Oligonucleotide Delivery across the Caco-2 Monolayer: The Design and Evaluation of Self-Emulsifying Drug Delivery Systems (SEDDS)
Abstract
1. Introduction
2. Materials and Methods
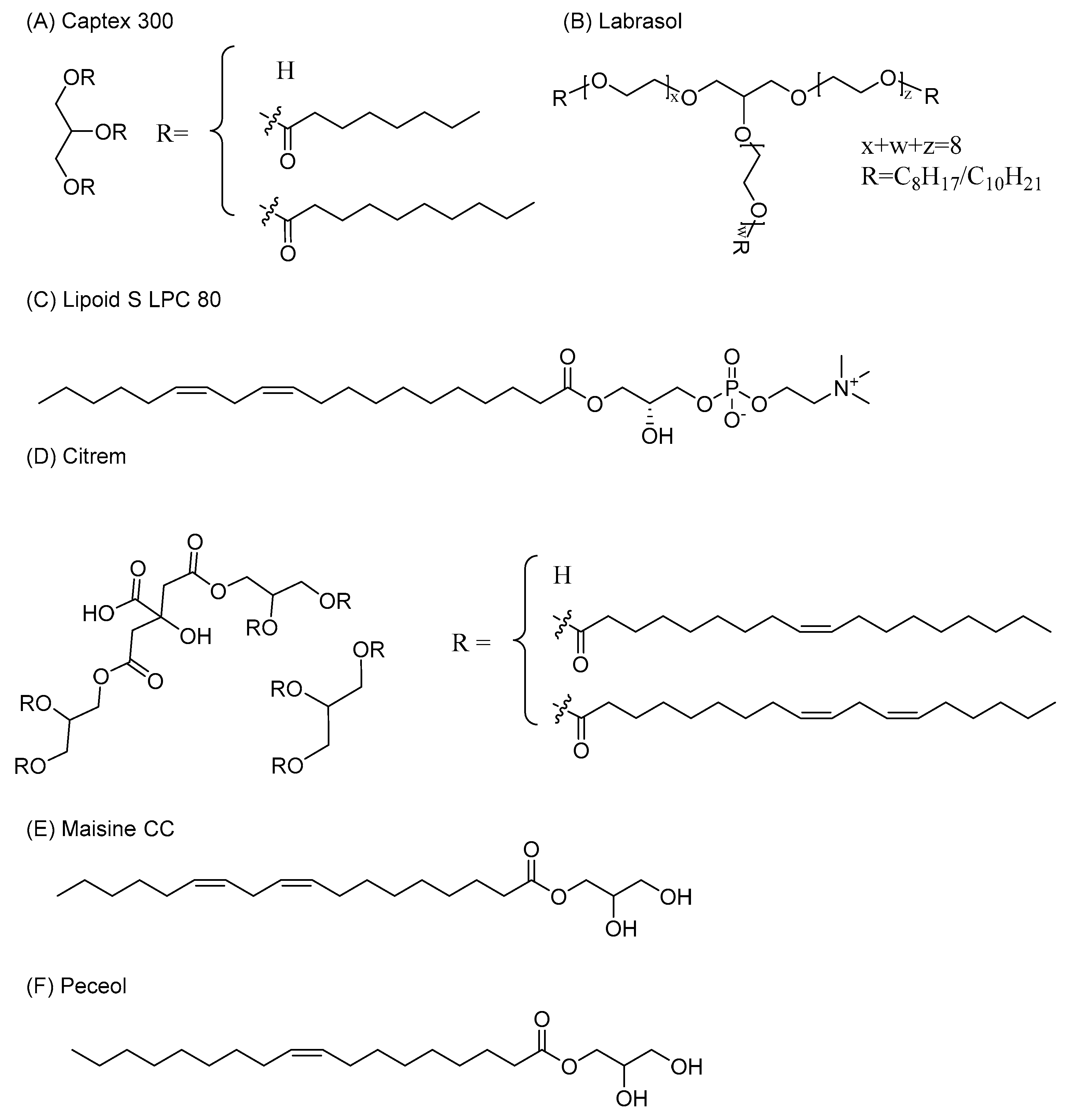
2.1. Materials
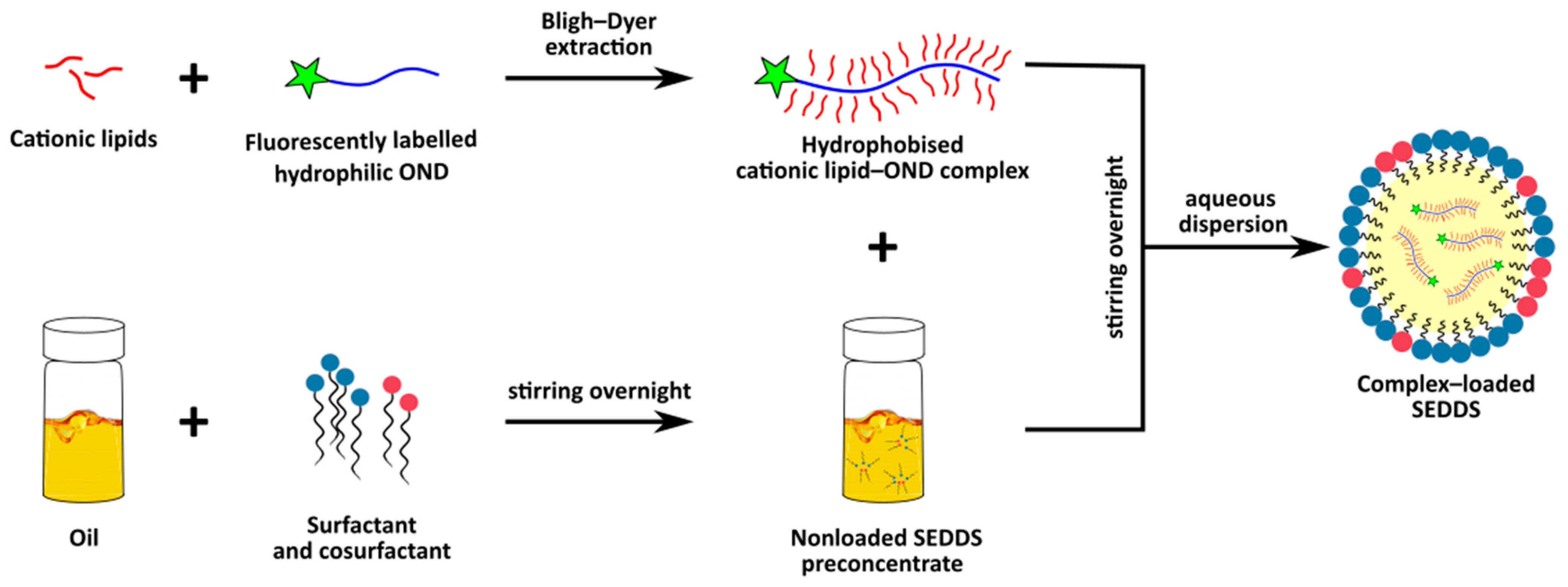
2.2. Complex Preparation
2.3. Effect of SEDDS Lipid Excipents on Complex Stability
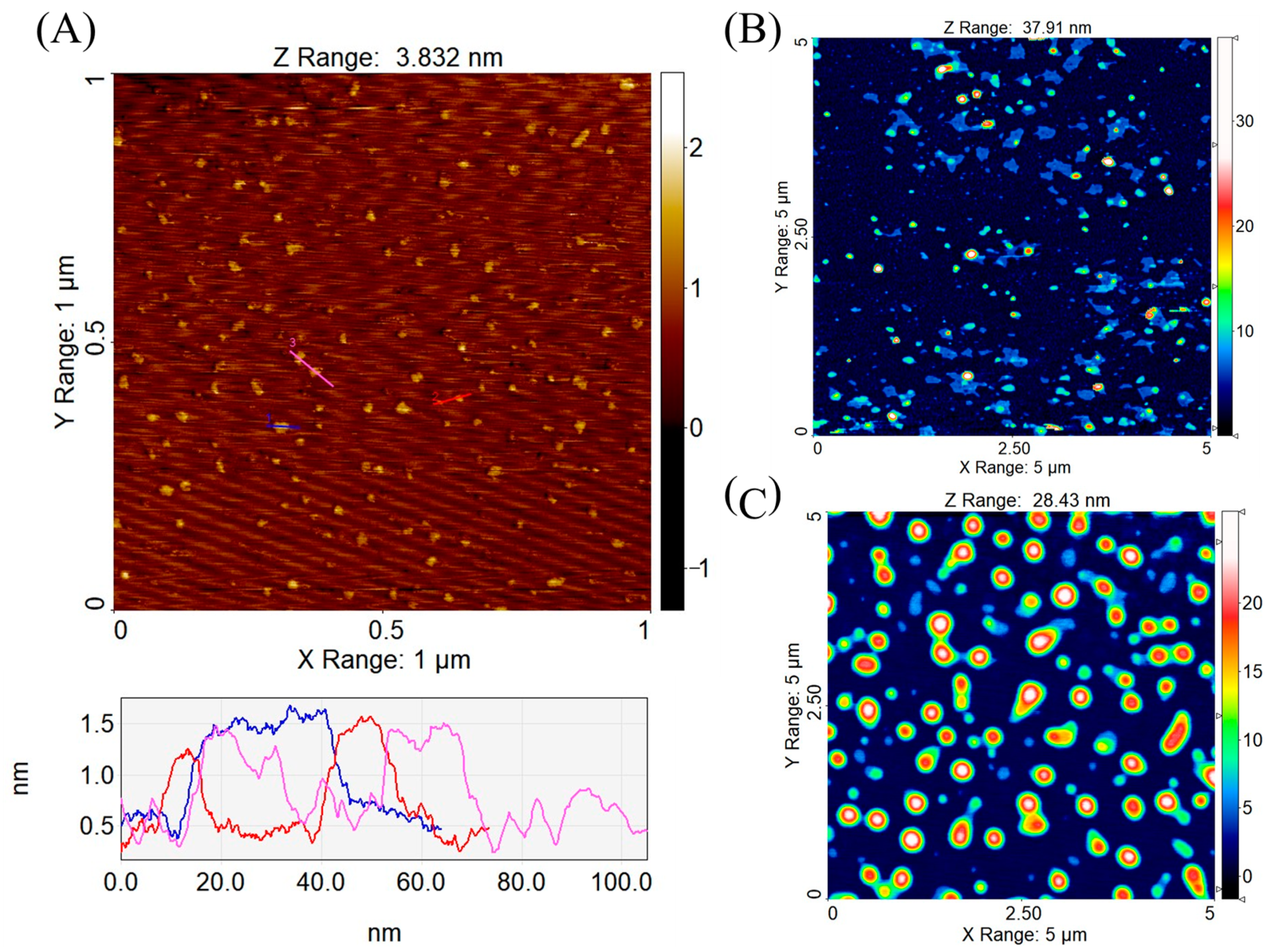
2.4. Atomic Force Microscopy (AFM)
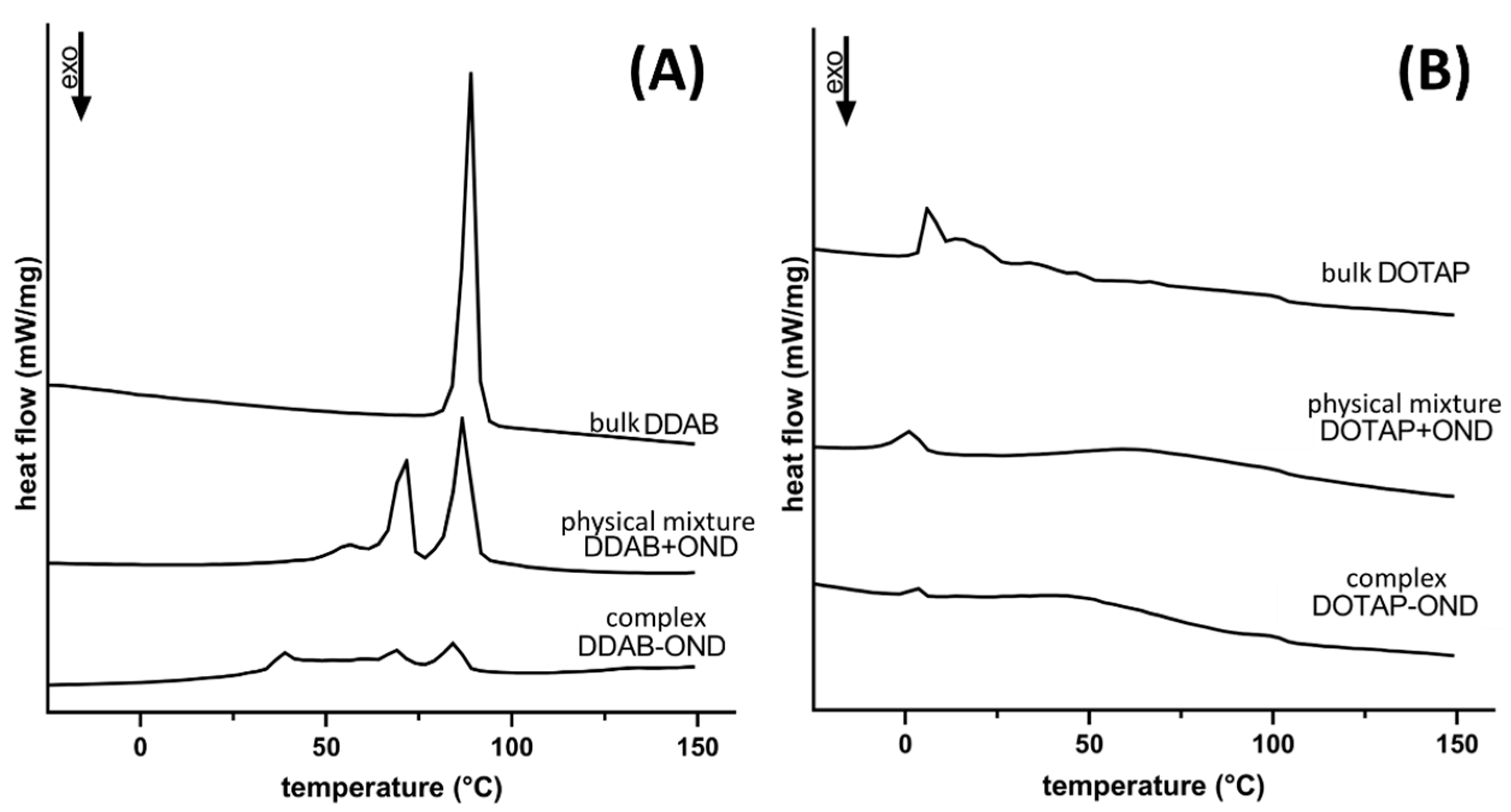
2.5. Differential Scanning Calorimetry (DSC)
2.6. Attenuated Total Reflectance-Fourier-Transform Infrared (ATR-FTIR) Spectroscopy
2.7. Preparation of SEDDS Formulations
2.8. Characterization of Dispersed SEDDS
2.9. Dynamic In Vitro Lipolysis of SEDDS
2.10. Cryogenic Transmission Electron Microscopy
2.11. Degradation by Nucleases
2.12. Caco-2 Cell Monolayer Transport Study
2.13. In Vitro Cytotoxicity Study
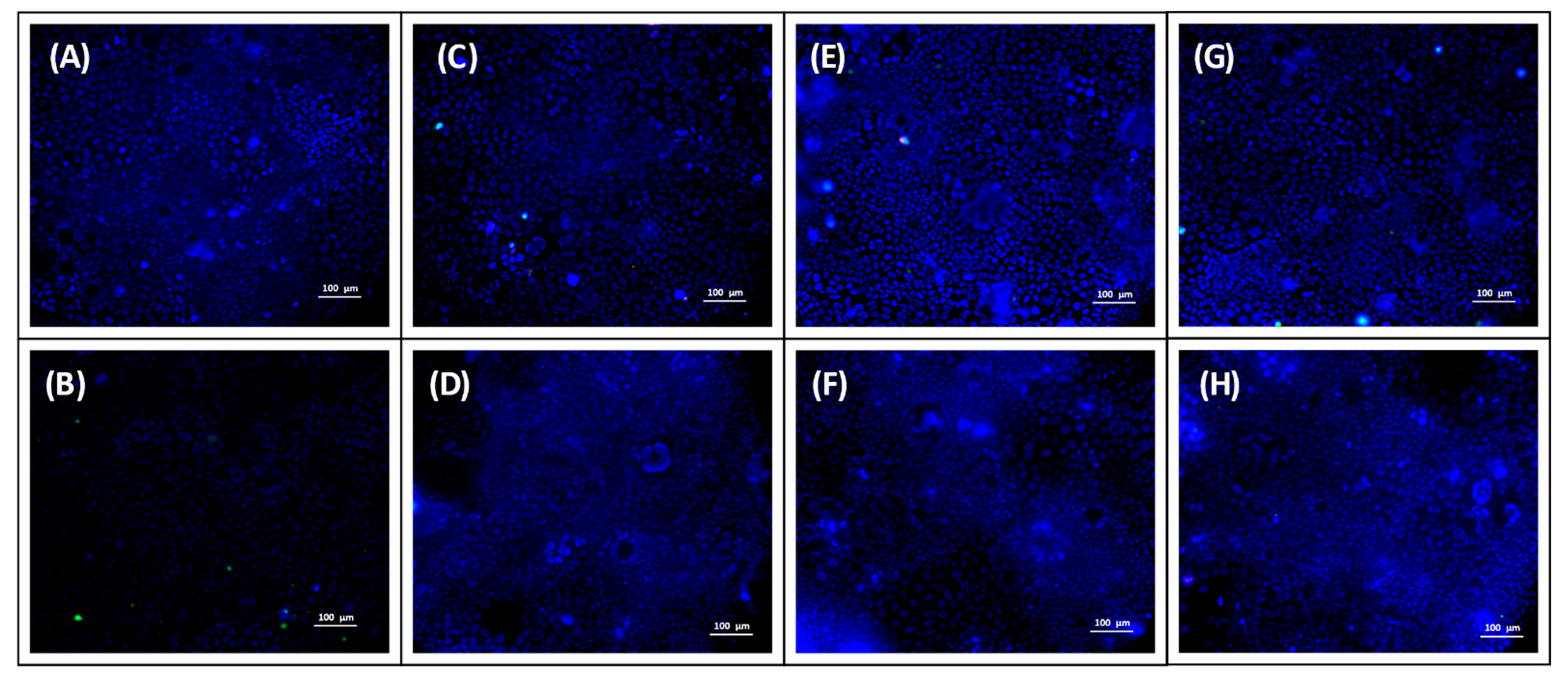
2.14. Uptake Study
2.15. Statistical Analysis
3. Results
3.1. Complex Preparation
3.2. Effect of SEDDS Lipid Excipents on Complex Stability
3.3. Atomic Force Microscopy (AFM)
3.4. Differential Scanning Calorimetry (DSC)
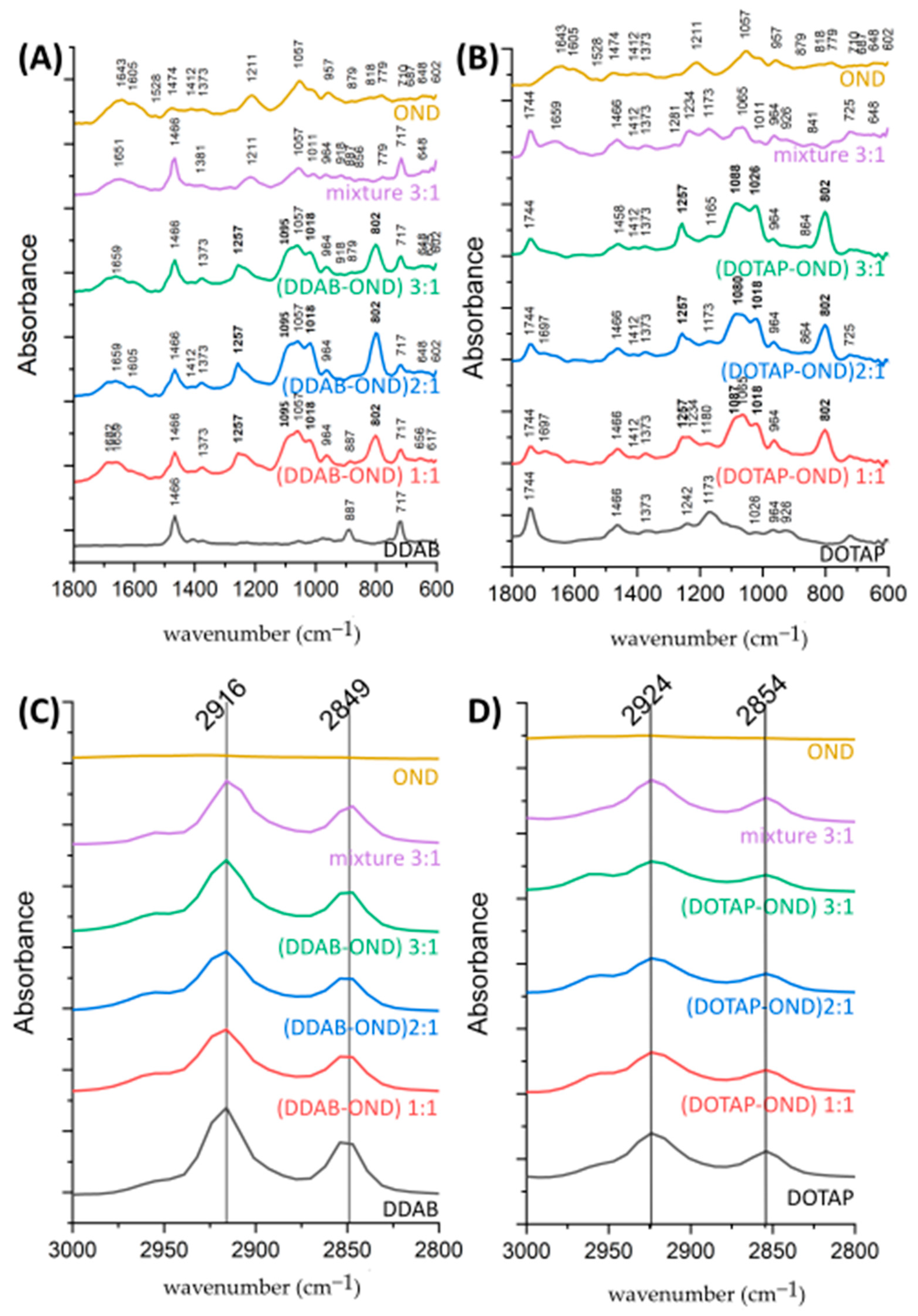
3.5. ATR-FTIR Spectroscopy
3.6. Characterization of Dispersed SEDDS
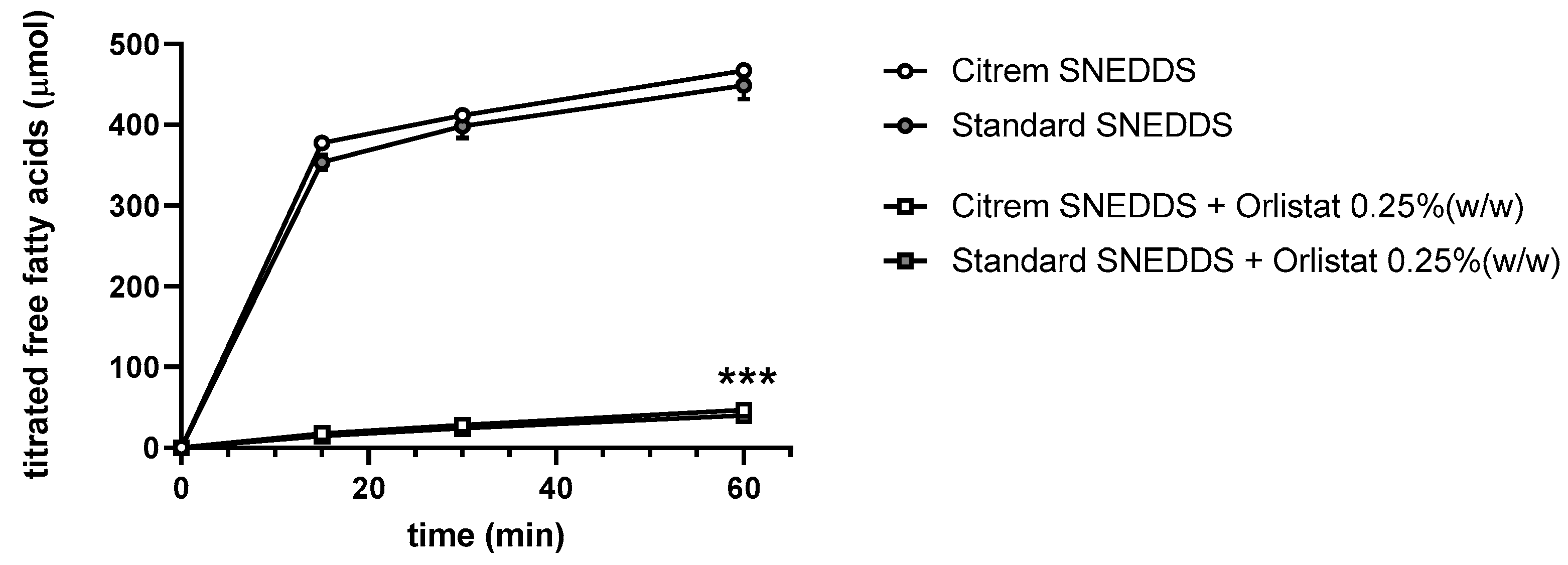
3.7. Dynamic In Vitro Lipolysis of SEDDS
3.8. Cryo-TEM
3.9. Protection Against Nuclease Degradation
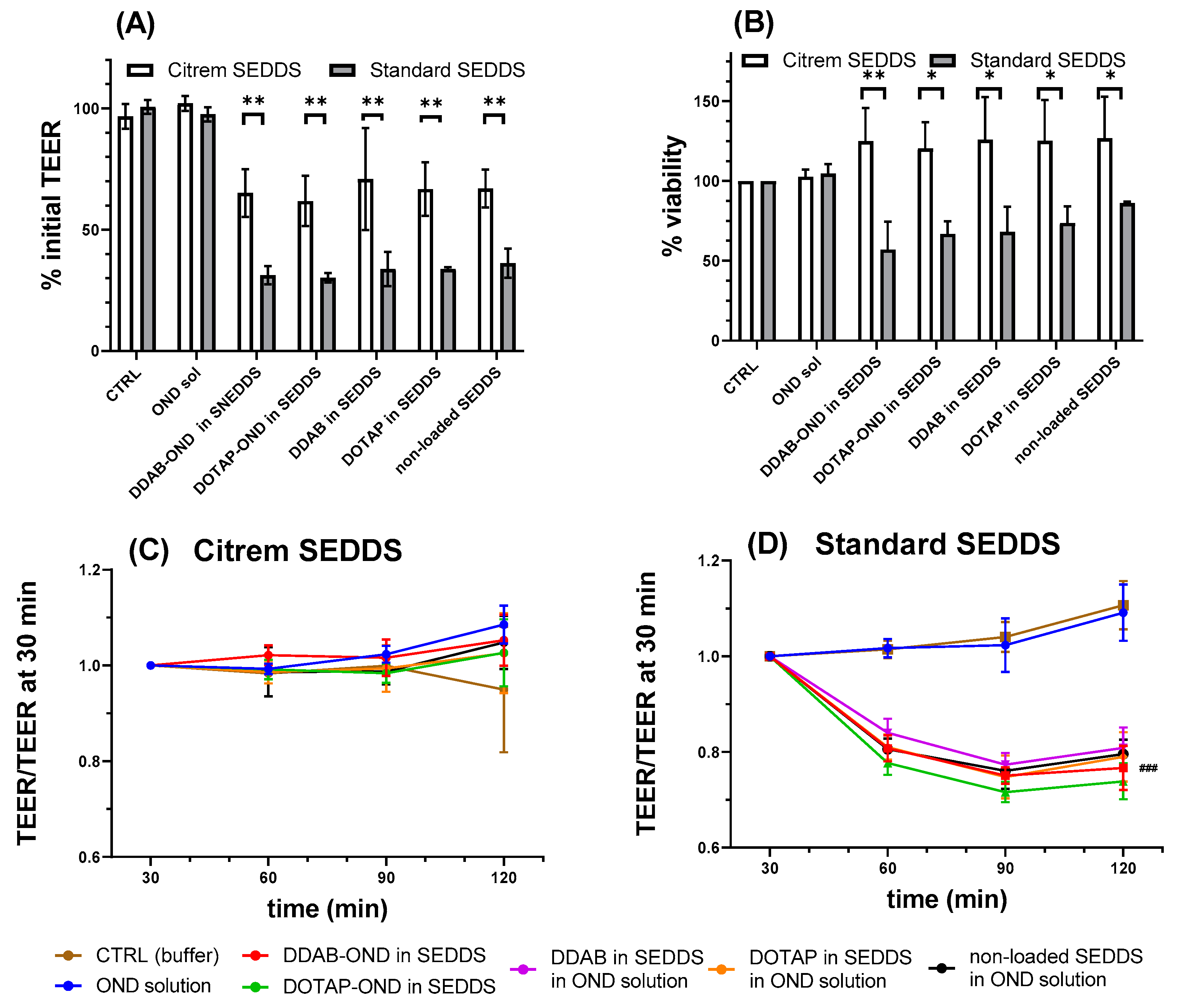
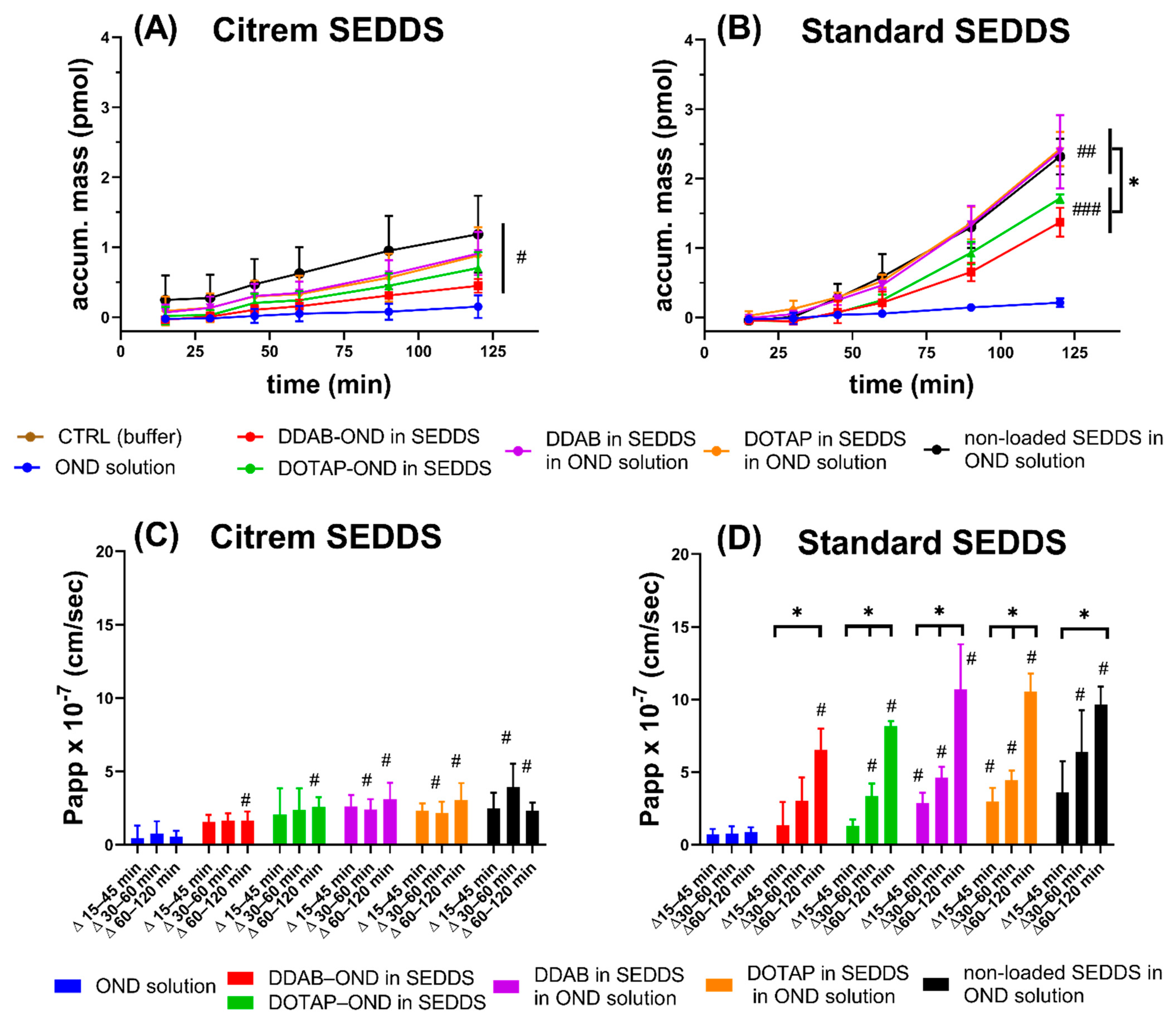
3.10. Caco-2 Cell Monolayer Transport Study
3.11. In Vitro Toxicity Study
3.12. Uptake Study
4. Discussion
4.1. Preparation of Cationic Lipid-OND Complexes
4.2. Loading the Complexes into Citrem and Standard SEDDS and Evaluation of the Formulations
4.3. In Vitro Performance of the Formulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, X.; Kim, K.; Liu, D. Nonviral gene delivery: What we know and what is next. AAPS J. 2007, 9, E92–E104. [Google Scholar] [CrossRef]
- Lam, J.K.W.; Chow, M.Y.T.; Zhang, Y.; Leung, S.W.S. siRNA Versus miRNA as Therapeutics for Gene Silencing. Mol. Ther. Nucleic Acids 2015, 4, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.; Antonsson, M.; Elebring, M.; Jansson-Löfmark, R.; Weidolf, L. Drug metabolism and pharmacokinetic strategies for oligonucleotide- and mRNA-based drug development. Drug Discov. Today 2018, 23, 1733–1745. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.M. Delivery of genes and oligonucleotides. In Drug Delivery: Principles and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 655–673. [Google Scholar]
- O’Driscoll, C.M.; Bernkop-Schnürch, A.; Friedl, J.D.; Péat, V.; Jannin, V. Oral delivery of non-viral nucleic acid-based therapeutics-do we have the guts for this? Eur. J. Pharm. Sci. 2019, 93, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Podolasky, D.K. Inflammatory bowel disease. N. Engl. J. Med. 2002, 347, 417–429. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Li, Y.Y. Inflammatory bowel disease: Pathogenesis. World J. Gastroenterol. 2014, 20, 91–99. [Google Scholar] [CrossRef]
- Eisenstein, M. Biology: A slow-motion epidemic. Nature 2016, 540, S98–S99. [Google Scholar] [CrossRef]
- Hua, S.; Marks, E.; Schneider, J.J.; Keely, S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1117–1132. [Google Scholar] [CrossRef]
- Youshia, J.; Lamprecht, A. Size-dependent nanoparticulate drug delivery in inflammatory bowel diseases. Expert Opin. Drug Deliv. 2016, 13, 281–294. [Google Scholar] [CrossRef]
- Collnot, E.M.; Ali, H.; Lehr, C.M. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J. Control. Release 2012, 161, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Jiang, X.; Gui, S. RNA interference-based nanosystems for inflammatory bowel disease therapy. Int. J. Nanomed. 2016, 11, 5287–5310. [Google Scholar] [CrossRef]
- Ball, R.L.; Bajaj, P.; Whitehead, K.A. Oral delivery of siRNA lipid nanoparticles: Fate in the GI tract. Sci. Rep. 2018, 8, 2178. [Google Scholar] [CrossRef]
- Knipe, J.M.; Strong, L.E.; Peppas, N.A. Enzyme- and pH-Responsive Microencapsulated Nanogels for Oral Delivery of siRNA to Induce TNF-α Knockdown in the Intestine. Biomacromolecules 2016, 17, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.S.; Dalmasso, G.; Wang, L.; Sitaraman, S.V.; Merlin, D.; Murthy, N. Orally delivered thioketal nanoparticles loaded with TNF-α-siRNA target inflammation and inhibit gene expression in the intestines. Nat. Mater. 2010, 9, 923–928. [Google Scholar] [CrossRef]
- Attarwala, H.; Han, M.; Kim, J.; Amiji, M. Oral nucleic acid therapy using multicompartmental delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1478. [Google Scholar] [CrossRef] [PubMed]
- Müllertz, A.; Ogbonna, A.; Ren, S.; Rades, T. New perspectives on lipid and surfactant based drug delivery systems for oral delivery of poorly soluble drugs. J. Pharm. Pharmacol. 2010, 62, 1622–1636. [Google Scholar] [CrossRef]
- Gursoy, R.N.; Benita, S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed. Pharmacother. 2004, 58, 173–182. [Google Scholar] [CrossRef]
- Mahmood, A.; Bernkop-Schnürch, A. SEDDS: A game changing approach for the oral administration of hydrophilic macromolecular drugs. Adv. Drug Deliv. Rev. 2019, 142, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.D.; Manning, M.C. Hydrophobic ion pairing: Altering the solubility properties of biomolecules. Pharm. Res. 1998, 15, 188–193. [Google Scholar] [CrossRef]
- Simberg, D.; Weisman, S.; Talmon, Y.; Barenholz, Y. DOTAP (and other cationic lipids): Chemistry, biophysics, and transfection. Crit. Rev. Ther. Drug Carr. Syst. 2004, 21, 257–317. [Google Scholar] [CrossRef]
- Lobovkina, T.; Jacobson, G.B.; Gonzalez-Gonzalez, E.; Hickerson, R.P.; Leake, D.; Kaspar, R.L.; Contag, C.H.; Zare, R.N. In vivo sustained release of siRNA from solid lipid nanoparticles. ACS Nano 2011, 5, 9977–9983. [Google Scholar] [CrossRef]
- Wong, F.M.P.; Reimer, D.L.; Bally, M.B. Cationic Lipid Binding to DNA: Characterization of Complex Formation. Biochemistry 1996, 35, 5756–5763. [Google Scholar] [CrossRef]
- Marty, R.; N’soukpoé-Kossi, C.N.; Charbonneau, D.; Weinert, C.M.; Kreplak, L.; Tajmir-Riahi, H.A. Structural analysis of DNA complexation with cationic lipids. Nucleic Acids Res. 2009, 37, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Szoka, F.C. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry 1996, 35, 5616–5623. [Google Scholar] [CrossRef]
- Liang, W.W.; Lam, J.K. Endosomal Escape Pathways for Non-Viral Nucleic Acid Delivery Systems. In Molecular Regulation of Endocytosis; InTech: London, UK, 2012; Volume 2, p. 64. ISBN 9789537619992. [Google Scholar]
- Kiptoo, P.; Calcagno, A.M.; Siahann, T.J. Drug delivery: Principles and applications. In Drug Delivery: Principles and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 19–34. ISBN 1118833236. [Google Scholar]
- Maher, S.; Brayden, D.J.; Casettari, L.; Illum, L. Application of permeation enhancers in oral delivery of macromolecules: An update. Pharmaceutics 2019, 11, 1–23. [Google Scholar] [CrossRef]
- Hayashi, M.; Sakai, T.; Hasegawa, Y.; Nishikawahara, T.; Tomioka, H.; Iida, A.; Shimizu, N.; Tomita, M.; Awazu, S. Physiological mechanism for enhancement of paracellular drug transport. J. Control. Release 1999, 62, 141–148. [Google Scholar] [CrossRef]
- Sha, X.; Yan, G.; Wu, Y.; Li, J.; Fang, X. Effect of self-microemulsifying drug delivery systems containing Labrasol on tight junctions in Caco-2 cells. Eur. J. Pharm. Sci. 2005, 24, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Hauptstein, S.; Prüfert, F.; Bernkop-Schnürch, A. Self-nanoemulsifying drug delivery systems as novel approach for pDNA drug delivery. Int. J. Pharm. 2015, 487, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, A.; Prüfert, F.; Efiana, N.A.; Ashraf, M.I.; Hermann, M.; Hussain, S.; Bernkop-Schnürch, A. Cell-penetrating self-nanoemulsifying drug delivery systems (SNEDDS) for oral gene delivery. Expert Opin. Drug Deliv. 2016, 13, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Gilmore, J.; Deguchi, K.; Takeyasu, K. Nanoimaging of RNA Molecules with Atomic Force Microscopy. Microsc. Imaging Sci. Pract. Approaches Appl. Res. Educ. 2017, 54, 300–306. [Google Scholar]
- Lyubchenko, Y.L.; Shlyakhtenko, L.S.; Ando, T. Imaging of nucleic acids with atomic force microscopy. Methods 2011, 54, 274–283. [Google Scholar] [CrossRef]
- Niu, Z.; Tedesco, E.; Benetti, F.; Mabondzo, A.; Montagner, I.M.; Marigo, I.; Gonzalez-Touceda, D.; Tovar, S.; Diéguez, C.; Santander-Ortega, M.J.; et al. Rational design of polyarginine nanocapsules intended to help peptides overcoming intestinal barriers. J. Control. Release 2017, 263, 4–17. [Google Scholar] [CrossRef]
- Amara, S.; Patin, A.; Giuffrida, F.; Wooster, T.J.; Thakkar, S.K.; Bénarouche, A.; Poncin, I.; Robert, S.; Point, V.; Molinari, S.; et al. In vitro digestion of citric acid esters of mono- and diglycerides (CITREM) and CITREM-containing infant formula/emulsions. Food Funct. 2014, 5, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Zangenberg, N.H.; Müllertz, A.; Gjelstrup, K.H.; Hovgaard, L. A dynamic in vitro lipolysis model. II: Evaluation of the model. Eur. J. Pharm. Sci. 2001, 14, 237–244. [Google Scholar] [CrossRef]
- Siqueira, J.S.D.; Al Sawaf, M.; Graeser, K.; Mu, H.; Müllertz, A.; Rades, T. The ability of two in vitro lipolysis models reflecting the human and rat gastro-intestinal conditions to predict the in vivo performance of SNEDDS dosing regimens. Eur. J. Pharm. Biopharm. 2018, 124, 116–124. [Google Scholar] [CrossRef]
- Berthelsen, R.; Klitgaard, M.; Rades, T.; Müllertz, A. In vitro digestion models to evaluate lipid based drug delivery systems; present status and current trends. Adv. Drug Deliv. Rev. 2019, 142, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, M.H.; Siqueira, J.S.D.; Abdi, I.M.; Wasan, K.M.; Rades, T.; Müllertz, A. Fenofibrate oral absorption from SNEDDS and super-SNEDDS is not significantly affected by lipase inhibition in rats. Eur. J. Pharm. Biopharm. 2019, 142, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Fountain, K.J.; Gilar, M.; Budman, Y.; Gebler, J.C. Purification of dye-labeled oligonucleotides by ion-pair reversed-phase high-performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2003, 783, 61–72. [Google Scholar] [CrossRef]
- Schön, P. Atomic force microscopy of RNA: State of the art and recent advancements. Semin. Cell Dev. Biol. 2018, 73, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Seeman, N.C. An Overview of Structural DNA Nanotechnology. Mol. Biotechnol. 2007, 37, 246–257. [Google Scholar] [CrossRef]
- Berg, J.; Tymoczko, J.; Stryer, L. Fatty Acids Are Key Constituents of Lipids. In Biochemistry, 5th ed.; WH Freeman: New York, NY, USA, 2002. [Google Scholar]
- Banyay, M.; Sarkar, M.; Gräslund, A. A library of IR bands of nucleic acids in solution. Biophys. Chem. 2003, 104, 477–488. [Google Scholar] [CrossRef]
- Choosakoonkriang, S.; Wiethoff, C.M.; Anchordoquy, T.J.; Koe, G.S.; Smith, J.G.; Middaugh, C.R. Infrared Spectroscopic Characterization of the Interaction of Cationic Lipids with Plasmid DNA. J. Biol. Chem. 2001, 276, 8037–8043. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. Encycl. Anal. Chem. 2004, 1–23. [Google Scholar]
- Taillandier, E.; Liquier, J. Vibrational Spectroscopy of Nucleic Acids. In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; ISBN 9780470027325. [Google Scholar]
- Wu, Y.; Manna, S.; Petrochenko, P.; Koo, B.; Chen, L.; Xu, X.; Choi, S.; Kozak, D.; Zheng, J. Coexistence of oil droplets and lipid vesicles in propofol drug products. Int. J. Pharm. 2020, 577, 118998. [Google Scholar] [CrossRef]
- de Ilarduya, C.T.; Sun, Y.; Düzgüneş, N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010, 40, 159–170. [Google Scholar] [CrossRef]
- Bouxsein, N.F.; McAllister, C.S.; Ewert, K.K.; Samuel, C.E.; Safinya, C.R. Structure and gene silencing activities of monovalent and pentavalent cationic lipid vectors complexed with siRNA. Biochemistry 2007, 46, 4785–4792. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Lobo, B.A.; Davis, A.; Koe, G.; Smith, J.G.; Middaugh, C.R. Isothermal Titration Calorimetric Analysis of the Interaction between Cationic Lipids and Plasmid DNA. Arch. Biochem. Biophys. 2001, 386, 95–105. [Google Scholar] [CrossRef]
- Reimer, D.L.; Zhang, Y.P.; Kong, S.; Wheeler, J.J.; Graham, R.W.; Bally, M.B. Formation of Novel Hydrophobic Complexes between Cationic Lipids and Plasmid DNA. Biochemistry 1995, 34, 12877–12883. [Google Scholar] [CrossRef]
- Belletti, D.; Tonelli, M.; Forni, F.; Tosi, G.; Vandelli, M.A.; Ruozi, B. AFM and TEM characterization of siRNAs lipoplexes: A combinatory tools to predict the efficacy of complexation. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 436, 459–466. [Google Scholar] [CrossRef]
- Scholz, C.; Wagner, E. Therapeutic plasmid DNA versus siRNA delivery: Common and different tasks for synthetic carriers. J. Control. Release 2012, 161, 554–565. [Google Scholar] [CrossRef]
- Kubackova, J.; Zbytovska, J.; Holas, O. Nanomaterials for direct and indirect immunomodulation: A review of applications. Eur. J. Pharm. Sci. 2020, 142, 105–139. [Google Scholar] [CrossRef]
- Tran, T.; Xi, X.; Rades, T.; Müllertz, A. Formulation and characterization of self-nanoemulsifying drug delivery systems containing monoacyl phosphatidylcholine. Int. J. Pharm. 2016, 502, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Fatouros, D.G.; Bergenstahl, B.; Mullertz, A. Morphological observations on a lipid-based drug delivery system during in vitro digestion. Eur. J. Pharm. Sci. 2007, 31, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Armand, M. Lipases and lipolysis in the human digestive tract: Where do we stand? Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 156–164. [Google Scholar] [CrossRef]
- McCartney, F.; Jannin, V.; Chevrier, S.; Boulghobra, H.; Hristov, D.R.; Ritter, N.; Miolane, C.; Chavant, Y.; Demarne, F.; Brayden, D.J. Labrasol® is an efficacious intestinal permeation enhancer across rat intestine: Ex vivo and in vivo rat studies. J. Control. Release 2019, 310, 115–126. [Google Scholar] [CrossRef]
- Liu, J.; Werner, U.; Funke, M.; Besenius, M.; Saaby, L.; Fanø, M.; Mu, H.; Müllertz, A. SEDDS for intestinal absorption of insulin: Application of Caco-2 and Caco-2/HT29 co-culture monolayers and intra-jejunal instillation in rats. Int. J. Pharm. 2019, 560, 377–384. [Google Scholar] [CrossRef]
- Maher, S.; Geoghegan, C.; Brayden, D.J. Intestinal permeation enhancers to improve oral bioavailability of macromolecules: Reasons for low efficacy in humans. Expert Opin. Drug Deliv. 2020, 18, 273–300. [Google Scholar] [CrossRef]
- Li, P.; Nielsen, H.M.; Müllertz, A. Impact of Lipid-Based Drug Delivery Systems on the Transport and Uptake of Insulin Across Caco-2 Cell Monolayers. J. Pharm. Sci. 2016, 105, 2743–2751. [Google Scholar] [CrossRef]
- Swain, S.; Patra, C.N.; Rao, M.E.B. Pharmaceutical Drug Delivery Systems and Vehicles; Woodhead Publishing India: New Delhi, India, 2016; ISBN 987-93-85059-00-1. [Google Scholar]
- van Hoogevest, P. Review–An update on the use of oral phospholipid excipients. Eur. J. Pharm. Sci. 2017, 108, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [PubMed]

| SEDDS | Captex 300 | Labrasol | Lipoid S LPC 80 | Citrem | Maisine CC | Peceol |
|---|---|---|---|---|---|---|
| (%w/w) | (%w/w) | (%w/w) | (%w/w) | (%w/w) | (%w/w) | |
| Citrem (negatively charged) | 20 | 40 | 20 | 20 | - | - |
| Standard (neutral) | 20 | 40 | 20 | - | 10 | 10 |
| Name of Formulation | Loaded Substance | Concentration of Loaded Substance in SEDDS |
|---|---|---|
| DDAB-OND in SEDDS | Complex of DDAB and OND at the charge ratio 3:1 | 100 nmol of OND/g SEDDS |
| DOTAP-OND in SEDDS | Complex of DOTAP and OND at the charge ratio 3:1 | 100 nmol of OND/g SEDDS |
| DDAB in SEDDS | DDAB | 3.8 mg/g SEDDS |
| DOTAP in SEDDS | DOTAP | 4.2 mg/g SEDDS |
| SEDDS + Orlistat | Orlistat | 0.25%(w/w) |
| Charge Ratio N+: PO2− | Molar Ratio Cationic Lipid: OND | CE (%) | |
|---|---|---|---|
| DDAB | DOTAP | ||
| 1:1 | 20:1 | 58.3 ± 0.7 | 84.6 ± 1.4 |
| 2:1 | 40:1 | 84.0 ± 2.8 | 100.1 ± 0.1 |
| 3:1 | 60:1 | 96.5 ± 2.0 | 100.4 ± 0.1 |
| SEDDS Excipient | CE (%) | |
|---|---|---|
| DDAB-OND | DOTAP-OND | |
| Citrem | 97.3 ± 0.2 *** | 97.7 ± 0.2 *** |
| LPC | 100.0 ± 0.2 | n.d. |
| Captex 300 | 100.3 ± 0.1 | 100.1 ± 0.1 |
| Labrasol | 100.5 ± 0.2 | 100.2 ± 0.1 |
| Maisine CC | 100.8 ± 0.1 | 100.4 ± 0.1 |
| Peceol | 100.2 ± 0.4 | 100.3 ± 0.1 |
| Complex-Specific Bands (cm−1) | Marker Bands Characteristic of | Assignment | Reference |
|---|---|---|---|
| 1257 | Phosphate-deoxyribose backbone | Organic phosphate P=O, vibrational asymmetric band | [24,46,48,49] |
| 1095 DDAB-OND 1088-1080 DOTAP-OND | Phosphate-deoxyribose backbone | Organic phosphate P=O, vibrational symmetric band | [24,46,49] |
| 1018 | Phosphate-deoxyribose backbone | P-O-C aliphatic phosphate | [48] |
| 802 | Deoxyribose conformation | N-type (C3′-endo) puckering mode | [46] |
| Size (nm) | PdI | Zeta Potential (mV) | |||||
|---|---|---|---|---|---|---|---|
| SEDDS | Loaded Substance | DI Water | MES-HBSS | DI Water | MES-HBSS | DI Water | MES-HBSS |
| Citrem | nonloaded | 201 ± 11 | 207 ± 16 | 0.36 | 0.27 | −35.5 ± 0.6 | −10.9 ± 0.7 |
| DDAB-OND | 209 ± 14 | 237 ± 11 | 0.30 | 0.23 | −24.1 ± 0.8 ### | −9.9 ± 0.7 | |
| DOTAP-OND | 195 ± 19 | 213 ± 5 | 0.30 | 0.22 | −26.5 ± 1.2 ### | −9.4 ± 0.5 | |
| Standard | nonloaded | 223 ± 10 | 213 ± 44 | 0.17 | 0.22 | −5.2 ± 2.1 | −1.2 ± 0.5 |
| DDAB-OND | 256 ± 22 | 267 ± 10 *** | 0.28 | 0.12 | 13.0 ± 1.3 ### | 0.2 ± 0.5 | |
| DOTAP-OND | 240 ± 9 * | 183 ± 27 | 0.23 | 0.25 | 14.0 ± 0.9 ### | 0.3 ± 0.3 | |
| SEDDS | Intact OND (%) | |
|---|---|---|
| DDAB-OND in SEDDS | DOTAP-OND in SEDDS | |
| Citrem | 16.0 ± 1.5 | 15.7 ± 1.0 |
| Standard | 59.9 ± 3.4 *** | 57.1 ± 3.0 *** |
| Formulation | Citrem SEDDS | Standard SEDDS | ||
|---|---|---|---|---|
| Transported OND Accumulated Basolaterally at 120 min | Transported OND Accumulated Basolaterally at 120 min | |||
| pmol | % | pmol | % | |
| OND solution | 0.15 ± 0.16 | 0.03 | 0.21 ± 0.06 | 0.04 |
| DDAB-OND in SEDDS | 0.45 ± 0.10 * | 0.09 | 1.37 ± 0.21 ** | 0.28 |
| DOTAP-OND in SEDDS | 0.70 ± 0.23 *** | 0.14 | 1.72 ± 0.06 *** | 0.34 |
| DDAB in SEDDS | 0.91 ± 0.31 ** | 0.18 | 2.40 ± 0.53 ** | 0.48 |
| DOTAP in SEDDS | 0.88 ± 0.41 ** | 0.17 | 2.43 ± 0.25 *** | 0.49 |
| Nonloaded SEDDS | 1.19 ± 0.55 ** | 0.24 | 2.32 ± 0.26 *** | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubackova, J.; Holas, O.; Zbytovska, J.; Vranikova, B.; Zeng, G.; Pavek, P.; Mullertz, A. Oligonucleotide Delivery across the Caco-2 Monolayer: The Design and Evaluation of Self-Emulsifying Drug Delivery Systems (SEDDS). Pharmaceutics 2021, 13, 459. https://doi.org/10.3390/pharmaceutics13040459
Kubackova J, Holas O, Zbytovska J, Vranikova B, Zeng G, Pavek P, Mullertz A. Oligonucleotide Delivery across the Caco-2 Monolayer: The Design and Evaluation of Self-Emulsifying Drug Delivery Systems (SEDDS). Pharmaceutics. 2021; 13(4):459. https://doi.org/10.3390/pharmaceutics13040459
Chicago/Turabian StyleKubackova, Jana, Ondrej Holas, Jarmila Zbytovska, Barbora Vranikova, Guanghong Zeng, Petr Pavek, and Anette Mullertz. 2021. "Oligonucleotide Delivery across the Caco-2 Monolayer: The Design and Evaluation of Self-Emulsifying Drug Delivery Systems (SEDDS)" Pharmaceutics 13, no. 4: 459. https://doi.org/10.3390/pharmaceutics13040459
APA StyleKubackova, J., Holas, O., Zbytovska, J., Vranikova, B., Zeng, G., Pavek, P., & Mullertz, A. (2021). Oligonucleotide Delivery across the Caco-2 Monolayer: The Design and Evaluation of Self-Emulsifying Drug Delivery Systems (SEDDS). Pharmaceutics, 13(4), 459. https://doi.org/10.3390/pharmaceutics13040459









