 (20), 451 (19), 573 (19) | + | + | + | ||||
| 3 | kaempferol-di-3-glycoside | 14.37 | 258,351 | 609 | - | + | ||||||
| 4 | Quercimeritrin | 14.94 | 259, 353 | 463 | 302 (100), 300 (64) | + | ||||||
| 5 | Isoquecitrin | 15.33 | 262, 347 | 463 | 302 (100), 300 (54) | + | ||||||
| 6 | Kaempferol-7-O-glycoside | 16.60–16.80 | 266, 347 | 447 | 284 (100), 285, (93), 255 (39) | + | + | |||||
| 7 | Astragalin (Kaempferol-3-O-glycoside) | 17.48 | 264, 347 | 447 | 284 (100), 285, (60), 255 (31) | + | ||||||
| 8 | 3,5-dicaffeoylquinic acid | 19.22 | 195, 216 e 329 | 515 | 353 (100), 299 (16), 255 (10), 203 (18), 173 (11) | + | ||||||
| 9 | Quercetin | 21.64–22.52 | 267, 370 | 301 | 273 (14), 179 (100), 151 (68) | + | + | + | ||||
| 10 | Kaempferol | 26.26–26.32 | 267, 366 | 285 | 239 (100), 229 (63), 241 (52), 242 (81), 257 (92) | + | + | + | ||||
| 11 | Ursolic acid | 47.54–47.59 | - | + | ||||||||
| 12 | Oleanolic acid | 47.98–48.91 | - | + |
| Sample | EC50 (μg/mL) | Emax 1 (% Relaxation) |
|---|---|---|
| MMEAF | 5.56 ± 1.13 a | 85.05 ± 3.64 a |
| MMEAF-A | ND 2 | ND |
| MMEAF-B | ND | ND |
| MMEAF-C | 7.37 ± 0.88 a | 87.03 ± 3.30 a |
| MMEAF-D | 22.43 ± 5.73 b | 63.20 ± 9.74 b |
| MMEAF-E | 2.77 ± 0.12 a | 97.02 ± 0.79 a |
| MMEAF-F | 4.12 ± 0.89 a | 92.33 ± 2.80 a |
| MMEAF-G | 3.33 ± 0.75 a | 90.62 ± 3.86 a |
| A-type procyanidin trimer | 18.22 ± 1.92 b | 75.77 ± 4.39 a |
| Sample | DPPH EC50 (μg/mL) | ORAC (mmol TE 1/g Sample) |
|---|---|---|
| MMEAF | 8.57 ± 0.23 b | 8.28 ± 0.25 a |
| MMEAF-A | 17.89 ± 1.48 d | 6.77 ± 0.03 a,d |
| MMEAF-B | 6.19 ± 0.16 b | 0.87 ± 0.02 c |
| MMEAF-C | 6.30 ± 0.25 b,e | 3.07 ± 0.19 b |
| MMEAF-D | 11.47 ± 0.30 f | 5.06 ± 0.13 b,d |
| MMEAF-E | 3.38 ± 0.12 a | 3.87 ± 0.25 b |
| MMEAF-F | 2.08 ± 0.28 a | 3.58 ± 0.02 b |
| MMEAF-G | 5.36 ± 0.24 e | 3.78 ± 0.89 b |
| EGB761® | 26.69 ± 1.26 c | 4.62 ± 0.22 b |
| Quercetin | 2.70 ± 0.10 a | 6.75 ± 0.76 a,d |
| Group | Phe | ACh | ||
|---|---|---|---|---|
| pEC50 | Emax (g) | pEC50 | Emax (%) | |
| CTL | 7.63 ± 0.07 | 1.84 ± 0.09 | 7.64 ± 0.05 | 76.91 ± 2.94 |
| CTL + MMEAF | 7.50 ± 0.07 | 1.95 ± 0.14 | 7.24 ± 0.14 | 78.74 ± 2.67 |
| MSG | 7.22 ± 0.13 * | 1.89 ± 0.13 | 7.29 ± 0.03 * | 31.60 ± 1.85 * |
| MSG + MMEAF | 7.28 ± 0.62 | 1.67 ± 0.09 | 7.08 ± 0.12 | 63.34 ± 5.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, L.L.D.M.; Leão, V.d.F.; Melo, C.M.d.; Machado, T.d.B.; Amaral, A.C.F.; Silva, L.L.d.; Simas, N.K.; Muzitano, M.F.; Leal, I.C.R.; Raimundo, J.M. Ethyl Acetate Fraction and Isolated Phenolics Derivatives from Mandevilla moricandiana Identified by UHPLC-DAD-ESI-MSn with Pharmacological Potential for the Improvement of Obesity-Induced Endothelial Dysfunction. Pharmaceutics 2021, 13, 1173. https://doi.org/10.3390/pharmaceutics13081173
Ferreira LLDM, Leão VdF, Melo CMd, Machado TdB, Amaral ACF, Silva LLd, Simas NK, Muzitano MF, Leal ICR, Raimundo JM. Ethyl Acetate Fraction and Isolated Phenolics Derivatives from Mandevilla moricandiana Identified by UHPLC-DAD-ESI-MSn with Pharmacological Potential for the Improvement of Obesity-Induced Endothelial Dysfunction. Pharmaceutics. 2021; 13(8):1173. https://doi.org/10.3390/pharmaceutics13081173
Chicago/Turabian StyleFerreira, Leticia L. D. M., Valéria de F. Leão, Cinthya M. de Melo, Thelma de B. Machado, Ana Claudia F. Amaral, Leandro L. da Silva, Naomi K. Simas, Michelle F. Muzitano, Ivana C. R. Leal, and Juliana M. Raimundo. 2021. "Ethyl Acetate Fraction and Isolated Phenolics Derivatives from Mandevilla moricandiana Identified by UHPLC-DAD-ESI-MSn with Pharmacological Potential for the Improvement of Obesity-Induced Endothelial Dysfunction" Pharmaceutics 13, no. 8: 1173. https://doi.org/10.3390/pharmaceutics13081173
APA StyleFerreira, L. L. D. M., Leão, V. d. F., Melo, C. M. d., Machado, T. d. B., Amaral, A. C. F., Silva, L. L. d., Simas, N. K., Muzitano, M. F., Leal, I. C. R., & Raimundo, J. M. (2021). Ethyl Acetate Fraction and Isolated Phenolics Derivatives from Mandevilla moricandiana Identified by UHPLC-DAD-ESI-MSn with Pharmacological Potential for the Improvement of Obesity-Induced Endothelial Dysfunction. Pharmaceutics, 13(8), 1173. https://doi.org/10.3390/pharmaceutics13081173







