Lactose-Gated Mesoporous Silica Particles for Intestinal Controlled Delivery of Essential Oil Components: An In Vitro and In Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Cell-Culture Media
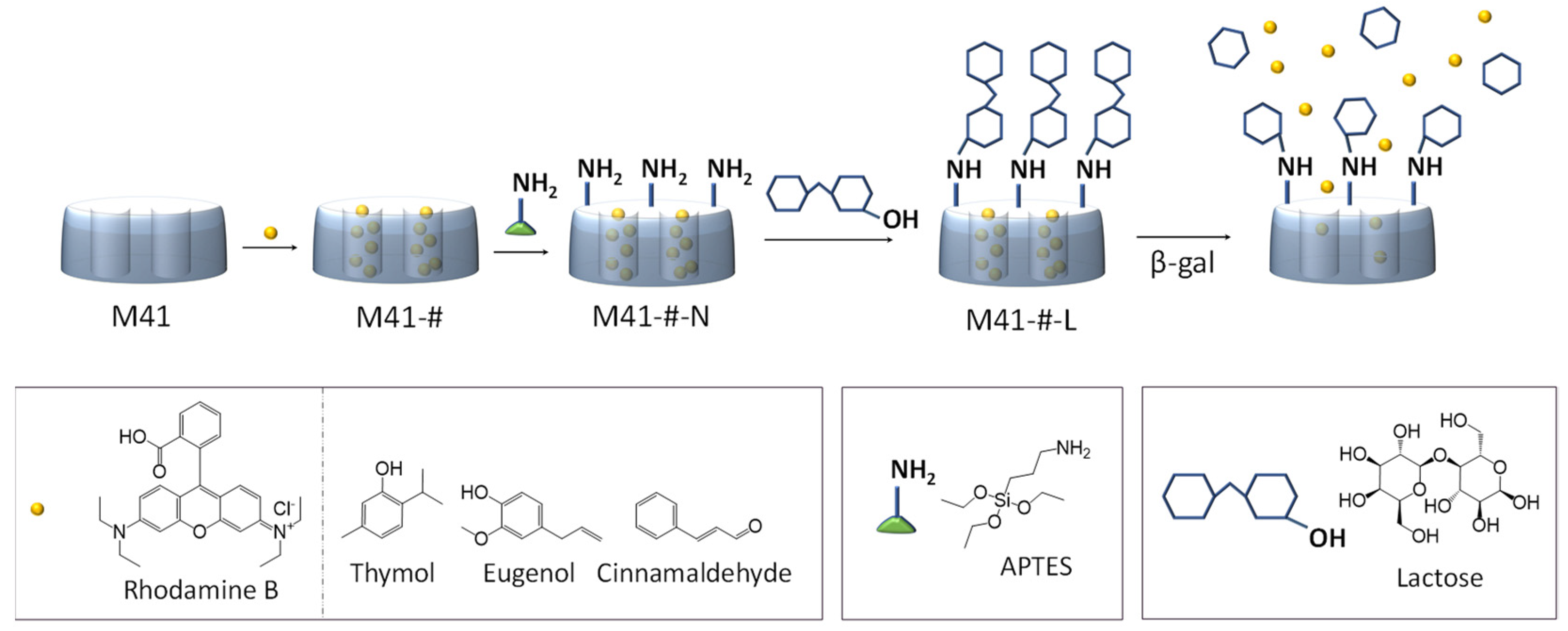
2.2. Synthesis of Mesoporous Silica
2.3. Synthesis of the Gated Materials
2.4. Characterization Techniques
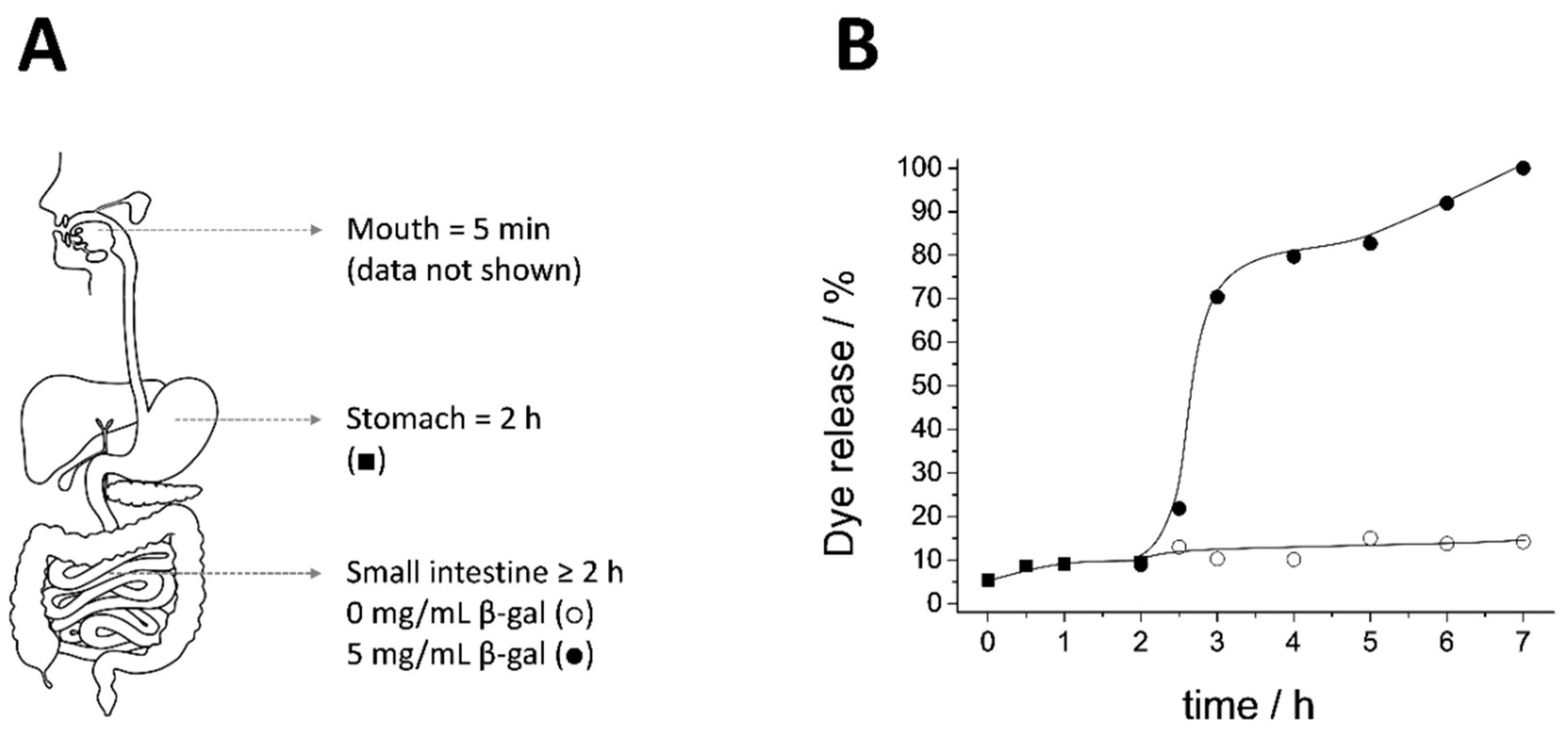
2.5. Biofluid Digestion Assay
2.6. Cargo Release Experiments
2.7. EOCs Quantification
2.8. Caco-2 Cell Culture Conditions
2.9. MTT Cell Viability Assay
2.10. Caco-2 Cell Monolayer Culture
2.11. Transepithelial Electrical Resistance (TEER) Measurements
2.12. Intestinal Barriers Treatment with M41-#-L and Free EOCs
2.13. EOCs Permeability across the Intestinal Barriers
2.14. Lucifer Yellow (LY) Assay
2.15. Inflammatory Response Measurement
2.16. Cell Staining and Confocal Microscopy
2.17. In Vivo Pharmacokinetic Assays
2.18. Statistical Analysis
3. Results and Discussion
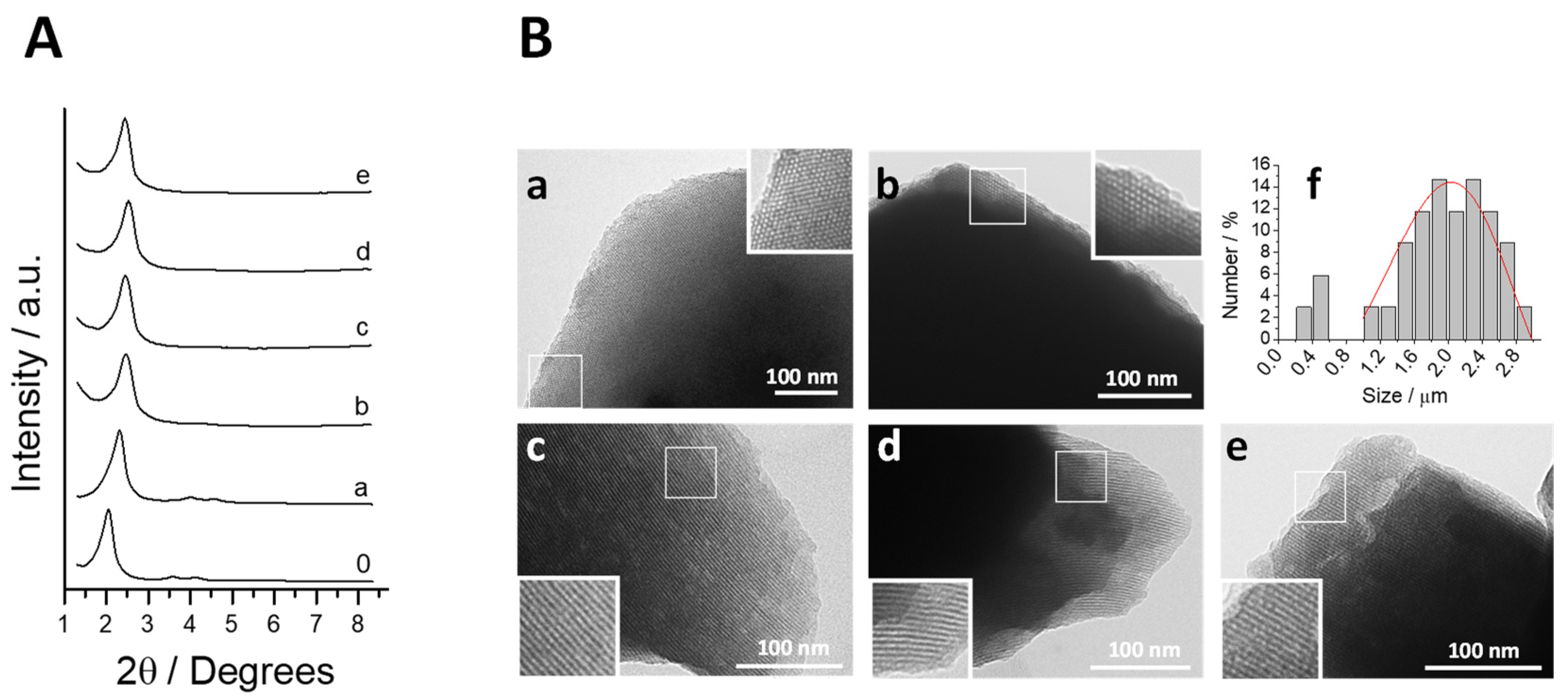
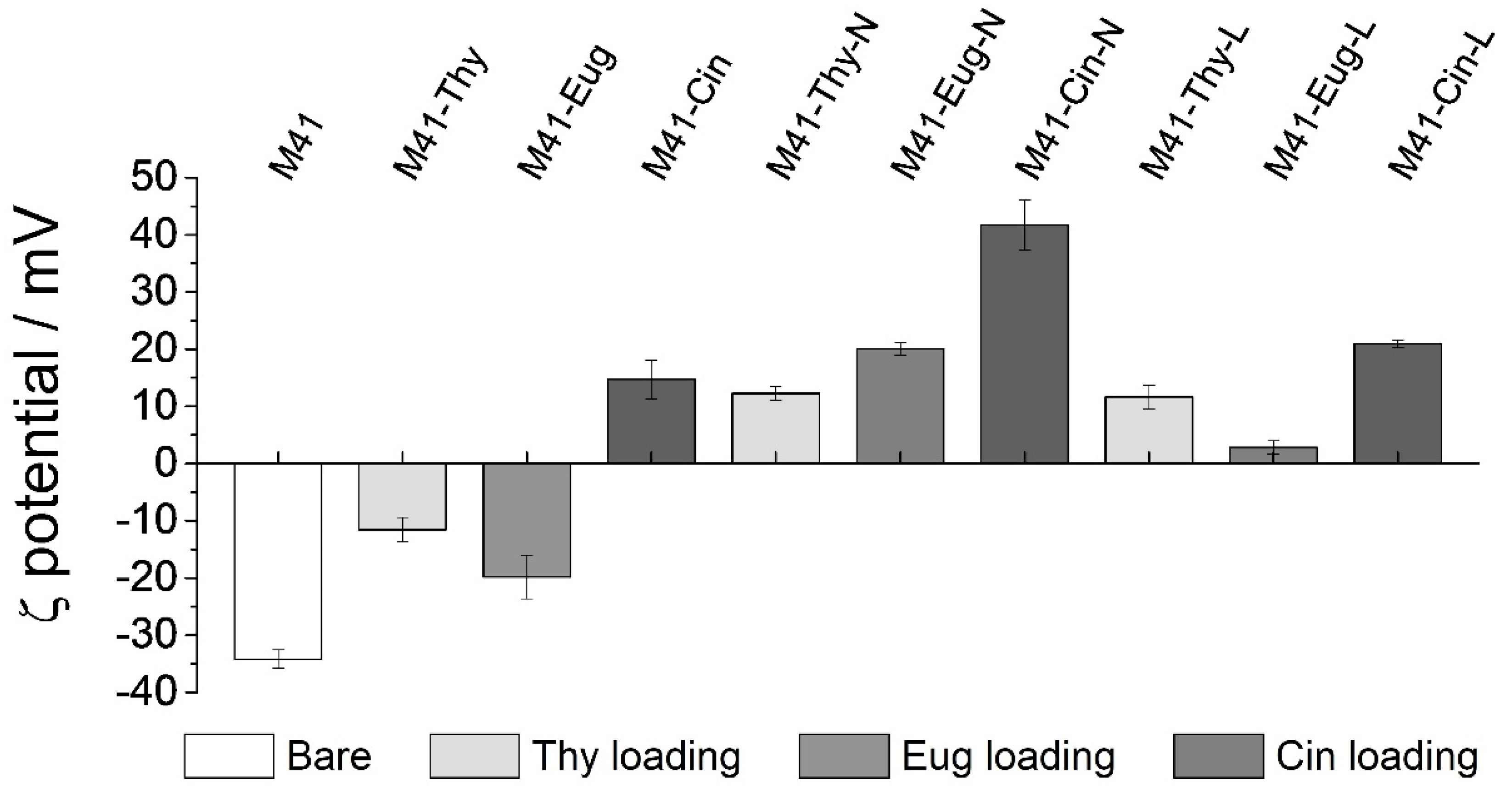
3.1. Design, Synthesis and Characterization of the Prepared Solids
3.2. Biofluid Digestion: Release Profile and Structure-Integrity
3.3. Cargo Release Experiments
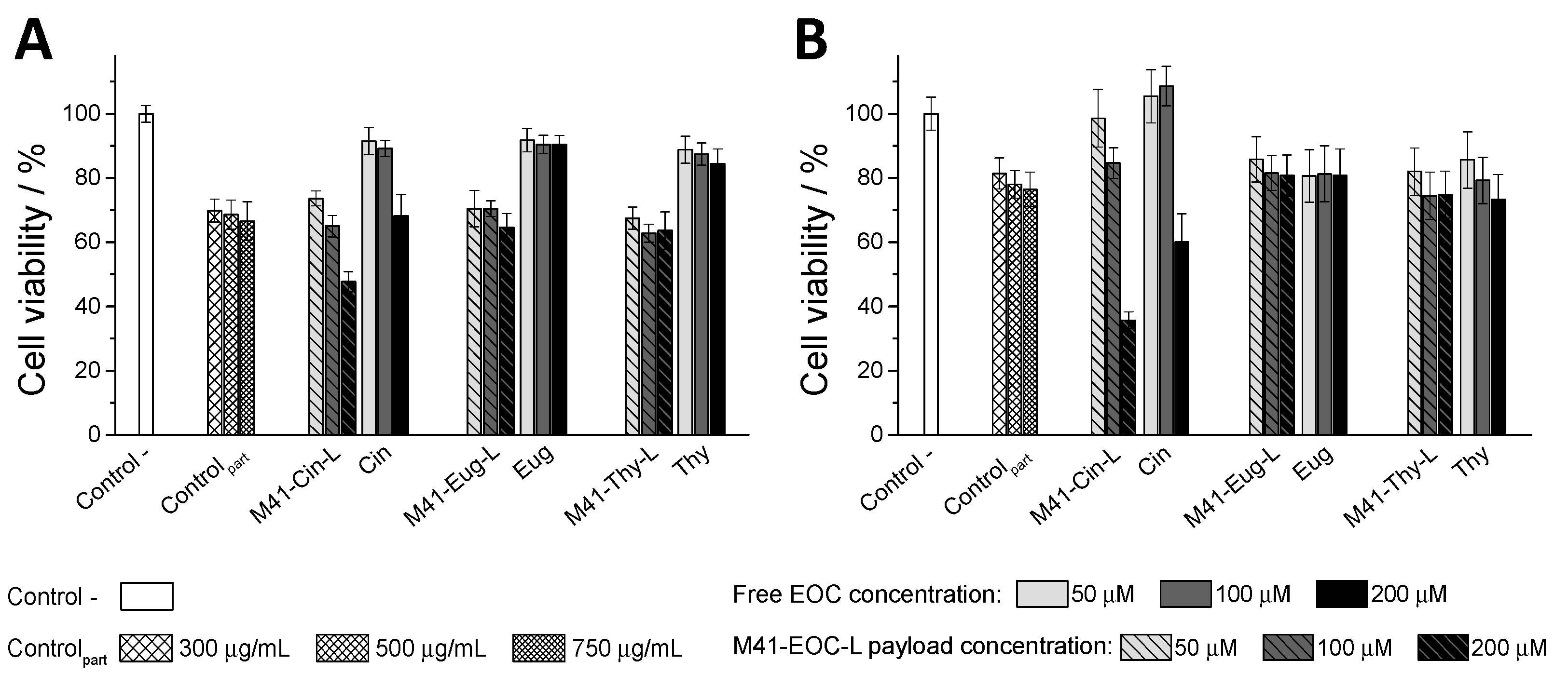
3.4. In Vitro Interaction Studies between Caco-2 Cells and M41-#-L Materials
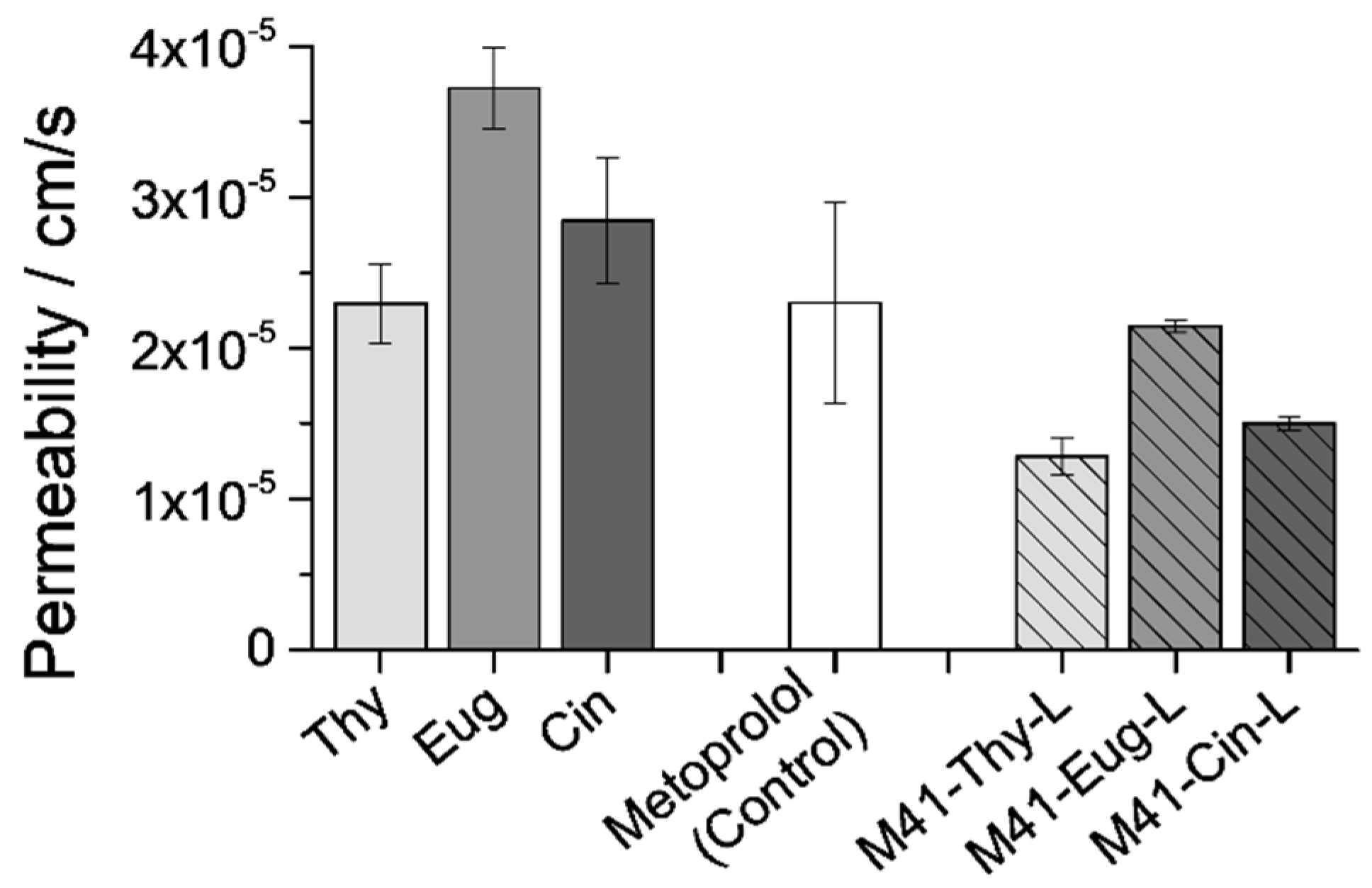
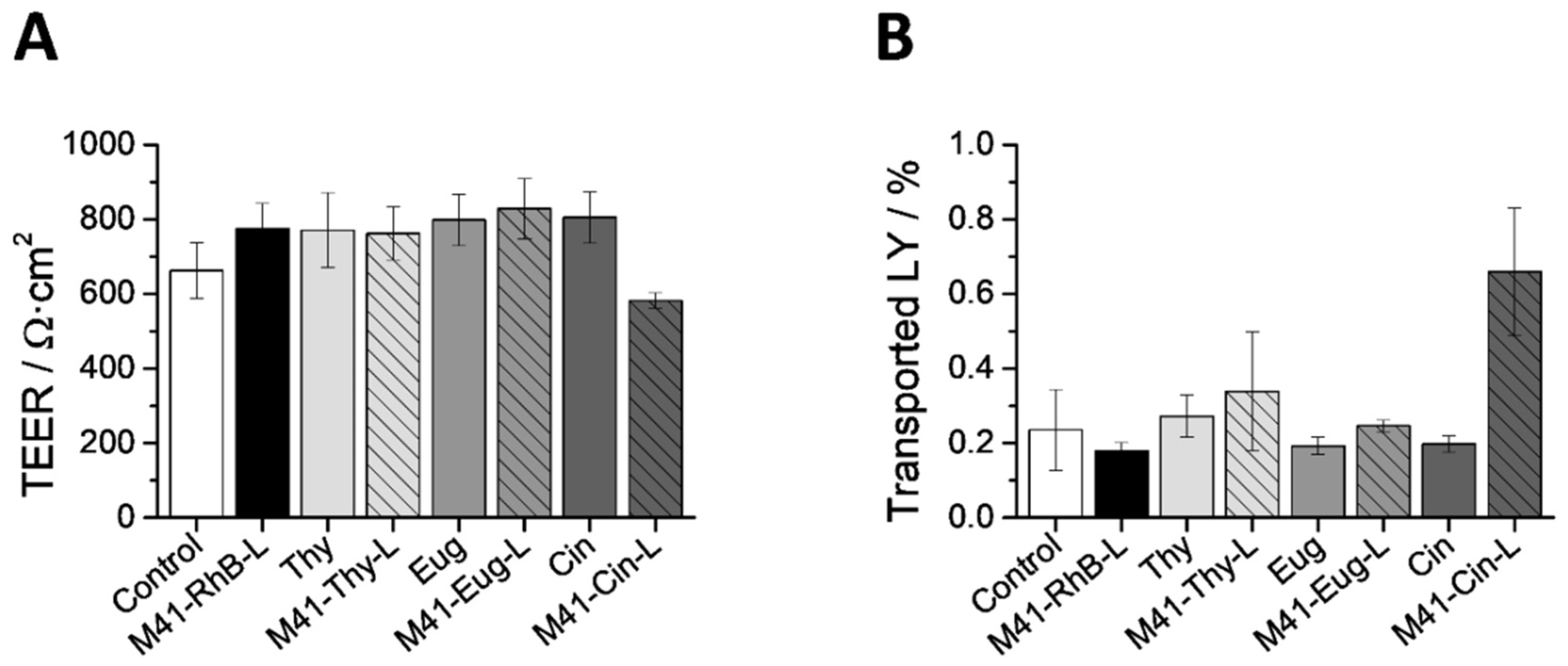
3.5. In Vitro Interaction Studies between Intestinal Barriers and M41-#-L
3.5.1. EOCs Permeability through the Intestinal Barriers
3.5.2. Intestinal Membrane Treatment with Free EOCs and M41-#-L
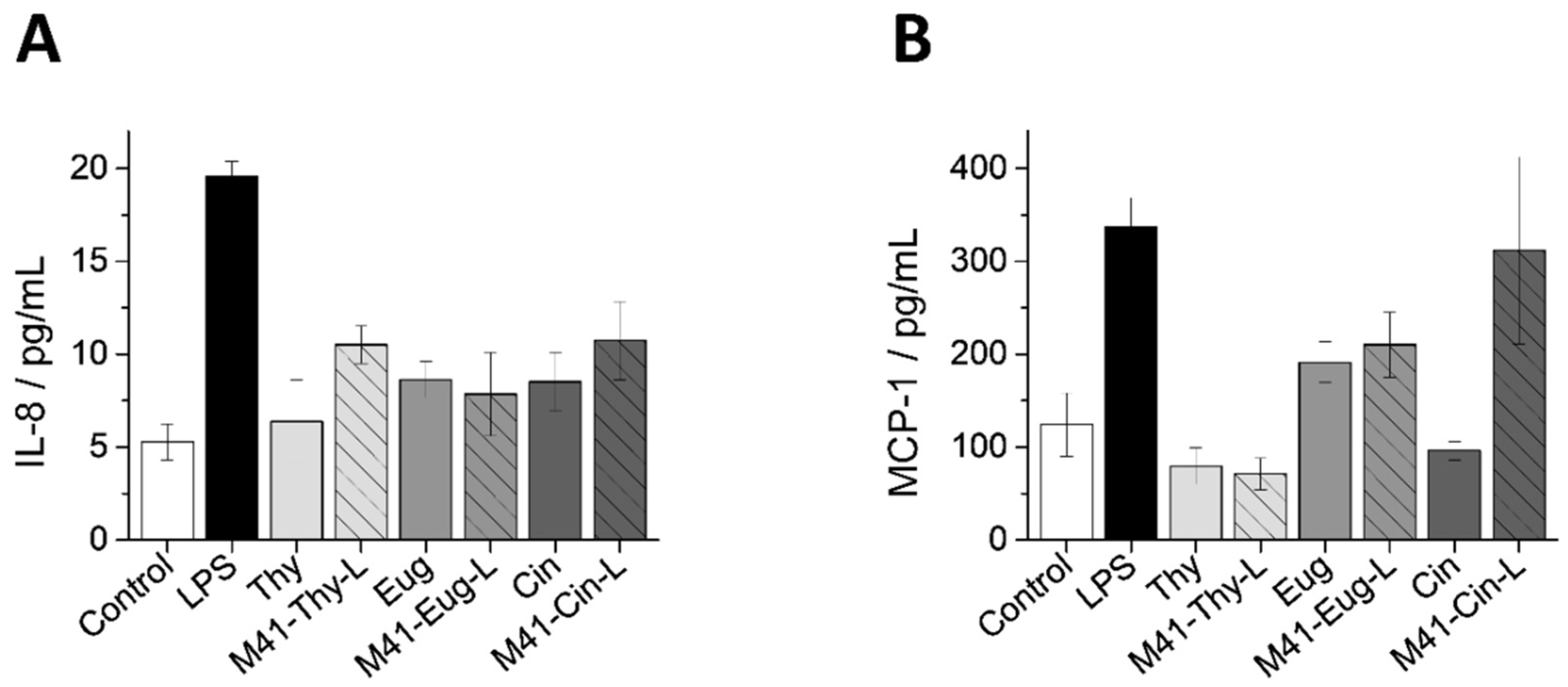
3.5.3. Inflammatory Response of Intestinal Epithelium to M41-EOC-L and Free EOCs
3.5.4. CLSM Imaging of the Intestinal Barrier’s Interaction with M41-#-L
3.6. In Vivo Pharmacokinetic Assays
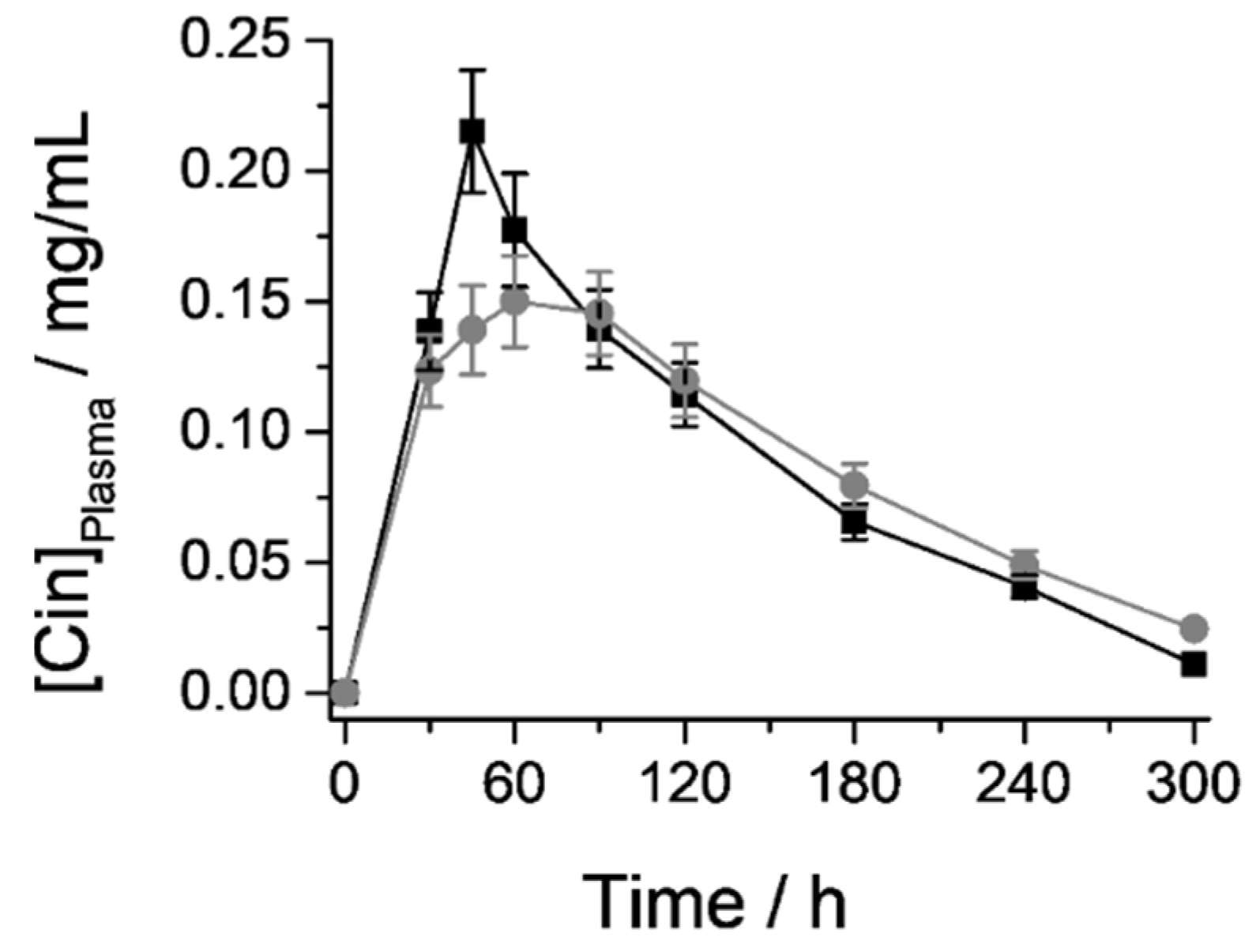
3.6.1. Cin Quantification in Plasma samples: Pharmacokinetic Parameters
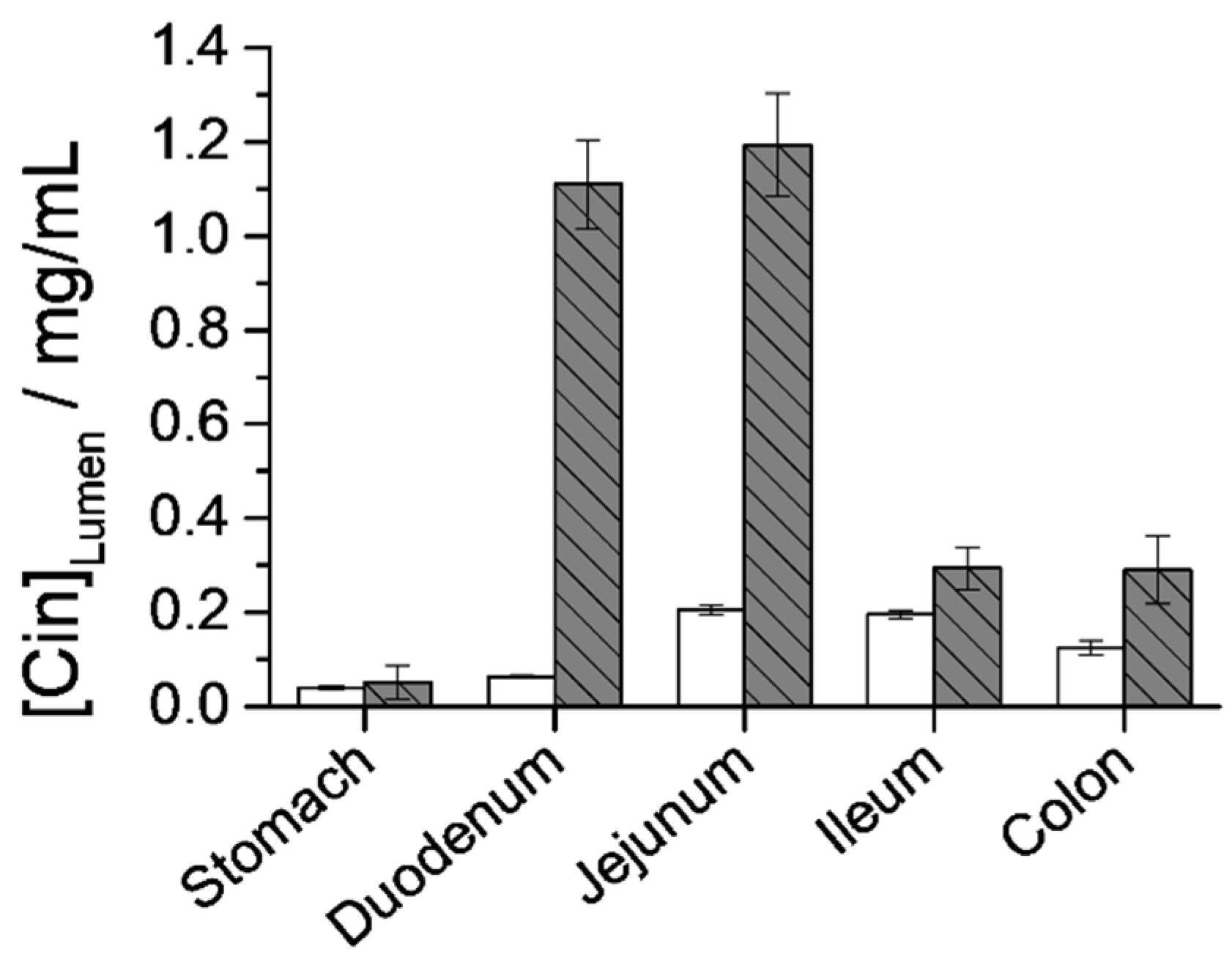
3.6.2. Cin Quantification in the GIT Lumen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribes, À.; Aznar, E.; Santiago-Felipe, S.; Xifre-Perez, E.; Tormo-Mas, M.Á.; Pemán, J.; Marsal, L.F.; Martínez-Máñez, R. Selective and Sensitive Probe Based in Oligonucleotide-Capped Nanoporous Alumina for the Rapid Screening of Infection Produced by Candida albicans. ACS Sens. 2019, 4, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Oroval, M.; Coll, C.; Bernardos, A.; Marcos, M.D.; Martínez-Máñez, R.; Shchukin, D.G.; Sancenón, F. Selective Fluorogenic Sensing of As(III) Using Aptamer-Capped Nanomaterials. ACS Appl. Mater. Interfaces 2017, 9, 11332–11336. [Google Scholar] [CrossRef]
- Giménez, C.; Climent, E.; Aznar, E.; Martánez-Máñez, R.; Sancenón, F.; Marcos, M.D.; Amorós, P.; Rurack, K. Towards chemical communication between gated nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 12629–12633. [Google Scholar] [CrossRef] [PubMed]
- de Luis, B.; Llopis-Lorente, A.; Rincón, P.; Gadea, J.; Sancenón, F.; Aznar, E.; Villalonga, R.; Murguía, J.R.; Martínez-Máñez, R. An Interactive Model of Communication between Abiotic Nanodevices and Microorganisms. Angew. Chem. Int. Ed. 2019, 58, 14986–14990. [Google Scholar] [CrossRef]
- Llopis-Lorente, A.; Díez, P.; Sánchez, A.; Marcos, M.D.; Sancenón, F.; Martínez-Ruiz, P.; Villalonga, R.; Martínez-Máñez, R. Interactive models of communication at the nanoscale using nanoparticles that talk to one another. Nat. Commun. 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, A.; Aznar, E.; Martínez-Máñez, R.; Sancenón, F. New Advances in In Vivo Applications of Gated Mesoporous Silica as Drug Delivery Nanocarriers. Small 2020, 16, 1–62. [Google Scholar] [CrossRef] [PubMed]
- Steed, J.W.; Atwood, J.L.; Gale, P.A. Definition and Emergence of Supramolecular Chemistry. In Supramolecular Chemistry: From Molecules to Nanomaterials; Gale, P.A., Steed, J.W., Eds.; Wiley: Hoboken, NJ, USA, 2012; pp. 3–8. ISBN 9780470661345. [Google Scholar]
- Giménez, C.; De La Torre, C.; Gorbe, M.; Aznar, E.; Sancenón, F.; Murguía, J.R.; Martínez-Máñez, R.; Marcos, M.D.; Amorós, P. Gated mesoporous silica nanoparticles for the controlled delivery of drugs in cancer cells. Langmuir 2015, 31, 3753–3762. [Google Scholar] [CrossRef] [PubMed]
- Pascual, L.; Cerqueira-Coutinho, C.; García-Fernández, A.; de Luis, B.; Bernardes, E.S.; Albernaz, M.S.; Missailidis, S.; Martínez-Máñez, R.; Santos-Oliveira, R.; Orzaez, M.; et al. MUC1 aptamer-capped mesoporous silica nanoparticles for controlled drug delivery and radio-imaging applications. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2495–2505. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, B.; Luo, Z.; Ding, X.; Li, J.; Dai, L.; Zhou, J.; Zhao, X.; Ye, J.; Cai, K. Enzyme responsive mesoporous silica nanoparticles for targeted tumor therapy in vitro and in vivo. Nanoscale 2015, 7, 3614–3626. [Google Scholar] [CrossRef]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous silica nanoparticles in medicine-Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef]
- Van Speybroeck, M.; Mellaerts, R.; Martens, J.A.; Annaert, P.; Van den Mooter, G.; Augustijns, P. Ordered Mesoporous Silica for the Delivery of Poorly Soluble Drugs. In Controlled Release in Oral Drug Delivery; Wilson, C.G., Crowley, P.J., Eds.; Springer: New York, NY, USA, 2011; pp. 203–219. [Google Scholar]
- Wang, S. Ordered mesoporous materials for drug delivery. Microporous Mesoporous Mater. 2009, 117, 1–9. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous materials for drug delivery. Angew. Chem. Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]
- Sabliov, C.M.; Astete, C.E. Encapsulation and controlled release of antioxidants and vitamins. In Delivery and Controlled Release of Bioactives in Foods and Nutraceuticals; Garti, N., Ed.; Woodhead Publishing: Cambridge, UK, 2008; pp. 297–330. ISBN 978-1-84569-145-5. [Google Scholar]
- Bernardos, A.; Aznar, E.; Coll, C.; Martínez-Mañez, R.; Barat, J.M.; Marcos, M.D.; Sancenón, F.; Benito, A.; Soto, J. Controlled release of vitamin B2 using mesoporous materials functionalized with amine-bearing gate-like scaffoldings. J. Control Release 2008, 131, 181–189. [Google Scholar] [CrossRef]
- Mai, Z.; Chen, J.; Hu, Y.; Liu, F.; Fu, B.; Zhang, H.; Dong, X.; Huang, W.; Zhou, W. Novel functional mesoporous silica nanoparticles loaded with Vitamin E acetate as smart platforms for pH responsive delivery with high bioactivity. J. Colloid Interface Sci. 2017, 508, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, L.; Vasheghani-Farahani, E.; Rostami, K.; Gangi, F.; Fallahpour, M. Mesoporous silica nanoparticles as a nanocarrier for delivery of vitamin C. Iran. J. Biotechnol. 2013, 11, 209–213. [Google Scholar] [CrossRef]
- Gargiulo, N.; Attianese, I.; Buonocore, G.G.; Caputo, D.; Lavorgna, M.; Mensitieri, G.; Lavorgna, M. α-Tocopherol release from active polymer films loaded with functionalized SBA-15 mesoporous silica. Microporous Mesoporous Mater. 2013, 167, 10–15. [Google Scholar] [CrossRef]
- Popova, M.; Szegedi, A.; Mavrodinova, V.; Tušar, N.N.; Mihály, J.; Klébert, S.; Benbassat, N.; Yoncheva, K. Preparation of resveratrol-loaded nanoporous silica materials with different structures. J. Solid State Chem. 2014, 219, 37–42. [Google Scholar] [CrossRef]
- Nairi, V.; Medda, L.; Monduzzi, M.; Salis, A. Adsorption and release of ampicillin antibiotic from ordered mesoporous silica. J. Colloid Interface Sci. 2017, 497, 217–225. [Google Scholar] [CrossRef]
- Shi, X.; Wang, Y.; Ren, L.; Zhao, N.; Gong, Y.; Wang, D.A. Novel mesoporous silica-based antibiotic releasing scaffold for bone repair. Acta Biomater. 2009, 5, 1697–1707. [Google Scholar] [CrossRef]
- Teruel, A.H.; Pérez-Esteve, É.; González-Álvarez, I.; González-Álvarez, M.; Costero, A.M.; Ferri, D.; Parra, M.; Gaviña, P.; Merino, V.; Martínez-Mañez, R.; et al. Smart gated magnetic silica mesoporous particles for targeted colon drug delivery: New approaches for inflammatory bowel diseases treatment. J. Control Release 2018, 281, 58–69. [Google Scholar] [CrossRef]
- García-Fernández, A.; García-Laínez, G.; Ferrándiz, M.L.; Aznar, E.; Sancenón, F.; Alcaraz, M.J.; Murguía, J.R.; Marcos, M.D.; Martínez-Máñez, R.; Costero, A.M.; et al. Targeting inflammasome by the inhibition of caspase-1 activity using capped mesoporous silica nanoparticles. J. Control Release 2017, 248, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.A.; Deng, T.; Lin, F.C.; Cai, Y.; Zink, J.I. Supramolecular nanomachines as stimuli-responsive gatekeepers on mesoporous silica nanoparticles for antibiotic and cancer drug delivery. Theranostics 2019, 9, 3341–3364. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wu, C.; Bai, A.; Liu, X.; Lv, H.; Liu, Y. Folate-Functionalized Mesoporous Silica Nanoparticles as a Liver Tumor-Targeted Drug Delivery System to Improve the Antitumor Effect of Paclitaxel. J. Nanomater. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Murugan, C.; Rayappan, K.; Thangam, R.; Bhanumathi, R.; Shanthi, K.; Vivek, R.; Thirumurugan, R.; Bhattacharyya, A.; Sivasubramanian, S.; Gunasekaran, P.; et al. Combinatorial nanocarrier based drug delivery approach for amalgamation of anti-tumor agents in bresat cancer cells: An improved nanomedicine strategies. Sci. Rep. 2016, 6, 1–22. [Google Scholar] [CrossRef]
- Bernardos, A.; Kourimská, L. Applications of mesoporous silica materials in food—A review. Czech J. Food Sci. 2013, 31, 99–107. [Google Scholar] [CrossRef]
- Florek, J.; Caillard, R.; Kleitz, F. Evaluation of mesoporous silica nanoparticles for oral drug delivery-current status and perspective of MSNs drug carriers. Nanoscale 2017, 9, 15252–15277. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Esteve, É.; Ruiz-Rico, M.; Martínez-Máñez, R.; Barat, J.M. Mesoporous Silica-Based Supports for the Controlled and Targeted Release of Bioactive Molecules in the Gastrointestinal Tract. J. Food Sci. 2015, 80, E2504–E2516. [Google Scholar] [CrossRef]
- Garti, N. (Ed.) Delivery and Controlled Release of Bioactives in Foods and Nutraceuticals; Woodhead Publishing: Cambridge, UK, 2008; ISBN 9781845691516. [Google Scholar]
- Clifford, N.W.; Iyer, K.S.; Raston, C.L. Encapsulation and controlled release of nutraceuticals using mesoporous silica capsules. J. Mater. Chem. 2008, 18, 162–165. [Google Scholar] [CrossRef]
- Pérez-Esteve, É.; Ruiz-Rico, M.; Martínez-Máñez, R.; Barat, J.M. Mesoporous silica particles as encapsulation and delivery systems for food ingredients and nutraceuticals. In Nanotechnology in Nutraceuticals: Production to Consumption; Sen, S., Pathak, Y., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 397–438. ISBN 9781498721899. [Google Scholar]
- Lea, T. Caco-2 cell line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Swiatecka, D., Requena, T., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 103–111. ISBN 9783319161044. [Google Scholar]
- Pérez-Esteve, É.; Fuentes, A.; Coll, C.; Acosta, C.; Bernardos, A.; Amorós, P.; Marcos, M.D.; Sancenón, F.; Martínez-Máñez, R.; Barat, J.M. Modulation of folic acid bioaccessibility by encapsulation in pH-responsive gated mesoporous silica particles. Microporous Mesoporous Mater. 2015, 202, 124–132. [Google Scholar] [CrossRef]
- Poyatos-Racionero, E.; Pérez-Esteve, É.; Marcos, M.D.; Barat, J.M.; Martínez-Máñez, R.; Aznar, E.; Bernardos, A. New Oleic Acid-Capped Mesoporous Silica Particles as Surfactant-Responsive Delivery Systems. ChemistryOpen 2019, 8, 1052–1056. [Google Scholar] [CrossRef]
- González-Alvarez, M.; Coll, C.; Gonzalez-Alvarez, I.; Giménez, C.; Aznar, E.; Martínez-Bisbal, M.C.; Lozoya-Agulló, I.; Bermejo, M.; Martínez-Máñez, R.; Sancenón, F. Gated Mesoporous Silica Nanocarriers for a “two-Step” Targeted System to Colonic Tissue. Mol. Pharm. 2017, 14, 4442–4453. [Google Scholar] [CrossRef]
- Teruel, A.H.; Coll, C.; Costero, A.M.; Ferri, D.; Parra, M.; Gaviña, P.; González-Álvarez, M.; Merino, V.; Marcos, M.D.; Martínez-Máñez, R.; et al. Functional magnetic mesoporous silica microparticles capped with an azo-derivative: A promising colon drug delivery device. Molecules 2018, 23, 375. [Google Scholar] [CrossRef] [PubMed]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Bernardos, A.; Piacenza, E.; Sancenón, F.; Hamidi, M.; Maleki, A.; Turner, R.J.; Martínez-Máñez, R. Mesoporous Silica-Based Materials with Bactericidal Properties. Small 2019, 15, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Bernardos, A.; Božik, M.; Montero, A.; Pérez-Esteve, É.; García-Casado, E.; Lhotka, M.; Fraňková, A.; Marcos, M.D.; Barat, J.M.; Martínez-Máñez, R.; et al. Secreted Enzyme-Responsive System for Controlled Antifungal Agent Release. Nanomaterials 2021, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Poyatos-Racionero, E.; Guarí-Borràs, G.; Ruiz-Rico, M.; Morellá-Aucejo, Á.; Aznar, E.; Barat, J.M.; Martínez-máñez, R.; Marcos, M.D.; Bernardos, A. Towards the enhancement of essential oil components’ antimicrobial activity using new zein protein-gated mesoporous silica microdevices. Int. J. Mol. Sci. 2021, 22, 3795. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef] [PubMed]
- Lingan, K. A Review on Major Constituents of Various Essential Oils and its Application. Transl. Med. 2018, 8, 1–5. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Inoue, M.; Hayashi, S.E.; Craker, L. Role of Medicinal and Aromatic Plants: Past, Present, and Future. In Pharmacognosy—Medicinal Plants; InTechOpen: Rijeka, Croatia, 2019; pp. 1–13. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Satzinger, S.; Civelek, M.; Feilhauer, K.; Köninger, J.; Bischoff, S.C.; Lorentz, A. Cinnamon reduces inflammatory response in intestinal fibroblasts in vitro and in colitis in vivo leading to decreased fibrosis. Mol. Nutr. Food Res. 2017, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, J.S.; Zaidi, S.F.; Shaharyar, S.; Refaat, A.; Usmanghani, K.; Saiki, I.; Sugiyama, T. Anti-inflammatory Effect of Cinnamaldehyde in Helicobacter pylori Induced Gastric Inflammation. Biol. Pharm. Bull. 2015, 38, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Bujňáková, D.; Juhás, Š.; Faix, Š. The anti-translocation and anti-inflammatory effect of cinnamon oil in mice with TNBS induced colitis. Biologia 2013, 68, 1000–1003. [Google Scholar] [CrossRef]
- Da Silveira, E.; Sá, R.D.C.; Andrade, L.N.; De Oliveira, R.D.R.B.; De Sousa, D.P. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules 2014, 19, 1459–1480. [Google Scholar] [CrossRef]
- Chao, L.K.; Hua, K.F.; Hsu, H.Y.; Cheng, S.S.; Lin, I.F.; Chen, C.J.; Chen, S.T.; Chang, S.T. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem. Toxicol. 2008, 46, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Bukovská, A.; Čikoš, Š.; Juhás, Š.; Il’ková, G.; Rehák, P.; Koppel, J. Effects of a combination of thyme and oregano essential oils on TNBS-induced colitis in mice. Mediat. Inflamm. 2007, 2007, 23296. [Google Scholar] [CrossRef]
- Juhás, Š.; Bujňáková, D.; Rehák, P.; Čikoš, Š.; Czikková, S.; Veselá, J.; Il’ková, G.; Koppel, J. Anti-inflammatory effects of thyme essential oil in mice. Acta Vet. Brno 2008, 77, 327–334. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Lu, Y.; Ma, C. Anti-inflammatory effects of eugenol on lipopolysaccharide-induced inflammatory reaction in acute lung injury via regulating inflammation and redox status. Int. Immunopharmacol. 2015, 26, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Ismail, I.A.; Kang, S.M.; Han, D.C.; Kwon, B.M. Cinnamaldehydes in Cancer Chemotherapy. Phytother. Res. 2016, 30, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Schmelz, E.M.; Bickford, L. Anti-Cancer Properties of Cinnamon Oil and Its Active Component, Trans-Cinnamaldehyde. J. Nutr. Food Sci. 2019, 9, 1–7. [Google Scholar] [CrossRef]
- Islam, M.T.; Khalipha, A.B.R.; Bagchi, R.; Mondal, M.; Smrity, S.Z.; Uddin, S.J.; Shilpi, J.A.; Rouf, R. Anticancer activity of thymol: A literature-based review and docking study with Emphasis on its anticancer mechanisms. IUBMB Life 2019, 71, 9–19. [Google Scholar] [CrossRef]
- Bezerra, D.P.; Militão, G.C.G.; De Morais, M.C.; De Sousa, D.P. The dual antioxidant/prooxidant effect of eugenol and its action in cancer development and treatment. Nutrients 2017, 9, 1367. [Google Scholar] [CrossRef] [PubMed]
- Suhr, K.I.; Nielsen, P.V. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J. Appl. Microbiol. 2003, 94, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Laird, K.; Phillips, C. Vapour phase: A potential future use for essential oils as antimicrobials? Lett. Appl. Microbiol. 2012, 54, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.J.; Yang, X.W.; Yang, X.; Wang, K. Studies of intestinal permeability of 36 flavonoids using Caco-2 cell monolayer model. Int. J. Pharm. 2009, 367, 58–64. [Google Scholar] [CrossRef]
- Chan, O.H.; Stewart, B.H. Physicochemical and drug-delivery considerations for oral drug bioavailability. Drug Discov. Today 1996, 1, 461–473. [Google Scholar] [CrossRef]
- Janatova, A.; Bernardos, A.; Smid, J.; Frankova, A.; Lhotka, M.; Kourimská, L.; Pulkrabek, J.; Kloucek, P. Long-term antifungal activity of volatile essential oil components released from mesoporous silica materials. Ind. Crop. Prod. 2015, 67, 216–220. [Google Scholar] [CrossRef]
- Jaganathan, H.; Godin, B. Biocompatibility assessment of Si-based nano- and micro-particles. Adv. Drug Deliv. Rev. 2012, 64, 1800–1819. [Google Scholar] [CrossRef]
- Rascol, E.; Pisani, C.; Dorandeu, C.; Nyalosaso, J.L.; Charnay, C.; Daurat, M.; Da Silva, A.; Devoisselle, J.M.; Gaillard, J.C.; Armengaud, J.; et al. Biosafety of mesoporous silica nanoparticles. Biomimetics 2018, 3, 22. [Google Scholar] [CrossRef]
- Fadeel, B.; Garcia-Bennett, A.E. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv. Drug Deliv. Rev. 2010, 62, 362–374. [Google Scholar] [CrossRef]
- Kim, I.Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size, dose, and cell type. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1407–1416. [Google Scholar] [CrossRef]
- Hudson, S.P.; Padera, R.F.; Langer, R.; Kohane, D.S. The biocompatibility of mesoporous silicates. Biomaterials 2008, 29, 4045–4055. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous silica nanoparticles: A comprehensive review on synthesis and recent advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, A.; Ozgur, E.; Bayindir, M. Impact of mesoporous silica nanoparticle surface functionality on hemolytic activity, thrombogenicity and non-specific protein adsorption. J. Mater. Chem. B 2013, 1, 1909–1920. [Google Scholar] [CrossRef]
- Yazdimamaghani, M.; Barber, Z.B.; Moghaddam, S.P.H.; Ghandehari, H. Influence of Silica Nanoparticle Density and Flow Conditions on Sedimentation, Cell Uptake, and Cytotoxicity. Mol. Pharm. 2018, 15, 2372–2383. [Google Scholar] [CrossRef]
- Fu, C.; Liu, T.; Li, L.; Liu, H.; Chen, D.; Tang, F. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials 2013, 34, 2565–2575. [Google Scholar] [CrossRef]
- Alegría, A.; Garcia-Llatas, G.; Cilla, A. Static digestion models: General introduction. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Swiatecka, D., Requena, T., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–12. ISBN 9783319161044. [Google Scholar]
- Mackie, A.; Rigby, N. InfoGest consensus method. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Swiatecka, D., Requena, T., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 13–22. ISBN 9783319161044. [Google Scholar]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the Human Colon Carcinoma Cell Line (Caco-2) as a Model System for Intestinal Epithelial Permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Meunier, V.; Bourrié, M.; Berger, Y.; Fabre, G. The human intestinal epithelial cell line Caco-2; pharmacological and pharmacokinetic applications. Cell Biol. Toxicol. 1995, 11, 187–194. [Google Scholar] [CrossRef]
- Bernardos, A.; Aznar, E.; Marcos, M.D.; Martínez-Máñez, R.; Sancenón, F.; Soto, J.; Barat, J.M.; Amorós, P. Enzyme-responsive controlled release using mesoporous silica supports capped with lactose. Angew. Chem. Int. Ed. 2009, 48, 5884–5887. [Google Scholar] [CrossRef]
- Van Beers, E.H.; Al, R.H.; Rings, E.H.H.M.; Einerhand, A.W.C.; Dekker, J.; Buller, H.A. Lactase and sucrase-isomaltase gene expression during Caco-2 cell differentiation. Biochem. J. 1995, 308, 769–775. [Google Scholar] [CrossRef]
- Fernandes, M.I.M.; Galvão, L.C.; Bortolozzi, M.F.; Oliveira, W.P.; Zucoloto, S.; Bianchi, M.L.P. Disaccharidase levels in normal epithelium of the small intestine of rats with iron-deficiency anemia. Braz. J. Med. Biol. Res. 1997, 30, 849–854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bolin, T.D.; McKern, A.; Davis, A.E. The Effect of Diet on Lactase Activity in the Rat. Gastroenterology 1971, 60, 432–437. [Google Scholar] [CrossRef]
- Cabrera, S.; El Haskouri, J.; Guillem, C.; Latorre, J.; Beltrán-Porter, A.; Beltrán-Porter, D.; Marcos, M.D.; Amorós, P. Generalised syntheses of ordered mesoporous oxides: The atrane route. Solid State Sci. 2000, 2, 405–420. [Google Scholar] [CrossRef]
- Popat, A.; Jambhrunkar, S.; Zhang, J.; Yang, J.; Zhang, H.; Meka, A.; Yu, C. Programmable drug release using bioresponsive mesoporous silica nanoparticles for site-specific oral drug delivery. Chem. Commun. 2014, 50, 5547–5550. [Google Scholar] [CrossRef]
- Versantvoort, C.H.M.; Oomen, A.G.; Van De Kamp, E.; Rompelberg, C.J.M.; Sips, A.J.A.M. Applicability of an in vitro digestion model in assessing the bioaccessibility of mycotoxins from food. Food Chem. Toxicol. 2005, 43, 31–40. [Google Scholar] [CrossRef]
- Guarnieri, D.; Sánchez-Moreno, P.; Castillo, A.E.D.R.; Bonaccorso, F.; Gatto, F.; Bardi, G.; Martín, C.; Vázquez, E.; Catelani, T.; Sabella, S.; et al. Biotransformation and Biological Interaction of Graphene and Graphene Oxide during Simulated Oral Ingestion. Small 2018, 14, 1–11. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Pérez-Esteve, É.; Ruiz-Rico, M.; De La Torre, C.; Llorca, E.; Sancenón, F.; Marcos, M.D.; Amorós, P.; Guillem, C.; Martínez-Máñez, R.; Barat, J.M. Stability of different mesoporous silica particles during an in vitro digestion. Microporous Mesoporous Mater. 2016, 230, 196–207. [Google Scholar] [CrossRef]
- Mateen, S.; Rehman, M.T.; Shahzad, S.; Naeem, S.S.; Faizy, A.F.; Khan, A.Q.; Khan, M.S.; Husain, F.M.; Moin, S. Anti-oxidant and anti-inflammatory effects of cinnamaldehyde and eugenol on mononuclear cells of rheumatoid arthritis patients. Eur. J. Pharmacol. 2019, 852, 14–24. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Liu, S.; Hui, Q.; Zhang, H.; Lahaye, L.; Bodin, J.C.; Gong, J.; Nyachoti, M.; Yang, C. Thymol Improves Barrier Function and Attenuates Inflammatory Responses in Porcine Intestinal Epithelial Cells during Lipopolysaccharide (LPS)-Induced Inflammation. J. Agric. Food Chem. 2019, 67, 615–624. [Google Scholar] [CrossRef]
- Liu, D.M.; Zhou, C.Y.; Meng, X.L.; Wang, P.; Li, W. Thymol exerts anti-inflammatory effect in dextran sulfate sodium-induced experimental murine colitis. Trop. J. Pharm. Res. 2018, 17, 1803–1810. [Google Scholar] [CrossRef]
- Huan, S.K.H.; Wang, K.T.; Yeh, S.D.; Lee, C.J.; Lin, L.C.; Liu, D.Z.; Wang, C.C. Scutellaria baicalensis alleviates cantharidin-induced rat hemorrhagic cystitis through inhibition of cyclooxygenase-2 overexpression. Molecules 2012, 17, 6277–6289. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Halamoda-Kenzaoui, B.; Ceridono, M.; Colpo, P.; Valsesia, A.; Urbán, P.; Ojea-Jiménez, I.; Gioria, S.; Gilliland, D.; Rossi, F.; Kinsner-Ovaskainen, A. Dispersion behaviour of silica nanoparticles in biological media and its influence on cellular uptake. PLoS ONE 2015, 10, e0141593. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, D.C.; Dignass, A.U. Intestinal barrier function. Curr. Opin. Clin. Nutr. Metab. Care 2002, 5, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Goll, R.; Granlund, A.V.B. Intestinal barrier homeostasis in inflammatory bowel disease. Scand. J. Gastroenterol. 2014, 50, 3–12. [Google Scholar] [CrossRef]
- Alhinai, E.A.; Walton, G.E.; Commane, D.M. The role of the gut microbiota in colorectal cancer causation. Int. J. Mol. Sci. 2019, 20, 5295. [Google Scholar] [CrossRef]
- Antoni, L.; Nuding, S.; Wehkamp, J.; Stange, E.F. Intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 1165–1179. [Google Scholar] [CrossRef]
- Laukoetter, M.G.; Nava, P.; Nusrat, A. Role of the intestinal barrier in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 401–407. [Google Scholar] [CrossRef]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Thoo, L.; Noti, M.; Krebs, P. Keep calm: The intestinal barrier at the interface of peace and war. Cell Death Dis. 2019, 10, 1–13. [Google Scholar] [CrossRef]
- Artursson, P.; Palm, K.; Luthman, K. Caco-2 monolayers in experimental and theoretical predictions of drug transport. Adv. Drug Deliv. Rev. 2001, 46, 27–43. [Google Scholar] [CrossRef]
- Morishige, T.; Yoshioka, Y.; Inakura, H.; Tanabe, A.; Narimatsu, S.; Yao, X.; Monobe, Y.; Imazawa, T.; Tsunoda, S.I.; Tsutsumi, Y.; et al. Suppression of nanosilica particle-induced inflammation by surface modification of the particles. Arch. Toxicol. 2012, 86, 1297–1307. [Google Scholar] [CrossRef]
- Fritsch-Decker, S.; An, Z.; Yan, J.; Hansjosten, I.; Al-Rawi, M.; Peravali, R.; Diabaté, S.; Weiss, C. Silica nanoparticles provoke cell death independent of p53 and BAX in human colon cancer cells. Nanomaterials 2019, 9, 1172. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, R.; Wang, B.; Cai, C.; Zheng, L.; Wang, H.; Wang, M.; Ouyang, H.; Zhou, X.; Chai, Z.; et al. The effects of orally administered Ag, TiO2 and SiO2 nanoparticles on gut microbiota composition and colitis induction in mice. NanoImpact 2017, 8, 80–88. [Google Scholar] [CrossRef]
- Kucharzik, T.; Lügering, N.; Pauels, H.G.; Domschke, W.; Stoll, R. IL-4, IL-10 and IL-13 down-regulate monocyte-chemoattracting protein-1 (MCP-1) production in activated intestinal epithelial cells. Clin. Exp. Immunol. 1998, 111, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Georgantzopoulou, A.; Serchi, T.; Cambier, S.; Leclercq, C.C.; Renaut, J.; Shao, J.; Kruszewski, M.; Lentzen, E.; Grysan, P.; Eswara, S.; et al. Effects of silver nanoparticles and ions on a co-culture model for the gastrointestinal epithelium. Part. Fibre Toxicol. 2016, 13, 9. [Google Scholar] [CrossRef]
- Uguccioni, M.; Gionchetti, P.; Robbiani, D.F.; Rizzello, F.; Peruzzo, S.; Campieri, M.; Baggiolini, M. Increased expression of IP-10, IL-8, MCP-1, and MCP-3 in ulcerative colitis. Am. J. Pathol. 1999, 155, 331–336. [Google Scholar] [CrossRef]
- Singh, U.P.; Singh, N.P.; Murphy, E.A.; Price, R.L.; Fayad, R.; Nagarkatti, M.; Nagarkatti, P.S. Chemokine and cytokine levels in inflammatory bowel disease patients. Cytokine 2016, 77, 44–49. [Google Scholar] [CrossRef]
- Sakai-Kato, K.; Hidaka, M.; Un, K.; Kawanishi, T.; Okuda, H. Physicochemical properties and in vitro intestinal permeability properties and intestinal cell toxicity of silica particles, performed in simulated gastrointestinal fluids. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Bramini, M.; Hristov, D.R.; Wan, S.; Salvati, A.; Åberg, C.; Dawson, K.A. Low uptake of silica nanoparticles in Caco-2 intestinal epithelial barriers. Beilstein J. Nanotechnol. 2017, 8, 1396–1406. [Google Scholar] [CrossRef] [PubMed]






| Solid | SBET (m2/g) | Pore Volume a (cm3/g) | Pore Size a,b (nm) |
|---|---|---|---|
| M41 | 1098.3 | 1.0 | 2.5 |
| M41-RhB-L | 2.6 | 0.0 | - |
| Solid | μgOM/mg SiO2 | μgEOC/mg M41-EOC-L Released |
|---|---|---|
| M41-Thy-L | 311 | 37.5 a |
| M41-Eug-L | 446 | 79.0 a |
| M41-Cin-L | 758 | 89.3 a |
| Parameter | Cin Free | M41-Cin-L | % Relative (M41-Cin-L/Cin Free) |
|---|---|---|---|
| tmax | 45 min | 60 min | ↑ 33.3% |
| Cmax | 0.22 mg/mL | 0.15 mg/mL | ↓ 31.8% |
| AUC | 31.02 | 30.76 mg·min/mL | ↓ 0.8% |
| t½ | 81.55 min | 93.67 min | ↑ 14.9% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poyatos-Racionero, E.; González-Álvarez, I.; Sánchez-Moreno, P.; Sitia, L.; Gatto, F.; Pompa, P.P.; Aznar, E.; González-Álvarez, M.; Martínez-Máñez, R.; Marcos, M.D.; et al. Lactose-Gated Mesoporous Silica Particles for Intestinal Controlled Delivery of Essential Oil Components: An In Vitro and In Vivo Study. Pharmaceutics 2021, 13, 982. https://doi.org/10.3390/pharmaceutics13070982
Poyatos-Racionero E, González-Álvarez I, Sánchez-Moreno P, Sitia L, Gatto F, Pompa PP, Aznar E, González-Álvarez M, Martínez-Máñez R, Marcos MD, et al. Lactose-Gated Mesoporous Silica Particles for Intestinal Controlled Delivery of Essential Oil Components: An In Vitro and In Vivo Study. Pharmaceutics. 2021; 13(7):982. https://doi.org/10.3390/pharmaceutics13070982
Chicago/Turabian StylePoyatos-Racionero, Elisa, Isabel González-Álvarez, Paola Sánchez-Moreno, Leopoldo Sitia, Francesca Gatto, Pier Paolo Pompa, Elena Aznar, Marta González-Álvarez, Ramón Martínez-Máñez, María Dolores Marcos, and et al. 2021. "Lactose-Gated Mesoporous Silica Particles for Intestinal Controlled Delivery of Essential Oil Components: An In Vitro and In Vivo Study" Pharmaceutics 13, no. 7: 982. https://doi.org/10.3390/pharmaceutics13070982
APA StylePoyatos-Racionero, E., González-Álvarez, I., Sánchez-Moreno, P., Sitia, L., Gatto, F., Pompa, P. P., Aznar, E., González-Álvarez, M., Martínez-Máñez, R., Marcos, M. D., & Bernardos, A. (2021). Lactose-Gated Mesoporous Silica Particles for Intestinal Controlled Delivery of Essential Oil Components: An In Vitro and In Vivo Study. Pharmaceutics, 13(7), 982. https://doi.org/10.3390/pharmaceutics13070982











