Dexamethasone-Loaded Nanostructured Lipid Carriers for the Treatment of Dry Eye Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Formulation of Nanostructured Lipid Carriers
2.2.2. Encapsulation Efficiency and Formulation Drug Retention
2.2.3. Particle Size and Zeta Potential Measurement
2.2.4. Cytotoxicity Study
2.2.5. Cellular Uptake Study
2.2.6. Ex Vivo Corneal Surface Distribution Study
2.2.7. Cytokine Profiling Using ELISA
2.2.8. Formulation Stability Studies
3. Results and Discussion
3.1. NLC Pre-Formulation Optimisation
3.2. Pilot Scale Batch Formulation of NLCs
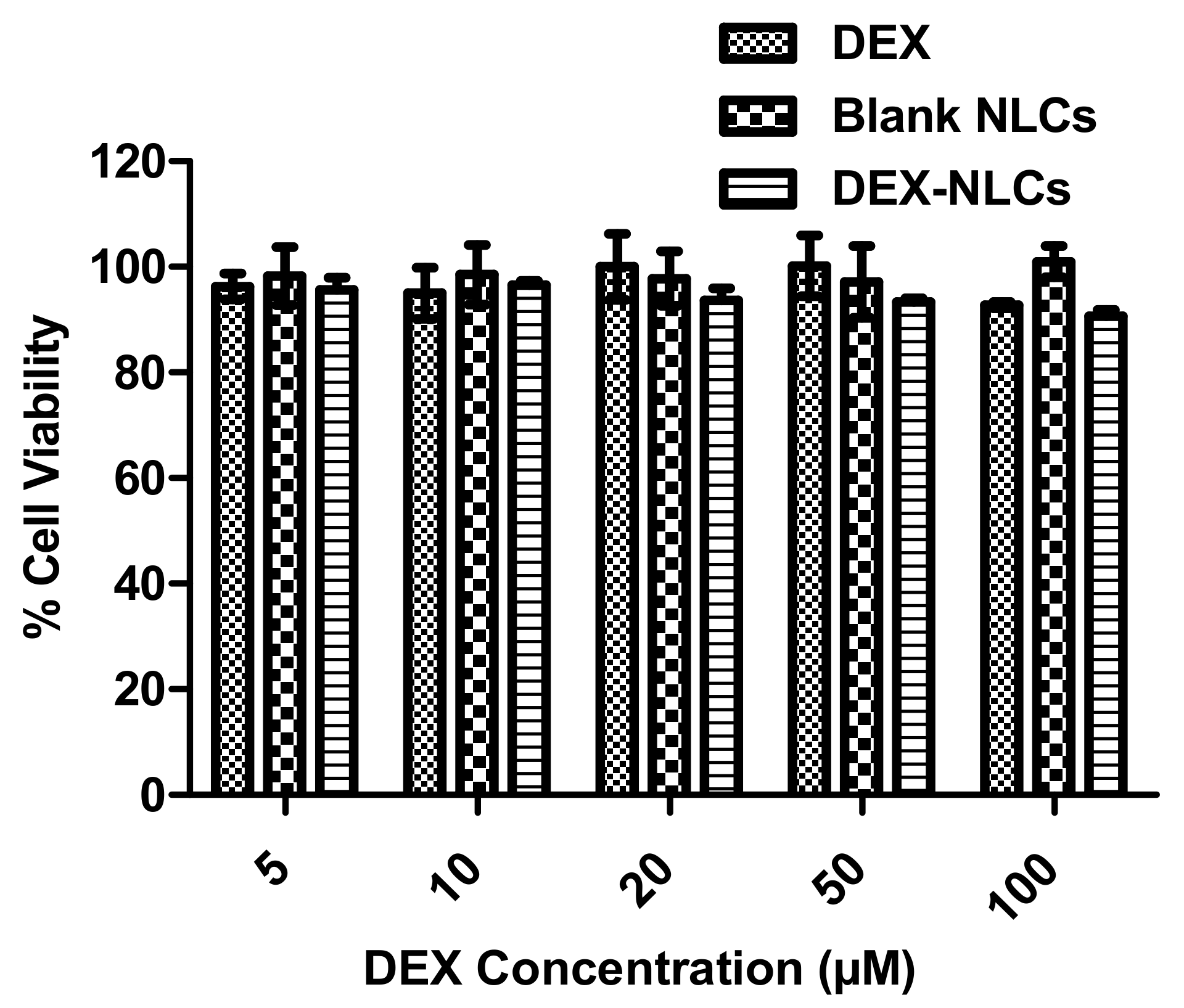
3.2.1. Cytotoxicity of PSF-NLCs on HCECs
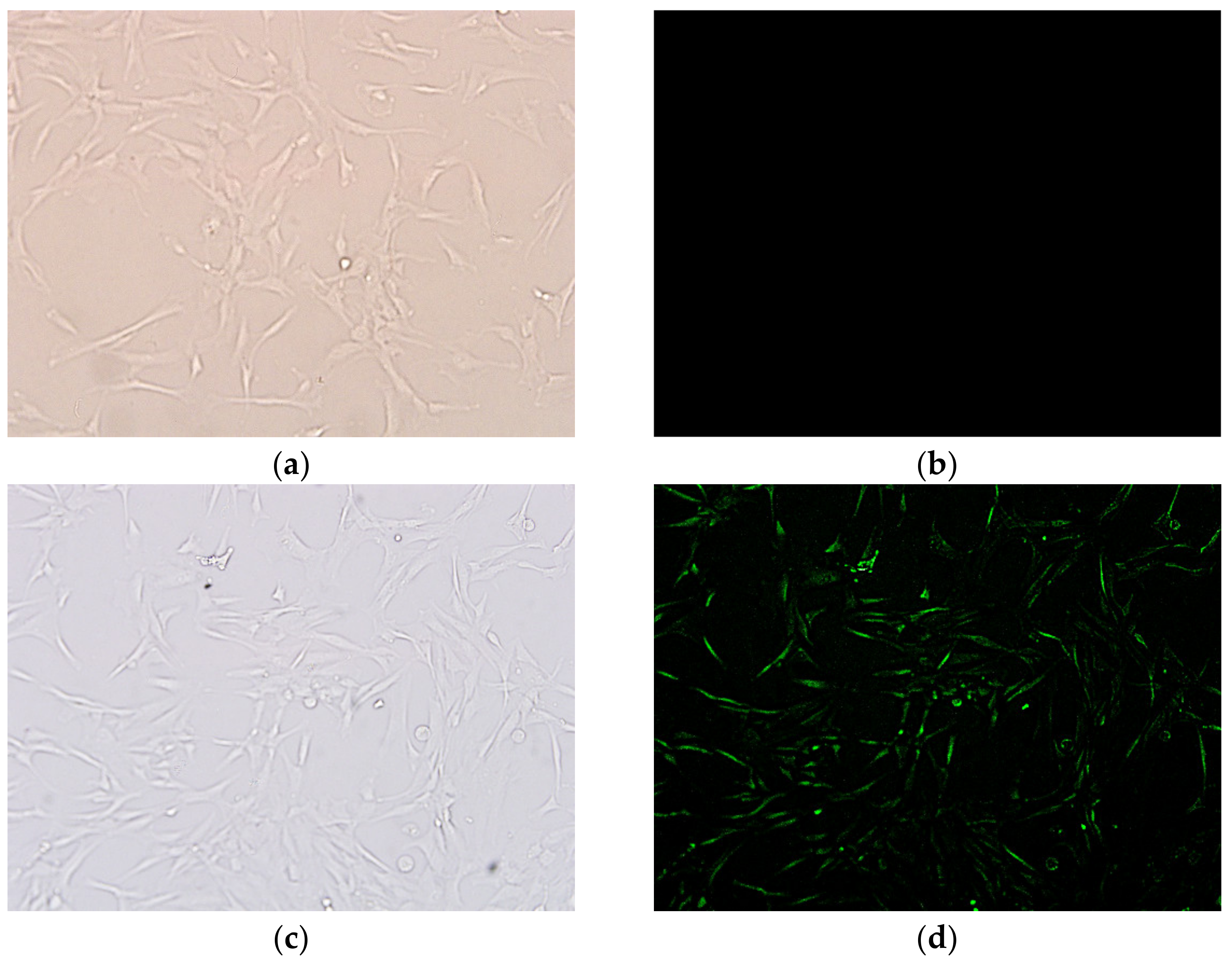
3.2.2. Cellular Internalization
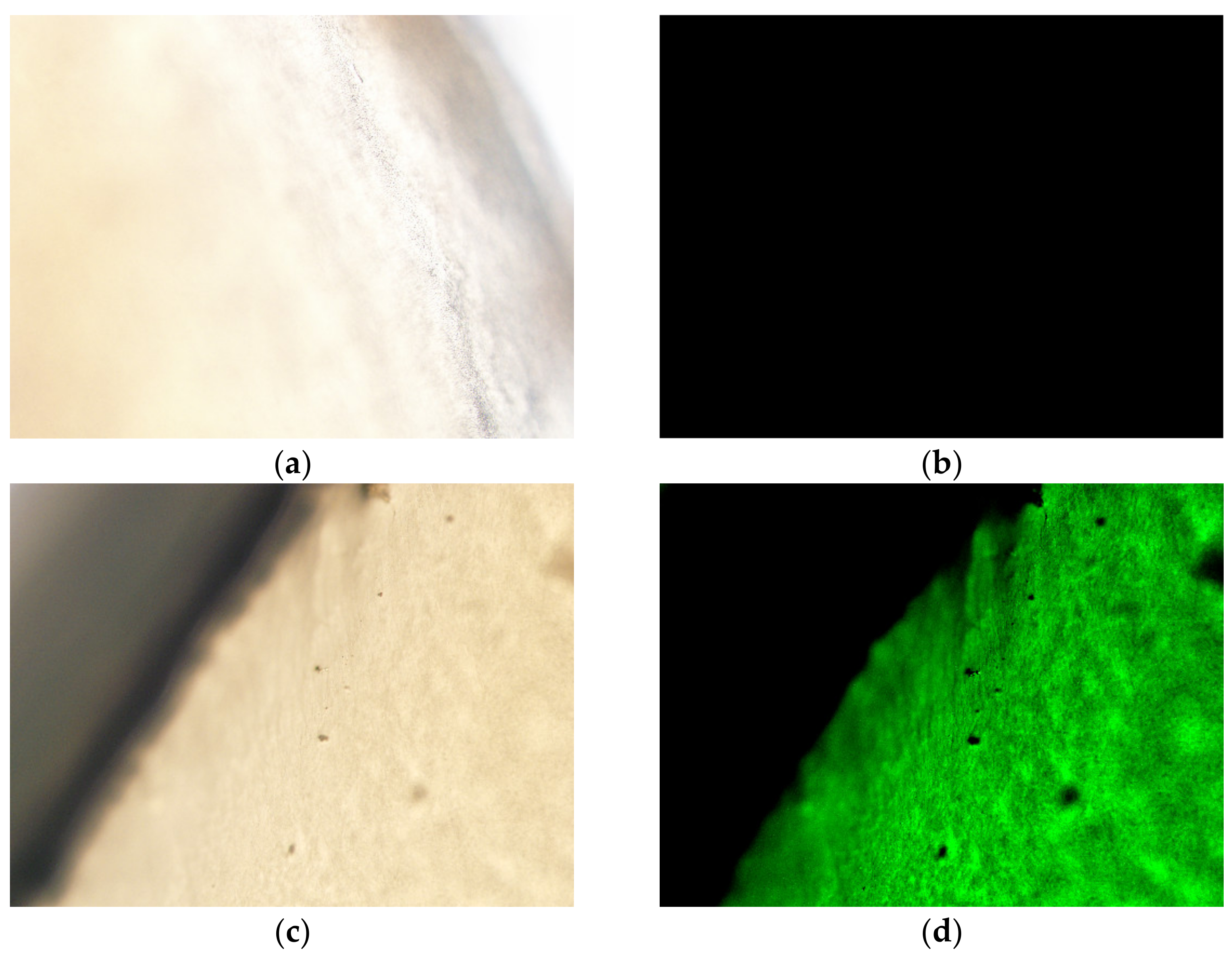
3.2.3. Ex Vivo Distribution
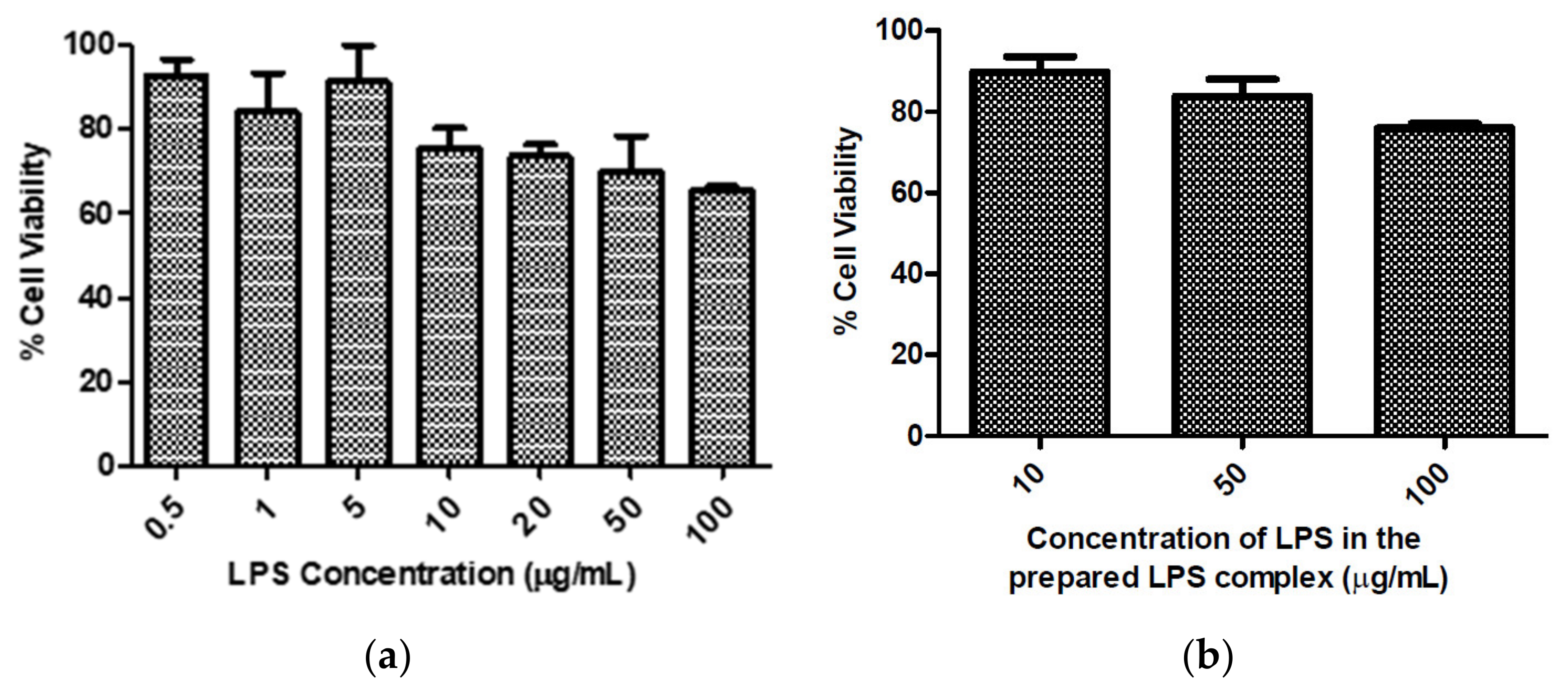
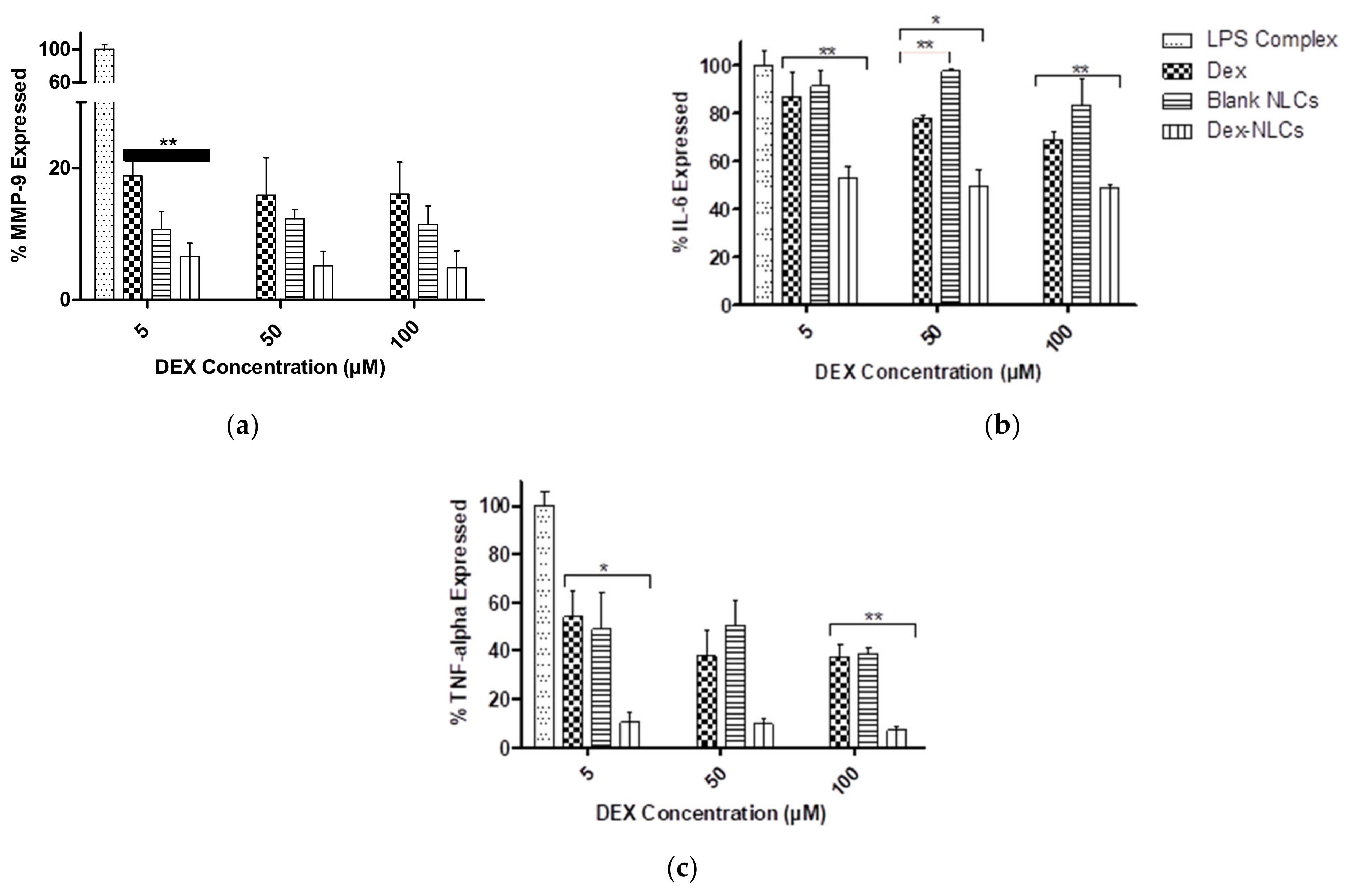
3.3. Evaluation of Cytokine Reduction in LPS-Induced Inflammation in HCECs by DEX-Loaded NLCs
3.4. Storage Stability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- TFOS Report of the International Dry Eye Workshop. Ocul. Surf. 2007, 5, 65–204.
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2019, 17, 842. [Google Scholar] [CrossRef]
- Gaballa, S.A.; Kompella, U.B.; Elgarhy, O.; Alqahtani, A.M.; Pierscionek, B.; Alany, R.G.; Abdelkader, H. Corticosteroids in ophthalmology: Drug delivery innovations, pharmacology, clinical applications, and future perspectives. Drug Deliv. Transl. Res. 2021, 11, 866–893. [Google Scholar] [CrossRef] [PubMed]
- CequaTM for Dry Eye Disease. Available online: https://cequapro.com/ (accessed on 30 December 2020).
- INVELTYS® (Loteprednol Etabonate Ophthalmic Suspension) 1%. Available online: https://inveltys.com/ (accessed on 30 December 2020).
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. Lipid Nanoparticles: Effect on Bioavailability and Pharmacokinetic Changes. Organotypic Models Drug Dev. 2009, 197, 115–141. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Z.; Zhang, C.; Zhang, B. Nanostructured lipid carriers as novel ophthalmic delivery system for mangiferin: Improving in vivo ocular bioavailability. J. Pharm. Sci. 2012, 101, 3833–3844. [Google Scholar] [CrossRef] [PubMed]
- Gasco, M.R. Solid Lipid Microspheres Having a Narrow Size Distribution and Method for Producing Them. European Patent Appl. EP0526666A1, 2 October 1993. [Google Scholar]
- Cavalli, R.; Morel, S.; Gasco, M.; Chetoni, P.; Saettone, M. Preparation and evaluation in vitro of colloidal lipospheres containing pilocarpine as ion pair. Int. J. Pharm. 1995, 117, 243–246. [Google Scholar] [CrossRef]
- Müller, R.; Radtke, M.; Wissing, S. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Sanchez-Lopez, E.; Espina, M.; Doktorovova, S.; Souto, E.B.; Garcia, M.L. Lipid nanoparticles (SLN, NLC): Overcoming the anatomical and physiological barriers of the eye. Part II—Ocular drug-loaded lipid nanoparticles. Eur. J. Pharm. Biopharm. 2017, 110, 58–69. [Google Scholar] [CrossRef]
- De Oliveira, I.F.; Barbosa, E.J.; Peters, M.C.C.; Henostroza, M.A.B.; Yukuyama, M.N.; Neto, E.D.S.; Löbenberg, R.; Bou-Chacra, N. Cutting-edge advances in therapy for the posterior segment of the eye: Solid lipid nanoparticles and nanostructured lipid carriers. Int. J. Pharm. 2020, 589, 119831. [Google Scholar] [CrossRef]
- Selvaraj, K.; Kuppusamy, G.; Krishnamurthy, J.; Mahalingam, R.; Singh, S.K.; Gulati, M. Repositioning of Itraconazole for the Management of Ocular Neovascularization Through Surface-Modified Nanostructured Lipid Carriers. ASSAY Drug Dev. Technol. 2019, 17, 178–190. [Google Scholar] [CrossRef]
- Zadeh, B.S.M.; Niro, H.; Rahim, F.; Esfahani, G. Ocular Delivery System for Propranolol Hydrochloride Based on Nanostructured Lipid Carrier. Sci. Pharm. 2018, 86, 16. [Google Scholar] [CrossRef]
- Lasoń, E.; Sikora, E.; Ogonowski, J. Influence of process parameters on properties of Nanostructured Lipid Carriers (NLC) formulation. Acta Biochim. Pol. 2013, 60, 773–777. [Google Scholar] [CrossRef]
- Kiss, E.L.; Berkó, S.; Gácsi, A.; Kovács, A.; Katona, G.; Soós, J.; Csányi, E.; Gróf, I.; Harazin, A.; Deli, M.A.; et al. Development and Characterization of Potential Ocular Mucoadhesive Nano Lipid Carriers Using Full Factorial Design. Pharmaceutics 2020, 12, 682. [Google Scholar] [CrossRef]
- Tan, G.; Li, J.; Song, Y.; Yu, Y.; Liu, D.; Pan, W. Phenylboronic acid-tethered chondroitin sulfate-based mucoadhesive nanostructured lipid carriers for the treatment of dry eye syndrome. Acta Biomater. 2019, 99, 350–362. [Google Scholar] [CrossRef]
- Mo, Z.; Ban, J.; Zhang, Y.; Du, Y.; Wen, Y.; Huang, X.; Xie, Q.; Shen, L.; Zhang, S.; Deng, H.; et al. Nanostructured lipid carriers-based thermosensitive eye drops for enhanced, sustained delivery of dexamethasone. Nanomedicine 2018, 13, 1239–1253. [Google Scholar] [CrossRef]
- Kuntsche, J.; Bunjes, H. Influence of preparation conditions and heat treatment on the properties of supercooled smectic cholesteryl myristate nanoparticles. Eur. J. Pharm. Biopharm. 2007, 67, 612–620. [Google Scholar] [CrossRef]
- Roda, M.; Corazza, I.; Reggiani, M.L.B.; Pellegrini, M.; Taroni, L.; Giannaccare, G.; Versura, P. Dry Eye Disease and Tear Cytokine Levels—A Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3111. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-Y.; Wang, M.-C.; Chen, Z.-Y.; Chiu, W.-Y.; Chen, K.-H.; Lin, I.-C.; Yang, W.-C.V.; Wu, C.-C.; Tseng, C.-L. Gelatin–epigallocatechin gallate nanoparticles with hyaluronic acid decoration as eye drops can treat rabbit dry-eye syndrome effectively via inflammatory relief. Int. J. Nanomed. 2018, 13, 7251–7273. [Google Scholar] [CrossRef]
- Emami, J.; Rezazadeh, M.; Varshosaz, J.; Tabbakhian, M.; Aslani, A. Formulation of LDL Targeted Nanostructured Lipid Carriers Loaded with Paclitaxel: A Detailed Study of Preparation, Freeze Drying Condition, and In Vitro Cytotoxicity. J. Nanomater. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- EMA. Guideline on the Sterilisation of the Medicinal Product, Active Substance, Excipient and Primary Container; EMA/CHMP/CVMP/QWP/850374/2015; EMA: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Abdelkader, H.; Fathalla, Z.; Moharram, H.; Ali, T.; Pierscionek, B. Cyclodextrin Enhances Corneal Tolerability and Reduces Ocular Toxicity Caused by Diclofenac. Oxidative Med. Cell. Longev. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Nirbhavane, P.; Sharma, G.; Singh, B.; Begum, G.; Jones, M.-C.; Rauz, S.; Vincent, R.; Denniston, A.K.; Hill, L.J.; Katare, O. Triamcinolone acetonide loaded-cationic nano-lipoidal formulation for uveitis: Evidences of improved biopharmaceutical performance and anti-inflammatory activity. Colloids Surf. B Biointerfaces 2020, 190, 110902. [Google Scholar] [CrossRef] [PubMed]
- Garrigue, J.-S.; Amrane, M.; Faure, M.-O.; Holopainen, J.M.; Tong, L. Relevance of Lipid-Based Products in the Management of Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2017, 33, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.S.; Chang, R.-K.; Hussain, M.A. Pharmaceutical Development and Regulatory Considerations for Nanoparticles and Nanoparticulate Drug Delivery Systems. J. Pharm. Sci. 2013, 102, 3867–3882. [Google Scholar] [CrossRef]
- Agrahari, V.; Hiremath, P. Challenges associated and approaches for successful translation of nanomedicines into commercial products. Nanomedicine 2017, 12, 819–823. [Google Scholar] [CrossRef]
- Paliwal, R.; Babu, R.J.; Palakurthi, S. Nanomedicine Scale-up Technologies: Feasibilities and Challenges. AAPS PharmSciTech 2014, 15, 1527–1534. [Google Scholar] [CrossRef]
- Waghule, T.; Rapalli, V.K.; Singhvi, G.; Manchanda, P.; Hans, N.; Dubey, S.K.; Hasnain, M.S.; Nayak, A.K. Voriconazole loaded nanostructured lipid carriers based topical delivery system: QbD based designing, characterization, in-vitro and ex-vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 52, 303–315. [Google Scholar] [CrossRef]
- Geng, Q.; Zhao, Y.; Wang, L.; Xu, L.; Chen, X.; Han, J. Development and Evaluation of Astaxanthin as Nanostructure Lipid Carriers in Topical Delivery. AAPS PharmSciTech 2020, 21, 1–12. [Google Scholar] [CrossRef]
- Naguib, S.S.; Hathout, R.M.; Mansour, S. Optimizing novel penetration enhancing hybridized vesicles for augmenting the in-vivo effect of an anti-glaucoma drug. Drug Deliv. 2017, 24, 99–108. [Google Scholar] [CrossRef]
- Deshkar, S.S.; Jadhav, M.S.; Shirolkar, S.V. Development of Carbamazepine Nanostructured Lipid Carrier Loaded Thermosensitive Gel for Intranasal Delivery. Adv. Pharm. Bull. 2020, 11, 150–162. [Google Scholar] [CrossRef]
- Salopek, B.; Krasic, D.; Filipovic, S. Measurement and Application of Zeta-Potential. Rud. Geol. Naft. Zb. 1992, 4, 147–151. [Google Scholar]
- Araujo, V.H.S.; da Silva, P.B.; Szlachetka, I.O.; da Silva, S.W.; Fonseca-Santos, B.; Chorilli, M.; Ganassin, R.; de Oliveira, G.R.T.; da Rocha, M.C.O.; Fernandes, R.P.; et al. The influence of NLC composition on curcumin loading under a physicochemical perspective and in vitro evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125070. [Google Scholar] [CrossRef]
- Behbahani, E.S.; Ghaedi, M.; Abbaspour, M.; Rostamizadeh, K.; Dashtian, K. Curcumin loaded nanostructured lipid carriers: In vitro digestion and release studies. Polyhedron 2019, 164, 113–122. [Google Scholar] [CrossRef]
- Pinto, F.; de Barros, D.P.; Fonseca, L.P. Design of multifunctional nanostructured lipid carriers enriched with α-tocopherol using vegetable oils. Ind. Crop. Prod. 2018, 118, 149–159. [Google Scholar] [CrossRef]
- González-Fernández, F.; Bianchera, A.; Gasco, P.; Nicoli, S.; Pescina, S. Lipid-Based Nanocarriers for Ophthalmic Administration: Towards Experimental Design Implementation. Pharmaceutics 2021, 13, 447. [Google Scholar] [CrossRef]
- Houacine, C.; Adams, D.; Singh, K. Impact of liquid lipid on development and stability of trimyristin nanostructured lipid carriers for oral delivery of resveratrol. J. Mol. Liq. 2020, 316, 113734. [Google Scholar] [CrossRef]
- Kanwar, R.; Gradzielski, M.; Prevost, S.; Kaur, G.; Appavou, M.-S.; Mehta, S.K. Physicochemical stimuli as tuning parameters to modulate the structure and stability of nanostructured lipid carriers and release kinetics of encapsulated antileprosy drugs. J. Mater. Chem. B 2019, 7, 6539–6555. [Google Scholar] [CrossRef]
- EMA. Note for Guidance on Process Validation, T.E.A.f.t.E.o.M. Products; EMA: London, UK, 2001. [Google Scholar]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part II—Production Scales and Clinically Compliant Production Methods. Nanomaterials 2020, 10, 455. [Google Scholar] [CrossRef]
- Manjunath, K.; Reddy, J.; Venkateswarlu, V. Solid lipid nanoparticles as drug delivery systems. Methods Find. Exp. Clin. Pharmacol. 2005, 27, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.; Costa, C.P.; Loureiro, J.A.; Alves, J.; Peixoto, A.F.; Forbes, B.; Lobo, J.M.S.S.; Silva, A.C. Double Optimization of Rivastigmine-Loaded Nanostructured Lipid Carriers (NLC) for Nose-to-Brain Delivery Using the Quality by Design (QbD) Approach: Formulation Variables and Instrumental Parameters. Pharmaceutics 2020, 12, 599. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: Effect on formulation and characterization parameters. Eur. J. Pharm. Sci. 2015, 78, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.; Calado, R.; Marto, J.; Bettencourt, A.; Almeida, A.J.; Gonçalves, L.M.D. Chitosan Nanoparticles as a Mucoadhesive Drug Delivery System for Ocular Administration. Mar. Drugs 2017, 15, 370. [Google Scholar] [CrossRef]
- Ban, J.; Zhang, Y.; Huang, X.; Deng, G.; Hou, D.; Chen, Y.; Lu, Z. Corneal permeation properties of a charged lipid nanoparticle carrier containing dexamethasone. Int. J. Nanomed. 2017, 12, 1329–1339. [Google Scholar] [CrossRef]
- Rapalli, V.K.; Kaul, V.; Waghule, T.; Gorantla, S.; Sharma, S.; Roy, A.; Dubey, S.K.; Singhvi, G. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: Optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur. J. Pharm. Sci. 2020, 152, 105438. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; Yu, S.; Li, J.; Tan, G.; Li, S.; Pan, W. A Hybrid Genipin-Cross-Linked Hydrogel/Nanostructured Lipid Carrier for Ocular Drug Delivery: Cellular, ex Vivo, and in Vivo Evaluation. ACS Biomater. Sci. Eng. 2020, 6, 1543–1552. [Google Scholar] [CrossRef]
- Sawaguchi, S.; Yue, B.Y.J.T.; Sugar, J.; Gilboy, J.E. Lysosomal Enzyme Abnormalities in Keratoconus. Arch. Ophthalmol. 1989, 107, 1507–1510. [Google Scholar] [CrossRef]
- Chuang, S.T.; Cruz, S.; Narayanaswami, V. Reconfiguring Nature’s Cholesterol Accepting Lipoproteins as Nanoparticle Platforms for Transport and Delivery of Therapeutic and Imaging Agents. Nanomaterials 2020, 10, 906. [Google Scholar] [CrossRef]
- Gokce, E.H.; Sandri, G.; Bonferoni, M.C.; Rossi, S.; Ferrari, F.; Güneri, T.; Caramella, C. Cyclosporine A loaded SLNs: Evaluation of cellular uptake and corneal cytotoxicity. Int. J. Pharm. 2008, 364, 76–86. [Google Scholar] [CrossRef]
- Robciuc, A.; Rantamäki, A.H.; Jauhiainen, M.; Holopainen, J.M. Lipid-Modifying Enzymes in Human Tear Fluid and Corneal Epithelial Stress Response. Investig. Ophthalmol. Vis. Sci. 2014, 55, 16–24. [Google Scholar] [CrossRef]
- Mantelli, F.; Argueso, P. Functions of ocular surface mucins in health and disease. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, J.; Cheng, B.; Wu, Q.; Pan, H. Ex Vivo and in Vivo Evaluation of the Effect of Coating a Coumarin-6-Labeled Nanostructured Lipid Carrier with Chitosan-N-acetylcysteine on Rabbit Ocular Distribution. Mol. Pharm. 2017, 14, 2639–2648. [Google Scholar] [CrossRef]
- Li, H.; Guissi, N.E.I.; Su, Z.; Ping, Q.; Sun, M. Effects of surface hydrophilic properties of PEG-based mucus-penetrating nanostructured lipid carriers on oral drug delivery. RSC Adv. 2016, 6, 84164–84176. [Google Scholar] [CrossRef]
- Hassan, H.; Bello, R.O.; Adam, S.K.; Alias, E.; Meor Mohd Affandi, M.M.R.; Shamsuddin, A.F.; Basir, R. Acyclovir-Loaded Solid Lipid Nanoparticles: Optimization, Characterization and Evaluation of Its Pharmacokinetic Profile. Nanomaterials 2020, 10, 1785. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, H. Upregulation of the IL-33/ST2 pathway in dry eye. Mol. Vis. 2019, 25, 583–592. [Google Scholar]
- Ogawa, Y.; Shimizu, E.; Tsubota, K. Interferons and Dry Eye in Sjogren’s Syndrome. Int. J. Mol. Sci. 2018, 19, 3548. [Google Scholar] [CrossRef]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry Eye Disease an Immune-Mediated Ocular Surface Disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef]
- Choi, W.; Noh, H.; Yeo, A.; Jang, H.; Ahn, H.K.; Song, Y.J.; Lee, H.K. The Effect of TNF-α Blocker HL036337 and Its Best Concentration to Inhibit Dry Eye Inflammation. Korean J. Ophthalmol. 2016, 30, 302–308. [Google Scholar] [CrossRef][Green Version]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear Cytokine Profiles in Dysfunctional Tear Syndrome. Am. J. Ophthalmol. 2009, 147, 198–205. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Yang, L.; Li, M.; Li, B.; Wang, W.; Sheng, M. Decreased PPAR-γ expression in the conjunctiva and increased expression of TNF-α and IL-1β in the conjunctiva and tear fluid of dry eye mice. Mol. Med. Rep. 2014, 9, 2015–2023. [Google Scholar] [CrossRef]
- Yoon, K.-C.; Jeong, I.-Y.; Park, Y.-G.; Yang, S.-Y. Interleukin-6 and Tumor Necrosis Factor-α Levels in Tears of Patients with Dry Eye Syndrome. Cornea 2007, 26, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Pflugfelder, S.C.; Bian, F.; de Paiva, C.S. Matrix metalloproteinase-9 in the pathophysiology and diagnosis of dry eye syndrome. Met. Med. 2017, 4, 37–46. [Google Scholar] [CrossRef]
- Thorley, A.J.; Ford, P.A.; Giembycz, M.A.; Goldstraw, P.; Young, A.; Tetley, T.D. Differential Regulation of Cytokine Release and Leukocyte Migration by Lipopolysaccharide-Stimulated Primary Human Lung Alveolar Type II Epithelial Cells and Macrophages. J. Immunol. 2006, 178, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Vantaku, V.R.; Gupta, G.; Rapalli, K.C.; Karnati, R. Lacritin Salvages Human Corneal Epithelial Cells from Lipopolysaccharide Induced Cell Death. Sci. Rep. 2015, 5, 18362. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.M. Dynamic lipopolysaccharide transfer cascade to TLR4/MD2 complex via LBP and CD. BMB Rep. 2017, 50, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Shin, C.S.; Wang, C.; Pflugfelder, S.C.; Acharya, G.; De Paiva, C.S. Dexamethasone Drug Eluting Nanowafers Control Inflammation in Alkali-Burned Corneas Associated with Dry Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Raffy, S.; Teissie, J. Control of Lipid Membrane Stability by Cholesterol Content. Biophys. J. 1999, 76, 2072–2080. [Google Scholar] [CrossRef]
- Yeagle, P.L. Cholesterol and the Cell-Membrane. Biochim. Biophys. Acta 1985, 822, 267–287. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Widomska, J.; Mainali, L.; Raguz, M. High Cholesterol/Low Cholesterol: Effects in Biological Membranes: A Review. Cell Biophys. 2017, 75, 369–385. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, W.; Gao, X.; Zhang, H.; Kong, N. Inhibition of Zymosan-Induced Inflammatory Factors Expression by ATRA Nanostructured Lipid Carriers. J. Ophthalmol. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Agrahari, V.; Agrahari, V. Facilitating the translation of nanomedicines to a clinical product: Challenges and opportunities. Drug Discov. Today 2018, 23, 974–991. [Google Scholar] [CrossRef]
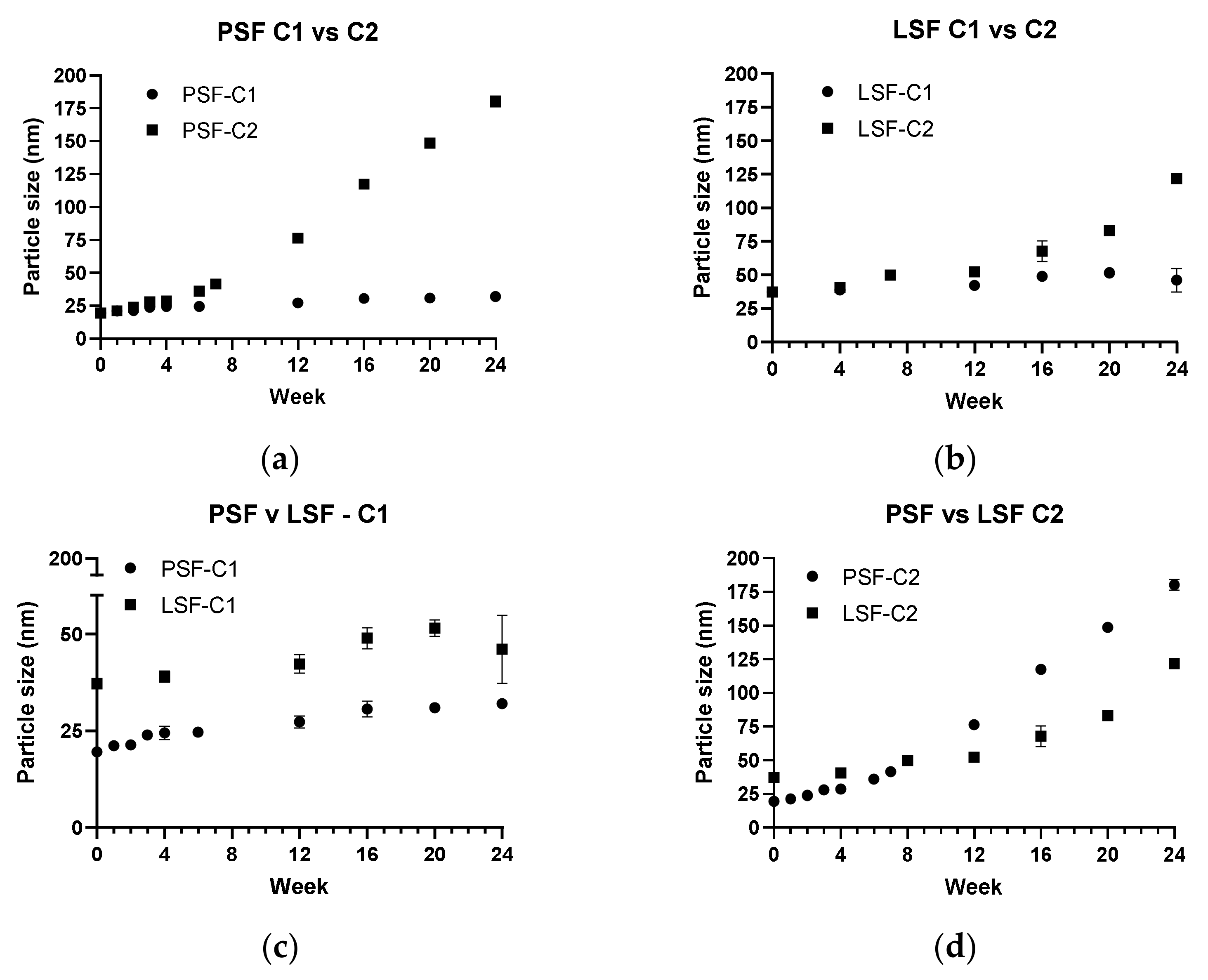
| NLC Batch | Storage Condition |
|---|---|
| LSF4-C1 | 2–8 °C |
| LSF4-C2 | 25 °C/60% RH |
| PSF-C1 | 2–8 °C |
| PSF-C2 | 25 °C/60% RH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumari, S.; Dandamudi, M.; Rani, S.; Behaeghel, E.; Behl, G.; Kent, D.; O’Reilly, N.J.; O’Donovan, O.; McLoughlin, P.; Fitzhenry, L. Dexamethasone-Loaded Nanostructured Lipid Carriers for the Treatment of Dry Eye Disease. Pharmaceutics 2021, 13, 905. https://doi.org/10.3390/pharmaceutics13060905
Kumari S, Dandamudi M, Rani S, Behaeghel E, Behl G, Kent D, O’Reilly NJ, O’Donovan O, McLoughlin P, Fitzhenry L. Dexamethasone-Loaded Nanostructured Lipid Carriers for the Treatment of Dry Eye Disease. Pharmaceutics. 2021; 13(6):905. https://doi.org/10.3390/pharmaceutics13060905
Chicago/Turabian StyleKumari, Sangeeta, Madhuri Dandamudi, Sweta Rani, Elke Behaeghel, Gautam Behl, David Kent, Niall J. O’Reilly, Orla O’Donovan, Peter McLoughlin, and Laurence Fitzhenry. 2021. "Dexamethasone-Loaded Nanostructured Lipid Carriers for the Treatment of Dry Eye Disease" Pharmaceutics 13, no. 6: 905. https://doi.org/10.3390/pharmaceutics13060905
APA StyleKumari, S., Dandamudi, M., Rani, S., Behaeghel, E., Behl, G., Kent, D., O’Reilly, N. J., O’Donovan, O., McLoughlin, P., & Fitzhenry, L. (2021). Dexamethasone-Loaded Nanostructured Lipid Carriers for the Treatment of Dry Eye Disease. Pharmaceutics, 13(6), 905. https://doi.org/10.3390/pharmaceutics13060905





