In Vitro–In Vivo Correlation in Dermal Delivery: The Role of Excipients
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. HPLC Analysis
2.3. Gas Chromatography (GC) Analysis
2.4. Preparation of Formulations
2.5. Dynamic Vapor Sorption Studies
2.6. In Vitro Finite Dose Permeation Studies
2.7. Confocal Raman Spectroscopy
2.8. Data Analysis
3. Results and Discussion
3.1. Dynamic Vapour Sorption Studies
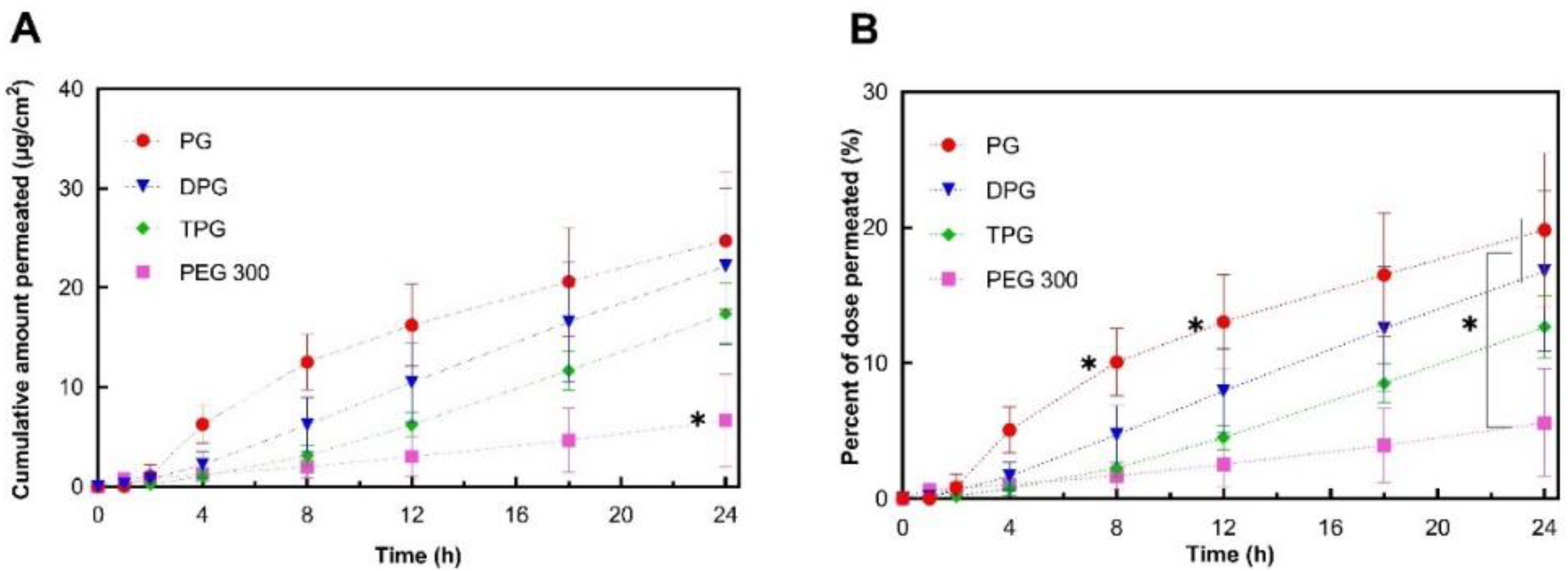
3.2. In Vitro Finite Dose Permeation and Mass Balance Studies
3.2.1. Skin Permeation of IBU
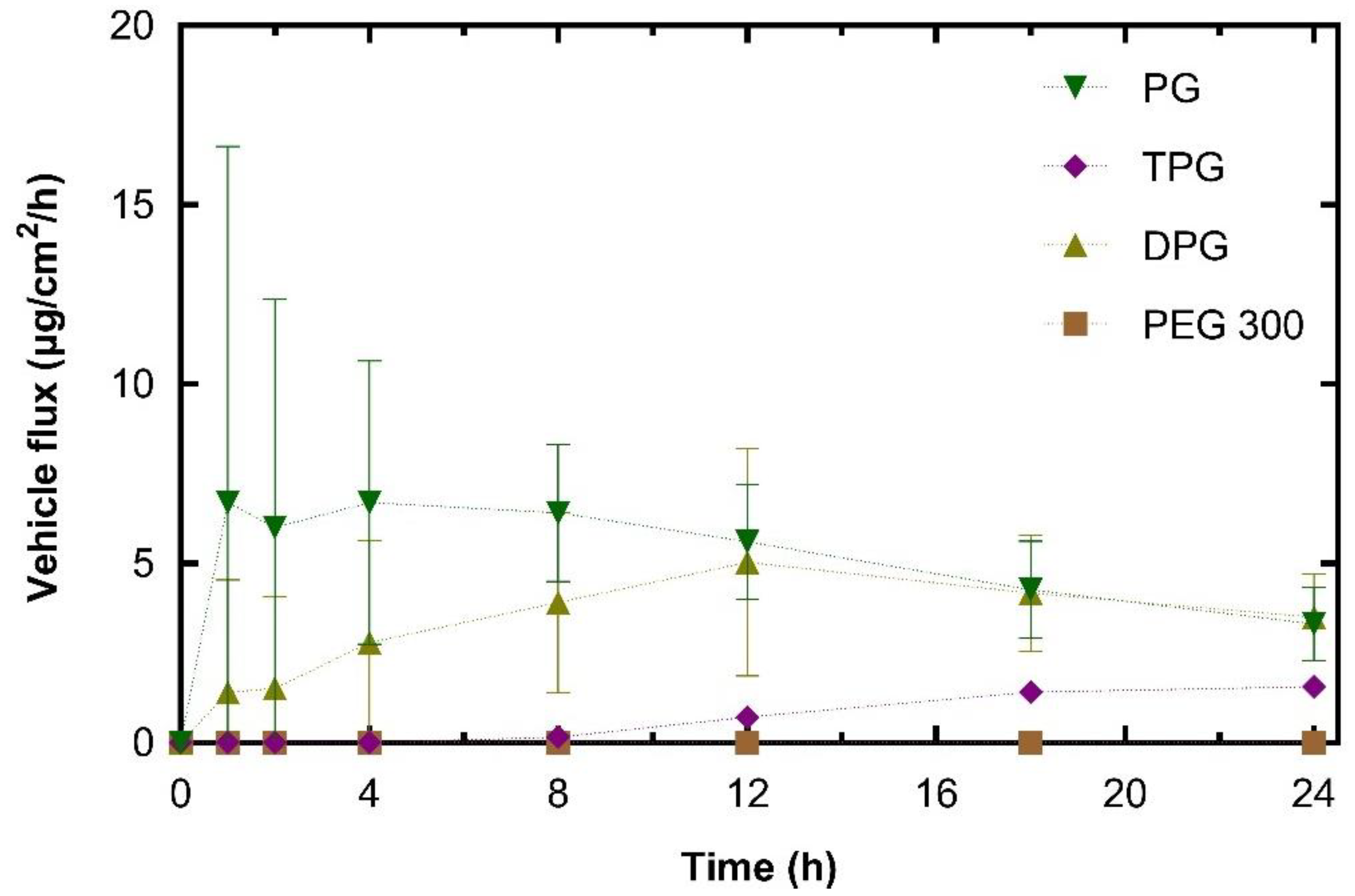
3.2.2. Skin Permeation of the Vehicle
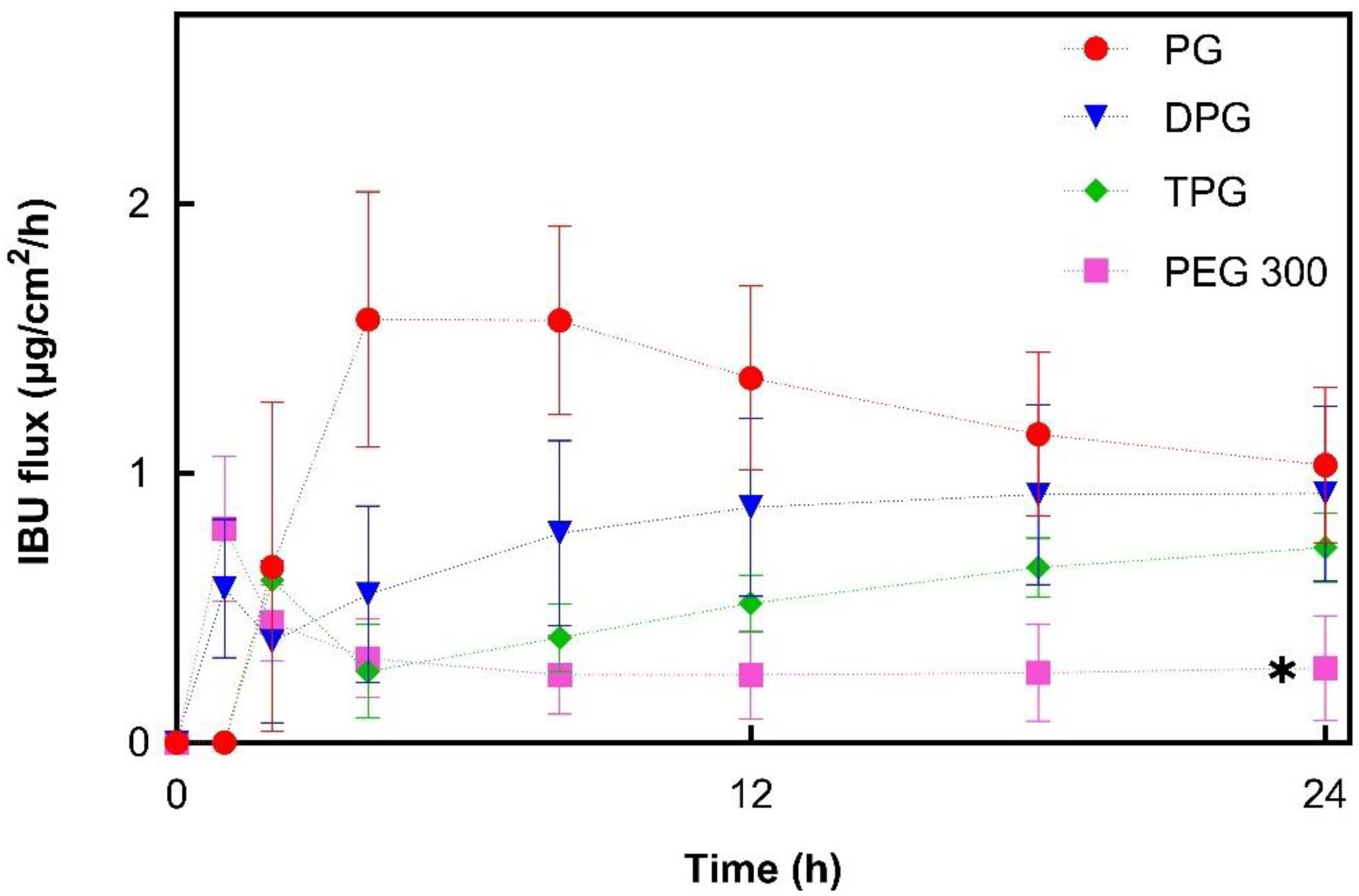
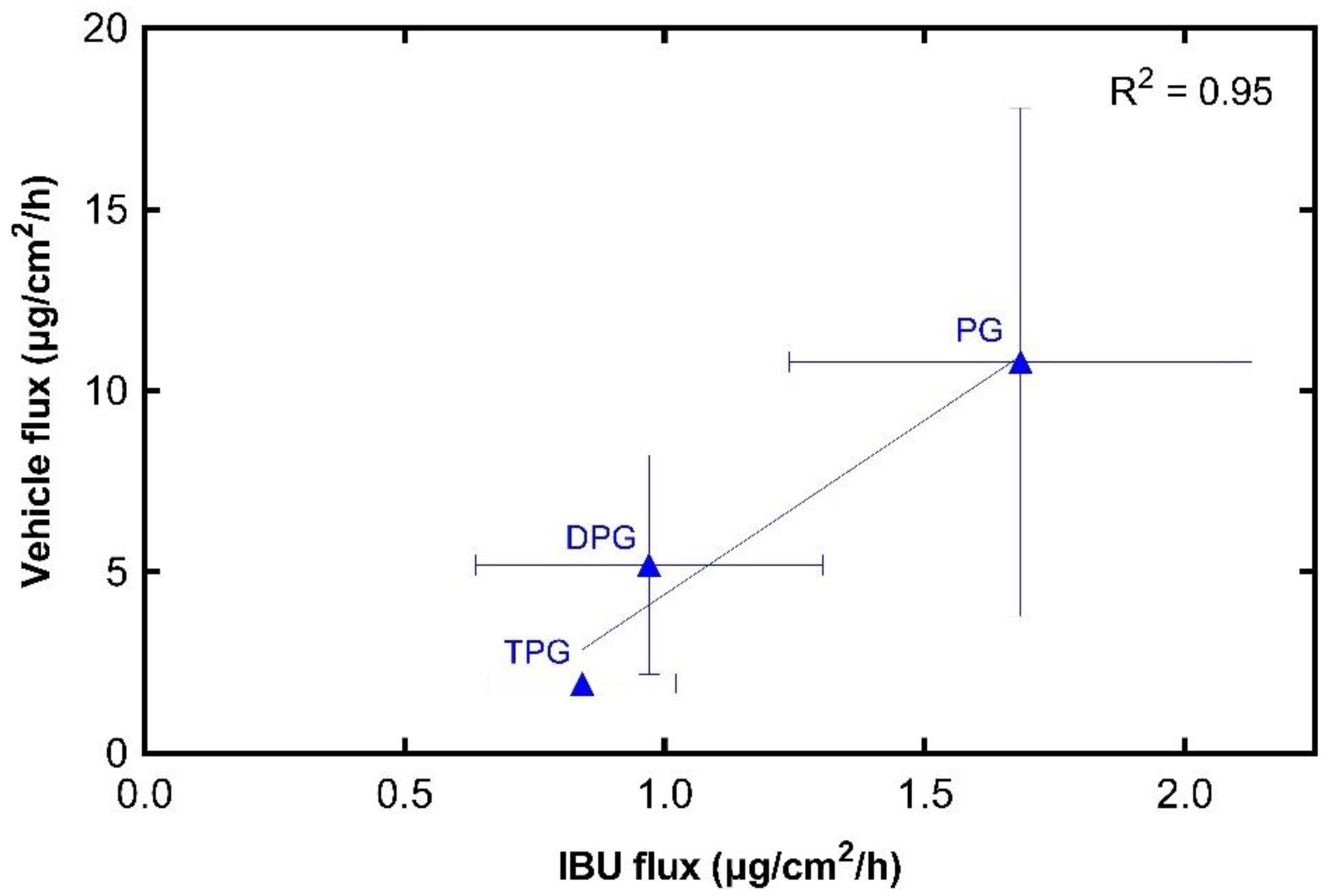
3.2.3. IBU and Vehicle Flux Correlation
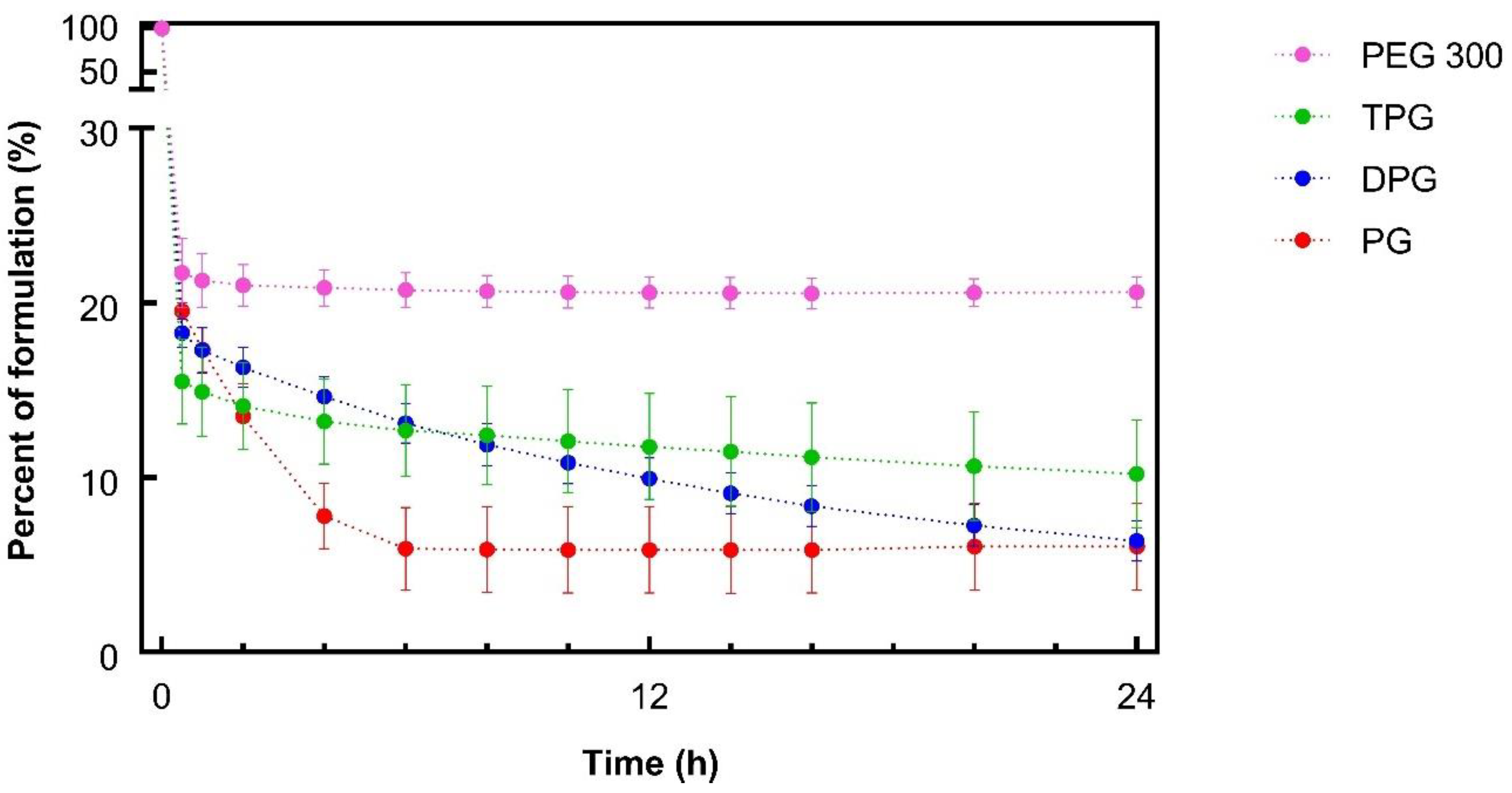
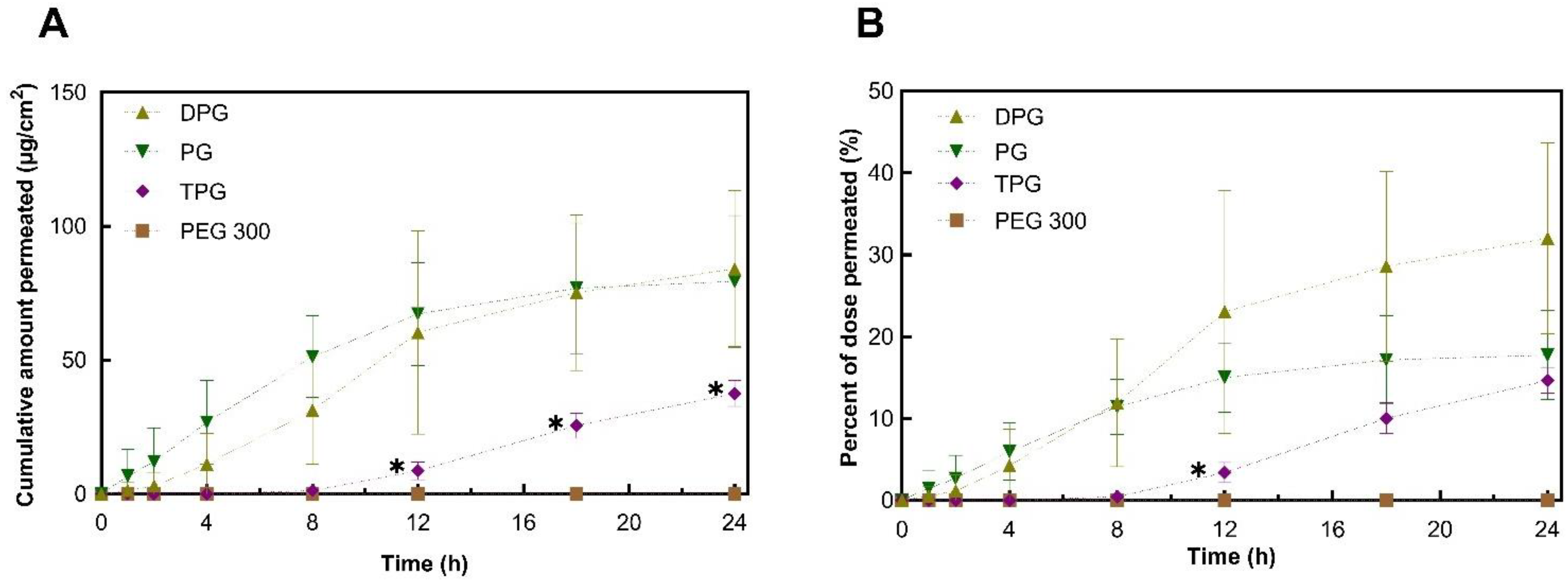
3.3. In Vivo Finite Dose Permeation Studies—Confocal Raman Spectroscopy
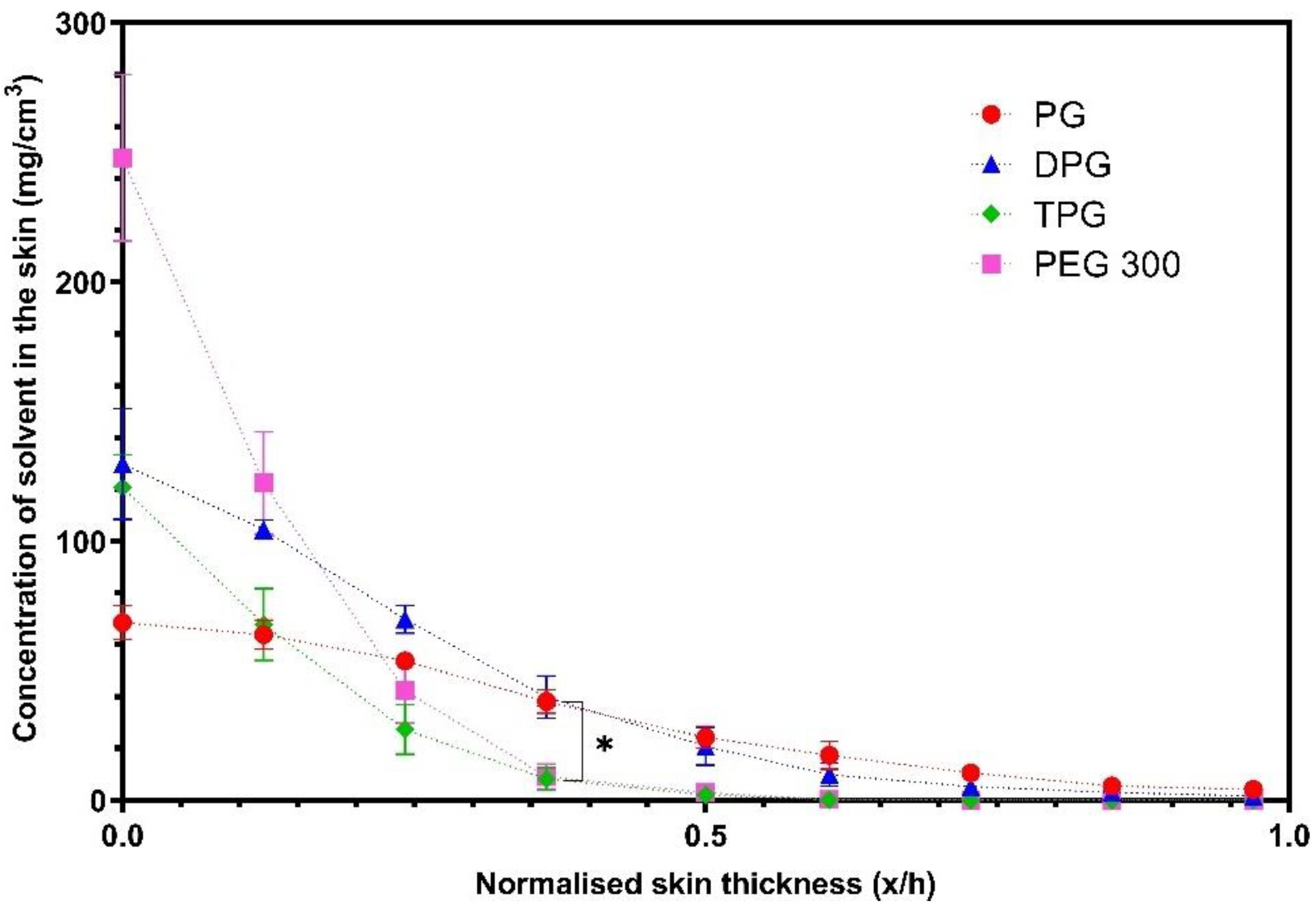
Skin Permeation of IBU and Vehicles
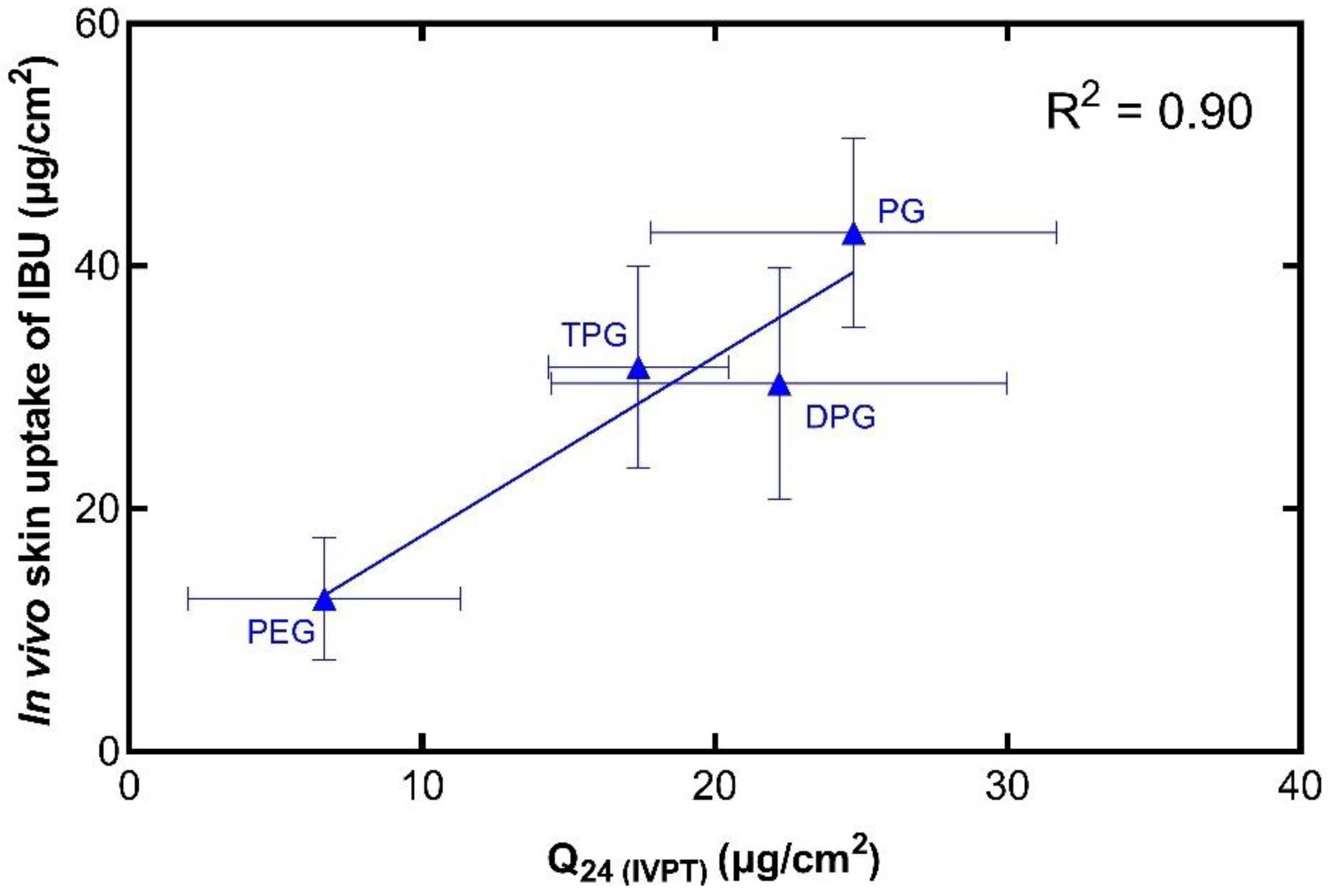
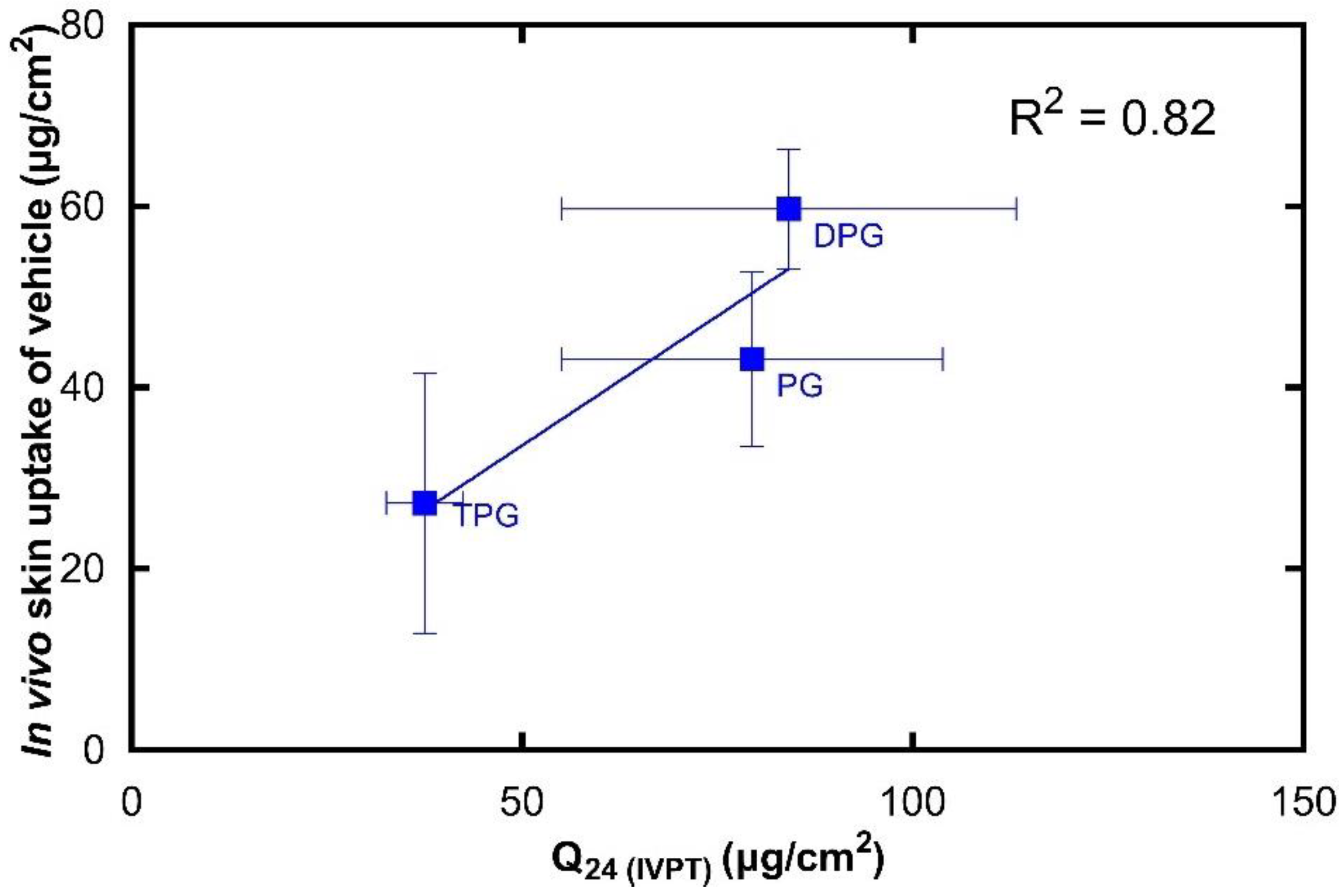
3.4. In Vitro–In Vivo Correlations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, I.; Müller-Goymann, C.C. An attempt to clarify the influence of glycerol, propylene glycol, isopropyl myristate and a combination of propylene glycol and isopropyl myristate on human stratum corneum. Pharmazie 2005, 60, 215–220. [Google Scholar] [PubMed]
- Moghadam, S.H.; Saliaj, E.; Wettig, S.D.; Dong, C.; Ivanova, M.V.; Huzil, J.T.; Foldvari, M. Effect of Chemical Permeation Enhancers on Stratum Corneum Barrier Lipid Organizational Structure and Interferon Alpha Permeability. Mol. Pharm. 2013, 10, 2248–2260. [Google Scholar] [CrossRef]
- Marjukka Suhonen, T.; Bouwstra, J.A.; Urtti, A. Chemical enhancement of percutaneous absorption in relation to stratum corneum structural alterations. J. Control. Release 1999, 59, 149–161. [Google Scholar] [CrossRef]
- Harrison, J.E.; Watkinson, A.C.; Green, D.M.; Hadgraft, J.; Brain, K. The Relative Effect of Azone® and Transcutol® on Permeant Diffusivity and Solubility in Human Stratum Corneum. Pharm. Res. 1996, 13, 542–546. [Google Scholar] [CrossRef]
- Hoppel, M.; Baurecht, D.; Holper, E.; Mahrhauser, D.; Valenta, C. Validation of the combined ATR-FTIR/tape stripping technique for monitoring the distribution of surfactants in the stratum corneum. Int. J. Pharm. 2014, 472, 88–93. [Google Scholar] [CrossRef]
- Russeau, W.; Mitchell, J.; Tetteh, J.; Lane, M.E.; Hadgraft, J. Investigation of the permeation of model formulations and a commercial ibuprofen formulation in Carbosil® and human skin using ATR-FTIR and multivariate spectral analysis. Int. J. Pharm. 2009, 374, 17–25. [Google Scholar] [CrossRef]
- Franz, T.J. The finite dose technique as a valid in vitro model for the study of percutaneous absorption in man. In Skin-Drug Application and Evaluation of Environmental Hazards; Karger Publishers: Basel, Switzerland, 1978; Volume 7, pp. 58–68. [Google Scholar]
- Franz, T.J.; Lehman, P.A.; Raney, S.G. Use of Excised Human Skin to Assess the Bioequivalence of Topical Products. Skin Pharmacol. Physiol. 2009, 22, 276–286. [Google Scholar] [CrossRef]
- Raney, S.G.; Franz, T.J.; Lehman, P.A.; Lionberger, R.; Chen, M.-L. Pharmacokinetics-Based Approaches for Bioequivalence Evaluation of Topical Dermatological Drug Products. Clin. Pharmacokinet. 2015, 54, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies; OECD Publishing: Paris, France, 2004. [Google Scholar] [CrossRef]
- OECD. Test No. 428: Skin Absorption: In Vitro Method; OECD Publishing: Paris, France, 2004. [Google Scholar] [CrossRef]
- SCCS. EU Scientific Committee on Consumer Safety Report SCCS/1358/10: Basic Criteria for the In Vitro Assessment of Dermal Absorption of Cosmetic Ingredients; European Commission: Brussels, Belgium, 2010; pp. 4–14. [Google Scholar]
- SCCS. EU Scientific Committee on Consumer Safety Report SCCS/1501/12: Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation; European Commission: Brussels, Belgium, 2012; pp. 4–14. [Google Scholar]
- Lehman, P.A.; Raney, S.G.; Franz, T.J. Percutaneous Absorption in Man: In vitro-in vivo Correlation. Skin Pharmacol. Physiol. 2011, 24, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical delivery of anthramycin I. Influence of neat solvents. Eur. J. Pharm. Sci. 2017, 104, 188–195. [Google Scholar] [CrossRef]
- Haque, T.; Rahman, K.M.; Thurston, D.E.; Hadgraft, J.; Lane, M.E. Topical delivery of anthramycin II. Influence of binary and ternary solvent systems. Eur. J. Pharm. Sci. 2018, 121, 59–64. [Google Scholar] [CrossRef]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The role of vehicle interactions on permeation of an active through model membranes and human skin. Int. J. Cosmet. Sci. 2012, 34, 536–545. [Google Scholar] [CrossRef]
- Kung, C.-P.; Zhang, Y.; Sil, B.C.; Hadgraft, J.; Lane, M.E.; Patel, B.; McCulloch, R. Investigation of binary and ternary solvent systems for dermal delivery of methadone. Int. J. Pharm. 2020, 586, 119538. [Google Scholar] [CrossRef] [PubMed]
- Caspers, P.J.; Lucassen, G.W.; Wolthuis, R.; Bruining, H.A.; Puppels, G.J. In vitro and in vivo Raman spectroscopy of human skin. Biospectroscopy 1998, 4, S31–S39. [Google Scholar] [CrossRef]
- Caspers, P.J.; Bruining, H.A.; Puppels, G.J.; Lucassen, G.W.; Carter, E.A. In Vivo Confocal Raman Microspectroscopy of the Skin: Noninvasive Determination of Molecular Concentration Profiles. J. Investig. Dermatol. 2001, 116, 434–442. [Google Scholar] [CrossRef]
- Binder, L.; Kulovits, E.M.; Petz, R.; Ruthofer, J.; Baurecht, D.; Klang, V.; Valenta, C. Penetration monitoring of drugs and additives by ATR-FTIR spectroscopy/tape stripping and confocal Raman spectroscopy—A comparative study. Eur. J. Pharm. Biopharm. 2018, 130, 214–223. [Google Scholar] [CrossRef]
- Tfaili, S.; Josse, G.; Angiboust, J.-F.; Manfait, M.; Piot, O. Monitoring caffeine and resveratrol cutaneous permeation by confocal Raman microspectroscopy. J. Biophotonics 2014, 7, 676–681. [Google Scholar] [CrossRef]
- Caspers, P.J.; Williams, A.C.; Carter, E.A.; Edwards, H.G.M.; Barry, B.W.; Bruining, H.A.; Puppels, G.J. Monitoring the Penetration Enhancer Dimethyl Sulfoxide in Human Stratum Corneum in Vivo by Confocal Raman Spectroscopy. Pharm. Res. 2002, 19, 1577–1580. [Google Scholar] [CrossRef]
- Caspers, P.J.; Nico, C.; Bakker Schut, T.C.; de Sterke, J.; Pudney, P.D.A.; Curto, P.R.; Illand, A.; Puppels, G.J. Method to quantify the in vivo skin penetration of topically applied materials based on confocal Raman spectroscopy. Transl. Biophotonics 2019, 1, e201900004. [Google Scholar] [CrossRef]
- Iliopoulos, F.; Caspers, P.J.; Puppels, G.J.; Lane, M.E. Franz Cell Diffusion Testing and Quantitative Confocal Raman Spectroscopy: In Vitro-In Vivo Correlation. Pharmaceutics 2020, 12, 887. [Google Scholar] [CrossRef]
- Coldman, M.F.; Poulsen, B.J.; Higuchi, T. Enhancement of percutaneous absorption by the use of volatile: Nonvolatile systems as vehicles. J. Pharm. Sci. 1969, 58, 1098–1102. [Google Scholar] [CrossRef]
- Oliveira, G.; Hadgraft, J.; Lane, M.E. The influence of volatile solvents on transport across model membranes and human skin. Int. J. Pharm. 2012, 435, 38–49. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Inactive Ingredient Search for Approved Drug Products; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2021. [Google Scholar]
- Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Final Report of the Cosmetic Ingredient Review Expert Panel on the Safety Assessment of Methyl Acetate. Int. J. Toxicol. 2012, 31, 112S–136S. [Google Scholar] [CrossRef]
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef]
- Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety Assessment of Propylene Glycol, Tripropylene Glycol, and PPGs as Used in Cosmetics. Int. J. Toxicol. 2012, 31, 245S–260S. [Google Scholar] [CrossRef]
- Hadgraft, J.; Whitefield, M.; Rosher, P.H. Skin Penetration of Topical Formulations of Ibuprofen 5%: An in vitro Comparative Study. Skin Pharmacol. Physiol. 2003, 16, 137–142. [Google Scholar] [CrossRef]
- Luo, L.; Patel, A.; Sinko, B.; Bell, M.; Wibawa, J.; Hadgraft, J.; Lane, M.E. A comparative study of the in vitro permeation of ibuprofen in mammalian skin, the PAMPA model and silicone membrane. Int. J. Pharm. 2016, 505, 14–19. [Google Scholar] [CrossRef]
- Patel, A.; Bell, M.; O’Connor, C.; Inchley, A.; Wibawa, J.; Lane, M.E. Delivery of ibuprofen to the skin. Int. J. Pharm. 2013, 457, 9–13. [Google Scholar] [CrossRef]
- Dias, M.; Hadgraft, J.; Lane, M.E. Influence of membrane-solvent-solute interactions on solute permeation in skin. Int. J. Pharm. 2007, 340, 65–70. [Google Scholar] [CrossRef]
- ICH. Validation of analytical procedures: Text and methodology. Q2 (R1) 2005, 1, 1–15. [Google Scholar]
- The National Institute for Health and Care Excellence. IBUPROFEN|Drug|BNF Content Published by NICE. Available online: https://bnf.nice.org.uk/medicinal-forms/ibuprofen.html#PHP77344 (accessed on 30 December 2020).
- Skelly, J.P.; Shah, V.P.; Maibach, H.I.; Guy, R.H.; Wester, R.C.; Flynn, G.; Yacobi, A. FDA and AAPS report of the workshop on principles and practices of in vitro percutaneous penetration studies: Relevance to bioavailability and bioequivalence. Pharm. Res. 1987, 4, 265–267. [Google Scholar] [CrossRef]
- Bialik, W.; Walters, K.A.; Brain, K.R.; Hadgraft, J. Some factors affecting the in vitro penetration of ibuprofen through human skin. Int. J. Pharm. 1993, 92, 219–223. [Google Scholar] [CrossRef]
- Zhang, Y.; Lane, M.E.; Moore, D.J. An Investigation of the Influence of PEG 400 and PEG-6-Caprylic/Capric Glycerides on Dermal Delivery of Niacinamide. Polymers 2020, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.S.M.M.A.; Sil, B.C.; Iliopoulos, F.; Lever, R.; Hadgraft, J.; Lane, M.E. Preparation, Characterisation, and Topical Delivery of Terbinafine. Pharmaceutics 2019, 11, 548. [Google Scholar] [CrossRef]
- Paz-Alvarez, M.; Pudney, P.D.A.; Hadgraft, J.; Lane, M.E. Topical delivery of climbazole to mammalian skin. Int. J. Pharm. 2018, 549, 317–324. [Google Scholar] [CrossRef]
- Intarakumhaeng, R.; Li, S.K. Effects of solvent on percutaneous absorption of nonvolatile lipophilic solute. Int. J. Pharm. 2014, 476, 266–276. [Google Scholar] [CrossRef]
- Zhang, Y.; Lane, M.E.; Hadgraft, J.; Heinrich, M.; Chen, T.; Lian, G.; Sinko, B. A comparison of the in vitro permeation of niacinamide in mammalian skin and in the Parallel Artificial Membrane Permeation Assay (PAMPA) model. Int. J. Pharm. 2019, 556, 142–149. [Google Scholar] [CrossRef]
- Hadgraft, J.; Lane, M.E. Drug crystallization—Implications for topical and transdermal delivery. Expert Opin. Drug Deliv. 2016, 13, 817–830. [Google Scholar] [CrossRef]
- Goh, C.F.; Boyd, B.J.; Craig, D.Q.M.; Lane, M.E. Profiling of drug crystallization in the skin. Expert Opin. Drug Deliv. 2020, 17, 1321–1334. [Google Scholar] [CrossRef] [PubMed]
- Goh, C.F.; Moffat, J.G.; Craig, D.Q.M.; Hadgraft, J.; Lane, M.E. Monitoring Drug Crystallization in Percutaneous Penetration Using Localized Nanothermal Analysis and Photothermal Microspectroscopy. Mol. Pharm. 2019, 16, 359–370. [Google Scholar] [CrossRef]
- Fasano, W.J.; ten Berge, W.F.; Banton, M.I.; Heneweer, M.; Moore, N.P. Dermal penetration of propylene glycols: Measured absorption across human abdominal skin in vitro and comparison with a QSAR model. Toxicol. In Vitro 2011, 25, 1664–1670. [Google Scholar] [CrossRef] [PubMed]
- Thombre, A.; Tse, S.; Yeoh, T.; Chen, R.; North, R.; Brown, M. Ex vivo (human skin) and in vivo (minipig) permeation of propylene glycol applied as topical crisaborole ointment, 2%. Int. J. Pharm. 2020, 576, 118847. [Google Scholar] [CrossRef]
- Møllgaard, B.; Hoelgaard, A. Permeation of estradiol through the skin—Effect of vehicles. Int. J. Pharm. 1983, 15, 185–197. [Google Scholar] [CrossRef]
- Cooper, E.R. Increased Skin Permeability for Lipophilic Molecules. J. Pharm. Sci. 1984, 73, 1153–1156. [Google Scholar] [CrossRef]
- Wotton, P.K.; Møllgaard, B.; Hadgraft, J.; Hoelgaard, A. Vehicle effect on topical drug delivery. III. Effect of Azone on the cutaneous permeation of metronidazole and propylene glycol. Int. J. Pharm. 1985, 24, 19–26. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, P.; Roberts, M.S. Maximum transepidermal flux for similar size phenolic compounds is enhanced by solvent uptake into the skin. J. Control. Release 2011, 154, 50–57. [Google Scholar] [CrossRef]
- Trottet, L.; Merly, C.; Mirza, M.; Hadgraft, J.; Davis, A.F. Effect of finite doses of propylene glycol on enhancement of in vitro percutaneous permeation of loperamide hydrochloride. Int. J. Pharm. 2004, 274, 213–219. [Google Scholar] [CrossRef]
- Watkinson, R.M.; Guy, R.H.; Hadgraft, J.; Lane, M.E. Optimisation of Cosolvent Concentration for Topical Drug Delivery—II: Influence of Propylene Glycol on Ibuprofen Permeation. Skin Pharmacol. Physiol. 2009, 22, 225–230. [Google Scholar] [CrossRef]
- Pudney, P.D.; Mélot, M.; Caspers, P.J.; Van Der Pol, A.; Puppels, G.J. An in vivo confocal Raman study of the delivery of trans retinol to the skin. Appl. Spectrosc. 2007, 61, 804–811. [Google Scholar] [CrossRef]
- Melot, M.; Pudney, P.D.; Williamson, A.M.; Caspers, P.J.; Van Der Pol, A.; Puppels, G.J. Studying the effectiveness of penetration enhancers to deliver retinol through the stratum cornum by in vivo confocal Raman spectroscopy. J. Control. Release 2009, 138, 32–39. [Google Scholar] [CrossRef]
- Mohammed, D.; Matts, P.J.; Hadgraft, J.; Lane, M.E. In vitro-in vivo correlation in skin permeation. Pharm. Res. 2014, 31, 394–400. [Google Scholar] [CrossRef] [PubMed]

| Solvent | PG | DPG | TPG | PEG 300 |
|---|---|---|---|---|
| Column | ZB-WAX | ZB-WAX | ZB-WAX | HP-5® |
| Injection Volume (μL) | 0.5 | 0.5 | 0.5 | 2 |
| Split Ratio | 1:1 | 1:1 | 1:1 | 6:1 |
| Septum Purge Flow (mL/min) | 3 | 3 | 3 | 3 |
| Inlet Temperature (°C) | 225 | 225 | 225 | 250 |
| Detector Temperature (°C) | 300 | 300 | 300 | 300 |
| Oven Conditions | 80 | 80 | 80 | 80 |
| 1 | 1 | 1 | 1 | |
| 200 | 200 | 250 | 300 | |
| 15 | 15 | 40 | 50 | |
| 0 | 0 | 2.25 | 2 | |
| Retention Time of Analyte (min) | 5.3 | 7 | 4.3 | 4.2 |
| Formulation | Vehicle | IBU | IPA |
|---|---|---|---|
| PG | 15.6 | 5.0 | 79.4 |
| DPG | 10.7 | 5.0 | 84.3 |
| TPG | 8.0 | 5.0 | 87.0 |
| PEG 300 | 15.3 | 5.0 | 79.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, A.; Iliopoulos, F.; Caspers, P.J.; Puppels, G.J.; Lane, M.E. In Vitro–In Vivo Correlation in Dermal Delivery: The Role of Excipients. Pharmaceutics 2021, 13, 542. https://doi.org/10.3390/pharmaceutics13040542
Patel A, Iliopoulos F, Caspers PJ, Puppels GJ, Lane ME. In Vitro–In Vivo Correlation in Dermal Delivery: The Role of Excipients. Pharmaceutics. 2021; 13(4):542. https://doi.org/10.3390/pharmaceutics13040542
Chicago/Turabian StylePatel, Avnish, Fotis Iliopoulos, Peter J. Caspers, Gerwin J. Puppels, and Majella E. Lane. 2021. "In Vitro–In Vivo Correlation in Dermal Delivery: The Role of Excipients" Pharmaceutics 13, no. 4: 542. https://doi.org/10.3390/pharmaceutics13040542
APA StylePatel, A., Iliopoulos, F., Caspers, P. J., Puppels, G. J., & Lane, M. E. (2021). In Vitro–In Vivo Correlation in Dermal Delivery: The Role of Excipients. Pharmaceutics, 13(4), 542. https://doi.org/10.3390/pharmaceutics13040542






