Enhanced Transport and Permeation of a Polymeric Nanocarrier across the Retina by Mixing with ATP upon Intravitreal Injection
Abstract
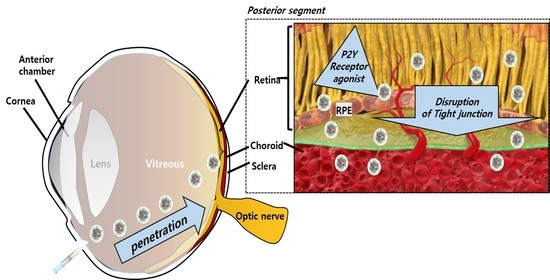
1. Introduction
2. Materials and Methods
2.1. Materials
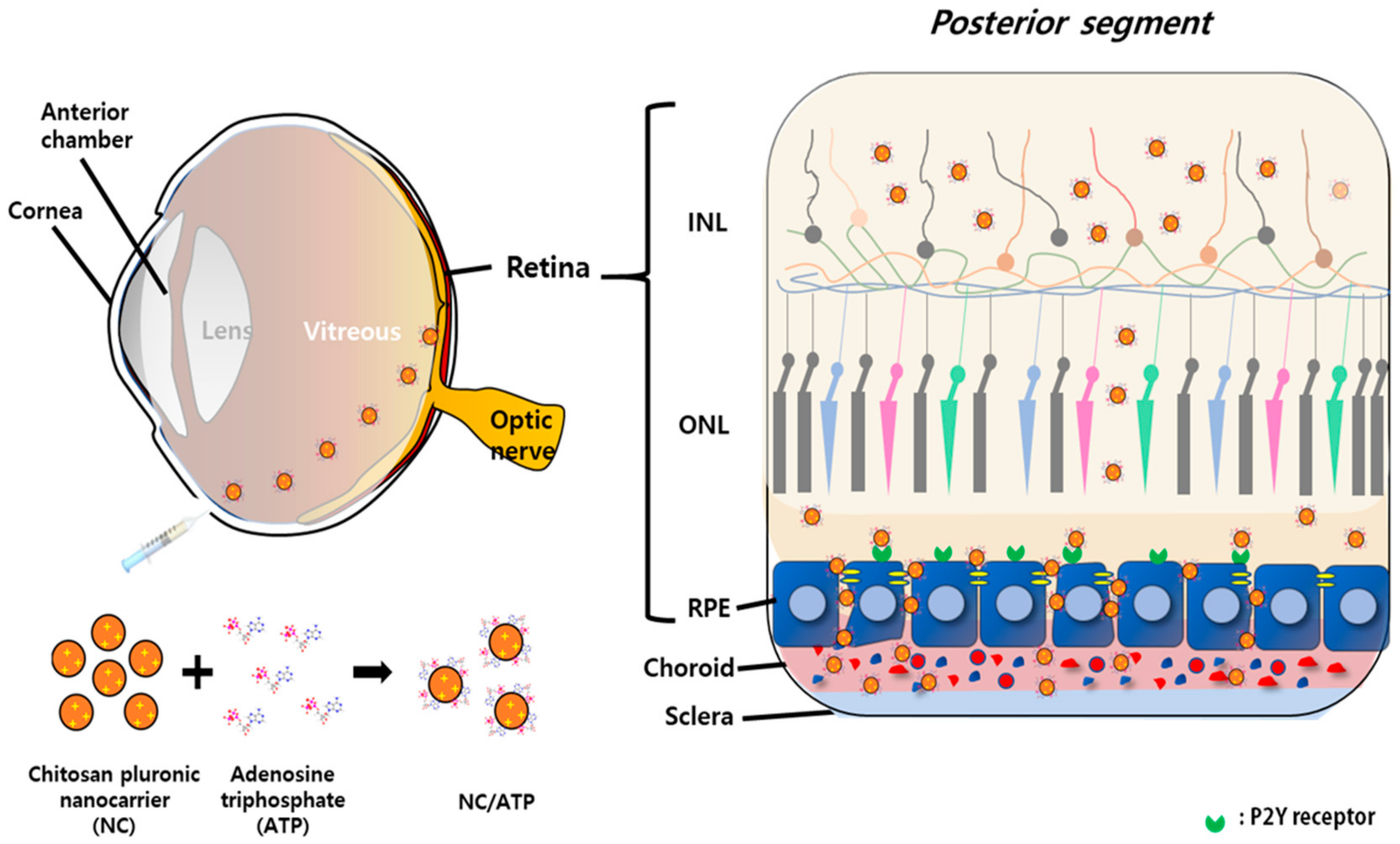
2.2. Preparation of Chitosan-Functionalized Pluronic-Based Nanocarrier
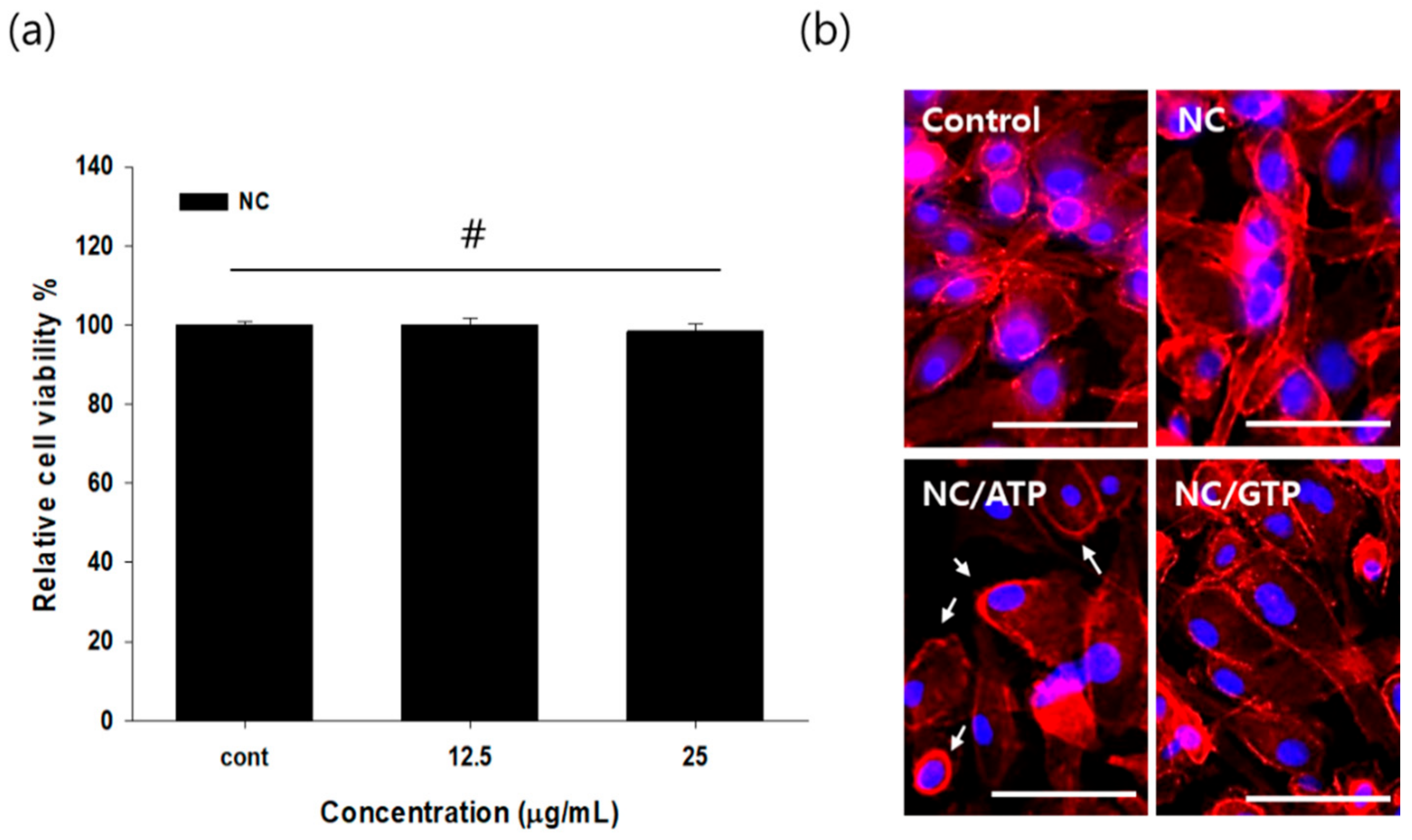
2.3. Cytotoxicity of NC
2.4. Actin Rearrangement Effect of NC–NTP on the Retinal Cell Layer
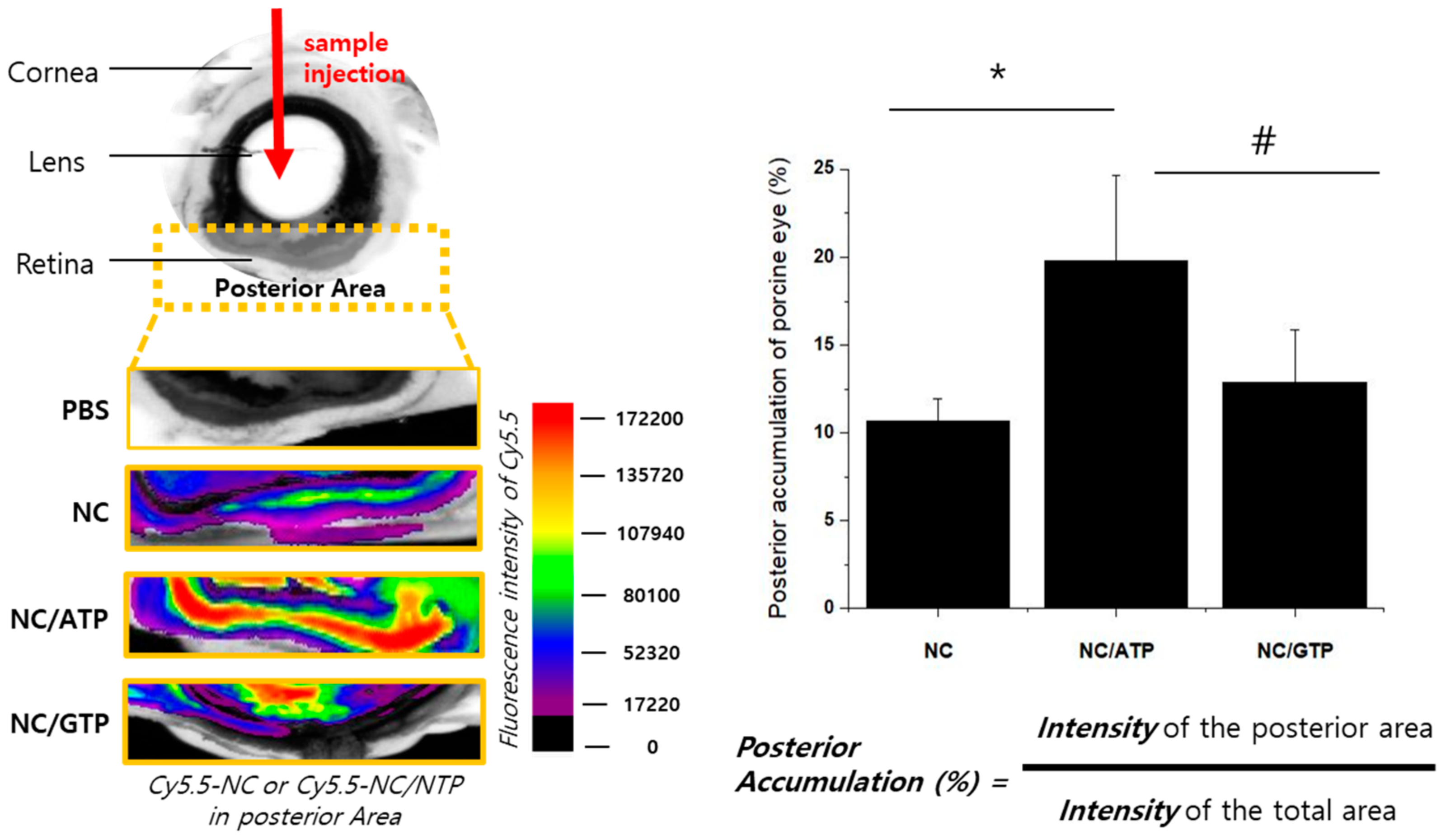
2.5. Distribution of NC and NC–ATP in the Porcine Eye Ex Vivo
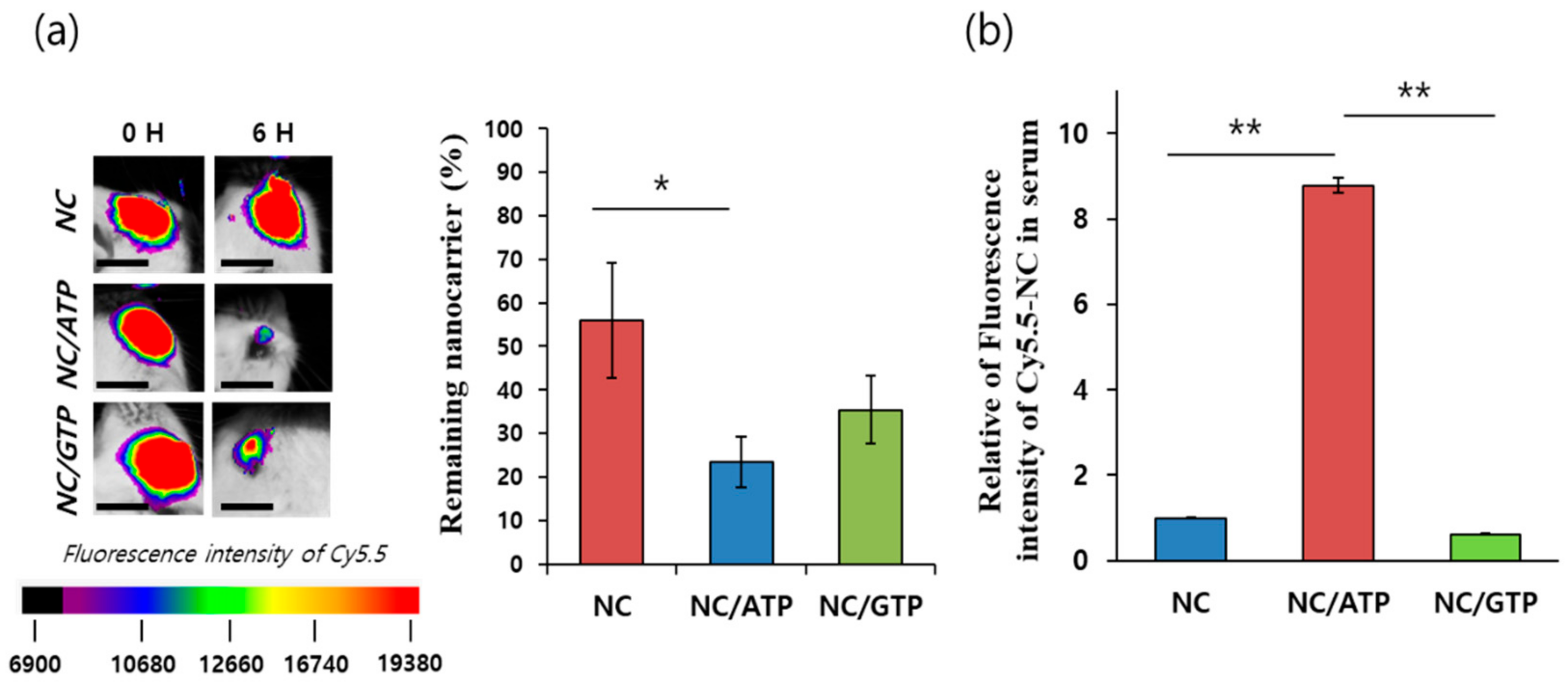
2.6. Retention of NC in the Eye and Permeation through the RPE to the Blood Vessels in Mice
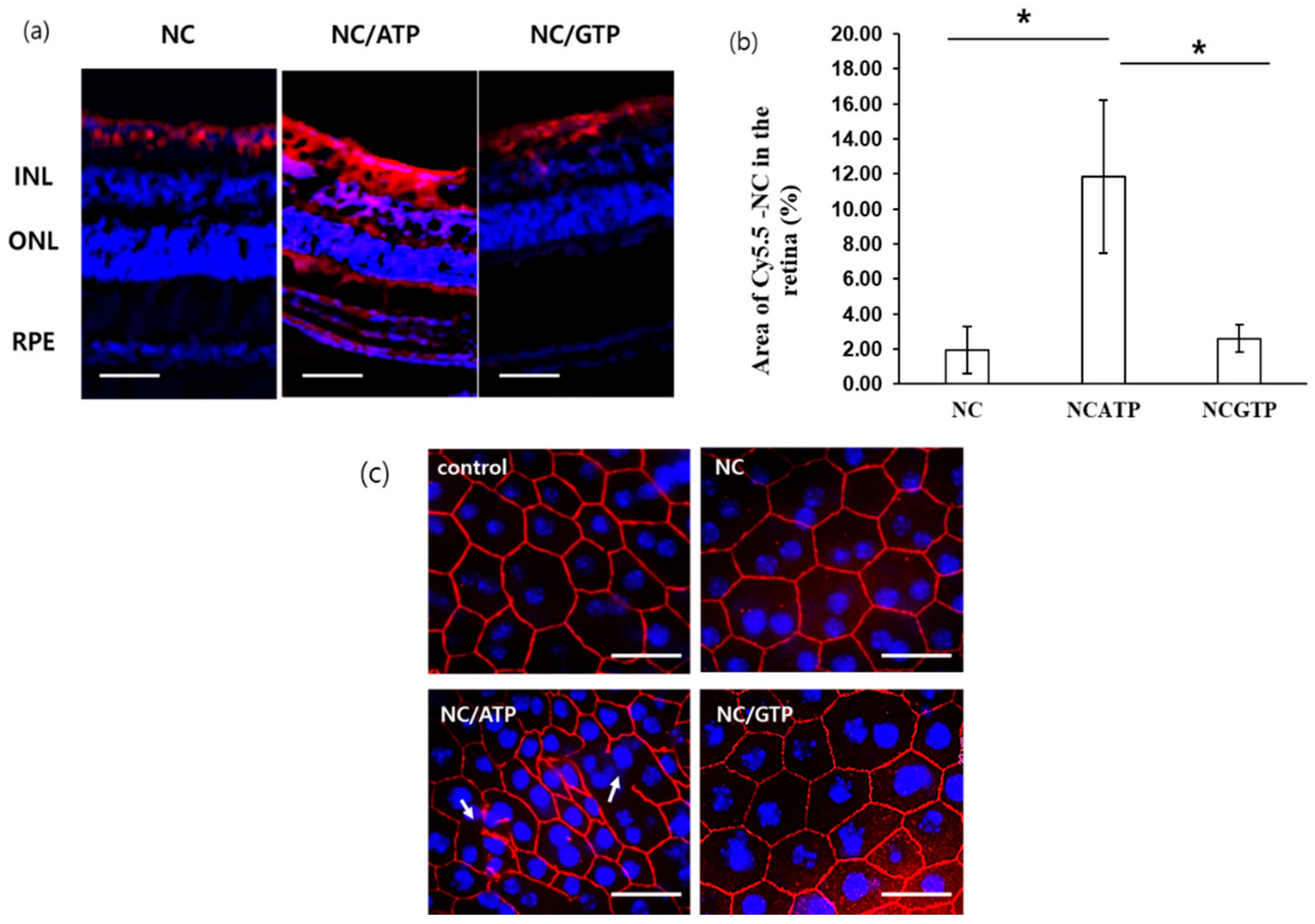
2.7. Histogolical Analysis of NC–NTP in the Retina
2.8. Statistical Analysis
3. Results and Discussion
3.1. Biocompatibility of NC and Bioactivity of ATP-Mixed Chitosan-Functionalized Pluronic-Based Nanocarrieron the Retinal Pigmented Epithelium
3.2. Distribution of NC and NC–NTP in the Porcine Eye Ex Vivo
3.3. Retention of NC–NTP in the Eye and Permeation of NC–NTP across the Retina In Vivo
3.4. Histological Analysis of NC–NTP in the Retina
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Patel, A.; Cholkar, K.; Agrahari, V.; Mitra, A.K. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47–64. [Google Scholar] [CrossRef]
- Mantelli, F.; Mauris, J.; Argueso, P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: From allergy to infectious diseases. Curr. Opin. Allergy Clin. Immunol. 2013, 13, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-Álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Con-siderations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- del Amo, E.M.; Rimpelä, A.-K.; Heikkinen, E.; Kari, O.K.; Ramsay, E.; Lajunen, T.; Schmitt, M.; Pelkonen, L.; Bhattacharya, M.; Richardson, D.; et al. Pharmacokinetic aspects of retinal drug delivery. Prog. Retin. Eye Res. 2017, 57, 134–185. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Shimazawa, M.; Hara, H. Retinal Diseases Associated with Oxidative Stress and the Effects of a Free Radical Scavenger (Edaravone). Oxidative Med. Cell. Longev. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Falavarjani, K.G.; Nguyen, Q.D. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: A review of literature. Eye 2013, 27, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Xu, H.; Ding, P.T.; Zheng, J.M. Thermosetting gels with modulated gelation temperature for ophtahlmic use_the rheological and gamma scintigraphic studies. J. Control Release 2002, 18, 65–74. [Google Scholar] [CrossRef]
- Bachu, R.D.; Chowdhury, P.; Al-Saedi, Z.H.; Karla, P.K.; Boddu, S.H. Ocular Drug Delivery Barriers-Role of Nanocarriers in the Treatment of Anterior Segment Ocular Diseas-es. Pharmaceutics 2018, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Chen, Y.S.; Green, C.R.; Rupenthal, I.D. Hyaluronic acid coated albumin nanoparticles for targeted peptide delivery in the treatment of retinal is-chaemia. Biomaterials 2018, 168, 10–23. [Google Scholar] [CrossRef]
- Gorantla, S.; Rapalli, V.K.; Waghule, T.; Singh, P.P.; Dubey, S.K.; Saha, R.N.; Singhvi, G. Nanocarriers for ocular drug delivery: Current status and translational opportunity. RSC Adv. 2020, 10, 27835–27855. [Google Scholar] [CrossRef]
- Koo, H.; Moon, H.; Han, H.; Na, J.H.; Huh, M.S.; Park, J.H.; Woo, S.J.; Park, K.H.; Kwon, I.C.; Kim, K.; et al. The movement of self-assembled amphiphilic polymeric nanoparticles in the vitreous and retina after intravi-treal injection. Biomaterials 2012, 33, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.A.; Dale, N.; Llaudet, E.; Mobbs, P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal pro-genitor proliferation. Neuron 2005, 46, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Zihni, C.; Mills, C.; Matter, K.; Balda, M.S. Tight junctions: From simple barriers to multifunctional molecular gates. Nat. Rev. Mol. Cell Biol. 2016, 17, 564–580. [Google Scholar] [CrossRef]
- Erb, L.; Weisman, G.A. Coupling of P2Y receptors to G proteins and other signaling pathways. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2012, 1, 789–803. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signalling: Therapeutic Developments. Front. Pharmacol. 2017, 8, 661. [Google Scholar] [CrossRef]
- Shen, H.H.; Chan, E.C.; Lee, J.H.; Bee, Y.S.; Lin, T.W.; Dusting, G.J.; Liu, G.S. Nanocarriers for treatment of ocular neovascularization in the back of the eye: New vehicles for opthal-mic drug delivery. Nanomedicine 2015, 10, 2093–2107. [Google Scholar] [CrossRef] [PubMed]
- Loma, P.; Guzman-Aranguez, A.; Pérez de Lara, M.J.; Pintor, J. Diadenosine tetraphosphate induces tight junction disassembly thus increasing corneal epithelial permeabil-ity. Br. J. Pharmacol. 2015, 172, 1045–1058. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Choi, W.I.; Kim, Y.H.; Tae, G.; Lee, S.-Y.; Kim, K.; Kwon, I.C. In-vivo tumor targeting of pluronic-based nano-carriers. J. Control Release 2010, 147, 109–117. [Google Scholar] [CrossRef]
- Jacobson, K.A.; Paoletta, S.; Katritch, V.; Wu, B.; Gao, Z.-G.; Zhao, Q.; Stevens, R.C.; Kiselev, E. Nucleotides Acting at P2Y Receptors: Connecting Structure and Function. Mol. Pharmacol. 2015, 88, 220–230. [Google Scholar] [CrossRef]
- Lee, J.S.; Hwang, Y.; Oh, H.; Kim, S.; Kim, J.H.; Lee, J.H.; Shin, Y.C.; Tae, G.; Choi, W.I. A novel chitosan nanocapsule for enhanced skin penetration of cyclosporin A and effective hair growth in vivo. Nano Res. 2019, 12, 3024–3030. [Google Scholar] [CrossRef]
- Lee, J.H.; Sahu, A.; Choi, W.I.; Lee, J.Y.; Tae, G. ZOT-derived peptide and chitosan functionalized nanocarrier for oral delivery of protein drug. Biomaterials 2016, 103, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Zemskov, E.; Lucas, R.; Verin, A.D.; Umapathy, N.S. P2Y receptors as regulators of lung endothelial barrier integrity. J. Cardiovasc. Dis. Res. 2011, 2, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Chadet, S.; Jelassi, B.; Wannous, R.; Angoulvant, D.; Chevalier, S.; Besson, P.; Roger, S. The activation of P2Y2 receptors increases MCF-7 breast cancer cells migration through the MEK-ERK1/2 signalling pathway. Carcinogenesis 2014, 35, 1238–1247. [Google Scholar] [CrossRef]
- Szrejder, M.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Rychłowski, M.; Angielski, S.; Piwkowska, A. Extracellular ATP modulates podocyte function through P2Y purinergic receptors and pleiotropic effects on AMPK and cAMP/PKA signaling pathways. Arch. Biochem. Biophys. 2020, 695, 108649. [Google Scholar] [CrossRef]
- Yu, N.; Erb, L.; Shivaji, R.; Weisman, G.A.; Seye, C.I. Binding of the P2Y 2 Nucleotide Receptor to Filamin A Regulates Migration of Vascular Smooth Muscle Cells. Circ. Res. 2008, 102, 581–588. [Google Scholar] [CrossRef]
- Dubyak, G.R. P2Y Receptors. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, W.J., Lane, M.D., Eds.; Academic Press: Waltham, MA, USA, 2013; pp. 375–378. [Google Scholar]
- Kennedy, C. P2Y Receptor Agents. In xPharm: The Comprehensive Pharmacology Reference; Enna, S.J., Bylund, D.B., Eds.; Elsevier: New York, NY, USA, 2007; pp. 1–3. [Google Scholar]
- von Kügelgen, I.; Harden, T.K. Chapter 12—Molecular Pharmacology, Physiology, and Structure of the P2Y Receptors. In Advances in Pharmacology; Jacobson, K.A., Linden, J., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 373–415. [Google Scholar]
- Ward, M.; Puthussery, T.; Fletcher, E. Localization and possible function of P2Y4 receptors in the rodent retina. Neuroscience 2008, 155, 1262–1274. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, K.; Hwang, Y.; Jung, J.; Tae, G. Enhanced Transport and Permeation of a Polymeric Nanocarrier across the Retina by Mixing with ATP upon Intravitreal Injection. Pharmaceutics 2021, 13, 463. https://doi.org/10.3390/pharmaceutics13040463
Kwon K, Hwang Y, Jung J, Tae G. Enhanced Transport and Permeation of a Polymeric Nanocarrier across the Retina by Mixing with ATP upon Intravitreal Injection. Pharmaceutics. 2021; 13(4):463. https://doi.org/10.3390/pharmaceutics13040463
Chicago/Turabian StyleKwon, Kiyoon, Youngmin Hwang, Junyoung Jung, and Giyoong Tae. 2021. "Enhanced Transport and Permeation of a Polymeric Nanocarrier across the Retina by Mixing with ATP upon Intravitreal Injection" Pharmaceutics 13, no. 4: 463. https://doi.org/10.3390/pharmaceutics13040463
APA StyleKwon, K., Hwang, Y., Jung, J., & Tae, G. (2021). Enhanced Transport and Permeation of a Polymeric Nanocarrier across the Retina by Mixing with ATP upon Intravitreal Injection. Pharmaceutics, 13(4), 463. https://doi.org/10.3390/pharmaceutics13040463