Abstract
Suprachoroidal drug delivery technology has advanced rapidly and emerged as a promising administration route for a variety of therapeutic candidates, in order to target multiple ocular diseases, ranging from neovascular age-related macular degeneration to choroidal melanoma. This review summarizes the latest preclinical and clinical progress in suprachoroidal delivery of therapeutic agents, including small molecule suspensions, polymeric entrapped small molecules, gene therapy (viral and nonviral nanoparticles), viral nanoparticle conjugates (VNCs), and cell therapy. Formulation customization is critical in achieving favorable pharmacokinetics, and sustained drug release profiles have been repeatedly observed for multiple small molecule suspensions and polymeric formulations. Novel therapeutic agents such as viral and nonviral gene therapy, as well as VNCs, have demonstrated promise in animal studies. Several of these suprachoroidally-administered therapies have been assessed in clinical trials, including small molecule suspensions of triamcinolone acetonide and axitinib, viral vector RGX-314 for gene therapy, and VNC AU-011. With continued drug delivery research and optimization, coupled with customized drug formulations, suprachoroidal drug delivery may address large unmet therapeutic needs in ophthalmology, targeting affected tissues with novel therapies for efficacy benefits, compartmentalizing therapies away from unaffected tissues for safety benefits, and achieving durability to relieve the treatment burden noted with current agents.
1. Current Treatment Landscape and Unmet Clinical Need
Highly active research and development efforts are ongoing to optimize ocular drug delivery for potential improvement in efficacy, safety, and durability benefits. The most common ocular drug delivery method for treating posterior eye diseases is intravitreal (IVT) injections [1,2,3,4]. It is estimated that 24.4 million IVT injections were performed globally in 2019 with 6.9 million of these injections performed in the United States, most often for neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME) [5]. While IVT injections of anti-vascular endothelial growth factor (anti-VEGF) therapies are commonly performed in an office setting, and have demonstrated remarkable therapeutic benefits in controlled clinical trials [6,7,8,9,10,11,12,13,14,15,16,17], real world data on patients receiving IVT injections of anti-VEGF therapies show a much more modest improvement in patients’ vision [18,19,20,21,22]. This disparity may relate to treatment burden, with patients undertreated due to an inability to maintain the frequent treatments in fixed regimens utilized in clinical trials. Furthermore, a subset of patients responds incompletely to anti-VEGF treatments regardless of the frequency of administration [23]. Other pharmacological agents, such as corticosteroids, that are commonly injected intravitreally for posterior eye diseases, may have undesirable side effects, including ocular hypertension and/or cataracts, due to their anterior chamber exposure [24,25,26,27,28,29,30,31].
In addition to posterior eye diseases that are currently treated with IVT injections, there remain significant unmet clinical needs in other ocular disorders, such as glaucoma, that are currently treated topically with eye drops and/or surgery. Glaucoma patients face challenges including compliance with proper administration of topical eye drops of beta blockers (e.g., timolol), alpha agonists (e.g., brimonidine), Rho kinase inhibitors (e.g., netarsudil), and prostaglandin analogs (e.g., latanoprost) [32,33,34]. These drugs act by decreasing the production and/or increasing the outflow of the aqueous humor. In some instances, combination treatments may also be required to provide adequate treatment. Specifically related to topical eye drops, in addition to patient compliance, there are challenges maintaining drug concentrations above therapeutic levels due to the high clearance rate via nasolacrimal drainage and/or dilution from production of reflex tears [35,36]. Taken together, there remains an unmet clinical need for improved delivery to targeted ocular tissues, compared to current topical or intravitreal delivery methods.
One possible solution to address this unmet need is the delivery of therapeutic agents into the suprachoroidal space (SCS). The SCS is the ‘potential space’ between the sclera and choroid that circumferentially spans the entire posterior segment of the eye from the ciliary body rearwards. Potential spaces in the body are areas between directly apposed organs or tissue layers. These potential spaces, such as the pleural space, the pericardial cavity, and the SCS, expand when fluid enters and flows within the space and collapse upon fluid egress. These potential spaces can serve as “druggable” targets for the delivery of therapeutic agents.
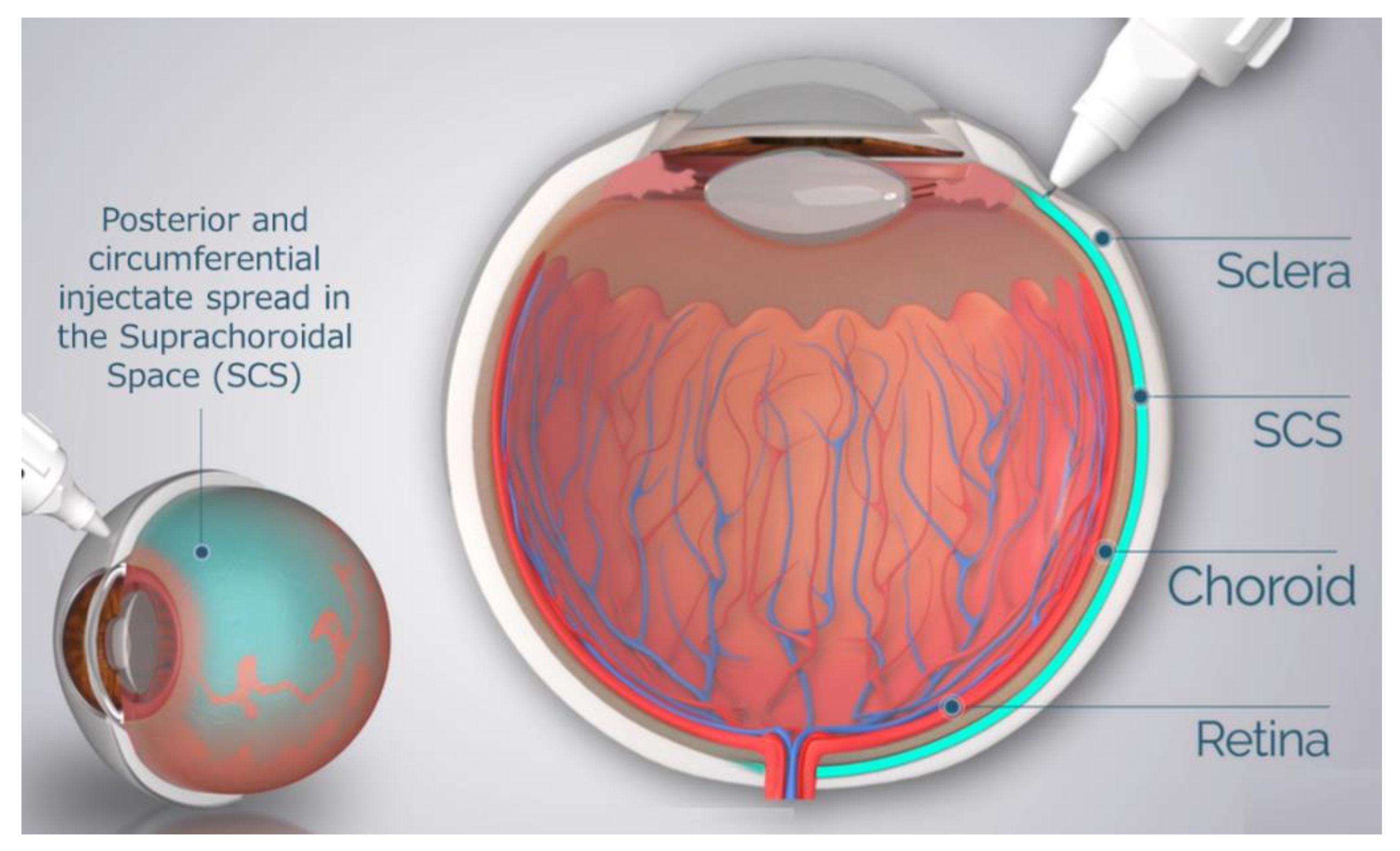
Drug delivery to the SCS has unique potential advantages in that (1) it specifically targets affected chorioretinal tissues with posterior and circumferential spread of the drug administered, (2) it may provide sustained drug kinetic release profiles, depending on the physio-chemical formulation properties, and (3) it may spare the unaffected anterior segment of the eye and the vitreous chamber, thus minimizing risks associated with off-target effects to potentiate safety [37,38]. To reliably access the SCS, microneedles have been designed to be long enough to penetrate through the sclera, delivering therapeutic agents into the SCS, without penetrating into the vitreous. The therapeutic agent flows both circumferentially and posteriorly towards the back of the eye, targeting chorioretinal tissues (Figure 1). In recent years, multiple clinical trials have evaluated this technique of suprachoroidal (SC) delivery with therapeutic agents ranging from small molecule suspensions to viral vector therapies.
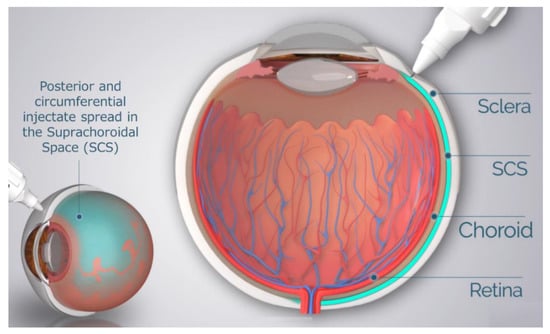
Figure 1.
Schematic of Microneedle Injection into the Suprachoroidal Space (SCS).
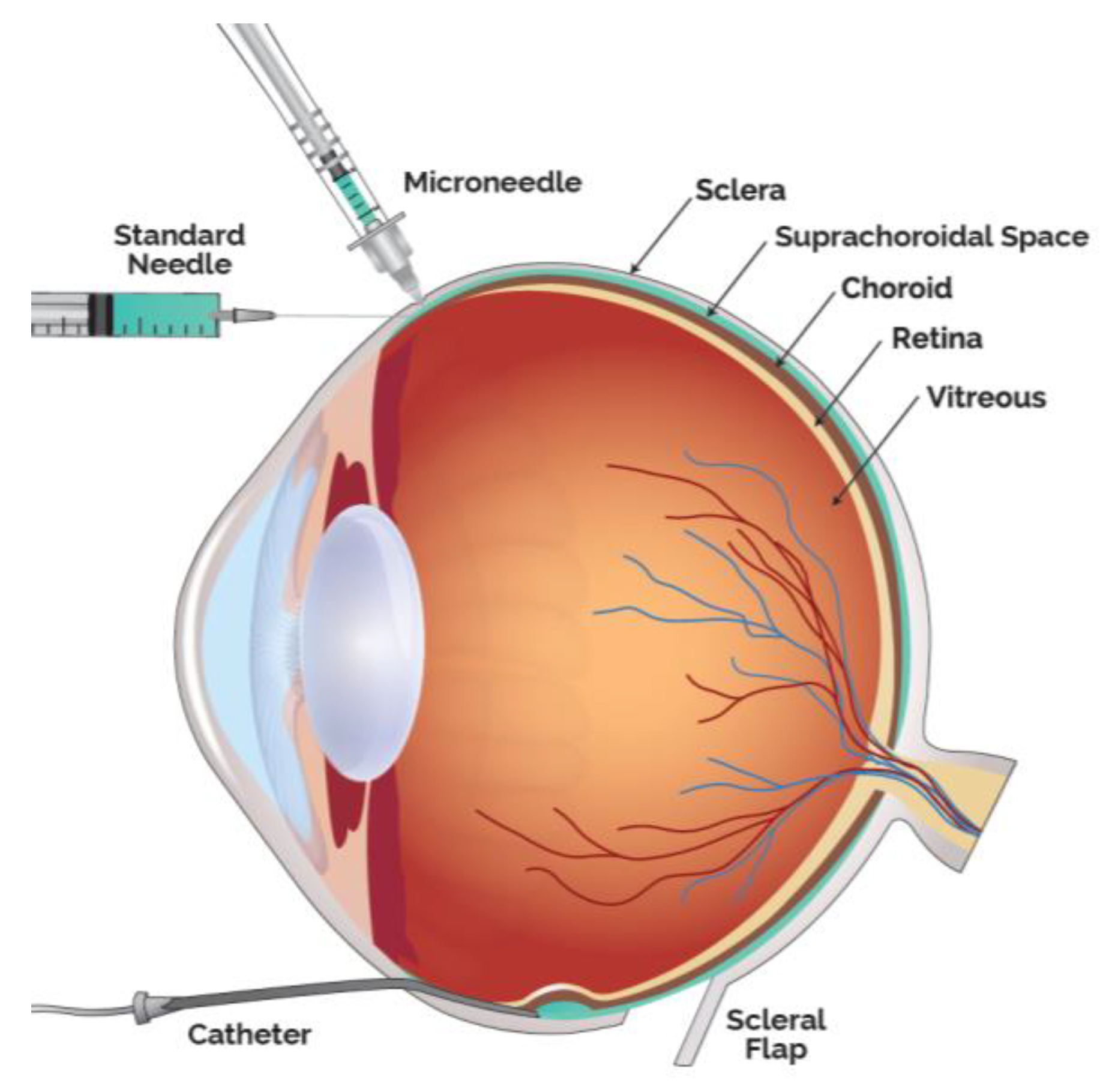
Drug delivery into the SCS has been achieved in multiple ways, in addition to the microneedle method, the predominate access technique clinically utilized. Small case series have also been performed by creating a scleral flap and a suprachoroidal (SC) pocket to insert autologous tissue. Preclinically, SC injections have been achieved with scleral flap technique, catheters as well as standard hypodermic needles (Figure 2).
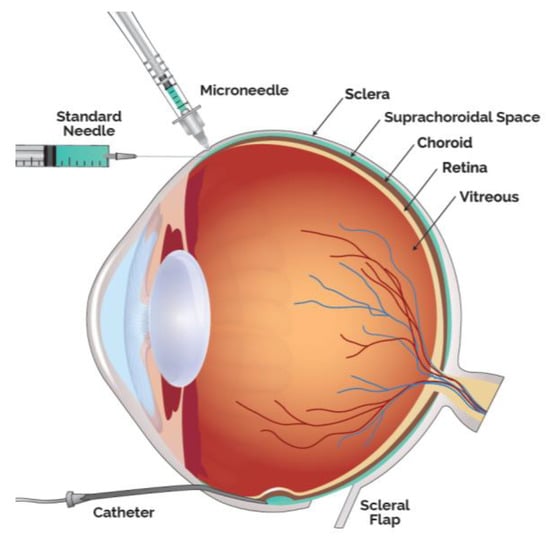
Figure 2.
Modalities to Administer Therapeutic Agents into the Suprachoroidal Space (SCS).
The objective of this review article is to summarize the current status of preclinical and clinical therapeutic agents that have been administered into the SCS. As synthesized in this review article, SC administration may offer unique advantages, such as compartmentalization away from the anterior segment and prolonged durability with appropriate formulations. Clinical trials with suprachoroidally administered triamcinolone acetonide, a small molecule suspension, have already demonstrated safety and efficacy. This route of administration may further play a significant role in ocular drug delivery of additional novel therapeutic agents for a wide range of ocular diseases, spanning from glaucoma and ocular melanoma to various common chorioretinal diseases, such as age-related macular degeneration (AMD) and diabetic retinopathy (DR).
2. Small Molecule Suspensions
Introduction of low-solubility therapeutic agents, such as small molecules in suspension, into the SCS is one strategy employed to promote both improved pharmacokinetic (PK) profiles and targeted drug delivery. Several well-characterized therapeutic agents, compounded as small molecule suspensions, have been administered suprachoroidally.
2.1. Corticosteroid
Safety and efficacy of an SC-delivered investigational formulation of triamcinolone acetonide (TA) has been evaluated in multiple controlled clinical trials [39,40,41,42,43] and its pharmacokinetic and pharmacodynamic characteristics have been evaluated in multiple animal studies.
Preclinically, favorable pharmacokinetics of SC TA has been demonstrated. This commonly used corticosteroid is a crystalline small molecule with low aqueous solubility (ranging between 0.02 mg/mL at 28 °C and 0.03 mg/mL at 50 °C [44]) and has historically been administered via topical, periocular or intravitreal routes, for the treatment of ocular inflammation. At a particle size distribution in the low micrometer range after micronization, it has been demonstrated that the concentration of TA in the SCS in various preclinical models can be maintained above therapeutic levels for an extended period of time [45].
With respect to ocular distribution, when TA was injected into the SCS in rabbits, TA concentrations in the retinal pigment epithelium (RPE)-choroid-sclera (RCS) and the retina were significantly higher than those in both the aqueous humor and the vitreous through the duration of the study (91 days) [46]. This study illustrated that drug delivery into the SCS can be compartmentalized, away from the anterior segment and the vitreous humor, preferentially targeting chorioretinal tissues and provide sustained PK profile.
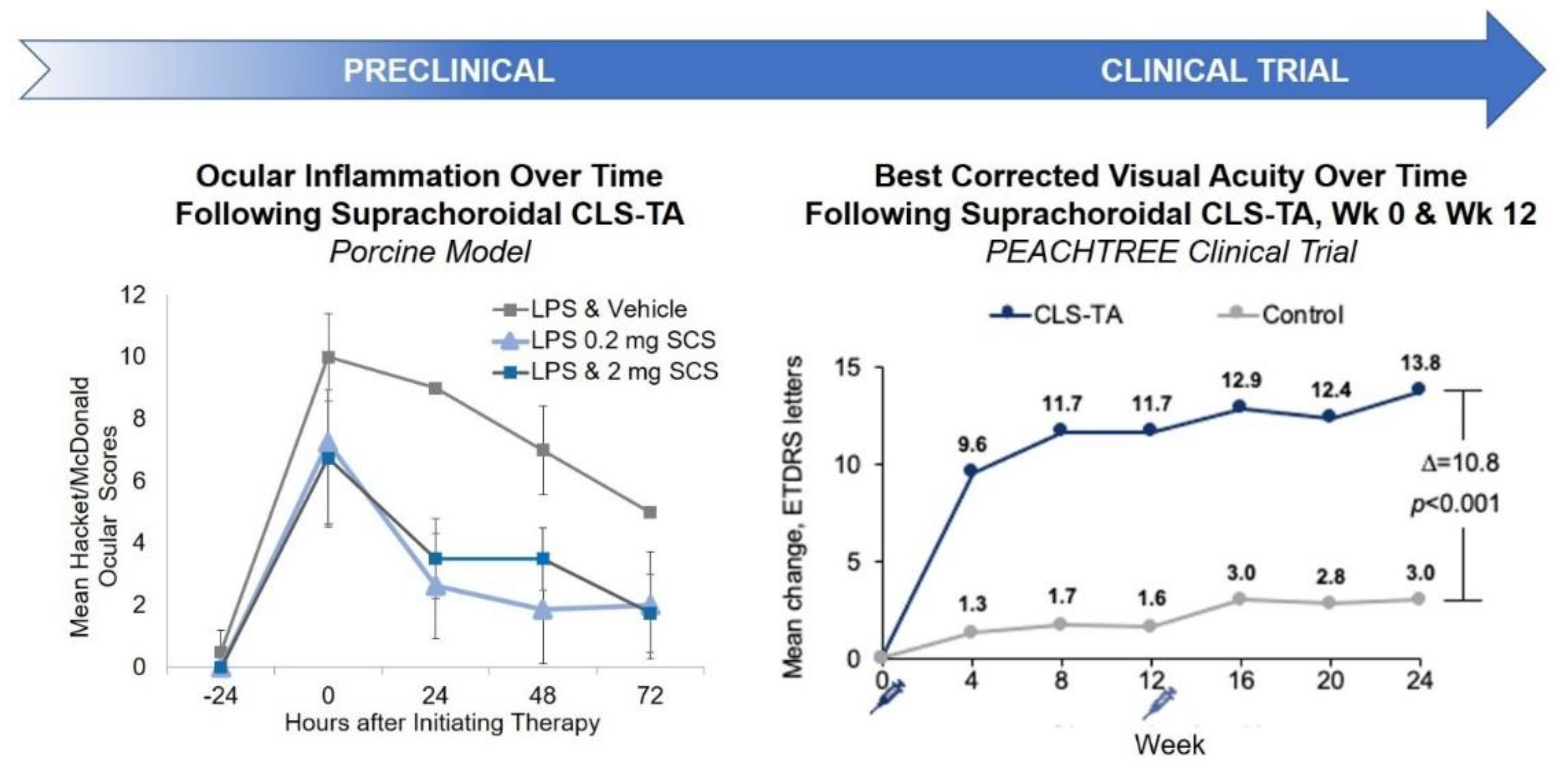
Pharmacodynamically, efficacy of SC TA was supported in a porcine model of uveitis. In this model, lipopolysaccharides (LPS) were introduced into pigs to induce uveitis [47]. Suprachoroidal administration of TA meaningfully reduced inflammation over time. In an in vivo model, Chen et al. showed that TA delivered via the SC route provided excellent targeting to the posterior retina; resulting in improved efficacy with significantly fewer aqueous humor cells and lower vitreous opacity scores compared to TA administered via the sub-Tenon route in rabbits [48]. Similar studies have also shown that TA administered via the SC or IVT routes in a model of endotoxin-induced panuveitis in albino rabbits led to comparable efficacy in the reduction of ocular inflammation [49]. Furthermore, an in vivo porcine model of acute uveitis demonstrated that 1/10th the dose of TA administered suprachoroidally was as effective as the full dose administered intravitreally, with no adverse effects [47]. Using a similar porcine model, another study showed that TA administered suprachoroidally was more effective in reducing ocular inflammation than low dose oral prednisone (0.1 mg/kg/day) administered for three days, and resulted in an improved rate of reduction in inflammation when compared to high dose oral prednisone (1 mg/kg/day) [50]. These results validate SC administration as a potential drug delivery route for small molecule suspensions.
These preclinical study results translated well into clinical trial outcomes. Specifically, in a phase 3 double-masked and randomized clinical trial for macula edema associated with noninfectious uveitis, 46.9% of the subjects treated with two SC injections of TA administered 12 weeks apart gained at least 15 Early Treatment Diabetic Retinopathy Study (ETDRS) letters from baseline in best corrected visual acuity (BCVA) at 24 weeks, compared to 15.6% in the sham control group [39] (Figure 3).
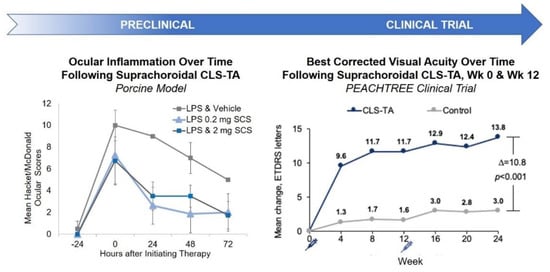
Figure 3.
Preclinical and Clinical Results of SC Administration of Small Molecule Suspensions. CLS-TA: investigational formulation of triamcinolone acetonide developed by Clearside Biomedical (Alpharetta, GA, USA); LPS: lipopolysaccharides.
2.2. Tyrosine Kinase Inhibitor
Another small molecule suspension that has been evaluated for potential SC delivery is axitinib. Axitinib has several properties with attractive therapeutic potential. It is a highly potent tyrosine kinase inhibitor (TKI) with pan-VEGF inhibition and high binding affinities [51]. Pan-VEGF inhibition may have benefits over current specific VEGF-A inhibition for the treatment of nAMD, DR, and DME, as other VEGF ligands such as VEGF-C and VEGF-D, have been shown to be upregulated after anti-VEGF-A administration both locally, after the treatment of nAMD, and systemically [52,53]. This upregulation of other VEGF ligands could contribute to tachyphylaxis, a form of treatment resistance, and lead to refractory cases clinically. Axitinib has also been shown to more effectively inhibit angiogenic sprouts than anti-VEGF-A inhibition in an in vitro angiogenesis model [54]. One recent phase 2 clinical trial demonstrated that broad VEGF inhibition yielded a statistically significantly better visual outcome in nAMD than focused VEGF-A inhibition [55]. In addition, axitinib is a more highly potent TKI than others that have undergone assessment in ocular clinical trials [56] and has demonstrated more potent inhibition of murine corneal neovascularization, compared to other TKIs at the same dose [57]. Axitinib has been shown to not only inhibit angiogenesis, but also regress established neovascularization in a preclinical choroidal neovascularization model, which may be more relevant to potential clinical use [58]. Finally, axitinib has shown better biocompatibility with ocular cells, including retinal pigment epithelial cells, compared to other TKIs in an in vitro study, suggesting the potential for intrinsic safety benefits [59].
While axitinib, as a poorly water-soluble small molecule (molecular weight: ~387 g/mol, solubility: 0.2 µg/mL in neutral pH aqueous media [60]), may not be well-suited for many forms of ocular delivery, it can be injected into the SCS as a stable suspension. In a rabbit PK and ocular tolerability study, a single bilateral SC injection of an axitinib suspension at 2 different doses (either 0.03 or 0.1 mg per eye) was administered to Dutch-Belted rabbits [61]. Axitinib was well-tolerated and optical coherence tomography (OCT) showed no evidence of choroidal/retinal degeneration through the duration of the study (10 weeks). More importantly, efficacious and sustained levels of axitinib, above the in vitro IC50 for the VEGF 2 receptor, were observed in the posterior ocular tissues—specifically in the RCS and retina—throughout the duration of the study, while no axitinib was detected in the plasma or aqueous humor. Furthermore, when compared to an equivalent dose of IVT axitinib in this rabbit model, SC axitinib resulted in RCS levels that were 11 times greater, supporting the potential for favorable targeting of chorioretinal tissues, which are affected in common causes of vision loss, such as AMD and DME. In a separate rabbit PK and ocular tolerability study, a single SC axitinib suspension was administered at a dose of 4 mg/eye. Over the duration of this 91-day study, axitinib was quantifiable at all timepoints in the RCS, retina and the vitreous humor with the highest concentration detected in the RCS. The administered dose was generally well tolerated. Currently, a phase 1/2a clinical trial of suprachoroidally-administered axitinib, for the treatment of nAMD, is underway [62].
2.3. Complement Inhibitor & Plasma Kallikrein Inhibitor
Additional small molecules have been investigated to target other signaling pathways, such as the complement system, a potential treatment target for non-neovascular age-related macular degeneration (dry AMD). Suprachoroidally administered A01017 (Achillion Pharamaceuticals, Blue Bell, PA, USA now AstraZeneca, Cambridge, UK), a potent small molecule complement factor D inhibitor [63], was well tolerated with sustained drug levels in posterior segment tissue of rabbits for up to 92 days. Similar to TA and axitinib, a high level of A01017 was observed in the RCS and retina throughout the entire duration of the study with an estimated half-life of approximately 66–76 days. Low or no quantifiable A01017 was detected in the vitreous humor, aqueous humor or plasma.
Yet another example of a small molecule suspension that has demonstrated preclinical ocular tolerability and sustained ocular drug levels is BCX4161 (BioCryst Pharmaceuticals, Durham, NC, USA), a potent and selective inhibitor of human plasma kallikrein, that is elevated in patients with diabetic macular edema [64]. BCX4161 was suprachoroidally administered to Dutch-Belted rabbits and was found to be well tolerated with sustained levels detected throughout the RCS and both central and peripheral retina over the 12-week study duration. Furthermore, the concentration of BCX4161 in the retina was 1 to 2 orders of magnitude higher than levels in the vitreous humor.
Collectively, these study results demonstrate that insoluble small molecule suspensions delivered via the SC route result in targeted high levels in chorioretinal tissues with potential efficacy benefits, compartmentalization away from unaffected tissues for potential safety benefits, and durability to potentially relieve treatment burden compared to current IVT therapies (Table 1).

Table 1.
Summary of Preclinical and Clinical Investigations of Small Molecule Suspensions Administered into the Suprachoroidal Space.
3. Polymeric Formulations
3.1. Corticosteroid
An additional approach to enhancing the durability of a therapeutic agent in the SCS is through polymeric entrapment of the active pharmaceutical ingredient (API). A sustained release formulation of dexamethasone, combined with a Laponite carrier, has been evaluated in rabbits [65,66]. Laponite is a synthetic clay used as a drug delivery carrier and has been demonstrated to be safe and biocompatible in vivo [67]. In this rabbit study, the dexamethasone/Laponite (DEX/LAP) formulation was administered suprachoroidally and dexamethasone levels were evaluated for up to 24 weeks. In the choroid and retina, dexamethasone concentration peaked at 1 week and a detectable concentration was maintained throughout the duration of the study, with an estimated half-life of 36.4 days.
Dexamethasone has also been incorporated into polyurethane implants and evaluated in a rat model of endotoxin-induced uveitis [68]. The sheet-like implants were implanted into the SCS, 4 mm posterior from the limbus and were found to be safe and biocompatible. The drug release profile, assessed in an in vitro release model, revealed a burst of dexamethasone the first week followed by a gradual sustained release as a function of the polymeric degradation, for up to 42 days. This release profile elicited an in vivo response, with a superior suppression of the inflammatory response, indicated by a reduction of the infiltration of polymorphonuclear cells and macrophages in the dexamethasone-laden implants, compared to placebo implants.
3.2. Neuroprotection
Furthermore, tauroursodeoxycholic acid (TUDCA), a potential neuroprotective agent, has been evaluated when administered suprachoroidally [69,70]. Olsen et al. loaded polycaprolactone-polyethylene glycol polymer with TUDCA and implanted it into the SCS or the vitreous humor (IVT) in pigs [71]. In in vitro drug release assays, a rapid burst of TUDCA from the implant surface was observed, followed by a slower, sustained release through diffusion. In vivo analysis revealed that higher serum levels of TUDCA were detected at week 2 and week 4 with SCS TUDCA implants, compared to IVT implants. Furthermore, TUDCA concentration rapidly decreased from week 1 to week 4 in the peri-retina and peri-choroid tissues and the levels remained low in the neurosensory retina through the duration of the study for the SCS TUDCA implants. These results suggest that while the TUDCA concentration was measurable in local ocular tissues for the SCS group, TUDCA may be rapidly cleared through the choroidal vasculature. Additional optimization may be required to slow the release of TUDCA from the implant.
3.3. Hypoxia-Inducible Factor (HIF) Inhibitor
Another polymer that has been used as a controlled drug release platform is poly (lactic-co-glycolic acid) (PLGA). Hackett et al. incorporated acriflavine (ACF), a highly aqueous-soluble molecule (solubility: 0.33 g/mL [72]), into PLGA and introduced the ACF-PLGA microspheres into the vitreous or the SCS in rats and rabbits [73]. The function of ACF is to inhibit HIF-1, a potent angiogenic factor that has been implicated in the pathogenesis of choroidal neovascularization [74,75]. By incorporating ACF into PLGA microparticles, the drug release profile was extended to up to 60 days in vitro. SC injections of the ACF-PLGA microparticles in rabbits were safe as demonstrated by fundus imaging, electroretinogram (ERG), intraocular pressure (IOP) monitoring, and retinal histology. SC injections of the ACF-PLGA microparticles suppressed laser-induced choroidal neovascularization for at least 18 weeks in Male Norway Brown rats.
3.4. Alpha-2-Agonist
Poly(lactic acid) microspheres loaded with brimonidine have also been introduced into the suprachoroidal/superciliary space to evaluate its impact on IOP [76]. Brimonidine, an alpha-2 adrenergic agonist, is a commonly used anti-ocular hypertensive treatment and is typically administered as a thrice daily eye drop. Chiang et al. developed microspheres with a sustained release profile of brimonidine and evaluated the effect of this formulation in the SCS in New Zealand White rabbits. The microspheres were suspended in a viscous fluid (5% carboxymethyl cellulose in Hank’s buffer salt solution) to localize the drug close to the injection site, 3 mm posterior to the limbus. The authors demonstrated IOP reductions of up to 6 mm Hg for up to 1 month, thus providing evidence of feasibility of sustained IOP reduction with brimonidine-laden microspheres delivered suprachoroidally.
3.5. In Situ Forming Hydrogel
Lastly, the administration of an in situ forming hyaluronic acid (HA) hydrogel into the supraciliary/suprachoroidal space has been shown to reduce IOP in normotensive rabbits for more than 4 months [77]. The IOP reduction was found to strongly correlate with the SCS expansion, facilitating the uveoscleral outflow pathway. This is a potential novel drug-free, surgery-free option for sustained IOP reduction.
Taken together, utilization of polymers to incorporate therapeutic agents is a promising method for sustained release of drug in the SCS (Table 2). It is foreseeable that the release profile may be tunable based on polymeric formulation customization.

Table 2.
Summary of Preclinical and Clinical Investigations of Small Molecules in Polymeric Formulation Administered into the Suprachoroidal Space.
5. Proteins and Peptides
5.1. Anti-VEGF
Another class of biologics that has been suprachoroidally administered includes peptides and proteins. Anti-VEGF therapy is commonly administered intravitreally, but no anti-VEGF therapy has been approved for administration into the SCS. Olsen et al. compared intravitreal and SC injections of bevacizumab in a porcine model [104]. From a safety perspective, no findings were observed for the SC group, while vitritis and granulomatous vasculitis were noted in 7 of 30 eyes in the intravitreal group. Given bevacizumab is frequently administered intravitreally in humans without ocular inflammation, the authors hypothesized that the introduction of a human antibody into porcine vitreous humor may result in a xenograft immune rejection. Pharmacokinetically, bevacizumab concentrations decreased gradually over 60 days in the intravitreal group while bevacizumab levels dropped off rapidly and were no longer detected after 7 days in the SC group. Disparate protein distribution patterns, corresponding to the expected levels depending on the routes of administration, were also observed for the two groups; while bevacizumab was primarily located within the inner retina and the internal limiting membrane in the intravitreal group, bevacizumab was mostly found in the RCS (dose depot) for the SC group. Taken together, these data suggest that rapid clearance of suprachoroidally injected bevacizumab, a soluble protein, may be occurring via the choroidal vasculature or other fluid outflow mechanisms such as the uveoscleral outflow pathway. However, the higher levels observed in the choroid after SC administration could yield some efficacy benefits in treating choroidal neovascularization, and further study with sustained release polymeric formulations is warranted.
Bevacizumab was also evaluated in the SCS in 8-week-old Lewis rats [105]. Saline (control group) or 0.2 mg of bevacizumab (treatment group) were injected into the SCS. Retinal thickness and choroidal vasculature were observed with histology after one month. Compared to the control group, reduced vascular area was observed in the choroidal vasculature in the treatment group. No difference in retinal morphology (retinal thickness, outer nuclear layer thickness) was observed. This study indicates that SC bevacizumab was able to elicit a biological response (i.e., reduced choroidal vascular area) with acceptable safety profile (i.e., normal retinal morphology).
Efficacy of another anti-VEGF agent, aflibercept, has also been investigated suprachoroidally in a laser-induced choroidal neovascularization model in rats [106]. Either aflibercept or saline was injected into the SCS and the area of neovascularization was measured after 3 weeks. Aflibercept-treated animals had significantly reduced neovascularization, compared to the saline control group. This result echoes the previous bevacizumab findings.
5.2. Vasoconstrictor
Another protein that has been administered into the SCS is endothelin-1, a potent vasoconstrictor that has been previously investigated as a model for optic nerve ischemia [107,108,109]. Nork et al. injected endothelin-1 into the SCS in rabbits as a model to reduce choroidal blood flow [110]. Compared to the vehicle control, endothelin-1 injected eyes had significantly lower choroidal blood flow. This study demonstrated that endothelin-1 in the SCS was able to elicit a targeted physiological response, measured by choroidal blood flow assessment. This study, in conjunction with an early complementary model of inner retinal ischemia after IVT endothelin-1 administration, illustrates the ability to target pharmacologic effect to ocular compartments, based on the route of administration [111].
As highlighted previously, one of the limitations of utilizing soluble proteins and peptides is that they are dispersed in an aqueous state, which facilitates rapid clearance therefore limiting their duration in the SCS, compared to insoluble small molecule therapeutic suspensions. Further formulation development is warranted to optimize durability of these biologics within the SCS, and one such effort is already underway with a hydrogel formulation of aflibercept currently undergoing preclinical assessment [112]. Another potential solution to enhance durability and improve retinal exposure is to leverage the advancements in nanoparticle systems, such as liposomes, lipid nanoparticles or lipid nanoparticles containing cell-penetrating peptide sequences, to encapsulate therapeutic agents and to target chorioretinal tissues [113,114,115].
6. Cell Therapy
6.1. Autologous Tissue–Trophic Factors
Beyond gene and protein-based therapies, cells and autologous tissue derivates have also been evaluated in the SCS for retinal diseases. These potential therapies include platelet rich plasma (PRP) and stromal vascular fractions (SVFs). Limoli et al. conducted multiple small clinical evaluations, placing autologous fat tissue, SVF, and PRP in the SCS through a deep 5 × 5 mm sclerotomy window 8 mm post-limbus [116]. The rationale for the tissue implantation was that SVF and PRP both contain growth factors that may play a role in promoting retinal health through paracrine signaling [117,118] and adipose-tissue-derived stem cells, albeit a small fraction of the cell population, have been found in SVFs [119]. In the first study, 12 eyes of 12 patients with dry AMD underwent the cell transplantation procedure; ERGs and BCVA were used as efficacy indicators. Scotopic rod-ERG response was found to be statistically different between baseline and 30 days post treatment. No statistical differences could be identified for BCVA, maximal rod-cone-ERG, or photopic cone-ERG [116]. The treatment was well-tolerated.
To further investigate the effect of the autologous tissue implantation, the authors conducted another study with 36 eyes in 25 patients with dry AMD [120,121]. Improvements in BCVA from baseline to 6 months post procedure were observed for patients with baseline retinal thickness greater than 250 µm. The authors hypothesized that the thicker retina may be indicative of higher cellularity in response to the paracrine signaling effects from the autologous tissue. No adverse events were documented. This treatment has also been evaluated for 34 eyes in 25 patients with retinitis pigmentosa (RP) [122,123]. No statistically significant improvements from baseline in BCVA to 6 months were observed.
6.2. Allogenic Cell Therapy
The same technique of transplanting autologous tissue has also been utilized for implanting mesenchymal stem cells derived from umbilical cords (UC-MSCs) [124] or adipose tissues (AD-MSCs) [125,126]. Twenty-nine eyes in 23 patients with optic atrophy were enrolled at a single center, received five million UC-MSCs per eye, and followed for a minimum of 12 months. The treatment was well-tolerated and improvements in visual field and BCVA were observed between baseline and 12 months.
While cell-based treatment yielded no serious safety signals in these initial small studies, the milieu of cytokines in PRP, SVF and cell-based products requires additional study to better understand the mechanism of action and characterize the safety, and when appropriate, to evaluate efficacy with a randomized clinical trial [127]. Given the unfortunate history of permanent vision loss after “stem cell” clinic treatment of AMD patients with unapproved cell-based products, the safety and efficacy of cell-based products for ocular diseases must be assessed through institutional review board (IRB) approved clinical trials [128].
Collective summary of the preclinical and clinical investigations of proteins, peptides and cell therapies is described in Table 5.

Table 5.
Summary of Preclinical and Clinical Investigations of Proteins, Peptides, and Cells Administered into the Suprachoroidal Space.
7. Conclusion and Future Perspective
An increasing number of preclinical and clinical investigations involve the delivery of existing and novel therapeutic agents into the SCS, the potential space between the sclera and choroid and a promising drug delivery route to the posterior segment of the eye.
The durability of therapeutic agents in the SCS appears to be highly dependent on the formulation attributes; while poorly aqueous soluble small molecule suspensions maintain a sustained drug concentration gradient in the target ocular tissues, highly soluble small molecules and proteins are rapidly cleared from the SCS through the choroidal vasculature. To improve durability of these highly soluble therapeutic agents, further studies with sustained release polymeric formulations or nanoparticles systems are underway. Proof of concept evaluations of novel biologics-based therapeutics have yielded promising preclinical outcomes, including transduction and transfection of chorioretinal tissues with viral and nonviral gene therapies, as well as reduction of the size of choroidal melanomas with VNCs. Suprachoroidal drug delivery is a promising drug delivery platform, with additional ongoing preclinical and clinical studies.
Expert Opinion
- Suprachoroidal drug delivery is a promising route of administration for ocular diseases. Taking advantage of the natural ocular anatomy, suprachoroidal delivery targets high level of therapy to affected chorioretinal layers for potential efficacy benefits while compartmentalizing them away from unaffected anterior structures for potential safety benefits.
- Numerous therapies have been assessed preclinically for suprachoroidal delivery, including small molecule suspensions, polymeric formulations, and biologics including gene therapy, proteins and peptides, and cell therapy. Small molecule suspensions and polymeric formulations injected suprachoroidally have the potential for favorable pharmacokinetics with prolonged durability.
- As clinical proof of concept, the safety and efficacy of suprachoroidal delivery of an investigational suspension formulation of triamcinolone acetonide has been demonstrated in a Phase 3 clinical trial. Clinical evaluation of additional therapeutic agents, from tyrosine kinase inhibitor to gene therapy and viral nanoparticle conjugates, are underway.
- Durability of suprachoroidal drug delivery is dependent on the formulation. While soluble proteins have been shown to be rapidly transported out of the suprachoroidal space through the choroidal vasculature and uveoscleral outflow pathways, small molecules in suspension form have repeatedly demonstrated efficacious drug concentrations (above IC50) in target ocular tissues.
- A synergistic effort to design drug formulation tailored for suprachoroidal delivery may optimize drug delivery efficacy without compromising safety. Drug formulation designs for hydrophilic therapeutic agents that leveraging polymeric or lipid-based drug delivery system may further enhance the pharmacokinetic profiles.
Author Contributions
C.W. and L.M. contributed equally to the conception, literature search and draft of the manuscript. V.K. assisted in edits and reviews of the manuscript. T.A.C. contributed in the conception, edits and reviews of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge editorial support from Shelley Hancock, Barry Kapik, Barbara Bauschka, and Leslie Zacks.
Conflicts of Interest
C.W., L.M., V.K. and T.A.C. are all employees of Clearside Biomedical and own stocks from Clearside Biomedical.
References
- Patel, A. Ocular drug delivery systems: An overview. World J. Pharmacol. 2013, 2, 47. [Google Scholar] [CrossRef]
- Edelhauser, H.F.; Rowe-Rendleman, C.L.; Robinson, M.R.; Dawson, D.G.; Chader, G.J.; Grossniklaus, H.E.; Rittenhouse, K.D.; Wilson, C.G.; Weber, D.A.; Kuppermann, B.D.; et al. Ophthalmic drug delivery systems for the treatment of retinal diseases: Basic research to clinical applications. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5403–5420. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.; Misra, M. A review on recent drug delivery systems for posterior segment of eye. Biomed. Pharmacother. 2018, 107, 1564–1582. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; Díaz-Tomé, V.; Luaces-Rodríguez, A.; Conde-Penedo, A.; García-Otero, X.; Luzardo-álvarez, A.; Fernández-Ferreiro, A.; Otero-Espinar, F.J. Drug delivery to the posterior segment of the eye: Biopharmaceutic and pharmacokinetic considerations. Pharmaceutics 2020, 12. [Google Scholar]
- Puliafito, C.A.; Wykoff, C.C. New Frontiers in Retina: Highlights of the 2020 angiogenesis, exudation and degeneration symposium. Int. J. Retin. Vitr. 2020, 6. [Google Scholar] [CrossRef] [PubMed]
- Korobelnik, J.F.; Holz, F.G.; Roider, J.; Ogura, Y.; Simader, C.; Schmidt-Erfurth, U.; Lorenz, K.; Honda, M.; Vitti, R.; Berliner, A.J.; et al. Intravitreal aflibercept injection for macular edema resulting from central retinal vein occlusion: One-year results of the phase 3 GALILEO study. Ophthalmology 2014, 121, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Clark, W.L.; Boyer, D.S.; Brown, D.M.; Vitti, R.; Berliner, A.J.; Kazmi, H.; Ma, Y.; Stemper, B.; Zeitz, O.; et al. Intravitreal aflibercept injection for macular edema due to central retinal vein occlusion: Two-year results from the COPERNICUS study. Ophthalmology 2014, 121, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Clark, W.L.; Boyer, D.S.; Heier, J.S.; Brown, D.M.; Haller, J.A.; Vitti, R.; Kazmi, H.; Berliner, A.J.; Erickson, K.; Chu, K.W.; et al. Intravitreal Aflibercept for Macular Edema Following Branch Retinal Vein Occlusion 52-Week Results of the VIBRANT Study. Ophthalmology 2016, 123, 330–336. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Brown, D.M.; Pearson, A.; Chen, S.; Boyer, D.; Ruiz-Moreno, J.; Garretson, B.; Gupta, A.; Hariprasad, S.M.; Bailey, C.; et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 2012, 119, 2125–2132. [Google Scholar] [CrossRef]
- Heier, J.S.; Korobelnik, J.-F.; Brown, D.M.; Schmidt-Erfurth, U.; Do, D.V.; Midena, E.; Boyer, D.S.; Terasaki, H.; Kaiser, P.K.; Marcus, D.M.; et al. Intravitreal Aflibercept for Diabetic Macular Edema: 148-Week Results from the VISTA and VIVID Studies. Ophthalmology 2016, 123, 2376–2385. [Google Scholar] [CrossRef]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S. Ranibizumab versus Verteporfin for Neovascular Age-Related Macular Degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.Y.; Cui, H.; Hashad, Y.; Whitcup, S.M. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef]
- Nguyen, Q.D.; Brown, D.M.; Marcus, D.M.; Boyer, D.S.; Patel, S.; Feiner, L.; Gibson, A.; Sy, J.; Rundle, A.C.; Hopkins, J.J.; et al. Ranibizumab for diabetic macular edema: Results from 2 phase iii randomized trials: RISE and RIDE. Ophthalmology 2012, 119, 789–801. [Google Scholar] [CrossRef]
- Martin, D.F.; Maguire, M.G.; Ying, G.S.; Grunwald, J.E.; Fine, S.L.; Jaffe, G.J. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2011, 364, 1897–1908. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Bressler, N.M.; Suñer, I.; Lee, P.; Dolan, C.M.; Ward, J.; Colman, S.; Rubio, R.G. Improved vision-related function after ranibizumab for macular edema after retinal vein occlusion: Results from the BRAVO and CRUISE trials. Ophthalmology 2012, 119, 2108–2118. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Diabetic Retinopathy Clinical Research Network; Wells, J.; Glassman, A.R.; Ayala, A.R.; Jampol, L.M.; Aiello, L.; Antoszyk, A.N.; Arnold-Bush, B.; Baker, C.; Bressler, N.M.; et al. Aflibercept, Bevacizumab, or Ranibizumab for Diabetic Macular Edema. N. Engl. J. Med. 2015, 372, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, T.A.; Bracha, P.; Pollack, J.; Williams, D.F. Real-world Outcomes of Anti-Vascular Endothelial Growth Factor Therapy in Diabetic Macular Edema in the United States. Ophthalmol. Retin. 2018, 2, 1179–1187. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Hussain, R.M.; Pollack, J.S.; Williams, D.F. Visual Acuity Outcomes and Anti–Vascular Endothelial Growth Factor Therapy Intensity in Neovascular Age-Related Macular Degeneration Patients: A Real-World Analysis of 49 485 Eyes. Ophthalmol. Retin. 2020, 4, 19–30. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Huang, F.; Westby, K.; Williams, D.F.; Zaveri, S.; Patel, S.C. Real-world Outcomes of Anti-Vascular Endothelial Growth Factor Therapy in Neovascular Age-Related Macular Degeneration in the United States. Ophthalmol. Retin. 2018, 2, 645–653. [Google Scholar] [CrossRef]
- Ciulla, T.; Pollack, J.S.; Williams, D.F. Visual acuity outcomes and anti-VEGF therapy intensity in macular oedema due to retinal vein occlusion: A real-world analysis of 15 613 patient eyes. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, T.A.; Pollack, J.S.; Williams, D.F. Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: A real-world analysis of 28 658 patient eyes. Br. J. Ophthalmol. 2020. [Google Scholar] [CrossRef]
- Amoaku, W.M.; Chakravarthy, U.; Gale, R.; Gavin, M.; Ghanchi, F.; Gibson, J.; Harding, S.; Johnston, R.L.; Kelly, S.; Lotery, A.; et al. Defining response to anti-VEGF therapies in neovascular AMD. Eye 2015, 29, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Kempen, J.H.; Altaweel, M.M.; Holbrook, J.T.; Jabs, D.A.; Louis, T.A.; Sugar, E.A.; Thorne, J.E. Randomized comparison of systemic anti-inflammatory therapy versus fluocinolone acetonide implant for intermediate, posterior, and panuveitis: The multicenter uveitis steroid treatment trial. Ophthalmology 2011, 118, 1916–1926. [Google Scholar] [CrossRef]
- Yap, Y.C.; Papathomas, T.; Kamal, A. Results of intravitreal dexamethasone implant 0.7 mg (Ozurdex®) in non-infectious posterior uveitis. Int. J. Ophthalmol. 2015, 8, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Kempen, J.H.; Jabs, D.A. Benefits of systemic anti-inflammatory therapy versus fluocinolone acetonide intraocular implant for intermediate uveitis, posterior uveitis, and panuveitis: Fifty-four-month results of the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study. Ophthalmology 2015, 122, 1967–1975. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.Y.; Kang, S.W.; Kim, Y.T.; Chung, S.E.; Lee, S.W. A three-year follow-up of intravitreal triamcinolone acetonide injection and macular laser photocoagulation for diffuse diabetic macular edema. Korean J. Ophthalmol. 2012, 26, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bailey, C.; Loewenstein, A.; Massin, P. Intravitreal corticosteroids in diabetic macular edema: Pharmacokinetic considerations. Retina 2015, 35, 2440–2449. [Google Scholar] [CrossRef] [PubMed]
- Sen, H.N.; Vitale, S.; Gangaputra, S.S.; Nussenblatt, R.B.; Liesegang, T.L.; Levy-Clarke, G.A.; Rosenbaum, J.T.; Suhler, E.B.; Thorne, J.E.; Foster, C.S.; et al. Periocular corticosteroid injections in uveitis: Effects and complications. Ophthalmology 2014, 121, 2275–2286. [Google Scholar] [CrossRef]
- Kothari, S.; Foster, C.S.; Pistilli, M.; Liesegang, T.L.; Daniel, E.; Sen, H.N.; Suhler, E.B.; Thorne, J.E.; Jabs, D.A.; Levy-Clarke, G.A.; et al. The risk of intraocular pressure elevation in pediatric noninfectious uveitis. Ophthalmology 2015, 122, 1987–2001. [Google Scholar] [CrossRef]
- James, E.R. The etiology of steroid cataract. J. Ocul. Pharmacol. Ther. 2007, 23, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA - J. Am. Med. Assoc. 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Burns, E.; Mulley, G.P. Practical problems with eye-drops among elderly ophthalmology outpatients. Age Ageing 1992, 21, 168–170. [Google Scholar] [CrossRef]
- Gurwitz, J.H.; Glynn, R.J.; Monane, M.; Everitt, D.E.; Gilden, D.; Smith, N.; Avorn, J. Treatment for glaucoma: Adherence by the elderly. Am. J. Public Health 1993, 83, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Djebli, N.; Khier, S.; Griguer, F.; Coutant, A.L.; Tavernier, A.; Fabre, G.; Leriche, C.; Fabre, D. Ocular Drug Distribution After Topical Administration: Population Pharmacokinetic Model in Rabbits. Eur. J. Drug Metab. Pharmacokinet. 2017, 42, 59–68. [Google Scholar] [CrossRef]
- Sakanaka, K.; Kawazu, K.; Tomonari, M.; Kitahara, T.; Nakashima, M.; Nishida, K.; Nakamura, J.; Sasaki, H.; Higuchi, S. Ocular pharmacokinetic/pharmacodynamic modeling for multiple anti-glaucoma drugs. Biol. Pharm. Bull. 2008, 31, 1590–1595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldstein, D.A.; Ciulla, T.A. Suprachoroidal Delivery of Suspensions of Tyrosine Kinase Inhibitor, Complement Inhibitor, and Corticosteroid: Preclinical and Clinical Correlates | IOVS | ARVO Journals. Invest. Ophthalmol. Vis. Sci. 2020, 61, 2898. [Google Scholar]
- Habot-Wilner, Z.; Noronha, G.; Wykoff, C.C. Suprachoroidally injected pharmacological agents for the treatment of chorio-retinal diseases: A targeted approach. Acta Ophthalmol. 2019, 97, 460–472. [Google Scholar] [CrossRef]
- Yeh, S.; Khurana, R.N.; Shah, M.; Henry, C.R.; Wang, R.C.; Kissner, J.M.; Ciulla, T.A.; Noronha, G. Efficacy and Safety of Suprachoroidal CLS-TA for Macular Edema Secondary to Noninfectious Uveitis: Phase 3 Randomized Trial. Ophthalmology 2020, 127, 948–955. [Google Scholar] [CrossRef]
- Yeh, S.; Kurup, S.K.; Wang, R.C.; Foster, C.S.; Noronha, G.; Nguyen, Q.D.; Do, D.V. Suprachoroidal Injection of Triamcinolone Acetonide, Cls-Ta, for Macular Edema due to Noninfectious Uveitis: A Randomized, Phase 2 Study (DOGWOOD). Retina 2019, 39, 1880–1888. [Google Scholar] [CrossRef]
- Campochiaro, P.A.; Wykoff, C.C.; Brown, D.M.; Boyer, D.S.; Barakat, M.; Taraborelli, D.; Noronha, G. Suprachoroidal Triamcinolone Acetonide for Retinal Vein Occlusion: Results of the Tanzanite Study. Ophthalmol. Retin. 2018, 2, 320–328. [Google Scholar] [CrossRef]
- Barakat, M.R.; Wykoff, C.C.; Gonzalez, V.; Hu, A.; Marcus, D.; Zavaleta, E.; Ciulla, T.A. Suprachoroidal CLS-TA plus Intravitreal Aflibercept for Diabetic Macular Edema: A Randomized, Double-Masked, Parallel-Design, Controlled Study. Ophthalmol. Retin. 2021, 5, 60–70. [Google Scholar] [CrossRef]
- Henry, C.R.; Shah, M.; Barakat, M.R.; Dayani, P.; Wang, R.C.; Khurana, R.N.; Rifkin, L.; Yeh, S.; Hall, C.; Ciulla, T. Suprachoroidal CLS-TA for non-infectious uveitis: An open-label, safety trial (AZALEA). Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Block, L.H.; Patel, R.N. Solubility and Dissolution of Triamcinolone Acetonide. J. Pharm. Sci. 1973, 62, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Edelhauser, H.F.; Patel, S.R.; Meschter, C.; Dean, R.; Powell, K.; Verhoeven, R. Suprachoroidal Microinjection Delivers Triamcinolone Acetonide to Therapeutically-Relevant Posterior Ocular Structures and Limits Exposure in the Anterior Segment. Invest. Ophthalmol. Vis. Sci. 2013, 54, 5063. [Google Scholar]
- Edelhauser, H.F.; Verhoeven, R.S.; Burke, B.; Struble, C.B.; Patel, S.R. Intraocular Distribution and Targeting of Triamcinolone Acetonide Suspension Administered Into the Suprachoroidal Space. Invest. Ophthalmol. Vis. Sci. 2014, 55, 5259. [Google Scholar]
- Gilger, B.C.; Abarca, E.M.; Salmon, J.H.; Patel, S. Treatment of acute posterior uveitis in a porcine model by injection of triamcinolone acetonide into the suprachoroidal space using microneedles. Invest. Ophthalmol. Vis. Sci. 2013, 54, 2483–2492. [Google Scholar] [CrossRef]
- Chen, M.; Li, X.; Liu, J.; Han, Y.; Cheng, L. Safety and pharmacodynamics of suprachoroidal injection of triamcinolone acetonide as a controlled ocular drug release model. J. Control. Release 2015, 203, 109–117. [Google Scholar] [CrossRef]
- Patel, S.; Carvalho, R.; Mundwiler, K.; Meschter, C.; Verhoeven, R. Evaluation of Suprachoroidal Microinjection of Triamcinolone Acetonide in a Model of Panuveitis in Albino Rabbits. Invest. Ophthalmol. Vis. Sci. 2013, 54, 2927. [Google Scholar]
- Noronha, G.; Blackwell, K.; Gilger, B.C.; Kissner, J.; Patel, S.R.; Walsh, K.T. Evaluation of suprachoroidal CLS-TA and oral prednisone in a porcine model of uveitis. Invest. Ophthalmol. Vis. Sci. 2015, 56, 3110. [Google Scholar]
- Lledó Riquelme, M.; Campos-Mollo, E.; Fernández-Sánchez, L. Topical axitinib is a potent inhibitor of corneal neovascularization. Clin. Experiment. Ophthalmol. 2018, 46, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Lieu, C.H.; Tran, H.; Jiang, Z.-Q.; Mao, M.; Overman, M.J.; Lin, E.; Eng, C.; Morris, J.; Ellis, L.; Heymach, J.V.; et al. The Association of Alternate VEGF Ligands with Resistance to Anti-VEGF Therapy in Metastatic Colorectal Cancer. PLoS ONE 2013, 8, e77117. [Google Scholar] [CrossRef] [PubMed]
- Cabral, T.; Lima, L.H.; Luiz, L.G.; Polido, J.; Correa, É.P.; Oshima, A.; Duong, J.; Serracarbassa, P.; Regatieri, C.V.; Mahajan, V.B.; et al. Bevacizumab Injection in Patients with Neovascular Age-Related Macular Degeneration Increases Angiogenic Biomarkers. Ophthalmol. Retin. 2018, 2, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Giddabasappa, A.; Lalwani, K.; Norberg, R.; Gukasyan, H.J.; Paterson, D.; Schachar, R.A.; Rittenhouse, K.; Klamerus, K.; Mosyak, L.; Eswaraka, J. Axitinib inhibits retinal and choroidal neovascularization in in vitro and in vivo models. Exp. Eye Res. 2016, 145, 373–379. [Google Scholar] [CrossRef]
- Jackson, T. OPT-302 Phase 2b in Wet AMD A Multicenter, Randomized, Double-Masked, Sham Controlled Study of Intravitreal OPT-302 in Combination with Ranibizumab, in Participants with Wet AMD. Available online: https://opthea.com/wp-content/uploads/2019/09/20190905-EuRetina-ASX-Slides_Final2.pdf (accessed on 1 February 2021).
- Bhargava, P.; Robinson, M.O. Development of Second-Generation VEGFR Tyrosine Kinase Inhibitors: Current Status. Curr. Oncol. Rep. 2011, 13, 103–111. [Google Scholar] [CrossRef]
- Yuan, X.; Marcano, D.C.; Shin, C.S.; Hua, X.; Isenhart, L.C.; Pflugfelder, S.C.; Acharya, G. Ocular Drug Delivery Nanowafer with Enhanced Therapeutic Efficacy. ACS Nano 2015, 9, 1749–1758. [Google Scholar] [CrossRef]
- Kang, S.; Roh, C.R.; Cho, W.-K.; Park, K.C.; Yang, K.-J.; Choi, H.-S.; Kim, S.-H.; Roh, Y.-J. Antiangiogenic Effects of Axitinib, an Inhibitor of Vascular Endothelial Growth Factor Receptor Tyrosine Kinase, on Laser-Induced Choroidal Neovascularization in Mice. Curr. Eye Res. 2013, 38, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.; Liegl, R.G.; König, S.; Siedlecki, J.; Langer, J.; Eibl, K.; Haritoglou, C.; Kampik, A.; Kernt, M. Multikinase inhibitors as a new approach in neovascular age-related macular degeneration (AMD) treatment: In vitro safety evaluations of axitinib, pazopanib and sorafenib for intraocular use. Klin. Monbl. Augenheilkd. 2013, 230, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Research, C. for D.E. and Clinical Pharmacology and Biopharmaceutics Review(s) Application Number: 202324Orig1s000. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202324Orig1s000ClinPharmR.pdf (accessed on 15 February 2021).
- Muya, L.; Kansara, V.; Ciulla, T. Pharmacokinetics and Ocular Tolerability of Suprachoroidal CLS-AX (axitinib injectable suspension) in rabbits. Invest. Ophthalmol. Vis. Sci. 2020, 61, 4925. [Google Scholar]
- Biomedical, C. Clearside Biomedical Announces First Patients Enrolled in Phase 1/2a Clinical Trial of CLS-AX (axitinib injectable suspension) for the Treatment of Wet AMD. Available online: https://ir.clearsidebio.com/news-releases/news-release-details/clearside-biomedical-announces-first-patients-enrolled-phase-12a (accessed on 1 February 2021).
- Hancock, S.; Phadke, A.; Kansara, V.; Boyer, D.; Rivera, J.; Marlor, C.; Prodos, S.; Wiles, J.; McElheny, R.; Ciulla, T.A.; et al. Ocular Pharmacokinetics and Safety of Suprachoroidal A01017, Small Molecule Complement Inhibitor, Injectable Suspension in Rabbits. Invest. Ophthalmol. Vis. Sci. 2020, 61, 3694. [Google Scholar]
- Muya, L. Pharmacokinetics and Ocular Tolerability of suprachoroidal BCX4161 suspension, a selective plasma kallikrein inhibitor, in rabbits. Invest. Ophthalmol. Vis. Sci. 2021, 61, 4925. [Google Scholar]
- Prieto, E.; Cardiel, M.J.; Vispe, E.; Idoipe, M.; Garcia-Martin, E.; Fraile, J.M.; Polo, V.; Mayoral, J.A.; Pablo, L.E.; Rodrigo, M.J. Dexamethasone delivery to the ocular posterior segment by sustained-release Laponite formulation. Biomed. Mater. 2020. [Google Scholar] [CrossRef]
- Prieto, E.; Vispe, E.; De Martino, A.; Idoipe, M.; Rodrigo, M.J.; Garcia-Martin, E.; Fraile, J.M.; Polo-Llorens, V.; Mayoral, J.A. Safety study of intravitreal and suprachoroidal Laponite clay in rabbit eyes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 535–546. [Google Scholar] [CrossRef]
- Das, S.S.; Neelam; Hussain, K.; Singh, S.; Hussain, A.; Faruk, A.; Tebyetekerwa, M. Laponite-based Nanomaterials for Biomedical Applications: A Review. Curr. Pharm. Des. 2019, 25, 424–443. [Google Scholar] [CrossRef]
- Saliba, J.B.; Vieira, L.; Fernandes-Cunha, G.M.; Da Silva, G.R.; Fialho, S.L.; Silva-Cunha, A.; Bousquet, E.; Naud, M.C.; Ayres, E.; Oréfice, R.L.; et al. Anti-inflammatory effect of dexamethasone controlled released from anterior suprachoroidal polyurethane implants on endotoxin-induced uveitis in rats. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1671–1679. [Google Scholar] [CrossRef]
- Alhasani, R.H.; Almarhoun, M.; Zhou, X.; Reilly, J.; Patterson, S.; Zeng, Z.; Shu, X. Tauroursodeoxycholic acid protects retinal pigment epithelial cells from oxidative injury and endoplasmic reticulum stress in vitro. Biomedicines 2020, 8, 367. [Google Scholar] [CrossRef]
- Mantopoulos, D.; Murakami, Y.; Comander, J.; Thanos, A.; Roh, M.; Miller, J.W.; Vavvas, D.G. Tauroursodeoxycholic acid (TUDCA) protects photoreceptors from cell death after experimental retinal detachment. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Olsen, T.W.; Dyer, R.B.; Mano, F.; Boatright, J.H.; Chrenek, M.A.; Paley, D.; Wabner, K.; Schmit, J.; Chae, J.B.; Sellers, J.T.; et al. Drug Tissue Distribution of TUDCA From a Biodegradable Suprachoroidal Implant versus Intravitreal or Systemic Delivery in the Pig Model. Transl. Vis. Sci. Technol. 2020, 9, 11. [Google Scholar] [CrossRef]
- PubChem—Acriflavine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acriflavine (accessed on 1 February 2021).
- Hackett, S.F.; Fu, J.; Kim, Y.C.; Tsujinaka, H.; Shen, J.; Lima e Silva, R.; Khan, M.; Hafiz, Z.; Wang, T.; Shin, M.; et al. Sustained delivery of acriflavine from the suprachoroidal space provides long term suppression of choroidal neovascularization. Biomaterials 2020, 243, 119935. [Google Scholar] [CrossRef]
- Zeng, M.; Shen, J.; Liu, Y.; Lu, L.Y.; Ding, K.; Fortmann, S.D.; Khan, M.; Wang, J.; Hackett, S.F.; Semenza, G.L.; et al. The HIF-1 antagonist acriflavine: Visualization in retina and suppression of ocular neovascularization. J. Mol. Med. 2017, 95, 417–429. [Google Scholar] [CrossRef]
- Shen, J.; Zeng, M.; Ding, K.; Lu, L.; Formica, R.; Liu, Y.; Hackett, S.; Campochiaro, P.A. The HIF-1 inhibitor acriflavine is visualized in retina after multiple modes of administration/doses that suppress ocular neovascularization. Invest. Ophthalmol. Vis. Sci. 2016, 57, 4537. [Google Scholar]
- Chiang, B.; Kim, Y.C.; Doty, A.C.; Grossniklaus, H.E.; Schwendeman, S.P.; Prausnitz, M.R. Sustained reduction of intraocular pressure by supraciliary delivery of brimonidine-loaded poly(lactic acid) microspheres for the treatment of glaucoma. J. Control. Release 2016, 228, 48–57. [Google Scholar] [CrossRef]
- Chae, J.J.; Jung, J.H.; Zhu, W.; Gerberich, B.G.; Bahrani Fard, M.R.; Grossniklaus, H.E.; Ethier, C.R.; Prausnitz, M.R. Drug-Free, Nonsurgical Reduction of Intraocular Pressure for Four Months after Suprachoroidal Injection of Hyaluronic Acid Hydrogel. Adv. Sci. 2020, 2001908. [Google Scholar] [CrossRef]
- Ochakovski, G.A.; Ulrich Bartz-Schmidt, K.; Fischer, M.D. Retinal gene therapy: Surgical vector delivery in the translation to clinical trials. Front. Neurosci. 2017, 11, 174. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, L.; Zhou, Y. Subretinal Injection: A Review on the Novel Route of Therapeutic Delivery for Vitreoretinal Diseases. Ophthalmic Res. 2017, 58, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kansara, V.; Muya, L.; Wan, C.; Ciulla, T.A. Suprachoroidal Delivery of Viral and Nonviral Gene Therapy for Retinal Diseases. J. Ocul. Pharmacol. Ther. 2020, 36, 384–392. [Google Scholar] [CrossRef]
- Kansara, V.; Yoo, J.; Cooper, M.J.; Laird, O.S.; Taraborelli, D.; Moen, R.; Noronha, G. Suprachoroidally delivered non-viral DNA nanoparticles transfect chorioretinal cells in non-human primates and rabbits. Invest. Ophthalmol. Vis. Sci. 2019, 60, 2909. [Google Scholar]
- Taraborelli, D.; Noronha, G.; Yoo, J.; Laird, O.; Cooper, M. A One-week Study to Evaluate Safety, Tolerability, and Retinal Cell Transfection of Non-viral DNA Nanoparticles Administered by Suprachoroidal Injection. Available online: https://www.clearsidebio.com/news/a-one-week-study-to-evaluate-safety-tolerability-and-retinal-cell-transfection-of-non-viral-dna-nanoparticles-administered-by-suprachoroidal-injection (accessed on 2 December 2020).
- Chung, S.H.; Mollhoff, I.N.; Mishra, A.; Sin, T.-N.; Ngo, T.; Ciulla, T.; Sieving, P.; Thomasy, S.M.; Yiu, G. Host immune responses after suprachoroidal delivery of AAV8 in nonhuman primate eyes. bioRxiv 2020. [Google Scholar] [CrossRef]
- Yiu, G.; Mollhoff, I.; Chung, S.H.; Nguyen, T.; Thomasy, S.; Yoo, J.; Taraborelli, D.; Noronha, G. Suprachoroidal Injection of AAV8 for Ocular Gene Delivery in the Nonhuman Primate. Invest. Ophthalmol. Vis. Sci. 2019, 60, 2904. [Google Scholar]
- Han, I.C.; Cheng, J.L.; Burnight, E.R.; Ralston, C.L.; Fick, J.L.; Thomsen, G.J.; Tovar, E.F.; Russell, S.R.; Sohn, E.H.; Mullins, R.F.; et al. Retinal Tropism and Transduction of Adeno-Associated Virus Varies by Serotype and Route of Delivery (Intravitreal, Subretinal, or Suprachoroidal) in Rats. Hum. Gene Ther. 2020. [Google Scholar] [CrossRef]
- Ding, K.; Shen, J.; Hafiz, Z.; Hackett, S.F.; Lima e Silva, R.; Khan, M.; Lorenc, V.E.; Chen, D.; Chadha, R.; Zhang, M.; et al. AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. J. Clin. Invest. 2019. [Google Scholar] [CrossRef]
- Shen, J.; Kim, J.; Tzeng, S.Y.; Ding, K.; Hafiz, Z.; Long, D.; Wang, J.; Green, J.J.; Campochiaro, P.A. Suprachoroidal gene transfer with nonviral nanoparticles. Sci. Adv. 2020, 6, eaba1606. [Google Scholar] [CrossRef]
- Woodard, K.T.; Vance, M.; Gilger, B.; Samulski, R.J.; Hirsch, M. 544. Comparison of AAV Serotype2 Transduction by Various Delivery Routes to the Mouse Eye. Mol. Ther. 2016, 24, S217–S218. [Google Scholar] [CrossRef]
- Martorana, G.; Levine, M.; Peden, M.C.; Boye, S.; Lukowski, Z.; Min, J.; Meyers, C.; Boye, S.L.; Sherwood, M.B. Comparison of Suprachoroidal delivery via an Ab-Externo approach with the iTrack Microcatheter versus Vitrectomy and subretinal delivery for 3 different AAV Serotypes for Gene Transfer to the Retina. Invest. Ophthalmol. Vis. Sci. 2012, 53, 1931. [Google Scholar]
- Peden, M.C.; Min, J.; Meyers, C.; Lukowski, Z.; Li, Q.; Boye, S.L.; Levine, M.; Hauswirth, W.W.; Ratnakaram, R.; Dawson, W.; et al. Ab-externo AAV-mediated gene delivery to the suprachoroidal space using a 250 micron flexible microcatheter. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed]
- Kompella, U.B.; Amrite, A.C.; Pacha Ravi, R.; Durazo, S.A. Nanomedicines for back of the eye drug delivery, gene delivery, and imaging. Prog. Retin. Eye Res. 2013, 36, 172–198. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.E.; Wan, C.-R.; Fisher, N.E.; Andino, R.V.; Ciulla, T.A. Biomechanics of suprachoroidal drug delivery: From benchtop to clinical investigation in ocular therapies. Expert Opin. Drug Deliv. 2021, 1–12. [Google Scholar] [CrossRef]
- REGENXBIO Announces Dosing of First Patient in Phase II AAVIATETM Trial of RGX-314 for the Treatment of Wet AMD Using Suprachoroidal Delivery | REGENXBIO Inc. Available online: https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-announces-dosing-first-patient-phase-ii-aaviatetm (accessed on 2 October 2020).
- REGENXBIO Reports Third Quarter 2020 Financial Results and Operational Highlights|REGENXBIO Inc. Available online: https://regenxbio.gcs-web.com/news-releases/news-release-details/regenxbio-reports-third-quarter-2020-financial-results-and (accessed on 2 December 2020).
- Kansara, V.S.; Cooper, M.; Sesenoglu-Laird, O.; Muya, L.; Moen, R.; Ciulla, T.A. Suprachoroidally Delivered DNA Nanoparticles Transfect Retina and Retinal Pigment Epithelium/Choroid in Rabbits. Transl. Vis. Sci. Technol. 2020, 9, 21. [Google Scholar] [CrossRef]
- Chiang, B.; Venugopal, N.; Edelhauser, H.F.; Prausnitz, M.R. Distribution of particles, small molecules and polymeric formulation excipients in the suprachoroidal space after microneedle injection. Exp. Eye Res. 2016, 153, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Chiang, B.; Wang, K.; Ross Ethier, C.; Prausnitz, M.R. Clearance kinetics and clearance routes of molecules from the suprachoroidal space after microneedle injection. Investig. Ophthalmol. Vis. Sci. 2017, 58, 545–554. [Google Scholar] [CrossRef]
- Savinainen, A.; Grossniklaus, H.; Kang, S.; Rasmussen, C.; Bentley, E.; Krakova, Y.; Struble, C.B.; Rich, C. Ocular distribution and efficacy after suprachoroidal injection of AU-011 for treatment of ocular melanoma. Invest. Ophthalmol. Vis. Sci. 2020, 61, 3615. [Google Scholar]
- Aura Biosciences Announces Dosing of First Patient in Phase 2 Study Evaluating Suprachoroidal Administration of AU-011 in Patients with Choroidal Melanoma | Aura Biosciences. Available online: http://www.aurabiosciences.com/news-archive/2020/6/12/aura-biosciences-presents-updated-au-011-clinical-data-at-arvo-2020-njm82 (accessed on 2 December 2020).
- Tzameret, A.; Ketter-Katz, H.; Edelshtain, V.; Sher, I.; Corem-Salkmon, E.; Levy, I.; Last, D.; Guez, D.; Mardor, Y.; Margel, S.; et al. In vivo MRI assessment of bioactive magnetic iron oxide/human serum albumin nanoparticle delivery into the posterior segment of the eye in a rat model of retinal degeneration. J. Nanobiotechnology 2019, 17, 3. [Google Scholar] [CrossRef]
- Chiang, B.; Kim, Y.C.; Edelhauser, H.F.; Prausnitz, M.R. Circumferential flow of particles in the suprachoroidal space is impeded by the posterior ciliary arteries. Exp. Eye Res. 2016, 145, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Chiang, B.; Grossniklaus, H.E.; Prausnitz, M.R. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J. Control. Release 2018, 277, 14–22. [Google Scholar] [CrossRef]
- Kim, Y.C.; Hee Oh, K.; Edelhauser, H.F.; Prausnitz, M.R.; Oh, K.H.; Edelhauser, H.F.; Prausnitz, M.R. Formulation to target delivery to the ciliary body and choroid via the suprachoroidal space of the eye using microneedles. Eur J Pharm Biopharm 2015, 95, 398–406. [Google Scholar] [CrossRef]
- Olsen, T.W.; Feng, X.; Wabner, K.; Csaky, K.; Pambuccian, S.; Douglas Cameron, J.; Cameron, J.D. Pharmacokinetics of Pars Plana Intravitreal Injections versus Microcannula Suprachoroidal Injections of Bevacizumab in a Porcine Model. Investig. Opthalmology Vis. Sci. 2011, 52, 4749. [Google Scholar] [CrossRef]
- Valamanesh, F.; Jeanny, J.-C.; Savoldelli, M.; Naud, M.C.; Behar-Cohen, F. Effects Of Suprachoroidal Bevacizumab (Avastin®) On The Posterior Segment Of The Eye. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3119. [Google Scholar]
- Patel, S.R.; Kissner, J.; Farjo, R.; Zarnitsyn, V.; Noronha, G. Efficacy of Suprachoroidal Aflibercept in a Laser Induced Choroidal Neovascularization Model. Invest. Ophthalmol. Vis. Sci. 2016, 57, 286. [Google Scholar]
- Orgül, S.; Cioffi, G.A.; Bacon, D.R.; Van Buskirk, E.M. An endothelin-1-induced model of chronic optic nerve ischemia in rhesus monkeys. J. Glaucoma 1996, 5, 135–138. [Google Scholar] [PubMed]
- MacCumber, M.W. Ocular Effects of the Endothelins. Arch. Ophthalmol. 1991, 109, 705. [Google Scholar] [CrossRef]
- MacCumber, M.W.; Ross, C.A.; Snyder, S.H. Endothelin in brain: Receptors, mitogenesis, and biosynthesis in glial cells. Proc. Natl. Acad. Sci. USA 1990, 87, 2359–2363. [Google Scholar] [CrossRef]
- Nork, T.M.; Katz, A.W.; Hennes-Beean, E.A.; Kim, C.B.Y. Suprachoroidal (SC) injection of endothelin-1 (ET-1) in rabbits: A new model of outer retinal ischemia. Invest. Ophthalmol. Vis. Sci. 2019, 60, 1637. [Google Scholar]
- CIULLA, T.A.; PAWLYK, B.S.; HARRIS, A.; OBEROI, A.; MILLER, J.W.; SANDBERG, M.A. Endothelin-1-Mediated Retinal Artery Vasospasm and the Rabbit Electroretinogram. J. Ocul. Pharmacol. Ther. 2000, 16, 393–398. [Google Scholar] [CrossRef]
- OTX-IVT (anti-VEGF antibody implant) – Ocular Therapeutix. Available online: https://www.ocutx.com/research/otx-ivt/ (accessed on 2 December 2020).
- del Pozo-Rodríguez, A.; Delgado, D.; Gascón, A.R.; Solinís, M.Á. Lipid Nanoparticles as Drug/Gene Delivery Systems to the Retina. J. Ocul. Pharmacol. Ther. 2013, 29, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Rajala, A.; Rajala, R. Lipid Nanoparticles for Ocular Gene Delivery. J. Funct. Biomater. 2015, 6, 379–394. [Google Scholar] [CrossRef]
- Tavakoli, S.; Peynshaert, K.; Lajunen, T.; Devoldere, J.; del Amo, E.M.; Ruponen, M.; De Smedt, S.C.; Remaut, K.; Urtti, A. Ocular barriers to retinal delivery of intravitreal liposomes: Impact of vitreoretinal interface. J. Control. Release 2020, 328, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Limoli, P.G.; Vingolo, E.M.; Morales, M.U.; Nebbioso, M.; Limoli, C. Preliminary study on electrophysiological changes after cellular autograft in age-related macular degeneration. Medicine (Baltimore). 2014, 93, e355. [Google Scholar] [CrossRef] [PubMed]
- Tobita, M.; Tajima, S.; Mizuno, H. Adipose tissue-derived mesenchymal stem cells and platelet-rich plasma: Stem cell transplantation methods that enhance stemness. Stem Cell Res. Ther. 2015, 6, 215. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, V.M.; Boyd, N.L. The Adipose Stromal Vascular Fraction as a Complex Cellular Source for Tissue Engineering Applications. Tissue Eng. Part B. Rev. 2018, 24, 289–299. [Google Scholar] [CrossRef]
- Dykstra, J.A.; Facile, T.; Patrick, R.J.; Francis, K.R.; Milanovich, S.; Weimer, J.M.; Kota, D.J. Concise Review: Fat and Furious: Harnessing the Full Potential of Adipose-Derived Stromal Vascular Fraction. Stem Cells Transl. Med. 2017, 6, 1096–1108. [Google Scholar] [CrossRef]
- Limoli, P.G.; Limoli, C.; Vingolo, E.M.; Scalinci, S.Z.; Nebbioso, M. Cell surgery and growth factors in dry age-related macular degeneration: Visual prognosis and morphological study. Oncotarget 2016, 7, 46913–46923. [Google Scholar] [CrossRef] [PubMed]
- Limoli, P.G.; Vingolo, E.M.; Limoli, C.; Scalinci, S.Z.; Nebbioso, M. Regenerative Therapy by Suprachoroidal Cell Autograft in Dry Age-related Macular Degeneration: Preliminary In Vivo Report. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Limoli, P.G.; Vingolo, E.M.; Limoli, C.; Nebbioso, M. Stem Cell Surgery and Growth Factors in Retinitis Pigmentosa Patients: Pilot Study after Literature Review. Biomedicines 2019, 7, 94. [Google Scholar] [CrossRef]
- Limoli, P.G.; Limoli, C.S.S.; Morales, M.U.; Vingolo, E.M. Mesenchymal stem cell surgery, rescue and regeneration in retinitis pigmentosa: Clinical and rehabilitative prognostic aspects. Restor. Neurol. Neurosci. 2020, 38, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Kahraman, N.S.; Öner, A. Umbilical cord-derived mesenchymal stem cell implantation in patients with optic atrophy. Eur. J. Ophthalmol. 2020, 112067212097782. [Google Scholar] [CrossRef]
- Oner, A.; Gonen, Z.B.; Sevim, D.G.; Smim Kahraman, N.; Unlu, M. Suprachoroidal Adipose Tissue-Derived Mesenchymal Stem Cell Implantation in Patients with Dry-Type Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: 6-Month Follow-Up Results of a Phase 2 Study. Cell. Reprogram. 2018, 20, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Oner, A.; Gonen, Z.B.; Sevim, D.G.; Sinim Kahraman, N.; Unlu, M. Six-month results of suprachoroidal adipose tissue-derived mesenchymal stem cell implantation in patients with optic atrophy: A phase 1/2 study. Int. Ophthalmol. 2019, 39, 2913–2922. [Google Scholar] [CrossRef] [PubMed]
- Oner, A.; Sevim, D. Complications of stem cell based therapies in retinal diseases. Stem Cell Res. Open Libr. 2017, 1, 1–7. [Google Scholar]
- Kuriyan, A.E.; Albini, T.A.; Townsend, J.H.; Rodriguez, M.; Pandya, H.K.; Leonard, R.E.; Parrott, M.B.; Rosenfeld, P.J.; Flynn, H.W.; Goldberg, J.L. Vision loss after intravitreal injection of autologous “stem Cells” for AMD. N. Engl. J. Med. 2017, 376, 1047–1053. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).