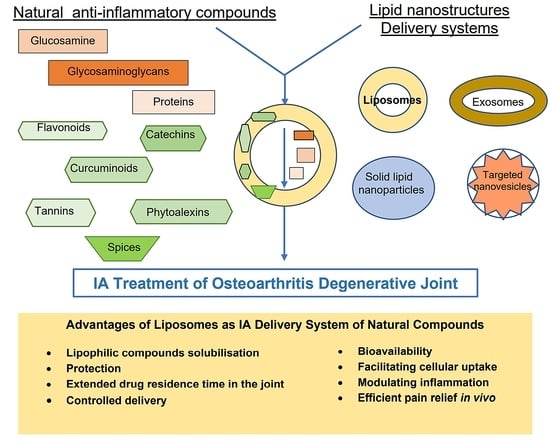
Mechanisms and Pharmaceutical Action of Lipid Nanoformulation of Natural Bioactive Compounds as Efficient Delivery Systems in the Therapy of Osteoarthritis
Abstract
1. Introduction
2. Natural Bioactive Compounds with Anti-Inflammatory Activity and Mechanisms of Action
2.1. Polysaccharides with Anti-Inflammatory Activity
2.2. Plant Polyphenols with Anti-Inflammatory Activity
3. Recent Advances in Liposomes Technology
3.1. Novel Methodological Approaches
3.2. Advantages and Limits of Using Liposomes as Efficient Delivery Systems
3.3. IA vs. Oral Administration
4. Lipid Nanostructures Loaded with Natural Anti-Inflammatory Compounds for OA Treatment
5. Anti-Inflammatory Activity and Mechanisms of Action Demonstrated in Experimental Models In Vitro and In Vivo
5.1. Redox Control
5.2. Modulation of Catabolic Mediators
5.3. Modulation of Cytokines Level
5.4. Modulation of Inflammasome and Toll-Like Receptors (TLR)
5.5. Control of Bone Remodelling Processes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sandell, L.J.; Aigner, T. Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001, 3, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.S.; Walsh, D.A. Osteoarthritis, angiogenesis and inflammation. Rheumatology 2005, 44, 7–16. [Google Scholar] [CrossRef]
- Bottini, M.; Bhattacharya, K.; Fadeel, B.; Magrini, A.; Bottini, N.; Rosato, N. Nanodrugs to target articular cartilage: An emerging platform for osteoarthritis therapy. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 255–268. [Google Scholar] [CrossRef]
- Mora, J.C.; Przkora, R.; Cruz-Almeida, Y. Knee osteoarthritis: Pathophysiology and current treatment modalities. J. Pain Res. 2018, 11, 2189–2196. [Google Scholar] [CrossRef]
- Kou, L.F.; Xiao, S.Y.; Sun, R.; Bao, S.H.; Yao, Q.; Chen, R.J. Biomaterial-engineered intra-articular drug delivery systems for osteoarthritis therapy. Drug Deliv. 2019, 26, 870–885. [Google Scholar] [CrossRef]
- Jin, G.Z. Current Nanoparticle-Based Technologies for Osteoarthritis Therapy. Nanomaterials 2020, 10, 2368. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, Z.; Shen, M.; Ma, Y.; Li, R.; Jin, X.; Gao, L.; Wang, Z. Changes of type II collagenase biomarkers on IL-1β-induced rat articular chondrocytes. Exp. Ther. Med. 2021, 21, 582. [Google Scholar] [CrossRef]
- Poulet, B.; Beier, F. Targeting oxidative stress to reduce osteoarthritis. Arthritis Res. Ther. 2016, 18. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J. Inflammation in osteoarthritis: Is it time to dampen the alarm(in) in this debilitating disease? Clin. Exp. Immunol. 2019, 195, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Mathiessen, A.; Conaghan, P.G. Synovitis in osteoarthritis: Current understanding with therapeutic implications. Arthritis Res. Ther. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Mobasheri, A.; Mozafari, M. Inflammatory mediators in osteoarthritis: A critical review of the state-of-the-art, current prospects, and future challenges. Bone 2016, 85, 81–90. [Google Scholar] [CrossRef]
- Liu-Bryan, R. Synovium and the Innate Inflammatory Network in Osteoarthritis Progression. Curr. Rheumatol. Rep. 2013, 15. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.; Jordan, O.; Allémann, E. Osteoarthritis In Vitro Models: Applications and Implications in Development of Intra-Articular Drug Delivery Systems. Pharmaceutics 2021, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Sellam, J.; Berenbaum, F. Is osteoarthritis a metabolic disease? Jt. Bone Spine 2013, 80, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Fuggle, N.R.; Cooper, C.; Oreffo, R.O.C.; Price, A.J.; Kaux, J.F.; Maheu, E.; Cutolo, M.; Honvo, G.; Conaghan, P.G.; Berenbaum, F.; et al. Alternative and complementary therapies in osteoarthritis and cartilage repair. Aging Clin. Exp. Res. 2020, 32, 547–560. [Google Scholar] [CrossRef]
- Birke, H.; Kurita, G.P.; Sjogren, P.; Hojsted, J.; Simonsen, M.K.; Juel, K.; Ekholm, O. Chronic non-cancer pain and the epidemic prescription of opioids in the Danish population: Trends from 2000 to 2013. Acta Anaesthesiol. Scand. 2016, 60, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.; Martel-Pelletier, J.; Rannou, F.; Cooper, C. Efficacy and safety of oral NSAIDs and analgesics in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin. Arthritis Rheum. 2016, 45, S22–S27. [Google Scholar] [CrossRef]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22. [Google Scholar] [CrossRef]
- Maudens, P.; Jordan, O.; Allemann, E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov. Today 2018, 23, 1761–1775. [Google Scholar] [CrossRef] [PubMed]
- Rahnfeld, L.; Thamm, J.; Steiniger, F.; van Hoogevest, P.; Luciani, P. Study on the in situ aggregation of liposomes with negatively charged phospholipids for use as injectable depot formulation. Colloids Surfaces B 2018, 168, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Trif, M.; Roseanu, A.; Brock, J.H.; Brewer, J.M. Designing lipid nanostructures for local delivery of biologically active macromolecules. J. Liposome Res. 2007, 17, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Wehling, P.; Evans, C.; Wehling, J.; Maixner, W. Effectiveness of intra-articular therapies in osteoarthritis: A literature review. Ther. Adv. Musculoskelet. Dis. 2017, 9, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Michaelis, M.; Ladel, C.; Siebuhr, A.S.; Bihlet, A.R.; Andersen, J.R.; Guehring, H.; Christiansen, C.; Bay-Jensen, A.C.; Kraus, V.B. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and hip: Lessons learned from failures and opportunities for the future. Osteoarthr. Cartil. 2016, 24, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S. Green tea polyphenol epigallocatechin 3-gallate in arthritis: Progress and promise. Arthritis Res. Ther. 2010, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Haqqi, T.M. Current nutraceuticals in the management of osteoarthritis: A review. Ther. Adv. Musculoskelet. Dis. 2012, 4, 181–207. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y. The spice for joint inflammation: Anti-inflammatory role of curcumin in treating osteoarthritis. Drug Des. Dev. Ther. 2016, 10, 3029–3042. [Google Scholar] [CrossRef]
- Hochberg, M.C.; Martel-Pelletier, J.; Monfort, J.; Moller, I.; Castillo, J.R.; Arden, N.; Berenbaum, F.; Blanco, F.J.; Conaghan, P.G.; Domenech, G.; et al. Combined chondroitin sulfate and glucosamine for painful knee osteoarthritis: A multicentre, randomised, double-blind, non-inferiority trial versus celecoxib. Ann. Rheum. Dis. 2016, 75, 37–44. [Google Scholar] [CrossRef]
- Kann, B.; Spengler, C.; Coradini, K.; Rigo, L.A.; Bennink, M.L.; Jacobs, K.; Offerhaus, H.L.; Beck, R.C.R.; Windbergs, M. Intracellular Delivery of Poorly Soluble Polyphenols: Elucidating the Interplay of Self-Assembling Nanocarriers and Human Chondrocytes. Anal. Chem. 2016, 88, 7014–7022. [Google Scholar] [CrossRef]
- Lim, H.; Min, D.S.; Park, H.; Kim, H.P. Flavonoids interfere with NLRP3 inflammasome activation. Toxicol. Appl. Pharm. 2018, 355, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Reginster, J.Y.; Veronese, N. Highly purified chondroitin sulfate: A literature review on clinical efficacy and pharmacoeconomic aspects in osteoarthritis treatment. Aging Clin. Exp. Res. 2021, 33, 37–47. [Google Scholar] [CrossRef]
- Trif, M.; Craciunescu, O. Liposomes as Delivery System of Chondroitin Sulfate to the Arthritic Joint by Intra-articular Administration. Austin Arthritis 2016, 1, 1011. [Google Scholar]
- Yagi, H.; Ulici, V.; Tuan, R.S. Polyphenols suppress inducible oxidative stress in human osteoarthritic and bovine chondrocytes. Osteoarthr. Cartil. Open 2020, 2, 100064. [Google Scholar] [CrossRef]
- Burt, H.M.; Tsallas, A.; Gilchrist, S.; Liang, L.S. Intra-articular drug delivery systems: Overcoming the shortcomings of joint disease therapy. Expert Opin. Drug Deliv. 2009, 6, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, T.E.; Werfel, T.A.; Cho, H.; Hasty, K.A.; Duvall, C.L. Particle-based technologies for osteoarthritis detection and therapy. Drug Deliv. Transl. Res. 2016, 6, 132–147. [Google Scholar] [CrossRef] [PubMed]
- Ghafari, M.; Haghiralsadat, F.; Falahati-pour, S.K.; Reza, J.Z. Development of a novel liposomal nanoparticle formulation of cisplatin to breast cancer therapy. J. Cell Biochem. 2020, 121, 3584–3592. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Cipollaro, L.; Trucillo, P.; Bragazzi, N.L.; Della Porta, G.; Reverchon, E.; Maffulli, N. Liposomes for Intra-Articular Analgesic Drug Delivery in Orthopedics: State-of-Art and Future Perspectives. Insights from a Systematic Mini-Review of the Literature. Medicina 2020, 56, 423. [Google Scholar] [CrossRef]
- Pawar, V.A.; Manjappa, A.S.; Murumkar, P.R.; Gajaria, T.K.; Devkar, R.V.; Mishra, A.K.; Yadav, M.R. Drug-fortified liposomes as carriers for sustained release of NSAIDs: The concept and its validation in the animal model for the treatment of arthritis. Eur. J. Pharm. Sci. 2018, 125, 11–22. [Google Scholar] [CrossRef]
- Gerwin, N.; Hops, C.; Lucke, A. Intraarticular drug delivery in osteoarthritis. Adv. Drug Deliv. Rev. 2006, 58, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Agiba, A.M. Nutraceutical formulations containing glucosamine and chondroitin sulphate in the treatment of osteoarthritis: Emphasis on clinical efficacy and formulation challenges. Int. J. Curr. Pharm. Res. 2017, 9, 1–7. [Google Scholar] [CrossRef][Green Version]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Orally Administered NSAIDs-General Characteristics and Usage in the Treatment of Temporomandibular Joint Osteoarthritis-A Narrative Review. Pharmaceuticals 2021, 14, 219. [Google Scholar] [CrossRef]
- Sharma, A.; Wood, L.D.; Richardson, J.B.; Roberts, S.; Kuiper, N.J. Glycosaminoglycan profiles of repair tissue formed following autologous chondrocyte implantation differ from control cartilage. Arthritis Res. Ther. 2007, 9. [Google Scholar] [CrossRef] [PubMed]
- Restaino, O.F.; Finamore, R.; Stellavato, A.; Diana, P.; Bedini, E.; Trifuoggi, M.; De Rosa, M.; Schiraldi, C. European chondroitin sulfate and glucosamine food supplements: A systematic quality and quantity assessment compared to pharmaceuticals. Carbohyd. Polym. 2019, 222. [Google Scholar] [CrossRef]
- Mishra, S.; Ganguli, M. Functions of, and replenishment strategies for, chondroitin sulfate in the human body. Drug Discov. Today 2021. [Google Scholar] [CrossRef]
- Hatano, S.; Watanabe, H. Regulation of Macrophage and Dendritic Cell Function by Chondroitin Sulfate in Innate to Antigen-Specific Adaptive Immunity. Front. Immunol. 2020, 11. [Google Scholar] [CrossRef]
- Monfort, J.; Nacher, M.; Montell, E.; Vila, J.; Verges, J.; Benito, P. Chondroitin sulfate and hyaluronic acid (500-730 kda) inhibit stromelysin-1 synthesis in human osteoarthritic chondrocytes. Drug Exp. Clin. Res. 2005, 31, 71–76. [Google Scholar]
- Martel-Pelletier, J.; Tat, S.K.; Pelletier, J.P. Effects of chondroitin sulfate in the pathophysiology of the osteoarthritic joint: A narrative review. Osteoarthr. Cartil. 2010, 18, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Towheed, T.E.; Maxwell, L.; Anastassiades, T.P.; Shea, B.; Houpt, J.; Robinson, V.; Hochberg, M.C.; Wells, G. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst. Rev. 2005. [Google Scholar] [CrossRef] [PubMed]
- Tio, L.; Orellana, C.; Perez-Garcia, S.; Piqueras, L.; Escudero, P.; Juarranz, Y.; Garcia-Giralt, N.; Montanes, F.; Farran, A.; Benito, P.; et al. Effect of chondroitin sulphate on synovitis of knee osteoarthritic patients. Med. Clin. 2017, 149, 9–16. [Google Scholar] [CrossRef]
- Lubis, A.M.T.; Siagian, C.; Wonggokusuma, E.; Marsetio, A.F.; Setyohadi, B. Comparison of Glucosamine-Chondroitin Sulfate with and without Methylsulfonylmethane in Grade I-II Knee Osteoarthritis: A Double Blind Randomized Controlled Trial. Acta Med. Indones 2017, 49, 105–111. [Google Scholar]
- Shortt, C.; Luyt, L.G.; Turley, E.A.; Cowman, M.K.; Kirsch, T. A Hyaluronan-binding Peptide (P15-1) Reduces Inflammatory and Catabolic Events in IL-1β-treated Human Articular Chondrocytes. Sci. Rep. 2020, 10, 1441. [Google Scholar] [CrossRef]
- Jensen, G.S.; Attridge, V.L.; Lenninger, M.R.; Benson, K.F. Oral Intake of a Liquid High-Molecular-Weight Hyaluronan Associated with Relief of Chronic Pain and Reduced Use of Pain Medication: Results of a Randomized, Placebo-Controlled Double-Blind Pilot Study. J. Med. Food. 2015, 18, 95–101. [Google Scholar] [CrossRef]
- Navarro-Sarabia, F.; Coronel, P.; Collantes, E.; Navarro, F.J.; de la Serna, A.R.; Naranjo, A.; Gimeno, M.; Herrero-Beaumont, G. A 40-month multicentre, randomised placebo-controlled study to assess the efficacy and carry-over effect of repeated intra-articular injections of hyaluronic acid in knee osteoarthritis: The AMELIA project. Ann. Rheum. Dis. 2011, 70, 1957–1962. [Google Scholar] [CrossRef]
- Natarajan, V.; Madhan, B.; Tiku, M.L. Intra-Articular Injections of Polyphenols Protect Articular Cartilage from Inflammation-Induced Degradation: Suggesting a Potential Role in Cartilage Therapeutics. PLoS ONE 2015, 10. [Google Scholar] [CrossRef]
- Lay, E.; Samiric, T.; Handley, C.J.; Ilic, M.Z. Short- and long-term exposure of articular cartilage to curcumin or quercetin inhibits aggrecan loss. J. Nutr. Biochem. 2012, 23, 106–112. [Google Scholar] [CrossRef]
- Csaki, C.; Keshishzadeh, N.; Fischer, K.; Shakibaei, M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008, 75, 677–687. [Google Scholar] [CrossRef]
- Zheng, W.H.; Feng, Z.H.; Lou, Y.T.; Chen, C.H.; Zhang, C.X.; Tao, Z.Y.; Li, H.; Cheng, L.; Ying, X.Z. Silibinin protects against osteoarthritis through inhibiting the inflammatory response and cartilage matrix degradation in vitro and in vivo. Oncotarget 2017, 8, 99649–99665. [Google Scholar] [CrossRef]
- Zheng, W.; Tao, Z.; Cai, L.; Chen, C.; Zhang, C.; Wang, Q.; Ying, X.; Hu, W.; Chen, H. Chrysin Attenuates IL-1β-Induced Expression of Inflammatory Mediators by Suppressing NF-κB in Human Osteoarthritis Chondrocytes. Inflammation 2017, 40, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, C.; Cai, L.; Xie, H.; Hu, W.; Wang, T.; Lu, D.; Chen, H. Baicalin suppresses IL-1β-induced expression of inflammatory cytokines via blocking NF-κB in human osteoarthritis chondrocytes and shows protective effect in mice osteoarthritis models. Int. Immunopharmacol. 2017, 52, 218–226. [Google Scholar] [CrossRef]
- Khan, N.M.; Haseeb, A.; Ansari, M.Y.; Devarapalli, P.; Haynie, S.; Haqqi, T.M. Wogonin, a plant derived small molecule, exerts potent anti-inflammatory and chondroprotective effects through the activation of ROS/ERK/Nrf2 signaling pathways in human Osteoarthritis chondrocytes. Free. Radical. Bio. Med. 2017, 106, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.; Clutterbuck, A.L.; Allaway, D.; Lodwig, E.M.; Harris, P.; Mathy-Hartert, M.; Shakibaei, M.; Mobasheri, A. Biological actions of curcumin on articular chondrocytes. Osteoarthr. Cartil. 2010, 18, 141–149. [Google Scholar] [CrossRef]
- Liang, J.; Chang, B.; Huang, M.; Huang, W.; Ma, W.; Liu, Y.; Tai, W.; Long, Y.; Lu, Y. Oxymatrine prevents synovial inflammation and migration via blocking NF-κB activation in rheumatoid fibroblast-like synoviocytes. Int. Immunopharmacol. 2018, 55, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Mobasheri, A.; Buhrmann, C. Curcumin synergizes with resveratrol to stimulate the MAPK signaling pathway in human articular chondrocytes in vitro. Genes Nutr. 2011, 6, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular Mechanisms and Therapeutic Trajectory. Front. Vet. Sci. 2019, 6. [Google Scholar] [CrossRef]
- Murata, M.; Yudoh, K.; Shimizu, H.; Beppu, M.; Nakamura, H.; Kato, T.; Masuko, K. Layilin, a talin-binding hyaluronan receptor, is expressed in human articular chondrocytes and synoviocytes and is down-regulated by interleukin-1β. Mod. Rheumatol. 2013, 23, 478–488. [Google Scholar] [CrossRef]
- Oe, M.; Tashiro, T.; Yoshida, H.; Nishiyama, H.; Masuda, Y.; Maruyama, K.; Koikeda, T.; Maruya, R.; Fukui, N. Oral hyaluronan relieves knee pain: A review. Nutr. J. 2016, 15. [Google Scholar] [CrossRef]
- Strauss, E.J.; Hart, J.A.; Miller, M.D.; Altman, R.D.; Rosen, J.E. Hyaluronic Acid Viscosupplementation and Osteoarthritis: Current Uses and Future Directions. Am. J. Sport Med. 2009, 37, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.F.; Pham, C.T.N. Intra-articular drug delivery systems for joint diseases. Curr. Opin. Pharmacol. 2018, 40, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Zhang, Y.J.; Xu, X.R.; Chen, Y.M.; Li, H.B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Madhan, B.; Muralidharan, C.; Jayakumar, R. Study on the stabilisation of collagen with vegetable tannins in the presence of acrylic polymer. Biomaterials 2002, 23, 2841–2847. [Google Scholar] [CrossRef]
- Shen, C.L.; Smith, B.J.; Lo, D.F.; Chyu, M.C.; Dunn, D.M.; Chen, C.H.; Kwun, I.S. Dietary polyphenols and mechanisms of osteoarthritis. J. Nutr. Biochem. 2012, 23, 1367–1377. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 897484. [Google Scholar] [CrossRef]
- Lim, H.; Heo, M.Y.; Kim, H.P. Flavonoids: Broad Spectrum Agents on Chronic Inflammation. Biomol. Ther. 2019, 27, 241–253. [Google Scholar] [CrossRef]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J. Immunol. Res. 2018, 2018. [Google Scholar] [CrossRef]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. Biomed Res. Int. 2018, 2018. [Google Scholar] [CrossRef]
- Soyocak, A.; Kurt, H.; Cosan, D.T.; Saydam, F.; Calis, I.U.; Kolac, U.K.; Koroglu, Z.O.; Degirmenci, I.; Mutlu, F.S.; Gunes, H.V. Tannic acid exhibits anti-inflammatory effects on formalin-induced paw edema model of inflammation in rats. Hum. Exp. Toxicol. 2019, 38, 1296–1301. [Google Scholar] [CrossRef]
- Marouf, B.H.; Hussain, S.A.; Ali, Z.S.; Ahmmad, R.S. Resveratrol Supplementation Reduces Pain and Inflammation in Knee Osteoarthritis Patients Treated with Meloxicam: A Randomized Placebo-Controlled Study. J. Med. Food. 2018, 21, 1253–1259. [Google Scholar] [CrossRef]
- Dai, J.P.; Wang, Q.W.; Su, Y.; Gu, L.M.; Deng, H.X.; Chen, X.X.; Li, W.Z.; Li, K.S. Oxymatrine Inhibits Influenza A Virus Replication and Inflammation via TLR4, p38 MAPK and NF-κB Pathways. Int. J. Mol. Sci. 2018, 19, 965. [Google Scholar] [CrossRef]
- Bartels, E.M.; Folmer, V.N.; Bliddal, H.; Altman, R.D.; Juhl, C.; Tarp, S.; Zhang, W.; Christensen, R. Efficacy and safety of ginger in osteoarthritis patients: A meta-analysis of randomized placebo-controlled trials. Osteoarthr. Cartil. 2015, 23, 13–21. [Google Scholar] [CrossRef]
- Ghasemian, M.; Owlia, S.; Owlia, M.B. Review of Anti-Inflammatory Herbal Medicines. Adv. Pharmacol. Sci. 2016, 2016. [Google Scholar] [CrossRef]
- Lee, H.; Zhao, X.Y.; Son, Y.O.; Yang, S.Y. Therapeutic Single Compounds for Osteoarthritis Treatment. Pharmaceuticals 2021, 14, 131. [Google Scholar] [CrossRef]
- Sharma, D.; Aara, A.; Ali, E.; Trivedi, L. An Updated Review On:Liposomes as Drug Delivery System. Pharmatutor 2018, 6. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Hussein, S.A.; Ali, A.H.; Korma, S.A.; Lipeng, Q.; Jinghua, C. Liposome: Composition, characterisation, preparation, and recent innovation in clinical applications. J. Drug Target. 2019, 27, 742–761. [Google Scholar] [CrossRef] [PubMed]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- van den Hoven, J.M.; Van Tomme, S.R.; Metselaar, J.M.; Nuijen, B.; Beijnen, J.H.; Storm, G. Liposomal Drug Formulations in the Treatment of Rheumatoid Arthritis. Mol. Pharm. 2011, 8, 1002–1015. [Google Scholar] [CrossRef]
- Vanniasinghe, A.S.; Bender, V.; Manolios, N. The Potential of Liposomal Drug Delivery for the Treatment of Inflammatory Arthritis. Semin. Arthritis Rheum. 2009, 39, 182–196. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.F.; Zhang, W.; Wei, K.; Pei, Y.P.; Zou, C.M.; Zhang, C.; Ding, J.H.; Fang, H.; Tan, S.W. Oxymatrine Liposomes for Intervertebral Disc Treatment: Formulation, in vitro and vivo Assessments. Drug Des. Dev. Ther. 2020, 14, 921–931. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G. Micro- and Nano-Carrier Mediated Intra-Articular Drug Delivery Systems for the Treatment of Osteoarthritis. J. Nanotechnol. 2012, 2012, 748909. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications. Int. J. Nanomed. 2020, 15, 3099–3120. [Google Scholar] [CrossRef]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mat. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to Supercritical Fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery Systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Otake, K.; Imura, T.; Sakai, H.; Abe, M. Development of a new preparation method of liposomes using supercritical carbon dioxide. Langmuir 2001, 17, 3898–3901. [Google Scholar] [CrossRef]
- Imura, T.; Otake, K.; Hashimoto, S.; Gotoh, T.; Yuasa, M.; Yokoyama, S.; Sakai, H.; Rathman, J.F.; Abe, M. Preparation and physicochemical properties of various soybean lecithin liposomes using supercritical reverse phase evaporation method. Colloids Surfaces B Biointerfaces 2003, 27, 133–140. [Google Scholar] [CrossRef]
- Shirakawa, M.; Nakai, K.; Sato, Y.; Nakamura, S.; Harada, M.; Ishihara, K.; Yoshida, F.; Matsumura, A.; Tomida, H. Optimization of preparation methods for high loading content and high encapsulation efficiency of BSH into liposomes. Appl. Radiat. Isot. 2021, 169. [Google Scholar] [CrossRef] [PubMed]
- Secolin, V.A.; Souza, C.R.; Oliveira, W.P. Spray drying of lipid-based systems loaded with Camellia sinensis polyphenols. J. Liposome Res. 2017, 27, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Toniazzo, T.; Peres, M.S.; Ramos, A.P.; Pinho, S.C. Encapsulation of quercetin in liposomes by ethanol injection and physicochemical characterization of dispersions and lyophilized vesicles. Food Biosci. 2017, 19, 17–25. [Google Scholar] [CrossRef]
- Franze, S.; Selmin, F.; Samaritani, E.; Minghetti, P.; Cilurzo, F. Lyophilization of Liposomal Formulations: Still Necessary, Still Challenging. Pharmaceutics 2018, 10, 139. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Supercritical CO2 assisted liposomes formation: Optimization of the lipidic layer for an efficient hydrophilic drug loading. J. Co2 Util. 2017, 18, 181–188. [Google Scholar] [CrossRef]
- Cosio, B.G.; Iglesias, A.; Rios, A.; Noguera, A.; Sala, E.; Ito, K.; Barnes, P.J.; Agusti, A. Low-dose theophylline enhances the anti-inflammatory effects of steroids during exacerbations of COPD. Thorax 2009, 64, 424–429. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. A versatile supercritical assisted process for the one-shot production of liposomes. J. Supercrit. Fluids 2019, 146, 136–143. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Control of liposomes diameter at micrometric and nanometric level using a supercritical assisted technique. J. Co2 Util. 2019, 32, 119–127. [Google Scholar] [CrossRef]
- Lim, C.B.; Abuzar, S.M.; Karn, P.R.; Cho, W.; Park, H.J.; Cho, C.W.; Hwang, S.J. Preparation, Characterization, and In Vivo Pharmacokinetic Study of the Supercritical Fluid-Processed Liposomal Amphotericin B. Pharmaceutics 2019, 11, 589. [Google Scholar] [CrossRef]
- Maeki, M.; Kimura, N.; Sato, Y.; Harashima, H.; Tokeshi, M. Advances in microfluidics for lipid nanoparticles and extracellular vesicles and applications in drug delivery systems. Adv. Drug Deliv. Rev. 2018, 128, 84–100. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Hussain, M.T.; Roces, C.B.; Anderluzzi, G.; Kastner, E.; Salmaso, S.; Kirby, D.J.; Perrie, Y. Microfluidics based manufacture of liposomes simultaneously entrapping hydrophilic and lipophilic drugs. Int. J. Pharm. 2016, 514, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.W.Z.; Wong, Y.S.; Czarny, B.; Venkatraman, S. Microfluidic-directed self-assembly of liposomes: Role of interdigitation. J. Colloid Interface Sci. 2020, 578, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2017, 6, 25876. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, T.R.; Straubinger, R.M. Chapter 12—Nanoparticles for Brain Tumor Delivery. In Nervous System Drug Delivery; Lonser, R.R., Sarntinoranont, M., Bankiewicz, K., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 229–250. [Google Scholar]
- Gonda, A.; Zhao, N.; Shah, J.V.; Calvelli, H.R.; Kantamneni, H.; Francis, N.L.; Ganapathy, V. Engineering Tumor-Targeting Nanoparticles as Vehicles for Precision Nanomedicine. Med. One 2019, 4. [Google Scholar] [CrossRef]
- Caracciolo, G. Clinically approved liposomal nanomedicines: Lessons learned from the biomolecular corona. Nanoscale 2018, 10, 4167–4172. [Google Scholar] [CrossRef]
- Bonicelli, M.G.; Giansanti, L.; Ierino, M.; Mancini, G. Interaction of cationic liposomes with cell membrane models. J. Colloid Interface Sci. 2011, 355, 1–8. [Google Scholar] [CrossRef]
- Cosimati, R.; Milardi, G.L.; Bombelli, C.; Bonincontro, A.; Bordi, F.; Mancini, G.; Risuleo, G. Interactions of DMPC and DMPC/gemini liposomes with the cell membrane investigated by electrorotation. Bba-Biomembranes 2013, 1828, 352–356. [Google Scholar] [CrossRef]
- Kotrotsiou, O.; Kotti, K.; Dini, E.; Kammona, O.; Kiparissides, C. Nanostructured materials for selective recognition and targeted drug delivery. J. Phys. Conf. Ser. 2005, 10, 281–284. [Google Scholar] [CrossRef]
- Ren, H.W.; He, Y.W.; Liang, J.M.; Cheng, Z.K.; Zhang, M.; Zhu, Y.; Hong, C.; Qin, J.; Xu, X.C.; Wang, J.X. Role of Liposome Size, Surface Charge, and PEGylation on Rheumatoid Arthritis Targeting Therapy. ACS Appl. Mater. Interfaces 2019, 11, 20304–20315. [Google Scholar] [CrossRef]
- Mignet, N.; Seguin, J.; Chabot, G.G. Bioavailability of polyphenol liposomes: A challenge ahead. Pharmaceutics 2013, 5, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil(R)--the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Goldberg, R.; Klein, J. Liposomes as lubricants: Beyond drug delivery. Chem. Phys. Lipids 2012, 165, 374–381. [Google Scholar] [CrossRef]
- Lin, W.F.; Liu, Z.; Kampf, N.; Klein, J. The Role of Hyaluronic Acid in Cartilage Boundary Lubrication. Cells 2020, 9, 1606. [Google Scholar] [CrossRef] [PubMed]
- McNary, S.M.; Athanasiou, K.A.; Reddi, A.H. Engineering Lubrication in Articular Cartilage. Tissue Eng. Part B Rev. 2012, 18, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Osorno, L.L.; Brandley, A.N.; Maldonado, D.E.; Yiantsos, A.; Mosley, R.J.; Byrne, M.E. Review of Contemporary Self-Assembled Systems for the Controlled Delivery of Therapeutics in Medicine. Nanomaterials 2021, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.T.; Kreutzberger, A.J.B.; Lee, J.; Kiessling, V.; Tamm, L.K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids 2016, 199, 136–143. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Lee, D.U.; Park, H.W.; Lee, S.C. Comparing the stability of retinol in liposomes with cholesterol, β-sitosterol, and stigmasterol. Food Sci. Biotechnol. 2021, 30, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.M.; Maxwell, J.S.; Weng, L.H.; Angelos, M.G.; Golzarian, J. Intra-articular treatment of knee osteoarthritis: From anti-inflammatories to products of regenerative medicine. Physician Sportsmed. 2016, 44, 101–108. [Google Scholar] [CrossRef]
- Lombardo, D.; Calandra, P.; Barreca, D.; Magazu, S.; Kiselev, M.A. Soft Interaction in Liposome Nanocarriers for Therapeutic Drug Delivery. Nanomaterials 2016, 6, 125. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.M.; Saleh, K.S.; Burdick, J.A.; Mauck, R.L. Bioactive factors for cartilage repair and regeneration: Improving delivery, retention, and activity. Acta Biomater. 2019, 93, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Migliore, A.; Paoletta, M.; Moretti, A.; Liguori, S.; Iolascon, G. The perspectives of intra-articular therapy in the management of osteoarthritis. Expert Opin. Drug Deliv. 2020, 17, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.P.; Hunter, D.J. Intra-articular therapies for osteoarthritis. Expert Opin. Pharmacother. 2016, 17, 2057–2071. [Google Scholar] [CrossRef] [PubMed]
- Kompel, A.J.; Roemer, F.W.; Murakami, A.M.; Diaz, L.E.; Crema, M.D.; Guermazi, A. Intra-articular Corticosteroid Injections in the Hip and Knee: Perhaps Not as Safe as We Thought? Radiology 2019, 293, 656–663. [Google Scholar] [CrossRef] [PubMed]
- Jay, G.D.; Fleming, B.C.; Watkins, B.A.; McHugh, K.A.; Anderson, S.C.; Zhang, L.X.; Teeple, E.; Waller, K.A.; Elsaid, K.A. Prevention of Cartilage Degeneration and Restoration of Chondroprotection by Lubricin Tribosupplementation in the Rat Following Anterior Cruciate Ligament Transection. Arthritis Rheum. 2010, 62, 2382–2391. [Google Scholar] [CrossRef]
- Colen, S.; van den Bekerom, M.P.J.; Mulier, M.; Haverkamp, D. Hyaluronic Acid in the Treatment of Knee Osteoarthritis A Systematic Review and Meta-Analysis with Emphasis on the Efficacy of Different Products. Biodrugs 2012, 26, 257–268. [Google Scholar] [CrossRef]
- Huang, H.-T.; Cheng, T.-L.; Yang, C.-D.; Chang, C.-F.; Ho, C.-J.; Chuang, S.-C.; Li, J.-Y.; Huang, S.-H.; Lin, Y.-S.; Shen, H.-Y.; et al. Intra-Articular Injection of (-)-Epigallocatechin 3-Gallate (EGCG) Ameliorates Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis. Antioxidants 2021, 10, 178. [Google Scholar] [CrossRef]
- Li, W.; Cai, L.; Zhang, Y.; Cui, L.; Shen, G. Intra-articular resveratrol injection prevents osteoarthritis progression in a mouse model by activating SIRT1 and thereby silencing HIF-2α. J. Orthop. Res. 2015, 33, 1061–1070. [Google Scholar] [CrossRef]
- Qin, N.; Wei, L.W.; Li, W.Y.; Yang, W.; Cai, L.T.; Qian, Z.; Wu, S.F. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J. Pharmacol. Sci. 2017, 134, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Nicoliche, T.; Maldonado, D.C.; Faber, J.; da Silva, M.C.P. Evaluation of the articular cartilage in the knees of rats with induced arthritis treated with curcumin. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Park, S.; Lee, L.R.; Seo, J.H.; Kang, S. Curcumin and tetrahydrocurcumin both prevent osteoarthritis symptoms and decrease the expressions of pro-inflammatory cytokines in estrogen-deficient rats. Genes Nutr. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.H.R.; Cake, M.A.; Spoelstra, G.; Read, R.A. Biodistribution and clearance of intra-articular Liposomes in a large animal model using a radiographic marker. J. Liposome Res. 2007, 17, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Coimbra, M.; Isacchi, B.; van Bloois, L.; Torano, J.S.; Ket, A.; Wu, X.; Broere, F.; Metselaar, J.M.; Rijcken, C.J.F.; Storm, G.; et al. Improving solubility and chemical stability of natural compounds for medicinal use by incorporation into liposomes. Int. J. Pharm. 2011, 416, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Kanwar, R.K.; Kanwar, J.R. Molecular targets in arthritis and recent trends in nanotherapy. Int. J. Nanomed. 2015, 10, 5407–5420. [Google Scholar] [CrossRef]
- Sivan, S.; Schroeder, A.; Verberne, G.; Merkher, Y.; Diminsky, D.; Priev, A.; Maroudas, A.; Halperin, G.; Nitzan, D.; Etsion, I.; et al. Liposomes Act as Effective Biolubricants for Friction Reduction in Human Synovial Joints. Langmuir 2010, 26, 1107–1116. [Google Scholar] [CrossRef]
- Ji, X.; Zhang, H. Current Strategies for the Treatment of Early Stage Osteoarthritis. Front. Mech. Eng. 2019, 5. [Google Scholar] [CrossRef]
- Trif, M.; Guillen, C.; Vaughan, D.M.; Telfer, J.M.; Brewer, J.M.; Roseanu, A.; Brock, J.H. Liposomes as Possible Carriers for Lactoferrin in the Local Treatment of Inflammatory Diseases. Exp. Biol. Med. 2001, 226, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.L.; Yan, Y.F.; Sun, T.; Zhang, Q.; Wang, Y.X.; Zhang, M.; Zhang, H.Y.; Zhao, X. Glucosamine sulphate-loaded distearoyl phosphocholine liposomes for osteoarthritis treatment: Combination of sustained drug release and improved lubrication. Biomater. Sci. 2019, 7, 2716–2728. [Google Scholar] [CrossRef]
- Craciunescu, O.; Moldovan, L.; Moisei, M.; Trif, M. Liposomal formulation of chondroitin sulfate enhances its antioxidant and anti-inflammatory potential in L929 fibroblast cell line. J. Liposome Res. 2013, 23, 145–153. [Google Scholar] [CrossRef]
- Craciunescu, O.; Gaspar, A.; Trif, M.; Moisei, M.; Oancea, A.; Moldovan, L.; Zarnescu, O. Preparation and Characterization of a Collagen-Liposome-Chondroitin Sulfate Matrix with Potential Application for Inflammatory Disorders Treatment. J. Nanomater. 2014, 2014. [Google Scholar] [CrossRef]
- Agiba, A.M.; Nasr, M.; Abdel-Hamid, S.; Eldin, A.B.; Geneidi, A.S. Enhancing the Intestinal Permeation of the Chondroprotective Nutraceuticals Glucosamine Sulphate and Chondroitin Sulphate Using Conventional and Modified Liposomes. Curr. Drug Deliv. 2018, 15, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, M.; Jain, A.; Singla, Y.; Shrivastava, B. Sublingual delivery of chondroitin sulfate conjugated tapentadol loaded nanovesicles for the treatment of osteoarthritis. J. Liposome Res. 2021, 31, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Mishra, S.K.; Vuddanda, P.R.; Singh, S.K.; Singh, R.; Singh, S. Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1031–1040. [Google Scholar] [CrossRef]
- Dong, J.; Jiang, D.H.; Wang, Z.; Wu, G.Z.; Miao, L.Y.; Huang, L.X. Intra-articular delivery of liposomal celecoxib-hyaluronate combination for the treatment of osteoarthritis in rabbit model. Int. J. Pharm. 2013, 441, 285–290. [Google Scholar] [CrossRef]
- Shakouri, A.; Adljouy, N.; Balkani, S.; Mohamadi, M.; Hamishehkar, H.; Abdolalizadeh, J.; Shakouri, S.K. Effectiveness of topical gel of medical leech (Hirudo medicinalis) saliva extract on patients with knee osteoarthritis: A randomized clinical trial. Complement. Ther. Clin. Pr. 2018, 31, 352–359. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernandez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Sultana, F.; Neog, M.K.; Rasool, M. Targeted delivery of morin, a dietary bioflavanol encapsulated mannosylated liposomes to the macrophages of adjuvant-induced arthritis rats inhibits inflammatory immune response and osteoclastogenesis. Eur. J. Pharm. Biopharm. 2017, 115, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Neog, M.K.; Rasool, M. Targeted delivery of p-coumaric acid encapsulated mannosylated liposomes to the synovial macrophages inhibits osteoclast formation and bone resorption in the rheumatoid arthritis animal model. Eur. J. Pharm. Biopharm. 2018, 133, 162–175. [Google Scholar] [CrossRef]
- Yeh, C.C.; Su, Y.H.; Lin, Y.J.; Chen, P.J.; Shi, C.S.; Chen, C.N.; Chang, H.I. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug Des. Dev. Ther. 2015, 9, 2285–2300. [Google Scholar] [CrossRef]
- Shi, H.S.; Gao, X.; Li, D.; Zhang, Q.W.; Wang, Y.S.; Zheng, Y.; Cai, L.L.; Zhong, R.M.; Rui, A.; Li, Z.Y.; et al. A systemic administration of liposomal curcumin inhibits radiation pneumonitis and sensitizes lung carcinoma to radiation. Int. J. Nanomed. 2012, 7, 2601–2611. [Google Scholar] [CrossRef]
- Chen, C.; Johnston, T.D.; Jeon, H.; Gedaly, R.; McHugh, P.P.; Burke, T.G.; Ranjan, D. An in vitro study of liposomal curcumin: Stability, toxicity and biological activity in human lymphocytes and Epstein-Barr virus-transformed human B-cells. Int. J. Pharm. 2009, 366, 133–139. [Google Scholar] [CrossRef]
- Sun, D.M.; Zhuang, X.Y.; Xiang, X.Y.; Liu, Y.L.; Zhang, S.Y.; Liu, C.R.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A Novel Nanoparticle Drug Delivery System: The Anti-inflammatory Activity of Curcumin Is Enhanced When Encapsulated in Exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef]
- Sun, Z.Y.; Wei, T.; Zhou, X.Y. Liposomes encapsulated dimethyl curcumin regulates dipeptidyl peptidase I activity, gelatinase release and cell cycle of spleen lymphocytes in-vivo to attenuate collagen induced arthritis in rats. Int. Immunopharmacol. 2018, 65, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Ye, J.H.; Augustin, M.A. Nano- and micro-particles for delivery of catechins: Physical and biological performance. Crit. Rev. Food Sci. 2019, 59, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Isailovic, B.D.; Kostic, I.T.; Zvonar, A.; Dordevic, V.B.; Gasperlin, M.; Nedovic, V.A.; Bugarski, B.M. Resveratrol loaded liposomes produced by different techniques. Innov. Food Sci. Emerg. Technol. 2013, 19, 181–189. [Google Scholar] [CrossRef]
- Coradini, K.; Friedrich, R.B.; Fonseca, F.N.; Vencato, M.S.; Andrade, D.F.; Oliveira, C.M.; Battistel, A.P.; Guterres, S.S.; da Rocha, M.I.U.M.; Pohlmann, A.R.; et al. A novel approach to arthritis treatment based on resveratrol and curcumin co-encapsulated in lipid-core nanocapsules: In vivo studies. Eur. J. Pharm. Sci. 2015, 78, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Ghosh, S.; Sil, P.C. Role of nanostructures in improvising oral medicine. Toxicol. Rep. 2019, 6, 358–368. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J. Osteoarthritis year in review 2020: Biology. Osteoarthr. Cartil. 2021, 29, 143–150. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Fukuda, K. Hyaluronic acid regulates a key redox control factor Nrf2 via phosphorylation of Akt in bovine articular chondrocytes. FEBS Open Bio. 2015, 5, 476–484. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef] [PubMed]
- Elron-Gross, I.; Glucksam, Y.; Margalit, R. Liposomal dexamethasone-diclofenac combinations for local osteoarthritis treatment. Int. J. Pharm. 2009, 376, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Yano, S.; Fujimura, Y.; Umeda, D.; Miyase, T.; Yamada, K.; Tachibana, H. Relationship between the biological activities of methylated derivatives of (-)-epigallocatechin-3-O-gallate (EGCG) and their cell surface binding activities. J. Agric. Food Chem. 2007, 55, 7144–7148. [Google Scholar] [CrossRef]
- Mobasheri, A.; Barrett-Jolley, R.; Staunton, C.A.; Ford, C.; Henrotin, Y. Nutrigenomics, Inflammaging, and Osteoarthritis. In Genomics, Proteomics and Metabolomics in Nutraceuticals and Functional Foods; John Wiley & Sons Ltd.: West Sussex, UK, 2015; pp. 71–84. [Google Scholar]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Coradini, K.; Lima, F.O.; Oliveira, C.M.; Chaves, P.S.; Athayde, M.L.; Carvalho, L.M.; Beck, R.C.R. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur. J. Pharm. Biopharm. 2014, 88, 178–185. [Google Scholar] [CrossRef]
- Jung, K.K.; Lee, H.S.; Cho, J.Y.; Shin, W.C.; Rhee, M.H.; Kim, T.G.; Kang, J.H.; Kim, S.H.; Hong, S.; Kang, S.Y. Inhibitory effect of curcumin on nitric oxide production from lipopolysaccharide-activated primary microglia. Life Sci. 2006, 79, 2022–2031. [Google Scholar] [CrossRef]
- Comblain, F.; Sanchez, C.; Lesponne, I.; Balligand, M.; Serisier, S.; Henrotin, Y. Curcuminoids extract, hydrolyzed collagen and green tea extract synergically inhibit inflammatory and catabolic mediator’s synthesis by normal bovine and osteoarthritic human chondrocytes in monolayer. PLoS ONE 2015, 10, e0121654. [Google Scholar] [CrossRef]
- Troeberg, L.; Nagase, H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochim. Biophys. Acta Proteins Proteom. 2012, 1824, 133–145. [Google Scholar] [CrossRef]
- Clutterbuck, A.L.; Allaway, D.; Harris, P.; Mobasheri, A. Curcumin reduces prostaglandin E2, matrix metalloproteinase-3 and proteoglycan release in the secretome of interleukin 1beta-treated articular cartilage. F1000Research 2013, 2, 147. [Google Scholar] [CrossRef]
- Ishikado, A.; Imanaka, H.; Takeuchi, T.; Harada, E.; Makino, T. Liposomalization of lactoferrin enhanced it’s anti-inflammatory effects via oral administration. Biol. Pharm. Bull. 2005, 28, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.P.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, H.; Wei, J.; Lin, S.; Zong, Z.; Gong, F.; Huang, X.; Sun, J.; Li, P.; Lin, H.; et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res. Ther. 2019, 21, 300. [Google Scholar] [CrossRef]
- Stabler, T.V.; Huang, Z.; Montell, E.; Vergés, J.; Kraus, V.B. Chondroitin sulphate inhibits NF-κB activity induced by interaction of pathogenic and damage associated molecules. Osteoarthr. Cartil. 2017, 25, 166–174. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. Hyaluronan, a crucial regulator of inflammation. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef]
- He, X.X.; Wei, Z.K.; Wang, J.J.; Kou, J.H.; Liu, W.J.; Fu, Y.H.; Yang, Z.T. Alpinetin attenuates inflammatory responses by suppressing TLR4 and NLRP3 signaling pathways in DSS-induced acute colitis. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.B.; Yan, Z.J.; Shui, X.L.; Qi, W.H.; Chen, Y.L.; Xu, X.X.; Hu, Y.Z.; Guo, W.J.; Shang, P. Astilbin prevents osteoarthritis development through the TLR4/MD-2 pathway. J. Cell Mol. Med. 2020, 24, 13104–13114. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; He, B.S.; Guo, J.; Li, S.L.; Wang, J. Involvement of TLR4 in the protective effect of intra-articular administration of curcumin on rat experimental osteoarthritis. Acta Cir. Bras. 2019, 34. [Google Scholar] [CrossRef]
- Boyce, B.F.; Xing, L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch. Biochem. Biophys. 2008, 473, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Pecchi, E.; Priam, S.; Mladenovic, Z.; Gosset, M.; Saurel, A.S.; Aguilar, L.; Berenbaum, F.; Jacques, C. A potential role of chondroitin sulfate on bone in osteoarthritis: Inhibition of prostaglandin E2 and matrix metalloproteinases synthesis in interleukin-1β-stimulated osteoblasts. Osteoarthr. Cartil. 2012, 20, 127–135. [Google Scholar] [CrossRef] [PubMed]
| Natural Compound | Main Class | Composition/Subclass | Administration | Activity [Reference] |
|---|---|---|---|---|
| Polysaccharides | ||||
| GLU | Polysaccharides | Amino sugar | Oral administration of 1500 mg/day in symptomatic OA | Pain relief and improvement of Lequesne index, but not WOMAC score [52] |
| CS | Polysaccharides | Repetitive sulfated amino sugar and glucuronic acid units | Oral administration of 800 mg/day, 6 months | Better than paracetamol to reduce synovitis [53] |
| CS | Polysaccharides | Repetitive sulfated amino sugar and glucuronic acid units | Oral administration of 1200 mg or 3 × 400 mg/day, 24 months | Improved joint swelling and WOMAC pain score; better than celecoxib in preserving cartilage volume [19] |
| CS and GLU | Polysaccharides | - | Oral administration of 400 mg CS and 500 mg GLU, 6 months in OA patients with moderate-severe pain | Reduced pain similar to celecoxib [29] |
| CS and GLU and MSM | Polysaccharides and sulfur compounds | - | Clinical trial in grade I-II knee OA | Reduced pain and improved WOMAC scores [54] |
| HA | Polysaccharides | Repetitive amino sugar and glucuronic acid units | Human IL-1 β treated chondrocytes from total knee replacement patients cultured with 1 mg/mL HA and 17 µg/mL P15-1 peptide | Inhibited the catabolic events via MAPK, CD44 clustering and TLR4 signaling, enhanced the protective environment for chondrocytes and stem cells [55] |
| HA | Polysaccharides | Repetitive amino sugar and glucuronic acid units | Oral HA, 225 mg/day for 2 weeks and 150 mg/day for 2 weeks in patients with knee OA | Relieved knee pain in synovitis [56] |
| HA | Polysaccharides | Repetitive amino sugar and glucuronic acid | Intra-articular HA for 6 months in patients with knee OA grade II-III | Improved OA symptoms and had a carry-over effect for 1 year [57] |
| Polyphenols | ||||
| EGCG | Flavonoids | Catechins | In vitro model of acute injury in H2O2-treated bovine chondrocytes | Reduced ROS and NO production [35] |
| EGCG | Flavonoids | Catechins | In vivo IA injection in CIA rats | Articular cartilage resistance to degradation in therapeutic groups [58] |
| Tannic acid | Phenolics | Tannins | In vivo IA injection in CIA rats | Reduction of cartilage degradation in prophylactic groups and therapeutic groups [58] |
| Quercetin | Flavonoids | Flavonols | In vitro incubation of bovine articular cartilage explants | Inhibited matrix-degrading enzymes and aggrecan loss [59] |
| Resveratrol | Phenolics | Phytoalexins (Stilbenoids) | In vitro primary human articular IL-1β treated chondrocytes with | Inhibited cell apoptosis and mitochondria degradation, blocked caspase pathway and reversed ROS up-regulated production [60] |
| Silibinin | Flavonoids | Flavones | Human chondrocytes from OA patients with total knee replacement surgery, OA model in mice | Alleviated cartilage damage and proteoglycan loss, decreased MMP-13 and increased collagen-II expression in OA mice [61] |
| Chrysin | Flavonoids | Flavonols | In vitro OA IL-1β treated chondrocytes | Inhibited NO production, MMP-1, -3 and -13 expression, but also suppressed ADAMTS-4, -5 and blocked NF-kB activation [62] |
| Baicalin | Flavonoids | Flavones | Human OA IL-1β treated chondrocytes, OA model in mice | Inhibited COX-2, iNOS, MMP-3, -13 expression and NO, PGE2 production; relieved synovitis in OA mice [63] |
| Wogonin | Flavonoids | Flavones | Human OA IL-1β treated chondrocytes, cartilage explants | Switched the catabolic action to elevated expression of anabolic factors, suppressed oxidative stress and inflammation [64] |
| Apigenin | Flavonoids | Flavones | Differentiated THP-1 cells activated with sodium urate | Inhibited IL-1 β production and apoptosis-associated speck-like protein oligomerization, and improved the inflammatory symptoms associated with inflammasome activation [31] |
| Kaempferol | Flavonols | |||
| 3′, 4′-Dichloroflavone | Flavones | |||
| Curcumin | Flavonoids | Curcuminoids | Orally taken capsules | Improved WOMAC and decreased inflammation in OA patients [28] |
| In vitro IL-1β-stimulated human chondrocytes | Anti-apoptotic effect in chondrocytes and regulation of cartilage degradation through MMP-3, caspase-3 and IL-1 β actions [65] | |||
| In vitro incubation in human osteoarthritic chondrocytes | Suppressed the oxidative stress-induced responses involved in OA pathogenesis [35] | |||
| Oxymatrine | Alkaloid | Quinolizidine alkaloids | In vitro incubation in human synoviocytes | Decreased IL-6 and IL-8 expression through of NF-kB inhibition [66] |
| Curcumin and resveratrol | Polyphenols combination | Curcuminoids and phytoalexins | In vitro incubation in human articular IL-1 β treated chondrocytes | Synergistically inhibited catabolic effect, MAPK pathway and apoptosis of chondrocytes [67] |
| Natural Compound | Lipid Nanostructure | Composition | Administration | Activity [Reference] |
|---|---|---|---|---|
| Polysaccharides | ||||
| GLU | Liposomes | DSPC:GLU 8:2 (molar ratio) | In vitro studies in primary mouse chondrocytes | Accelerated cell viability and proliferation; down-regulation of pro-inflammatory cytokines; up-regulation of anabolic components [151] |
| CS | Positive Liposomes | PC:DOPE:Chol:SA 4:2:3:1 (molar ratio) Size—170.3 nm; PDI—0.218; ζ—10.44 mV | In vitro studies in stressed L929 mouse fibroblasts | Protective effect against oxidative damage and decrease of pro-inflammatory cytokines production [152] |
| CS | Positive liposomes embedded in type I collagen and freeze-dried | PC:DOPE:Chol:SA 4:2:3:1 (molar ratio) E.E.—68.2% Size—523.83 nm; PDI—0.40; ζ—10.44 mV | L929 mouse fibroblasts injected in sterile freeze-dried matrices | Better control of CS release compared to liposomal CS; allowed cell penetration for regenerative activity [153] |
| CS and GLU | Liposomes | Epikuron 200©, Epikuron 200© SH | Oral administration to rabbits | High permeation through intestinal mucosa; no histopathological alterations of the intestinal tissue [154] |
| CS and tapentadol | CS surface modified nanovesicles | PC:Chol:SA 7:3:1.5 (molar ratio) | Sublingual administration in OA-induced Wistar rats | Improved bioavailability and reduction of pain [155] |
| CS and diacerein | CS modified SLN | Lecithin:SA 1:6.25 (mass ratio) | IA administration in femoro-tibial joint of rat knee | Increased drug bioavailability [156] |
| HA | Liposomes | PC: HA solution (1:1, volume ratio) | Surface force balance measurements | The model suggested that multiple lipid layers formed on the surface increased lubrication, while HA could be complexed by lipids in the synovial fluid [125] |
| HA and celecoxib | Liposomes embedded in HA gel | PC:Chol 5:1 (mass ratio) | Single IA administration in rabbit OA knee model | Effective in pain control and cartilage protection [157] |
| Proteins | ||||
| Leech saliva extract rich in proteins and peptides | Liposomes | PC:Chol 95:5 (mass ratio) | Topical administration in human OA patients for 1 month | Enhanced skin absorption; 50% pain relief; reduction of joint inflammation and stiffness [158] |
| Lactoferrin | Positive MVL (Multivesicular liposomes) Liposomes | DPPE:Chol:SA 5:5:1 (molar ratio) | IA administration in CIA DBA1 mice | Prolonged the residence time for better reduction of inflammation, compared to free lactofferin; decreased pro-inflammatory cytokines production (TNF-α, IFN-γ); increased anti-inflammatory cytokines (IL-5, IL-10) [23,150] |
| Polyphenols | ||||
| Quercetin and resveratrol | Liposomes | Lipoid S75:oleic acid 10:1 (mass ratio) | Oxidative stressed fibroblasts | High cellular uptake and superior ROS scavenging, compared to free polyphenols [159] |
| Resveratrol and curcumin | Lipid-core nanocapsules | Polycaprolactone:seed oil:sorbitan stearate 1:1.65:0.385 (mass ratio) | In vitro model of human primary chondrocytes treated with nitric oxide-donor to mimic OA joint conditions | Higher dose delivery, protective effect on cell morphology and membrane surface [30] |
| Morin | Mannose decorated Liposomes | DSPC:Chol: F-DHPE 60:35:5 (molar ratio) | Arthritic rats treated intravenously for 3 days | Preferential internalization into macrophages, inhibited the osteoclastogenesis, pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-17), VEGF angiogenic factor and iNOS inflammatory enzyme production; suppressed RANKL and STAT-3 expression, but increased osteoprotegerin expression [160] |
| p-Coumaric acid | Mannose decorated Liposomes | DSPC:Chol:mannose 60:35:5 (molar ratio) | Ex vivo studies in macrophages; arthritic rats treated intravenously, for 3 days | Targeted synovial macrophages, inhibited osteoclasts differentiation, suppressed expression of MMP-9 and inflammatory cytokines [161] |
| Oxymatrine | Positive MV liposomes | EPC:Chol:DSPE-PEG 4:1:1 (mass ratio) E.E.—73.4%; Size—178 nm; PDI—0.167; ζ—13.30 mV | Intraperitoneal administration in intervertebral disc degeneration (IVDD) mice model | Reduced the mRNA and protein level of MMP3, MMP-9 and IL-6 [92] |
| Curcumin | Liposomes | PC:Chol 30:70 (molar ratio) | In vitro studies in 7F2 osteoblasts and RAW 234.7 macrophages | High cellular uptake, favored osteoblast differentiation and mineralization, increased OPG:RANKL ratio and prevented osteoclastogenesis [162] |
| Curcumin | Liposomes | Lecithin:Chol 18:1 (molar ratio) | Sistemic administration in C57BL/6J mice with hemi-lung radiation | Inhibited NF-kB pathway, down-regulated pro-inflammatory cytokines TNF-α, IL-6 and IL-8, and TGF-β [163] |
| Curcumin | Liposomes | DMPC:DMPG:Chol 7:1:8 (molar ratio) | In vitro studies of liposomal curcumin in human blood, plasma and culture medium of human lymphocytes, splenocytes and virus-transformed human B-cells | Higher stability and inhibitory effects on concanavalin A-stimulated human lymphocytes, splenocytes and B-cells proliferation; better bioavailability and efficacy, compared to free curcumin, recommending its clinical application [164] |
| Curcumin (curcuminoids) | Exosomes | Exosomes: curcumin 1:2:9 (mass ratio) | In vitro studies in RAW 264.7 macrophages and in vivo studies in C57BL/6J mice with oral administered or injected system | Increased bioavailability and anti-inflammatory activity of curcumin [165] |
| Dimethyl curcumin | Liposomes | PC:Chol 4:4 (molar ratio) | IA injections (six times) in CIA rats model | Regulated gelatinases release and cell cycle of spleen lymphocytes [166] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craciunescu, O.; Icriverzi, M.; Florian, P.E.; Roseanu, A.; Trif, M. Mechanisms and Pharmaceutical Action of Lipid Nanoformulation of Natural Bioactive Compounds as Efficient Delivery Systems in the Therapy of Osteoarthritis. Pharmaceutics 2021, 13, 1108. https://doi.org/10.3390/pharmaceutics13081108
Craciunescu O, Icriverzi M, Florian PE, Roseanu A, Trif M. Mechanisms and Pharmaceutical Action of Lipid Nanoformulation of Natural Bioactive Compounds as Efficient Delivery Systems in the Therapy of Osteoarthritis. Pharmaceutics. 2021; 13(8):1108. https://doi.org/10.3390/pharmaceutics13081108
Chicago/Turabian StyleCraciunescu, Oana, Madalina Icriverzi, Paula Ecaterina Florian, Anca Roseanu, and Mihaela Trif. 2021. "Mechanisms and Pharmaceutical Action of Lipid Nanoformulation of Natural Bioactive Compounds as Efficient Delivery Systems in the Therapy of Osteoarthritis" Pharmaceutics 13, no. 8: 1108. https://doi.org/10.3390/pharmaceutics13081108
APA StyleCraciunescu, O., Icriverzi, M., Florian, P. E., Roseanu, A., & Trif, M. (2021). Mechanisms and Pharmaceutical Action of Lipid Nanoformulation of Natural Bioactive Compounds as Efficient Delivery Systems in the Therapy of Osteoarthritis. Pharmaceutics, 13(8), 1108. https://doi.org/10.3390/pharmaceutics13081108