Potential Applications of Chitosan-Based Nanomaterials to Surpass the Gastrointestinal Physiological Obstacles and Enhance the Intestinal Drug Absorption
Abstract
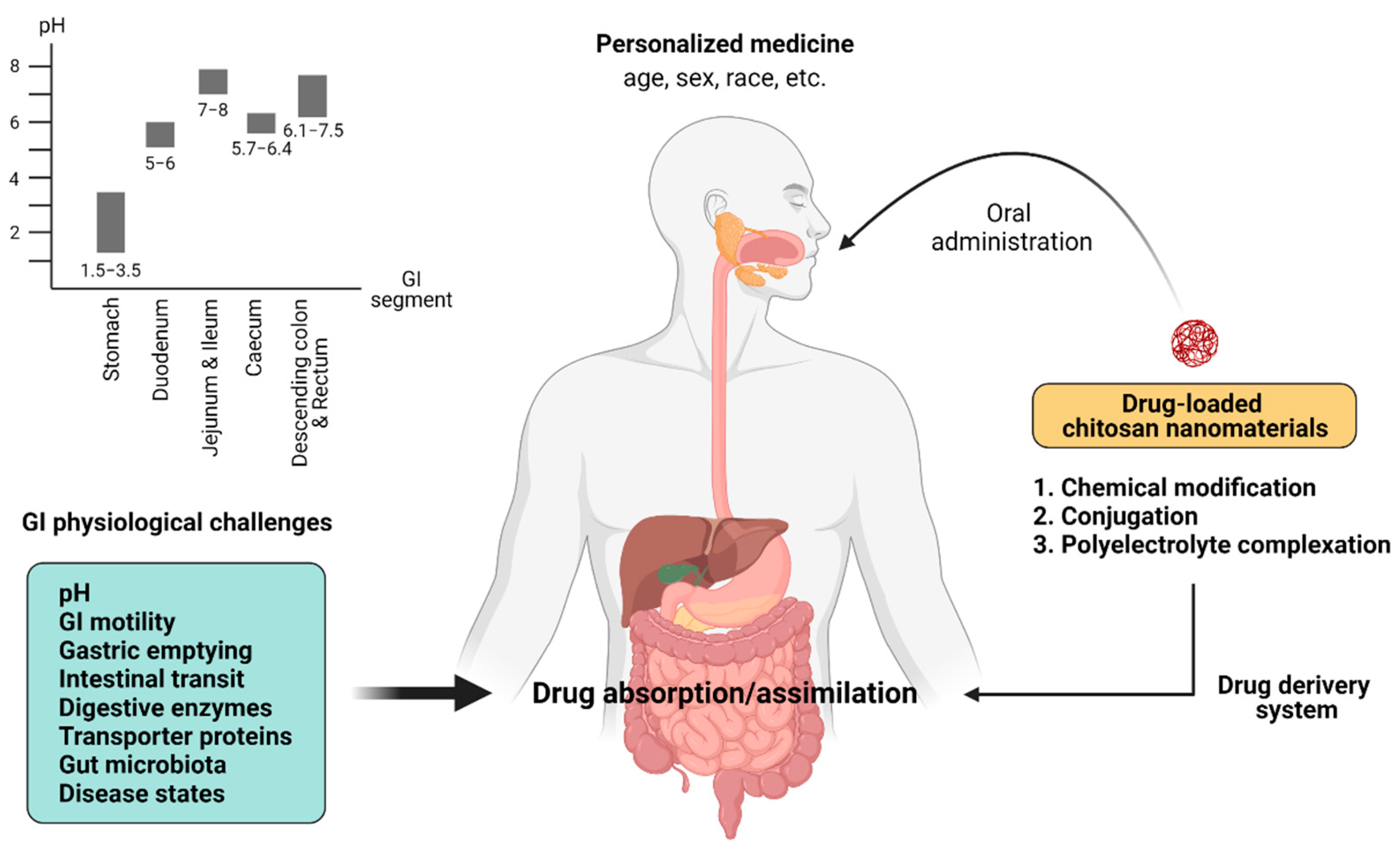
1. Introduction
2. The GI Challenges of Intestinal Drug Absorption
2.1. Gastric pH
2.2. GI Motility
2.3. Transport Proteins and Enzymes
2.4. Gut Microbiota
2.5. Disease Conditions
3. Chitosan-Based Nanomaterials for Improving Intestinal Drug Absorption and Their Pharmacological Applications
3.1. Chitosan-Based Polymeric Nanoparticles with Chemical Modifications
3.2. Conjugated Chitosan
3.3. Chitosan-Based Polyelectrolyte Complex Nanoparticles/Nanocapsules
| Nanocarrier | Drug | Pharmacological Effect(s) | Reference(s) |
|---|---|---|---|
| 1. Chemical modification | |||
| 1.1 Thiolated chitosan | Insulin | mucoadhesion, permeation enhancement, controlled drug release | [118] |
| Docetaxel | mucoadhesion, permeation enhancement, controlled drug release, efflux inhibition | [148] | |
| α-mangostin | mucoadhesion, controlled drug release | [149] | |
| LMWH | protection against GI luminal degradation, mucoadhesion, permeation enhancement, controlled drug release | [150] | |
| Leuprolide | mucoadhesion, permeation enhancement | [154] | |
| 1.2 Trimethyl chitosan (TMC) | Insulin | mucoadhesion, permeation enhancement, controlled drug release | [160] |
| Paclitaxel | mucoadhesion, permeation enhancement, controlled drug release | [161] | |
| Calcitonin | mucoadhesion, permeation enhancement, prolongation of residence time | [162] | |
| OPBP-1 | mucoadhesion, permeation enhancement, controlled drug release | [163] | |
| Curcumin | mucoadhesion, permeation enhancement, controlled drug release | [164,165,166] | |
| 1.3 Carboxymethyl chitosan | Doxorubicin | mucoadhesion, permeation enhancement, controlled drug release, efflux inhibition | [131,132,133,169,170] |
| Clarithromycin | controlled drug release, prolongation of residence time | [171] | |
| 5-FU | controlled drug release | [192] | |
| Curcumin | mucoadhesion, controlled drug release, efflux inhibition | [85,165] | |
| Omeprazole | protection against gastric degradation, controlled drug release | [172] | |
| 2. Conjugation | |||
| Poly(vinyl alcohol) (PVA) | Ascorbic acid | controlled drug release | [173] |
| Poly(γ-glutamic acid) (PGA | Insulin | protection against GI luminal degradation, mucoadhsion, permeation enhancement | [175,176] |
| Poly(ethylene glycol) (PEG) | Insulin | mucoadhsion, permeation enhancement, controlled drug release | [197] |
| BSA | mucoadhsion, permeation enhancement, controlled drug release | [190] | |
| 3. Polyelectrolyte complexation | Insulin | protection against GI luminal degradation, mucoadhesion, permeation enhancement, controlled drug release | [119,197] |
| Doxorubicin | mucoadhesion, permeation enhancement, controlled drug release, efflux inhibition | [131,132,133,169,170] | |
| 5-FU | controlled drug release | [192] | |
| Quercetin | protection against GI luminal degradation, controlled drug release | [193] | |
| Curcumin | mucoadhesion, controlled drug release | [165,191,195] | |
| Rutin | mucoadhesion, permeation enhancement, controlled drug release | [194] | |
| Gentamicin | mucoadhesion, permeation enhancement, controlled drug release | [134] | |
| Paracetamol | permeation enhancement, controlled drug release | [137] | |
| Ibuprofen | controlled drug release | [138] | |
| Omeprazole | protection against GI luminal degradation, controlled drug release | [172] | |
| Furosemide | mucoadhesion, permeation enhancement, controlled drug release | [189] | |
| Theophylline | controlled drug release | [196] | |
| Delafloxacin | controlled drug release | [200] | |
| Ciprofloxacin | efflux inhibition | [201] | |
| Tobramycin | mucoadhesion, permeation enhancement | [202] | |
4. Future Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khafagy, E.-S.; Morishita, M. Oral biodrug delivery using cell-penetrating peptide. Adv. Drug Deliv. Rev. 2012, 64, 531–539. [Google Scholar] [CrossRef]
- Chen, M.C.; Mi, F.L.; Liao, Z.X.; Hsiao, C.W.; Sonaje, K.; Chung, M.F.; Hsu, L.W.; Sung, H.W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef]
- Hatton, G.B.; Madla, C.M.; Rabbie, S.C.; Basit, A.W. Gut reaction: Impact of systemic diseases on gastrointestinal physiology and drug absorption. Drug Discov. Today 2019, 24, 417–427. [Google Scholar] [CrossRef]
- Lang, X.; Wang, T.; Sun, M.; Chen, X.; Liu, Y. Advances and applications of chitosan-based nanomaterials as oral delivery carriers: A review. Int. J. Biol. Macromol. 2020, 154, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Bajracharya, R.; Song, J.G.; Back, S.Y.; Han, H.K. Recent Advancements in Non-Invasive Formulations for Protein Drug Delivery. Comput. Struct. Biotechnol. J. 2019, 17, 1290–1308. [Google Scholar] [CrossRef]
- Sung, Y.K.; Kim, S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Grenha, A. Chitosan nanoparticles: A survey of preparation methods. J. Drug Target. 2012, 20, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Sun, Y.; Wu, Y. Advances in chitosan-based drug delivery vehicles. Nanoscale 2013, 5, 3103–3111. [Google Scholar] [CrossRef]
- Debotton, N.; Dahan, A. A mechanistic approach to understanding oral drug absorption in pediatrics: An overview of fundamentals. Drug Discov. Today 2014, 19, 1322–1336. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Roberts, M.S. Challenges and innovations of drug delivery in older age. Adv. Drug Deliv. Rev. 2018, 135, 3–38. [Google Scholar] [CrossRef]
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex differences in drug disposition. J. Biomed. Biotechnol. 2011, 2011, 187103. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Matera, M.G.; Hanania, N.A.; Rogliani, P. How does race/ethnicity influence pharmacological response to asthma therapies? Expert Opin. Drug Metab. Toxicol. 2018, 14, 435–446. [Google Scholar] [CrossRef]
- Williams, K.; Thomson, D.; Seto, I.; Contopoulos-Ioannidis, D.G.; Ioannidis, J.P.; Curtis, S.; Constantin, E.; Batmanabane, G.; Hartling, L.; Klassen, T. Standard 6: Age groups for pediatric trials. Pediatrics 2012, 129 (Suppl. 3), S153–S160. [Google Scholar] [CrossRef]
- Klotz, U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 2009, 41, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–157. [Google Scholar] [CrossRef]
- Messing, K.; Mager Stellman, J. Sex, gender and women’s occupational health: The importance of considering mechanism. Environ. Res. 2006, 101, 149–162. [Google Scholar] [CrossRef]
- Yasuda, S.U.; Zhang, L.; Huang, S.M. The role of ethnicity in variability in response to drugs: Focus on clinical pharmacology studies. Clin. Pharmacol. Ther. 2008, 84, 417–423. [Google Scholar] [CrossRef]
- Huang, T.; Shu, Y.; Cai, Y.D. Genetic differences among ethnic groups. BMC Genom. 2015, 16, 1093. [Google Scholar] [CrossRef]
- Pontoriero, A.C.; Trinks, J.; Hulaniuk, M.L.; Caputo, M.; Fortuny, L.; Pratx, L.B.; Frías, A.; Torres, O.; Nuñez, F.; Gadano, A.; et al. Influence of ethnicity on the distribution of genetic polymorphisms associated with risk of chronic liver disease in South American populations. BMC Genet. 2015, 16, 93. [Google Scholar] [CrossRef]
- Stillhart, C.; Vučićević, K.; Augustijns, P.; Basit, A.W.; Batchelor, H.; Flanagan, T.R.; Gesquiere, I.; Greupink, R.; Keszthelyi, D.; Koskinen, M.; et al. Impact of gastrointestinal physiology on drug absorption in special populations—An UNGAP review. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2020, 147, 105280. [Google Scholar] [CrossRef]
- Liu, L.; Yao, W.; Rao, Y.; Lu, X.; Gao, J. pH-Responsive carriers for oral drug delivery: Challenges and opportunities of current platforms. Drug Deliv. 2017, 24, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Manallack, D.T. The pK(a) Distribution of Drugs: Application to Drug Discovery. Perspect. Med. Chem. 2007, 1, 25–38. [Google Scholar]
- Kataoka, M.; Fukahori, M.; Ikemura, A.; Kubota, A.; Higashino, H.; Sakuma, S.; Yamashita, S. Effects of gastric pH on oral drug absorption: In vitro assessment using a dissolution/permeation system reflecting the gastric dissolution process. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2016, 101, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Kesisoglou, F. Impaired drug absorption due to high stomach pH: A review of strategies for mitigation of such effect to enable pharmaceutical product development. Mol. Pharm. 2013, 10, 3970–3979. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Bertz, R.; Ren, S.; Boulton, D.W.; Någård, M. A Systematic Review of Gastric Acid-Reducing Agent-Mediated Drug-Drug Interactions with Orally Administered Medications. Clin. Pharmacokinet. 2020, 59, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wu, F.; Lee, S.C.; Zhao, H.; Zhang, L. pH-dependent drug-drug interactions for weak base drugs: Potential implications for new drug development. Clin. Pharmacol. Ther. 2014, 96, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Neal-Kluever, A.; Fisher, J.; Grylack, L.; Kakiuchi-Kiyota, S.; Halpern, W. Physiology of the Neonatal Gastrointestinal System Relevant to the Disposition of Orally Administered Medications. Drug Metab. Dispos. Biol. Fate Chem. 2019, 47, 296–313. [Google Scholar] [CrossRef]
- van den Anker, J.; Reed, M.D.; Allegaert, K.; Kearns, G.L. Developmental Changes in Pharmacokinetics and Pharmacodynamics. J. Clin. Pharmacol. 2018, 58 (Suppl. 10), S10–S25. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.D. Sex and racial differences in pharmacological response: Effect of route of administration and drug delivery system on pharmacokinetics. J. Women Health 2005, 14, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Sansone-Parsons, A.; Krishna, G.; Simon, J.; Soni, P.; Kantesaria, B.; Herron, J.; Stoltz, R. Effects of age, gender, and race/ethnicity on the pharmacokinetics of posaconazole in healthy volunteers. Antimicrob. Agents Chemother. 2007, 51, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- TM, M.W.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Botelho da Silva, S.; Krolicka, M.; van den Broek, L.A.M.; Frissen, A.E.; Boeriu, C.G. Water-soluble chitosan derivatives and pH-responsive hydrogels by selective C-6 oxidation mediated by TEMPO-laccase redox system. Carbohydr. Polym. 2018, 186, 299–309. [Google Scholar] [CrossRef]
- Dhaliwal, S.; Jain, S.; Singh, H.P.; Tiwary, A.K. Mucoadhesive microspheres for gastroretentive delivery of acyclovir: In vitro and in vivo evaluation. AAPS J. 2008, 10, 322–330. [Google Scholar] [CrossRef]
- Bonengel, S.; Bernkop-Schnürch, A. Thiomers—From bench to market. J. Control Release Off. J. Control Release Soc. 2014, 195, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Park, T.E.; Reesor, E.; Cherukula, K.; Hasan, A.; Firdous, J.; Singh, B.; Kang, S.K.; Choi, Y.J.; Park, I.K.; et al. Mucoadhesive Chitosan Derivatives as Novel Drug Carriers. Curr. Pharm. Des. 2015, 21, 4285–4309. [Google Scholar] [CrossRef]
- Kuo, P.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Pathophysiology and management of diabetic gastropathy: A guide for endocrinologists. Drugs 2007, 67, 1671–1687. [Google Scholar] [CrossRef]
- Huang, W.; Lee, S.L.; Yu, L.X. Mechanistic approaches to predicting oral drug absorption. AAPS J. 2009, 11, 217–224. [Google Scholar] [CrossRef]
- Shekhawat, P.B.; Pokharkar, V.B. Understanding peroral absorption: Regulatory aspects and contemporary approaches to tackling solubility and permeability hurdles. Acta Pharm. Sin. B 2017, 7, 260–280. [Google Scholar] [CrossRef] [PubMed]
- Back, H.M.; Song, B.; Pradhan, S.; Chae, J.W.; Han, N.; Kang, W.; Chang, M.J.; Zheng, J.; Kwon, K.I.; Karlsson, M.O.; et al. A mechanism-based pharmacokinetic model of fenofibrate for explaining increased drug absorption after food consumption. BMC Pharmacol. Toxicol. 2018, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Sarosiek, I.; Selover, K.H.; Katz, L.A.; Semler, J.R.; Wilding, G.E.; Lackner, J.M.; Sitrin, M.D.; Kuo, B.; Chey, W.D.; Hasler, W.L.; et al. The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment. Pharmacol. Ther. 2010, 31, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Roland, B.C.; Ciarleglio, M.M.; Clarke, J.O.; Semler, J.R.; Tomakin, E.; Mullin, G.E.; Pasricha, P.J. Small Intestinal Transit Time Is Delayed in Small Intestinal Bacterial Overgrowth. J. Clin. Gastroenterol. 2015, 49, 571–576. [Google Scholar] [CrossRef]
- Koziolek, M.; Grimm, M.; Garbacz, G.; Kühn, J.P.; Weitschies, W. Intragastric volume changes after intake of a high-caloric, high-fat standard breakfast in healthy human subjects investigated by MRI. Mol. Pharm. 2014, 11, 1632–1639. [Google Scholar] [CrossRef]
- Koziolek, M.; Garbacz, G.; Neumann, M.; Weitschies, W. Simulating the postprandial stomach: Physiological considerations for dissolution and release testing. Mol. Pharm. 2013, 10, 1610–1622. [Google Scholar] [CrossRef]
- Grimm, M.; Koziolek, M.; Kühn, J.P.; Weitschies, W. Interindividual and intraindividual variability of fasted state gastric fluid volume and gastric emptying of water. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2018, 127, 309–317. [Google Scholar] [CrossRef]
- Mudie, D.M.; Murray, K.; Hoad, C.L.; Pritchard, S.E.; Garnett, M.C.; Amidon, G.L.; Gowland, P.A.; Spiller, R.C.; Amidon, G.E.; Marciani, L. Quantification of gastrointestinal liquid volumes and distribution following a 240 mL dose of water in the fasted state. Mol. Pharm. 2014, 11, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zheng, Q.S.; Li, G.F. Similarities and differences in gastrointestinal physiology between neonates and adults: A physiologically based pharmacokinetic modeling perspective. AAPS J. 2014, 16, 1162–1166. [Google Scholar] [CrossRef] [PubMed]
- Allegaert, K.; van den Anker, J. Neonatal drug therapy: The first frontier of therapeutics for children. Clin. Pharmacol. Ther. 2015, 98, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Moyer, A.M.; Matey, E.T.; Miller, V.M. Individualized medicine: Sex, hormones, genetics, and adverse drug reactions. Pharmacol. Res. Perspect. 2019, 7, e00541. [Google Scholar] [CrossRef] [PubMed]
- Whitley, H.; Lindsey, W. Sex-based differences in drug activity. Am. Fam. Phys. 2009, 80, 1254–1258. [Google Scholar]
- Torrado, S.; Prada, P.; de la Torre, P.M.; Torrado, S. Chitosan-poly(acrylic) acid polyionic complex: In vivo study to demonstrate prolonged gastric retention. Biomaterials 2004, 25, 917–923. [Google Scholar] [CrossRef]
- Modi, J.; Joshi, G.; Sawant, K. Chitosan based mucoadhesive nanoparticles of ketoconazole for bioavailability enhancement: Formulation, optimization, in vitro and ex vivo evaluation. Drug Dev. Ind. Pharm. 2013, 39, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Deeley, R.G.; Cole, S.P. Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol. Appl. Pharmacol. 2005, 204, 216–237. [Google Scholar] [CrossRef]
- Gutmann, H.; Hruz, P.; Zimmermann, C.; Beglinger, C.; Drewe, J. Distribution of breast cancer resistance protein (BCRP/ABCG2) mRNA expression along the human GI tract. Biochem. Pharmacol. 2005, 70, 695–699. [Google Scholar] [CrossRef]
- Stappaerts, J.; Annaert, P.; Augustijns, P. Site dependent intestinal absorption of darunavir and its interaction with ketoconazole. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 49, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.A.; Jones, C.R.; Ungell, A.L.; Hatley, O.J. Predicting Drug Extraction in the Human Gut Wall: Assessing Contributions from Drug Metabolizing Enzymes and Transporter Proteins using Preclinical Models. Clin. Pharmacokinet. 2016, 55, 673–696. [Google Scholar] [CrossRef]
- Gameiro, M.; Silva, R.; Rocha-Pereira, C.; Carmo, H.; Carvalho, F.; Bastos, M.L.; Remião, F. Cellular Models and In Vitro Assays for the Screening of modulators of P-gp, MRP1 and BCRP. Molecules 2017, 22, 600. [Google Scholar] [CrossRef] [PubMed]
- Estudante, M.; Morais, J.G.; Soveral, G.; Benet, L.Z. Intestinal drug transporters: An overview. Adv. Drug Deliv. Rev. 2013, 65, 1340–1356. [Google Scholar] [CrossRef]
- Hilgendorf, C.; Ahlin, G.; Seithel, A.; Artursson, P.; Ungell, A.L.; Karlsson, J. Expression of thirty-six drug transporter genes in human intestine, liver, kidney, and organotypic cell lines. Drug Metab. Dispos. Biol. Fate Chem. 2007, 35, 1333–1340. [Google Scholar] [CrossRef]
- Murakami, T.; Takano, M. Intestinal efflux transporters and drug absorption. Expert Opin. Drug Metab. Toxicol. 2008, 4, 923–939. [Google Scholar] [CrossRef]
- Grandvuinet, A.S.; Steffansen, B. Interactions between organic anions on multiple transporters in Caco-2 cells. J. Pharm. Sci. 2011, 100, 3817–3830. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Arias, I.M. Intracellular trafficking of P-glycoprotein. Int. J. Biochem. Cell Biol. 2012, 44, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lindquist, S.; Lowe, M.; Noppa, L.; Hernell, O. Bile salt-stimulated lipase and pancreatic lipase-related protein 2 are the dominating lipases in neonatal fat digestion in mice and rats. Pediatric Res. 2007, 62, 537–541. [Google Scholar] [CrossRef]
- Ianiro, G.; Pecere, S.; Giorgio, V.; Gasbarrini, A.; Cammarota, G. Digestive Enzyme Supplementation in Gastrointestinal Diseases. Curr. Drug Metab. 2016, 17, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Li, Y. Interplay of Drug-Metabolizing Enzymes and Transporters in Drug Absorption and Disposition. Curr. Drug Metab. 2014, 15, 915–941. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Trzoss, L.; Yang, D.; Yan, B. Ontogenic expression of human carboxylesterase-2 and cytochrome P450 3A4 in liver and duodenum: Postnatal surge and organ-dependent regulation. Toxicology 2015, 330, 55–61. [Google Scholar] [CrossRef]
- Brussee, J.M.; Yu, H.; Krekels, E.H.J.; de Roos, B.; Brill, M.J.E.; van den Anker, J.N.; Rostami-Hodjegan, A.; de Wildt, S.N.; Knibbe, C.A.J. First-Pass CYP3A-Mediated Metabolism of Midazolam in the Gut Wall and Liver in Preterm Neonates. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 374–383. [Google Scholar] [CrossRef]
- Kearns, G.L.; Abdel-Rahman, S.M.; Alander, S.W.; Blowey, D.L.; Leeder, J.S.; Kauffman, R.E. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N. Engl. J. Med. 2003, 349, 1157–1167. [Google Scholar] [CrossRef]
- Takashima, T.; Yokoyama, C.; Mizuma, H.; Yamanaka, H.; Wada, Y.; Onoe, K.; Nagata, H.; Tazawa, S.; Doi, H.; Takahashi, K.; et al. Developmental changes in P-glycoprotein function in the blood-brain barrier of nonhuman primates: PET study with R-11C-verapamil and 11C-oseltamivir. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2011, 52, 950–957. [Google Scholar] [CrossRef]
- Lam, J.; Koren, G. P-glycoprotein in the developing human brain: A review of the effects of ontogeny on the safety of opioids in neonates. Ther. Drug Monit. 2014, 36, 699–705. [Google Scholar] [CrossRef]
- Toornvliet, R.; van Berckel, B.N.; Luurtsema, G.; Lubberink, M.; Geldof, A.A.; Bosch, T.M.; Oerlemans, R.; Lammertsma, A.A.; Franssen, E.J. Effect of age on functional P-glycoprotein in the blood-brain barrier measured by use of (R)-[(11)C]verapamil and positron emission tomography. Clin. Pharmacol. Ther. 2006, 79, 540–548. [Google Scholar] [CrossRef]
- Marzolini, C.; Tirona, R.G.; Kim, R.B. Pharmacogenomics of the OATP and OAT families. Pharmacogenomics 2004, 5, 273–282. [Google Scholar] [CrossRef]
- Shah, R.R.; Gaedigk, A.; LLerena, A.; Eichelbaum, M.; Stingl, J.; Smith, R.L. CYP450 genotype and pharmacogenetic association studies: A critical appraisal. Pharmacogenomics 2016, 17, 259–275. [Google Scholar] [CrossRef]
- Hoffmeyer, S.; Burk, O.; von Richter, O.; Arnold, H.P.; Brockmöller, J.; Johne, A.; Cascorbi, I.; Gerloff, T.; Roots, I.; Eichelbaum, M.; et al. Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 3473–3478. [Google Scholar] [CrossRef] [PubMed]
- Cascorbi, I.; Gerloff, T.; Johne, A.; Meisel, C.; Hoffmeyer, S.; Schwab, M.; Schaeffeler, E.; Eichelbaum, M.; Brinkmann, U.; Roots, I. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin. Pharmacol. Ther. 2001, 69, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Sansone-Parsons, A.; Krishna, G.; Calzetta, A.; Wexler, D.; Kantesaria, B.; Rosenberg, M.A.; Saltzman, M.A. Effect of a nutritional supplement on posaconazole pharmacokinetics following oral administration to healthy volunteers. Antimicrob. Agents Chemother. 2006, 50, 1881–1883. [Google Scholar] [CrossRef]
- Vendelbo, J.; Olesen, R.H.; Lauridsen, J.K.; Rungby, J.; Kleinman, J.E.; Hyde, T.M.; Larsen, A. Increasing BMI is associated with reduced expression of P-glycoprotein (ABCB1 gene) in the human brain with a stronger association in African Americans than Caucasians. Pharm. J. 2018, 18, 121–126. [Google Scholar] [CrossRef]
- Hu, K.; Xie, X.; Zhao, Y.N.; Li, Y.; Ruan, J.; Li, H.R.; Jin, T.; Yang, X.L. Chitosan Influences the Expression of P-gp and Metabolism of Norfloxacin in Grass Carp. J. Aquat. Anim. Health 2015, 27, 104–111. [Google Scholar] [CrossRef]
- Ni, J.; Tian, F.; Dahmani, F.Z.; Yang, H.; Yue, D.; He, S.; Zhou, J.; Yao, J. Curcumin-carboxymethyl chitosan (CNC) conjugate and CNC/LHR mixed polymeric micelles as new approaches to improve the oral absorption of P-gp substrate drugs. Drug Deliv. 2016, 23, 3424–3435. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Fu, Y.; Li, J.; Yu, X.; Li, Y.; Wang, Y.; Wu, X.; Zhang, K.; Kong, M.; Feng, C.; et al. Multifunctional quercetin conjugated chitosan nano-micelles with P-gp inhibition and permeation enhancement of anticancer drug. Carbohydr. Polym. 2019, 203, 10–18. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 2014, 35, 7654–7665. [Google Scholar] [CrossRef] [PubMed]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, G.P.; Lee, S.M.; Mazmanian, S.K. Gut biogeography of the bacterial microbiota. Nat. Rev. Microbiol. 2016, 14, 20–32. [Google Scholar] [CrossRef]
- Kim, S.; Covington, A.; Pamer, E.G. The intestinal microbiota: Antibiotics, colonization resistance, and enteric pathogens. Immunol. Rev. 2017, 279, 90–105. [Google Scholar] [CrossRef]
- Noh, K.; Kang, Y.R.; Nepal, M.R.; Shakya, R.; Kang, M.J.; Kang, W.; Lee, S.; Jeong, H.G.; Jeong, T.C. Impact of gut microbiota on drug metabolism: An update for safe and effective use of drugs. Arch. Pharmacal Res. 2017, 40, 1345–1355. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; Wang, R. Gut microbiota modulates drug pharmacokinetics. Drug Metab. Rev. 2018, 50, 357–368. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Zhang, S.H.; Wang, Y.Z.; Meng, F.Y.; Li, Y.L.; Li, C.X.; Duan, F.P.; Wang, Q.; Zhang, X.T.; Zhang, C.N. Studies of the microbial metabolism of flavonoids extracted from the leaves of Diospyros kaki by intestinal bacteria. Arch. Pharmacal Res. 2015, 38, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Tozaki, H.; Emi, Y.; Horisaka, E.; Fujita, T.; Yamamoto, A.; Muranishi, S. Degradation of insulin and calcitonin and their protection by various protease inhibitors in rat caecal contents: Implications in peptide delivery to the colon. J. Pharm. Pharmacol. 1997, 49, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Clayton, T.A.; Baker, D.; Lindon, J.C.; Everett, J.R.; Nicholson, J.K. Pharmacometabonomic identification of a significant host-microbiome metabolic interaction affecting human drug metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 14728–14733. [Google Scholar] [CrossRef]
- Saitta, K.S.; Zhang, C.; Lee, K.K.; Fujimoto, K.; Redinbo, M.R.; Boelsterli, U.A. Bacterial β-glucuronidase inhibition protects mice against enteropathy induced by indomethacin, ketoprofen or diclofenac: Mode of action and pharmacokinetics. Xenobiotica Fate Foreign Compd. Biol. Syst. 2014, 44, 28–35. [Google Scholar] [CrossRef]
- Fujiwara, R.; Maruo, Y.; Chen, S.; Tukey, R.H. Role of extrahepatic UDP-glucuronosyltransferase 1A1: Advances in understanding breast milk-induced neonatal hyperbilirubinemia. Toxicol. Appl. Pharmacol. 2015, 289, 124–132. [Google Scholar] [CrossRef]
- Kim, Y.S.; Unno, T.; Kim, B.Y.; Park, M.S. Sex Differences in Gut Microbiota. World J. Men Health 2020, 38, 48–60. [Google Scholar] [CrossRef]
- Gupta, V.K.; Paul, S.; Dutta, C. Geography, Ethnicity or Subsistence-Specific Variations in Human Microbiome Composition and Diversity. Front. Microbiol. 2017, 8, 1162. [Google Scholar] [CrossRef]
- Dwiyanto, J.; Hussain, M.H.; Reidpath, D.; Ong, K.S.; Qasim, A.; Lee, S.W.H.; Lee, S.M.; Foo, S.C.; Chong, C.W.; Rahman, S. Ethnicity influences the gut microbiota of individuals sharing a geographical location: A cross-sectional study from a middle-income country. Sci. Rep. 2021, 11, 2618. [Google Scholar] [CrossRef]
- Stojančević, M.; Bojić, G.; Salami, H.A.; Mikov, M. The Influence of Intestinal Tract and Probiotics on the Fate of Orally Administered Drugs. Curr. Issues Mol. Biol. 2014, 16, 55–68. [Google Scholar]
- Matuskova, Z.; Anzenbacherova, E.; Vecera, R.; Tlaskalova-Hogenova, H.; Kolar, M.; Anzenbacher, P. Administration of a probiotic can change drug pharmacokinetics: Effect of E. coli Nissle 1917 on amidarone absorption in rats. PLoS ONE 2014, 9, e87150. [Google Scholar] [CrossRef]
- Feng, D.; Zhang, M.; Tian, S.; Wang, J.; Zhu, W. Chitosan-chelated zinc modulates cecal microbiota and attenuates inflammatory response in weaned rats challenged with Escherichia coli. J. Microbiol. 2020, 58, 780–792. [Google Scholar] [CrossRef]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef]
- Owu, D.U.; Obembe, A.O.; Nwokocha, C.R.; Edoho, I.E.; Osim, E.E. Gastric ulceration in diabetes mellitus: Protective role of vitamin C. ISRN Gastroenterol. 2012, 2012, 362805. [Google Scholar] [CrossRef]
- Eliasson, B.; Björnsson, E.; Urbanavicius, V.; Andersson, H.; Fowelin, J.; Attvall, S.; Abrahamsson, H.; Smith, U. Hyperinsulinaemia impairs gastrointestinal motility and slows carbohydrate absorption. Diabetologia 1995, 38, 79–85. [Google Scholar] [CrossRef]
- Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Relationships between gastric emptying, postprandial glycemia, and incretin hormones. Diabetes Care 2013, 36, 1396–1405. [Google Scholar] [CrossRef]
- Zhao, M.; Liao, D.; Zhao, J. Diabetes-induced mechanophysiological changes in the small intestine and colon. World J. Diabetes 2017, 8, 249–269. [Google Scholar] [CrossRef]
- Redan, B.W.; Buhman, K.K.; Novotny, J.A.; Ferruzzi, M.G. Altered Transport and Metabolism of Phenolic Compounds in Obesity and Diabetes: Implications for Functional Food Development and Assessment. Adv. Nutr. 2016, 7, 1090–1104. [Google Scholar] [CrossRef] [PubMed]
- Kobori, T.; Harada, S.; Nakamoto, K.; Tokuyama, S. Functional alterations of intestinal P-glycoprotein under diabetic conditions. Biol. Pharm. Bull. 2013, 36, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Dostalek, M.; Sam, W.J.; Paryani, K.R.; Macwan, J.S.; Gohh, R.Y.; Akhlaghi, F. Diabetes mellitus reduces the clearance of atorvastatin lactone: Results of a population pharmacokinetic analysis in renal transplant recipients and in vitro studies using human liver microsomes. Clin. Pharmacokinet. 2012, 51, 591–606. [Google Scholar] [CrossRef]
- Zhelyazkova-Savova, M.; Gancheva, S.; Sirakova, V. Potential statin-drug interactions: Prevalence and clinical significance. SpringerPlus 2014, 3, 168. [Google Scholar] [CrossRef]
- Mahajan, V.; Hashmi, J.; Singh, R.; Samra, T.; Aneja, S. Comparative evaluation of gastric pH and volume in morbidly obese and lean patients undergoing elective surgery and effect of aspiration prophylaxis. J. Clin. Anesth. 2015, 27, 396–400. [Google Scholar] [CrossRef]
- Foucaud-Vignault, M.; Soayfane, Z.; Ménez, C.; Bertrand-Michel, J.; Martin, P.G.; Guillou, H.; Collet, X.; Lespine, A. P-glycoprotein dysfunction contributes to hepatic steatosis and obesity in mice. PLoS ONE 2011, 6, e23614. [Google Scholar] [CrossRef]
- Rodríguez-Morató, J.; Goday, A.; Langohr, K.; Pujadas, M.; Civit, E.; Pérez-Mañá, C.; Papaseit, E.; Ramon, J.M.; Benaiges, D.; Castañer, O.; et al. Short- and medium-term impact of bariatric surgery on the activities of CYP2D6, CYP3A4, CYP2C9, and CYP1A2 in morbid obesity. Sci. Rep. 2019, 9, 20405. [Google Scholar] [CrossRef]
- Sudhakar, S.; Chandran, S.V.; Selvamurugan, N.; Nazeer, R.A. Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int. J. Biol. Macromol. 2020, 150, 281–288. [Google Scholar] [CrossRef]
- Li, L.; Jiang, G.; Yu, W.; Liu, D.; Chen, H.; Liu, Y.; Tong, Z.; Kong, X.; Yao, J. Preparation of chitosan-based multifunctional nanocarriers overcoming multiple barriers for oral delivery of insulin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 278–286. [Google Scholar] [CrossRef]
- Lu, P.J.; Hsu, P.I.; Chen, C.H.; Hsiao, M.; Chang, W.C.; Tseng, H.H.; Lin, K.H.; Chuah, S.K.; Chen, H.C. Gastric juice acidity in upper gastrointestinal diseases. World J. Gastroenterol. 2010, 16, 5496–5501. [Google Scholar] [CrossRef]
- Ghosh, T.; Lewis, D.I.; Axon, A.T.; Everett, S.M. Review article: Methods of measuring gastric acid secretion. Aliment. Pharmacol. Ther. 2011, 33, 768–781. [Google Scholar] [CrossRef]
- Chang, F.Y.; Chen, C.Y.; Lu, C.L.; Luo, J.C.; Jiun, K.L.; Lee, S.D.; Wu, C.W. Undisturbed water gastric emptying in patients of stomach cancer. Hepato-Gastroenterology 2004, 51, 1219–1224. [Google Scholar]
- Kim, D.H.; Yun, H.Y.; Song, Y.J.; Ryu, D.H.; Han, H.S.; Han, J.H.; Kim, K.B.; Yoon, S.M.; Youn, S.J. Clinical features of gastric emptying after distal gastrectomy. Ann. Surg. Treat. Res. 2017, 93, 310–315. [Google Scholar] [CrossRef]
- Joshi, G.; Kumar, A.; Sawant, K. Enhanced bioavailability and intestinal uptake of Gemcitabine HCl loaded PLGA nanoparticles after oral delivery. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2014, 60, 80–89. [Google Scholar] [CrossRef]
- Louisa, M.; Soediro, T.M.; Suyatna, F.D. In vitro modulation of P-glycoprotein, MRP-1 and BCRP expression by mangiferin in doxorubicin-treated MCF-7 cells. Asian Pac. J. Cancer Prev. APJCP 2014, 15, 1639–1642. [Google Scholar] [CrossRef]
- Nanayakkara, A.K.; Follit, C.A.; Chen, G.; Williams, N.S.; Vogel, P.D.; Wise, J.G. Targeted inhibitors of P-glycoprotein increase chemotherapeutic-induced mortality of multidrug resistant tumor cells. Sci. Rep. 2018, 8, 967. [Google Scholar] [CrossRef]
- Weiss, R.B. The anthracyclines: Will we ever find a better doxorubicin? Semin. Oncol. 1992, 19, 670–686. [Google Scholar]
- Cortés-Funes, H.; Coronado, C. Role of anthracyclines in the era of targeted therapy. Cardiovasc. Toxicol. 2007, 7, 56–60. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharm. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Kalaria, D.R.; Sharma, G.; Beniwal, V.; Ravi Kumar, M.N. Design of biodegradable nanoparticles for oral delivery of doxorubicin: In vivo pharmacokinetics and toxicity studies in rats. Pharm. Res. 2009, 26, 492–501. [Google Scholar] [CrossRef]
- Feng, C.; Li, J.; Mu, Y.; Kong, M.; Li, Y.; Raja, M.A.; Cheng, X.J.; Liu, Y.; Chen, X.G. Multilayer micro-dispersing system as oral carriers for co-delivery of doxorubicin hydrochloride and P-gp inhibitor. Int. J. Biol. Macromol. 2017, 94, 170–180. [Google Scholar] [CrossRef]
- Deng, L.; Dong, H.; Dong, A.; Zhang, J. A strategy for oral chemotherapy via dual pH-sensitive polyelectrolyte complex nanoparticles to achieve gastric survivability, intestinal permeability, hemodynamic stability and intracellular activity. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2015, 97, 107–117. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Z.; Jiang, C.; Kong, M.; Zhou, X.; Li, Y.; Cheng, X.; Chen, X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013, 457, 158–167. [Google Scholar] [CrossRef]
- Iannuccelli, V.; Montanari, M.; Bertelli, D.; Pellati, F.; Coppi, G. Microparticulate polyelectrolyte complexes for gentamicin transport across intestinal epithelia. Drug Deliv. 2011, 18, 26–37. [Google Scholar] [CrossRef]
- Eftaiha, A.F.; Qinna, N.; Rashid, I.S.; Al Remawi, M.M.; Al Shami, M.R.; Arafat, T.A.; Badwan, A.A. Bioadhesive controlled metronidazole release matrix based on chitosan and xanthan gum. Mar. Drugs 2010, 8, 1716–1730. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.J. Stress and the gut: Pathophysiology, clinical consequences, diagnostic approach and treatment options. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2011, 62, 591–599. [Google Scholar]
- Treenate, P.; Monvisade, P. In vitro drug release profiles of pH-sensitive hydroxyethylacryl chitosan/sodium alginate hydrogels using paracetamol as a soluble model drug. Int. J. Biol. Macromol. 2017, 99, 71–78. [Google Scholar] [CrossRef]
- Ofokansi, K.C.; Kenechukwu, F.C. Formulation Development and Evaluation of Drug Release Kinetics from Colon-Targeted Ibuprofen Tablets Based on Eudragit RL 100-Chitosan Interpolyelectrolyte Complexes. ISRN Pharm. 2013, 2013, 838403. [Google Scholar] [CrossRef]
- Ahmed, T.A.; Aljaeid, B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Dev. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef]
- Fabiano, A.; Piras, A.M.; Uccello-Barretta, G.; Balzano, F.; Cesari, A.; Testai, L.; Citi, V.; Zambito, Y. Impact of mucoadhesive polymeric nanoparticulate systems on oral bioavailability of a macromolecular model drug. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2018, 130, 281–289. [Google Scholar] [CrossRef]
- Blanquet, S.; Zeijdner, E.; Beyssac, E.; Meunier, J.P.; Denis, S.; Havenaar, R.; Alric, M. A dynamic artificial gastrointestinal system for studying the behavior of orally administered drug dosage forms under various physiological conditions. Pharm. Res. 2004, 21, 585–591. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Kast, C.E.; Guggi, D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: Thiomer/GSH systems. J. Control Release Off. J. Control Release Soc. 2003, 93, 95–103. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Recent advances of polysaccharide-based nanoparticles for oral insulin delivery. Int. J. Biol. Macromol. 2018, 120, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Bayat, A.; Larijani, B.; Ahmadian, S.; Junginger, H.E.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and its quaternized derivatives. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Arbit, E. The physiological rationale for oral insulin administration. Diabetes Technol. Ther. 2004, 6, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C.; Silbernagl, J.; Laffleur, F.; Leichner, C.; Jelkmann, M.; Huck, C.W.; Hussain, S.; Bernkop-Schnürch, A. 2,2′Dithiodinicotinyl ligands: Key to more reactive thiomers. Int. J. Pharm. 2016, 503, 199–206. [Google Scholar] [CrossRef]
- Sajjad, M.; Khan, M.I.; Naveed, S.; Ijaz, S.; Qureshi, O.S.; Raza, S.A.; Shahnaz, G.; Sohail, M.F. Folate-Functionalized Thiomeric Nanoparticles for Enhanced Docetaxel Cytotoxicity and Improved Oral Bioavailability. AAPS PharmSciTech 2019, 20, 81. [Google Scholar] [CrossRef]
- Samprasit, W.; Opanasopit, P.; Chamsai, B. Mucoadhesive chitosan and thiolated chitosan nanoparticles containing alpha mangostin for possible Colon-targeted delivery. Pharm. Dev. Technol. 2021, 26, 362–372. [Google Scholar] [CrossRef]
- Fan, B.; Xing, Y.; Zheng, Y.; Sun, C.; Liang, G. pH-responsive thiolated chitosan nanoparticles for oral low-molecular weight heparin delivery: In vitro and in vivo evaluation. Drug Deliv. 2016, 23, 238–247. [Google Scholar] [CrossRef]
- Gradauer, K.; Vonach, C.; Leitinger, G.; Kolb, D.; Fröhlich, E.; Roblegg, E.; Bernkop-Schnürch, A.; Prassl, R. Chemical coupling of thiolated chitosan to preformed liposomes improves mucoadhesive properties. Int. J. Nanomed. 2012, 7, 2523–2534. [Google Scholar] [CrossRef]
- Dünnhaupt, S.; Barthelmes, J.; Rahmat, D.; Leithner, K.; Thurner, C.C.; Friedl, H.; Bernkop-Schnürch, A. S-protected thiolated chitosan for oral delivery of hydrophilic macromolecules: Evaluation of permeation enhancing and efflux pump inhibitory properties. Mol. Pharm. 2012, 9, 1331–1341. [Google Scholar] [CrossRef]
- Dünnhaupt, S.; Barthelmes, J.; Iqbal, J.; Perera, G.; Thurner, C.C.; Friedl, H.; Bernkop-Schnürch, A. In vivo evaluation of an oral drug delivery system for peptides based on S-protected thiolated chitosan. J. Control. Release Off. J. Control Release Soc. 2012, 160, 477–485. [Google Scholar] [CrossRef]
- Iqbal, J.; Shahnaz, G.; Perera, G.; Hintzen, F.; Sarti, F.; Bernkop-Schnürch, A. Thiolated chitosan: Development and in vivo evaluation of an oral delivery system for leuprolide. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2012, 80, 95–102. [Google Scholar] [CrossRef]
- Dünnhaupt, S.; Barthelmes, J.; Hombach, J.; Sakloetsakun, D.; Arkhipova, V.; Bernkop-Schnürch, A. Distribution of thiolated mucoadhesive nanoparticles on intestinal mucosa. Int. J. Pharm. 2011, 408, 191–199. [Google Scholar] [CrossRef]
- Mourya, V.K.; Inamdar, N.N. Trimethyl chitosan and its applications in drug delivery. J. Mater. Sci. Mater. Med. 2009, 20, 1057–1079. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Patel, H.M.; Surana, S.J.; Vanjari, Y.H.; Belgamwar, V.S.; Pardeshi, C.V. N,N,N-Trimethyl chitosan: An advanced polymer with myriad of opportunities in nanomedicine. Carbohydr. Polym. 2017, 157, 875–902. [Google Scholar] [CrossRef]
- Sheng, J.; Han, L.; Qin, J.; Ru, G.; Li, R.; Wu, L.; Cui, D.; Yang, P.; He, Y.; Wang, J. N-trimethyl chitosan chloride-coated PLGA nanoparticles overcoming multiple barriers to oral insulin absorption. ACS Appl. Mater. Interfaces 2015, 7, 15430–15441. [Google Scholar] [CrossRef]
- Tsai, L.C.; Chen, C.H.; Lin, C.W.; Ho, Y.C.; Mi, F.L. Development of mutlifunctional nanoparticles self-assembled from trimethyl chitosan and fucoidan for enhanced oral delivery of insulin. Int. J. Biol. Macromol. 2019, 126, 141–150. [Google Scholar] [CrossRef]
- Ghavimishamekh, A.; Ziamajidi, N.; Dehghan, A.; Goodarzi, M.T.; Abbasalipourkabir, R. Study of Insulin-Loaded Chitosan Nanoparticle Effects on TGF-β1 and Fibronectin Expression in Kidney Tissue of Type 1 Diabetic Rats. Indian J. Clin. Biochem. IJCB 2019, 34, 418–426. [Google Scholar] [CrossRef]
- Song, R.F.; Li, X.J.; Cheng, X.L.; Fu, A.R.; Wang, Y.H.; Feng, Y.J.; Xiong, Y. Paclitaxel-loaded trimethyl chitosan-based polymeric nanoparticle for the effective treatment of gastroenteric tumors. Oncol. Rep. 2014, 32, 1481–1488. [Google Scholar] [CrossRef][Green Version]
- Huang, A.; Makhlof, A.; Ping, Q.; Tozuka, Y.; Takeuchi, H. N-trimethyl chitosan-modified liposomes as carriers for oral delivery of salmon calcitonin. Drug Deliv. 2011, 18, 562–569. [Google Scholar] [CrossRef]
- Li, W.; Zhu, X.; Zhou, X.; Wang, X.; Zhai, W.; Li, B.; Du, J.; Li, G.; Sui, X.; Wu, Y.; et al. An orally available PD-1/PD-L1 blocking peptide OPBP-1-loaded trimethyl chitosan hydrogel for cancer immunotherapy. J. Control Release Off. J. Control Release Soc. 2021, 334, 376–388. [Google Scholar] [CrossRef]
- Martins, A.F.; Bueno, P.V.; Almeida, E.A.; Rodrigues, F.H.; Rubira, A.F.; Muniz, E.C. Characterization of N-trimethyl chitosan/alginate complexes and curcumin release. Int. J. Biol. Macromol. 2013, 57, 174–184. [Google Scholar] [CrossRef]
- Saheb, M.; Fereydouni, N.; Nemati, S.; Barreto, G.E.; Johnston, T.P.; Sahebkar, A. Chitosan-based delivery systems for curcumin: A review of pharmacodynamic and pharmacokinetic aspects. J. Cell. Physiol. 2019, 234, 12325–12340. [Google Scholar] [CrossRef]
- Ramalingam, P.; Ko, Y.T. Enhanced oral delivery of curcumin from N-trimethyl chitosan surface-modified solid lipid nanoparticles: Pharmacokinetic and brain distribution evaluations. Pharm. Res. 2015, 32, 389–402. [Google Scholar] [CrossRef]
- Kalliola, S.; Repo, E.; Srivastava, V.; Zhao, F.; Heiskanen, J.P.; Sirviö, J.A.; Liimatainen, H.; Sillanpää, M. Carboxymethyl Chitosan and Its Hydrophobically Modified Derivative as pH-Switchable Emulsifiers. Langmuir ACS J. Surf. Colloids 2018, 34, 2800–2806. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Cheng, X.; Kong, M.; Liu, Y.; Feng, C.; Chen, X. Positive/negative surface charge of chitosan based nanogels and its potential influence on oral insulin delivery. Carbohydr. Polym. 2016, 136, 867–874. [Google Scholar] [CrossRef]
- Li, J.; Jiang, C.; Lang, X.; Kong, M.; Cheng, X.; Liu, Y.; Feng, C.; Chen, X. Multilayer sodium alginate beads with porous core containing chitosan based nanoparticles for oral delivery of anticancer drug. Int. J. Biol. Macromol. 2016, 85, 1–8. [Google Scholar] [CrossRef]
- Feng, C.; Song, R.; Sun, G.; Kong, M.; Bao, Z.; Li, Y.; Cheng, X.; Cha, D.; Park, H.; Chen, X. Immobilization of coacervate microcapsules in multilayer sodium alginate beads for efficient oral anticancer drug delivery. Biomacromolecules 2014, 15, 985–996. [Google Scholar] [CrossRef]
- Cong, Y.; Geng, J.; Wang, H.; Su, J.; Arif, M.; Dong, Q.; Chi, Z.; Liu, C. Ureido-modified carboxymethyl chitosan-graft-stearic acid polymeric nano-micelles as a targeted delivering carrier of clarithromycin for Helicobacter pylori: Preparation and in vitro evaluation. Int. J. Biol. Macromol. 2019, 129, 686–692. [Google Scholar] [CrossRef]
- Huang, G.Q.; Zhang, Z.K.; Cheng, L.Y.; Xiao, J.X. Intestine-targeted delivery potency of O-carboxymethyl chitosan-coated layer-by-layer microcapsules: An in vitro and in vivo evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 105, 110129. [Google Scholar] [CrossRef]
- Le, T.N.; Her, J.; Sim, T.; Jung, C.E.; Kang, J.K.; Oh, K.T. Preparation of Gastro-retentive Tablets Employing Controlled Superporous Networks for Improved Drug Bioavailability. AAPS PharmSciTech 2020, 21, 320. [Google Scholar] [CrossRef]
- Park, H.; Park, K.; Kim, D. Preparation and swelling behavior of chitosan-based superporous hydrogels for gastric retention application. J. Biomed. Mater. Res. Part A 2006, 76, 144–150. [Google Scholar] [CrossRef]
- Su, F.Y.; Lin, K.J.; Sonaje, K.; Wey, S.P.; Yen, T.C.; Ho, Y.C.; Panda, N.; Chuang, E.Y.; Maiti, B.; Sung, H.W. Protease inhibition and absorption enhancement by functional nanoparticles for effective oral insulin delivery. Biomaterials 2012, 33, 2801–2811. [Google Scholar] [CrossRef]
- Sung, H.W.; Sonaje, K.; Liao, Z.X.; Hsu, L.W.; Chuang, E.Y. pH-responsive nanoparticles shelled with chitosan for oral delivery of insulin: From mechanism to therapeutic applications. Acc. Chem. Res. 2012, 45, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.Y.; Lin, C.W.; Hsu, C.H.; Ho, Y.C.; Chuang, E.Y.; Sung, H.W.; Mi, F.L. FRET-based dual-emission and pH-responsive nanocarriers for enhanced delivery of protein across intestinal epithelial cell barrier. ACS Appl. Mater. Interfaces 2014, 6, 18275–18289. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.I.; Jin, S.G.; Kim, I.Y.; Pei, J.; Wen, M.; Jung, T.Y.; Moon, K.S.; Jung, S. Doxorubicin-incorporated nanoparticles composed of poly(ethylene glycol)-grafted carboxymethyl chitosan and antitumor activity against glioma cells in vitro. Colloids Surf. B Biointerfaces 2010, 79, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hauptstein, S.; Bonengel, S.; Griessinger, J.; Bernkop-Schnürch, A. Synthesis and characterization of pH tolerant and mucoadhesive (thiol-polyethylene glycol) chitosan graft polymer for drug delivery. J. Pharm. Sci. 2014, 103, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, S.A.; Achilias, D.S.; Bikiaris, D.N. Chitosan-g-PEG nanoparticles ionically crosslinked with poly(glutamic acid) and tripolyphosphate as protein delivery systems. Int. J. Pharm. 2012, 430, 318–327. [Google Scholar] [CrossRef]
- Mo, R.; Jin, X.; Li, N.; Ju, C.; Sun, M.; Zhang, C.; Ping, Q. The mechanism of enhancement on oral absorption of paclitaxel by N-octyl-O-sulfate chitosan micelles. Biomaterials 2011, 32, 4609–4620. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Swarnakar, N.K.; Das, M.; Godugu, C.; Singh, R.P.; Rao, P.R.; Jain, S. Augmented anticancer efficacy of doxorubicin-loaded polymeric nanoparticles after oral administration in a breast cancer induced animal model. Mol. Pharm. 2011, 8, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Gaucher, G.; Satturwar, P.; Jones, M.C.; Furtos, A.; Leroux, J.C. Polymeric micelles for oral drug delivery. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2010, 76, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Nornoo, A.O.; Zheng, H.; Lopes, L.B.; Johnson-Restrepo, B.; Kannan, K.; Reed, R. Oral microemulsions of paclitaxel: In situ and pharmacokinetic studies. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. Verfahr. EV 2009, 71, 310–317. [Google Scholar] [CrossRef]
- Al-Hilal, T.A.; Alam, F.; Byun, Y. Oral drug delivery systems using chemical conjugates or physical complexes. Adv. Drug Deliv. Rev. 2013, 65, 845–864. [Google Scholar] [CrossRef]
- Soares, S.F.; Fernandes, T.; Daniel-da-Silva, A.L.; Trindade, T. The controlled synthesis of complex hollow nanostructures and prospective applications(†). Proc. Math. Phys. Eng. Sci. 2019, 475, 20180677. [Google Scholar] [CrossRef] [PubMed]
- Sultan, Y.; DeRosa, M.C. Target binding influences permeability in aptamer-polyelectrolyte microcapsules. Small 2011, 7, 1219–1226. [Google Scholar] [CrossRef]
- Sultan, Y.; Walsh, R.; Monreal, C.; DeRosa, M.C. Preparation of functional aptamer films using layer-by-layer self-assembly. Biomacromolecules 2009, 10, 1149–1154. [Google Scholar] [CrossRef]
- Radwan, S.E.; Sokar, M.S.; Abdelmonsif, D.A.; El-Kamel, A.H. Mucopenetrating nanoparticles for enhancement of oral bioavailability of furosemide: In vitro and in vivo evaluation/sub-acute toxicity study. Int. J. Pharm. 2017, 526, 366–379. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Pan, D.; Wan, Y.; Wang, Z. pH-sensitive interpenetrating network hydrogels based on chitosan derivatives and alginate for oral drug delivery. Carbohydr. Polym. 2013, 92, 719–725. [Google Scholar] [CrossRef]
- Oshi, M.A.; Lee, J.; Naeem, M.; Hasan, N.; Kim, J.; Kim, H.J.; Lee, E.H.; Jung, Y.; Yoo, J.W. Curcumin Nanocrystal/pH-Responsive Polyelectrolyte Multilayer Core-Shell Nanoparticles for Inflammation-Targeted Alleviation of Ulcerative Colitis. Biomacromolecules 2020, 21, 3571–3581. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. Optimization of chitosan nanoparticles for colon tumors using experimental design methodology. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1917–1926. [Google Scholar] [CrossRef]
- Caddeo, C.; Díez-Sales, O.; Pons, R.; Carbone, C.; Ennas, G.; Puglisi, G.; Fadda, A.M.; Manconi, M. Cross-linked chitosan/liposome hybrid system for the intestinal delivery of quercetin. J. Colloid Interface Sci. 2016, 461, 69–78. [Google Scholar] [CrossRef]
- Konecsni, K.; Low, N.H.; Nickerson, M.T. Chitosan-tripolyphosphate submicron particles as the carrier of entrapped rutin. Food Chem. 2012, 134, 1775–1779. [Google Scholar] [CrossRef]
- Sareen, R.; Jain, N.; Rajkumari, A.; Dhar, K.L. pH triggered delivery of curcumin from Eudragit-coated chitosan microspheres for inflammatory bowel disease: Characterization and pharmacodynamic evaluation. Drug Deliv. 2016, 23, 55–62. [Google Scholar] [CrossRef]
- Pandey, S.; Mishra, A.; Raval, P.; Patel, H.; Gupta, A.; Shah, D. Chitosan-pectin polyelectrolyte complex as a carrier for colon targeted drug delivery. J. Young Pharm. JYP 2013, 5, 160–166. [Google Scholar] [CrossRef]
- Liu, C.; Kou, Y.; Zhang, X.; Dong, W.; Cheng, H.; Mao, S. Enhanced oral insulin delivery via surface hydrophilic modification of chitosan copolymer based self-assembly polyelectrolyte nanocomplex. Int. J. Pharm. 2019, 554, 36–47. [Google Scholar] [CrossRef]
- Liu, C.; Kou, Y.; Zhang, X.; Cheng, H.; Chen, X.; Mao, S. Strategies and industrial perspectives to improve oral absorption of biological macromolecules. Expert Opin. Drug Deliv. 2018, 15, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Zhong, Y.; Cheng, R.; Deng, C.; Zhong, Z. pH-sensitive polymeric nanoparticles for tumor-targeting doxorubicin delivery: Concept and recent advances. Nanomedicine 2014, 9, 487–499. [Google Scholar] [CrossRef]
- Anwer, M.K.; Iqbal, M.; Muharram, M.M.; Mohammad, M.; Ezzeldin, E.; Aldawsari, M.F.; Alalaiwe, A.; Imam, F. Development of Lipomer Nanoparticles for the Enhancement of Drug Release, Anti-microbial Activity and Bioavailability of Delafloxacin. Pharmaceutics 2020, 12, 252. [Google Scholar] [CrossRef]
- Khare, T.; Mahalunkar, S.; Shriram, V.; Gosavi, S.; Kumar, V. Embelin-loaded chitosan gold nanoparticles interact synergistically with ciprofloxacin by inhibiting efflux pumps in multidrug-resistant Pseudomonas aeruginosa and Escherichia coli. Environ. Res. 2021, 199, 111321. [Google Scholar] [CrossRef]
- Maisetta, G.; Piras, A.M.; Motta, V.; Braccini, S.; Mazzantini, D.; Chiellini, F.; Zambito, Y.; Esin, S.; Batoni, G. Antivirulence Properties of a Low-Molecular-Weight Quaternized Chitosan Derivative against Pseudomonas aeruginosa. Microorganisms 2021, 9, 912. [Google Scholar] [CrossRef]
- Traverso, G.; Langer, R. Perspective: Special delivery for the gut. Nature 2015, 519, S19. [Google Scholar] [CrossRef]
- Pan, J.; Rostamizadeh, K.; Filipczak, N.; Torchilin, V.P. Polymeric Co-Delivery Systems in Cancer Treatment: An Overview on Component Drugs’ Dosage Ratio Effect. Molecules 2019, 24, 1035. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Liang, J.; Wu, A.; Chen, Y.; Zhao, P.; Lin, T.; Zhang, M.; Xu, Q.; Wang, J.; Huang, Y. Co-Delivery of Trichosanthin and Albendazole by Nano-Self-Assembly for Overcoming Tumor Multidrug-Resistance and Metastasis. ACS Appl. Mater. Interfaces 2017, 9, 26648–26664. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Zhang, M.; Wei, D.; Stueber, D.; Taratula, O.; Minko, T.; He, H. Co-delivery of doxorubicin and Bcl-2 siRNA by mesoporous silica nanoparticles enhances the efficacy of chemotherapy in multidrug-resistant cancer cells. Small 2009, 5, 2673–2677. [Google Scholar] [CrossRef] [PubMed]
- Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. [Google Scholar] [CrossRef]
- Jo, S.D.; Ku, S.H.; Won, Y.Y.; Kim, S.H.; Kwon, I.C. Targeted Nanotheranostics for Future Personalized Medicine: Recent Progress in Cancer Therapy. Theranostics 2016, 6, 1362–1377. [Google Scholar] [CrossRef]
- Hong, S.C.; Yoo, S.Y.; Kim, H.; Lee, J. Chitosan-Based Multifunctional Platforms for Local Delivery of Therapeutics. Mar. Drugs 2017, 15, 60. [Google Scholar] [CrossRef]
- Liu, Y.; Kong, M.; Cheng, X.J.; Wang, Q.Q.; Jiang, L.M.; Chen, X.G. Self-assembled nanoparticles based on amphiphilic chitosan derivative and hyaluronic acid for gene delivery. Carbohydr. Polym. 2013, 94, 309–316. [Google Scholar] [CrossRef]
- Tan, C.S.S.; Lee, S.W.H. Warfarin and food, herbal or dietary supplement interactions: A systematic review. Br. J. Clin. Pharmacol. 2021, 87, 352–374. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathomthongtaweechai, N.; Muanprasat, C. Potential Applications of Chitosan-Based Nanomaterials to Surpass the Gastrointestinal Physiological Obstacles and Enhance the Intestinal Drug Absorption. Pharmaceutics 2021, 13, 887. https://doi.org/10.3390/pharmaceutics13060887
Pathomthongtaweechai N, Muanprasat C. Potential Applications of Chitosan-Based Nanomaterials to Surpass the Gastrointestinal Physiological Obstacles and Enhance the Intestinal Drug Absorption. Pharmaceutics. 2021; 13(6):887. https://doi.org/10.3390/pharmaceutics13060887
Chicago/Turabian StylePathomthongtaweechai, Nutthapoom, and Chatchai Muanprasat. 2021. "Potential Applications of Chitosan-Based Nanomaterials to Surpass the Gastrointestinal Physiological Obstacles and Enhance the Intestinal Drug Absorption" Pharmaceutics 13, no. 6: 887. https://doi.org/10.3390/pharmaceutics13060887
APA StylePathomthongtaweechai, N., & Muanprasat, C. (2021). Potential Applications of Chitosan-Based Nanomaterials to Surpass the Gastrointestinal Physiological Obstacles and Enhance the Intestinal Drug Absorption. Pharmaceutics, 13(6), 887. https://doi.org/10.3390/pharmaceutics13060887






