Activity of Povidone in Recent Biomedical Applications with Emphasis on Micro- and Nano Drug Delivery Systems
Abstract
1. Introduction
2. Popular Biomedical Applications of pVP
3. pVP in Micro- and Nano Drug Delivery Systems
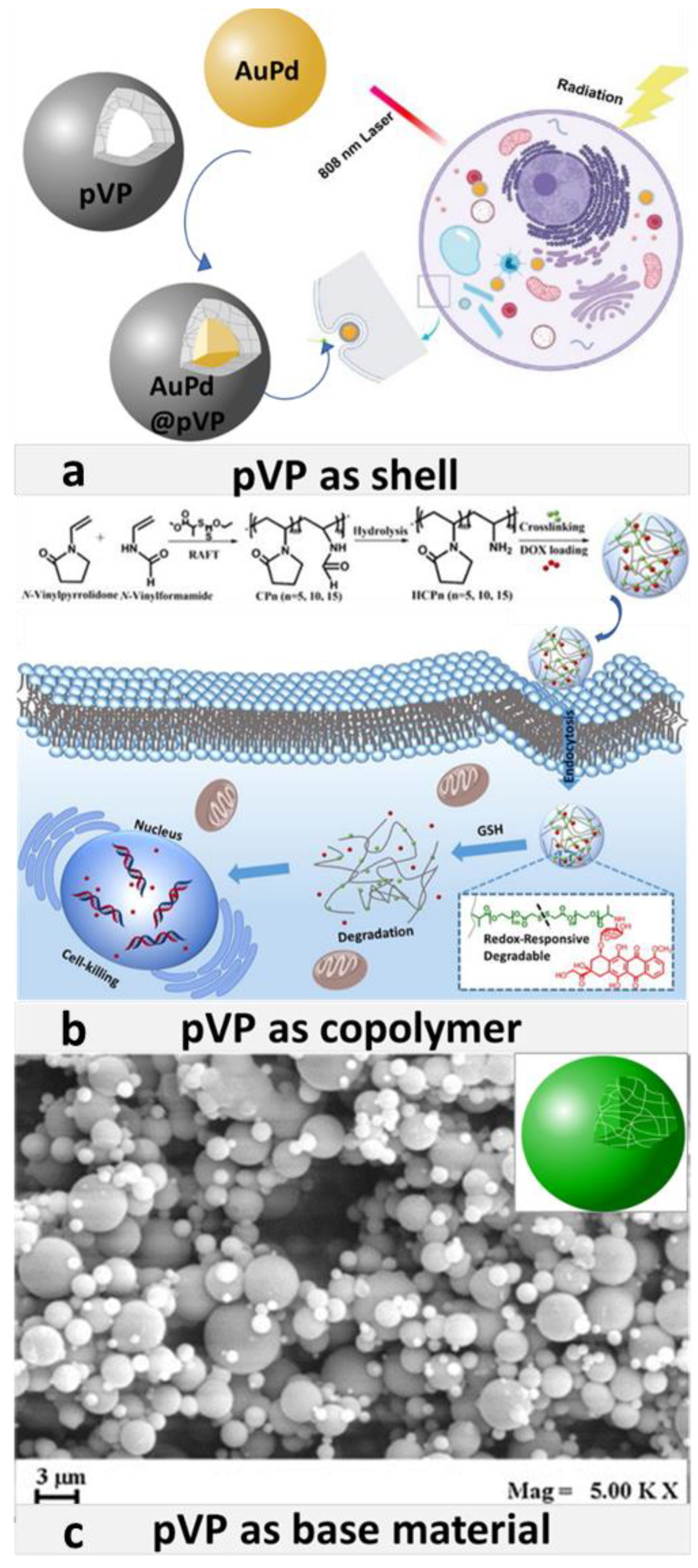
3.1. pVP Coating
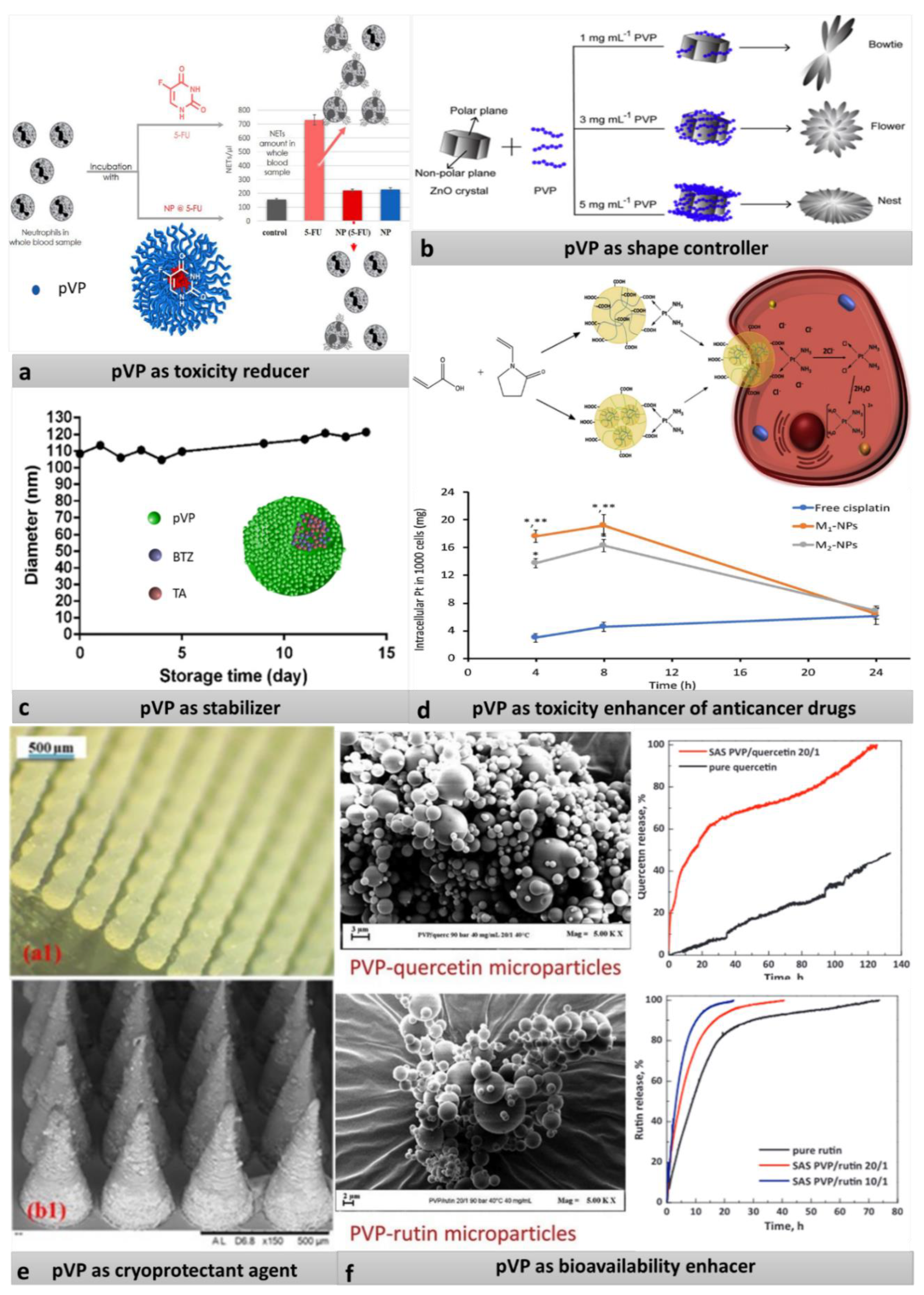
3.1.1. pVP as Stabilizer and Size Limiter
3.1.2. pVP as Shape Controller
3.1.3. pVP as Cryoprotective Agent
3.1.4. pVP as Unwanted-Toxicity Reducer
3.1.5. pVP for Protection and Solubilization of Substances and for Enhancing Solubility
3.2. pVP in Copolymers
3.2.1. pVP as LCST Stabilizer
3.2.2. pVP as Solubilizing Agent
3.2.3. pVP as Base Material
4. Limitations of pVP
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Dox@pVP-AuNPs | doxorubicin loaded povidone coated gold nanoparticles |
| 5FU | 5-fluorouracil |
| AAM | acrylamide |
| Ag-NPs | silver nanoparticles |
| Amph-pVP | amphiphilic poly-N-vinylpyrrolidone |
| Au-NPs | gold nanoparticles |
| AuPd@pVP NP | povidone coated gold-palladium nanoparticles |
| BTZ | bortezomib |
| CDDP | cis-diamminedichloroplatinum II |
| CTAB | cetyl trimethylammonium bromide |
| Cur | curcumin |
| DDSs | drug delivery systems |
| DEAEMA | 2-(diethylamino)ethyl methacrylate |
| FA–CurAu-PVP NPs | folate–curcumin-loaded gold–polyvinylpyrrolidone nanoparticles |
| Gd2O3 NPs | gadolin oxide nanoparticles |
| GEF | gefitnib |
| GO-NPs | graphene oxide nanoparticles |
| HPMC | hydroxypropyl methylcellulose |
| HPβCD | hydroxypropyl-β-cyclodextrin |
| LCST | lower critical solution temperature |
| NaBH4 | sodium borohydride |
| NAC | N-acetyl-l-cysteine |
| NC | nanocapsules |
| NE | nanoemulsion |
| NETs | neutrophil extracellular traps |
| NIPAAm/NVP | poly(N-isopropylacrylamide-co-N-vinylpyrrolidon |
| NVP | 1-vinyl-2-pyrrolidone |
| PEO | poly(ethylene oxide) |
| PEO-b-P2VP | 2-vinyl pyridine poly(ethylene oxide)-b-poly(2-vin(2-vinyl pyridine |
| PLGA-H | polylactide-co-glycolide |
| PLGA-PEG | polylactide-co-glycolide-co-polyethylenglycol |
| PTT | photothermal therapy |
| pVP | poly (N-vinyl pyrrolidone) |
| pVP-I | povidone iodine |
| pVP-Ag-NPs | povidone coated silver nanoparticles |
| QSR | quercetin |
| RAFT | the reversible addition–fragmentation chain-transfer polymerization |
| SAS | supercritical antisolvent process |
| THP-1 | human monocytic cell line |
References
- Laffleur, F.; Keckeis, V. Advances in drug delivery systems: Work in progress still needed? Int. J. Pharm. 2020, 2, 100050. [Google Scholar]
- LaSala, R.; LoGreco, A.; Romagnoli, A.; Santoleri, F.; Musicco, F.; Costantini, A. Cancer drugs for solid tumors approved by the EMA since 2014: An overview of pivotal clinical trials. Eur. J. Clin. Pharmacol. 2020, 76, 843–850. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.E.R.; Borthwick, A.; Rigg, A.; Leary, A.; Assersohn, L.; Last, K.; Tan, S.; Milan, S.; Tait, D.; E Smith, I. Mortality within 30 days of chemotherapy: A clinical governance benchmarking issue for oncology patients. Br. J. Cancer 2006, 95, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Drug Delivery Systems; Humana Press: Totowa, NJ, USA, 2008. [Google Scholar]
- Boverhof, D.R.; Bramante, C.M.; Butala, J.H.; Clancy, S.F.; Lafranconi, M.; West, J.; Gordon, S.C. Comparative assessment of na-nomaterial definitions and safety evaluation considerations. Regul. Toxicol. Pharmacol. 2015, 73, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Hossain, M.K.; Basher, M.; Mia, M.; Rahman, M.; Uddin, M.J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. [Google Scholar] [CrossRef]
- Hamidi, M.; Azadi, A.; Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1638–1649. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]
- Constantin, M.; Bucatariu, S.; Ascenzi, P.; Butnaru, M.; Fundueanu, G. Smart drug delivery system activated by specific biomole-cules. Mater. Sci. Eng. C 2020, 108, 110466. [Google Scholar] [CrossRef]
- Karbarz, M.; Mackiewicz, M.; Kaniewska, K.; Marcisz, K.; Stojek, Z. Recent developments in design and functionalization of micro- and nanostructural environmentally-sensitive hydrogels based on N-isopropylacrylamide. Appl. Mater. Today 2017, 9, 516–532. [Google Scholar] [CrossRef]
- Rosso, A.P.; Martinelli, M. Preparation and characterization of dendronized chitosan/gelatin-based nanogels. Eur. Polym. J. 2020, 124, 109506. [Google Scholar] [CrossRef]
- Mahmoodzadeh, F.; Ghorbani, M.; Jannat, B. Glutathione and pH-responsive chitosan-based nanogel as an efficient nanoplatform for controlled delivery of doxorubicin. J. Drug Deliv. Sci. Technol. 2019, 54, 101315. [Google Scholar] [CrossRef]
- Rao, K.M.; Ramanjaneyulu, G.; Ha, C.-S. Curcumin encapsulated pH sensitive gelatin based interpenetrating polymeric network nanogels for anti cancer drug delivery. Int. J. Pharm. 2015, 478, 788–795. [Google Scholar] [CrossRef]
- Suraiya, A.B.; Hun, M.L.; Truong, V.X.; Forsythe, J.S.; Chidgey, A.P. Gelatin-based 3D microgels for in vitro T lineage cell generation. ACS Biomater. Sci. Eng. 2020, 6, 2198–2208. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Saha, S. Microencapsulated zero valent iron nanoparticles in polylactic acid matrix for in situ remediation of contaminated water. J. Environ. Chem. Eng. 2020, 8, 103909. [Google Scholar] [CrossRef]
- Lu, T.; Hu, F.; Yue, H.; Yang, T.; Ma, G. The incorporation of cationic property and immunopotentiator in poly (lactic acid) microparticles promoted the immune response against chronic hepatitis B. J. Control. Release 2020, 321, 576–588. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Li, B.; Hu, T.; Yang, Y.; Li, L.; Zhang, J. Environmentally benign and durable superhydrophobic coatings based on SiO2 nanoparticles and silanes. J. Colloid Interface Sci. 2019, 542, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Han, J.-Y.; Gao, Y.-J.; Jiang, S.-L.; Sun, F. Surface morphology and property of UV-cured film containing photopolymerizable polysiloxane-based nanogels with initiating capability. Int. J. Ind. Chem. 2019, 10, 281–289. [Google Scholar] [CrossRef]
- Ghorbani, M.; Nezhad-Mokhtari, P.; Ramazani, S. Aloe vera-loaded nanofibrous scaffold based on Zein/Polycaprolactone/Collagen for wound healing. Int. J. Biol. Macromol. 2020, 153, 921–930. [Google Scholar] [CrossRef]
- Lazurko, C.; Khatoon, Z.; Goel, K.; Sedlakova, V.; Cimenci, C.E.; Ahumada, M.; Zhang, L.; Mah, T.-F.; Franco, W.; Suuronen, E.J.; et al. Multifunctional nano and collagen-based therapeutic materials for skin repair. ACS Biomater. Sci. Eng. 2020, 6, 1124–1134. [Google Scholar] [CrossRef]
- Manuja, A.; Raguvaran, R.; Kumar, B.; Kalia, A.; Tripathi, B. Accelerated healing of full thickness excised skin wound in rabbits using single application of alginate/acacia based nanocomposites of ZnO nanoparticles. Int. J. Biol. Macromol. 2020, 155, 823–833. [Google Scholar] [CrossRef]
- Singh, I.; Lacko, C.S.; Zhao, Z.; Schmidt, C.E.; Rinaldi, C. Preparation and evaluation of microfluidic magnetic alginate micropar-ticles for magnetically templated hydrogels. J. Colloid Interface Sci. 2020, 561, 647–658. [Google Scholar] [CrossRef]
- Waleka, E.; Mackiewicz, M.; Romanski, J.; Dybko, A.; Stojek, Z.; Karbarz, M. Degradable nanohydrogel with high doxorubicin loadings exhibiting controlled drug release and decreased toxicity against healthy cells. Int. J. Pharm. 2020, 579, 119188. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, N.; Peng, Q.; Cheng, X.; Li, W. Temperature/pH dual-responsive and luminescent drug carrier based on PNIPAM-MAA/lanthanide-polyoxometalates for controlled drug delivery and imaging in HeLa cells. Mater. Chem. Phys. 2020, 239, 121994. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Stojek, Z.; Karbarz, M. Synthesis of cross-linked poly(acrylic acid) nanogels in an aqueous environment using precipitation polymerization: Unusually high volume change. R. Soc. Open Sci. 2019, 6, 190981. [Google Scholar] [CrossRef]
- Kalasin, S.; Sangnuang, P.; Khownarumit, P.; Tang, I.M.; Surareungchai, W. Evidence of Cu(I) coupling with creatinine using cuprous nanoparticles encapsulated with polyacrylic acid (PAA) Gel-Cu (II) in facilitating the determination of advanced kidney dysfunctions. ACS Biomater. Sci. Eng. 2020, 6, 1247–1258. [Google Scholar] [CrossRef]
- Cerda-Sumbarda, Y.D.; Domínguez-González, C.; Zizumbo-López, A.; Licea-Claverie, A. Thermoresponsive nanocomposite hydrogels with improved properties based on poly(N-vinylcaprolactam). Mater. Today Commun. 2020, 24, 10104. [Google Scholar] [CrossRef]
- Xu, F.; Zhu, J.; Lin, L.; Zhang, C.; Sun, W.; Fan, Y.; Yin, F.; Van Hest, J.C.M.; Wang, H.; Du, L.; et al. Multifunctional PVCL nanogels with redox-responsiveness enable enhanced MR imaging and ultrasound-promoted tumor chemotherapy. Theranostics 2020, 10, 4349–4358. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Sun, Y.; Cai, C.; Fan, R.; Guo, R.; Xie, D. Targeted delivery of DOX by transferrin conjugated DSPE-PEG nano-particles in leukemia therapy. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 27–36. [Google Scholar] [CrossRef]
- Kaldybekov, D.B.; Filippov, S.K.; Radulescu, A.; Khutoryanskiy, V.V. Maleimide-functionalised PLGA-PEG nanoparticles as mucoadhesive carriers for intravesical drug delivery. Eur. J. Pharm. Biopharm. 2019, 143, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Quérette, T.; Fleury, E.; Sintes-Zydowicz, N. Non-isocyanate polyurethane nanoparticles prepared by nanoprecipitation. Eur. Polym. J. 2019, 114, 434–445. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, D.; Bai, H.; Ming, W. ZnO nanoparticles coated with amphiphilic polyurethane for transparent polyurethane nanocomposites with enhanced mechanical and UV-shielding performance. ACS Appl. Nano Mater. 2019, 3, 59–67. [Google Scholar] [CrossRef]
- Morkhade, D.M. Comparative impact of different binder addition methods, binders and diluents on resulting granule and tablet attributes via high shear wet granulation. Powder Technol. 2017, 320, 114–124. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.-S.; Xu, Z.-K.; Huang, X.-J.; Wang, Z.-G.; Wang, J.-L. Copolymerization of acrylonitrile with N-vinyl-2-pyrrolidone to improve the hemocompatibility of polyacrylonitrile. Polymer 2005, 46, 7715–7723. [Google Scholar] [CrossRef]
- Awasthi, R.; Manchanda, S.; Das, P.; Velu, V.; Malipeddi, H.; Pabreja, K.; Pinto, T.D.J.A.; Gupta, G.; Dua, K. Poly(vinylpyrrolidone). In Engineering of Biomaterials for Drug Delivery Systems: Beyond Polyethylene Glycol; Woodhead Publishing Series in Biomaterials: Cambridge, UK, 2018. [Google Scholar]
- Wen, T.; Lu, L.; Dong, C. Enhancing the dehumidification performance of LiCl solution with surfactant PVP-K30. Energy Build. 2018, 171, 183–195. [Google Scholar] [CrossRef]
- Lee, J. Intrinsic adhesion properties of poly(vinyl pyrrolidone) to pharmaceutical materials: Humidity effect. Macromol. Biosci. 2005, 5, 1085–1093. [Google Scholar] [CrossRef]
- Tavlarakis, P.; Urban, J.J.; Snow, N. Determination of total polyvinylpyrrolidone (PVP) in ophthalmic solutions by size exclusion chromatography with ultraviolet-visible detection. J. Chromatogr. Sci. 2011, 49, 457–462. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A versatile polymer for biomedical and beyond medical applications. Polym. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Song, G.; Lin, Y.; Zhu, Z.; Zheng, H.; Qiao, J.; He, C.; Wang, H. Strong fluorescence of poly(nvinylpyrrolidone) and its oxidized hydrolyzate. Macromol. Rapid Commun. 2015, 26, 278–285. [Google Scholar] [CrossRef]
- Hua, T.I.; Sundari, R.; Lintang, H.O.; Yuliati, L. Polyvinylpyrrolidone as a new fluorescent sensor for nitrate ion. Malays. J. Anal. Sci. 2016, 20, 288–295. [Google Scholar] [CrossRef]
- Sütekin, S.D.; Güven, O. Application of radiation for the synthesis of poly(n-vinyl pyrrolidone) nanogels with controlled sizes from aqueous solutions. Appl. Radiat. Isot. 2019, 145, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Fiscella, R. Ophthalmic Drug Formulations in Clinical Ocular Pharmacology, 5th ed.; Bartlett, J.D., Jaanus, S.D., Eds.; Butter-worth-Heinemann: Oxford, UK, 2008; pp. 17–37. [Google Scholar]
- Darouiche, R.O.; Wall, M.J.; Itani, K.M.F.; Otterson, M.F.; Webb, A.L.; Carrick, M.M.; Miller, H.J.; Award, S.S.; Crosby, C.T.; Mosier, M.C.; et al. Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N. Engl. J. Med. 2010, 362, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G.S.N.K. Pharmaceutical assessment of polyvinylpyrrolidone (pvp): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Pinto, R.D.P.; Lira, R.P.C.; Abe, R.Y.; Zacchia, R.S.; Felix, J.P.F.; Pereira, A.V.F.; Arieta, C.E.L.; De Castro, R.S.; Bonon, S.H.A. Dexamethasone/povidone eye drops versus artificial tears for treatment of presumed viral conjunctivitis: A randomized clinical trial. Curr. Eye Res. 2014, 40, 870–877. [Google Scholar] [CrossRef]
- Liccioli, G.; Mori, F.; Barni, S.; Pucci, N.; Novembre, E. Anaphylaxis to polyvinylpyrrolidone in eye drops administered to an adolescent. J. Investig. Allergol. Clin. Immunol. 2018, 28, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.-K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef]
- Stupak, E.I.; Bates, T.R. Enhanced absorption and dissolution of reserpine from reserpine-polyvinylpyrrolidone coprecipitates. J. Pharm. Sci. 1972, 61, 400–404. [Google Scholar] [CrossRef]
- Manju, S.; Sreenivasan, K. Synthesis and characterization of a cytotoxic cationic polyvinylpyrrolidone-curcumin conjugate. J. Pharm. Sci. 2011, 100, 504–511. [Google Scholar] [CrossRef]
- Hackl, E.; Ermolina, I. Application of texture analysis technique in formulation development of lyophilized orally disintegrating tablets containing mannitol, polyvinylpyrrolidone and amino acids. AAPS PharmSciTech 2019, 20, 71. [Google Scholar] [CrossRef]
- Khokhar, P.; Shukla, V. Formulation and evaluation of fast dissolving tablets of diclofenac sodium using PVP. Int. J. Pharma Res. Rev. 2014, 3, 12–19. [Google Scholar]
- Arndt, O.-R.; Kleinebudde, P. Influence of binder properties on dry granules and tablets. Powder Technol. 2018, 337, 68–77. [Google Scholar] [CrossRef]
- Guo, Y.; Hao, Z.; Wan, C. Tribological characteristics of polyvinylpyrrolidone (PVP) as a lubrication additive for artificial knee joint. Tribol. Int. 2016, 93, 214–219. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, N. Design of sterculia gum based double potential antidiarrheal drug delivery system. Colloids Surf. B Biointerfaces 2011, 82, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Schafer, J.; Reindel, W.; Steffen, R.; Mosehauer, G.; Chinn, J. Use of a novel extended blink test to evaluate the performance of two polyvinylpyrrolidone-containing, silicone hydrogel contact lenses. Clin. Ophthalmol. 2018, 12, 819–825. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, W.; Lei, Y.; Dang, M. Novel polyvinyl pyrrolidone-loaded olopatadine HCl-laden doughnut contact lens to treat allergic conjunctivitis. J. Pharm. Sci. 2020, 109, 1714–1724. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, E.A. Blood substitutes. Can. J. Anesth. 1975, 22, 12–19. [Google Scholar] [CrossRef]
- Lecce, J.G.; Matrone, G.; Morgan, D.O. Porcine neonatal nutrition: Absorption of unaltered nonporcine proteins and polyvi-nylpyrrolidone from the gut of piglets and the subsequent effect on the maturation of the serum protein profile. J. Nutr. 1961, 73, 158–166. [Google Scholar] [CrossRef]
- Kadajji, V.G.; Betageri, G.V. Water soluble polymers for pharmaceutical applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef]
- Gangwar, R.K.; Dhumale, V.A.; Kumari, D.; Nakate, U.T.; Gosavi, S.; Sharma, R.B.; Kale, S.; Datar, S. Conjugation of curcumin with PVP capped gold nanoparticles for improving bioavailability. Mater. Sci. Eng. C 2012, 32, 2659–2663. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R.; Yadav, K.S. Formulations for polymer coatings. Polym. Coat. 2020, 415–443. [Google Scholar] [CrossRef]
- Foldbjerg, R.; Olesen, P.; Hougaard, M.; Dang, D.A.; Hoffmann, H.J.; Autrup, H. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol. Lett. 2009, 190, 156–162. [Google Scholar] [CrossRef]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y.; et al. Anti-leukemia activity of PVP-coated silver nanoparticles via generation of reactive oxygen species and release of silver ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef]
- Fahrenholtz, C.D.; Swanner, J.; Ramirez-Perez, M.; Singh, R.N. Heterogeneous responses of ovarian cancer cells to silver nanoparticles as a single agent and in combination with cisplatin. J. Nanomater. 2017, 2017, 5107485. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.; Kollenda, S.; Epple, M. Nanoparticle-protein interactions: Therapeutic approaches and supramolecular chemistry. Acc. Chem. Res. 2017, 50, 1383–1390. [Google Scholar] [CrossRef]
- Batista, C.C.; Albuquerque, L.J.; Jägerb, A.; Stepánekb, P.; Giacomelli, F.C. Probing protein adsorption onto polymer-stabilized silver nanocolloids towards a better understanding on the evolution and consequences of biomolecular coronas. Mater. Sci. Eng. C 2020, 111, 110850. [Google Scholar] [CrossRef]
- Mohamed, T.; Matou-Nasri, S.; Farooq, A.; Whitehead, D.; Azzawi, M. Polyvinylpyrrolidone-coated gold nanoparticles inhibit endothelial cell viability, proliferation, and ERK1/2 phosphorylation and reduce the magnitude of endothelial-independent dilator responses in isolated aortic vessels. Int. J. Nanomed. 2017, 12, 8813–8830. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, S.; Yu, Y.; Zhang, H.; He, Q. Structural-engineering rationales of gold nanoparticles for cancer theranostics. Adv. Mater. 2016, 28, 8567–8585. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qiao, X.; Chen, J.; Wang, X.; Ding, S. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005, 94, 449–453. [Google Scholar] [CrossRef]
- Seo, K.; Sinha, K.; Novitskaya, E.; Graeve, O.A. Polyvinylpyrrolidone (PVP) effects on iron oxide nanoparticle formation. Mater. Lett. 2018, 215, 203–206. [Google Scholar] [CrossRef]
- Ramalingam, V.; Varunkumar, K.; Ravikumar, V.; Rajaram, R. Target delivery of doxorubicin tethered with PVP stabilized gold nanoparticles for effective treatment of lung cancer. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Tao, A.R.; Habas, S.; Yang, P. Shape control of colloidal metal nanocrystals. Small 2008, 4, 310–325. [Google Scholar] [CrossRef]
- Song, Y.-J.; Wang, M.; Zhang, X.-Y.; Wu, J.-Y.; Zhang, T. Investigation on the role of the molecular weight of polyvinyl pyrrolidone in the shape control of high-yield silver nanospheres and nanowires. Nanoscale Res. Lett. 2014, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Oliver, S.; Chen, Y.; Boyer, C.; Chandrawati, R. Tuning, crystallization and morphology of zinc oxide with polyvi-nylpyrrolidone: Formation mechanisms and antimicrobial activity. J. Colloid Interface Sci. 2019, 546, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Rajan, R.; Matsumura, K. Development and application of cryoprotectants. Adv. Exp. Med. Biol. 2018, 1081, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Dorati, R.; DeTrizio, A.; Spalla, M.; Migliavacca, R.; Pagani, L.; Pisani, S.; Chiesa, E.; Conti, B.; Modena, T.; Genta, I. Gentamicin sulfate PEG-PLGA/PLGA-H nanoparticles: Screening design and antimicrobial effect evaluation toward clinic bacterial isolates. Nanomaterials 2018, 8, 37. [Google Scholar] [CrossRef]
- Mir, M.; Dian Permana, A.; Ahmed, N.; Majid Khan, G.; ur Rehman, A.; Donnelly, R.F. Enhancement in site-specific delivery of carvacrol for potential treatment of infected wounds using infection responsive nanoparticles loaded into dissolving microneedles: A proof of concept study. Eur. J. Pharm. Biopharm. 2020, 147, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.; Hany, M.; Mokhtar, S.; Helmy, M.W.; Elkodairy, K.A.; Elzoghby, A.O. Boronic-targeted albumin-shell oily-core nanocapsules for synergistic aromatase inhibitor/herbal breast cancer therapy. Mater. Sci. Eng. C 2019, 105, 110099. [Google Scholar] [CrossRef]
- Işçi, S.; Işçi, Y.; Bekaroğlu, M.G. Particle interactions of polyvinylpyrrolidone-coated iron oxide particles as magnetic drug delivery agents. Appl. Phys. A 2017, 123, 534. [Google Scholar] [CrossRef]
- Basyreva, L.Y.; Voinova, E.V.; Gusev, A.A.; Mikhalchik, E.V.; Kuskov, A.N.; Goryachaya, A.V.; Gusev, S.A.; Shtilman, M.I.; Velonia, K.; Tsatsakis, A.M. Fluorouracil neutrophil extracellular traps formation inhibited by polymer nanoparticle shielding. Mater. Sci. Eng. C 2020, 108, 110382. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Xiao, Z.-K.; Lei, X.-P.; Li, J.-X.; Yu, X.-Y.; Zhang, J.-Y.; Ye, G.-D.; Guo, Y.-J.; Mo, G.; Li, C.-W.; et al. Preparation, characterization and in vitro-in vivo evaluation of bortezomib supermolecular aggregation nanovehicles. J. Nanobiotechnol. 2020, 18, 1–12. [Google Scholar] [CrossRef]
- Praphanwittaya, P.; Saokham, P.; Jansook, P.; Loftsson, T. Aqueous solubility of kinase inhibitors: I the effect of hydrophilic polymers on their γ-cyclodextrin solubilization. J. Drug Deliv. Sci. Technol. 2020, 55, 101462. [Google Scholar] [CrossRef]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surf. B Biointerfaces 2019, 178, 452–459. [Google Scholar] [CrossRef]
- Zuchowska, A.; Buta, A.; Dabrowski, B.; Jastrzebska, E.; Zukowski, K.; Brzozka, Z. 3D and 2D cell models in a novel microfluidic tool for evaluation of highly chemically and microbiologically pure graphene oxide (GO) as an effective drug carrier. Sens. Actuators B Chem. 2020, 302, 127064. [Google Scholar] [CrossRef]
- Ozkan, G.; Franco, P.; Capanoglu, E.; De Marco, I. PVP/flavonoid coprecipitation by supercritical antisolvent process. Chem. Eng. Process. Process. Intensif. 2019, 146, 107689. [Google Scholar] [CrossRef]
- Homayouni, A.; Sohrabi, M.; Amini, M.; Varshosaz, J.; Nokhodchi, A. Effect of high pressure homogenization on physicochemical properties of curcumin nanoparticles prepared by antisolvent crystallization using HPMC or PVP. Mater. Sci. Eng. C 2019, 98, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Evangelopoulos, A.; Schizas, N.; Kazazis, C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015, 35, 645–651. [Google Scholar] [PubMed]
- Mahalunkar, S.; Yadav, A.S.; Gorain, M.; Pawar, V.; Braathen, R.; Weiss, S.; Bogen, B.; Gosavi, S.W.; Kundu, G.C. Functional design of pH-responsive folate-targeted polymer-coated gold nanoparticles for drug delivery and in vivo therapy in breast cancer. Int. J. Nanomed. 2019, 14, 8285–8302. [Google Scholar] [CrossRef]
- Tsatsakis, A.; Stratidakis, A.K.; Goryachaya, A.V.; Tzatzarakis, M.N.; Stivaktakis, P.D.; Docea, A.O.; Berdiaki, A.; Nikitovic, D.; Velonia, K.; Shtilman, M.I.; et al. In vitro blood compatibility and in vitro cytotoxicity of amphiphilic poly-Nvinylpyrrolidone nanoparticles. Food Chem. Toxicol. 2019, 127, 42–52. [Google Scholar] [CrossRef]
- Pornpitchanarong, C.; Rojanarata, T.; Opanasopit, P.; Ngawhirunpat, T.; Patrojanasophon, P. Synthesis of novel N-vinylpyrrolidone/acrylic acid nanoparticles as drug delivery carriers of cisplatin to cancer cells. Colloids Surf. B Biointerfaces 2020, 185, 110566. [Google Scholar] [CrossRef]
- Xiang, Y.; Peng, X.; Kong, X.; Tang, Z.; Quan, H. Biocompatible AuPd@PVP core-shell nanoparticles for enhancement of radiosen-sitivity and photothermal cancer therapy. Colloids Surf. A 2020, 594, 124652. [Google Scholar] [CrossRef]
- Mahdavi, B.; Shokrani, P.; Hejazi, S.H.; Talebi, A.; Taheri, A. Doxorubicin-loaded PVP coated Gd2O3 NPs for effective chemora-diotherapy in melanoma. J. Drug Deliv. Sci. Technol. 2019, 53, 101189. [Google Scholar] [CrossRef]
- Thenmozhi, T. Functionalization of iron oxide nanoparticles with clove extract to induce apoptosis in MCF-7 breast cancer cells. 3 Biotech 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Fundueanu, G.; Constantin, M.; Bucatariu, S.; Ascenzi, P. Poly(N-isopropylacrylamide-co-N-vinylpyrrolidone) thermoresponsive microspheres: The low drug loading ensures the pulsatile release mechanism. Express Polym. Lett. 2020, 14, 63–76. [Google Scholar] [CrossRef]
- Özbaş, Z.; Özkahraman, B.; Bal Öztürk, A. Controlled release profile of 5-fluorouracil loaded P(AAM-co-NVP-co-DEAEMA) microgel prepared via free radical precipitation polymerization. Polym. Bull. 2018, 75, 3053–3067. [Google Scholar] [CrossRef]
- Swilem, A.E.; Elshazly, A.H.; Hamed, A.A.; Hegazy, E.-S.A.; El-Rehim, H.A.A. Nanoscale poly(acrylic acid)-based hydrogels prepared via a green single-step approach for application as low-viscosity biomimetic fluid tears. Mater. Sci. Eng. C 2020, 110, 110726. [Google Scholar] [CrossRef]
- Peng, H.; Huang, X.; Melle, A.; Karperien, M.; Pich, A. Redox-responsive degradable prodrug nanogels for intracellular drug delivery by crosslinking of amine-functionalized poly(N-vinylpyrrolidone) copolymers. J. Colloid Interface Sci. 2019, 540, 612–622. [Google Scholar] [CrossRef]
- Prosapio, V.; Reverchon, E.; De Marco, I. Formation of PVP/nimesulide microspheres by supercritical antisolvent coprecipitation. J. Supercrit. Fluids 2016, 118, 19–26. [Google Scholar] [CrossRef]
- Magee, P. Antiseptic drugs and disinfectants. In Side Effects of Drugs Annual 32; Aronson, J.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 437–443. [Google Scholar]
- Adachi, A.; Fukunaga, A.; Hayashi, K.; Kunisada, M.; Horikawa, T. Anaphylaxis to polyvinylpyrrolidone after vaginal application of povidone-iodine. Contact Dermat. 2003, 48, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Nagao, Y. Effect of PVP on sperm capacitation status and embryonic development in cattle. Theriogenology 2009, 72, 624–635. [Google Scholar] [CrossRef]
- Robinson, B.V.; Sullivan, F.M.; Borzelleca, J.F.; Schwartz, S.L. PVP: A Critical Review of the Kinetics and Toxicology of Polyvinylprrolidone (Povidone); Lewis Publishers: New York, NY, USA, 1990. [Google Scholar]

| No. | Name of Drug | pVP Type | Role of pVP | Therapeutic Effect of Drug |
|---|---|---|---|---|
| 1 | FRESHKOTE® Preservative Free | povidone K30 | lubricant | delivers relief from dry eye symptoms |
| 2 | BD PurPrep™ | povidone-iodine | antiseptic | patient preoperative skin preparation |
| 3 | Braunol 2000 | povidone-iodine | antiseptic | wound antiseptic |
| 4 | Betadine® VAG | povidone-iodine | germicidal | Antiseptic in acute and chronic vaginitis |
| 5 | FUCIDIN® | Crospovidone and povidone 90f | super-disintegrant and binder | antibiotic with antimicrobial activity |
| 6 | Neozipine XL | povidone K25 and crospovidone | bioavailability improver and super-disintegrant | used to treat high blood pressure |
| 7 | Cefdinir | povidone K30 | binder | used to treat a wide variety of bacterial infections |
| 8 | OXYCONTIN® | povidone K30 | binder, solublizer | used to mitigate persistent pain |
| 9 | Pantoprazole Sodium Delayed Release | povidone K25 and crospovidone | Solubilizer and used to allow absorption of the active drug. | used to treat gastroesophageal reflux disease |
| 10 | Tylenol (chewables) | crospovidone | taste masking | painkiller for children |
| Functions of pVP | Name of Drug Carrier | Name of Drug | Therapeutic Effect of Drug | Reference | |
|---|---|---|---|---|---|
| Action Functions | Bulding Functions | ||||
| drug carrier | base material | pVP microspheres | nimesulide | analgesic, anti-inflammatory, antipyretic effect | [100] |
| LCST stabilizer | copolymer | p(AAM-co-NVP-co-DEAEMA) microgel | 5-FU | anticancer | [97] |
| solubilizing agent | copolymer | pVP/AAc nanogels | - | delivers relief from dry eye symptoms | [98] |
| solublizing agent | shell | pVP GO-NPs | QSR | anticancer | [86] |
| toxicity enhancer | shell | pVP/AAc core-shell nanogels | cis-platin | anticancer | [92] |
| toxicity reducer (prevention from side effects of drugs) | shell | Amph-pVP | 5-FU | anticancer | [82] |
| bioavailability enhancer | shell | pVP coated Fe3O4-NPs | S. aromaticum extract | anticancer | [95] |
| stabilizer: Prevention from aggregation | shell | pVP coated BTZ/TA nanoparticles | BTZ | anticancer | [83] |
| stabilizer: Shape control | - | ZnO nanoparticles | - | - | [76] |
| stabilizer: Size limiter | shell | pVP coated Au-NPs | DOX | anticancer | [73] |
| cryoprotectant agent | PEG-PLGA/PLGA-H Nanoparticles | gentamycin sulfate | anticancer | [78] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waleka, E.; Stojek, Z.; Karbarz, M. Activity of Povidone in Recent Biomedical Applications with Emphasis on Micro- and Nano Drug Delivery Systems. Pharmaceutics 2021, 13, 654. https://doi.org/10.3390/pharmaceutics13050654
Waleka E, Stojek Z, Karbarz M. Activity of Povidone in Recent Biomedical Applications with Emphasis on Micro- and Nano Drug Delivery Systems. Pharmaceutics. 2021; 13(5):654. https://doi.org/10.3390/pharmaceutics13050654
Chicago/Turabian StyleWaleka, Ewelina, Zbigniew Stojek, and Marcin Karbarz. 2021. "Activity of Povidone in Recent Biomedical Applications with Emphasis on Micro- and Nano Drug Delivery Systems" Pharmaceutics 13, no. 5: 654. https://doi.org/10.3390/pharmaceutics13050654
APA StyleWaleka, E., Stojek, Z., & Karbarz, M. (2021). Activity of Povidone in Recent Biomedical Applications with Emphasis on Micro- and Nano Drug Delivery Systems. Pharmaceutics, 13(5), 654. https://doi.org/10.3390/pharmaceutics13050654






