Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Preparation of Ocular Tissues
2.2. Permeability Studies with the Ussing Chamber
2.3. Selection of Ophthalmic Drugs for Permeability Experiments
2.4. Determination of Permeability Coefficients
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geroski, D.H.; Edelhauser, H.F. Drug delivery for posterior segment eye disease. Investig. Ophthalmol. Vis. Sci. 2000, 41, 961–964. [Google Scholar]
- Novack, G.D.; Robin, A.L. Ocular pharmacology. J. Clin. Pharmacol. 2015, 56, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef] [PubMed]
- Delplace, V.; Ortin-Martinez, A.; Tsai, E.L.S.; Amin, A.N.; Wallace, V.; Shoichet, M.S. Controlled release strategy designed for intravitreal protein delivery to the retina. J. Control. Release Off. J. Control. Release Soc. 2019, 293, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Kang-Mieler, J.J.; Rudeen, K.M.; Liu, W.; Mieler, W.F. Advances in ocular drug delivery systems. Eye 2020, 34, 1371–1379. [Google Scholar] [CrossRef]
- Cunha-Vaz, J.G. The blood–retinal barriers system. Basic concepts and clinical evaluation. Exp. Eye Res. 2004, 78, 715–721. [Google Scholar] [CrossRef]
- Gaudreault, J.; Fei, D.; Beyer, J.C.; Ryan, A.; Rangell, L.; Shiu, V.; Damico, L.A. Pharmacokinetics and Retinal Distribution of Ranibizumab, A Humanized Antibody Fragment Directed Against Vegf-A, Following Intravitreal Administration In Rabbits. Retina 2007, 27, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- I Sinapis, A.; Sinapis, C.I.; Sinapis, D.I.; Routsias, J.G.; Pantopoulou, A.; Baltatzis, S.; Patsouris, E.; Perrea, D. Pharmacokinetics of intravitreal bevacizumab (Avastin®) in rabbits. Clin. Ophthalmol. 2011, 5, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Vellonen, K.-S.; Kidron, H.; Urtti, A. Intravitreal clearance and volume of distribution of compounds in rabbits: In silico prediction and pharmacokinetic simulations for drug development. Eur. J. Pharm. Biopharm. 2015, 95, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.B. Extracellular matrix alterations precede vascularization of the retinal pigment epithelium in dystrophic rats. Curr. Eye Res. 1989, 8, 907–921. [Google Scholar] [PubMed]
- Nishihara, H. Studies on the ultrastructure of the inner limiting membrane of the retina--distribution of anionic sites in the inner limiting membrane of the retina. Nippon. Ganka Gakkai zasshi 1991, 95, 951–958. [Google Scholar]
- Ambati, J.; Canakis, C.S.; Miller, J.W.; Gragoudas, E.S.; Edwards, A.; Weissgold, D.J.; Kim, I.; Delori, F.C.; Adamis, A.P. Diffusion of high molecular weight compounds through sclera. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1181–1185. [Google Scholar]
- Pitkänen, L.; Ranta, V.-P.; Moilanen, H.; Urtti, A. Permeability of Retinal Pigment Epithelium: Effects of Permeant Molecular Weight and Lipophilicity. Investig. Opthalmol. Vis. Sci. 2005, 46, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Park, K.H.; Chung, J.Y.; Woo, S.J. A Prediction Model for the Intraocular Pharmacokinetics of Intravitreally Injected Drugs Based on Molecular Physicochemical Properties. Ophthalmic Res. 2019, 63, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ussing, H.H. Transport of Water and Solutes through Living Membranes. Clin. Sci. 1972, 42, 23–24. [Google Scholar] [CrossRef]
- Kimura, M.; Araie, M.; Koyano, S. Movement of Carboxyfluorescein across Retinal Pigment Epithelium–Choroid. Exp. Eye Res. 1996, 63, 51–56. [Google Scholar] [CrossRef]
- Kadam, R.S.; Cheruvu, N.P.S.; Edelhauser, H.F.; Kompella, U.B. Sclera-Choroid-RPE Transport of Eight β-Blockers in Human, Bovine, Porcine, Rabbit, and Rat Models. Investig. Opthalmol. Vis. Sci. 2011, 52, 5387–5399. [Google Scholar] [CrossRef]
- Loch, C.; Zakelj, S.; Kristl, A.; Nagel, S.; Guthoff, R.; Weitschies, W.; Seidlitz, A. Determination of permeability coefficients of ophthalmic drugs through different layers of porcine, rabbit and bovine eyes. Eur. J. Pharm. Sci. 2012, 47, 131–138. [Google Scholar] [CrossRef]
- Ramsay, E.; Hagström, M.; Vellonen, K.-S.; Boman, S.; Toropainen, E.; del Amo, E.M.; Kidron, H.; Urtti, A.; Ruponen, M. Role of retinal pigment epithelium permeability in drug transfer between posterior eye segment and systemic blood circulation. Eur. J. Pharm. Biopharm. Off. J. Arb. Pharm. 2019, 143, 18–23. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, H.; Woo, S.J.; Park, J.H.; Park, S.; Hwang, D.J.; Park, K.H. Pharmacokinetics of Intravitreally Injected Bevacizumab in Vitrectomized Eyes. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2013, 29, 612–618. [Google Scholar] [CrossRef]
- Ahn, S.J.; Ahn, J.; Park, S.; Kim, H.; Hwang, D.J.; Park, J.H.; Park, J.Y.; Chung, J.Y.; Park, K.H.; Woo, S.J. Intraocular Pharmacokinetics of Ranibizumab in Vitrectomized Versus Nonvitrectomized Eyes. Investig. Opthalmol. Vis. Sci. 2014, 55, 567–573. [Google Scholar] [CrossRef]
- Park, S.J.; Oh, J.; Kim, Y.-K.; Park, J.H.; Hong, H.K.; Park, K.H.; Lee, J.-E.; Kim, H.M.; Chung, J.Y.; Woo, S.J. Intraocular pharmacokinetics of intravitreal vascular endothelial growth factor-Trap in a rabbit model. Eye 2015, 29, 561–568. [Google Scholar] [CrossRef]
- Park, S.J.; Choi, Y.; Na, Y.M.; Hong, H.K.; Park, J.Y.; Park, K.H.; Chung, J.Y.; Woo, S.J. Intraocular Pharmacokinetics of Intravitreal Aflibercept (Eylea) in a Rabbit Model. Investig. Opthalmol. Vis. Sci. 2016, 57, 2612–2617. [Google Scholar] [CrossRef]
- Joo, K.; Park, S.J.; Choi, Y.; Lee, J.E.; Na, Y.M.; Hong, H.K.; Park, K.H.; Kim, H.M.; Chung, J.-Y.; Woo, S.J. Role of the Fc Region in the Vitreous Half-Life of Anti-VEGF Drugs. Investig. Opthalmol. Vis. Sci. 2017, 58, 4261–4267. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wheeler, L.; WoldeMussie, E.; Lai, R. Role of Alpha-2 Agonists in Neuroprotection. Surv. Ophthalmol. 2003, 48, S47–S51. [Google Scholar] [CrossRef]
- Kuppermann, B.D.; Patel, S.S.; Boyer, D.S.; Augustin, A.J.; Freeman, W.R.; Kerr, K.J.; Guo, Q.; Schneider, S.; López, F.J. Phase 2 study of the safety and efficacy of brimonidine drug delivery system (brimo dds) generation 1 in patients with geographic atrophy secondary to age-related macular degeneration. Retina 2021, 41, 144–155. [Google Scholar] [CrossRef]
- Henderly, D.E.; Freeman, W.R.; Causey, D.M.; Rao, N.A. Cytomegalovirus Retinitis and Response to Therapy with Ganciclovir. Ophthalmology 1987, 94, 425–434. [Google Scholar] [CrossRef]
- Macha, S.; Mitra, A.K. Ocular Disposition of Ganciclovir and Its Monoester Prodrugs following Intravitreal Administration Using Microdialysis. Drug Metab. Dispos. 2002, 30, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Durairaj, C.; Lin, T.; Liu, Y.; Burke, J. Ocular Pharmacokinetics of Intravitreally Administered Brimonidine and Dexamethasone in Animal Models with and Without Blood–Retinal Barrier Breakdown. Investig. Opthalmol. Vis. Sci. 2014, 55, 1056–1066. [Google Scholar] [CrossRef]
- Bakri, S.J.; Snyder, M.R.; Reid, J.M.; Pulido, J.S.; Singh, R.J. Pharmacokinetics of Intravitreal Bevacizumab (Avastin). Ophthalmology 2007, 114, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Barza, M.; McCue, M. Pharmacokinetics of aztreonam in rabbit eyes. Antimicrob. Agents Chemother. 1983, 24, 468–473. [Google Scholar] [CrossRef]
- Velez, G.; Yuan, P.; Sung, C.; Tansey, G.; Reed, G.F.; Chan, C.-C.; Nussenblatt, R.B.; Robinson, M.R. Pharmacokinetics and toxicity of intravitreal chemotherapy for primary intraocular lymphoma. Arch. Ophthalmol. 2001, 119, 1518–1524. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Q.F.; Perkins, R.; Drusano, G.; Louie, A.; Madu, A.; Mian, U.; Mayers, M.; Miller, M.H. Pharmacokinetics of Sparfloxacin in the Serum and Vitreous Humor of Rabbits: Physicochemical Properties That Regulate Penetration of Quinolone Antimicrobials. Antimicrob. Agents Chemother. 1998, 42, 1417–1423. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gupta, S.K.; Velpandian, T.; Dhingra, N.; Jaiswal, J. Intravitreal Pharmacokinetics of Plain and Liposome-Entrapped Fluconazole in Rabbit Eyes. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2000, 16, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Wang, M.-Y.; Wang, C.-Y.; Tsai, T.-C.; Tsai, H.-Y.; Lee, Y.-F.; Wei, L.-C. Clearance of Intravitreal Voriconazole. Investig. Opthalmol. Vis. Sci. 2007, 48, 2238–2241. [Google Scholar] [CrossRef]
- Kwak, H.W. Evaluation of the Retinal Toxicity and Pharmacokinetics of Dexamethasone after Intravitreal Injection. Arch. Ophthalmol. 1992, 110, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Komarowska, I.; Heilweil, G.; Rosenfeld, P.J.; Perlman, I.; Loewenstein, A. Retinal toxicity of commercially available intravitreal ketorolac in albino rabbits. Retina 2009, 29, 98–105. [Google Scholar] [CrossRef]
- Zhang, N.; Kannan, R.; Okamoto, C.T.; Ryan, S.J.; Lee, V.H.L.; Hinton, D.R. Characterization of Brimonidine Transport in Retinal Pigment Epithelium. Investig. Opthalmol. Vis. Sci. 2006, 47, 287–294. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Skarphedinsdottir, S.B.; Eysteinsson, T.; Árnason, S.S. Mechanisms of Ion Transport across the Mouse Retinal Pigment Epithelium Measured In Vitro. Investig. Opthalmol. Vis. Sci. 2020, 61, 31. [Google Scholar] [CrossRef]
- Hutton-Smith, L.A.; Gaffney, E.A.; Byrne, H.M.; Maini, P.K.; Gadkar, K.; Mazer, N.A. Ocular Pharmacokinetics of Therapeutic Antibodies Given by Intravitreal Injection: Estimation of Retinal Permeabilities Using a 3-Compartment Semi-Mechanistic Model. Mol. Pharm. 2017, 14, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Haghjou, N.; Abdekhodaie, M.J.; Cheng, Y.-L. Retina-Choroid-Sclera Permeability for Ophthalmic Drugs in the Vitreous to Blood Direction: Quantitative Assessment. Pharm. Res. 2012, 30, 41–59. [Google Scholar] [CrossRef] [PubMed]
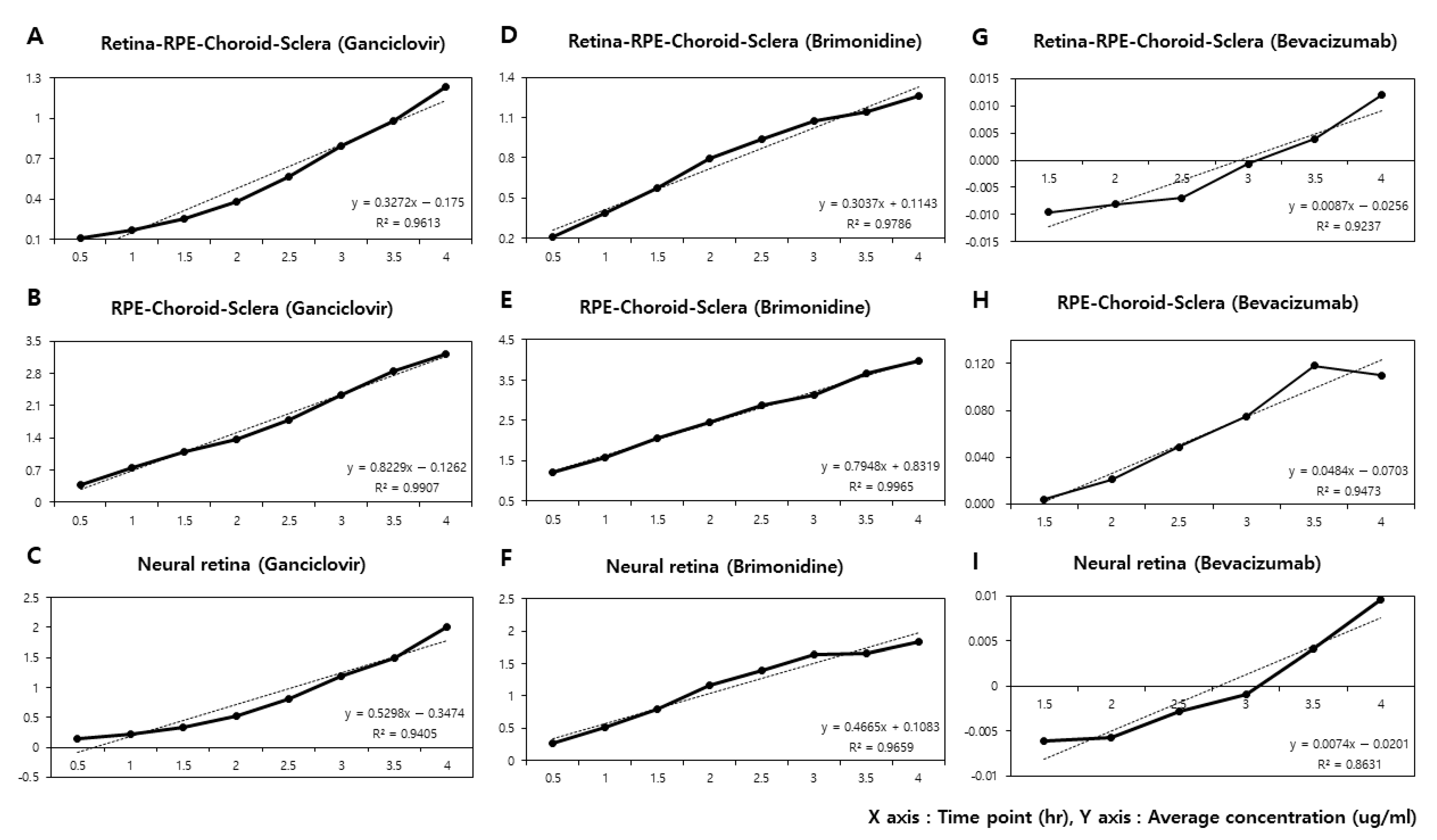
| Time Point (h) | Ganciclovir (n = 5) | Brimonidine (n = 5) | Bevacizumab (n = 5) | |||
|---|---|---|---|---|---|---|
| RCS (μg/mL) | CS (μg/mL) | RCS (μg/mL) | CS (μg/mL) | RCS (μg/mL) | CS (μg/mL) | |
| 0.5 h | 0.108 | 0.382 | 0.215 | 1.216 | −0.009 | −0.009 |
| 1.0 h | 0.172 | 0.753 | 0.386 | 1.578 | −0.007 | −0.10 |
| 1.5 h | 0.254 | 1.084 | 0.571 | 2.057 | −0.010 | 0.004 |
| 2.0 h | 0.383 | 1.375 | 0.793 | 2.465 | −0.008 | 0.021 |
| 2.5 h | 0.562 | 1.792 | 0.937 | 2.873 | −0.007 | 0.049 |
| 3.0 h | 0.793 | 2.341 | 1.074 | 3.124 | −0.001 | 0.075 |
| 3.5 h | 0.981 | 2.859 | 1.146 | 3.681 | 0.004 | 0.118 |
| 4.0 h | 1.237 | 3.217 | 1.258 | 3.967 | 0.012 | 0.110 |
| (μg/mL·h) | 0.3272 | 0.8229 | 0.3037 | 0.7948 | 0.0087 | 0.0484 |
| Parameters | Ganciclovir (n = 5) | Brimonidine (n = 5) | Bevacizumab (n = 5) |
|---|---|---|---|
| Molecular weight (Da) | 255.23 | 292.13 | 149,000 |
| Log P | −1.66 | 1.7 | - |
| Water solubility (mg/mL) | 4.3 | 1.5 | - |
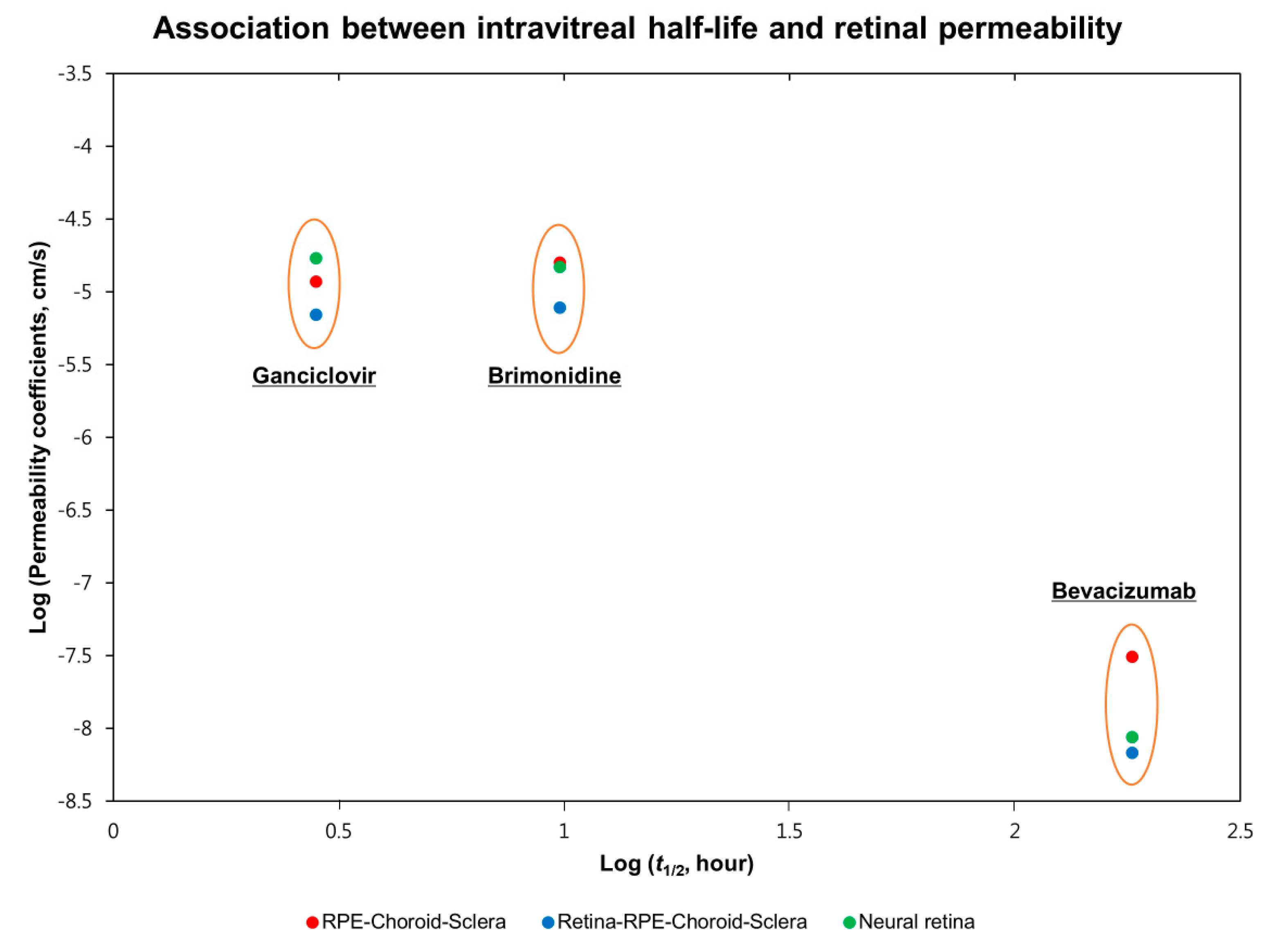
| Intravitreal half-life (t1/2, h) | 2.83 ‡ [28] | 9.9 † [29] | 181.4 ‡ [30] |
| Permeability coefficients (Papp) (×10−6 cm/s) | |||
| Retina-RPE-Choroid-Sclera (RCS) | 13.78 ± 5.82 | 15.34 ± 7.64 | 0.0136 ± 0.0059 |
| RPE-Choroid-Sclera (CS) | 23.22 ± 9.74 | 31.56 ± 12.46 | 0.0612 ± 0.0264 |
| Neural Retina (R) * | 33.89 ± 12.64 | 29.83 ± 11.58 | 0.0205 ± 0.0074 |
| Molecules | Molecular Weight (Da) | Log P | Intravitreal Half-Life (t1/2, h) * | Permeability Coefficients (Papp) (×10−6 cm/s) # | Species | Reference |
|---|---|---|---|---|---|---|
| Aztreonam | 434.44 | −4.4 | 7.5 [31] | 5.37 ± 5.19 | Bovine | Ramsay et al., 2019 [19] |
| Methotrexate | 454.45 | −0.241 | 7.6 [32] | 9.39 ± 2.74 | Bovine | Ramsay et al., 2019 [19] |
| Ciprofloxacin | 331.3 | 1.313 | 4.41 [33] | 9.52 ± 5.28 | Bovine | Ramsay et al., 2019 [19] |
| Fluconazole | 306.27 | 0.5 | 3.18 [34] | 15.64 ± 4.66 | Bovine | Ramsay et al., 2019 [19] |
| Voriconazole | 349.31 | 0.927 | 2.5 [35] | 25.00 ± 6.12 | Bovine | Ramsay et al., 2019 [19] |
| Dexamethasone | 472 | 0.65 | 3.5 [36] | 8.90 ± 1.6 | Porcine | Loch et al., 2012 [18] |
| Ketorolac | 376.41 | 2.1 | 3.09 [37] | 69.21 ± 31.9 | Bovine | Ramsay et al., 2019 [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Han, H.; Hong, H.K.; Park, J.H.; Park, K.H.; Kim, H.; Woo, S.J. Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors. Pharmaceutics 2021, 13, 655. https://doi.org/10.3390/pharmaceutics13050655
Kim HM, Han H, Hong HK, Park JH, Park KH, Kim H, Woo SJ. Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors. Pharmaceutics. 2021; 13(5):655. https://doi.org/10.3390/pharmaceutics13050655
Chicago/Turabian StyleKim, Hyeong Min, Hyounkoo Han, Hye Kyoung Hong, Ji Hyun Park, Kyu Hyung Park, Hyuncheol Kim, and Se Joon Woo. 2021. "Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors" Pharmaceutics 13, no. 5: 655. https://doi.org/10.3390/pharmaceutics13050655
APA StyleKim, H. M., Han, H., Hong, H. K., Park, J. H., Park, K. H., Kim, H., & Woo, S. J. (2021). Permeability of the Retina and RPE-Choroid-Sclera to Three Ophthalmic Drugs and the Associated Factors. Pharmaceutics, 13(5), 655. https://doi.org/10.3390/pharmaceutics13050655







