Abstract
Acute pneumonia is an inflammatory disease caused by several pathogens, with symptoms such as fever and chest pain, to which children are particularly vulnerable. Gancaonin N is a prenylated isoflavone of Glycyrrhiza uralensis that has been used in the treatment of various diseases in oriental medicine. There are little data on the anti-inflammatory efficacy of Gancaonin N, and its effects and mechanisms on acute pneumonia are unknown. Therefore, this study was conducted as a preliminary analysis of the anti-inflammatory effect of Gancaonin N in lipopolysaccharide (LPS)-induced RAW264.7 cells, and to identify its preventive effect on the lung inflammatory response and the molecular mechanisms underlying it. In this study, Gancaonin N inhibited the production of NO and PGE2 in LPS-induced RAW264.7 cells and significantly reduced the expression of iNOS and COX-2 proteins at non-cytotoxic concentrations. In addition, in LPS-induced A549 cells, Gancaonin N significantly reduced the expression of COX-2 and pro-inflammatory cytokines, such as TNF-α, IL-1β, and IL-6. Moreover, Gancaonin N reduced MAPK signaling pathway phosphorylation and NF-κB nuclear translocation. Therefore, Gancaonin N relieved the inflammatory response by inactivating the MAPK and NF-κB signaling pathways; thus, it is a potential natural anti-inflammatory agent that can be used in the treatment of acute pneumonia.
1. Introduction
Pneumonia, a lower respiratory tract infection disease, is an inflammatory disease caused by pathogens, such as various bacteria, viruses, fungi, and other factors [1,2,3]. Clinical symptoms include fever, cough, phlegm, drowsiness, chest pain, and shortness of breath [4,5]. Despite the advances in antibacterial therapy and improvement in supportive therapy, it is a major infectious disease that is common in all age groups [6]. It is one of the main causes of death in the United States and also has a fatal effect on children in developing countries [7]. In particular, acute pneumonia is a major cause of mortality and disease rates in children under the age of 5, and it has been reported that 1.1 million to 1.4 million children are diagnosed with pneumonia every year [8,9,10]. Children’s acute pneumonia includes aspiration pneumonia and infectious pneumonia, and a recent study showed that the highest incidence and severity occurs at 6 months after birth [11]. Currently, when pneumonia is suspected or confirmed, antibiotics are commonly used, but sometimes symptoms are not relieved, and one of the most important factors is the specific inflammatory response of the host [12,13,14]. Lipopolysaccharide (LPS) present in Gram-negative bacteria is a factor characterized by an extreme inflammatory reaction, leading to inflammatory diseases in various organs [15]. The mechanism by which LPS affects pneumonia is not fully understood, but there is a lot of evidence that it causes inflammation in the lungs, contributing to the development of acute pneumonia [16,17]. In addition, A549 cells, a human type II lung cell line, along with the human bronchial epithelial cell line (BEAS-2B), is the most commonly used cell line for various studies related to respiratory diseases, including pneumonia. Several studies have shown that LPS-treated A549 cells can promote and amplify the expression of inflammatory mediators by activating the nuclear factor kappa light chain enhancer of activated B cells/mitogen-activated protein kinase (NF-κB/MAPK) signaling pathways [18,19,20]. In addition, by LPS induction, the phosphoinositide-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling pathways are activated to promote cell cycles and inhibit apoptosis, which affects inflammatory reactions [17,21]. Transcription factors such as AP-1 and STAT-3 are also activated to facilitate the production of inflammatory mediator cyclooxygenase-2 (COX-2) [22]. Therefore, modulation of these signaling pathways may help in the treatment of acute pneumonia by suppressing the inflammatory response. Accordingly, natural products that have few side effects and are inexpensive are receiving more attention as potential anti-inflammatory drugs that can improve the inflammatory state of the lungs and many natural products that can alleviate the inflammatory state of the lungs are continuously being discovered [23]. As an example, Labib et al. reported that the essential oil of Pinus roxburghii bark containing palmitic acid as the main component could improve the inflammatory state of the lungs [24], and Gao et al. reported that neochlorogenic acid isolated from Morus alba L. relieved oxidative stress and inflammatory response in inflamed lungs [18]. In addition, terpenoids, phenylpropanoid glycosides, and polyphenols, which are naturally derived components isolated from plant extracts, showed the ability to alleviate oxidative control damage and inflammatory responses in the lungs [25,26,27].
The roots and rhizomes of Glycyrrhiza uralensis are the oldest medicinal herbs in oriental medicine and are mainly prescribed as herbal plants to treat cough, bronchitis, peptic ulcer, and dermatitis [28]. In addition, according to recently published research results, Glycyrrhiza uralensis protects the liver from alcoholic fatty liver disease [29], and relieves airway hypersensitivity reactions and oxidative stress, thereby preventing asthma symptoms [30]. In addition, it has been proven to be effective in immunomodulation [31] and has anti-inflammatory [32], anti-obesity [33], anti-viral [34], and antibacterial effects [35]. Therefore, based on the efficacy of Glycyrrhiza uralensis, various pharmacological activities have been reported for various compounds (triterpenoids, flavones, isoflavones, among others) derived from Glycyrrhiza uralensis [36,37].
Gancaonin N is a prenylated isoflavone-based organic compound extracted from the roots of Glycyrrhiza uralensis [38]. In a recent study, Gancaonin N was shown to have antiproliferative activity in human-derived tumor cell lines and an inhibitory effect on the production of nitric oxide (NO) in LPS-induced RAW264.7 cell lines [39]. However, no clear study of the therapeutic mechanism of Gancaonin N in lung inflammation has been revealed. Therefore, in this study, before verifying the effect of Gancaonin N in inflammatory conditions in the lungs, it was confirmed that Gancaonin N inhibits the production of inflammatory mediators, NO and prostglandin E2 (PGE2), and their biosynthetic enzymes, inducible nitric oxide synthase (iNOS) and COX-2, in RAW264.7 cells exposed to LPS. Later, it was confirmed that Gancaonin N has an anti-inflammatory effect in alveolar epithelial A549 cells in the LPS-induced inflammatory state, and further we analyzed its effect on the intracellular inflammatory signal transduction pathway, to suggest the possibility of an acute pneumonia treatment.
2. Materials and Methods
2.1. Reagents
Gancaonin N was purchased from Chemfaces (Wuhan Chemfaces Biochemical Co., Ltd., Wuhan, China). LPS (Escherichia coli 055:B5) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Primary antibodies for COX-2, NF-κB p65, extracellular signal-regulated kinase (ERK), p-ERK, p38, p-p38, and Lamin B1 were purchased from Cell Signaling Technology (Beverly, MA, USA), interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α) were purchased from Proteintech (Rosemont, IL, USA), PGE2 was purchased from Bioss (Woburn, MA, USA), and iNOS was purchased from R&D Systems Inc. (Minneapolis, MN, USA). β-Actin and secondary antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, USA).
2.2. Cell Culture
The RAW264.7 cell line was purchased from the Korean Cell Line Bank (Seoul, Korea), while the A549 cell line was purchased from American Type Culture Collection (ATCC, MD, USA). Each cell line was cultured using DMEM containing 10% FBS (Gibco, NY, USA) and 1% antibiotics (Corning Inc., New York, NY, USA) at a temperature of 37°C, 5% CO2 environment [40].
2.3. Cytotoxicity Assay
To measure the cytotoxicity of Gancaonin N in RAW264.7 and A549 cells, the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed according to Ko et al.’s paper method described previously [41]. Briefly, cells were seeded in a 96-well plate (1 × 104 cells/well), followed by treatment with Gancaonin N (0–40 μM) for 24 h. The absorbance of formazan produced by the MTT solution was quantified at 540 nm using a 96-well microplate reader (Bio-Rad, Hercules, CA, USA).
2.4. NO Assay
NO assay was performed according to our method previously described [42]. Briefly, RAW264.7 cells were seeded in a 6-well plate (6 × 105 cells/well). The cultured medium was exchanged for each Gancaonin N dose 2 h prior to treatment with 1 μg/mL LPS. The cells were then incubated for 24 h. Thereafter, the cultured medium of each well and Griess reagent were mixed and reacted for 10 min, and absorbance was measured at 540 nm.
2.5. Immunoblotting
The preparation of whole cell lysates and nuclear fractions and the whole immunoblotting process were performed based on the previously detailed description of Ko et al. [41]. To identify a specific protein band, ImageQuant LAS 500 (GE Healthcare Life Sciences, Sydney, NSW, Australia) was used by treating the EZ-Western Lumi Femto kit (DoGen, Seoul, Korea) and Image J software (NIH, Bethesda, MD, USA) was used to quantify the band [43].
2.6. Immunofluorescence Assay
A549 cells were seeded in 4-well culture slides. Cells were pretreated with Gancaonin N at 40 μM 2 h before stimulation with LPS and incubated for 6 h. After that, to confirm that NF-κB p65 was translocated to the nucleus, it was performed according to our previously described method [44,45]. Fluorescence images of each slide were obtained using an EVOSR Cell Imaging system (Thermo Fisher Scientific, Waltham, MA, USA).
2.7. Isolation of the Total RNA and Real-Time PCR
The methods of RNA acquisition, cDNA synthesis, and real-time PCR were previously described in detail [41,42,44]. The relative mRNA expression level of each target gene was normalized with GAPDH [46]. The primer sequences used for real-time PCR analysis are as below.
TNF-α: (F) 5′-GCAGGTCTACTTTGGGTCATTG-3′ and (R) 5′-GCGTTTGGGAAGGTTGGA-3′.
IL-1β: (F) 5′-TCAGCCAATCTTCATTGCTCAA-3′ and (R) 5′- TGGCGAGCTCAGGTACTTCTG -3′.
IL-6: (F) 5′-AGGGCTCTTCGGCAAATGTA-3′ and (R) 5′- GAAGGAATGCCCATTAACAACAA-3′.
GAPDH (F) 5′-GCCACATCGCTCAGACACC-3′ and (R) 5′-CCCAATACGACCAAATCCGT-3′.
2.8. Statistical Analysis
All data were expressed as mean ± SEM through repeated experiments. An unpaired t-test (one-tailed) was used to analyze the statistical significance between each group.
3. Results
3.1. Effects of Gancaonin N on RAW264.7 and A549 Cell Viability
Before analyzing the anti-inflammatory effects of Gancaonin N, the researchers used an MTT assay for evaluating the cytotoxicity of Gancaonin N in RAW264.7 and A549 cells. As shown in Figure 1B,C, when Gancaonin N was used for treatment at a concentration of 5 to 40 μM for 24 h in each cell, it was confirmed that no cytotoxicity was observed. Therefore, we set the concentration of Gancaonin N to up to 40 μM for the subsequent experiments under conditions that did not affect the cells (Figure 1B,C).
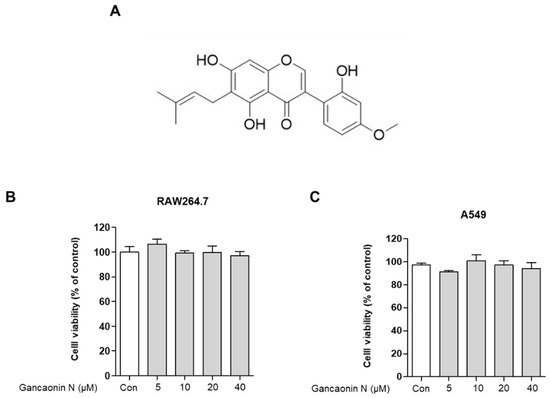
Figure 1.
Chemical structure of Gancaonin N (A) and its effects on RAW264.7 (B) and A549 (C) cell viability. Cells were seeded in 96-well plate and treated with Gancaonin N (5–40 μM) for 24 h. The cytotoxicity was confirmed by MTT assay. Values are represented as means ± SEM.
3.2. Effect of Gancaonin N on Pro-Inflammatory Mediators in RAW264.7 Cells
Before confirming the acute pneumonia prophylactic effect of Gancaonin N, we evaluated the inhibitory effect of the inflammatory response in LPS-stimulated RAW264.7 cells. NO is an inflammatory mediator and is expressed only when cells are exposed to pro-inflammatory conditions. Therefore, in RAW264.7 cells stimulated with LPS, the amount of NO production was excessively increased compared to that in the untreated group. However, in the group pretreated with Gancaonin N before LPS treatment, NO production significantly decreased, depending on the concentration of Gancaonin N (Figure 2A). The expression of PGE2, a major mediator of chronic inflammatory diseases, was confirmed at the protein level using immunoblotting. As shown in Figure 2B, the level of PGE2 was significantly increased by LPS induction, and it was confirmed that Gancaonin N at a concentration of 10–40 μM significantly suppressed the level of PGE2. Additionally, the effect of Gancaonin N on the expression of pro-inflammatory proteins was analyzed. iNOS is frequently produced in mononuclear phagocytes and among the three types of nitric oxide synthase (NOS)-related enzymes it produces the largest amount of NO, causing severe inflammation. COX-2 is an enzyme that stimulates the synthesis and secretion of prostaglandin E2 (PGE2) by inducing an inflammatory mediator. Therefore, we analyzed the protein expression levels of iNOS and COX-2 (Figure 2C,D). Consequently, Gancaonin N inhibited the expression of iNOS and COX-2 in a treatment concentration-dependent manner in LPS-induced RAW264.7 cells. In particular, the expression level of iNOS was significantly decreased at a Gancaonin N concentration of 5–40 μM, and the expression level of COX-2 was significantly decreased at a concentration of 20–40 μM.
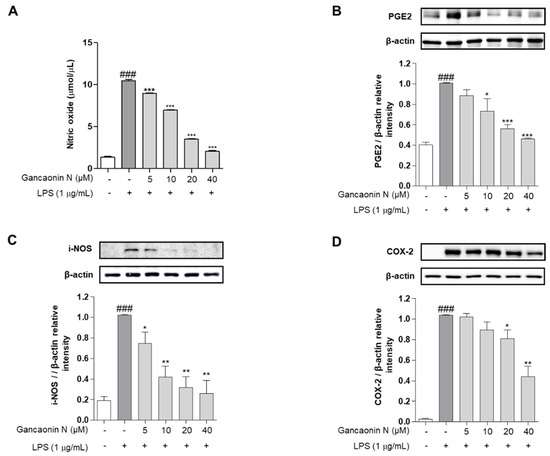
Figure 2.
Effects of Gancaonin N on nitric oxide production (A) and protein levels of PGE2 (B), iNOS (C) and COX-2 (D) in LPS-induced RAW264.7 cells. Gancaonin N (5–40 μM) was pretreated 2 h prior to LPS-induced inflammation in RAW264.7 cells, incubated for 24 h with LPS (1 μg/mL). The protein levels of PGE2, iNOS and COX-2 were investigated by immunoblotting assay. Ratio of each protein was determined by ImageJ. Values are represented as means ± SEM. ### p < 0.001 is significantly different from non-treated group; * p < 0.05, ** p < 0.01, *** p < 0.001 are significantly different from only LPS-treated group.
3.3. Effects of Gancaonin N on Pro-Inflammatory Cytokine and COX-2 Expression in LPS-Induced A549 Cells
Based on the mechanism of lung inflammation, several studies are being conducted with the purpose of developing therapeutic agents for respiratory diseases, such as pneumonia. Therefore, we confirmed the anti-inflammatory effect of Gancaonin N in LPS induced-A549 cells and evaluated its potential as a therapeutic agent for lung disease prevention. First, we measured the expression levels of pro-inflammatory cytokines using real-time PCR (Figure 3A–C). As a result, it was confirmed that in LPS-induced A549 cells, Gancaonin N significantly reduced the mRNA expression level of each pro-inflammatory cytokine. Moreover, to further confirm the anti-inflammatory effect of Gancaonin N at the protein level, immunoblotting analysis was performed to confirm the effect on the expression of each pro-inflammatory cytokine and COX-2. As shown in Figure 4A–D, it was confirmed that pretreatment with Gancaonin N clearly decreased the protein levels of TNF-α, IL-1β, IL-6, and COX-2, which were increased by LPS induction.
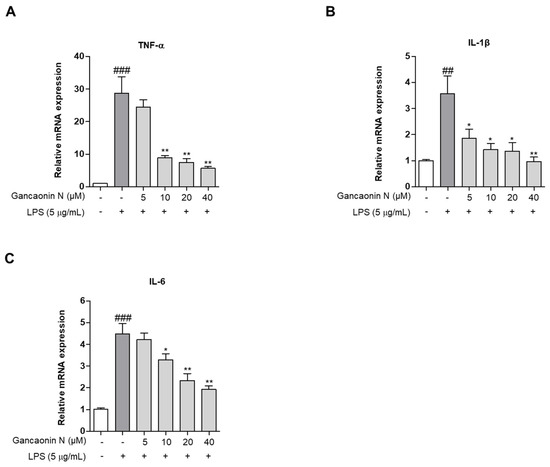
Figure 3.
(A–C) Effects of Gancaonin N on pro-inflammatory cytokine mRNA expression in LPS-induced A549 cells. Gancaonin N (5–40 μM) was pretreated 2 h prior to LPS-induced inflammation in A549 cells, incubated for 24 h with LPS (5 μg/mL). The mRNA expressions of cytokine were investigated by real-time PCR analysis. Values are represented as means ± SEM. ## p < 0.01, ### p < 0.001 are significantly different from non-treated group; * p < 0.05, ** p < 0.01 are significantly different from only LPS-treated group.
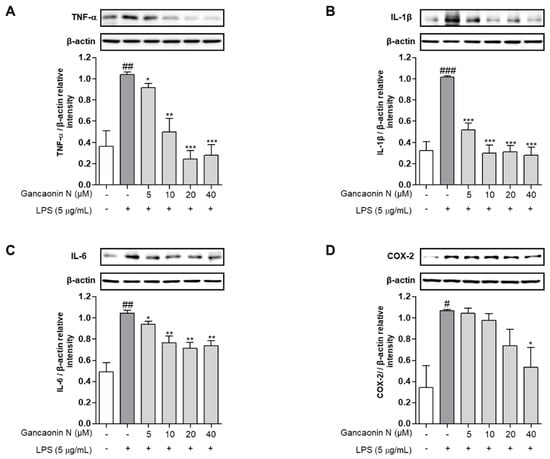
Figure 4.
Effects of Gancaonin N on protein levels of pro-inflammatory cytokines (A–C) and COX-2 (D) in LPS-induced A549 cells. Gancaonin N (5–40 μM) was pretreated 2 h prior to LPS-induced inflammation in A549 cells, incubated for 24 h with LPS (5 μg/mL). The protein level of inflammatory biomarkers was investigated by immunoblotting assay. Ratio of each protein was determined by ImageJ. Values are represented as means ± SEM. # p < 0.05, ## p < 0.01, ### p < 0.001 are significantly different from non-treated group; * p < 0.05, ** p < 0.01, *** p < 0.001 are significantly different from only LPS-treated group.
3.4. Effect of Gancaonin N on MAPK/NF-κB Signaling Pathway in LPS-Induced A549 Cells
Based on the inhibitory effect of Gancaonin N on the expression of pro-inflammatory cytokines and COX-2, to further analyze the mechanism of anti-inflammatory effects, the protein expression level of the MAPK/NF-κB signaling pathway associated with inflammation was evaluated using immunoblotting. As a result, the phosphorylation of ERK and p38 was increased in A549 cells induced by LPS alone, but the phosphorylation of ERK and p38 was effectively suppressed in the 10–40 μM Gancaonin N pretreatment group (Figure 5A,B). In the nuclear fraction, it was confirmed that the nuclear translocation of NF-κB p65 was inhibited by Gancaonin N in LPS-induced A549 cells (Figure 5C). To further demonstrate the following procedure, we measured the expression of NF-κB p65 in the nucleus using immunofluorescence analysis and we found that 40 μM Gancaonin N significantly inhibited the nuclear translocation of NF-κB p65 via LPS stimulation (Figure 5D). Thus, Gancaonin N regulates the expression of pro-inflammatory cytokines and inflammatory mediators, such as COX-2, through inhibition of the MAPK and NF-κB signaling pathways.
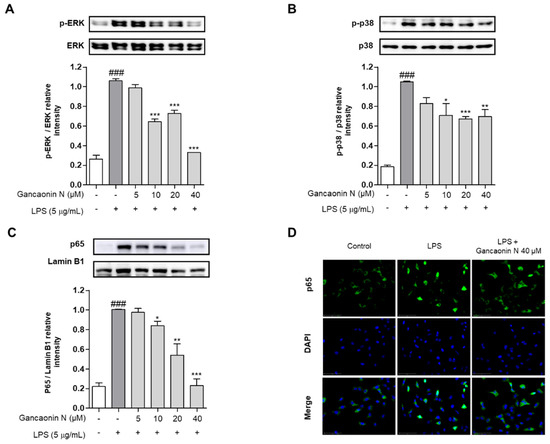
Figure 5.
Effect of Gancaonin N on MAPK/NF-κB signaling pathway in LPS-induced A549 cells. Gancaonin N (5–40 μM) was pretreated 2 h prior to LPS-induced inflammation in A549 cells, incubated for 6 h with LPS (5 μg/mL). The protein levels of ERK (A), p38 (B), and NF-κB p65 (C) were investigated by immunoblotting assay. Ratio of each protein was determined by ImageJ. The expression of NF-κB p65 (D) in the nucleus was confirmed using immunofluorescence assay (magnification: 400×, scale bar:75 μm). Values are represented as means ± SEM. ### p < 0.001 is significantly different from non-treated group; * p < 0.05, ** p < 0.01, *** p < 0.001 are significantly different from only LPS-treated group.
4. Discussion
Acute pneumonia, a major respiratory disease, is an inflammatory disease of the lungs caused by various pathogen infections and is a major infectious disease, to which infants are particularly susceptible [47]. Since this disease has a high mortality rate and a high incidence rate, various treatment methods are being developed, along with antibiotic treatment [48]. Among them, compounds derived from natural products that can improve the inflammatory response of the lungs and have relatively few side effects have been attracting much attention for the development of therapeutic agents. Previously, many studies suggested that secondary metabolites such as polyphenols, flavonoids, alkaloids, and terpenoids have the ability to prevent and treat inflammatory responses induced in the lungs [18,24,27,49].
Gancaonin N, a prenylated isoflavone isolated from Glycyrrhiza uralensis, has been reported to have anti-inflammatory activity in previous studies [38,39]. However, there are little data on its anti-inflammatory activity and anti-inflammatory mechanisms in pulmonary inflammatory conditions. Therefore, in this study, to evaluate the possibility of preventing and treating pneumonia, the anti-inflammatory effect of Gancaonin N was investigated through experiments using LPS-stimulated RAW264.7 and A549 cells.
Macrophages are the most widely distributed cell type in all tissues in the animal body and are immune cells that play an essential role in innate immune responses, including inflammation in the human body [50]. When macrophages are activated by external stimuli, such as pathogens, they produce various types of inflammatory mediators, such as arachidonic acid metabolites, NO, and pro-inflammatory cytokines [51]. An excessive production of these inflammatory mediators accelerates the development of chronic inflammatory diseases [52]. LPS is a major component of the outer membrane of Gram-negative bacteria and plays a pivotal role in inducing the inflammatory response associated with pneumonia [15,17]. Therefore, many studies have been conducted to analyze the anti-inflammatory effect of drugs by evaluating the inhibitory activity of inflammatory mediators secreted from macrophages activated by LPS stimulation. First, in order to explore the anti-inflammatory effect of Gancaonin N in LPS-stimulated RAW264.7 cells, we examined the inhibitory effect of Gancaonin N on NO and PGE2 production. NO, which is most easily observed in macrophages of inflammatory disease patients, is produced from L-arginine by nitric oxide synthases and contributes to anti-inflammatory activity under normal physiological conditions [53]. However, an excessive production of NO due to a physiological disorder promotes the biosynthesis of inflammatory mediators and intensifies inflammation [53,54]. This leads to serious inflammatory diseases. In addition, PGE2, a key inflammatory mediator, is a major product of COX-2 and actively participates in inflammatory responses, contributing to chronic inflammation and various diseases, including cancer [55,56]. Therefore, chemicals that inhibit NO and PGE2 production are known to have anti-inflammatory effects and may be an alternative strategy for treating inflammatory diseases. In this study, pretreatment with Gancaonin N dose-dependently reduced the NO production, induced via LPS stimulation, which was similar to that reported in a recent study [39]. Therefore, we further investigated whether Gancaonin N can reduce the protein expression levels of iNOS and COX-2. iNOS is a nitric oxide synthase (NOS), an enzyme that produces NO, and unlike endothelial NOS (eNOS) and neuronal NOS (nNOS), it is non-dependent on calcium/calmodulin [57]. iNOS is not normally expressed in cells, but is expressed via transcriptional regulation when it is exposed to external stimulation or pro-inflammatory stimulation, and produces a large amount of NO [58]. Cyclooxygenase (COX) is divided into two isoforms, of which COX-2 is directly involved in the generation of PGE2, causing pain and fever, and large amounts are expressed in inflammatory cells via LPS, pro-inflammatory cytokines, growth factors, and tumor promotors [59]. Therefore, the discovery of natural compounds that inhibit the production of NO and PG by inhibiting the expression of iNOS and COX-2 can be an indicator of the development of natural anti-inflammatory drugs with fewer side effects. The results of this study confirmed that the protein levels of iNOS and COX-2 were increased in RAW264.7 cells stimulated with LPS alone, but the protein expression levels of iNOS and COX-2 were suppressed when Gancaonin N was used for pretreatment. The results show that Gancaonin N relieves the inflammatory condition caused by LPS stimulation.
Despite the use of antibiotics to treat pneumonia, sometimes they do not relieve symptoms, and one of the most important factors is the specific inflammatory response of the host [13,14]. Therefore, based on the anti-inflammatory activity of Gancaonin N described above, in order to confirm whether Gancaonin N can help relieve the symptoms of pneumonia by improving the pulmonary inflammatory response, we additionally demonstrated the effect of Gancaonin N on LPS-stimulated A549 alveolar epithelial cells. A549 cells, which have the properties of type 2 alveolar epithelial cells, have been used in many studies to investigate the treatment mechanisms related to lung inflammation due to the limitation of the use of primary cultured human alveolar epithelial cells [60,61,62]. When LPS is used to stimulate A549 cells, the expression of pro-inflammatory cytokines is induced and various inflammatory mediators are produced, making it a widely used pneumonia model because there is evidence that it causes and worsens lung damage [18,19,63]. Therefore, suppressing the production of pro-inflammatory cytokines and COX-2 in LPS-induced A549 cells is a representative treatment method applicable to inflammatory symptoms caused by pneumonia. Inflammatory cytokines, such as TNF-α, IL-1β, and IL-6, are essential cell signaling proteins in the inflammatory response. TNF-α is expressed due to various external stimuli, such as LPS and viruses, and when expressed it causes the influx of inflammatory cells and various biological reactions, such as cell death, proliferation, and migration [64]. IL-1β is a cytokine responsible for initiating and amplifying the inflammatory response and activating NF-κB [65]. IL-6, which plays a role as a major pro-inflammatory mediator for the induction of acute phase reactions, is known to regulate the differentiation and activation of T lymphocytes by inducing the ERK pathway and is considered an important biomarker in respiratory inflammatory diseases [66,67]. As a result of the experiment, we confirmed that the expression of TNF-α, IL-1β IL-6, and COX-2 was upregulated in A549 cells stimulated with LPS alone. This is consistent with previous research findings in A549 cells stimulated with LPS [20]. Furthermore, the pretreatment with Gancaonin N significantly inhibited the expression of TNF-α, IL-1β, IL-6, and COX-2 in LPS-stimulated A549 cells. These results suggest that Gancaonin N inhibits the production of inflammatory mediators produced by LPS stimulation in A549 cells and, thus, has a preventive effect against the inflammatory response.
To characterize these effects, we further investigated whether Gancaonin N could inhibit the activation of MAPK and NF-κB signaling pathways via immunoblotting. MAPK is activated by various inflammatory and stress stimuli and is an intracellular signaling pathway that regulates the immune response by regulating the expression of inflammatory cytokines and other inflammatory mediators in various cells [68]. The MAPK pathway is largely classified into three subtypes: ERK, c-Jun N-terminal kinase (JNK), and p38. Phosphorylation in these pathways can be easily detected in lung diseases related to lung inflammation, such as pneumonia and chronic obstructive pulmonary disease (COPD) [69,70]. Among them, ERK is involved in cell proliferation, growth, differentiation, cell migration, and survival, and is related to pathological conditions, such as cancer and chronic inflammation [71]. The p38 MAPK pathway, which acts as a link for signaling, has a strong association with inflammation and is known to play an essential role in the production of TNF-α, IL-1β, and IL-6 [72,73]. This study showed that Gancaonin N pretreatment inhibited the phosphorylation of ERK and p38, which was increased by LPS stimulation in A549 cells. The NF-κB pathway is an important transcriptional regulator involved in the regulation of the immune system and inflammatory response [74]. NF-κB is present in the cytoplasm in a state bound to IκB in an inactive state; however, when activated by LPS stimulation, NF-κB separates from IκB and moves into the nucleus, inducing the expression of inflammatory cytokines and inducible enzymes, such as COX-2 and iNOS [75]. Therefore, many studies have demonstrated the anti-inflammatory effects of natural drugs on lung inflammation caused by LPS stimulation by inhibiting NF-κB nuclear translocation [18,19,20]. The results of this study show that Gancaonin N significantly inhibits the NF-κB nuclear translocation increased by LPS stimulation in A549 cells. To clarify this, we further confirmed that Gancaonin N clearly inhibited the nuclear translocation of NF-κB p65 in cells induced by LPS stimulation, using immunofluorescence. Therefore, we presume that in LPS-stimulated A549 cells, Gancaonin N exhibits anti-inflammatory effects by inhibiting the activation of the MAPK/NF-κB signaling pathway, which leads to the production of inflammatory mediators.
5. Conclusions
We demonstrated the anti-inflammatory activity of Gancaonin N in LPS-stimulated RAW264.7 and A549 cells, providing the potential to prevent acute pneumonia. Gancaonin N was shown to inhibit inflammatory mediators such as NO, PGE2, iNOS, and COX-2 in LPS-stimulated RAW264.7 cells. In addition, in LPS-stimulated A549 cells, Gancaonin N inhibited the expression of TNF-α, IL-1β, IL-6, and COX-2, which is closely related to the inactivation of the MAPK/NF-κB signaling pathway. These findings provide an experimental basis for the anti-inflammatory effect of Gancaonin N; therefore, it has been demonstrated that Gancaonin N may be a natural anti-inflammatory agent that can prevent acute pneumonia. A limitation of this study is that the cytotoxic and anti-inflammatory effects of Gancaonin N were not sufficiently analyzed using lung cell lines other than A549, and anti-inflammatory activity and stability should also be confirmed in an in vivo model in the future.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by H.M.K., S.-H.L. and W.J. The first draft of the manuscript was written by H.M.K., J.H.J., K.-I.K., H.-J.J. (Hee-Jae Jung) and H.-J.J. (Hyeung-Jin Jang) commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant number: HF20C0030020020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented this study are available from the corresponding author, upon reasonable request.
Acknowledgments
We thank for members of Jang laboratory for active discussion.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hanada, S.; Pirzadeh, M.; Carver, K.Y.; Deng, J.C. Respiratory viral infection-induced microbiome alterations and secondary bacterial pneumonia. Front. Immunol. 2018, 9, 2640. [Google Scholar] [CrossRef] [Green Version]
- Hussell, T.; Cavanagh, M.M. The Innate Immune Rheostat: Influence on Lung Inflammatory Disease and Secondary Bacterial Pneumonia; Portland Press: London, UK, 2009. [Google Scholar]
- Raghavendran, K.; Mylotte, J.M.; Scannapieco, F. Nursing home-associated pneumonia, hospital-acquired pneumonia and ventilator-associated pneumonia: The contribution of dental biofilms and periodontal inflammation. Periodontol. 2000 2007, 44, 164–177. [Google Scholar] [CrossRef]
- Hopstaken, R.; Muris, J.; Knottnerus, J.; Kester, A.; Rinkens, P.; Dinant, G. Contributions of symptoms, signs, erythrocyte sedimentation rate, and C-reactive protein to a diagnosis of pneumonia in acute lower respiratory tract infection. Br. J. Gen. Pract. 2003, 53, 358–364. [Google Scholar]
- Lutfiyya, M.N.; Henley, E.; Chang, L.F.; Reyburn, S.W. Diagnosis and treatment of community-acquired pneumonia. Am. Fam. Physician 2006, 73, 442–450. [Google Scholar] [PubMed]
- Izadnegahdar, R.; Cohen, A.L.; Klugman, K.P.; Qazi, S.A. Childhood pneumonia in developing countries. Lancet Respir. Med. 2013, 1, 574–584. [Google Scholar] [CrossRef]
- Morris, S.K.; Bassani, D.G.; Awasthi, S.; Kumar, R.; Shet, A.; Suraweera, W.; Jha, P. Diarrhea, Pneumonia, and Infectious Disease Mortality in Children Aged 5 to 14 Years in India. PLoS ONE 2011, 6, e20119. [Google Scholar] [CrossRef] [Green Version]
- Rudan, I.; O’Brien, K.L.; Nair, H.; Liu, L.; Theodoratou, E.; Qazi, S.; Lukšić, I.; Walker, C.L.F.; Black, R.E.; Campbell, H. Epidemiology and etiology of childhood pneumonia in 2010: Estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J. Glob. Health 2013, 3, 3. [Google Scholar]
- O’Brien, K.L.; Wolfson, L.; Watt, J.P.; Henkle, E.; Knoll, M.D.; McCall, N.; Lee, E.; Mulholland, K.; Levine, O.S.; Cherian, T. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: Global estimates. Lancet 2009, 374, 893–902. [Google Scholar] [CrossRef]
- Liu, L.; Johnson, H.L.; Cousens, S.; Perin, J.; Scott, S.; Lawn, J.; Rudan, I.; Campbell, H.; Cibulskis, R.; Li, M.; et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. Lancet 2012, 379, 2151–2161. [Google Scholar] [CrossRef]
- Zar, H.J.; Barnett, W.; Stadler, A.; Gardner-Lubbe, S.; Myer, L.; Nicol, M.P. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: A nested case-control study of the Drakenstein Child Health Study. Lancet Respir. Med. 2016, 4, 463–472. [Google Scholar] [CrossRef] [Green Version]
- Graham, K.; Sinyangwe, C.; Nicholas, S.; King, R.; Mukupa, S.; Källander, K.; Counihan, H.; Montague, M.; Tibenderana, J.; Hamade, P. Rational use of antibiotics by community health workers and caregivers for children with suspected pneumonia in Zambia: A cross-sectional mixed methods study. BMC Public Health 2016, 16, 897. [Google Scholar] [CrossRef] [Green Version]
- Dockrell, D.H.; Whyte, M.K.; Mitchell, T. Pneumococcal Pneumonia. Chest 2012, 142, 482–491. [Google Scholar] [CrossRef] [Green Version]
- Bordon, J.; Aliberti, S.; Fernandez-Botran, R.; Uriarte, S.M.; Rane, M.J.; Duvvuri, P.; Peyrani, P.; Morlacchi, L.C.; Blasi, F.; Ramirez, J. Understanding the roles of cytokines and neutrophil activity and neutrophil apoptosis in the protective versus deleterious inflammatory response in pneumonia. Int. J. Infect. Dis. 2013, 17, e76–e83. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.-L.; Wang, G.-Q.; Yang, L.-M.; Huang, Z.-B.; Zhang, W.-Q.; Yu, L.-Z. Endotoxin molecule lipopolysaccharide-induced zebrafish inflammation model: A novel screening method for anti-inflammatory drugs. Molecules 2014, 19, 2390–2409. [Google Scholar] [CrossRef] [Green Version]
- Shan, M.R.; Zhou, S.N.; Fu, C.N.; Song, J.W.; Wang, X.Q.; Bai, W.W.; Li, P.; Song, P.; Zhu, M.L.; Ma, Z.M. Vitamin B6 inhibits macrophage activation to prevent lipopolysaccharide-induced acute pneumonia in mice. J. Cell. Mol. Med. 2020, 24, 3139–3148. [Google Scholar] [CrossRef]
- Fei, S.; Cao, L.; Pan, L. microRNA-3941 targets IGF2 to control LPS-induced acute pneumonia in A549 cells. Mol. Med. Rep. 2018, 17, 4019–4026. [Google Scholar] [CrossRef]
- Gao, X.-H.; Zhang, S.-D.; Wang, L.-T.; Yu, L.; Zhao, X.-L.; Ni, H.-Y.; Wang, Y.-Q.; Wang, J.-D.; Shan, C.-H.; Fu, Y.-J. Anti-Inflammatory Effects of Neochlorogenic Acid Extract from Mulberry Leaf (Morus alba L.) Against LPS-Stimulated Inflammatory Response through Mediating the AMPK/Nrf2 Signaling Pathway in A549 Cells. Molecules 2020, 25, 1385. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Wang, H.; Liu, J.; Zhang, Y.; Luo, J.; Li, Y.; Yang, C.; Jiang, J. Ganoderic acid B attenuates LPS-induced lung injury. Int. Immunopharmacol. 2020, 88, 106990. [Google Scholar] [CrossRef]
- Shao, L.; Meng, D.; Yang, F.; Song, H.; Tang, D. Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem. Biophys. Res. Commun. 2017, 487, 194–200. [Google Scholar] [CrossRef]
- Jiang, M.; Zhou, L.; Xu, N.; An, Q. Hydroxysafflor yellow A inhibited lipopolysaccharide-induced non-small cell lung cancer cell proliferation, migration, and invasion by suppressing the PI3K/AKT/mTOR and ERK/MAPK signaling pathways. Thorac. Cancer 2019, 10, 1319–1333. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.; Lim, J.W.; Kim, H. Effect of thiol antioxidants on lipopolysaccharide-induced cyclooxygenase-2 expression in pulmonary epithelial cells. J. Physiol. Pharmacol. 2018, 69. [Google Scholar] [CrossRef]
- Batiha, G.E.S.; Beshbishy, A.M.; El-Mleeh, A.; Abdel-Daim, M.M.; Devkota, H.P. Traditional uses, bioactive chemical constituents, and pharmacological and toxicological activities of Glycyrrhiza glabra L. (Fabaceae). Biomolecules 2020, 10, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labib, R.M.; Youssef, F.S.; Ashour, M.L.; Abdel-Daim, M.M.; Ross, S.A. Chemical composition of Pinus roxburghii bark volatile oil and validation of its anti-inflammatory activity using molecular modelling and bleomycin-induced inflammation in Albino mice. Molecules 2017, 22, 1384. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Alsahli, M.A.; Aljasir, M.A.; Syed, M.A.; Rahmani, A.H. Epigallocatechin-3-Gallate (EGCG), an Active Compound of Green Tea Attenuates Acute Lung Injury Regulating Macrophage Polarization and Krüpple-Like-Factor 4 (KLF4) Expression. Molecules 2020, 25, 2853. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Zhang, P.; Ruan, W.; Zhang, L.; Yuan, S.; Pang, T.; Jia, A.-Q. Smiglaside A ameliorates LPS-induced acute lung injury by modulating macrophage polarization via AMPK-PPARγ pathway. Biochem. Pharmacol. 2018, 156, 385–395. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, Y.J.; Jang, H.J.; Lee, S.J.; Lee, S.; Yun, B.S.; Lee, S.W.; Rho, M.C. Rugosic acid A, derived from Rosa rugosa Thunb., is novel inhibitory agent for NF-κB and IL-6/STAT3 axis in acute lung injury model. Phytother. Res. 2020, 34, 3200–3210. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, K.; Han, S.; Zhang, L.; Bai, H.; Bao, F.; Zeng, Y.; Wang, J.; Du, H.; Liu, Y. Constituents isolated from the leaves of Glycyrrhiza uralansis and their anti-inflammatory activities on LPS-induced RAW264. 7 cells. Molecules 2019, 24, 1923. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.-C.; Lee, Y.-H.; Kim, S.H.; Kim, K.-J.; Kim, K.-M.; Oh, S.; Jung, Y.-S. Hepatoprotective effect of licorice, the root of Glycyrrhiza uralensis Fischer, in alcohol-induced fatty liver disease. BMC Complement. Altern. Med. 2015, 16, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.-C.; Liu, C.-Y.; Shen, S.-C.; Chen, L.-C.; Yeh, K.-W.; Liu, S.-H.; Liou, C.-J. Protective effects of licochalcone A improve airway hyper-responsiveness and oxidative stress in a mouse model of asthma. Cells 2019, 8, 617. [Google Scholar] [CrossRef] [Green Version]
- Ayeka, P.A.; Bian, Y.; Githaiga, P.M.; Zhao, Y. The immunomodulatory activities of licorice polysaccharides (Glycyrrhiza uralensis Fisch.) in CT 26 tumor-bearing mice. BMC Complement. Altern. Med. 2017, 17, 536. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Yuan, B.-C.; Ma, Y.-S.; Zhou, S.; Liu, Y. The anti-inflammatory activity of licorice, a widely used Chinese herb. Pharm. Biol. 2016, 55, 5–18. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.E.; Yang, G.; Han, S.-H.; Lee, J.-H.; An, T.-J.; Jang, J.-K.; Lee, J.Y. Anti-obesity potential of Glycyrrhiza uralensis and licochalcone A through induction of adipocyte browning. Biochem. Biophys. Res. Commun. 2018, 503, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.F.; Wang, K.C.; Chiang, L.C.; Shieh, D.E.; Yen, M.H.; San Chang, J. Water extract of licorice had anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 148, 466–473. [Google Scholar]
- He, J.; Chen, L.; Heber, D.; Shi, W.; Lu, Q.-Y. Antibacterial Compounds from Glycyrrhiza uralensis. J. Nat. Prod. 2006, 69, 121–124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Li, Z.; Song, W.; Wang, Y.; Liang, W.; Li, K.; Tang, S.; Wang, Q.; Qiao, X.; Zhou, D.; et al. Bioactive Constituents of Glycyrrhiza uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 2016, 79, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Fukai, T.; Wang, Q.-H.; Takayama, M. Structure of Five New Prenylated Flavonoids L, M, N, O, and P from Aerial Parts of Glycyrrhiza uralensis. Heterocycles 1990, 31, 373. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Guo, J.-M.; Yan, G.; Zhang, M.-M.; Zhang, W.-H.; Qiang, L.; Fu, Y.-H. Anti-inflammatory and antiproliferative prenylated isoflavone derivatives from the fruits of Ficus carica. J. Agric. Food Chem. 2019, 67, 4817–4823. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Chen, J.; Yang, F.; Li, G. Separation of Macrophages Using a Dielectrophoresis-Based Microfluidic Device. BioChip J. 2020, 14, 185–194. [Google Scholar] [CrossRef]
- Ko, H.M.; Choi, S.-H.; Kim, Y.; An, E.-J.; Lee, S.-H.; Kim, K.; Jung, H.-J.; Jang, H.-J. Effect of Rosa laevigata on PM10-Induced Inflammatory Response of Human Lung Epithelial Cells. Evid. Based Complement. Altern. Med. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; Lee, I.-S.; Kim, K.-H.; Kim, Y.; Na, Y.-C.; Lee, J.-H.; Jang, H.-J. The therapeutic effects of Yongdamsagan-tang on autoimmune hepatitis models. Biomed. Pharmacother. 2017, 94, 244–255. [Google Scholar] [CrossRef]
- Lim, M.H.; Lim, M.J.; Yun, W.-S.; Jin, S.; Lee, D.; Kim, S.W. Development of a Human Respiratory Mucosa-on-a-chip using Decellularized Extracellular Matrix. BioChip J. 2020, 14, 279–289. [Google Scholar] [CrossRef]
- An, E.-J.; Kim, Y.; Lee, S.-H.; Ko, H.M.; Chung, W.-S.; Jang, H.-J. Anti-cancer potential of Oxialis obtriangulata in pancreatic cancer cell through regulation of the erk/src/stat3-mediated pathway. Molecules 2020, 25, 2301. [Google Scholar] [CrossRef] [PubMed]
- An, E.-J.; Kim, Y.; Lee, S.-H.; Choi, S.-H.; Chung, W.S.; Jang, H.-J. Ophiopogonin D ameliorates DNCB-induced atopic dermatitis-like lesions in BALB/c mice and TNF-α- inflamed HaCaT cell. Biochem. Biophys. Res. Commun. 2020, 522, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-H.; Tong, Y.-W.; Yu, J.-F.; Lei, K.F.; Chen, A.C.-Y. Osteogenesis and Chondrogenesis of Primary Rabbit Periosteal Cells under Non-uniform 2-Axial Tensile Strain. BioChip J. 2020, 14, 438–446. [Google Scholar] [CrossRef]
- Mani, C.S. Acute pneumonia and its complications. In Principles and Practice of Pediatric Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2018; p. 238. [Google Scholar]
- Kolek, V.; Jakubec, P.; Losse, S. Diagnostics and treatment of community-acquired pneumonia—Simplicity is the key to success. Vnitrni Lek. 2017, 63, 770–775. [Google Scholar] [CrossRef]
- Hu, Y.; Yang, H.; Ding, X.; Liu, J.; Wang, X.; Hu, L.; Liu, M.; Zhang, C. Anti-inflammatory octahydroindolizine alkaloid enantiomers from Dendrobium crepidatum. Bioorg. Chem. 2020, 100, 103809. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Goncalves, R.; Mosser, D.M. The Isolation and Characterization of Murine Macrophages. Curr. Protoc. Immunol. 2008, 83, 14.1.1–14.1.14. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Zou, X.-B.; Chai, Y.-F.; Yao, Y.-M. Macrophage Polarization in Inflammatory Diseases. Int. J. Biol. Sci. 2014, 10, 520. [Google Scholar] [CrossRef]
- Montecucco, F.; Mach, F. Common inflammatory mediators orchestrate pathophysiological processes in rheumatoid arthritis and atherosclerosis. Rheumatology 2008, 48, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Raykova, V.D.; Glibetic, M.; Ofenstein, J.P.; Aranda, J.V. Nitric oxide-dependent regulation of pro-inflammatory cytokines in group B streptococcal inflammation of rat lung. Ann. Clin. Lab. Sci. 2003, 33, 62–67. [Google Scholar]
- Echizen, K.; Hirose, O.; Maeda, Y.; Oshima, M. Inflammation in gastric cancer: Interplay of the COX-2/prostaglandin E2and Toll-like receptor/MyD88 pathways. Cancer Sci. 2016, 107, 391–397. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.-Y.; Ju, J.-M.; Mo, L.-H.; Ma, L.; Hu, W.-H.; You, R.-R.; Chen, X.-Q.; Chen, Y.; Liu, Z.-Q.; Qiu, S.-Q.; et al. Anti-inflammation action of xanthones from Swertia chirayita by regulating COX-2/NF-κB/MAPKs/Akt signaling pathways in RAW 264.7 macrophage cells. Phytomedicine 2019, 55, 214–221. [Google Scholar] [CrossRef]
- Bredt, D.S. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic. Res. 1999, 31, 577–596. [Google Scholar] [CrossRef]
- Dai, J.-N.; Zong, Y.; Zhong, L.-M.; Li, Y.-M.; Zhang, W.; Bian, L.-G.; Ai, Q.-L.; Liu, Y.-D.; Sun, J.; Lu, D. Gastro-din inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS ONE 2011, 6, e21891. [Google Scholar] [CrossRef]
- Fosslien, E. Biochemistry of cyclooxygenase (COX)-2 inhibitors and molecular pathology of COX-2 in neoplasia. Crit. Rev. Clin. Lab. Sci. 2000, 37, 431–502. [Google Scholar] [CrossRef]
- Foster, K.A.; Oster, C.G.; Mayer, M.M.; Avery, M.L.; Audus, K.L. Characterization of the A549 Cell Line as a Type II Pulmonary Epithelial Cell Model for Drug Metabolism. Exp. Cell Res. 1998, 243, 359–366. [Google Scholar] [CrossRef]
- Song, L.; Zhu, Y.; Jin, M.; Zang, B. Hydroxysafflor yellow a inhibits lipopolysaccharide-induced inflammatory signal transduction in human alveolar epithelial A549 cells. Fitoterapia 2013, 84, 107–114. [Google Scholar] [CrossRef]
- Lee, I.-S.; Uh, I.; Kim, K.-S.; Kim, K.-H.; Park, J.; Kim, Y.; Jung, J.-H.; Jung, H.-J.; Jang, H.-J. Anti-inflammatory effects of ginsenoside Rg3 via NF-κB pathway in A549 cells and human asthmatic lung tissue. J. Immunol. Res. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Cheng, Y. MicroRNA-1247 inhibits lipopolysaccharides-induced acute pneumonia in A549 cells via targeting CC chemokine ligand 16. Biomed. Pharmacother. 2018, 104, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-D.; Zhou, B. TNF-α/NF-κ B/Snail pathway in cancer cell migration and invasion. Br. J. Cancer 2010, 102, 639–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M. Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-κB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1β-exposed Caco-2 cells. Br. J. Nutr. 2014, 111, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Neurath, M.F.; Finotto, S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011, 22, 83–89. [Google Scholar] [CrossRef]
- Grubek-Jaworska, H.; Paplińska, M.; Hermanowicz-Salamon, J.; Białek-Gosk, K.; Dąbrowska, M.; Grabczak, E.; Domagała-Kulawik, J.; Stępień, J.; Chazan, R. IL-6 and IL-13 in induced sputum of COPD and asthma patients: Correlation with respiratory tests. Respiration 2012, 84, 101–107. [Google Scholar] [CrossRef]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy—From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta Proteins Proteom. BBA Proteins Proteom. 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Wong, J.; Magun, B.; Wood, L. Lung inflammation caused by inhaled toxicants: A review. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1391–1401. [Google Scholar] [CrossRef] [Green Version]
- Renda, T.; Baraldo, S.; Pelaia, G.; Bazzan, E.; Turato, G.; Papi, A.; Maestrelli, P.; Maselli, R.; Vatrella, A.; Fabbri, L.; et al. Increased activation of p38 MAPK in COPD. Eur. Respir. J. 2008, 31, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Suzuki, M.; Tetsuka, T.; Yoshida, S.; Watanabe, N.; Kobayashi, M.; Matsui, N.; Okamoto, T. The role of p38 mitogen-activated protein kinase in IL-6 and IL-8 production from the TNF-α-or IL-1β-stimulated rheumatoid synovial fibroblasts. FEBS Lett. 2000, 465, 23–27. [Google Scholar] [CrossRef] [Green Version]
- Yin, F.; Wang, Y.-Y.; Du, J.-H.; Li, C.; Lu, Z.-Z.; Han, C.; Zhang, Y.-Y. Noncanonical cAMP pathway and p38 MAPK mediate β2-adrenergic receptor-induced IL-6 production in neonatal mouse cardiac fibroblasts. J. Mol. Cell. Cardiol. 2006, 40, 384–393. [Google Scholar] [CrossRef]
- Li, Q.; Verma, I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, Y.; Shen, P. NF-kappaB and its regulation on the immune system. Cell Mol. Immunol. 2004, 1, 343–350. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).