Inhalable Jojoba Oil Dry Nanoemulsion Powders for the Treatment of Lipopolysaccharide- or H2O2-Induced Acute Lung Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Preparation and Characterization of JJBO Nanoemulsions
2.4. Preparation and Characterization of JJBO Nanoemulsion Dry Powders
2.5. Simulated Lung Deposition of JNDs
2.6. Measurement of Polyphenols in JJBO Formulations
2.7. Pharmacodynamics Study In Vivo
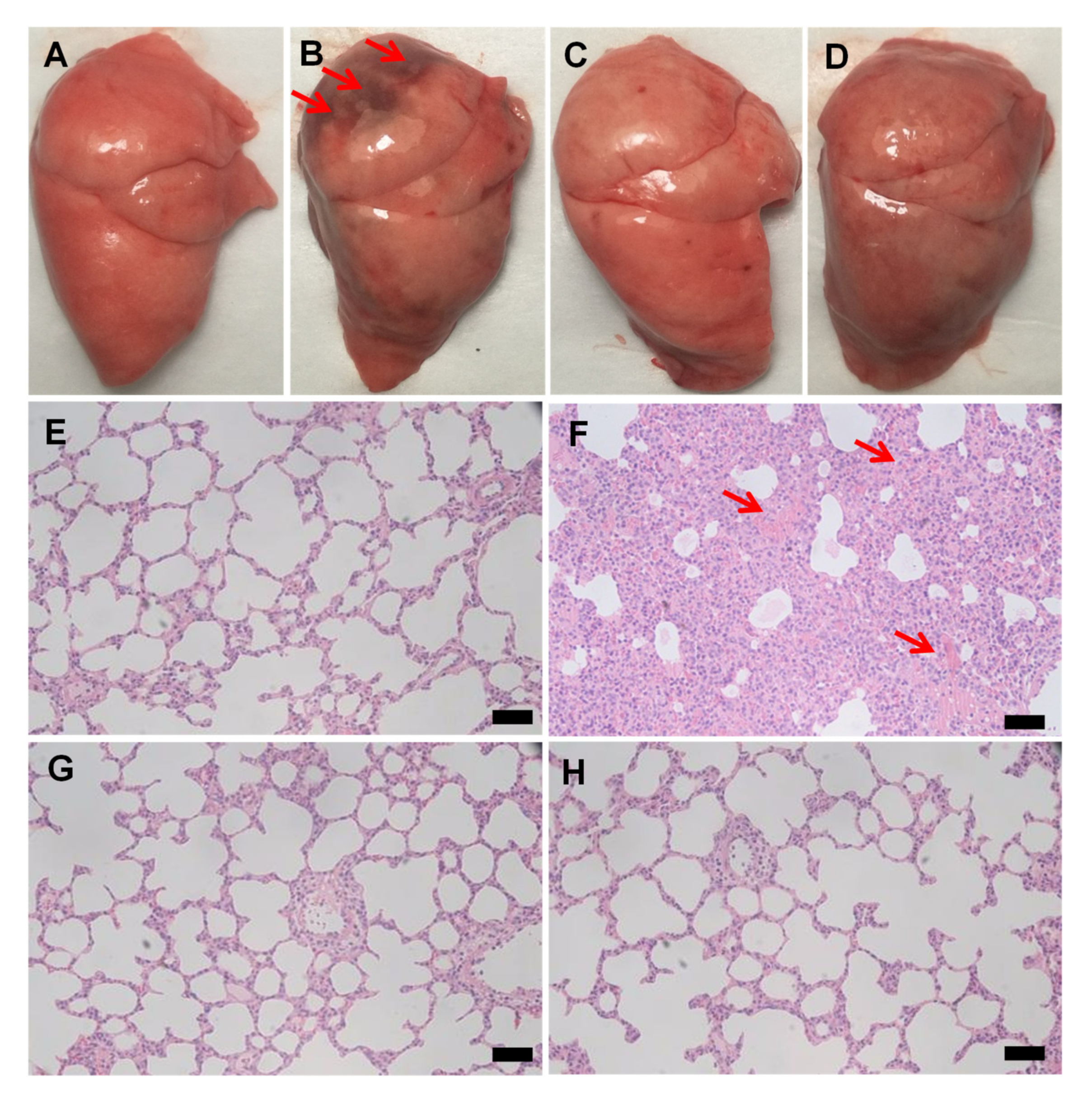
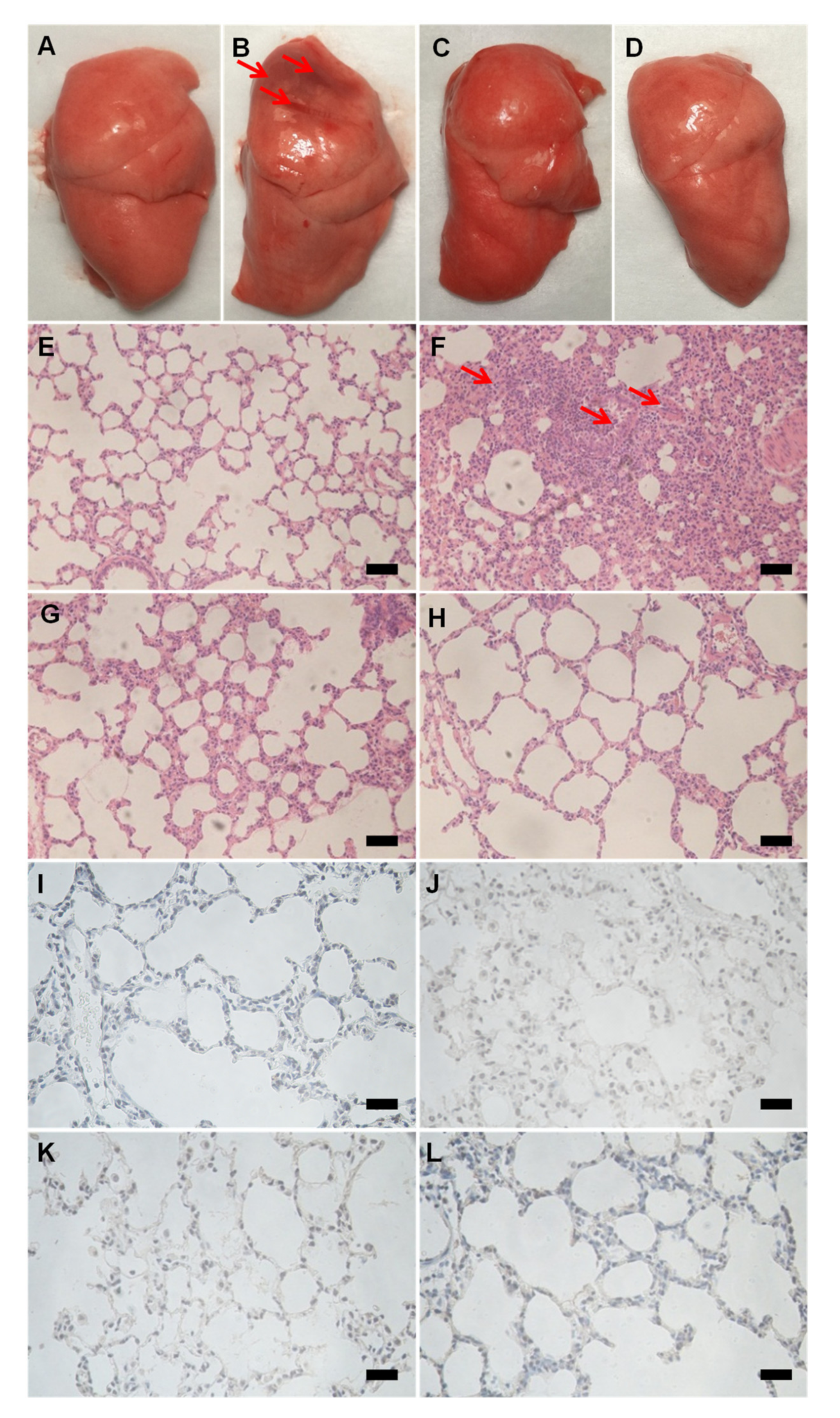
2.8. Histological Observation
2.9. Caspase-3 Evaluation with Immunohistochemistry
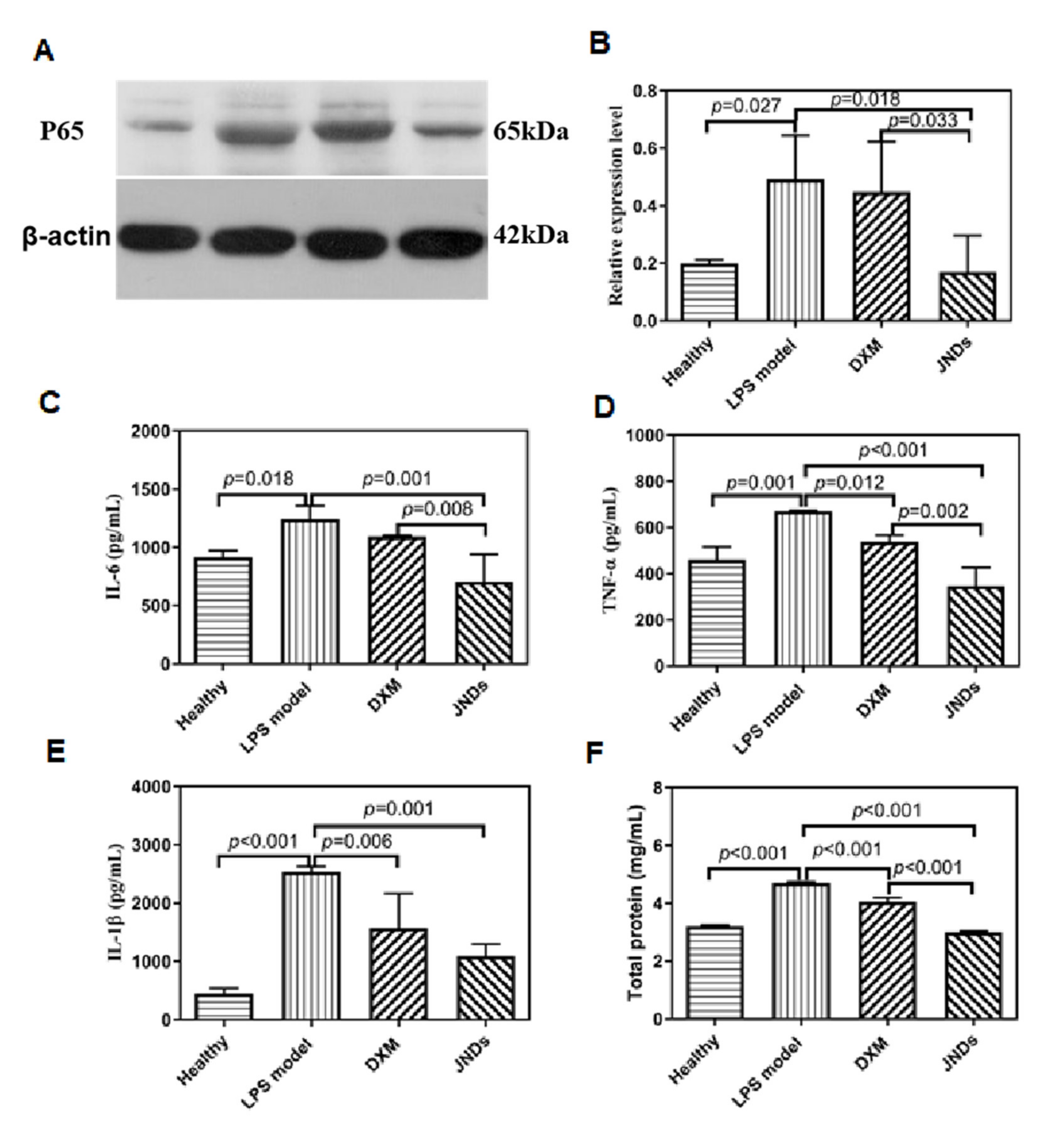
2.10. Expression of NF-κB p65 with Western Blotting
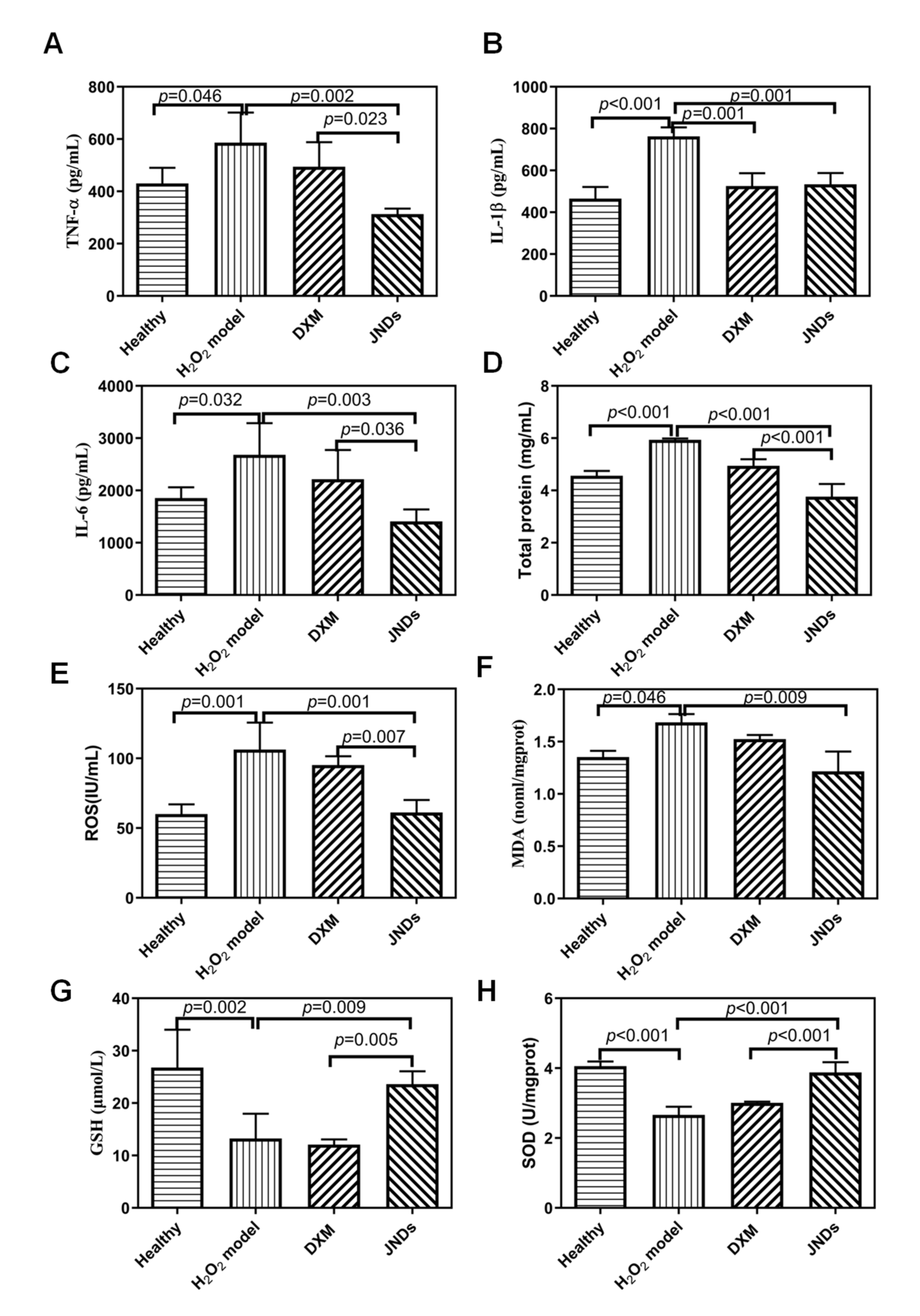
2.11. Measurement of Inflammatory and Oxidative Factors
2.12. Assay of Elimination of Free Radical
2.13. Statistical Analysis
3. Results
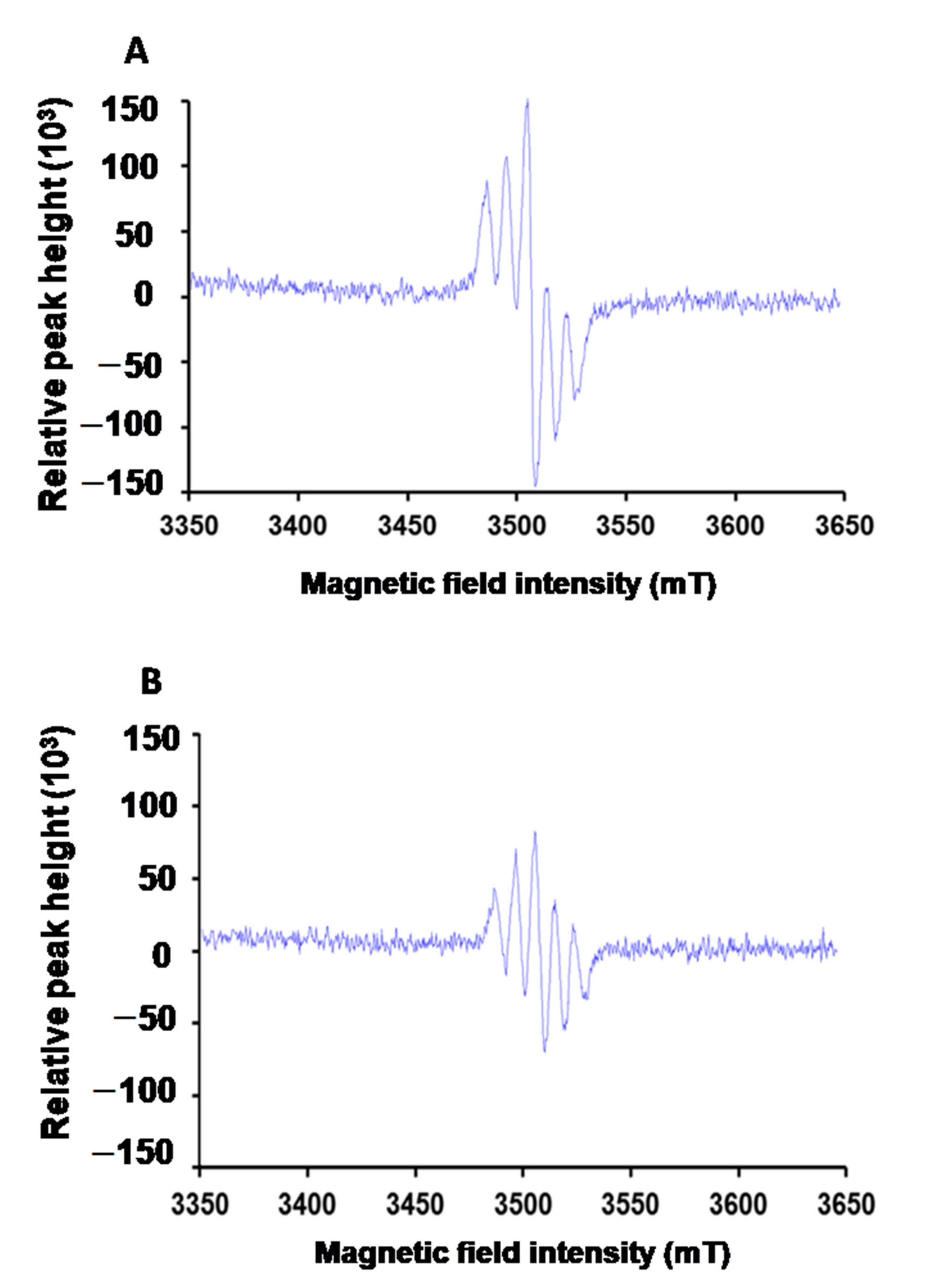
3.1. Characteristics of JNEs and JNDs
3.2. JNDs Attenuate LPS-Induced ALI
3.3. JNDs Attenuate H2O2-Induced ALI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El-Mallah, M.H.; El-Shami, S.M. Investigation of liquid wax components of Egyptian jojoba seeds. J. Oleo Sci. 2009, 58, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Elshazly, M.O.; Morgan, A.M.; Ali, M.E.; Abdel-mawla, E.; El-Rahman, S.S.A. The mitigative effect of Raphanus sativus oil on chromium-induced geno-and hepatotoxicity in male rats. J. Adv. Res. 2016, 7, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Ranzato, E.; Martinotti, S.; Burlando, B. Wound healing properties of jojoba liquid wax: An in vitro study. J. Ethnopharmacol. 2011, 134, 443–449. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int. J. Mol. Sci. 2018, 19. [Google Scholar] [CrossRef]
- Habashy, R.R.; Abdel-Naim, A.B.; Khalifa, A.E.; Al-Azizi, M.M. Anti-inflammatory effects of jojoba liquid wax in experimental models. Pharmacol. Res. 2005, 51, 95–105. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Abou-Arab, A.A.; Salem, F.M.A. Antioxidant and antimicrobial effect of some natural plant extracts added to lamb patties during storage. Grasas Y Aceites 2011, 62, 139–148. [Google Scholar] [CrossRef][Green Version]
- Loy, H.; Kuok, D.I.; Hui, K.P.; Choi, M.H.; Yuen, W.; Nicholls, J.M.; Peiris, J.M.; Chan, M.C. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A/H5N1-associated acute lung injury. J. Infect. Dis. 2018, 2, 186–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, R.; Hu, X.; Jiang, L.; Xiao, D.; Sun, Y.; Zhao, J.; Bao, Q.; Xie, J. The role of IL-33 on LPS-induced acute lung injury in mice. Inflammation 2017, 40, 285–294. [Google Scholar] [CrossRef]
- Wheeler, A.P.; Bernard, G.R. Acute lung injury and the acute respiratory distress syndrome: A clinical review. Lancet 2007, 369, 1553–1564. [Google Scholar] [CrossRef]
- Whitea, A.F.B.; Demchenko, A.V. Modulating LPS signal transduction at the LPS receptor complex with synthetic Lipid A analogues. Adv. Carbohydr. Chem. Biochem. 2014, 71, 339–389. [Google Scholar] [CrossRef]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P.C. pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J. Adv. Res. 2019, 18, 161–172. [Google Scholar] [CrossRef]
- Schreiber, M.P.; Colantuoni, E.; Bienvenu, O.J.; Neufeld, K.J.; Chen, K.-F.; Shanholtz, C.; Mendez-Tellez, P.A.; Needham, D.M. Corticosteroids and transition to delirium in patients with acute lung injury. Crit. Care Med. 2014, 42, 1480–1486. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chan, J.G.Y.; Chan, H.-K. Pulmonary drug delivery by powder aerosols. J. Control. Release 2014, 193, 228–240. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Liu, M.; Wang, S.; Cao, Z. Inhibitory effect of Zanthoxylum bungeanum seed oil on ovalbumin-induced lung inflammation in a murine model of asthma. Mol. Med. Rep. 2016, 13, 4289–4302. [Google Scholar] [CrossRef]
- Tu, G.; Shi, Y.; Zheng, Y.; Ju, M.; He, H.; Ma, G.; Hao, G.; Luo, Z. Glucocorticoid attenuates acute lung injury through induction of type 2 macrophage. J. Transl. Med. 2017, 15, 181–188. [Google Scholar] [CrossRef]
- Perlman, S. Another Decade, Another Coronavirus. N. Engl. J. Med. 2019, 382, 760–762. [Google Scholar] [CrossRef]
- Silvaa, D.M.; Palecob, R.; Trainib, D.; Sencadas, V. Development of ciprofloxacin-loaded poly(vinyl alcohol) dry powder formulations for lung delivery. Int. J. Pharm. 2018, 547, 114–121. [Google Scholar] [CrossRef]
- Zhu, L.; Li, M.; Dong, J.; Jin, Y. Dimethyl silicone dry nanoemulsion inhalations: Formulation study and anti-acute lung injury effect. Int. J. Pharm. 2015, 491, 292–298. [Google Scholar] [CrossRef]
- Cheng, S.; Kourmatzis, A.; Mekonnen, T.; Gholizadeh, H.; Raco, J.; Chen, L.; Tang, P.; Chan, H.-K. Does upper airway deformation affect drug deposition? Int. J. Pharm. 2019, 572, 118773–118782. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, M.; Zhang, M.; Jin, Y. Inhalation treatment of idiopathic pulmonary fibrosis with curcumin large porous microparticles. Int. J. Pharm. 2018, 551, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Lercker, G.; Rodriguez-Estrada, M.T. Chromatographic analysis of unsaponifiable compounds of olive oils and fat-containing foods. J. Chromatogr. A 2000, 881, 105–129. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Wan, L.; Tan, J.; Wan, S.; Meng, D.; Yu, P. Anti-inflammatory and anti-oxidative effects of dexpanthenol on lipopolysaccharide induced acute lung injury in mice. Inflammation 2016, 39, 1757–1763. [Google Scholar] [CrossRef]
- Belhadj, S.; Hentati, O.; Hamdaoui, G.; Fakhreddine, K.; Maillard, E.; Dal, S.; Sigrist, S. Beneficial effect of jojoba seed extracts on hyperglycemia-Induced oxidative stress in RINm5f beta cells. Nutrients 2018, 10, 384. [Google Scholar] [CrossRef]
- Li, M.; Zhu, L.; Liu, B.; Du, L.; Jia, X.; Han, L.; Jin, Y. Tea tree oil nanoemulsions for inhalation therapies of bacterial and fungal pneumonia. Colloids Surf. B Biointerfaces 2016, 141, 408–416. [Google Scholar] [CrossRef]
- Fernández, D.J.; Lamkanfi, M. Inflammatory caspases: Key regulators of inflammation and cell death. Biol. Chem. 2015, 396, 193–203. [Google Scholar] [CrossRef]
- Sani, D.; Khatab, N.I.O.; Kirby, B.P.; Yong, A.; Hasan, S.; Basri, H.; Stanslas, J. A standardised Andrographis paniculata Burm. Nees aqueous extract prevents Lipopolysaccharide-induced cognitive deficits through suppression of inflammatory cytokines and oxidative stress mediators. J. Adv. Res. 2018, 16, 87–97. [Google Scholar] [CrossRef]
- Bhatia, M.; Moochhala, S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004, 202, 145–156. [Google Scholar] [CrossRef]
- Jiao, C.; Chen, W.; Tan, X.; Liang, H.; Li, J.; Yun, H.; He, C.; Chen, J.; Ma, X.; Xie, Y.; et al. Ganoderma lucidum spore oil induces apoptosis of breast cancer cells in vitro and in vivo by activating caspase-3 and caspase-9. J. Ethnopharmacol. 2020, 247, 112256–112264. [Google Scholar] [CrossRef]
- Zhao, H.; Zeng, Z.; Liu, L.; Chen, J.; Zhou, H.; Huang, L.; Huang, J.; Xu, H.; Xu, Y.; Chen, Z.; et al. Polydopamine nanoparticles for treatment of acute inflammationinduced injury. Nanoscale 2018, 10, 6981–6991. [Google Scholar] [CrossRef]
- Badr, A.N.; Shehata, M.G.; Abdel-Razek, A.G. Antioxidant activities and potential impacts to reduce aflatoxins utilizing jojoba and jatropha oils and extracts. Int. J. Pharmacol. 2017, 13, 1103–1114. [Google Scholar] [CrossRef]
- Pło´ciennikowska, A.; Hromada-Judycka, A.; Borzecka, K.; Kwiatkowska, K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol. Life Sci. 2015, 72, 557–581. [Google Scholar] [CrossRef]
- Lu, Y.; Yeh, W.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Schwarz, A.; Bonaterra, G.A.; Schwarzbach, H.; Kinscherf, R. Oxidized LDL-induced JAB1 influences NF-kappaB independent inflammatory signaling in human macrophages during foam cell formation. J. Biomed. Sci. 2017, 24, 12. [Google Scholar] [CrossRef]
- Yang, H.; Zhuo, J.; Sun, C.; Nie, J.; Yuan, J.; Liu, Y.; Lin, R.; Lai, X.; Sua, Z.; Li, Y. Pogostone attenuates TNF-alpha-induced injury in A549 cells via inhibiting NF-kappaB and activating Nrf2 pathways. Int. Immunopharmacol. 2018, 62, 15–22. [Google Scholar] [CrossRef]
- Shojaie, M.; Ghanbari, F.; Shojaie, N. Intermittent fasting could ameliorate cognitive function against distress by regulation of inflammatory response pathway. J. Adv. Res. 2017, 8, 697–701. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Abdel-Mageed, W.M.; Bayoumi, S.A.L.H.; Salama, A.A.R.; Salem-Bekhit, M.M.; Abd-Alrahman, S.H.; Sayed, H.M. Antioxidant lipoxygenase inhibitors from the leaf extracts of Simmondsia chinensis. Asian Pac. J. Trop. Med. 2014, 7, 521–526. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Xie, F.; Sun, Y.; Yu, X.; Xiao, Z.; Fang, R.; Li, J.; Li, Q.; Du, L.; Jin, Y. Inhalable Jojoba Oil Dry Nanoemulsion Powders for the Treatment of Lipopolysaccharide- or H2O2-Induced Acute Lung Injury. Pharmaceutics 2021, 13, 486. https://doi.org/10.3390/pharmaceutics13040486
Zhang G, Xie F, Sun Y, Yu X, Xiao Z, Fang R, Li J, Li Q, Du L, Jin Y. Inhalable Jojoba Oil Dry Nanoemulsion Powders for the Treatment of Lipopolysaccharide- or H2O2-Induced Acute Lung Injury. Pharmaceutics. 2021; 13(4):486. https://doi.org/10.3390/pharmaceutics13040486
Chicago/Turabian StyleZhang, Guoli, Fei Xie, Yunbo Sun, Xiang Yu, Zhimei Xiao, Rongzhen Fang, Jingfei Li, Qian Li, Lina Du, and Yiguang Jin. 2021. "Inhalable Jojoba Oil Dry Nanoemulsion Powders for the Treatment of Lipopolysaccharide- or H2O2-Induced Acute Lung Injury" Pharmaceutics 13, no. 4: 486. https://doi.org/10.3390/pharmaceutics13040486
APA StyleZhang, G., Xie, F., Sun, Y., Yu, X., Xiao, Z., Fang, R., Li, J., Li, Q., Du, L., & Jin, Y. (2021). Inhalable Jojoba Oil Dry Nanoemulsion Powders for the Treatment of Lipopolysaccharide- or H2O2-Induced Acute Lung Injury. Pharmaceutics, 13(4), 486. https://doi.org/10.3390/pharmaceutics13040486





