Challenges in the Physical Characterization of Lipid Nanoparticles
Abstract
1. Introduction
2. Nanoscale Carriers
2.1. Drug Delivery
2.1.1. Liposomes
2.1.2. Ethosomes
2.1.3. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC)
2.1.4. Monoolein Aqueous Dispersions (MAD) and Cubosomes
2.1.5. Gene Delivery
2.2. Diagnostic Applications
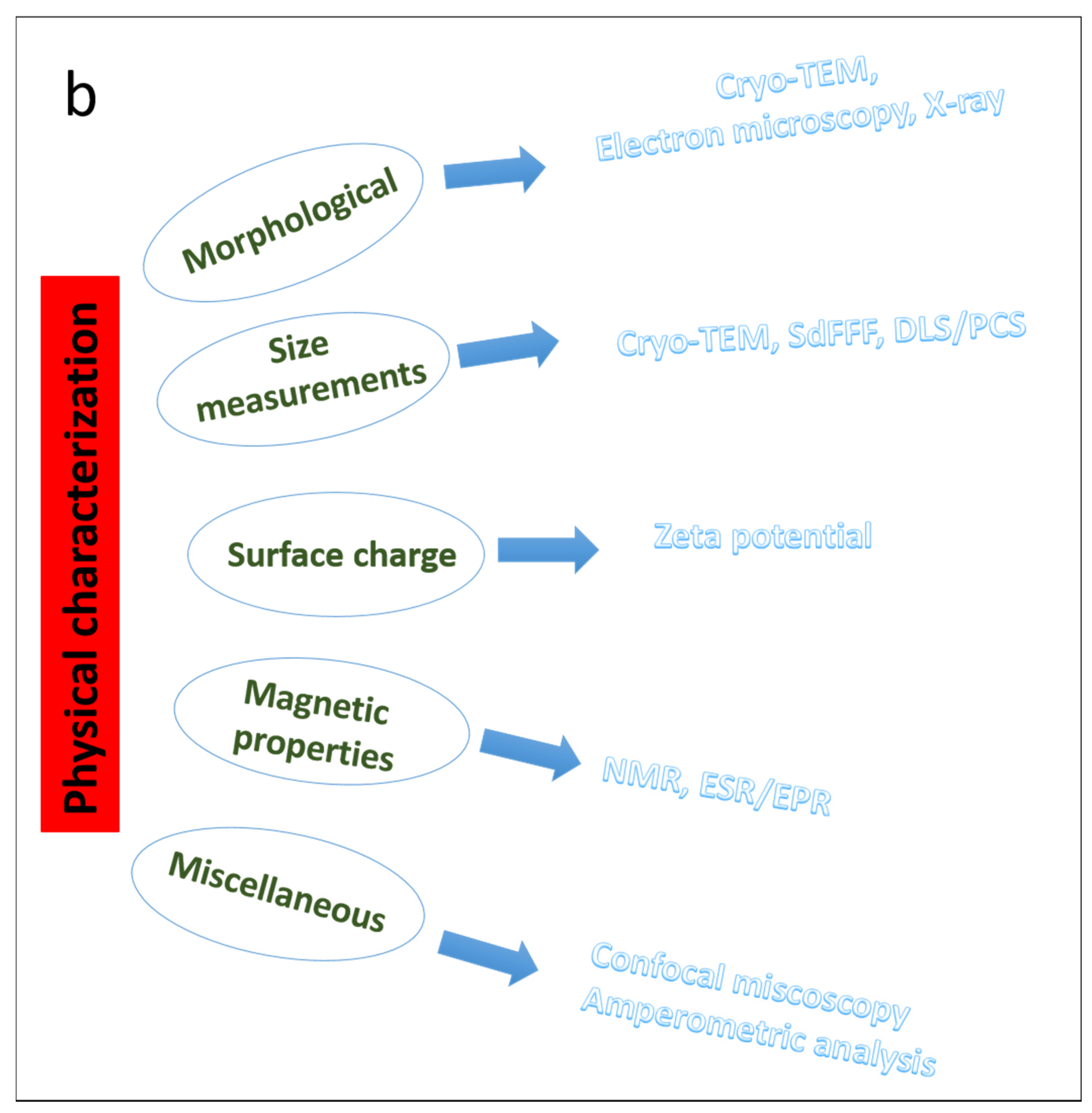
3. Parameters to Consider for the Characterization of Nanoparticles
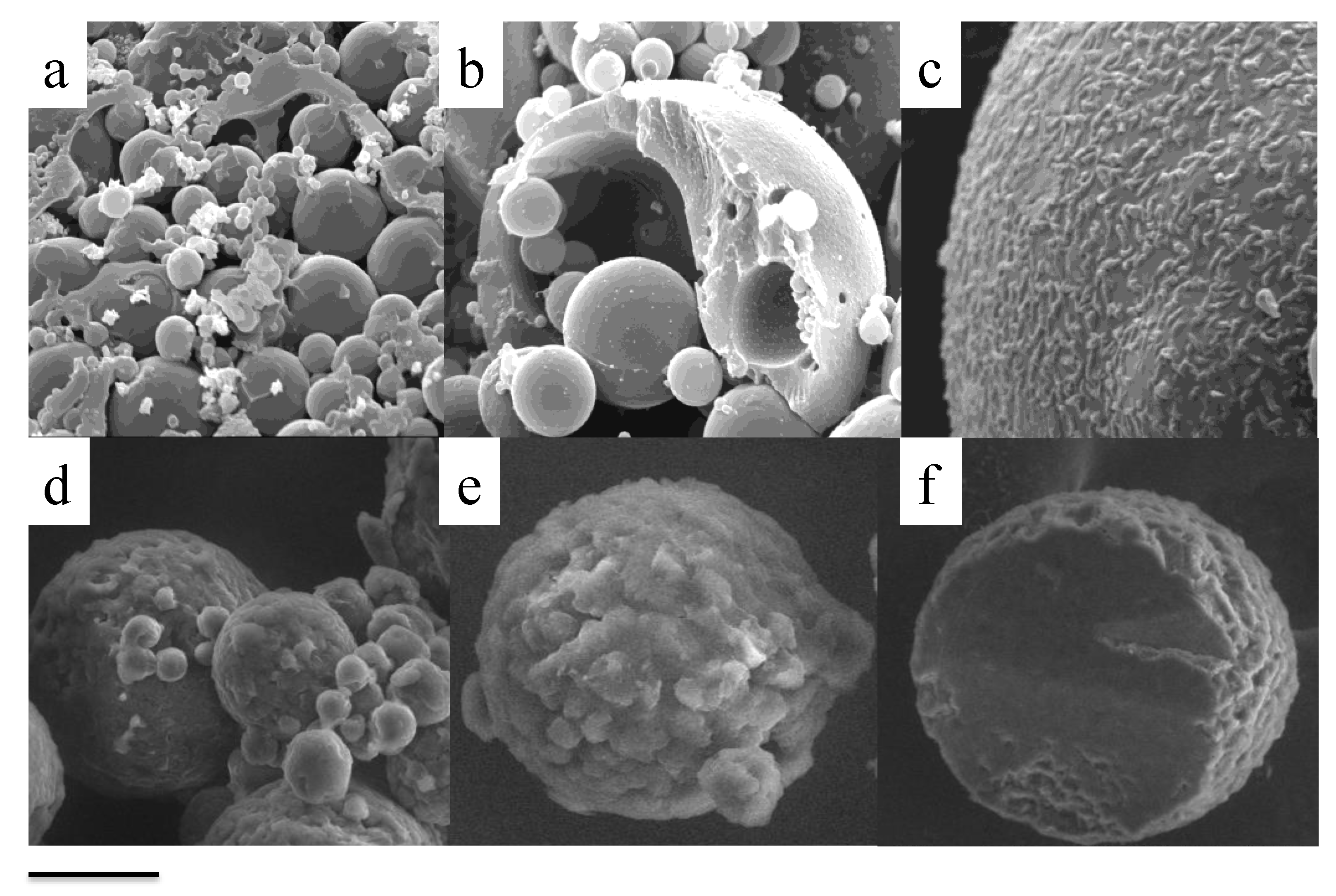
4. Morphological Characterization of Nanoparticles
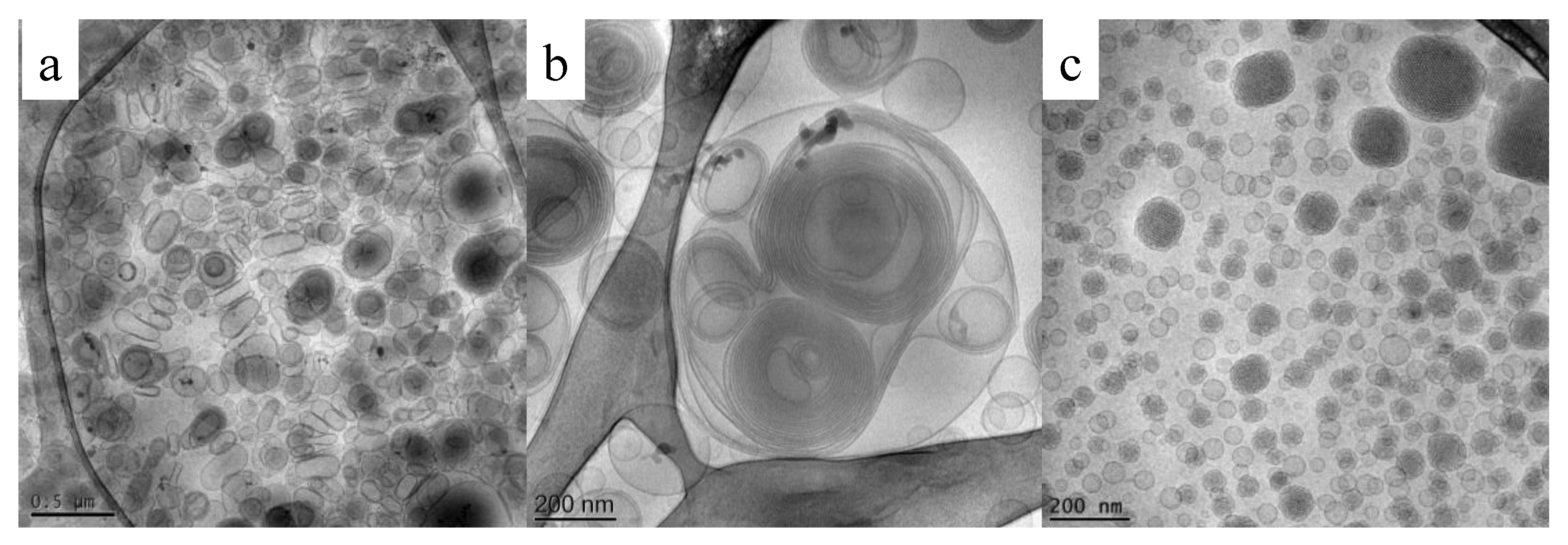
4.1. Cryo-Transmission Electron Microscopy
4.2. X-rays Diffraction Studies
5. Size Measurements
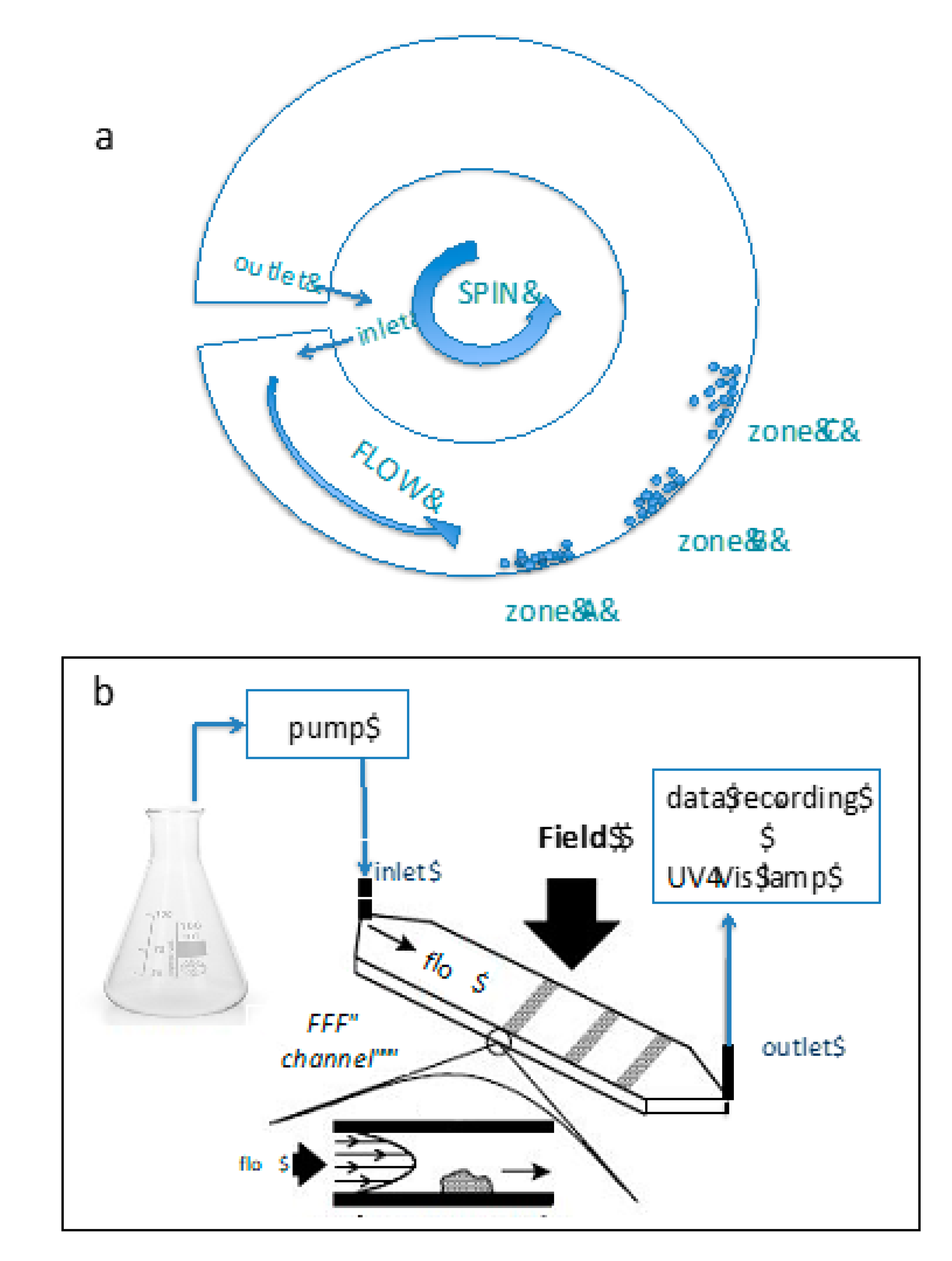
5.1. SdFFF
5.2. Dynamic Light Scattering/ Photon Correlation Spectrometry
5.3. Cryo-TEM
6. Zeta Potential and Surface Charge
7. Characterization of Magnetic Nanoparticles
7.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
7.2. Electron Spin Resonance (ESR)/Electrone Paramagnetic Resoanance (EPR)
8. Miscellaneous
8.1. Confocal Microscopy
8.2. Amperometric Approach
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallan, S.S.; Kaur, P.; Kaur, V.; Mishra, N.; Vaidya, B. Lipid Polymer Hybrid as Emerging Tool in Nanocarriers for Oral Drug Delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 334–349. [Google Scholar] [CrossRef] [PubMed]
- Vega-Vásquez, P.; Mosier, N.S.; Irudayaraj, J. Nanoscale Drug Delivery Systems: From Medicine to Agriculture. Front. Bioeng. Biotechnol. 2020, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, G.; Tiwari, R.; Sriwastawa, B.; Bhati, L.; Pandey, S.; Pandey, P.; Bannerjee, S.K. Drug Delivery Systems: An Updated Review. Int. J. Pharm. Investig. 2012, 2, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.; Jones, S.; Dobson, J. Applications of Magnetic Nanoparticles in Biomedicine: The Story so Far. J. Phys. D Appl. Phys. 2016, 49, 501002. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.; Try, C.; Pellequer, Y.; Lamprecht, A. Nanomedicine Strategies for Targeting Skin Inflammation. Nanomedicine 2014, 9, 1727–1743. [Google Scholar] [CrossRef]
- Bonam, S.R.; Kotla, N.G.; Bohara, R.A.; Rochev, Y.; Webster, T.J.; Bayry, J. Potential Immuno-Nanomedicine Strategies to Fight COVID-19 like Pulmonary Infections. Nano Today 2021, 36, 101051. [Google Scholar] [CrossRef] [PubMed]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Valencia, P.M.; Zhang, L.; Langer, R.; Farokhzad, O.C. Polymeric Nanoparticles for Drug Delivery. In Cancer Nanotechnology; Grobmyer, S.R., Moudgil, B.M., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 624, pp. 163–175. ISBN 978-1-60761-608-5. [Google Scholar]
- Tanbour, R.; Martins, A.M.; Pitt, W.G.; Husseini, A.G. Drug Delivery Systems Based on Polymeric Micelles and Ultrasound: A Review. Curr. Pharm. Des. 2016, 22, 2796–2807. [Google Scholar] [CrossRef] [PubMed]
- Reichel, D.; Tripathi, M.; Perez, J.M. Biological Effects of Nanoparticles on Macrophage Polarization in the Tumor Microenvironment. Nanotheranostics 2019, 3, 66–88. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.; Porter, T.M. Thermosensitive Liposomes for Localized Delivery and Triggered Release of Chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Pollock, S.; Antrobus, R.; Newton, L.; Kampa, B.; Rossa, J.; Latham, S.; Nichita, N.B.; Dwek, R.A.; Zitzmann, N. Uptake and Trafficking of Liposomes to the Endoplasmic Reticulum. FASEB J. 2010, 24, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Huang, L. Polyhistidine Mediates an Acid-Dependent Fusion of Negatively Charged Liposomes. Biochemistry 1984, 23, 4409–4416. [Google Scholar] [CrossRef] [PubMed]
- Zylberberg, C.; Matosevic, S. Pharmaceutical Liposomal Drug Delivery: A Review of New Delivery Systems and a Look at the Regulatory Landscape. Drug Deliv. 2016, 23, 3319–3329. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Sultana, Y.; Aqil, M. Liposomal Drug Delivery Systems: An Update Review. Curr. Drug Deliv. 2007, 4, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal Nanocarriers: The Impact of Constituents and Formulation Techniques on Ethosomal Properties, in Vivo Studies, and Clinical Trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.; de Freitas, V.; Mateus, N.; Fernandes, I.; Oliveira, J. Solid Lipid Nanoparticles as Carriers of Natural Phenolic Compounds. Antioxidants 2020, 9, 998. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Hsu, H.-J.; Bugno, J.; Lee, S.; Hong, S. Dendrimer-Based Nanocarriers: A Versatile Platform for Drug Delivery: Dendrimer-Based Nanocarriers. Wires Nanomed. Nanobiotechnol. 2017, 9, e1409. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Gill, G.S.; Jeet, K. Applications of Carbon Nanotubes in Drug Delivery. In Characterization and Biology of Nanomaterials for Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2019; pp. 113–135. ISBN 978-0-12-814031-4. [Google Scholar]
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.; Wang, B.; Zhang, T.; Tao, J. Advanced Review of Graphene-Based Nanomaterials in Drug Delivery Systems: Synthesis, Modification, Toxicity and Application. Mater. Sci. Eng. C 2017, 77, 1363–1375. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef]
- Chen, P.; Song, H.; Yao, S.; Tu, X.; Su, M.; Zhou, L. Magnetic Targeted Nanoparticles Based on β-Cyclodextrin and Chitosan for Hydrophobic Drug Delivery and a Study of Their Mechanism. RSC Adv. 2017, 7, 29025–29034. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. J. Nanomater. 2019, 2019, 1–26. [Google Scholar] [CrossRef]
- Shaker, S.; Gardouh, A.R.; Ghorab, M.M. Factors Affecting Liposomes Particle Size Prepared by Ethanol Injection Method. Res. Pharm. Sci. 2017, 12, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Charcosset, C.; Juban, A.; Valour, J.-P.; Urbaniak, S.; Fessi, H. Preparation of Liposomes at Large Scale Using the Ethanol Injection Method: Effect of Scale-up and Injection Devices. Chem. Eng. Res. Des. 2015, 94, 508–515. [Google Scholar] [CrossRef]
- Sahoo, S.K.; Labhasetwar, V. Nanotech Approaches to Drug Delivery and Imaging. Drug Discov. Today 2003, 8, 1112–1120. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel Methods for Liposome Preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as Nanomedical Devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Ali, A.A.E.; Trivedi, L.R. An Updated Review On: Liposomes as Drug Delivery System. PharmaTutor 2018, 6, 50. [Google Scholar] [CrossRef]
- Sankar, V.; Ramesh, S.; Siram, K. Ethosomes: An Exciting and Promising Alcoholic Carrier System for Treating Androgenic Alopecia. Alopecia 2018. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Mariani, P.; Cortesi, R.; Huang, N.; Simelière, F.; Marchetti, N.; Drechsler, M.; Ruzgas, T.; Esposito, E. Design and Characterization of Ethosomes for Transdermal Delivery of Caffeic Acid. Pharmaceutics 2020, 12, 740. [Google Scholar] [CrossRef] [PubMed]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Preparation of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Drug Delivery and the Effects of Preparation Parameters of Solvent Injection Method. Molecules 2020, 25, 4781. [Google Scholar] [CrossRef]
- Mishra, D.K.; Dhote, V.; Bhatnagar, P.; Mishra, P.K. Engineering Solid Lipid Nanoparticles for Improved Drug Delivery: Promises and Challenges of Translational Research. Drug Deliv. Transl. Res. 2012, 2, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Mudshinge, S.R.; Deore, A.B.; Patil, S.; Bhalgat, C.M. Nanoparticles: Emerging Carriers for Drug Delivery. Saudi Pharm. J. 2011, 19, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Harde, H.; Das, M.; Jain, S. Solid Lipid Nanoparticles: An Oral Bioavailability Enhancer Vehicle. Expert Opin. Drug Deliv. 2011, 8, 1407–1424. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable Drug Delivery Potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Um, J.Y.; Chung, H.; Kim, K.S.; Kwon, I.C.; Jeong, S.Y. In Vitro Cellular Interaction and Absorption of Dispersed Cubic Particles. Int. J. Pharm. 2003, 253, 71–80. [Google Scholar] [CrossRef]
- Wörle, G.; Drechsler, M.; Koch, M.H.J.; Siekmann, B.; Westesen, K.; Bunjes, H. Influence of Composition and Preparation Parameters on the Properties of Aqueous Monoolein Dispersions. Int. J. Pharm. 2007, 329, 150–157. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.; D’Souza, W.; Simone, C., II; Raghavan, S.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal Drug Delivery Systems: From Concept to Clinical Applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Chandrawati, R.; Caruso, F. Biomimetic Liposome- and Polymersome-Based Multicompartmentalized Assemblies. Langmuir 2012, 28, 13798–13807. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Gabizon, A.A.; de Rosales, R.T.M.; La-Beck, N.M. Translational Considerations in Nanomedicine: The Oncology Perspective. Adv. Drug Deliv. Rev. 2020, 158, 140–157. [Google Scholar] [CrossRef]
- Cruz-Leal, Y.; Lucatelli Laurindo, M.F.; Osugui, L.; del Carmen Luzardo, M.; López-Requena, A.; Alonso, M.E.; Álvarez, C.; Popi, A.F.; Mariano, M.; Pérez, R.; et al. Liposomes of Phosphatidylcholine and Cholesterol Induce an M2-like Macrophage Phenotype Reprogrammable to M1 Pattern with the Involvement of B-1 Cells. Immunobiology 2014, 219, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, P.J.; Adolph, S.K.; Subramanian, A.K.; Arouri, A.; Andresen, T.L.; Mouritsen, O.G.; Madsen, R.; Madsen, M.W.; Peters, G.H.; Clausen, M.H. Liposomal Formulation of Retinoids Designed for Enzyme Triggered Release. J. Med. Chem. 2010, 53, 3782–3792. [Google Scholar] [CrossRef]
- Lai, F.; Caddeo, C.; Manca, M.L.; Manconi, M.; Sinico, C.; Fadda, A.M. What’s New in the Field of Phospholipid Vesicular Nanocarriers for Skin Drug Delivery. Int. J. Pharm. 2020, 583, 119398. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel Vesicular Carriers for Enhanced Delivery: Characterization and Skin Penetration Properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Mishra, D.; Mishra, P.K.; Dabadghao, S.; Dubey, V.; Nahar, M.; Jain, N.K. Comparative Evaluation of Hepatitis B Surface Antigen–Loaded Elastic Liposomes and Ethosomes for Human Dendritic Cell Uptake and Immune Response. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 110–118. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Spinozzi, F.; Benedusi, M.; Cervellati, F.; Cortesi, R.; Drechsler, M.; Prieux, R.; Valacchi, G.; Esposito, E. Ethosomes for Coenzyme Q10 Cutaneous Administration: From Design to 3D Skin Tissue Evaluation. Antioxidants 2020, 9, 485. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Therapeutic and Cosmeceutical Potential of Ethosomes: An Overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274. [Google Scholar] [CrossRef]
- Benson, H.A.E. Vesicles for Transdermal Delivery of Peptides and Proteins. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 297–307. ISBN 978-3-662-47861-5. [Google Scholar]
- Kumar, L.; Verma, S.; Singh, K.; Prasad, D.N.; Jain, A.K. Ethanol Based Vesicular Carriers in Transdermal Drug Delivery: Nanoethosomes and Transethosomes in Focus. Nanoworld J. 2016, 2. [Google Scholar] [CrossRef]
- Bellefroid, C.; Lechanteur, A.; Evrard, B.; Mottet, D.; Debacq-Chainiaux, F.; Piel, G. In Vitro Skin Penetration Enhancement Techniques: A Combined Approach of Ethosomes and Microneedles. Int. J. Pharm. 2019, 572, 118793. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Bansal, K.; Verma, A.; Yadav, N.; Thakur, S.; Sudhakar, K.; Rosenholm, J. Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10, 191. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Ray, S.; Thakur, R. Solid Lipid Nanoparticles: A Modern Formulation Approach in Drug Delivery System. Indian J. Pharm. Sci. 2009, 71, 349. [Google Scholar] [CrossRef] [PubMed]
- Garcês, A.; Amaral, M.H.; Sousa Lobo, J.M.; Silva, A.C. Formulations Based on Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for Cutaneous Use: A Review. Eur. J. Pharm. Sci. 2018, 112, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Fuochi, V.; Zielińska, A.; Musumeci, T.; Souto, E.B.; Bonaccorso, A.; Puglia, C.; Petronio Petronio, G.; Furneri, P.M. Dual-Drugs Delivery in Solid Lipid Nanoparticles for the Treatment of Candida Albicans Mycosis. Colloids Surf. B Biointerfaces 2020, 186, 110705. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.R.; Pawar, P. Nanostructured Lipid Carriers (NLC) System: A Novel Drug Targeting Carrier. J. Drug Deliv. Sci. Technol. 2019, 51, 255–267. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid Nanoparticles (SLN, NLC) in Cosmetic and Pharmaceutical Dermal Products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Tetyczka, C.; Hodzic, A.; Kriechbaum, M.; Juraić, K.; Spirk, C.; Hartl, S.; Pritz, E.; Leitinger, G.; Roblegg, E. Comprehensive Characterization of Nanostructured Lipid Carriers Using Laboratory and Synchrotron X-Ray Scattering and Diffraction. Eur. J. Pharm. Biopharm. 2019, 139, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Üner, M. Characterization and Imaging of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers. In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 117–141. ISBN 978-3-319-15338-4. [Google Scholar]
- Pardeshi, C.; Rajput, P.; Belgamwar, V.; Tekade, A.; Patil, G.; Chaudhary, K.; Sonje, A. Solid Lipid Based Nanocarriers: An Overview / Nanonosači Na Bazi Čvrstih Lipida: Pregled. Acta Pharm. 2012, 62, 433–472. [Google Scholar] [CrossRef] [PubMed]
- Wissing, S.A.; Müller, R.H. The Influence of Solid Lipid Nanoparticles on Skin Hydration and Viscoelasticity—In Vivo Study. Eur. J. Pharm. Biopharm. 2003, 56, 67–72. [Google Scholar] [CrossRef]
- Lademann, J.; Richter, H.; Teichmann, A.; Otberg, N.; Blume-Peytavi, U.; Luengo, J.; Weiß, B.; Schaefer, U.F.; Lehr, C.-M.; Wepf, R. Nanoparticles—An Efficient Carrier for Drug Delivery into the Hair Follicles. Eur. J. Pharm. Biopharm. 2007, 66, 159–164. [Google Scholar] [CrossRef]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Cubic Lipid−Water Phase Dispersed into Submicron Particles. Langmuir 1996, 12, 4611–4613. [Google Scholar] [CrossRef]
- Larsson, K. Aqueous Dispersions of Cubic Lipid–Water Phases. Curr. Opin. Colloid Interface Sci. 2000, 5, 64–69. [Google Scholar] [CrossRef]
- Nakano, K.; Tozuka, Y.; Yamamoto, H.; Kawashima, Y.; Takeuchi, H. A Novel Method for Measuring Rigidity of Submicron-Size Liposomes with Atomic Force Microscopy. Int. J. Pharm. 2008, 355, 203–209. [Google Scholar] [CrossRef]
- Cortesi, R.; Cappellozza, E.; Drechsler, M.; Contado, C.; Baldisserotto, A.; Mariani, P.; Carducci, F.; Pecorelli, A.; Esposito, E.; Valacchi, G. Monoolein Aqueous Dispersions as a Delivery System for Quercetin. Biomed. Microdevices 2017, 19, 41. [Google Scholar] [CrossRef]
- Sguizzato, M.; Esposito, E.; Drechsler, M.; Hallan, S.S.; Cortesi, R. Monolein Aqueous Dispersions as a Tool to Increase Flavonoid Solubility: A Preliminary Study. Proceedings 2020, 78, 25. [Google Scholar] [CrossRef]
- Esposito, E.; Sticozzi, C.; Ravani, L.; Drechsler, M.; Muresan, X.M.; Cervellati, F.; Cortesi, R.; Valacchi, G. Effect of New Curcumin-Containing Nanostructured Lipid Dispersions on Human Keratinocytes Proliferative Responses. Exp. Dermatol. 2015, 24, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cortesi, R.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Contado, C.; Stellin, E.; Menegatti, E.; Bonina, F.; Puglia, C. Cubosome Dispersions as Delivery Systems for Percutaneous Administration of Indomethacin. Pharm. Res. 2005, 22, 2163–2173. [Google Scholar] [CrossRef]
- Esposito, E.; Drechsler, M.; Mariani, P.; Panico, A.M.; Cardile, V.; Crascì, L.; Carducci, F.; Graziano, A.C.E.; Cortesi, R.; Puglia, C. Nanostructured Lipid Dispersions for Topical Administration of Crocin, a Potent Antioxidant from Saffron (Crocus Sativus L.). Mater. Sci. Eng. C 2017, 71, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D.; Templeton, N.S. Liposomes in Gene Therapy. Adv. Drug Deliv. Rev. 1996, 20, 221–266. [Google Scholar] [CrossRef]
- Prabu, S.L.; Suriyaprakash, T.N.K.; Thirumurugan, R. Medicated Nanoparticle for Gene Delivery. In Advanced Technology for Delivering Therapeutics; Maiti, S., Sen, K.K., Eds.; InTech: London, UK, 2017; ISBN 978-953-51-3121-2. [Google Scholar]
- Adler, A.F.; Leong, K.W. Emerging Links between Surface Nanotechnology and Endocytosis: Impact on Nonviral Gene Delivery. Nano Today 2010, 5, 553–569. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Alwani, S.; Badea, I. Polymeric Nanoparticles in Gene Therapy: New Avenues of Design and Optimization for Delivery Applications. Polymers 2019, 11, 745. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Esposito, E.; Menegatti, E.; Gambari, R.; Nastruzzi, C. Effect of Cationic Liposome Composition on in Vitro Cytotoxicity and Protective Effect on Carried DNA. Int. J. Pharm. 1996, 139, 69–78. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.; Aifantis, K.E.; Fan, Y.; Feng, Q.; Cui, F.-Z.; Watari, F. Current Investigations into Magnetic Nanoparticles for Biomedical Applications: Magnetic Nanoparticles for Biomedical Applications. J. Biomed. Mater. Res. 2016, 104, 1285–1296. [Google Scholar] [CrossRef]
- Avval, Z.M.; Malekpour, L.; Raeisi, F.; Babapoor, A.; Mousavi, S.M.; Hashemi, S.A.; Salari, M. Introduction of Magnetic and Supermagnetic Nanoparticles in New Approach of Targeting Drug Delivery and Cancer Therapy Application. Drug Metab. Rev. 2020, 52, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Dulińska-Litewka, J.; Łazarczyk, A.; Hałubiec, P.; Szafrański, O.; Karnas, K.; Karewicz, A. Superparamagnetic Iron Oxide Nanoparticles—Current and Prospective Medical Applications. Materials 2019, 12, 617. [Google Scholar] [CrossRef]
- Hervault, A.; Thanh, N.T.K. Magnetic Nanoparticle-Based Therapeutic Agents for Thermo-Chemotherapy Treatment of Cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef]
- Montiel Schneider, M.G.; Lassalle, V.L. Magnetic Iron Oxide Nanoparticles as Novel and Efficient Tools for Atherosclerosis Diagnosis. Biomed. Pharmacother. 2017, 93, 1098–1115. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Huh, Y.-M.; Jun, Y.; Seo, J.; Jang, J.; Song, H.-T.; Kim, S.; Cho, E.-J.; Yoon, H.-G.; Suh, J.-S.; et al. Artificially Engineered Magnetic Nanoparticles for Ultra-Sensitive Molecular Imaging. Nat. Med. 2007, 13, 95–99. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.; Akram, M.; Udego, I.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef]
- Byoun, W.; Jang, M.; Yoo, H. Fabrication of Highly Fluorescent Multiple Fe3O4 Nanoparticles Core-Silica Shell Nanoparticles. J. Nanopart. Res. 2019, 21, 1. [Google Scholar] [CrossRef]
- Ling, W.; Wang, M.; Xiong, C.; Xie, D.; Chen, Q.; Chu, X.; Qiu, X.; Li, Y.; Xiao, X. Synthesis, Surface Modification, and Applications of Magnetic Iron Oxide Nanoparticles. J. Mater. Res. 2019, 34, 1828–1844. [Google Scholar] [CrossRef]
- Ghadiri, M.; Vasheghani-Farahani, E.; Atyabi, F.; Kobarfard, F.; Mohamadyar-Toupkanlou, F.; Hosseinkhani, H. Transferrin-Conjugated Magnetic Dextran-Spermine Nanoparticles for Targeted Drug Transport across Blood-Brain Barrier: Transferrin-Conjugated Magnetic Dextran-Spermine Nanoparticles. J. Biomed. Mater. Res. 2017, 105, 2851–2864. [Google Scholar] [CrossRef]
- Shanavas, A.; Sasidharan, S.; Bahadur, D.; Srivastava, R. Magnetic Core-Shell Hybrid Nanoparticles for Receptor Targeted Anti-Cancer Therapy and Magnetic Resonance Imaging. J. Colloid Interface Sci. 2017, 486, 112–120. [Google Scholar] [CrossRef]
- Qasim, S.B.; Zafar, M.S.; Najeeb, S.; Khurshid, Z.; Shah, A.H.; Husain, S.; Rehman, I.U. Electrospinning of Chitosan-Based Solutions for Tissue Engineering and Regenerative Medicine. Int. J. Mol. Sci. 2018, 19, 407. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, X.; Cao, D. Nanoparticle Hardness Controls the Internalization Pathway for Drug Delivery. Nanoscale 2015, 7, 2758–2769. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Shi, X.; Gao, H. Cellular Uptake of Elastic Nanoparticles. Phys. Rev. Lett. 2011, 107, 098101. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Nastruzzi, C.; Valacchi, G.; Cortesi, R. Lipid Nanostructures for Antioxidant Delivery: A Comparative Preformulation Study. Beilstein J. Nanotechnol. 2019, 10, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Gessner, A.; Lieske, A.; Paulke, B.R.; Müller, R.H. Influence of Surface Charge Density on Protein Adsorption on Polymeric Nanoparticles: Analysis by Two-Dimensional Electrophoresis. Eur. J. Pharm. Biopharm. 2002, 54, 165–170. [Google Scholar] [CrossRef]
- Boyd, R.D.; Pichaimuthu, S.K.; Cuenat, A. New Approach to Inter-Technique Comparisons for Nanoparticle Size Measurements; Using Atomic Force Microscopy, Nanoparticle Tracking Analysis and Dynamic Light Scattering. Colloids Surf. A Physicochem. Eng. Asp. 2011, 387, 35–42. [Google Scholar] [CrossRef]
- Kuchibhatla, S.V.N.T.; Karakoti, A.S.; Baer, D.R.; Samudrala, S.; Engelhard, M.H.; Amonette, J.E.; Thevuthasan, S.; Seal, S. Influence of Aging and Environment on Nanoparticle Chemistry: Implication to Confinement Effects in Nanoceria. J. Phys. Chem. C 2012, 116, 14108–14114. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.-S.; Lee, H.J.; Dong, Z. Caffeic Acid, a Phenolic Phytochemical in Coffee, Directly Inhibits Fyn Kinase Activity and UVB-Induced COX-2 Expression. Carcinogenesis 2008, 30, 321–330. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis Spectroscopy in Characterization of Polydisperse Nanoparticles Colloids. J. Nanomater. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Goldstein, J.; Newbury, D.E.; Joy, D.C.; Lyman, C.E.; Echlin, P.; Lifshin, E.; Sawyer, L.; Michael, J.R. Scanning Electron Microscopy and X-Ray Microanalysis, 3rd ed.; Springer: Boston, MA, USA, 2003; ISBN 978-0-306-47292-3. [Google Scholar]
- Kuntsche, J.; Horst, J.C.; Bunjes, H. Cryogenic Transmission Electron Microscopy (Cryo-TEM) for Studying the Morphology of Colloidal Drug Delivery Systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Hallan, S.S.; Marchetti, P.; Bortolotti, D.; Sguizzato, M.; Esposito, E.; Mariani, P.; Trapella, C.; Rizzo, R.; Cortesi, R. Design of Nanosystems for the Delivery of Quorum Sensing Inhibitors: A Preliminary Study. Molecules 2020, 25, 5655. [Google Scholar] [CrossRef]
- Dubochet, J.; Adrian, M.; Chang, J.-J.; Homo, J.-C.; Lepault, J.; McDowall, A.W.; Schultz, P. Cryo-Electron Microscopy of Vitrified Specimens. Quart. Rev. Biophys. 1988, 21, 129–228. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Fantin, M.; Marti, M.; Drechsler, M.; Paccamiccio, L.; Mariani, P.; Sivieri, E.; Lain, F.; Menegatti, E.; Morari, M.; et al. Solid Lipid Nanoparticles as Delivery Systems for Bromocriptine. Pharm. Res. 2008, 25, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Bunjes, H. Structural Properties of Solid Lipid Based Colloidal Drug Delivery Systems. Curr. Opin. Colloid Interface Sci. 2011, 16, 405–411. [Google Scholar] [CrossRef]
- Jores, K.; Mehnert, W.; Drechsler, M.; Bunjes, H.; Johann, C.; Mäder, K. Investigations on the Structure of Solid Lipid Nanoparticles (SLN) and Oil-Loaded Solid Lipid Nanoparticles by Photon Correlation Spectroscopy, Field-Flow Fractionation and Transmission Electron Microscopy. J. Control. Release 2004, 95, 217–227. [Google Scholar] [CrossRef]
- Spicer, P. Cubosome ProcessingIndustrial Nanoparticle Technology Development. Chem. Eng. Res. Des. 2005, 83, 1283–1286. [Google Scholar] [CrossRef]
- Spicer, P.T.; Hayden, K.L.; Lynch, M.L.; Ofori-Boateng, A.; Burns, J.L. Novel Process for Producing Cubic Liquid Crystalline Nanoparticles (Cubosomes). Langmuir 2001, 17, 5748–5756. [Google Scholar] [CrossRef]
- Almgren, M.; Edwards, K.; Karlsson, G. Cryo Transmission Electron Microscopy of Liposomes and Related Structures. Colloids Surf. A Physicochem. Eng. Asp. 2000, 174, 3–21. [Google Scholar] [CrossRef]
- Esposito, E. Production, Physico-Chemical Characterization and Biodistribution Studies of Lipid Nanoparticles. J. Nanomed. Nanotechnol 2015, 6. [Google Scholar] [CrossRef]
- Esposito, E.; Ravani, L.; Drechsler, M.; Mariani, P.; Contado, C.; Ruokolainen, J.; Ratano, P.; Campolongo, P.; Trezza, V.; Nastruzzi, C.; et al. Cannabinoid Antagonist in Nanostructured Lipid Carriers (NLCs): Design, Characterization and in Vivo Study. Mater. Sci. Eng. C 2015, 48, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Luzzati, V.; Delacroix, H.; Gulik, A.; Gulik-Krzywicki, T.; Mariani, P.; Vargas, R. Chapter 1 The Cubic Phases of Lipids. In Current Topics in Membranes; Epand, R.M., Ed.; Lipid Polymorphism and Membrane Properties; Academic Press: Cambridge, MA, USA, 1997; Volume 44, pp. 3–24. [Google Scholar]
- Lazar, T. The Structure of Biological Membranes (2nd Edn) P. Yeagle (Ed.), CRC Press, 540 Pp., ISBN 0-8493-1403-8 (2004). Cell Biochem. Funct. 2005, 23, 294–295. [Google Scholar] [CrossRef]
- Esposito, E.; Mariani, P.; Drechsler, M.; Cortesi, R. Structural Studies of Lipid-Based Nanosystems for Drug Delivery: X-ray Diffraction (XRD) and Cryogenic Transmission Electron Microscopy (Cryo-TEM). In Handbook of Nanoparticles; Aliofkhazraei, M., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 861–889. ISBN 978-3-319-15338-4. [Google Scholar]
- Luzzati, V.; Vargas, R.; Mariani, P.; Gulik, A.; Delacroix, H. Cubic Phases of Lipid-Containing Systems. Elements of a Theory and Biological Connotations. J. Mol. Biol. 1993, 229, 540–551. [Google Scholar] [CrossRef]
- Mariani, P.; Luzzati, V.; Delacroix, H. Cubic Phases of Lipid-Containing Systems. Structure Analysis and Biological Implications. J. Mol. Biol. 1988, 204, 165–189. [Google Scholar] [CrossRef]
- Esposito, E.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Carducci, F.; Nastruzzi, C.; Cortesi, R. Progesterone Lipid Nanoparticles: Scaling up and in Vivo Human Study. Eur. J. Pharm. Biopharm. 2017, 119, 437–446. [Google Scholar] [CrossRef]
- Singh Hallan, S.; Sguizzato, M.; Pavoni, G.; Baldisserotto, A.; Drechsler, M.; Mariani, P.; Esposito, E.; Cortesi, R. Ellagic Acid Containing Nanostructured Lipid Carriers for Topical Application: A Preliminary Study. Molecules 2020, 25, 1449. [Google Scholar] [CrossRef]
- Hallan, S.S.; Sguizzato, M.; Drechsler, M.; Mariani, P.; Montesi, L.; Cortesi, R.; Björklund, S.; Ruzgas, T.; Esposito, E. The Potential of Caffeic Acid Lipid Nanoparticulate Systems for Skin Application: In Vitro Assays to Assess Delivery and Antioxidant Effect. Nanomaterials 2021, 11, 171. [Google Scholar] [CrossRef]
- Taha, M.S.; Padmakumar, S.; Singh, A.; Amiji, M.M. Critical Quality Attributes in the Development of Therapeutic Nanomedicines toward Clinical Translation. Drug Deliv. Transl. Res. 2020, 10, 766–790. [Google Scholar] [CrossRef]
- Dieckmann, Y.; Cölfen, H.; Hofmann, H.; Petri-Fink, A. Particle Size Distribution Measurements of Manganese-Doped ZnS Nanoparticles. Anal. Chem. 2009, 81, 3889–3895. [Google Scholar] [CrossRef]
- von der Kammer, F.; Legros, S.; Hofmann, T.; Larsen, E.H.; Loeschner, K. Separation and Characterization of Nanoparticles in Complex Food and Environmental Samples by Field-Flow Fractionation. TrAC Trends Anal. Chem. 2011, 30, 425–436. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Shen, S.; Lee, S.; Dou, H. Field-Flow Fractionation: A Gentle Separation and Characterization Technique in Biomedicine. TrAC Trends Anal. Chem. 2018, 108, 231–238. [Google Scholar] [CrossRef]
- Metreau, J.M.; Gallet, S.; Cardot, P.J.P.; Maire, V.L.; Dumas, F.; Hernvann, A.; Loric, S. Sedimentation Field-Flow Fractionation of Cellular Species. Anal. Biochem. 1997, 251, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Koppel, D.E. Analysis of Macromolecular Polydispersity in Intensity Correlation Spectroscopy: The Method of Cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Ritz, S.; Schöttler, S.; Kotman, N.; Baier, G.; Musyanovych, A.; Kuharev, J.; Landfester, K.; Schild, H.; Jahn, O.; Tenzer, S.; et al. Protein Corona of Nanoparticles: Distinct Proteins Regulate the Cellular Uptake. Biomacromolecules 2015, 16, 1311–1321. [Google Scholar] [CrossRef]
- Olenick, L.L.; Troiano, J.M.; Vartanian, A.; Melby, E.S.; Mensch, A.C.; Zhang, L.; Hong, J.; Mesele, O.; Qiu, T.; Bozich, J.; et al. Lipid Corona Formation from Nanoparticle Interactions with Bilayers. Chem 2018, 4, 2709–2723. [Google Scholar] [CrossRef]
- Gilroy, E.L.; Hicks, M.R.; Smith, D.J.; Rodger, A. Viscosity of Aqueous DNA Solutions Determined Using Dynamic Light Scattering. Analyst 2011, 136, 4159. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical Evaluation of Nanoparticle Tracking Analysis (NTA) by NanoSight for the Measurement of Nanoparticles and Protein Aggregates. Pharm. Res. 2010, 27, 796–810. [Google Scholar] [CrossRef]
- Maulucci, G.; De Spirito, M.; Arcovito, G.; Boffi, F.; Castellano, A.C.; Briganti, G. Particle Size Distribution in DMPC Vesicles Solutions Undergoing Different Sonication Times. Biophys. J. 2005, 88, 3545–3550. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Yuana, Y.; Grootemaat, A.E.; van der Pol, E.; Gollwitzer, C.; Krumrey, M.; Nieuwland, R. Towards Traceable Size Determination of Extracellular Vesicles. J. Extracell. Vesicles 2014, 3, 23298. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, J.A.; Jacobs, D.J. Light Scattering from Benzene, Toluene, Carbon Disulphide and Carbon Tetrachloride. Chem. Phys. Lett. 1970, 6, 141–143. [Google Scholar] [CrossRef]
- Noday, D.A.; Steif, P.S.; Rabin, Y. Viscosity of Cryoprotective Agents Near Glass Transition: A New Device, Technique, and Data on DMSO, DP6, and VS55. Exp. Mech. 2009, 49, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Cherubin, P.; Cilenti, L.; Teter, K.; Huo, Q. A Simple and Fast Method to Study the Hydrodynamic Size Difference of Protein Disulfide Isomerase in Oxidized and Reduced Form Using Gold Nanoparticles and Dynamic Light Scattering. Analyst 2016, 141, 934–938. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.H.; Motskin, M.; Philpott, A.J.; Routh, A.F.; Shanahan, C.M.; Duer, M.J.; Skepper, J.N. The Effect of Particle Agglomeration on the Formation of a Surface-Connected Compartment Induced by Hydroxyapatite Nanoparticles in Human Monocyte-Derived Macrophages. Biomaterials 2014, 35, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- He, H.X.; Zhang, H.; Li, Q.G.; Zhu, T.; Li, S.F.Y.; Liu, Z.F. Fabrication of Designed Architectures of Au Nanoparticles on Solid Substrate with Printed Self-Assembled Monolayers as Templates. Langmuir 2000, 16, 3846–3851. [Google Scholar] [CrossRef]
- Pethkar, S.; Aslam, M.; Mulla, I.S.; Ganeshan, P.; Vijayamohanan, K. Preparation and Characterisation of Silver Quantum Dot Superlattice Using Self-Assembled Monolayers of Pentanedithiol. J. Mater. Chem. 2001, 11, 1710–1714. [Google Scholar] [CrossRef]
- Contado, C.; Blo, G.; Fagioli, F.; Dondi, F.; Beckett, R. Characterisation of River Po Particles by Sedimentation Field-Flow Fractionation Coupled to GFAAS and ICP-MS. Colloids Surf. A Physicochem. Eng. Asp. 1997, 120, 47–59. [Google Scholar] [CrossRef]
- Egelhaaf, S.U.; Wehrli, E.; Müller, M.; Adrian, M.; Schurtenberger, P. Determination of the Size Distribution of Lecithin Liposomes: A Comparative Study Using Freeze Fracture, Cryoelectron Microscopy and Dynamic Light Scattering. J. Microsc. 1996, 184, 214–228. [Google Scholar] [CrossRef]
- Weiner, B.B.; Tscharnuter, W.W.; Fairhurst, D. Zeta Potential: A New Approach. In Proceedings of the Canadian Mineral Analysts Meeting, Winnipeg, MB, Canada, 8–12 September 1993; Volume 12. [Google Scholar]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to Improve the Scientific Value of Zeta-Potential Measurements in NanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Tantra, R.; Schulze, P.; Quincey, P. Effect of Nanoparticle Concentration on Zeta-Potential Measurement Results and Reproducibility. Particuology 2010, 8, 279–285. [Google Scholar] [CrossRef]
- Cortesi, R.; Marastoni, M.; Tomatis, R.; Menegatti, E.; Esposito, E.; Nastruzzi, C. Peptide-Based Cationic Molecules for the Production of Positive Charged Liposomes and Micelles. J. Microencapsul. 2008, 25, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, R.; Mischiati, C.; Borgatti, M.; Breda, L.; Romanelli, A.; Saviano, M.; Pedone, C.; Gambari, R.; Nastruzzi, C. Formulations for Natural and Peptide Nucleic Acids Based on Cationic Polymeric Submicron Particles. AAPS J. 2004, 6, 10–21. [Google Scholar] [CrossRef][Green Version]
- Hallan, S.S.; Kaur, V.; Jain, V.; Mishra, N. Development and Characterization of Polymer Lipid Hybrid Nanoparticles for Oral Delivery of LMWH. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1631–1639. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Singh, D.; Rashid, M.; Hallan, S.S.; Mehra, N.K.; Prakash, A.; Mishra, N. Pharmacological Evaluation of Nasal Delivery of Selegiline Hydrochloride-Loaded Thiolated Chitosan Nanoparticles for the Treatment of Depression. Artif. Cells Nanomed. Biotechnol. 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V.; Castiglione, Z.; Cubas, P.; Zhu, L.; Li, W.; Habelitz, S. Zeta-Potential and Particle Size Analysis of Human Amelogenins. J. Dent. Res. 2010, 89, 149–153. [Google Scholar] [CrossRef]
- Kirkwood, J.; Hargreaves, D.; O’Keefe, S.; Wilson, J. Using Isoelectric Point to Determine the PH for Initial Protein Crystallization Trials. Bioinformatics 2015, 31, 1444–1451. [Google Scholar] [CrossRef]
- Lu, L.T. Water-Dispersible Magnetic Nanoparticles for Biomedical Applications: Synthesis and Characterisation. Doctoral Dissertation, University of Liverpool, Liverpool, UK, 2019. [Google Scholar] [CrossRef]
- Jenning, V.; Gysler, A.; Schäfer-Korting, M.; Gohla, S.H. Vitamin A Loaded Solid Lipid Nanoparticles for Topical Use: Occlusive Properties and Drug Targeting to the Upper Skin. Eur. J. Pharm. Biopharm. 2000, 49, 211–218. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H.; Manthei, L.; Mayer, C. Structural Characterization of Q10-Loaded Solid Lipid Nanoparticles by NMR Spectroscopy. Pharm. Res. 2004, 21, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Fuentes, M.; Torres, D.; Martín-Pastor, M.; Alonso, M.J. Application of NMR Spectroscopy to the Characterization of PEG-Stabilized Lipid Nanoparticles. Langmuir 2004, 20, 8839–8845. [Google Scholar] [CrossRef] [PubMed]
- Coelho, S.C.; Rangel, M.; Pereira, M.C.; Coelho, M.A.N.; Ivanova, G. Structural Characterization of Functionalized Gold Nanoparticles for Drug Delivery in Cancer Therapy: A NMR Based Approach. Phys. Chem. Chem. Phys. 2015, 17, 18971–18979. [Google Scholar] [CrossRef] [PubMed]
- Sguizzato, M.; Esposito, E.; Drechsler, M.; Gallerani, E.; Gavioli, R.; Mariani, P.; Carducci, F.; Cortesi, R.; Bergamini, P. Nafion®-Containing Solid Lipid Nanoparticles as a Tool for Anticancer Pt Delivery: Preliminary Studies. J. Chem. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Sunandana, C.S. Techniques and Applications of Electron Spin Resonance. Bull. Mater. Sci. 1998, 21, 1. [Google Scholar] [CrossRef]
- Lucarini, M.; Pasquato, L. ESR Spectroscopy as a Tool to Investigate the Properties of Self-Assembled Monolayers Protecting Gold Nanoparticles. Nanoscale 2010, 2, 668. [Google Scholar] [CrossRef] [PubMed]
- Chertok, B.; Moffat, B.A.; David, A.E.; Yu, F.; Bergemann, C.; Ross, B.D.; Yang, V.C. Iron Oxide Nanoparticles as a Drug Delivery Vehicle for MRI Monitored Magnetic Targeting of Brain Tumors. Biomaterials 2008, 29, 487–496. [Google Scholar] [CrossRef]
- He, W.; Liu, Y.; Wamer, W.G.; Yin, J.-J. Electron Spin Resonance Spectroscopy for the Study of Nanomaterial-Mediated Generation of Reactive Oxygen Species. J. Food Drug Anal. 2014, 22, 49–63. [Google Scholar] [CrossRef]
- Yakimovich, N.O.; Ezhevskii, A.A.; Guseinov, D.V.; Smirnova, L.A.; Gracheva, T.A.; Klychkov, K.S. Antioxidant Properties of Gold Nanoparticles Studied by ESR Spectroscopy. Russ. Chem. Bull. 2008, 57, 520–523. [Google Scholar] [CrossRef]
- Köseoğlu, Y. Effect of Surfactant Coating on Magnetic Properties of Fe3O4 Nanoparticles: ESR Study. J. Magn. Magn. Mater. 2006, 300, e327–e330. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Krist, B.; Schroeder, G.; Kurczewska, J.; Bluyssen, H.A.R. ESR Method in Monitoring of Nanoparticle Endocytosis in Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4388. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, R.M.; Casadei, B.R.; Duarte, E.L.; Severino, P.; Barbosa, L.R.S.; Duran, N.; de Paula, E. Electron Paramagnetic Resonance and Small-Angle X-Ray Scattering Characterization of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Dibucaine Encapsulation. Langmuir 2018, 34, 13296–13304. [Google Scholar] [CrossRef]
- Lu, P.J. Confocal Scanning Optical Microscopy and Nanotechnology. In Handbook of Microscopy for Nanotechnology; Yao, N., Wang, Z.L., Eds.; Springer: Boston, MA, USA, 2005; pp. 3–24. ISBN 978-1-4020-8006-7. [Google Scholar]
- Zhang, L.W.; Monteiro-Riviere, N.A. Use of Confocal Microscopy for Nanoparticle Drug Delivery through Skin. J. Biomed. Opt. 2012, 18, 061214. [Google Scholar] [CrossRef] [PubMed]
- Strachan, J.B.; Dyett, B.P.; Nasa, Z.; Valery, C.; Conn, C.E. Toxicity and Cellular Uptake of Lipid Nanoparticles of Different Structure and Composition. J. Colloid Interface Sci. 2020, 576, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, H.S.; Palanikumar, L.; Go, E.M.; Jana, B.; Park, S.A.; Kim, H.Y.; Kim, K.; Seo, J.K.; Kwak, S.K.; et al. Cloaking Nanoparticles with Protein Corona Shield for Targeted Drug Delivery. Nat. Commun. 2018, 9, 4548. [Google Scholar] [CrossRef] [PubMed]
- Saber, M.M.; Bahrainian, S.; Dinarvand, R.; Atyabi, F. Targeted Drug Delivery of Sunitinib Malate to Tumor Blood Vessels by CRGD-Chiotosan-Gold Nanoparticles. Int. J. Pharm. 2017, 517, 269–278. [Google Scholar] [CrossRef]
- Nocchi, S.; Björklund, S.; Svensson, B.; Engblom, J.; Ruzgas, T. Electrochemical Monitoring of Native Catalase Activity in Skin Using Skin Covered Oxygen Electrode. Biosens. Bioelectron. 2017, 93, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, M.; Rembiesa, J.; Startaitė, L.; Holefors, A.; Valančiūtė, A.; Faridbod, F.; Ganjali, M.R.; Engblom, J.; Ruzgas, T. Polyphenol-Hydrogen Peroxide Reactions in Skin: In Vitro Model Relevant to Study ROS Reactions at Inflammation. Anal. Chim. Acta 2019, 1075, 91–97. [Google Scholar] [CrossRef]
- Hernández, A.R.; Vallejo, B.; Ruzgas, T.; Björklund, S. The Effect of UVB Irradiation and Oxidative Stress on the Skin Barrier—A New Method to Evaluate Sun Protection Factor Based on Electrical Impedance Spectroscopy. Sensors 2019, 19, 2376. [Google Scholar] [CrossRef]
- Jankovskaja, S.; Labrousse, A.; Prévaud, L.; Holmqvist, B.; Brinte, A.; Engblom, J.; Rezeli, M.; Marko-Varga, G.; Ruzgas, T. Visualisation of H2O2 Penetration through Skin Indicates Importance to Develop Pathway-Specific Epidermal Sensing. Microchim. Acta 2020, 187, 656. [Google Scholar] [CrossRef] [PubMed]





| Nano-Carrier | Method of Preparation | Merits | Demerits | References |
|---|---|---|---|---|
| Liposomes | Ethanol injection | - Simple and safe - Reproducibility - No oxidative degradation of lipid | - Heterogeneous population - Very diluted liposomes - Difficulty in complete ethanol removal | [25,26,27] |
| Direct hydration | - Simple - Production speed | - Not transposable on industrial scale - Require further sonication or extrusion | [28,29] | |
| Reverse phase | - Uniformity of size and Lamellarity - High aqueous space-to-lipid ratio - More embedding of aqueous material | - Denaturation of loaded proteins due to exposure of organic solvents | [27,30] | |
| Ethosomes | Cold Method | - Simple approach - No sophisticated instrumentation required | N/A | [31,32] |
| Hot method | - Ehanolic mixture is heated to 40 °C - Useful for both hydrophilic/hydrophobic drugs | N/A | ||
| SLN/ NLC | Hot high-Pressure Homogenization | - Scaling up feasible - No use of organic solvent | - Extremely energy intensive process - Drug degradation owing to high temperature - Limited drug loading | [33] |
| Cold high-pressure homogenization | - No drug degradation - Useful to load hydrophilic drugs -No alteration in crystallization | - Larger diameter of particles - Extensive size distributions | [34,35] | |
| Micro emulsion-based method | - No energy required - Good theoretical stability | - Very much susceptible towards alterations - Low yield of nanoparticles - High dilution of final dispersion | [35,36] | |
| MAD/ Cubosomes | Top-down approach | - No aggregation - Higher encapsulation efficiency | - High energy input - Not applicable for thermo-sensitive moieties - Poor cubosomes quality due to use of high temperature (40 and 60 °C) | [37,38,39] |
| Bottom-up approach | - Required less energy inputs - No high temperature requirements - Long term stability - Homogenicity | - Higher dilution used | [37] |
| Phase | Type | Structure Elements | Class |
|---|---|---|---|
| 1D: lamellar | - | Lamellae | - |
| 2D: hexagonal | I or II | Infinitely long rods | Rod-like |
| 3D: Cubic, P4332 | II | Rod network and micelles | Mixed rod-like and micellar |
| 3D: Cubic, Pm3n | I | Micelles | Micellar |
| 3D: Cubic, Pn3m | II | Intertwined rod networks | Bicontinuous (IPMS * Diamond-surface) |
| 3D: Cubic, Fd3m | II | Micelles | Micellar |
| 3D: Cubic, Im3m | II | Intertwined rod networks | Bicontinuous (IPMS * Primitive-surface) |
| 3D: Cubic, Ia3d | I or II | Intertwined rod networks | Bicontinuous (IPMS * Gyroid-surface) |
| Factor | Description | References |
|---|---|---|
| Solvent used in sample | It should be underlined that some solvents (toluene) have tendency to scatter the light up to certain extent, which can create background noise in results. Secondly, dimethyl sulfoxide can alter viscosity of the sample at different temperature conditions | [133,134] |
| Concentration of sample | Higher concentration of sample corresponds to higher number of particles. It means that light hit so many times before reaching to detector and finally loses intensity. Too diluted sample cannot produce scattered light to be analyzed. DLS is only useful in case of diluted samples. | [135,136] |
| Agglomeration | Nanomaterials have tendency to undergo agglomeration. The bigger size of agglomerate scatter light with great extent. Even it can destroy the detector also. | [99] |
| Type of cuvette | Use of organic solvent or temperature conditions higher than 50 °C can interfere with Cuvette made up of plastic. Cuvette should be clean properly with detergent or distilled water. | [103,131] |
| Technique | Principle | References |
|---|---|---|
| SdFFF | Based on separation of nano/ micro scale particles as a function of their specific mass with known particle density by assuming that particles are spherical. The dimensions illustrate the diameter of an equivalent sphere. It gives higher resolution comparably to PCS because it fractionates different sized particles first more specific for the dispersions having multimodal size distributions. | [110,139] |
| PCS/DLS | It measures the particle diameter by light scattering could give misleading interpretation with systems having non-spherical particles. Moreover, larger spheres monopolize the scattering behavior of the sample, small numbers of large nanoparticles result in a considerable enlargement of size and dispersity of nano-dispersion. | [110,140] |
| Cryo-TEM | It is not possible for all the particles to be imaged because larger particles can be neglected in the analysis. Therefore, usage of cryo-TEM for estimating diameter could give uncertain results because distances or geometries may be over- or under assessed. | [103,110] |
| Type of Nanoparticle (np) | Active Molecules | Z-Ave (nm) 1 | ζ Potential (mV) 2 | Reference |
|---|---|---|---|---|
| SLN | caffeic acid | 201 ± 11 | −4.92 ± 0.01 | [119] |
| ethosomes | caffeic acid | 219 ± 21 | +1.99 ± 2.48 | [119] |
| liposomes | QSi 3 | 230 ± 12 | +55.8 ± 0.4 | [102] |
| liposomes | Peptides | 200–350 | 17.8 ±13 | [144] |
| cationic particles | Peptide Nucleic Acids | 870–1140 | +27.9 ± 4.2 | [145] |
| chitosan | Enoxaparin | 135.2 ± 3.1 | 31.67 ± 4.6 | [146] |
| stearylamine lipid | 180.3 ± 3.6 | −13.52 ± 2.3 | ||
| polymer lipid hybrid | Selegiline | 178.7 ± 3.4 | −25.07 ± 3.4 | [147] |
| thiolated chitosan | 215 ± 34.7 | +17.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hallan, S.S.; Sguizzato, M.; Esposito, E.; Cortesi, R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics 2021, 13, 549. https://doi.org/10.3390/pharmaceutics13040549
Hallan SS, Sguizzato M, Esposito E, Cortesi R. Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics. 2021; 13(4):549. https://doi.org/10.3390/pharmaceutics13040549
Chicago/Turabian StyleHallan, Supandeep Singh, Maddalena Sguizzato, Elisabetta Esposito, and Rita Cortesi. 2021. "Challenges in the Physical Characterization of Lipid Nanoparticles" Pharmaceutics 13, no. 4: 549. https://doi.org/10.3390/pharmaceutics13040549
APA StyleHallan, S. S., Sguizzato, M., Esposito, E., & Cortesi, R. (2021). Challenges in the Physical Characterization of Lipid Nanoparticles. Pharmaceutics, 13(4), 549. https://doi.org/10.3390/pharmaceutics13040549









