1. Introduction
Ophthalmic drug delivery continues to pose challenges to formulation scientists due to the complex biochemical, anatomical, and physiological ocular barriers. The biological membranes that protects the anterior and posterior segments of the eye, in addition to the unique structure of the cornea, result in poor ocular bioavailability [
1]. Topical drug administration to the anterior segment of the eye causes large pre-corneal clearance because of their high tear turn over (0.5–2.2 μL/min) and rapid blinking rate (6–15 times/min) [
2]. Consequently, conventional ophthalmic dosage forms must be applied with frequent instillations to attain and/or control targeted drug levels within the anterior segment of the eye. Delivering the drug molecules to the posterior segment of the eye is challenging primarily due to the long diffusion pathway and the cellular characteristics of the vitreous humor [
3]. Therefore, alternative techniques are utilized to transport drugs to the posterior vitreous, the uveal tract, retina, or choroid [
4]. Endophthalmitis is a severe intraocular inflammatory infection that affects the vitreous and aqueous fluids within the anterior and posterior region of eye [
5]. It is the infection generally caused due to organisms such as bacteria, fungi, or parasites that enter the eye through the blood stream, surgery in the eye or other parts near the eye, or sepsis [
6]. A literature review indicated that few drug delivery systems have been developed and their efficacy evaluated in animals. In one attempt, the therapeutic effect demonstrated by an intravitreal drug delivery system of voriconazole was superior to intravitreal injection in rabbits [
7]. Chitosan nanoparticles containing daptomycin were developed in ocular treatment of bacterial endophthalmitis. It was reported that these nanoparticles had appropriate characteristics to recommend as a non-invasive method for the ocular delivery of daptomycin to the eye [
8].
Clarithromycin is a broad spectrum macrolide antibiotic and shows potential action against various organisms, including
Staphylococcus aureus,
Streptococcus pneumoniae,
Legionella pneumophila,
Moraxella catarrhalis,
Chlamydia trachomatis, and
Mycobacterium avium [
9]. Clarithromycin is chemically 6-
O-methylerythromycin (C
38H
69NO
13), with a molecular weight of 747.95 Dalton, pKa value of 8.99, and melting point ranges between 217 and 220 °C [
10]. Different solubility studies demonstrated that clarithromycin is soluble in acetone, slightly soluble in methanol, ethanol, and acetonitrile, and practically insoluble in water (0.33 mg/L). Lipophilicity is a major determining factor in a compound’s absorption, distribution in the body, penetration across vital membranes and biological barriers, metabolism, and excretion. The pharmacokinetics of clarithromycin in adults showed average oral bioavailability of 53%, plasma concentration between 2.41 and 2.85 µg/L after 500 mg dose, and a half-life of ~4 h [
10,
11]. Pharmacokinetic and pharmacodynamic investigations suggest that clarithromycin exhibits a similar interaction profile as that of erythromycin [
12]. Lipid-based drug delivery system have the potential capacity to entrap both lipophobic and lipophilic drugs, enhance the bioavailability of low aqueous soluble drugs, and protect them against degradation. In the last few decades, liquid nanoemulsions have been increasingly used as drug carriers for lipophilic drugs [
13]. However, the feasibility of controlled drug release from nanoemulsions is restricted due to the submicron globule size and the fluid state of the carrier. Alternatively, solid matrices of nanostructured lipid carriers (NLCs) and solid lipid nanoparticles (SLNs) help to improve the stability and safety and provide controlled drug release, and they can incorporate both hydrophilic and hydrophobic molecules and can be used in various routes [
14]. The basic difference between NLCs and SLNs is the type of lipids used, where liquid lipids are used in NLCs and solid lipids in SLNs [
14].
SLNs are nano-sized (10–1000 nm) particles formed when the solid lipids are dispersed in aqueous media containing surfactants as stabilizers [
15]. They widely accepted as a prospective delivery system similar to other colloidal carriers, including liposomes and other polymeric nanoparticles [
16,
17]. Due to the inclusion of physiologically compatible lipids of either natural or synthetic origin, nonirritant and nontoxic properties in SLNs reduce the potential threats of acute or chronic toxicity [
15]. Furthermore, advantages such as sustained and controlled drug release, good physical stability, resistance to degradation of lipids, in vivo acceptability, and ability to increase pre-corneal retention time make SLNs a very adaptive carrier for various drug delivery systems [
18]. Indeed, formulations of SLNs can be performed in the absence of organic solvents, can be sterilized, and can also use excipients that are “generally recognized as safe” (GRAS), and hence can be easily scaled-up in industry [
19]. The incorporation of lipids in the solid state compared to the liquid form is very effective to obtain controlled drug release, since drug mobility is significantly retarded in solid lipid when compared to liquid oil. Extensive research has been carried in the last two decades to tap the potential of SLNs in delivering drugs through various routes, including the ocular route [
19,
20,
21,
22]. The literature suggests that SLNs have been widely studied in the treatment of ocular inflammations, infections, glaucoma, cataracts, age related macular degeneration, and as gene therapy carriers [
23]. In light of this, the objective of the present study was to design, formulate, and evaluate the potential of drug-loaded SLNs to improve the therapeutic efficacy of clarithromycin in ocular therapy. Fractional factorial design was applied for preliminary screening of various factors utilized in the SLN formulation. A 3
2 full factorial design was employed to evaluate the influence of independent variables on the dependent variables. Selected SLN were assessed for drug permeation using goat cornea and evaluated in vivo in rabbits.
2. Materials and Methods
2.1. Materials
Clarithromycin was provided by Century Pharmaceuticals Ltd., Vadodara, India. Stearic acid, glyceryl monostearate, Tween 20, Tween 80, Span 80, sodium lauryl sulphate, polyethylene glycol 400 (PEG 400), and propylene glycol were commercially procured from Central Drug House, New Delhi, India. Compritol 888ATO, Gelucire 43/01, Precirol ATO5, Geleol, and Transcutol P were procured from Gattefosse India, Mumbai, India. Monegyl T18 and Softemul® were purchased from Mohini Organics, Mumbai, India. Stearylamine was obtained from Hi-Media, Mumbai, India.
2.2. High Performance Liquid Chromatography (HPLC)
Estimation of clarithromycin was done using a Jasco HPLC system (LC–4000, Easton, MD, USA) containing a degasser unit, pump, an automatic injector, as well as an MD–4010 UV–Visible detector and a Phenomenex C-18 column (150 × 4.6 mm, i.d 5 μm). Chromatographic separation of clarithromycin was performed with the help of a mobile phase consisting of acetonitrile: potassium dihydrogen ortho-phosphate (0.02 mM) 40:60
v/
v, adjusted to pH 5.5 with phosphoric acid [
24]. The temperature in the HPLC column was set at 40 °C, and an isocratic elution of clarithromycin was achieved by allowing the solvent to flow at a fixed rate (1 mL/min) and was monitored at 210 nm. Regression analysis indicated good linearity when clarithromycin concentration was 30–400 ng/mL (r
2 = 0.990).
2.3. Solubility of Drug in Lipids and Surfactants
Preliminary screenings were carried out to evaluate the solubility of clarithromycin in various solid lipids and surfactants according to a method described in the literature [
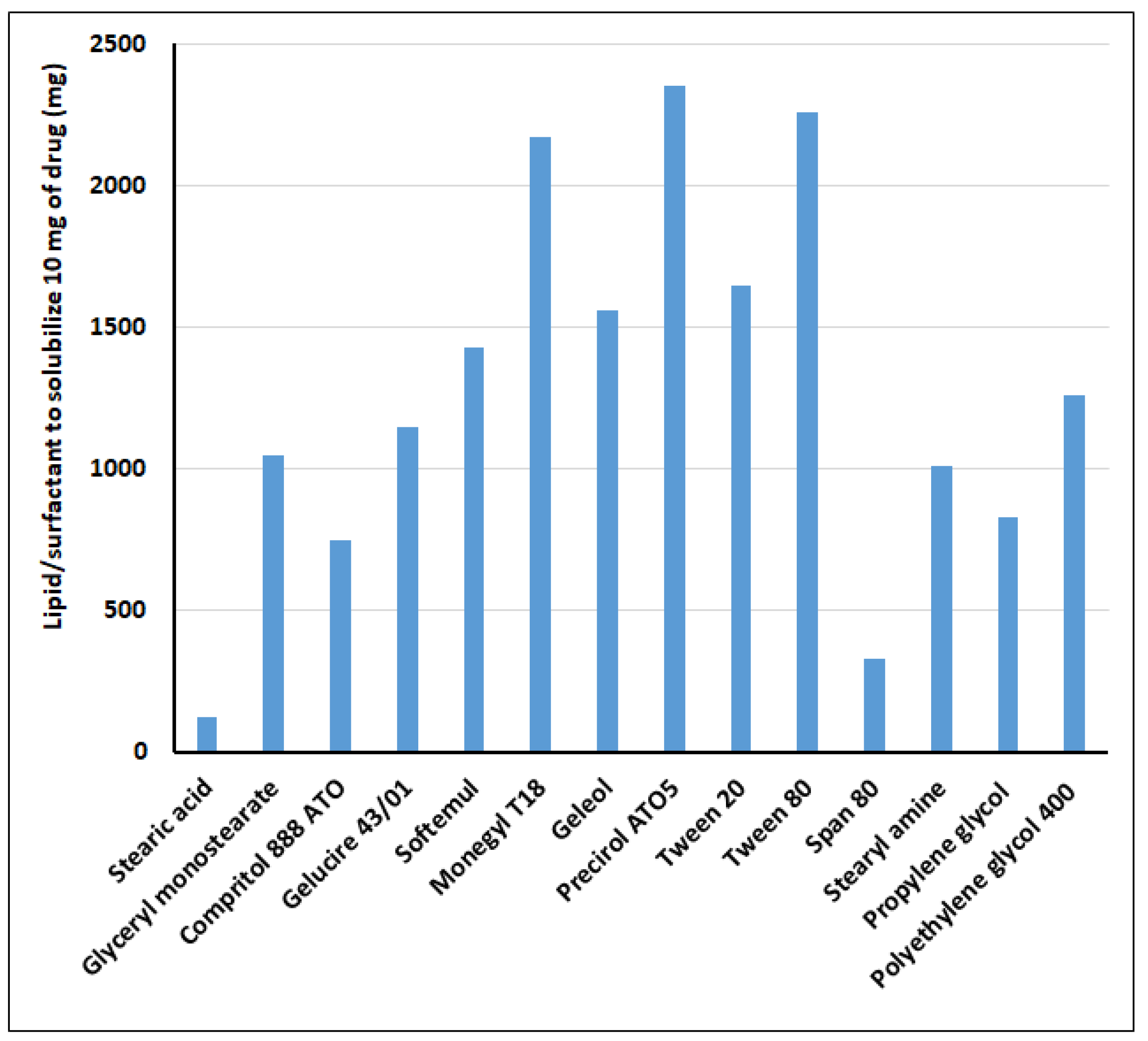
25]. Briefly, a weighed quantity (10 mg) of lipid or surfactant was taken in glass vials and melted above its melting point on a water bath (EIE Instruments, Ahmedabad, India). To the melted solution of lipids or surfactant, 10 mg clarithromycin was added and subsequently vortexed. Then, additional increments of lipid or surfactant were added until the drug was completely solubilized or form a homogenous mixture. The lipid in which clarithromycin showed maximum solubility, and the surfactant where it exhibited minimum solubility were selected for the development of an SLN formulation.
2.4. Formulation of SLN Using Fractional Factorial Screening Design
SLNs containing clarithromycin, lipid, surfactant, and cosurfactant were formulated by high-speed stirring and the ultra-sonication method described in the literature [
26]. High speed mixing generally produces an emulsion, which on cooling forms SLNs. Based on the solubility study of drug in lipid, the amounts of lipid used for the screening design were 150 mg and 200 mg, and the concentrations of surfactants selected were 1 and 3%. Preliminary studies were carried out using different surfactant ratios (surfactant to co-surfactant; 1:9, 3:7, 5:5, 7:3, and 9:1) and evaluated for percent entrapment efficiency and percent drug loading. The selection of the surfactant ratio was based on the target set for percent entrapment efficiency (>85%) and for percent drug loading (>30%).
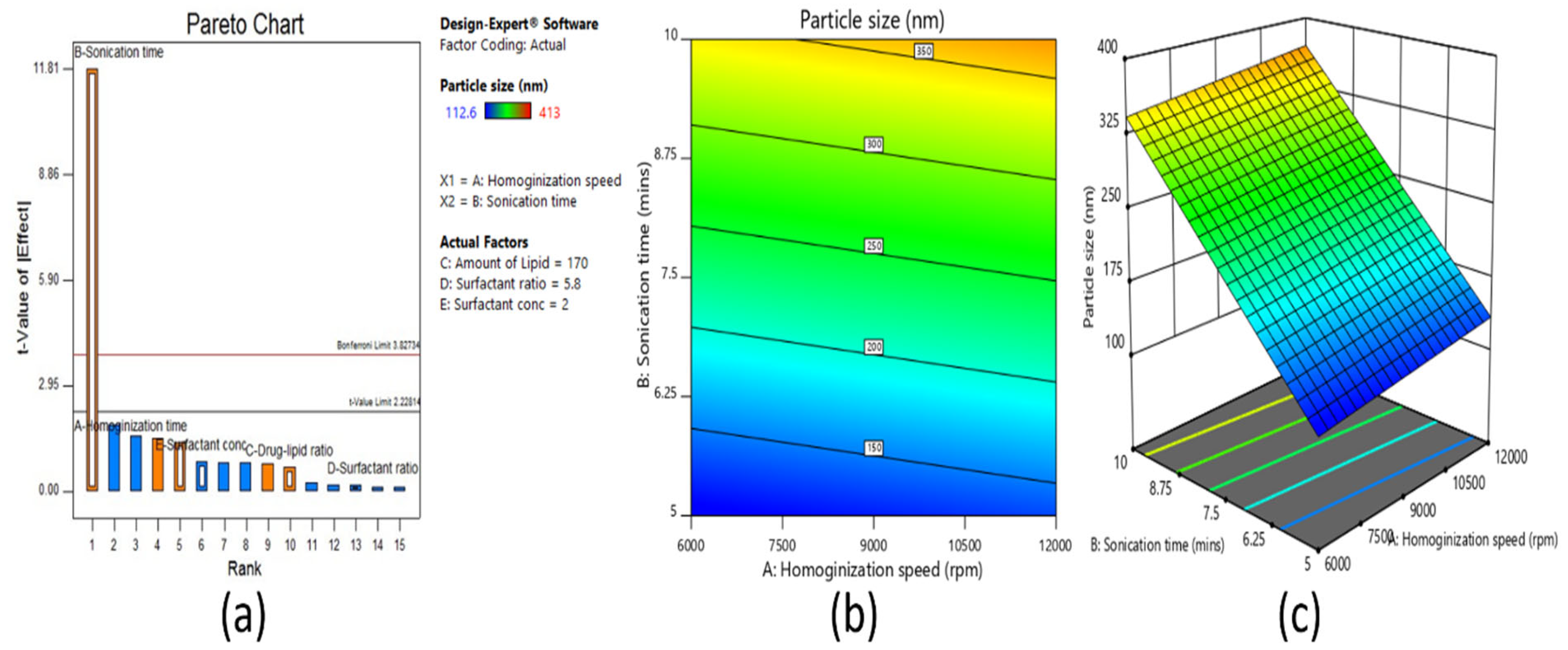
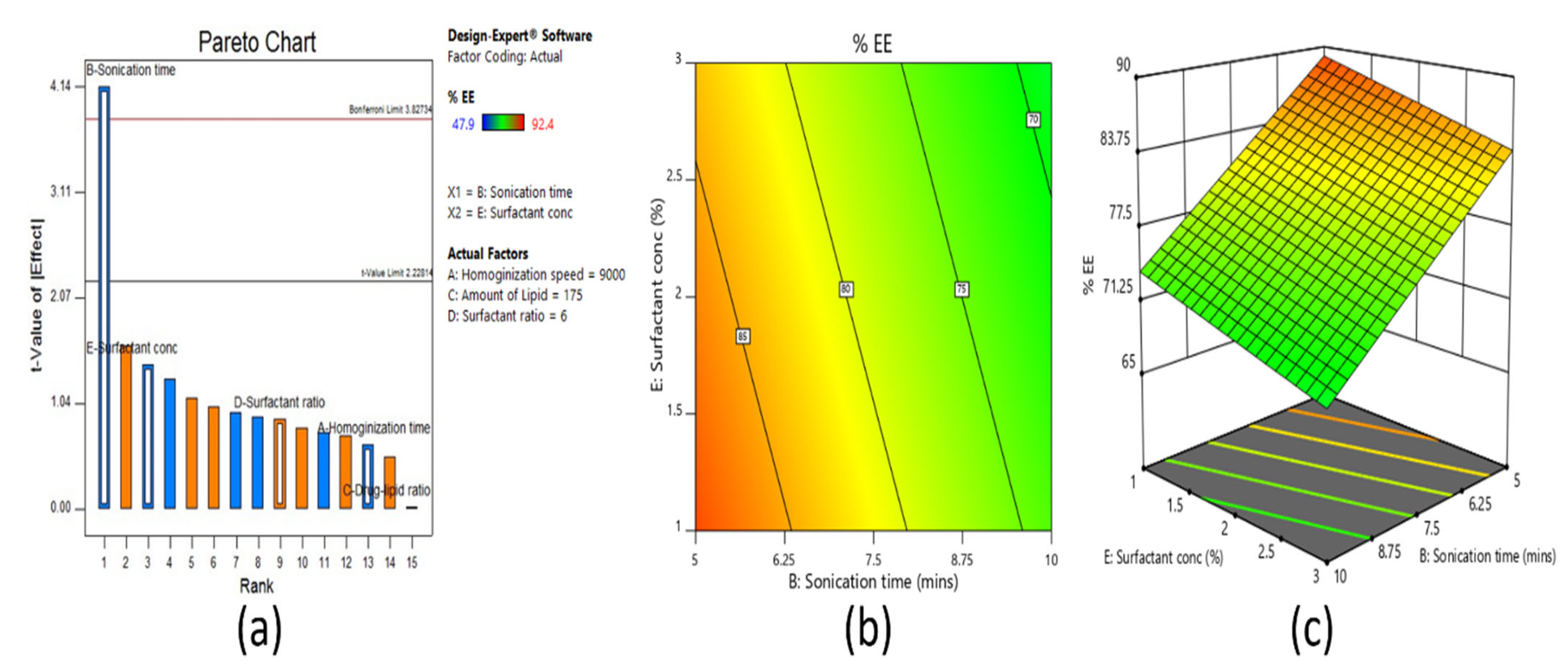
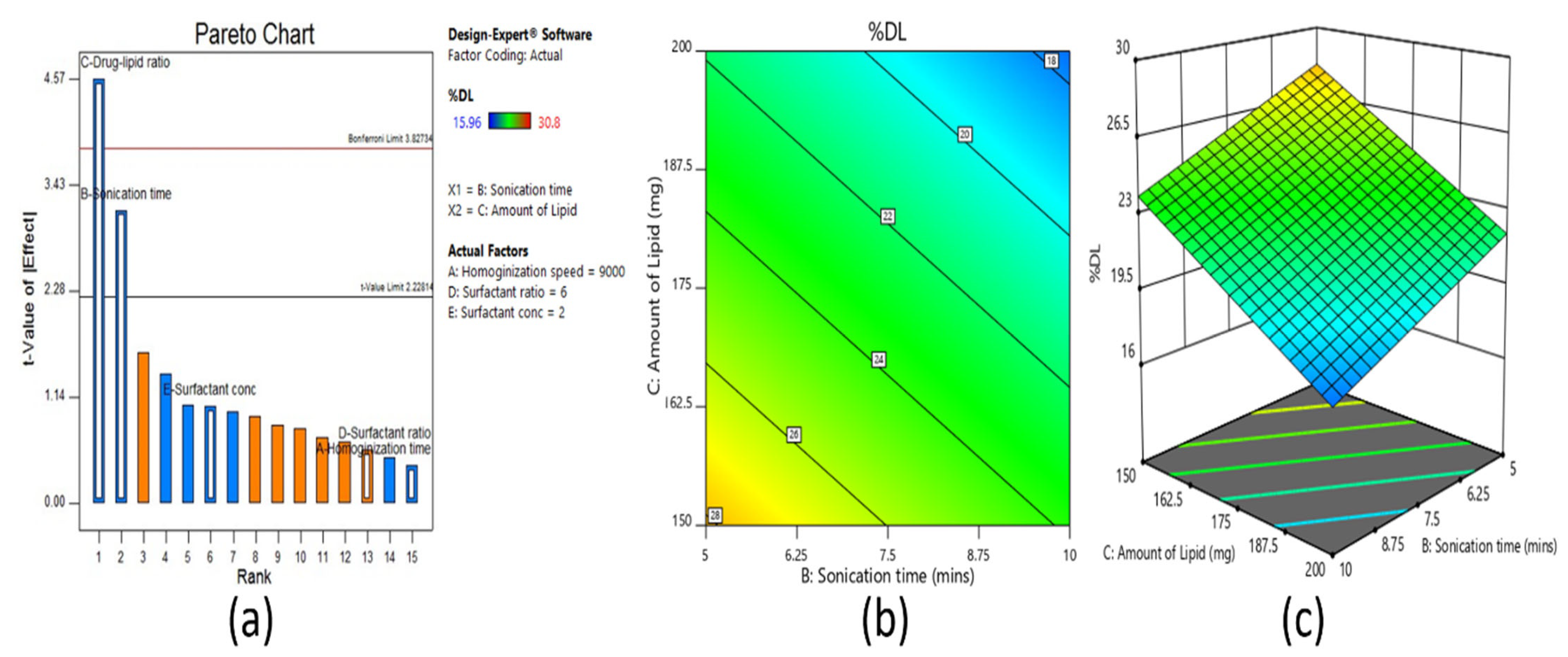
Screening designs were used to screen the main significant effects from various potential factors that can affect the formulation. Fractional factorial design is one type of screening design. It was applied for screening of all the selected factors utilized for the development of a clarithromycin-loaded SLN formulation. Based on the number of factors, the total number of experimental runs was calculated as sixteen by the equation, 2
n−1, where
n = number of factors. Selected independent variables included for the screening method were process-related factors, such as homogenization speed and sonication time, in addition to formulation variables like amount of lipid, surfactant concentration, and surfactant ratio. The dependent variables investigated were particle size (R
1), entrapment efficiency (R
2), and drug loading (R
3). The coded and actual values of independent variables for fractional factorial screening design are shown in
Table 1.
In brief, an accurately weighed quantity of stearic acid and clarithromycin were solubilized in ethanol by heating on a water bath at 65–70 °C, while surfactant and co-surfactant were dissolved in aqueous phase. SLNs were obtained by slowly adding the molten organic phase drop wise into the aqueous phase under continuous stirring using a homogenizer (Remi Motors, Mumbai, India) set at respective speeds for screening design batches, as shown in
Table 2. The precipitated SLN-containing dispersions were subjected to further mixing in cold water using a probe sonicator (Brookfield Engineering Laboratories, MA, USA). The SLNs formed were washed with distilled water and filtered.
Table 2 shows the coded and actual values for 16 batches of fractional factorial design.
2.5. Evaluation of Screening Design Batches
2.5.1. Particle Size Characterization and Zeta Potential
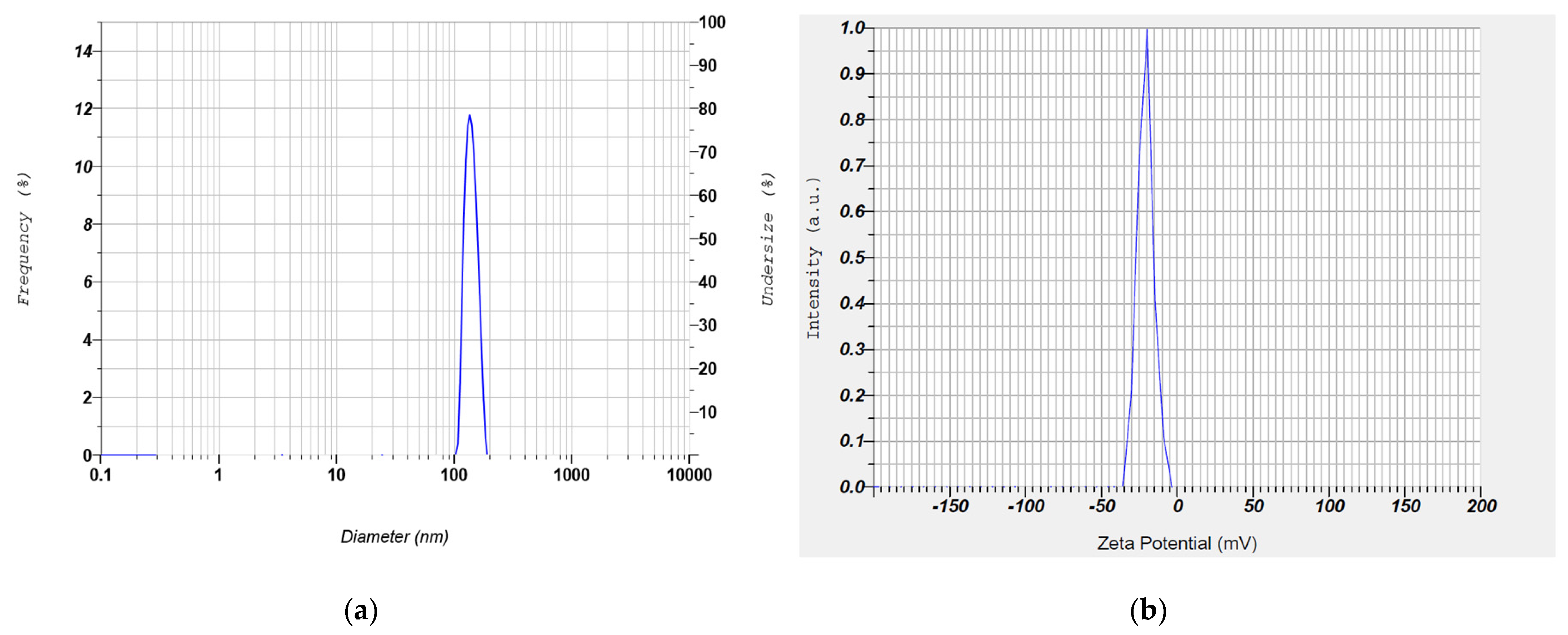
Particle size analysis, polydispersity index, and zeta potential of the clarithromycin-loaded SLN were determined using a zetasizer (Nanopartica SZ-100, Horiba, Kyoto, Japan). To perform the test, drops (2–3) of test samples placed in the disposable cuvette were exposed to the laser beam. The intensity and physical characteristics of scattered light were measured with the detector, and the particle sizes were subsequently determined [
27]. The electrophoretic mobility values were measured after suitable dilution with water at 25 °C.
2.5.2. Percent Drug Entrapment Efficiency and Percent Drug Loading
Formulations were filled in vials and centrifuged at 15,000 rpm using an ultracentrifuge (Remi Motors, Mumbai, India) for 25 min to separate the supernatant and pellets. The pellets were dissolved in methanol, and the drug contents were measured using HPLC. Entrapment efficiency and drug loading were calculated using the formula described in the literature [
28].
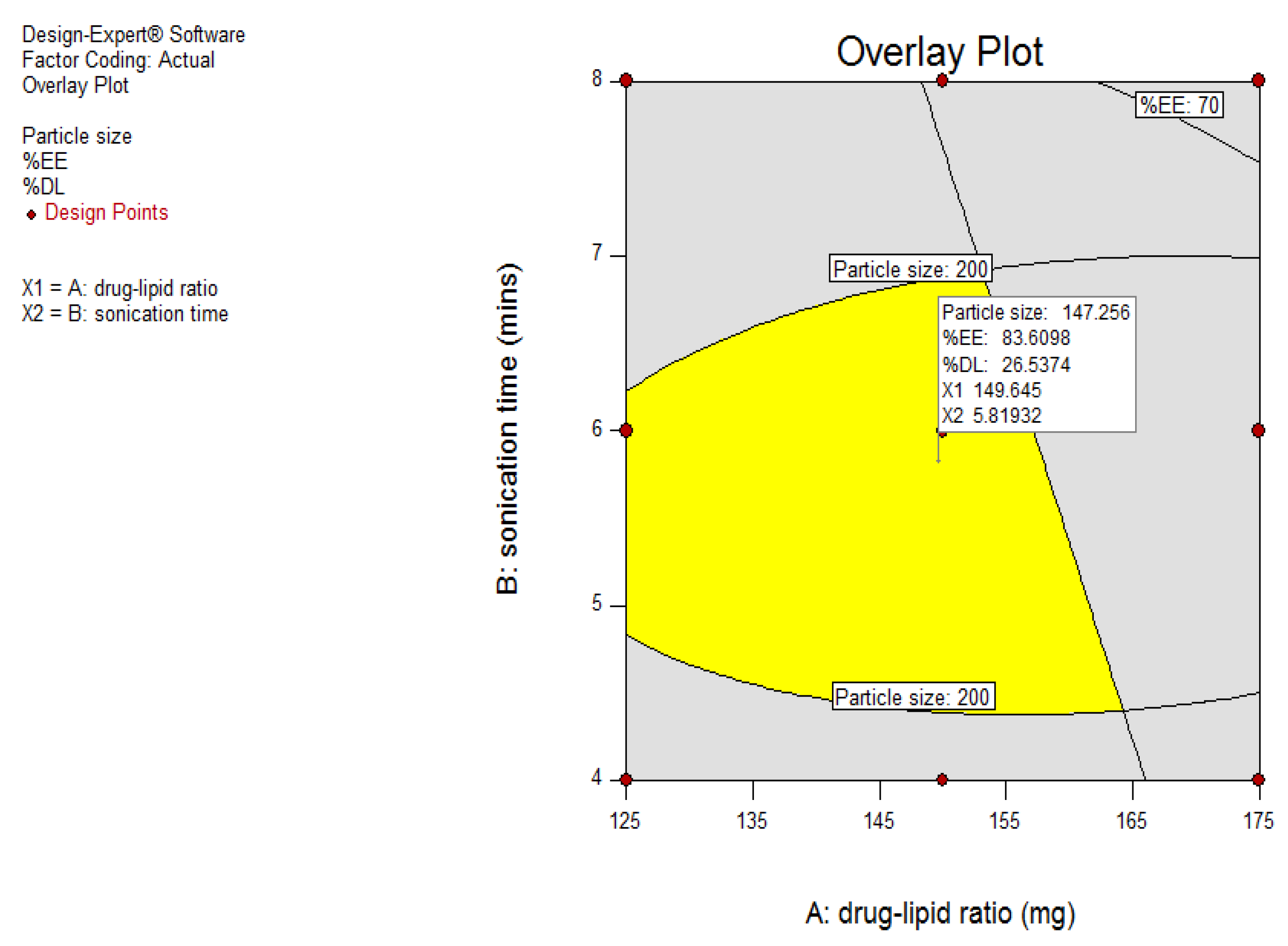
2.6. Optimization Design
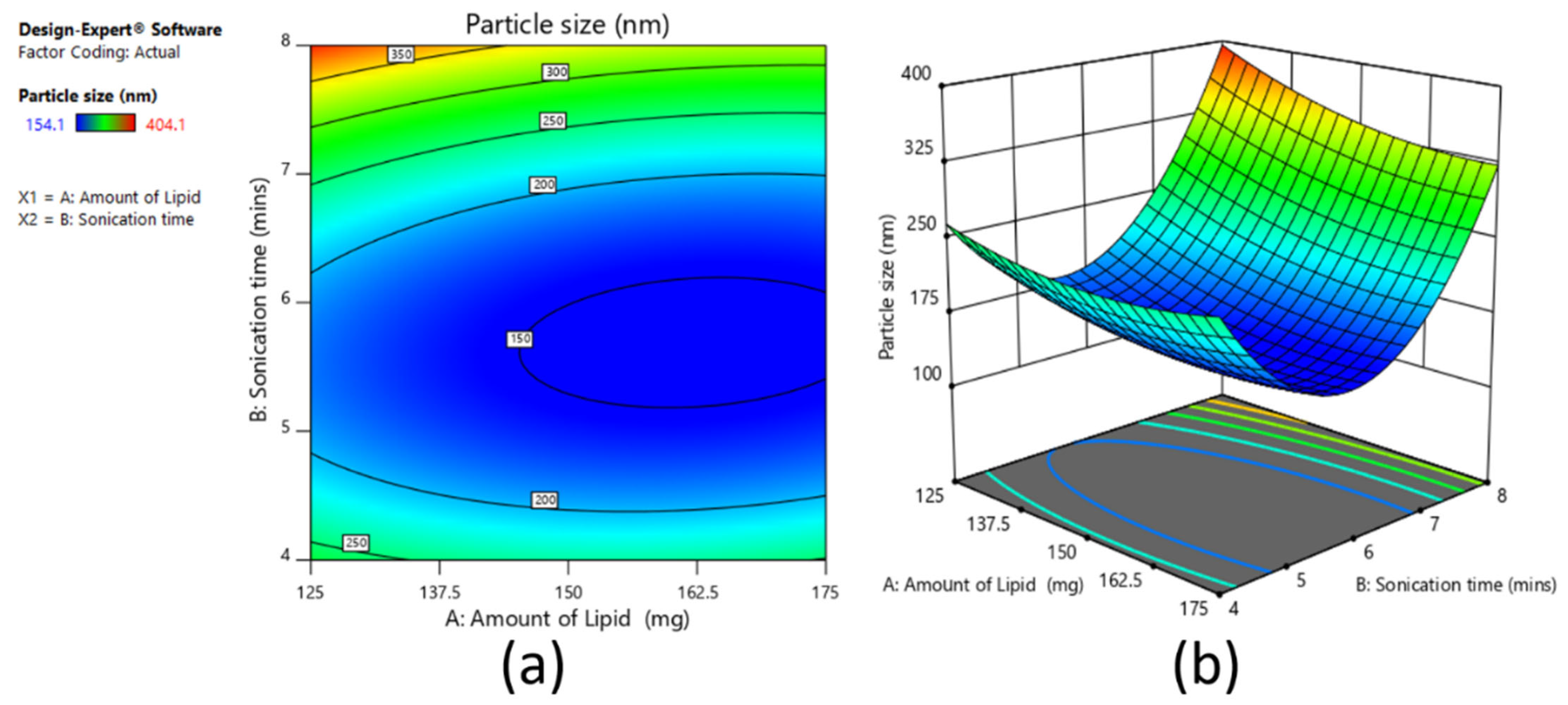
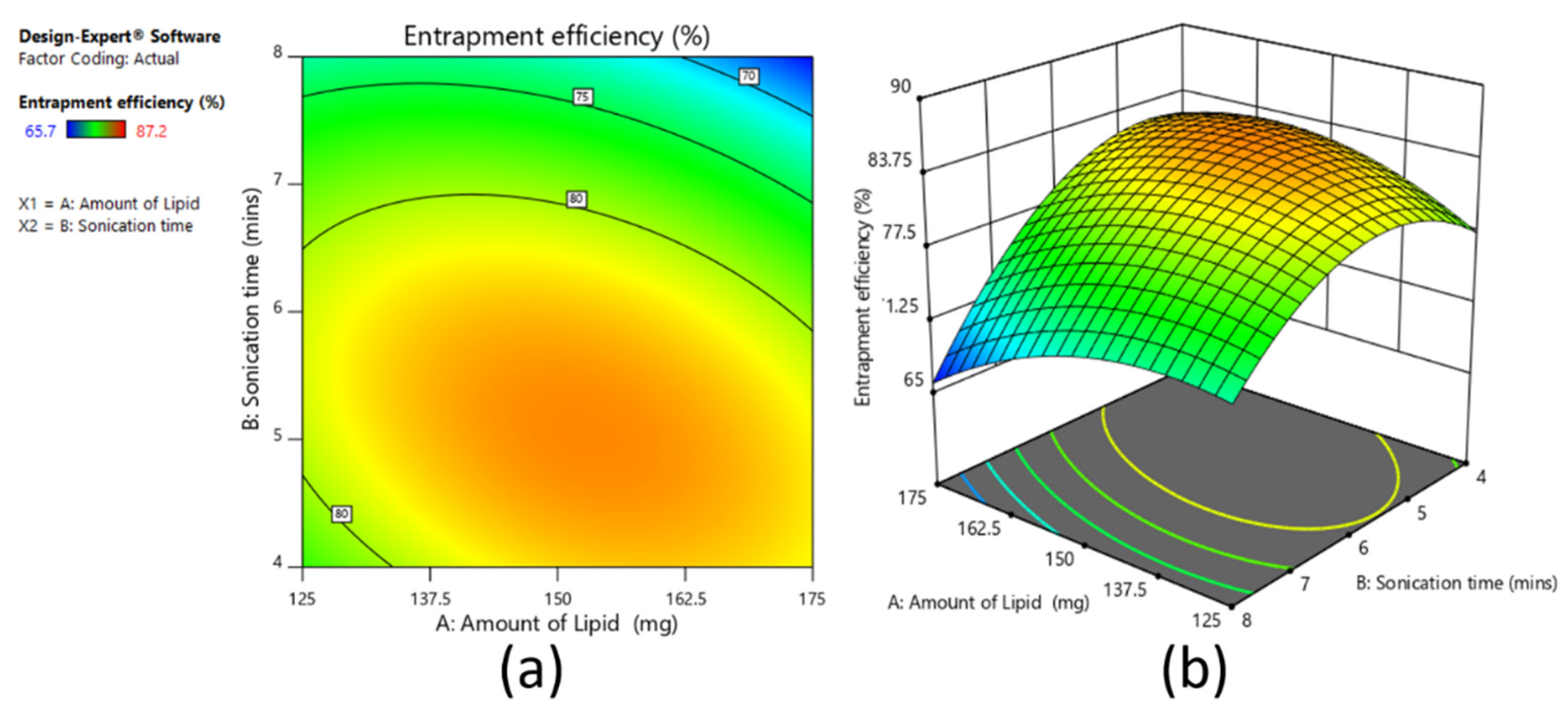
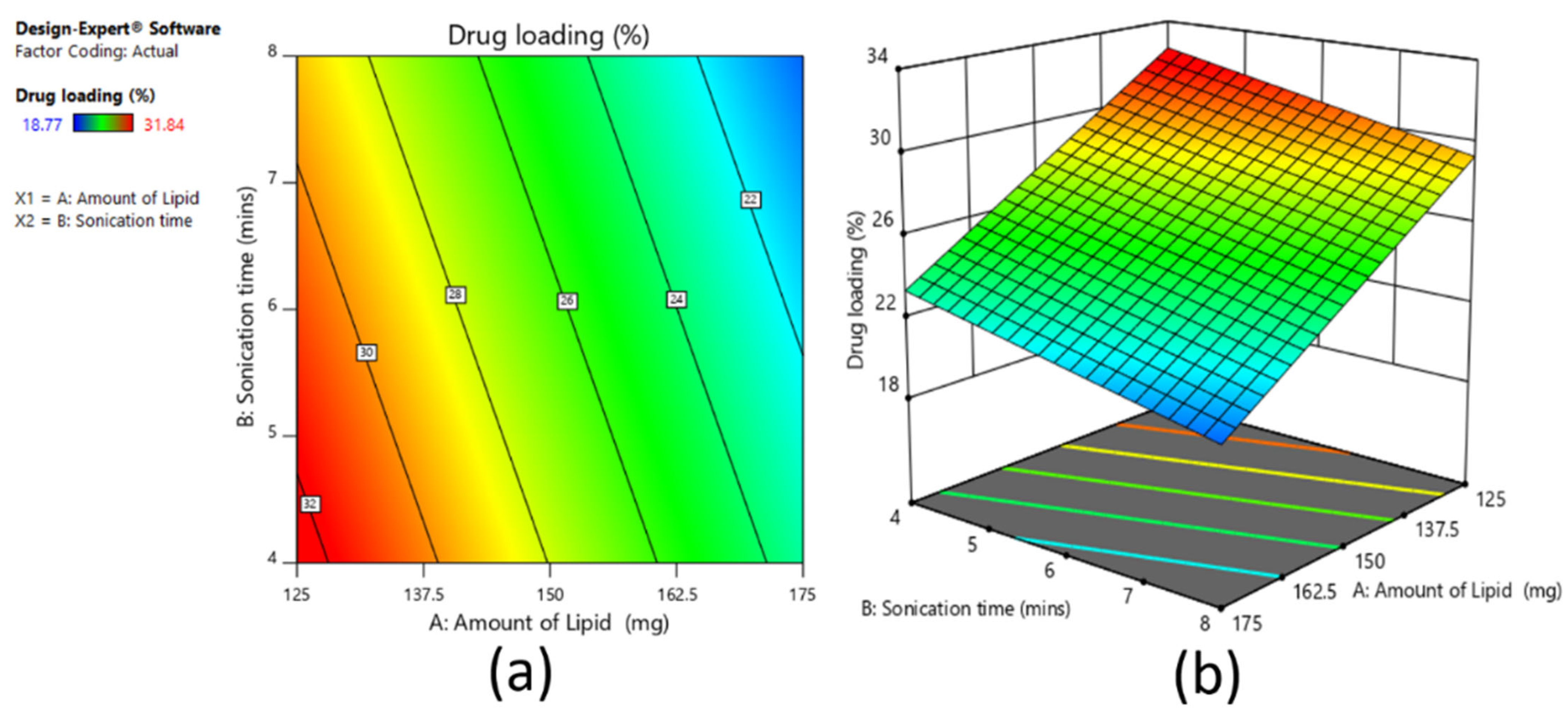
A 3
2 full factorial design was used to assess the influence of independent variables on the dependent variables for the prepared clarithromycin SLNs. Based on the fractional factorial design data, the two factors of amount of lipid (mg) (X1) and sonication time (min) (X2) showed substantial effects on the particle size, drug entrapment efficiency, and drug loading, and hence were applied at 3 levels. In addition, the other three factors, namely homogenization speed, surfactant ratio, and surfactant concentration, were kept constant at 9000 rpm, 5:5, and 3%, respectively, during the experimental run of nine experiments. The dependent variables were Y
1: particle size; Y
2: percent entrapment efficiency; and Y
3: percent drug loading.
Table 3 shows coded and actual values of the independent variables of the 3
2 full factorial design.
The responses Y
1, Y
2, and Y
3 were evaluated using the equation Y = b
0 + b
1X
1 + b
2X
2 + b
12 X
1X
2 + b
11X
11 + b
22X
22. This equation represents the main factors (X
1 and X
2), interaction factors (X
1X
2), and polynomial factors (X
11 and X
22). The b
0 is the mathematic mean value of total runs, and b
1 and b
2 are the projected coefficients for the factors X
1 and X
2, respectively. For validation of selected statistical design, polynomial equations were generated using Design Expert software, and one-way analysis of variance (ANOVA) was tested. With the help of response surface plots, the correlation between variables and response parameters was established. The method of preparation was the same as per the screening design batches.
Table 4 shows the coded and actual values for 9 batches of the 3
2 full factorial design.
2.7. Evaluation of Design Batches of SLN
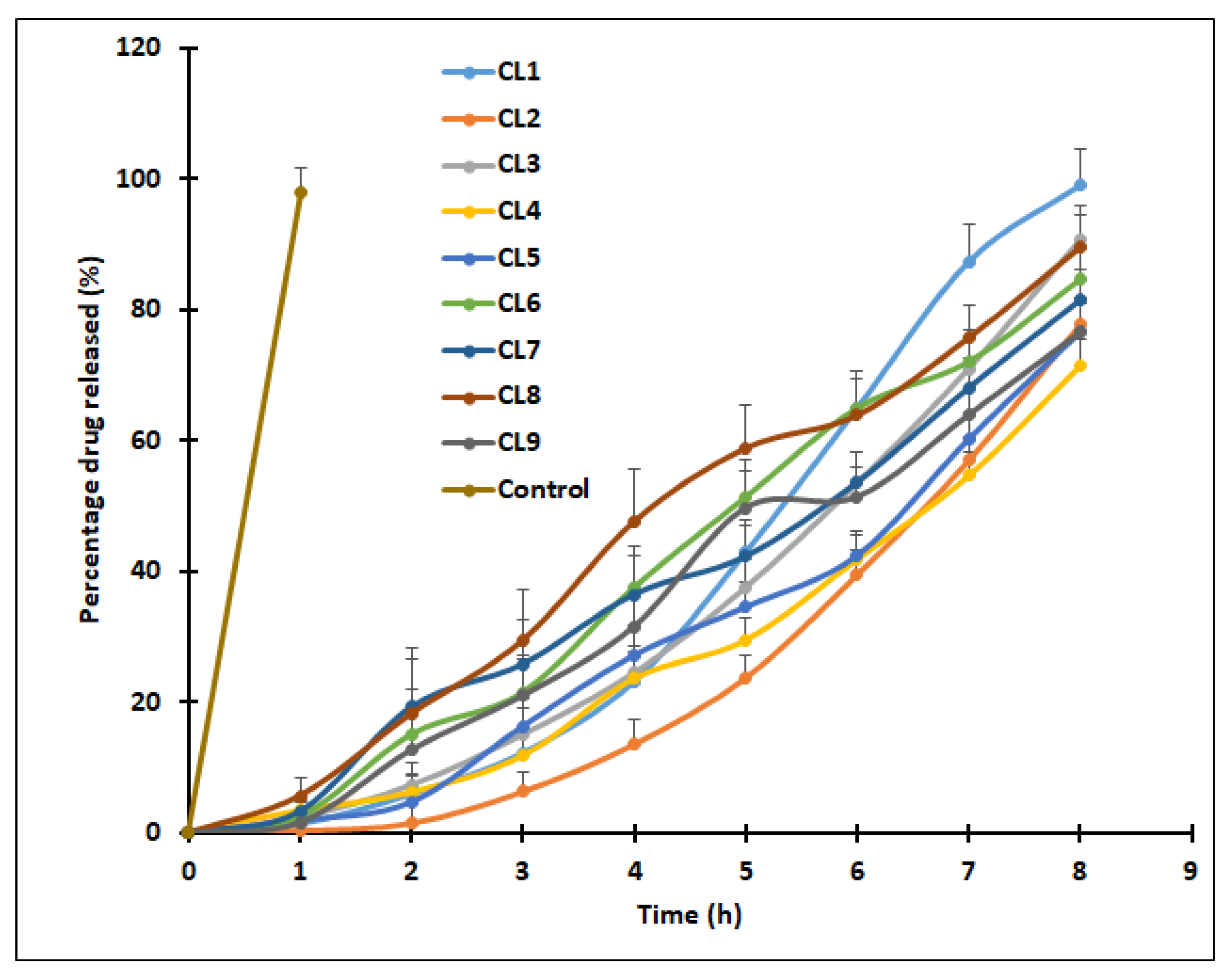
2.7.1. Drug Release
A Franz diffusion cell (Logan Instruments Ltd., Somerset, NJ, USA) having an exposed surface area of 0.79 cm
2, was used to carry out in vitro drug release. In short, a semipermeable membrane (MWCO 12–14 kDa) was held between the donor and receptor compartment. Clarithromycin-loaded SLN dispersion (CL1–CL9, 2.5 mg/mL of drug) or control (drug solution in 10%
v/
v span 80) was added in the upper compartment. The acceptor compartment (20 mL) contained simulated tear fluid (pH 7.4) with 1% Tween 80 (to maintain sink conditions) [
29] and was stirred at 50 rpm. The temperature of the entire assembly was controlled at 37 ± 0.5 °C by means of a thermostatic water bath. At various time intervals, aliquots of samples (1 mL) were drawn and substituted with the equivalent amount of buffer held at the same temperature. A control experiment was performed at the same experimental conditions using a similar strength of clarithromycin solution. The samples were later diluted with mobile phase and quantified for clarithromycin. The data collected were analyzed to calculate regression coefficient (r
2) and interpret various mathematical models [
30].
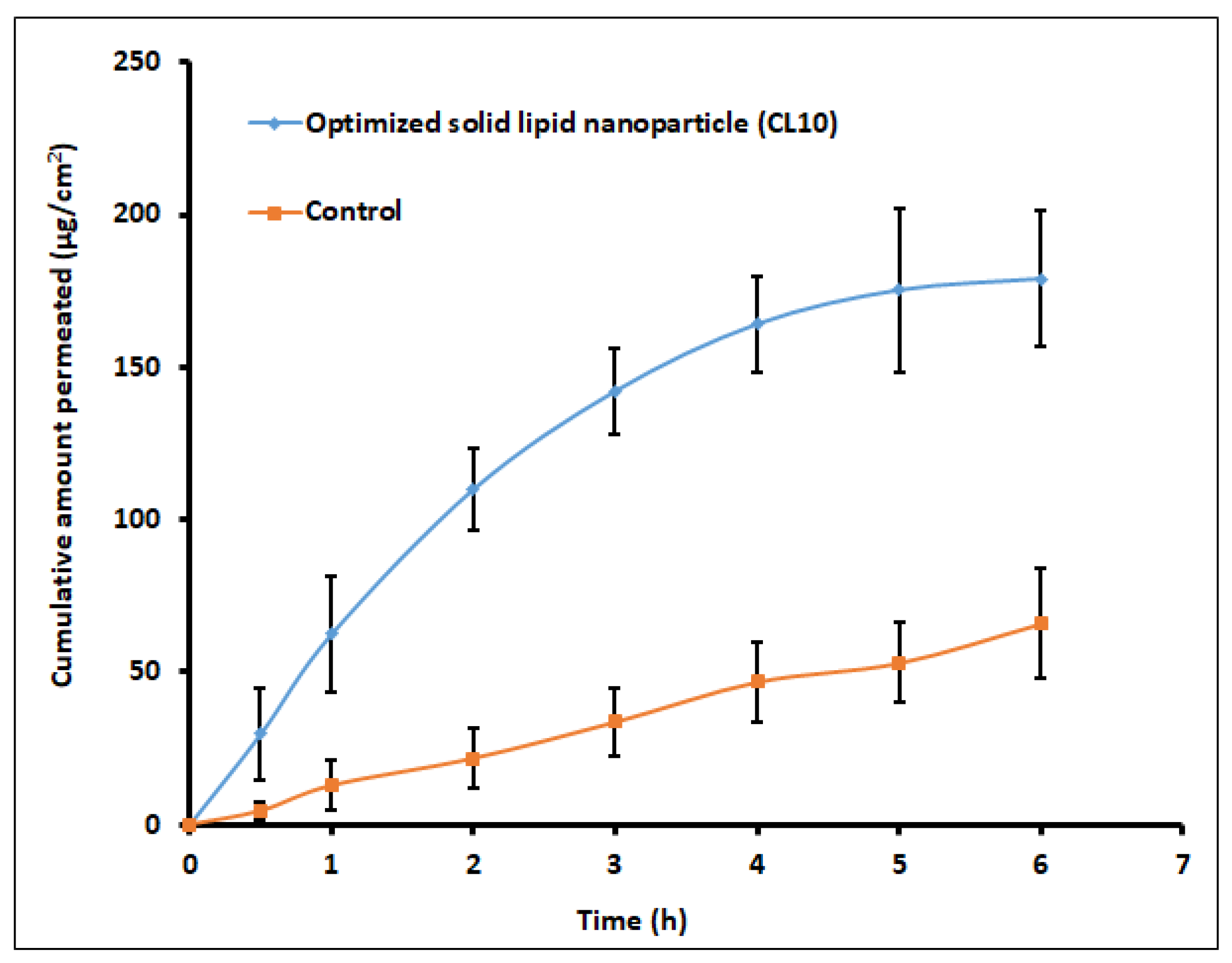
2.7.2. Trans-Corneal Permeation
Ex vivo permeation experiments were performed utilizing the vertical Franz diffusion cell using an isolated goat corneal membrane obtained from a local abattoir (Ahmedabad, India) held between the donor and receptor cell [
31]. Testing were done using either optimized formulation (CL10) or control (0.25% drug solution equivalent to 2.5 mg of clarithromycin/mL). The receptor compartment was filled with simulated tear fluid (pH 7.4) with 1% Tween 80 (to maintain sink conditions), and temperature was set at 37 ± 0.5 °C. Suitable volumes of the samples were taken at specific time intervals and similar volumes of fluids at the same temperature. The samples withdrawn from the receptor compartment were appropriately diluted and estimated for clarithromycin by HPLC. The flux was determined as described in the literature [
32].
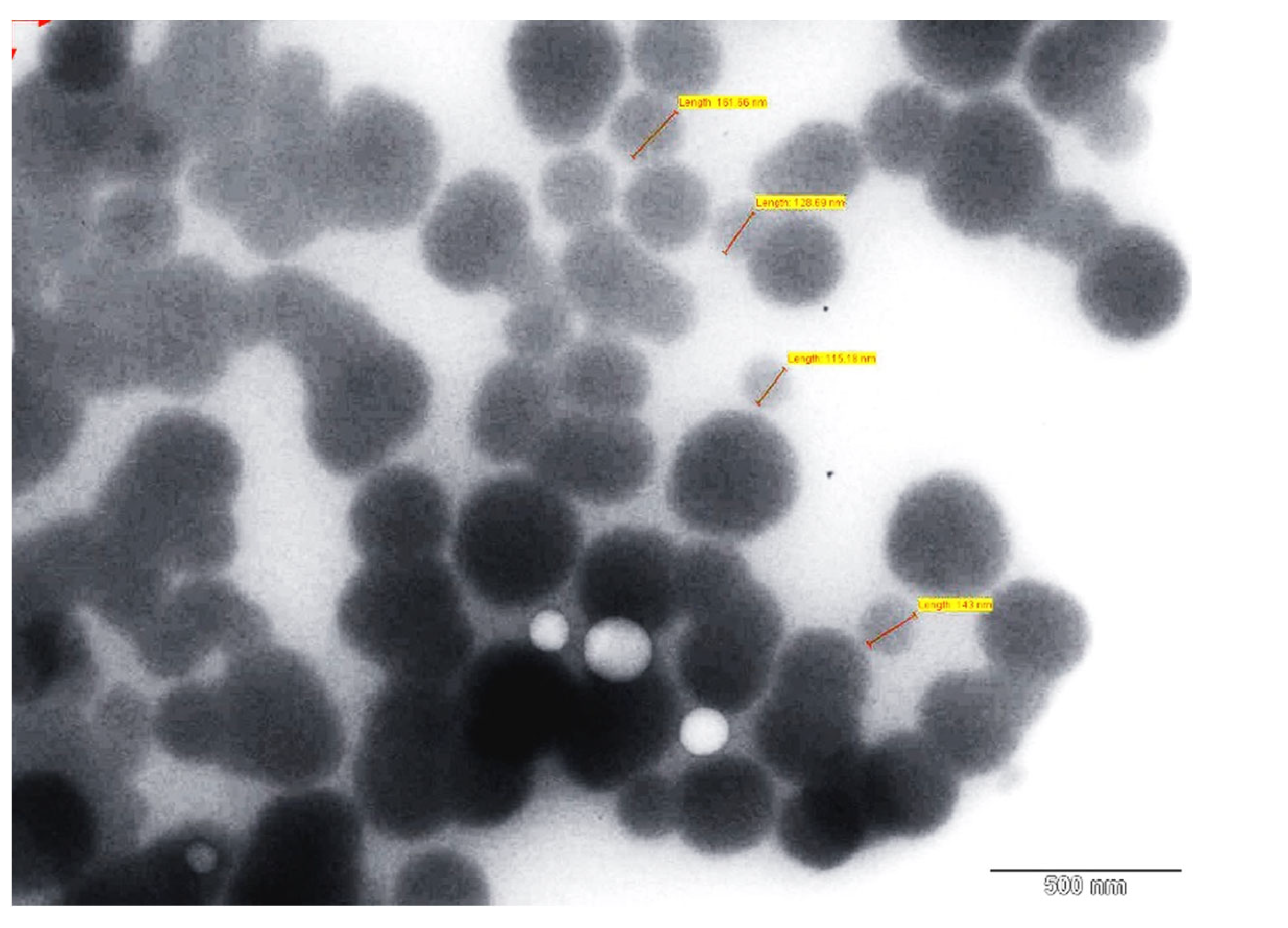
2.7.3. Transmission Electron Microscopy (TEM)
TEM (Holland Technai 20, Phillips, Amsterdam, The Netherlands) with bright field imaging and magnification technique was employed to examine the physical characteristics of SLN, such as particle size, shape, and surface morphology. A drop of the SLN dispersion was allowed to fix to the surface of a carbon coated copper grid, and the excess of dispersion was then wiped off using the tip of a filter paper. A drop of negative staining solution, 1% (
w/
v) phosphotungstic acid, was applied to the affixed SLNs, and extra stain was removed with a tissue paper [
33]. The films were formed on grids by air drying at room temperature, and images were captured at 80 kV by TEM.
2.8. Ocular Compatibility
The biocompatibility of the optimized formulation (CL10) was performed in six albino rabbits (2–3 kg). The experiments were performed in accordance with the guidelines stated by the Committee for the Purpose of Control and Supervision of Experiments on Animals (approval number IP/PCEU/FAC/21/029, dated; 9 June 2017). Eye irritation score was measured according to the guidelines based on the Draize technique [
34]. Single topical instillation of 50 µL of product (CL10) was applied on the left eye of each rabbit, whereas the same volume of physiological saline (reference) was applied on the right eye. The sterile formulation was tested two times a day for 21 days. The cornea, iris, and conjunctiva of rabbits were frequently observed during the study period. Similarly, signs of sensitivity reactions, particularly redness, swelling, cloudiness, edema, hemorrhage, discharge, and blindness, were checked [
35].
2.9. Pharmacokinetics
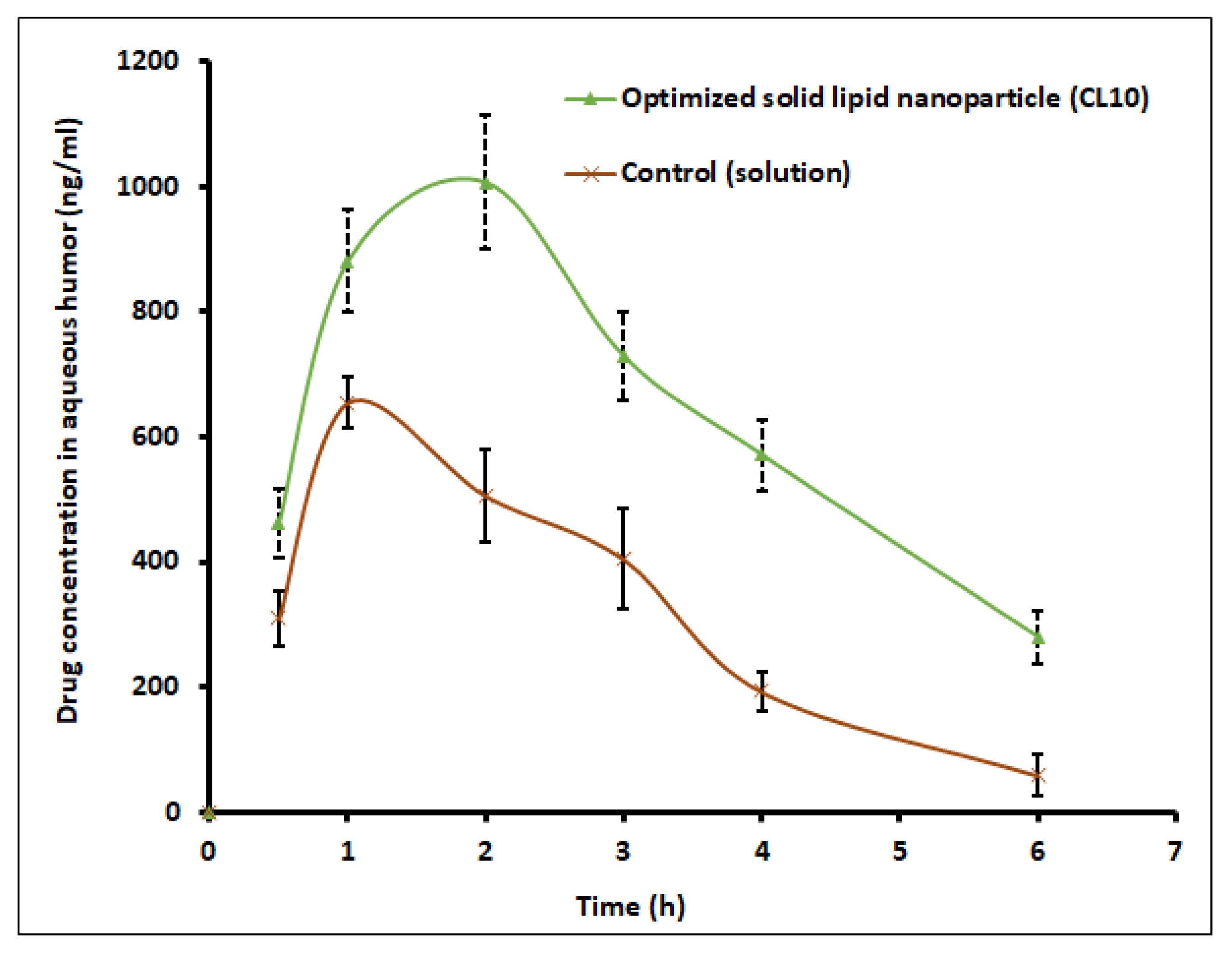
The amount of clarithromycin diffused into the aqueous humor of the rabbit eyes after ophthalmic administration was determined to compare the ocular bioavailability between CL10 and control (solution). In vivo pharmacokinetic investigations were performed in New Zealand Albino rabbits (2–3 kg) with two groups (
n = 6). The experiments were carried out by strictly following the guidelines for animal care at Nirma University (IP/PCEU/FAC/21/029; dated 9 June 2017). Single topical instillation (60 μL of 0.25%
w/
v drug) of SLN was dropped in one eye of individual rabbit in the first group, while the second eye remained untreated. A similar strength and volume of control solution was instilled into the second group of rabbits. Both eyelids of all rabbits were lightly closed for 2 min to increase the contact of drug with the corneal membrane. Before aqueous humor withdrawal, individual animals were anaesthetized by intramuscular administration of xylazine and ketamine [
36]. The aqueous humor was collected using 29-gauge insulin syringe needle [
37], and 20 μL of sample was mixed with acetonitrile and centrifuged, and the organic layer was assessed for clarithromycin content by HPLC.
2.10. Stability
An accurately weighed quantity of optimized SLN (CL10) was sealed separately in light resistant glass vials at ambient temperature (25 ± 1 °C) and under refrigerated conditions (4 ± 0.5 °C) up to 3 months [
38]. The stability of SLN was examined by assessing the drug content, particle size, and drug release during the storage period.
2.11. Statistical Analysis
The software used for Statistical data analysis was GraphPad Prism software (version 6, GraphPad, San Diego, CA, USA). The difference in values at p < 0.05 was considered statistically significant.
4. Conclusions
The solubility of clarithromycin in various lipid and surfactants was studied, and based on solubility criteria, stearic acid (lipid), Tween 80 (nonionic surfactant), and Transcutol P (cosurfactant) were selected for the preparation of SLNs. Initial fractional factorial design suggests that the sonication time and amount of lipid significantly influence the SLN formulation. Further, 32 full factorial design data also confirms that both the factors significantly affect the particle size, drug entrapment efficiency, and drug loading. The drug content and entrapment efficiency of SLNs were enhanced by increasing the concentration of lipid since higher hydrophobicity of the longer chain of stearic acid resulted in a high-loaded clarithromycin. Ex vivo permeation and in vivo pharmacokinetics data signify greater efficacy by the optimized clarithromycin-loaded SLN (CL10). Indeed, the clarithromycin observed in the aqueous humor was significantly higher than the minimum effective concentration of clarithromycin in bacterial endophthalmitis. Further, the topical ocular therapy of clarithromycin could be more advantageous than its oral counterpart because topical administration could minimize the side effects as well as diminish the possibility of producing resistant strains of bacteria. Being a noninvasive approach, topical ocular therapy is more preferred and possesses higher patient compliance in the treatment of various diseases in the anterior segment. Thus, the developed SLN of clarithromycin-loaded nanoparticles has greater potential and can be a promising alternative to conventional therapy for the effective management of bacterial endophthalmitis. Further studies need to be carried out to understand the interaction of SLNs with the biological environment for successful commercialization and regulatory approval.