Application of Machine-Learning Algorithms for Better Understanding of Tableting Properties of Lactose Co-Processed with Lipid Excipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Co-Processing of Excipients
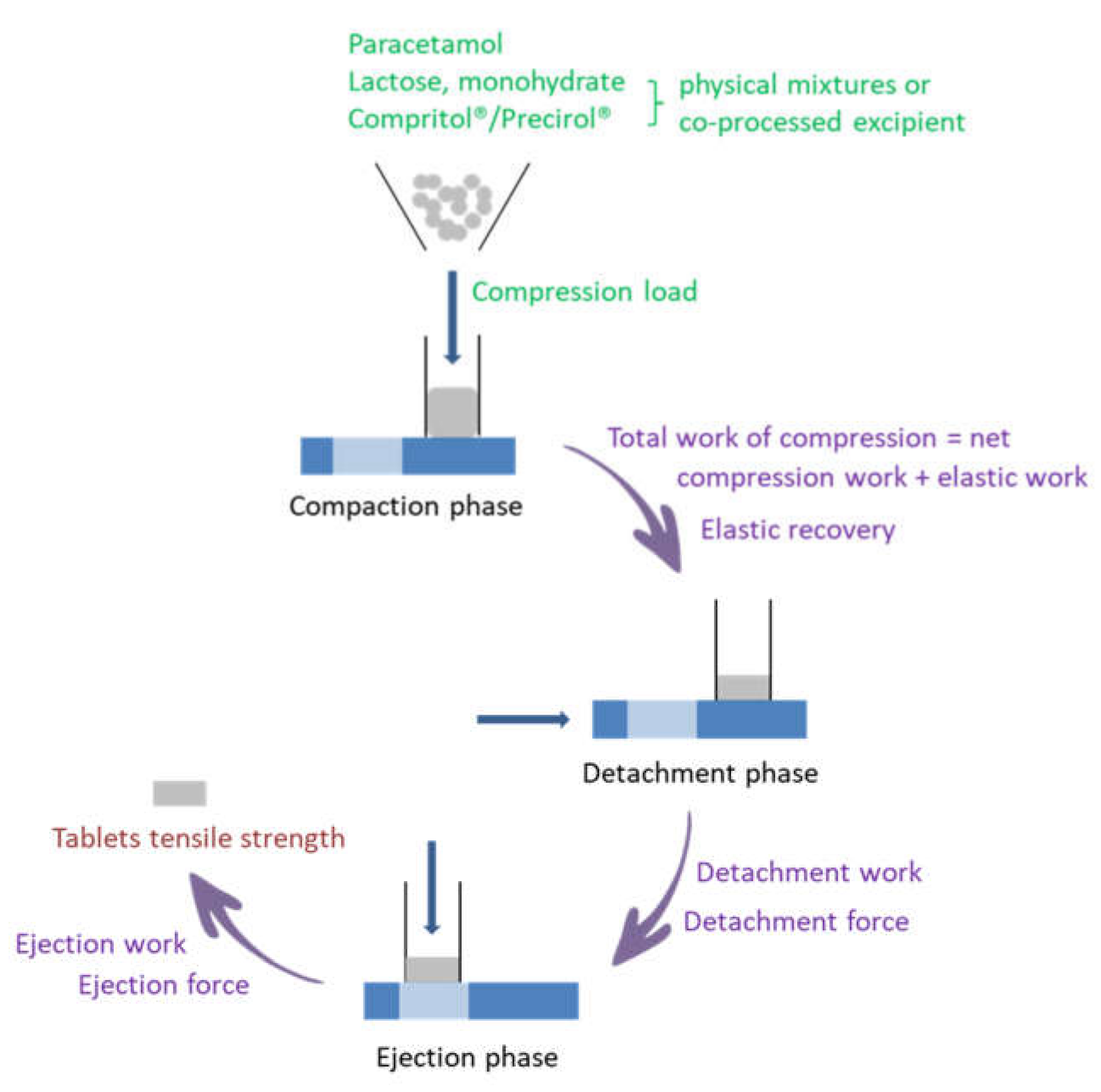
2.3. Dynamic Compaction Analysis
2.4. Artificial Neural Networks
3. Results and Discussion
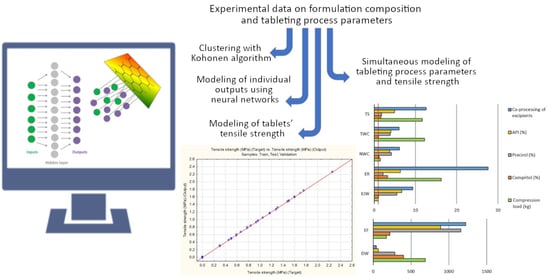
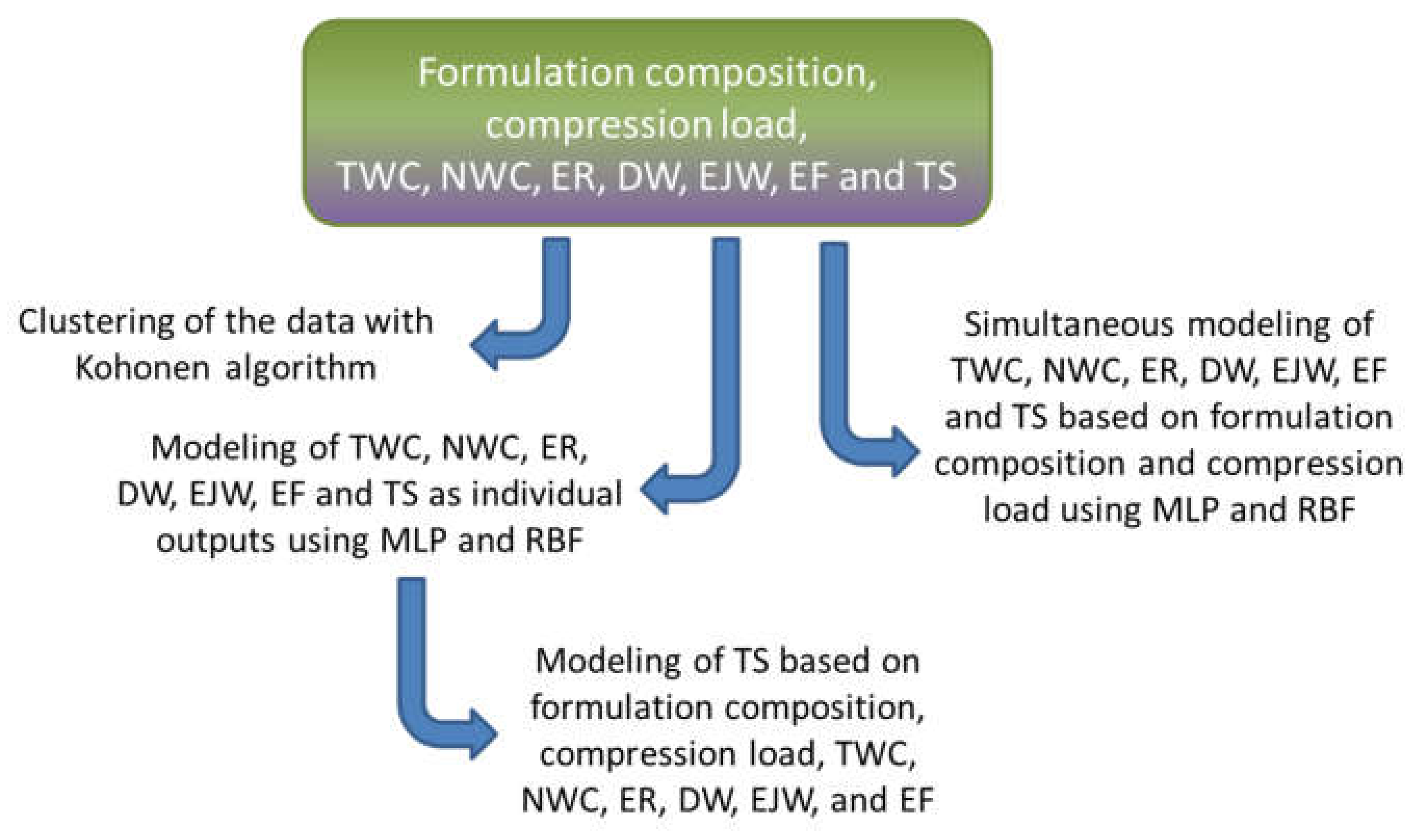
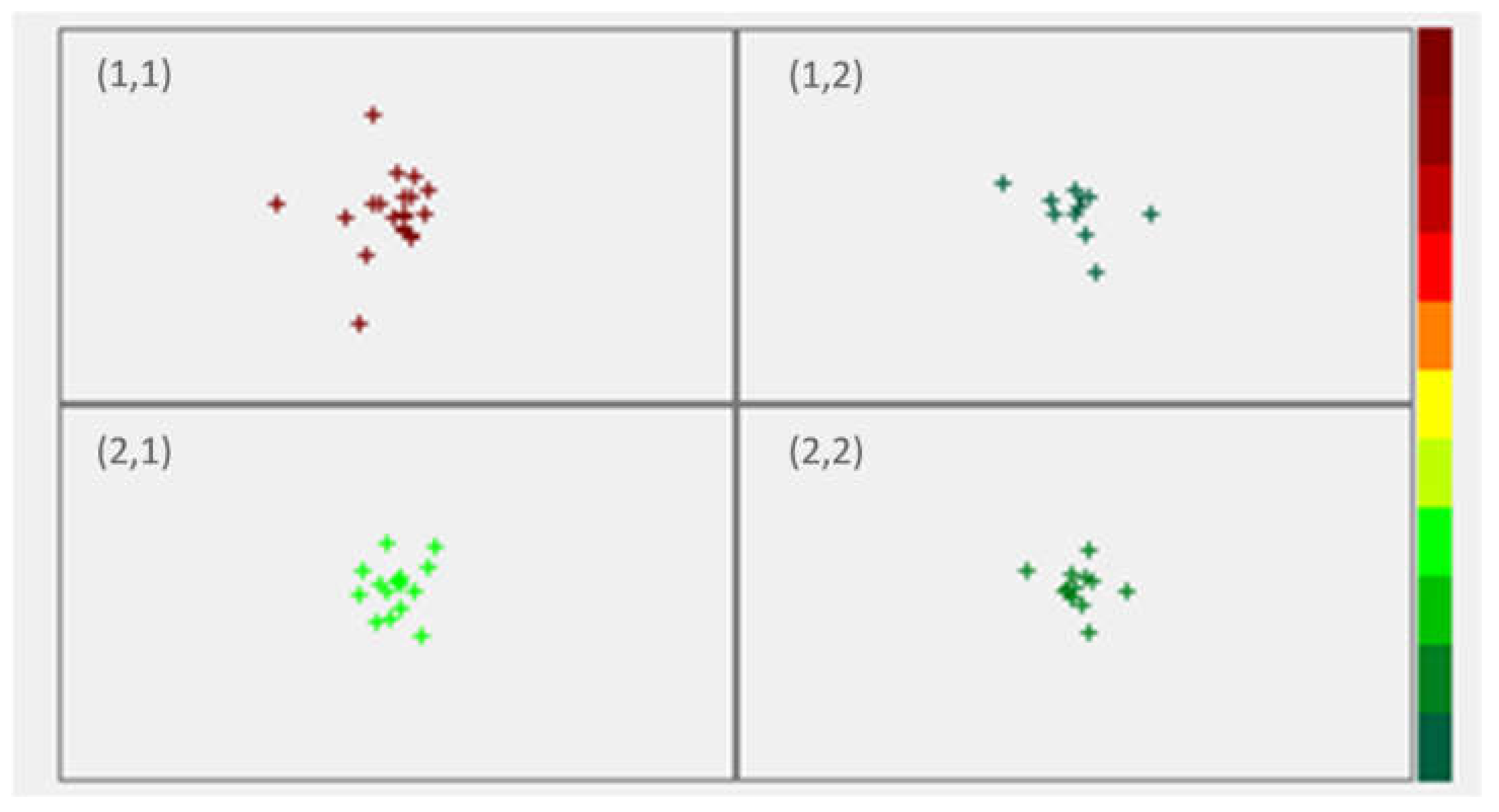
3.1. Clustering with Kohonen Neural Networks
3.2. Modeling of the Individual Outputs
3.3. Modeling of Tablets Tensile Strength
3.4. Development of Models for Simultaneous Predictions of Seven Outputs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Co-Processed Excipients Market Size and Forecast. Available online: https://www.verifiedmarketresearch.com/product/co-processed-excipients-market/ (accessed on 20 March 2021).
- Schaller, B.E.; Moroney, K.M.; Castro-Dominguez, B.; Cronin, P.; Belen-Girona, J.; Ruane, P.; Croker, D.M.; Walker, G.M. Systematic Development of a High Dosage Formulation to Enable Direct Compression of a Poorly Flowing API: A Case Study. Int. J. Pharm. 2019, 566, 615–630. [Google Scholar] [CrossRef]
- Franc, A.; Vetchý, D.; Vodáčková, P.; Kubaľák, R.; Jendryková, L.; Goněc, R. Co-processed excipients for direct compression of tablets. Čes. Slov. Farm. 2018, 67, 175–181. [Google Scholar]
- Rojas, J.; Buckner, I.; Kumar, V. Co-Proccessed Excipients with Enhanced Direct Compression Functionality for Improved Tableting Performance. Drug Dev. Ind. Pharm. 2012, 38, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Dureja, H.; Kaushik, D. Co-Processed Excipients: A Patent Review. Recent Pat. Drug Deliv. Formul. 2013, 7, 73–83. [Google Scholar] [CrossRef]
- Roopwani, R.; Buckner, I.S. Co-Processed Particles: An Approach to Transform Poor Tableting Properties. J. Pharm. Sci. 2019, 108, 3209–3217. [Google Scholar] [CrossRef]
- Benabbas, R.; Sanchez-Ballester, N.M.; Bataille, B.; Sharkawi, T.; Soulairol, I. Development and Pharmaceutical Performance of a Novel Co-Processed Excipient of Alginic Acid and Microcrystalline Cellulose. Powder Technol. 2021, 378, 576–584. [Google Scholar] [CrossRef]
- Lakshman, J.P.; Kowalski, J.; Vasanthavada, M.; Tong, W.-Q.; Joshi, Y.M.; Serajuddin, A.T.M. Application of Melt Granulation Technology to Enhance Tabletting Properties of Poorly Compactible High-Dose Drugs. J. Pharm. Sci. 2011, 100, 1553–1565. [Google Scholar] [CrossRef]
- Dureja, H.; Garg, N.; Pandey, P.; Kaushik, D. Development of Novel Multifunction Directly Compressible Co-Processed Excipient by Melt Granulation Technique. Int. J. Pharm. Investig. 2015, 5, 266. [Google Scholar] [CrossRef]
- Apeji, Y.E.; Olayemi, O.J.; Anyebe, S.N.; Oparaeche, C.; Orugun, O.A.; Olowosulu, A.K.; Oyi, A.R. Impact of Binder as a Formulation Variable on the Material and Tableting Properties of Developed Co-Processed Excipients. SN Appl. Sci. 2019, 1, 1. [Google Scholar] [CrossRef]
- Drašković, M.; Djuriš, J.; Ibrić, S.; Parojčić, J. Functionality and Performance Evaluation of Directly Compressible Co-Processed Excipients Based on Dynamic Compaction Analysis and Percolation Theory. Powder Technol. 2018, 326, 292–301. [Google Scholar] [CrossRef]
- Osamura, T.; Takeuchi, Y.; Onodera, R.; Kitamura, M.; Takahashi, Y.; Tahara, K.; Takeuchi, H. Characterization of Tableting Properties Measured with a Multi-Functional Compaction Instrument for Several Pharmaceutical Excipients and Actual Tablet Formulations. Int. J. Pharm. 2016, 510, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, A.; Leong, K.H.; Tsuji, H.; Hayashi, Y.; Kumada, S.; Okada, K.; Onuki, Y. Characterization of Powder- and Tablet Properties of Different Direct Compaction Grades of Mannitol Using a Kohonen Self-Organizing Map and a Lasso Regression Model. J. Pharm. Sci. 2020, 109, 2585–2593. [Google Scholar] [CrossRef] [PubMed]
- Chattoraj, S.; Daugherity, P.; McDermott, T.; Olsofsky, A.; Roth, W.J.; Tobyn, M. Sticking and Picking in Pharmaceutical Tablet Compression: An IQ Consortium Review. J. Pharm. Sci. 2018, 107, 2267–2282. [Google Scholar] [CrossRef]
- Kesavan, J.G.; Peck, G.E. Pharmaceutical Granulation and Tablet Formulation Using Neural Networks. Pharm. Dev. Technol. 1996, 1, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Ibrić, S.; Jovanović, M.; Djurić, Z.; Parojčić, J.; Solomun, L. The Application of Generalized Regression Neural Network in the Modeling and Optimization of Aspirin Extended Release Tablets with Eudragit® RS PO as Matrix Substance. J. Control. Release. 2002, 82, 213–222. [Google Scholar] [CrossRef]
- Mandal, U.; Gowda, V.; Ghosh, A.; Bose, A.; Bhaumik, U.; Chatterjee, B.; Pal, T.K. Optimization of Metformin HCl 500 Mg Sustained Release Matrix Tablets Using Artificial Neural Network (ANN) Based on Multilayer Perceptrons (MLP) Model. Chem. Pharm. Bull. 2008, 56, 150–155. [Google Scholar] [CrossRef]
- Ivić, B.; Ibrić, S.; Cvetković, N.; Petrović, A.; Trajković, S.; Djurić, Z. Application of design of experiments and multilayer perceptrons neural network in the optimization of diclofenac sodium extended release tablets with Carbopol 71G. Chem. Pharm. Bull. 2010, 58, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Aksu, B.; Paradkar, A.; de Matas, M.; Özer, Ö.; Güneri, T.; York, P. A Quality by Design Approach Using Artificial Intelligence Techniques to Control the Critical Quality Attributes of Ramipril Tablets Manufactured by Wet Granulation. Pharm. Dev. Technol. 2012, 18, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Petra, D.; Galata, D.L.; Démuth, B.; Borbás, E.; Marosi, G.; Nagy, Z.K.; Farkas, A. Application of Artificial Neural Networks for Process Analytical Technology-Based Dissolution Testing. Int. J. Pharm. 2019, 567, 118464. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.F.; Silva, G.; Pinto, A.C.; Fonseca, M.; Silva, N.E.; Pinto, R.M.A.; Simões, S. Artificial Neural Networks Applied to Quality-By-Design: From Formulation Development to Clinical Outcome. Eur. J. Pharm. Biopharm. 2020, 152, 282–295. [Google Scholar] [CrossRef]
- Sun, Y.; Peng, Y.; Chen, Y.; Shukla, A.J. Application of Artificial Neural Networks in the Design of Controlled Release Drug Delivery Systems. Adv. Drug Deliv. Rev. 2003, 55, 1201–1215. [Google Scholar] [CrossRef]
- Takayama, K.; Fujikawa, M.; Obata, Y.; Morishita, M. Neural Network Based Optimization of Drug Formulations. Adv. Drug Deliv. Rev. 2003, 55, 1217–1231. [Google Scholar] [CrossRef]
- Colbourn, E.A.; Rowe, R.C. Novel Approaches to Neural and Evolutionary Computing in Pharmaceutical Formulation: Challenges and New Possibilities. Future Med. Chem. 2009, 1, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Ibrić, S.; Djuriš, J.; Parojčić, J.; Djurić, Z. Artificial Neural Networks in Evaluation and Optimization of Modified Release Solid Dosage Forms. Pharmaceutics 2012, 4, 531–550. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.A. Digital Pharmaceutical Sciences. AAPS PharmSciTech 2020, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bourquin, J.; Schmidli, H.; van Hoogevest, P.; Leuenberger, H. Advantages of Artificial Neural Networks (ANNs) as Alternative Modelling Technique for Data Sets Showing Non-Linear Relationships Using Data from a Galenical Study on a Solid Dosage Form. Eur. J. Pharm. Sci. 1998, 7, 5–16. [Google Scholar] [CrossRef]
- Belic, A.; Skrjanc, I.; Bozic, D.Z.; Karba, R.; Vrecer, F. Minimisation of the Capping Tendency by Tableting Process Optimisation with the Application of Artificial Neural Networks and Fuzzy Models. Eur. J. Pharm. Biopharm. 2009, 73, 172–178. [Google Scholar] [CrossRef]
- Khalid, M.H.; Tuszyński, P.K.; Kazemi, P.; Szlek, J.; Jachowicz, R.; Mendyk, A. Transparent Computational Intelligence Models for Pharmaceutical Tableting Process. Comp. Adapt. Syst. Model. 2016, 4, 7. [Google Scholar] [CrossRef][Green Version]
- Lou, H.; Chung, J.I.; Kiang, Y.-H.; Xiao, L.-Y.; Hageman, M.J. The Application of Machine Learning Algorithms in Understanding the Effect of Core/Shell Technique on Improving Powder Compactability. Int. J. Pharm. 2019, 555, 368–379. [Google Scholar] [CrossRef]
- Millen, N.; Kovačević, A.; Khera, L.; Djuriš, J.; Ibric, S. Machine Learning Modelling of Wet Granulation Scale-up Using Compressibility, Compactibility and Manufacturability Parameters. Chem. Ind. 2019, 73, 155–168. [Google Scholar] [CrossRef]
- Khalid, G.M.; Usman, A.G. Application of Data-Intelligence Algorithms for Modeling the Compaction Performance of New Pharmaceutical Excipients. Future J. Pharm. Sci. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Koo, O.M.Y. Pharmaceutical Excipients: Properties, Functionality, and Applications in Research and Industry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Corzo, C.; Lopes, D.G.; Lochmann, D.; Reyer, S.; Stehr, M.; Salar-Behzadi, S. Novel Approach for Overcoming the Stability Challenges of Lipid-Based Excipients. Part 1: Screening of Solid-State and Physical Properties of Polyglycerol Esters of Fatty Acids as Advanced Pharmaceutical Excipients. Eur. J. Pharm. Biopharm. 2020, 148, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Hamdani, J.; Moës, A.J.; Amighi, K. Physical and Thermal Characterisation of Precirol® and Compritol® as Lipophilic Glycerides Used for the Preparation of Controlled-Release Matrix Pellets. Int. J. Pharm. 2003, 260, 47–57. [Google Scholar] [CrossRef]
- Çınar, M.; Engin, M.; Engin, E.Z.; Ziya Ateşçi, Y. Early Prostate Cancer Diagnosis by Using Artificial Neural Networks and Support Vector Machines. Expert Syst. Appl. 2009, 36, 6357–6361. [Google Scholar] [CrossRef]
- TIBCO Statistica® User’s Guide. Available online: https://docs.tibco.com/pub/stat/14.0.0/doc/html/UsersGuide/GUID-058F49FC-F4EF-4341-96FB-A785C2FA76E9-homepage.html (accessed on 1 March 2021).
- Pitt, K.G.; Webber, R.J.; Hill, K.A.; Dey, D.; Gamlen, M.J. Compression Prediction Accuracy from Small Scale Compaction Studies to Production Presses. Powder Technol. 2015, 270, 490–493. [Google Scholar] [CrossRef]
- Dwivedi, S.K. Analysis of Particle Deformation Mechanisms and Compact Expansion during Compaction on a High Speed Rotary Tablet Press. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1992. [Google Scholar]
- Iurian, S.; Ilie, L.; Achim, M.; Tomuta, I. The evaluation of dynamic compaction analysis as a QbD tool for paediatric orodispersible minitablet formulation. Farmacia 2020, 68, 999–1010. [Google Scholar] [CrossRef]
- Salar-Behzadi, S.; Karrer, J.; Demiri, V.; Barrios, B.; Corzo, C.; Meindl, C.; Lochmann, D.; Reyer, S. Polyglycerol Esters of Fatty Acids as Safe and Stable Matrix Forming Tableting Excipients: A Structure-Function Analysis. J. Drug Deliv. Sci. Technol. 2020, 60, 102019. [Google Scholar] [CrossRef]

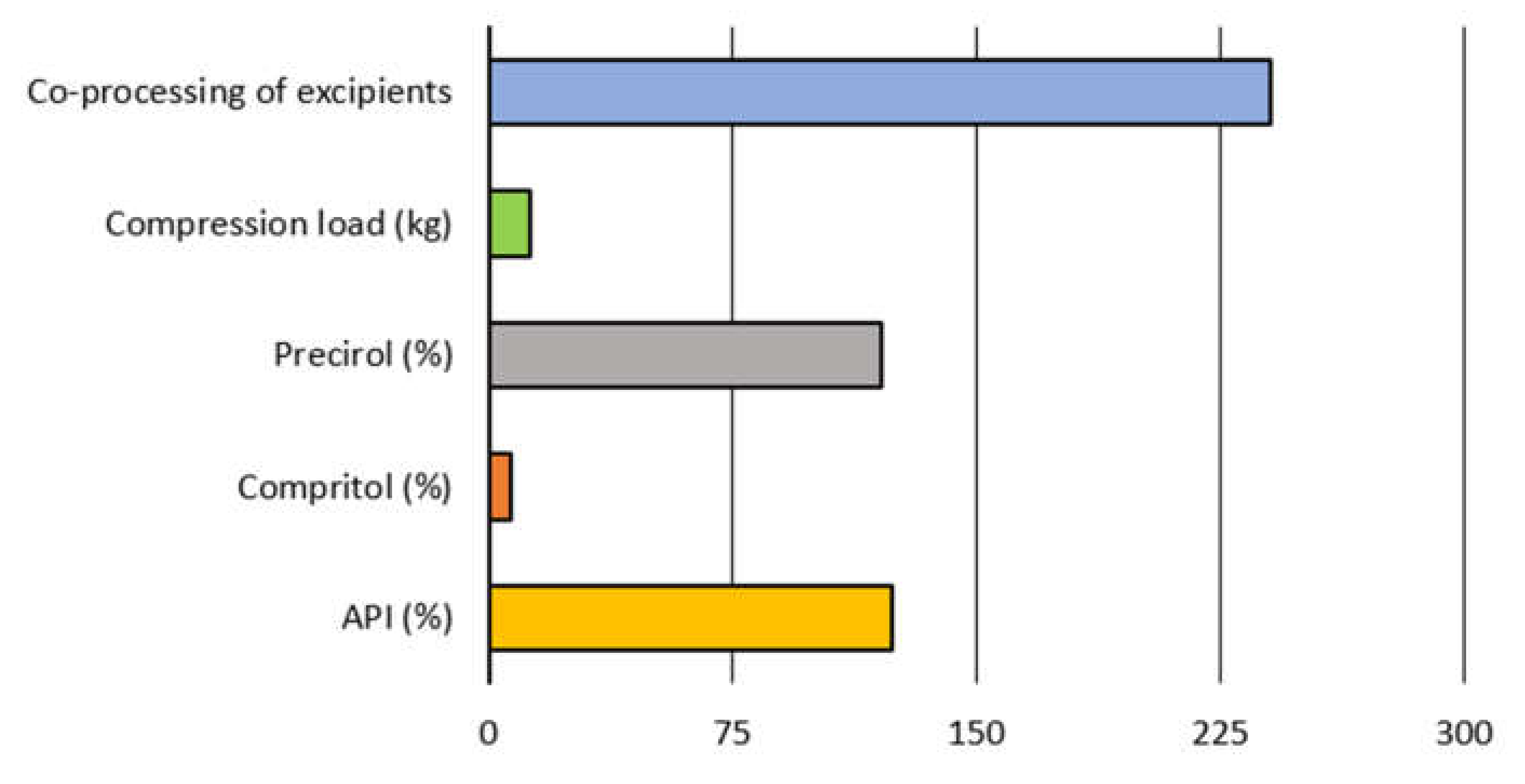
| Datasets | API (%) | Compritol® (%) | Precirol® (%) | Lactose Monohydrate (%) | Compression Load (kg) | State of Excipients |
|---|---|---|---|---|---|---|
| Training, testing, and validation data | 0 | 0 | 0 | 100 | 500 | PM |
| 10 | 0 | 90 | ||||
| 0 | 10 | 90 | ||||
| 15 | 0 | 85 | ||||
| 0 | 15 | 85 | ||||
| 25 | 11.25 | 0 | 63.75 | |||
| 50 | 7.50 | 0 | 42.50 | |||
| 25 | 0 | 11.25 | 63.75 | |||
| 50 | 0 | 7.50 | 42.50 | |||
| 0 | 15 | 0 | 85 | CS | ||
| 0 | 15 | 85 | ||||
| 25 | 11.25 | 0 | 63.75 | |||
| 50 | 7.5 | 0 | 42.50 | |||
| 25 | 0 | 11.25 | 63.75 | |||
| 50 | 0 | 7.5 | 42.50 | |||
| 0 | 0 | 0 | 100 | 100 | PM | |
| 10 | 0 | 90 | ||||
| 0 | 10 | 90 | ||||
| 15 | 0 | 85 | ||||
| 0 | 15 | 85 | ||||
| 25 | 11.25 | 0 | 63.75 | |||
| 50 | 7.5 | 0 | 42.50 | |||
| 25 | 0 | 11.25 | 63.75 | |||
| 50 | 0 | 7.5 | 42.50 | |||
| 0 | 15 | 0 | 85 | CS | ||
| 0 | 15 | 85 | ||||
| 25 | 11.25 | 0 | 63.75 | |||
| 50 | 7.5 | 0 | 42.50 | |||
| 25 | 0 | 11.25 | 63.75 | |||
| 50 | 0 | 7.5 | 42.50 | |||
| External validation dataset | 25 | 11.25 | 0 | 63.75 | 250 | PM |
| 0 | 11.25 | 63.75 | ||||
| 11.25 | 0 | 63.75 | CS | |||
| 0 | 11.25 | 63.75 | ||||
| 50 | 7.5 | 0 | 42.50 | PM | ||
| 0 | 7.5 | 42.50 | ||||
| 7.5 | 0 | 42.50 | CS | |||
| 0 | 7.5 | 42.50 |
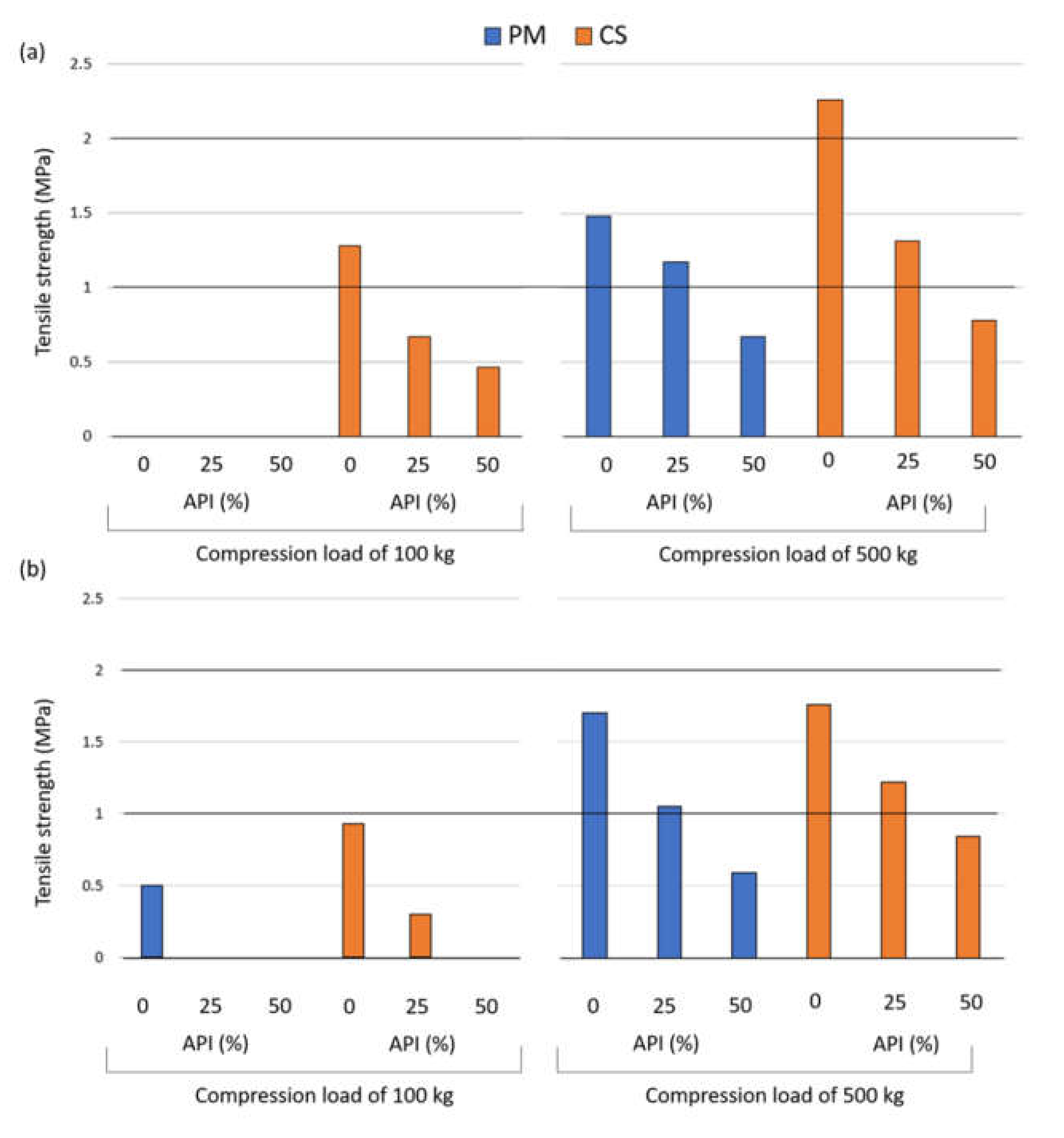
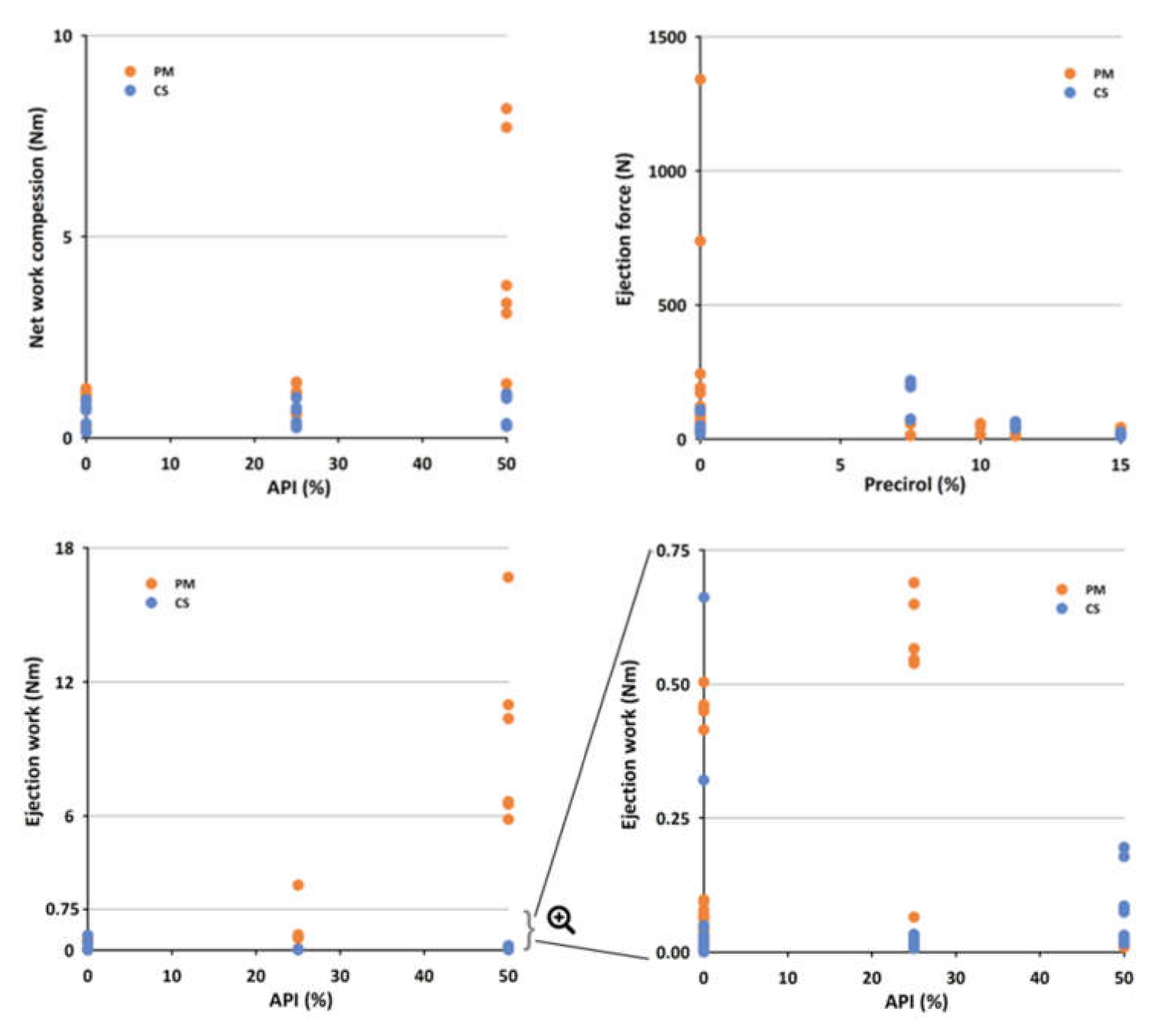
| API (%) | Compritol® (%) | Precirol® (%) | State | TS (MPa) | TWC (Nm) | NWC (Nm) | ER (%) | DW (Nm) | EJW (Nm) | EF (N) | Neuron Position |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 | 11.25 | 0 | PM | 0.64 | 1.10 | 0.93 | 19.3 | 0.14 | 0.58 | 123 | (2,1) |
| 25 | 0 | 11.25 | PM | 0.57 | 0.81 | 0.63 | 21.5 | 0.09 | 0.01 | 30 | (2,1) |
| 25 | 11.25 | 0 | CS | 1.05 | 0.86 | 0.69 | 22.5 | 0.10 | 0.01 | 35 | (2,2) |
| 25 | 0 | 11.25 | CS | 0.53 | 0.68 | 0.51 | 21.6 | 0.18 | 0.03 | 54 | (2,2) |
| 50 | 7.5 | 0 | PM | 0.51 | 4.05 | 3.89 | 17.5 | 0.12 | 8.00 | 692 | (2,1) |
| 50 | 0 | 7.5 | PM | 0.30 | 0.83 | 0.64 | 20.9 | 0.10 | 0.01 | 36 | (2,1) |
| 50 | 7.5 | 0 | CS | 0.70 | 0.85 | 0.68 | 21.6 | 0.25 | 0.01 | 50 | (2,2) |
| 50 | 0 | 7.5 | CS | 0.00 | 0.76 | 0.58 | 21.0 | 0.30 | 0.19 | 190 | (2,2) |
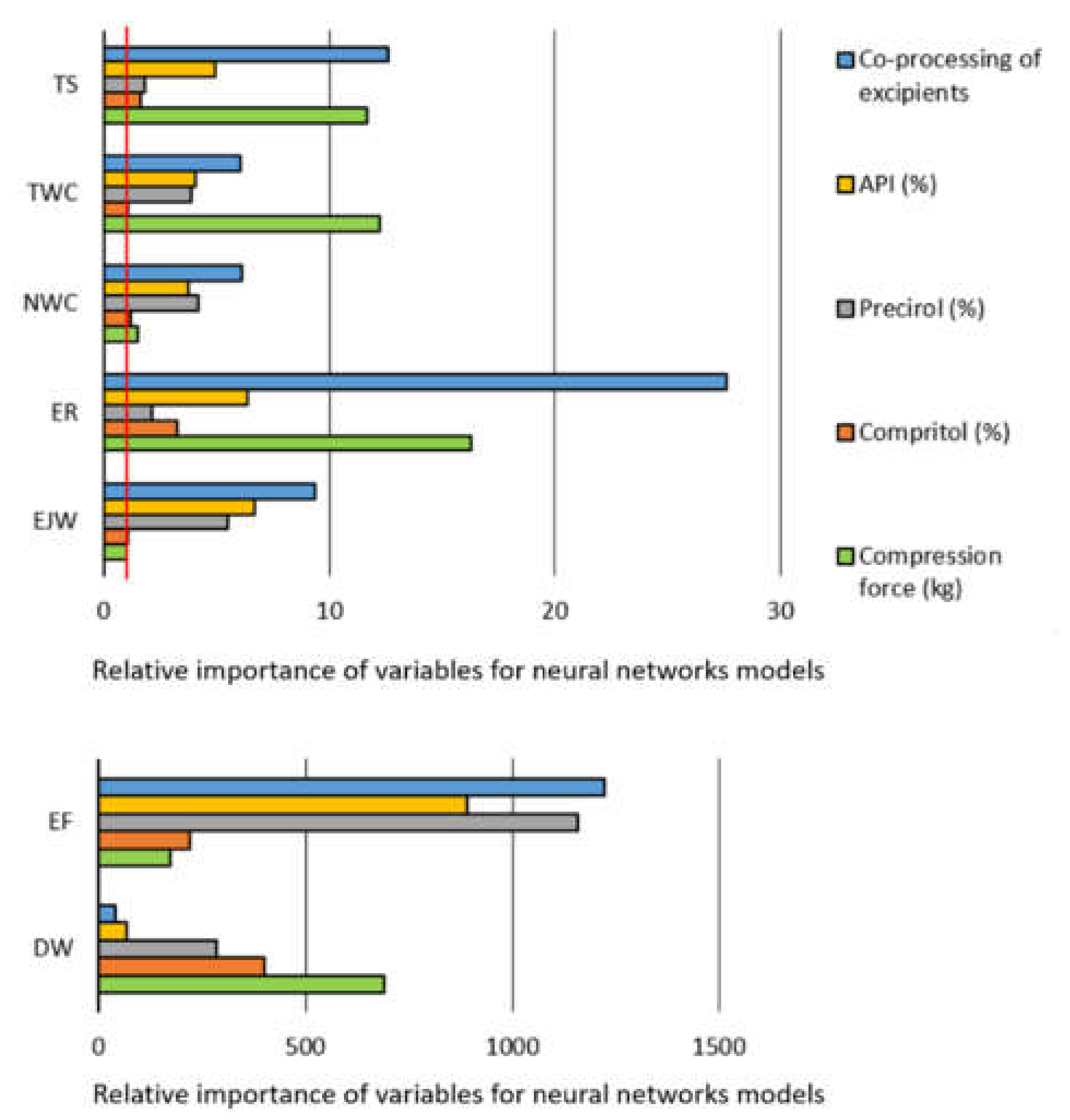
| Target Output | Optimal Neural Network | Correlation Coefficients | Training Algorithm | Activation Functions | ||||
|---|---|---|---|---|---|---|---|---|
| Training Data | Test Data | Validation Data | External Validation Data | Hidden Layer | Output Layer | |||
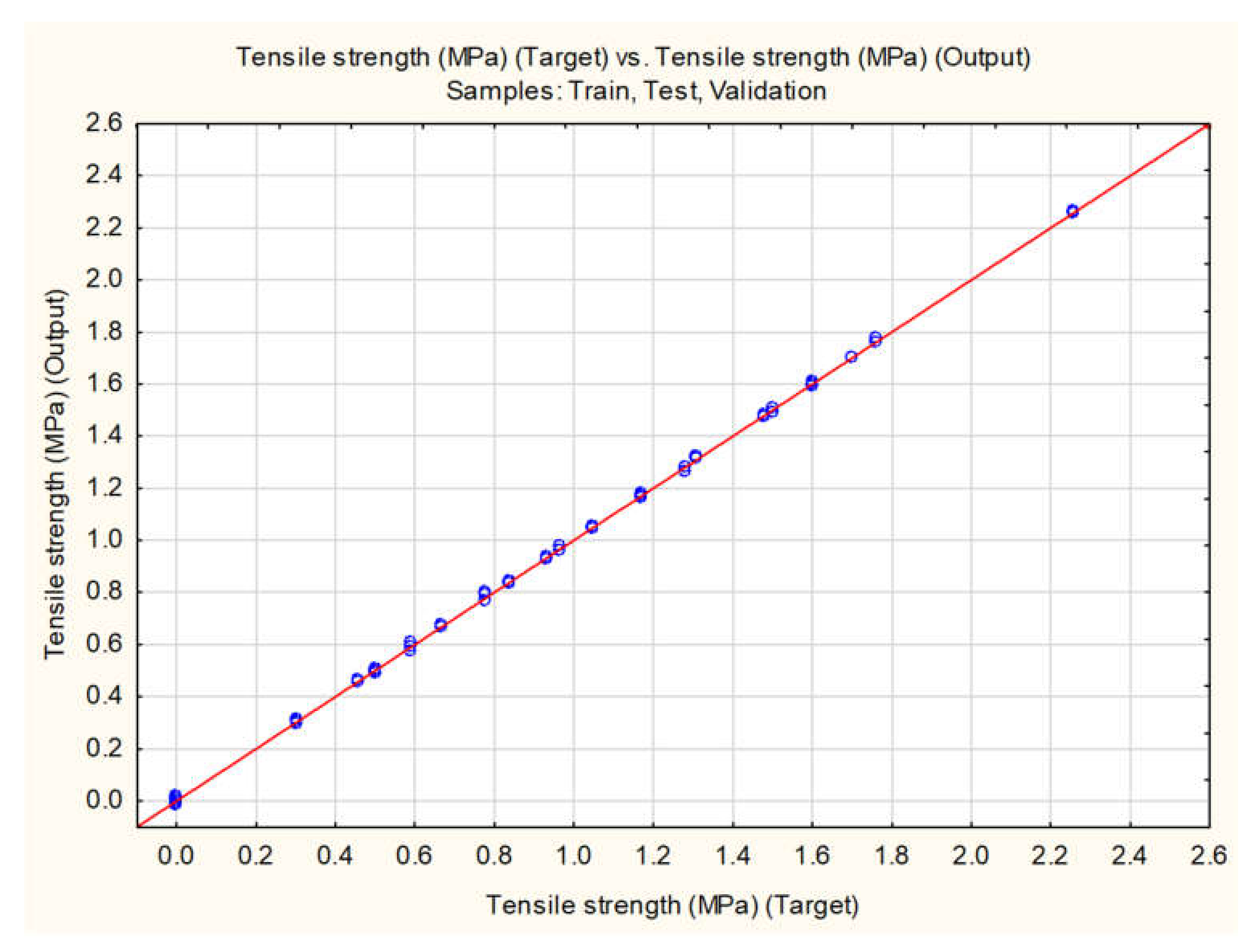
| TS | MLP 6-10-1 | 0.9958 | 0.9943 | 0.9994 | 0.6978 | BFGS 98 | Exp | Exp |
| RBF 6-16-1 | 0.9685 | 0.9830 | 0.9852 | 0.9218 | RBF | Gaussian | Identity | |
| TWC | MLP 6-3-1 | 0.8920 | 0.9932 | 0.9934 | 0.9996 | BFGS 146 | Logistic | Exp |
| RBF 6-18-1 | 0.8628 | 0.9089 | 0.8553 | 0.9890 | RBF | Gaussian | Identity | |
| NWC | MLP 6-7-1 | 0.8772 | 0.9973 | 0.9944 | 0.9998 | BFGS 177 | Logistic | Identity |
| RBF 6-16-1 | 0.8570 | 0.7915 | 0.8095 | 0.9736 | RBF | Gaussian | Identity | |
| ER | MLP 6-11-1 | 0.9786 | 0.9699 | 0.9557 | 0.9554 | BFGS 49 | Logistic | Tanh |
| RBF 6-13-1 | 0.9114 | 0.9462 | 0.9354 | 0.9448 | RBF | Gaussian | Identity | |
| DW | MLP 6-4-1 | 0.9839 | 0.9951 | 0.9736 | 0.9720 | BFGS 107 | Exp | Exp |
| RBF 6-15-1 | 0.9534 | 0.9585 | 0.8769 | 0.9379 | RBF | Gaussian | Identity | |
| EJW | MLP 6-5-1 | 0.9151 | −0.1924 | 0.8487 | 0.9997 | BFGS 6 | Exp | Logistic |
| RBF 6-17-1 | 0.9531 | 0.2711 | 0.8236 | 0.9715 | RBF | Gaussian | Identity | |
| EF | MLP 6-5-1 | 0.9994 | 0.9805 | 0.9772 | 0.9945 | BFGS 147 | Tanh | Logistic |
| RBF 6-17-1 | 0.9566 | 0.6982 | 0.6248 | 0.9726 | RBF | Gaussian | Identity | |
| Correlation Coefficients | Errors | Training Algorithm | Activation Functions | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Training Data | Test Data | Validation Data | External Validation Data | Training Data | Test Data | Validation Data | Hidden Layer | Output Layer | |
| 0.9999 | 0.9999 | 0.9999 | 0.9263 | 0.00002 | 0.00005 | 0.00006 | BFGS 140 | Logistic | Identity |
| Artificial Neural Networks | Correlation Coefficients | Training Algorithm | Activation Functions | ||||
|---|---|---|---|---|---|---|---|
| Training Data | Test Data | Validation Data | External Validation Data | Hidden Layer | Output Layer | ||
| MLP 6-11-7 | 0.9538 | 0.8857 | 0.9328 | 0.9048 | BFGS 151 | Logistic | Tanh |
| MLP 6-11-7 | 0.9553 | 0.8716 | 0.9416 | 0.9416 | BFGS 215 | Exponential | Tanh |
| MLP 6-6-7 | 0.9451 | 0.8510 | 0.9163 | 0.8572 | BFGS 158 | Logistic | Exponential |
| RBF 6-14-7 | 0.9199 | 0.6477 | 0.8200 | 0.8915 | RBF | Gaussian | Identity |
| RBF 6-16-7 | 0.9162 | 0.7197 | 0.8746 | 0.9111 | RBF | Gaussian | Identity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djuris, J.; Cirin-Varadjan, S.; Aleksic, I.; Djuris, M.; Cvijic, S.; Ibric, S. Application of Machine-Learning Algorithms for Better Understanding of Tableting Properties of Lactose Co-Processed with Lipid Excipients. Pharmaceutics 2021, 13, 663. https://doi.org/10.3390/pharmaceutics13050663
Djuris J, Cirin-Varadjan S, Aleksic I, Djuris M, Cvijic S, Ibric S. Application of Machine-Learning Algorithms for Better Understanding of Tableting Properties of Lactose Co-Processed with Lipid Excipients. Pharmaceutics. 2021; 13(5):663. https://doi.org/10.3390/pharmaceutics13050663
Chicago/Turabian StyleDjuris, Jelena, Slobodanka Cirin-Varadjan, Ivana Aleksic, Mihal Djuris, Sandra Cvijic, and Svetlana Ibric. 2021. "Application of Machine-Learning Algorithms for Better Understanding of Tableting Properties of Lactose Co-Processed with Lipid Excipients" Pharmaceutics 13, no. 5: 663. https://doi.org/10.3390/pharmaceutics13050663
APA StyleDjuris, J., Cirin-Varadjan, S., Aleksic, I., Djuris, M., Cvijic, S., & Ibric, S. (2021). Application of Machine-Learning Algorithms for Better Understanding of Tableting Properties of Lactose Co-Processed with Lipid Excipients. Pharmaceutics, 13(5), 663. https://doi.org/10.3390/pharmaceutics13050663