The Alteration of L-Carnitine Transport and Pretreatment Effect under Glutamate Cytotoxicity on Motor Neuron-Like NSC-34 Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Uptake Study on NSC-34 Cell Lines
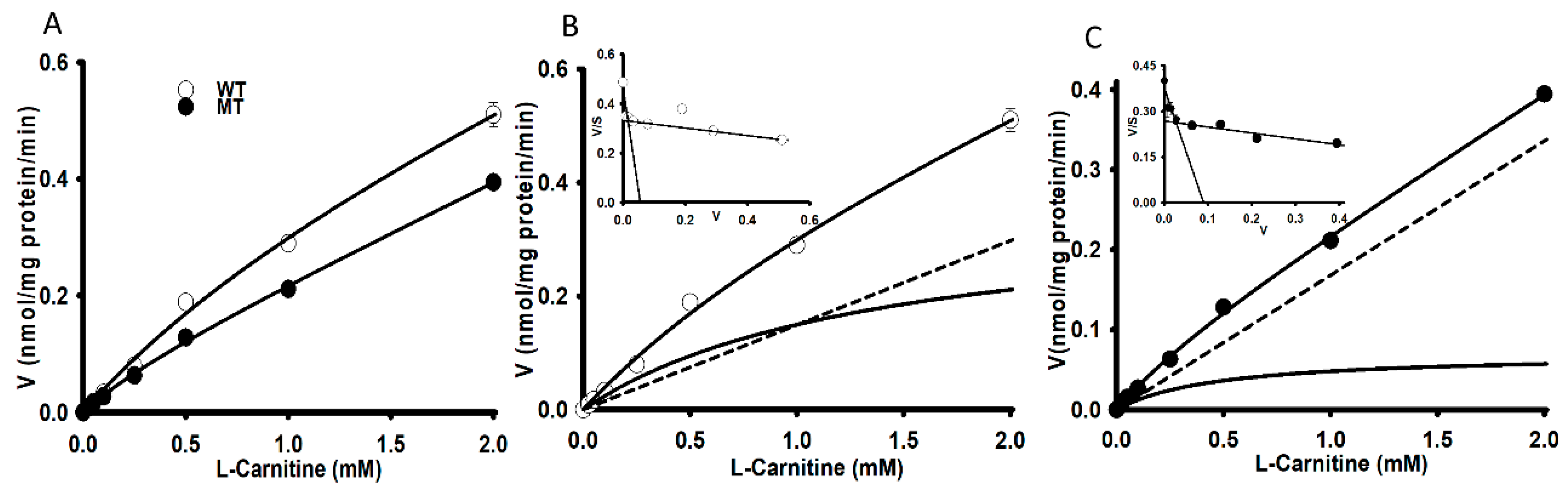
2.4. Kinetic Analysis
2.5. OCTN1 and 2 Small Interfering RNA (siRNA) Transfection Study
2.6. Quantitative Real Time-Polymerase Chain Reaction (qRT-PCR) Study
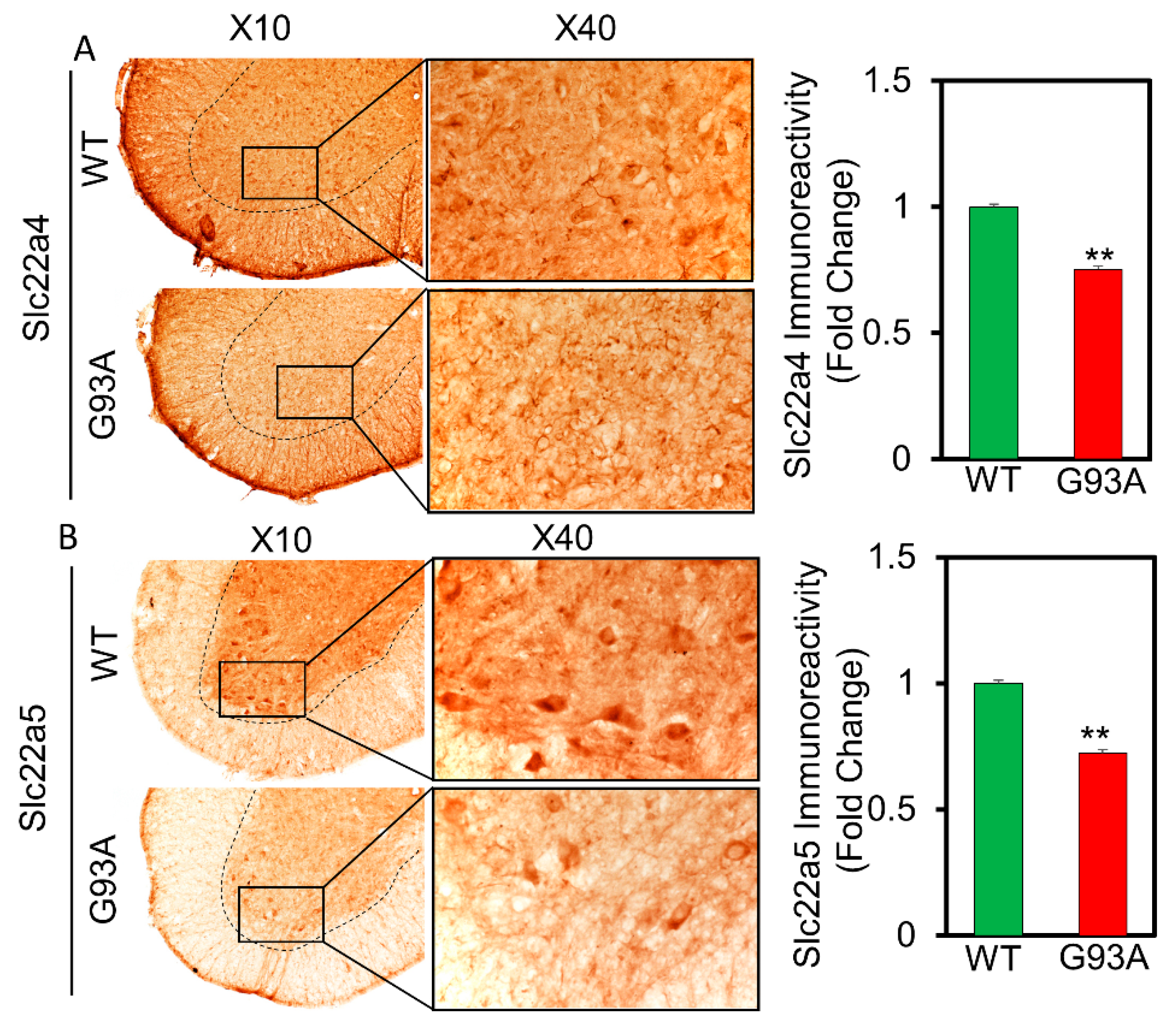
2.7. ALS Mouse Model
2.8. Histopathology and Bright Field Microscopy
2.9. Glutamate Neurotoxicity in NSC-34 Cell Lines
2.10. Data Analysis
3. Results
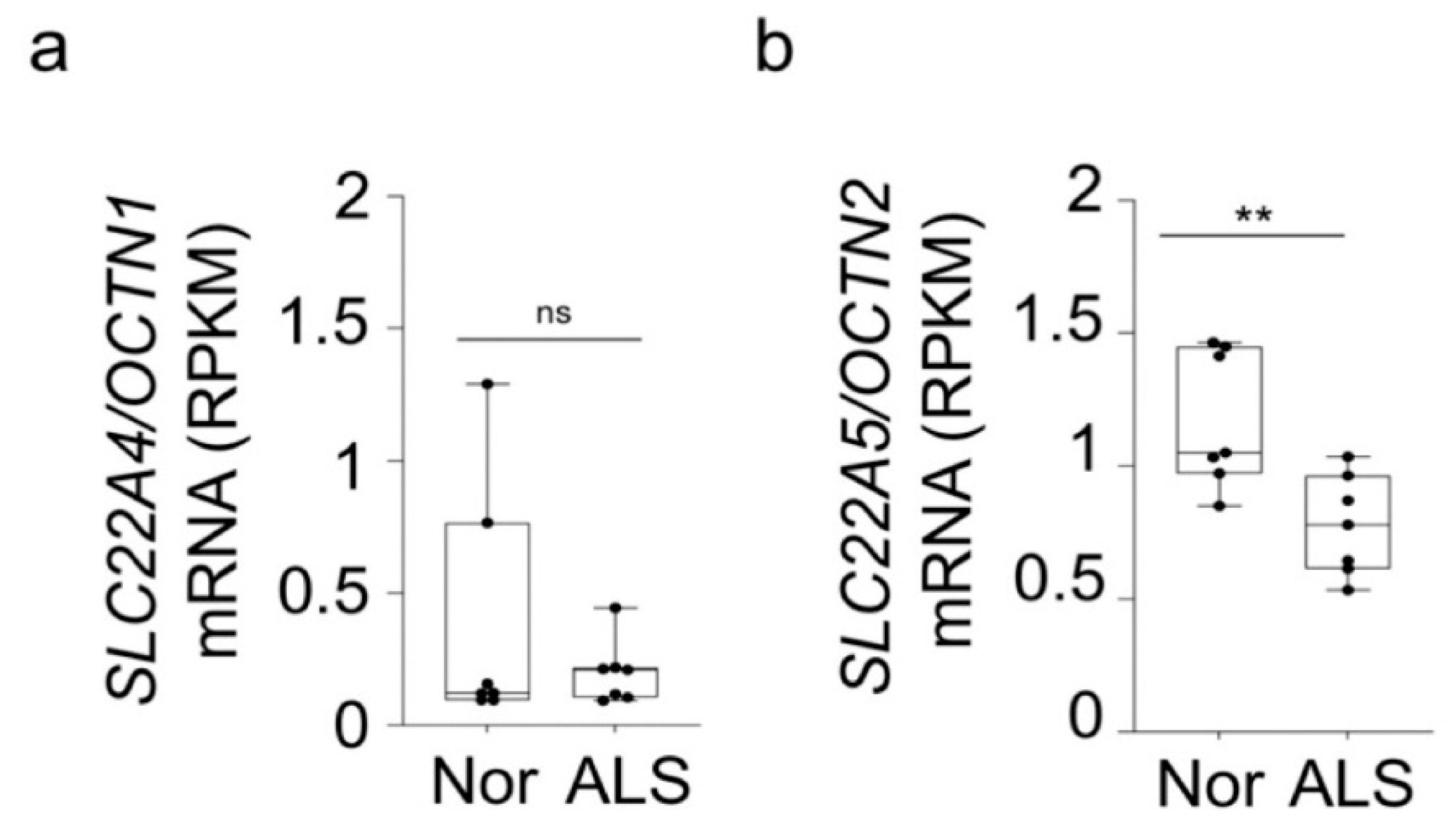
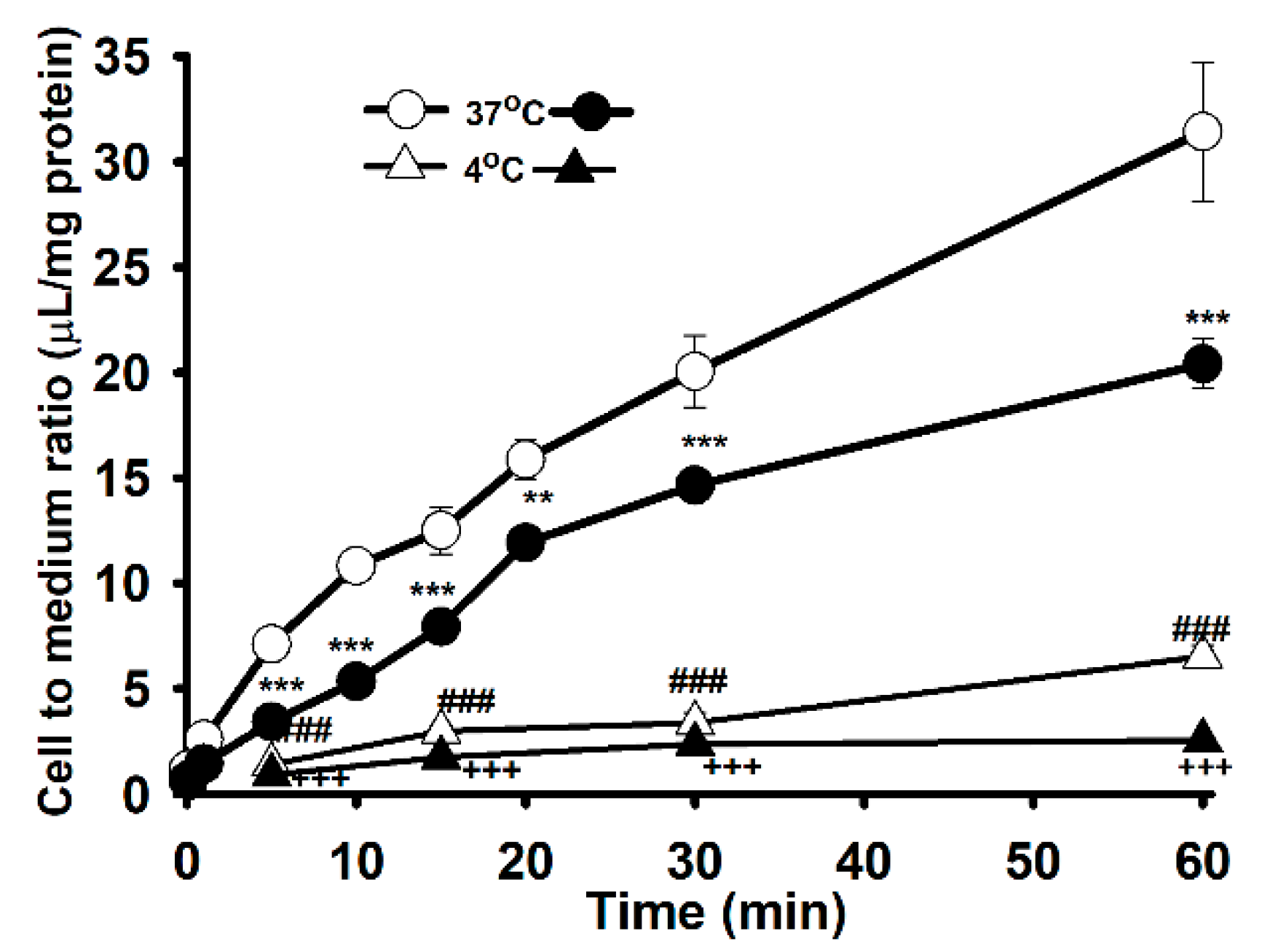
3.1. The Characteristics of [3H]L-Carnitine Transport in NSC-34 Cell Lines
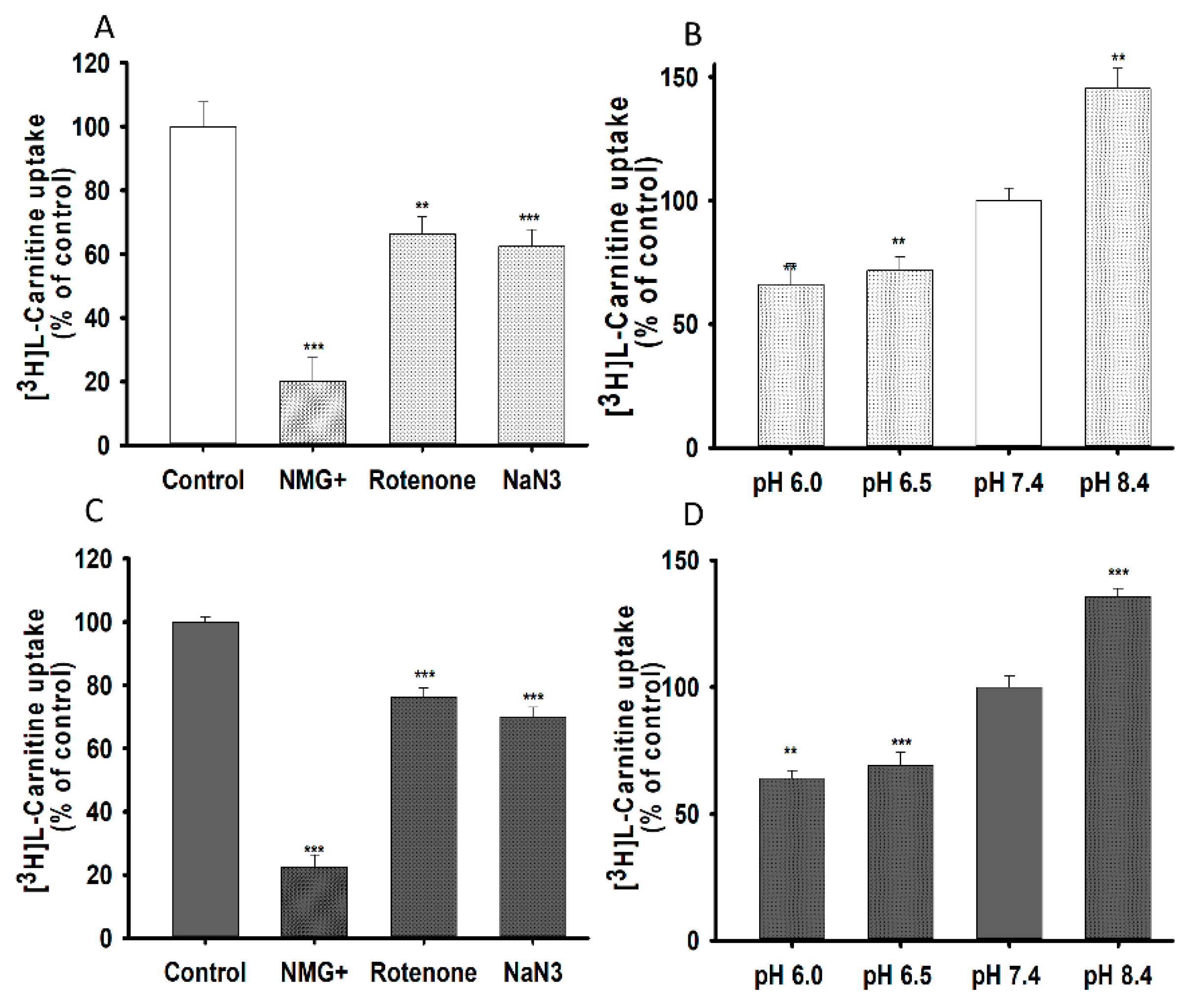
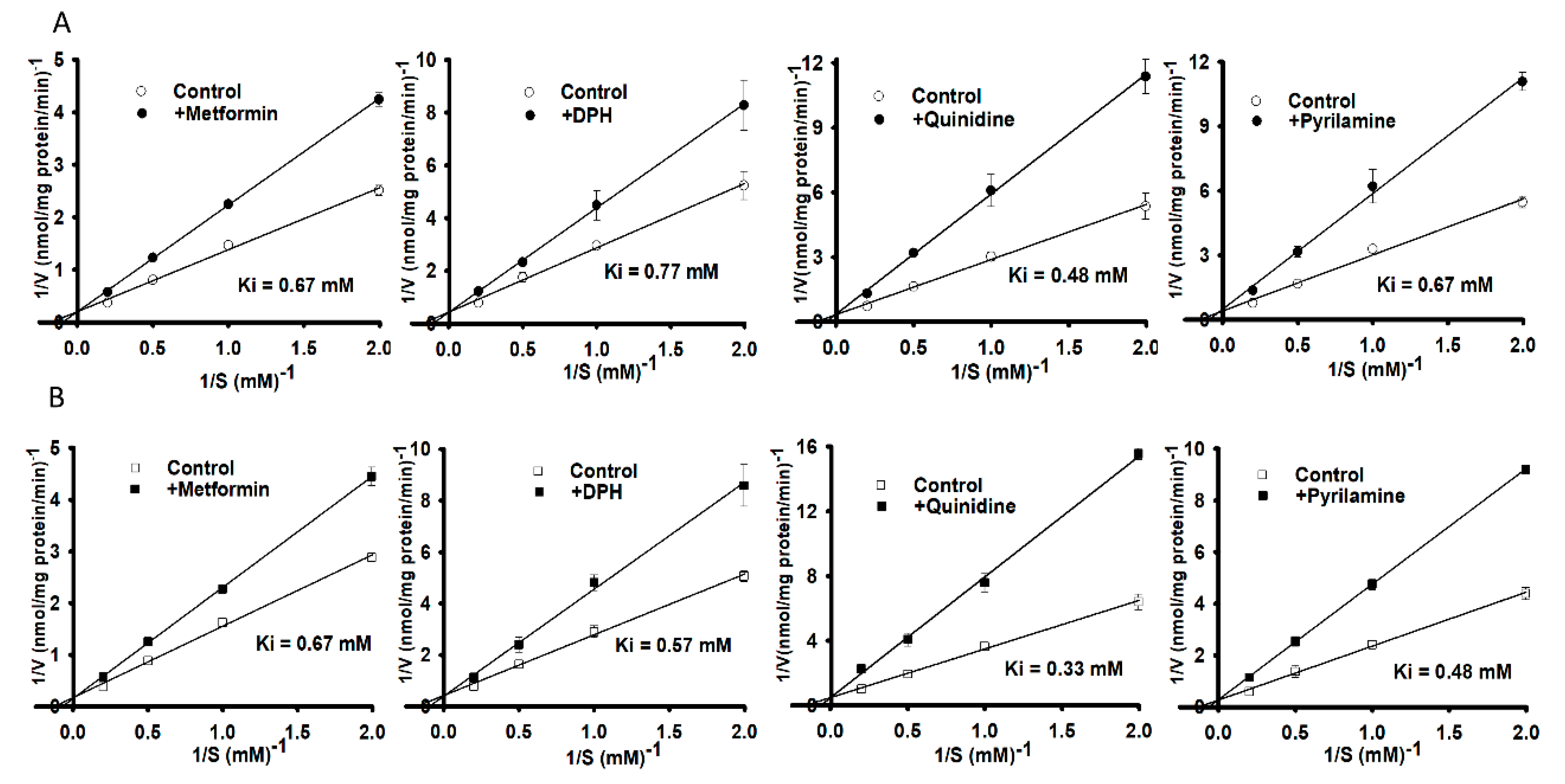
3.2. Structural Inhibition Analog Study in the Uptake of [3H]L-Carnitine by NSC-34 Cell Lines
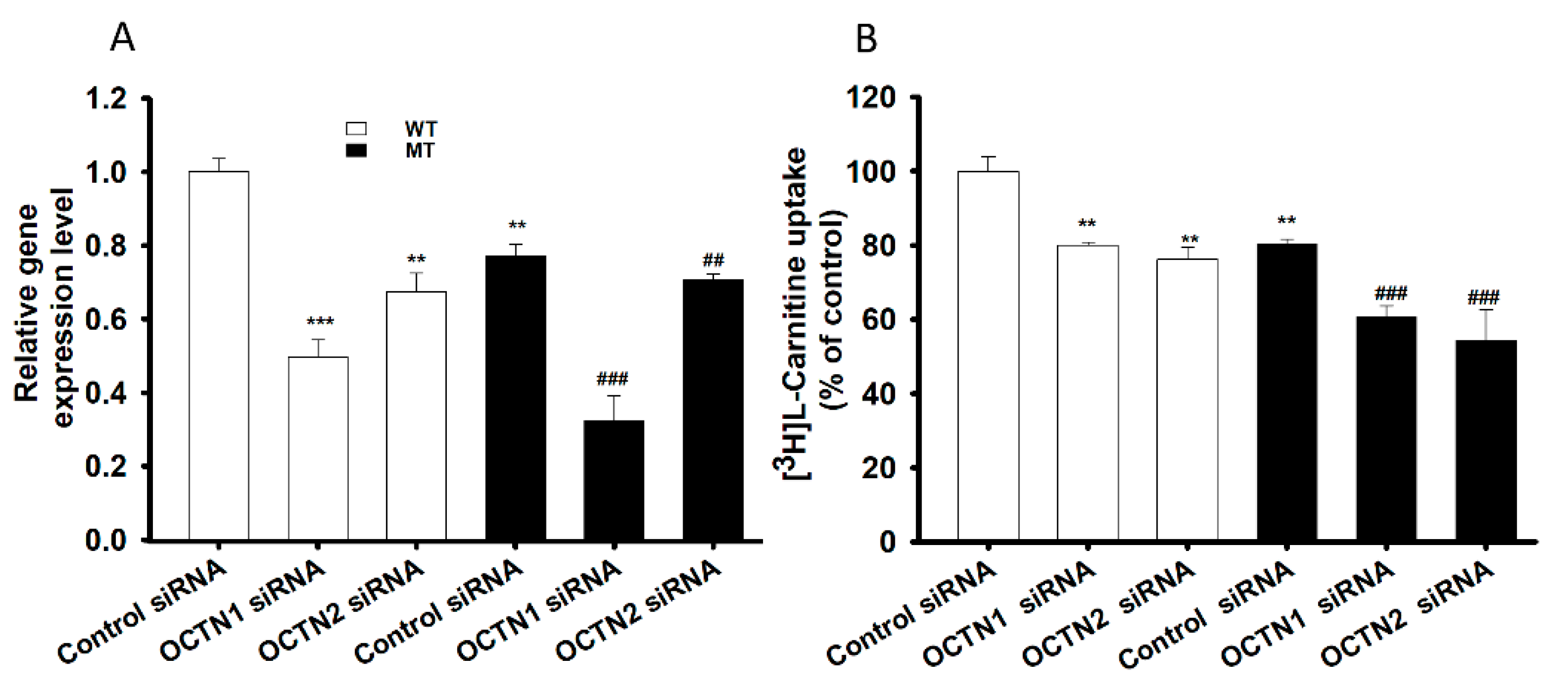
3.3. SiRNA Transfection and Carnitine Transporter Alteration Study in NSC-34 Cell Lines
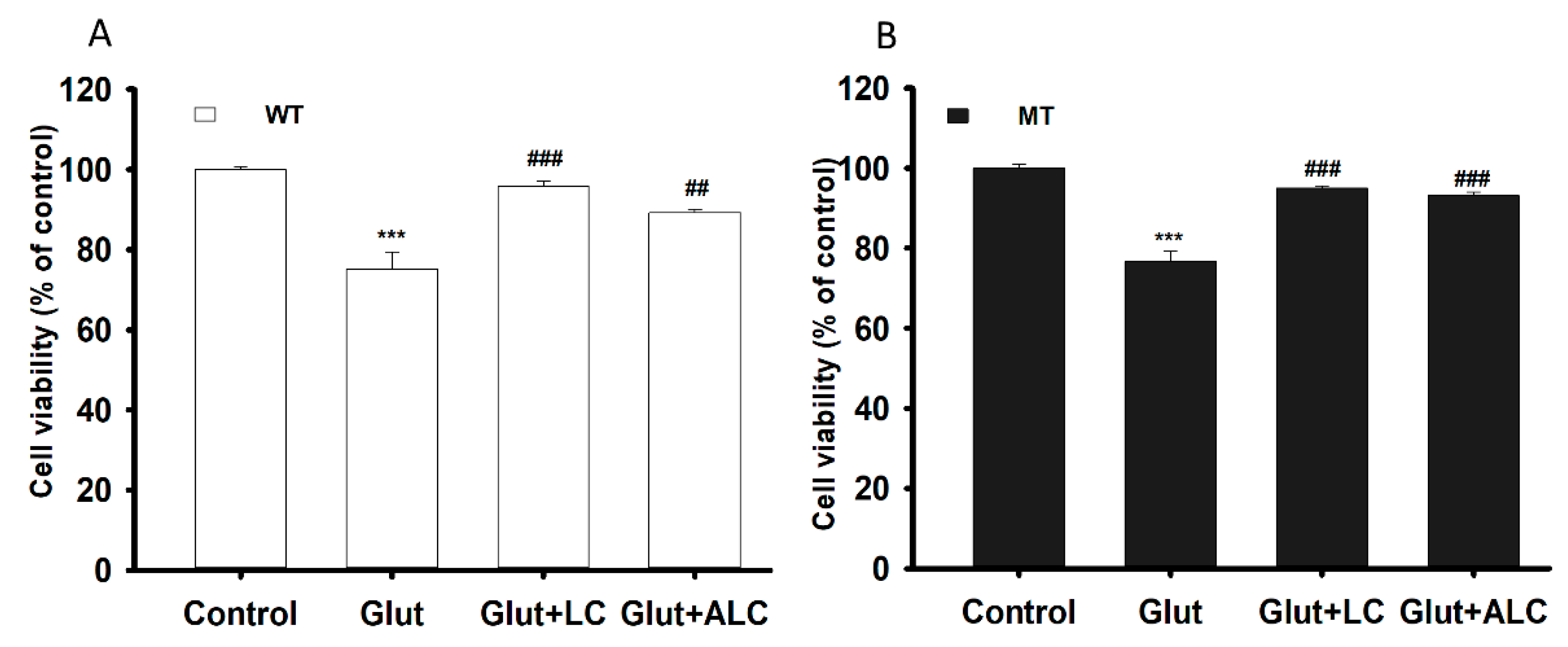
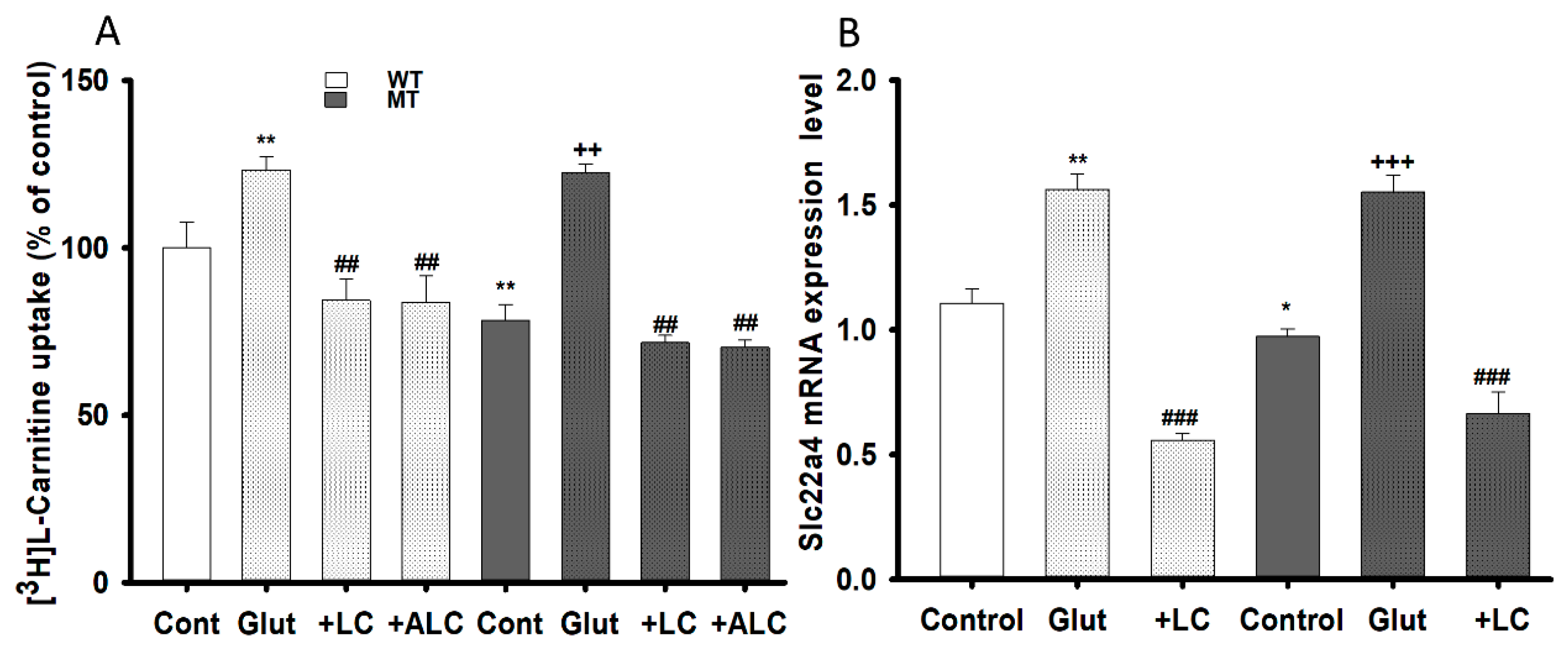
3.4. Glutamate Cytotoxicity and Neuroprotective Effect on the NSC-34 Cell Lines
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Ngo, S.; Mi, J.D.; Henderson, R.; McCombe, P.A.; Steyn, F. Exploring targets and therapies for amyotrophic lateral sclerosis: Current insights into dietary interventions. Degener. Neurol. Neuromuscul. Dis. 2017, 7, 95–108. [Google Scholar] [CrossRef]
- Ralli, M.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Amyotrophic lateral sclerosis: Autoimmune pathogenic mechanisms, clinical features, and therapeutic perspectives. Isr. Med. Assoc. J. 2019, 21, 438–443. [Google Scholar] [PubMed]
- Barber, S.C.; Shaw, P.J. Oxidative stress in ALS: Key role in motor neuron injury and therapeutic target. Free Radic. Biol. Med. 2010, 48, 629–641. [Google Scholar] [CrossRef]
- Ajroud-Driss, S.; Siddique, T. Sporadic and hereditary amyotrophic lateral sclerosis (ALS). Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 679–684. [Google Scholar] [CrossRef]
- Tefera, T.W.; Borges, K. Metabolic dysfunctions in amyotrophic lateral sclerosis pathogenesis and potential metabolic treatments. Front. Neurosci. 2017, 10. [Google Scholar] [CrossRef]
- Kira, Y.; Nishikawa, M.; Ochi, A.; Sato, E.; Inoue, M. L-Carnitine suppresses the onset of neuromuscular degeneration and increases the life span of mice with familial amyotrophic lateral sclerosis. Brain Res. 2006, 1070, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Isse, N.; Miura, Y.; Obata, T.; Takahara, N. Carnitine deficiency presenting with a decreased mental state in a patient with amyotrophic lateral sclerosis receiving long-term tube feeding: A case report. J. Med. Case Rep. 2013, 7, 7–9. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 2422–2435. [Google Scholar] [CrossRef] [PubMed]
- Geier, D.A.; Geier, M.R. L-carnitine exposure and mitochondrial function in human neuronal cells. Neurochem. Res. 2013, 38, 2336–2341. [Google Scholar] [CrossRef]
- Vamos, E.; Voros, K.; Vecsei, L.; Klivenyi, P. Neuroprotective effects of L-carnitine in a transgenic animal model of Huntington’s disease. Biomed. Pharmacother. 2010, 64, 282–286. [Google Scholar] [CrossRef]
- Ferreira, G.C.; Mckenna, M.C. L-Carnitine and Acetyl-l-carnitine Roles and Neuroprotection in Developing Brain. Neurochem. Res. 2017, 42, 1661–1675. [Google Scholar] [CrossRef]
- Pochini, L.; Galluccio, M.; Scalise, M.; Console, L.; Indiveri, C. OCTN: A Small Transporter Subfamily with Great Relevance to Human Pathophysiology, Drug Discovery, and Diagnostics. SLAS Discov. 2019, 24, 89–110. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I. Pharmacological and pathophysiological roles of carnitine/organic cation transporters (OCTNs: SLC22A4, SLC22A5 and Slc22a21). Biopharm. Drug Dispos. 2013, 34, 29–44. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Kato, Y. Physiological Roles of Carnitine/Organic Cation Transporter OCTN1/SLC22A4 in Neural Cells Noritaka. Biol. Pharm. Bull. 2017, 40, 1129. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.K.; Kim, K.Y.; Lee, N.Y.; Kang, Y.S.; Hwang, Y.J.; Kim, Y.; Sung, J.J.; McKee, A.; Kowall, N.; Lee, J.; et al. Expression of taurine transporter (TauT) is modulated by heat shock factor 1 (HSF1) in motor neurons of ALS. Mol. Neurobiol. 2013, 47, 699–710. [Google Scholar] [CrossRef]
- Gyawali, A.; Gautam, S.; Hyeon, S.J.; Ryu, H.; Kang, Y.S. L-Citrulline Level and Transporter Activity Are Altered in Experimental Models of Amyotrophic Lateral Sclerosis. Mol. Neurobiol. 2021, 58, 647–657. [Google Scholar] [CrossRef]
- Gyawali, A.; Kang, Y.S. Transport Alteration of 4-Phenyl Butyric Acid Mediated by a Sodium- and Proton-Coupled Monocarboxylic Acid Transporter System in ALS Model Cell Lines (NSC-34) Under Inflammatory States. J. Pharm. Sci. 2021, 110, 1374–1384. [Google Scholar] [CrossRef]
- Kang, Y.S.; Ohtsuki, S.; Takanaga, H.; Tomi, M.; Hosoya, K.; Terasaki, T. Regulation of taurine transport at the blood-brain barrier by tumor necrosis factor-α, taurine and hypertonicity. J. Neurochem. 2002, 83, 1188–1195. [Google Scholar] [CrossRef]
- Gyawali, A.; Kang, Y.S. Blood-to-retina transport of imperatorin involves the carrier-mediated transporter system at the inner blood-retinal barrier. J. Pharm. Sci. 2019, 108, 1619–1626. [Google Scholar] [CrossRef]
- Gyawali, A.; Kang, Y.S. Pretreatment effect of inflammatory stimuli and characteristics of tryptophan transport on brain capillary endothelial (Tr-bbb) and motor neuron like (nsc-34) cell lines. Biomedicines 2021, 9, 9. [Google Scholar] [CrossRef]
- Lee, N.Y.; Kang, Y.S. In vivo and in vitro evidence for brain uptake of 4-phenylbutyrate by the monocarboxylate transporter 1 (MCT1). Pharm. Res. 2016, 33, 1711–1722. [Google Scholar] [CrossRef]
- Lee, K.E.; Kang, Y.S. L-Citrulline restores nitric oxide level and cellular uptake at the brain capillary endothelial cell line (TR-BBB cells) with glutamate cytotoxicity. Microvasc. Res. 2018, 120, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Batra, R.; Hutt, K.; Vu, A.; Rabin, S.J.; Baughn, M.W.; Libby, R.T.; Hoon, S.; Ravits, J.; Yeo, G.W. Gene expression signatures of sporadic ALS motor neuron populations. bioRxiv 2016. [Google Scholar] [CrossRef]
- Vandoorne, T.; De Bock, K.; Van Den Bosch, L. Energy metabolism in ALS: An underappreciated opportunity? Acta Neuropathol. 2018, 135, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Tamai, I.; Ohashi, R.; Nezu, J.I.; Sai, Y.; Kobayashi, D.; Oku, A.; Shimane, M.; Tsuji, A. Molecular and functional characterization of organic cation/carnitine transporter family in mice. J. Biol. Chem. 2000, 275, 40064–40072. [Google Scholar] [CrossRef] [PubMed]
- Nakamichi, N.; Taguchi, T.; Hosotani, H.; Wakayama, T.; Shimizu, T.; Sugiura, T.; Iseki, S.; Kato, Y. Functional expression of carnitine/organic cation transporter OCTN1 in mouse brain neurons: Possible involvement in neuronal differentiation. Neurochem. Int. 2012, 61, 1121–1132. [Google Scholar] [CrossRef]
- Ohno, Y.; Otsuka, Y.; Nohara, M.; Furihata, T.; Kuse, Y.; Itoh, Y.; Hara, H.; Anzai, N. Characterization of an l-carnitine transport system in murine photoreceptor cell line. Biol. Pharm. Bull. 2017, 40, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- Jong, N.N.; Nakanishi, T.; Liu, J.J.; Tamai, I.; McKeage, M.J. Oxaliplatin transport mediated by organic cation/carnitine transporters OCTN1 and OCTN2 in overexpressing human embryonic kidney 293 cells and rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2011, 338, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, N.; Suzuki, F.; Tamai, I.; Nikaido, H.; Kuwajima, M.; Hayakawa, J.I.; Tsuji, A. Gene-dose effect on carnitine transport activity in embryonic fibroblasts of JVS mice as a model of human carnitine transporter deficiency. Biochem. Pharmacol. 1998, 55, 1729–1732. [Google Scholar] [CrossRef]
- Januszewicz, E.; Bekisz, M.; Mozrzymas, J.W.; Nałecz, K.A. High affinity carnitine transporters from OCTN family in neural cells. Neurochem. Res. 2010, 35, 743–748. [Google Scholar] [CrossRef]
- Hagenbuch, B. Drug uptake systems in liver and kidney: A historic perspective. Clin. Pharmacol. Ther. 2010, 87, 39–47. [Google Scholar] [CrossRef]
- Kato, Y.; Sugiura, M.; Sugiura, T.; Wakayama, T.; Kubo, Y.; Kobayashi, D.; Sai, Y.; Tamai, I.; Iseki, S.; Tsuji, A. Organic cation/carnitine transporter OCTN2 (Slc22a5) is responsible for carnitine transport across apical membranes of small intestinal epithelial cells in mouse. Mol. Pharmacol. 2006, 70, 829–837. [Google Scholar] [CrossRef]
- Kobayashi, D.; Tamai, I.; Sai, Y.; Yoshida, K.; Wakayama, T.; Kido, Y.; Nezu, J.I.; Iseki, S.; Tsuji, A. Transport of carnitine and acetylcarnitine by carnitine/organic cation transporter (OCTN) 2 and OCTN3 into epididymal spermatozoa. Reproduction 2007, 134, 651–658. [Google Scholar] [CrossRef]
- Peltekova, V.D.; Wintle, R.F.; Rubin, L.A.; Amos, C.I.; Huang, Q.; Gu, X.; Newman, B.; Van Oene, M.; Cescon, D.; Greenberg, G.; et al. Functional variants of OCTN cation transporter genes are associated with Crohn disease. Nat. Genet. 2004, 36, 471–475. [Google Scholar] [CrossRef]
- The International Transporter Consortium; Giacomini, K.M.; Huang, S.-M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; et al. Membrane transporters in drug development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar]
- Wawrzenczyk, A.; Sacher, A.; Mac, M.; Nalecz, M.J.; Nałecz, K.A. Transport of L-carnitine in isolated cerebral cortex neurons. Eur. J. Biochem. 2001, 268, 2091–2098. [Google Scholar] [CrossRef]
- Shaw, P.J.; Ince, P.G. Excitotoxicity and amyotrophic lateral sclerosis. J. Neurol. 1997, 244, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Llansola, M.; Erceg, S.; Hernández-Viadel, M.; Felipo, V. Prevention of ammonia and glutamate neurotoxicity by carnitine: Molecular mechanisms. Metab. Brain Dis. 2002, 17, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Llansola, M.; Felipo, V. Carnitine prevents NMDA receptor-mediated activation of MAP-kinase and phosphorylation of microtubule-associated protein 2 in cerebellar neurons in culture. Brain Res. 2002, 947, 50–56. [Google Scholar] [CrossRef]
- Lee, N.Y.; Kang, Y.S. Taurine Protects Glutamate Neurotoxicity in Motor Neuron Cells. Adv. Exp. Med. Biol. 2017, 975, 887–895. [Google Scholar] [PubMed]
- Tastekin, A.; Gepdiremen, A.; Ors, R.; Buyukokuroglu, M.E.; Halici, Z. Protective effect of l-carnitine against bilirubin-induced neuronal cell death. Brain Dev. 2006, 28, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, I. Antioxidant and antiradical activities of L-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
| Parameters | WT | MT |
|---|---|---|
| Km1 (µM) | 1.94 ± 0.34 | 1.96 ± 0.27 |
| Km2 (µM) | 994 ± 34 | 374 ± 89 *** |
| Vmax1 (pmol/mg protein/min) | 0.295 ± 0.059 | 0.191 ± 0.032 |
| Vmax2 (pmol/mg protein/min) | 259 ± 9 | 62.3 ± 13.3 *** |
| Kd (µL/(min/mg protein)) | 0.149 | 0.168 |
| Concentration | [3H]Carnitine Uptake (% of Control) | ||
|---|---|---|---|
| Compounds | (mM) | WT (hSOD1WT) | MT (hSOD1G93A) |
| Control | 1 | 100 ± 6 | 100 ± 4 |
| +Carnitine | 1 | 60.6 ± 2.4 *** | 56.4 ± 3.8 *** |
| +Palmitoyl-L-carnitine | 0.5 | 36.7 ± 1.4 *** | 51.2 ± 5.8 *** |
| +γ-Butyro betaine | 0.5 | 37.3 ± 2.8 *** | 55.3 ± 5.8 *** |
| +ALC | 1 | 51.2 ± 7.5 *** | 57.9 ± 4.8 *** |
| +Quinidine | 1 | 44.7 ± 7.7 *** | 40.0 ± 8.3 *** |
| +Verapamil | 1 | 30.1 ± 8.7 *** | 43.1 ± 7.9 *** |
| +Metformin | 1 | 65.4 ± 9.3 ** | 51.8± 1.6 *** |
| +Pyrilamine | 1 | 50.2 ± 7.7 *** | 49.5 ± 8.2 *** |
| +TEA | 1 | 45.9 ± 2.5 *** | 50.0 ± 7.1 *** |
| +Clonidine | 1 | 57.3 ± 3.8 *** | 63.6 ± 1.7 *** |
| +Diphenhydramine | 1 | 21.9 ± 2.2 *** | 50.0 ± 5.2 *** |
| +Donepezil | 0.5 | 29.7 ± 2.8 *** | 46.7 ± 6.9 *** |
| +Nicotine | 1 | 71.6 ± 8.7 ** | 69.9 ± 2.3 *** |
| +Paeonol | 1 | 50.6 ± 2.8 *** | 50.5 ± 1.2 *** |
| +Propranolol | 1 | 72.8 ± 7.9 ** | 48.2 ± 1.5 *** |
| +MPP+ | 1 | 71.5 ± 2.4 *** | 64.4 ± 9.1 *** |
| +Cimetidine | 1 | 78.9 ± 5.7 ** | 73.2 ± 6.7 *** |
| +Choline | 1 | 88.4 ± 7.3 | 88.3 ± 9.7 |
| +Dopamine | 1 | 90.4 ± 8.7 | 87.9 ± 6.3 |
| +GABA | 1 | 90.2 ± 9.6 | 91.4 ± 4.7 |
| +Gabapentin | 1 | 109 ± 8 | 101 ± 6 |
| +Histidine | 1 | 91.9 ± 6.6 | 110 ± 8 |
| +Probenecid | 1 | 90.3 ± 7.9 | 90.2 ± 10 |
| +Estron-3 sulphate | 1 | 93.0 ± 4.6 | 94.9 ± 5.6 |
| +PAH | 1 | 83.1 ± 5.3 | 107 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gyawali, A.; Hyeon, S.J.; Ryu, H.; Kang, Y.-S. The Alteration of L-Carnitine Transport and Pretreatment Effect under Glutamate Cytotoxicity on Motor Neuron-Like NSC-34 Lines. Pharmaceutics 2021, 13, 551. https://doi.org/10.3390/pharmaceutics13040551
Gyawali A, Hyeon SJ, Ryu H, Kang Y-S. The Alteration of L-Carnitine Transport and Pretreatment Effect under Glutamate Cytotoxicity on Motor Neuron-Like NSC-34 Lines. Pharmaceutics. 2021; 13(4):551. https://doi.org/10.3390/pharmaceutics13040551
Chicago/Turabian StyleGyawali, Asmita, Seung Jae Hyeon, Hoon Ryu, and Young-Sook Kang. 2021. "The Alteration of L-Carnitine Transport and Pretreatment Effect under Glutamate Cytotoxicity on Motor Neuron-Like NSC-34 Lines" Pharmaceutics 13, no. 4: 551. https://doi.org/10.3390/pharmaceutics13040551
APA StyleGyawali, A., Hyeon, S. J., Ryu, H., & Kang, Y.-S. (2021). The Alteration of L-Carnitine Transport and Pretreatment Effect under Glutamate Cytotoxicity on Motor Neuron-Like NSC-34 Lines. Pharmaceutics, 13(4), 551. https://doi.org/10.3390/pharmaceutics13040551






